CHAPTER 129 Central Nervous System Lymphoma
Primary Central Nervous System Lymphoma
Primary CNS lymphoma (PCNSL) is an uncommon variant of extranodal non-Hodgkin’s lymphoma (NHL) that can affect multiple parts of the CNS, including the eyes, brain, leptomeninges, or spinal cord. PCNSL accounts for about 3% of all the primary CNS tumors diagnosed each year in the United States. The incidence of PCNSL increased significantly from 1970 to 2000 largely owing to the human immunodeficiency virus (HIV) pandemic. However, the incidence has stabilized or decreased during the past decade to about 0.47 cases per 100,000 persons.1,2 Congenital or acquired immunodeficiency is the only established risk factor for PCNSL, and HIV-infected individuals are at greater risk for developing this tumor. About 2% to 13% of patients with a previous diagnosis of acquired immunodeficiency syndrome (AIDS) develop PCNSL. Because PCNSL is a rare malignancy, it has been challenging to study, and an effective standard of care has been difficult to establish. Although durable remissions may be achieved for a few years, the tumor relapses in most cases.
Pathobiology
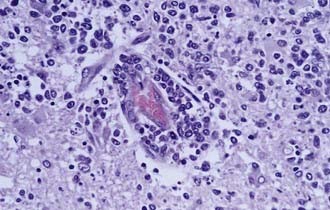
Ninety percent of non–HIV-associated PCNSL is diffuse large B-cell (DLBCL) type, and the remaining 10% are poorly characterized low-grade lymphomas, Burkitt’s lymphomas, or T-cell lymphomas.3 Less is known about these latter, rare variants of PCNSL. The DLBCL type of PCNSL is composed of immunoblasts or centroblasts that have a predilection for blood vessels, resulting in lymphoid clustering around small cerebral vessels (Fig. 129-1). This angiocentric pattern of tumor growth in the CNS is unique for diffuse large B-cell lymphoma. Reactive T-cell infiltrates may also be present in varying degrees, making it difficult for a neuropathologist to discriminate between PCNSL and a reactive process.
PCNSL likely arises from late germinal center or post–germinal center lymphoid cells and may localize to the CNS because of a poorly understood neurotropism.4 Systematic studies of the molecular pathology of PCNSL have rarely been performed largely because of the lack of adequate tumor specimens for research purposes. Most PCNSL patients are diagnosed by stereotactic needle biopsy so that most of the tissue specimen is consumed by the diagnostic evaluation. Despite this limitation, gene expression studies have demonstrated three gene “signatures” associated with PCNSL: germinal center B-cell; activated B-cell, and type 3 large B-cell lymphoma.5 Although these three gene expression patterns parallel systemic DLBCL, there are unique molecular features of PCNSL. For example, extracellular matrix–related genes are upregulated in PCNSL compared with systemic DLBCL.6 Interaction between tumor cells and extracellular matrix proteins specific to the CNS may offer an explanation for the neurotropism of PCNSL.
Several genes associated with interleukin-4 (IL-4), a B-cell growth factor expressed by both tumor vessels and tumor cells, are highly expressed in PCNSL, including X-box binding protein 1 (XBP-1), a regulator of the unfolded protein response (UPR) signaling pathway. The expression of UPR-related genes is important for cell survival under stressful conditions such as hypoxia so that activation of this pathway may promote tumor cell survival in the CNS. STAT6, a mediator of IL-4 signaling, is expressed by tumor cells and tumor endothelia in PCNSL. High expression levels of STAT6 are associated with short survival in PCNSL patients treated with methotrexate (MTX).5
HIV-related PCNSL is typically a large cell lymphoma with immunoblastic and more aggressive features.7,8 These patients are often severely immunocompromised, with CD4 counts less than 50 cells/mm3 at the time of diagnosis, and have had prior AIDS-defining illnesses. Infection by Epstein-Barr virus (EBV), a ubiquitous pathogen normally suppressed by the intact immune system, likely has a role in the pathogenesis of HIV-related PCNSL through transformation of normal B cells into lymphoma cells.
Clinical Features
The presentations of PCNSL in both immunocompromised and immunocompetent patients are similar, with signs of a focal mass lesion in 70% and 61.3% of patients, respectively.9,10 In 248 immunocompetent patients, 43% had neuropsychiatric signs, 33% had increased intracranial pressure, 14% had seizures, and 4% had ocular symptoms.9 In a series of 111 HIV-related PCNSL cases, 43.2% had headache, 21.6% had seizures, and 18% had ataxia.10 HIV-related PCNSL occurs in younger persons with a median age of 31 years, compared with a median age of 60 years in immunocompetent patients.10 Seizures are less common than with other types of brain tumors probably because PCNSL involves predominantly subcortical white matter rather then epileptogenic gray matter. Typically, patients do not present with B symptoms such as fever, weight loss, or night sweats.
Diagnostic Evaluation
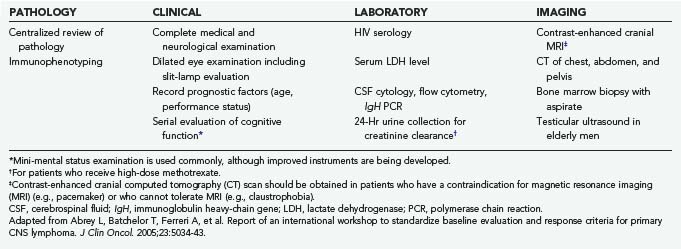
The International PCNSL Collaborative Group (IPCG) has established guidelines for the diagnostic evaluation of a patient with suspected PCNSL (Table 129-1).11 These guidelines establish the extent of disease and confirm that the disease is restricted to the CNS. Physical examination should include palpation of the lymphatic chain as well as testicular examination in males. Contrast-enhanced cranial magnetic resonance imaging (MRI) (contrast cranial computed tomography [CT] if MRI is contraindicated); lumbar puncture if not contraindicated (for cell count, protein, glucose, cytology, immunoglobulin heavy-chain [IgH] gene rearrangement and flow cytometry studies); ophthalmologic examination including slit-lamp evaluation; CT of the chest, abdomen, and pelvis; and bone marrow biopsy should be performed. Blood tests for HIV, complete blood count, basic metabolic panel, and lactate dehydrogenase (LDH) level are also recommended. Testicular ultrasound should be considered in men because testicular lymphoma has a predilection to spread to the CNS.
TABLE 129-1 International Primary Central Nervous System Lymphoma Collaborative Group (IPCG) Guidelines for Baseline Evaluation for Clinical Trials
The search for occult systemic disease has become increasingly important because recent evidence suggests that lymphoma may not be restricted to the nervous system in a subpopulation of patients with CNS lymphoma. Identical polymerase chain reaction (PCR) products of clonally rearranged IgH genes were identified in the bone marrow aspirates, blood samples, and brain tumor biopsy specimens in 2 of 24 patients with “primary” CNS lymphoma in one study. In one of these patients, follow-up IgH PCR 24 months after diagnosis yielded a persistent monoclonal blood product despite a complete radiographic response in the CNS.12 In a retrospective study of 49 PCNSL patients evaluated with body fluorodeoxyglucose (FDG) position emission tomography (PET) studies, extraneural hypermetabolic lesions were identified in 15% of subjects. Subsequent tissue biopsy was performed, and 11% of the lesions were found to be lymphoma, whereas 4% were other types of cancer. PET may play an increasingly important role in evaluating patients with PCNSL for subclinical systemic disease.13 Prospective, long-term follow-up studies are necessary to further elucidate the frequency and importance of subclinical systemic disease in CNS lymphoma patients and whether the presence of these monoclonal cell populations increase the risk for relapse.
Neuroimaging
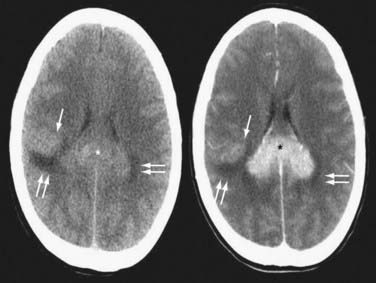
Contrast-enhanced cranial MRI is the imaging modality of choice in evaluating a patient with a suspected diagnosis of PCNSL. If MRI is not possible or is contraindicated, a contrast-enhanced cranial CT scan is recommended. PCNSL tends to enhance homogeneously on both MRI and CT, although in HIV-associated disease, lesions are often ring enhancing (Figs. 129-2 and 129-3; Figs. 129E-1 to 129E-3).7 In immunocompetent PCNSL patients, lesions are solitary in 65% of cases and are located in a cerebral hemisphere (38%), thalamus or basal ganglia (16%), corpus callosum (14%), periventricular region (12%), and cerebellum (9%).14 HIV-related PCNSL is solitary in 48.6% of cases and is localized to the cerebral cortex in 65%, the periventricular region in 56%, the basal ganglia in 33%, the cerebellum in 7%, and the brainstem in 4%.10 Isolated spinal cord involvement is rare and is observed in less than 1% of cases, so spinal imaging is necessary only if warranted based on clinical suspicion or to screen for leptomeningeal involvement if lumbar puncture cannot be performed.
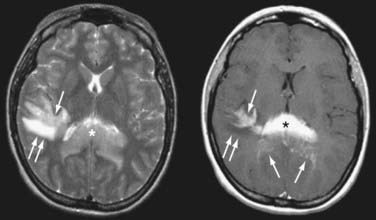
FIGURE 129-3 Axial magnetic resonance imaging (MRI) of the same patient in Figure 129-2. Axial T2-weighted MRI (left) shows that the splenial (asterisk) and right parietal (single arrow) lesions are isointense with respect to gray matter. Right parietal edema is T2-hyperintense (double arrows). Axial T1-weighted MRI after contrast (right) shows that the lesions enhance intensely. Linear-enhancing structures emanating from the corpus callosum indicate tumor spread through the Virchow-Robin perivascular spaces in a pattern characteristic of primary central nervous system lymphoma (single arrows). Adjacent edema is T1-hypointense (double arrows).
(From Batchelor TT, Buchbinder BR, Harris NL: case records of Massachusetts General Hospital. Weekly Clinicopathological exercises. Case 1-2005. A 35-year-old woman with difficulty walking, headache, and nausea. N Engl J Med. 2005;352:185-194.)
Prognostic Markers
Different prognostic scoring systems have been proposed. In a large retrospective review, the International Extranodal Lymphoma Study Group (IELSG) identified age older than 60 years, Eastern Cooperative Oncology Group (ECOG) performance status higher than 1, elevated serum LDH level, high cerebrospinal fluid (CSF) protein concentration, and involvement of deep regions of the brain as independent predictors of worse prognosis.15 In patients with 0 to 1 factors, the 2-year survival rate was 80%; in patients with 2 or 3 factors, the 2-year survival was 48%; and in patients with 4 or 5 factors, the 2-year survival was 15%. Another group of investigators has proposed a prognostic model that divides PCNSL patients into three groups based on age and performance status: those younger than 50 years; those older than 50 years with a Karnofsky performance score (KPS) higher than 70; and those younger than 50 years with a KPS lower than 70. Based on these divisions, significant differences in overall and failure-free survival were observed. These variables are easily obtained, so this model may prove useful for risk stratification in future clinical trials.16
The search for biomarkers of prognosis for patients with PCNSL is an active area of investigation. BCL-6, a tumor suppressor gene expressed in 22% to 100% of patients, has been associated with improved prognosis.17–19 Both progression-free survival (20.5 months versus 10.1 months)20 and overall survival (101 months versus 14.7 months)17,21 are longer in PCNSL patients with BCL-6 expression. These findings are consistent with the observation that BCL-6 expression is a favorable prognostic marker in patients with systemic NHL.19,22,23
Treatment of Immunocompetent Patients with Primary Central Nervous System Lymphoma
Surgery
Gross-total resection of tumor is typically not possible in patients with PCNSL because of the infiltrative nature of the tumor.24 In addition, PCNSL may be multifocal, involving the leptomeninges, eyes, or deep regions of the brain and making complete removal unfeasible. Median survival after surgery alone is only 1 to 4 months.25 Consequently, when the diagnosis is achieved after a stereotactic biopsy, further surgery is not necessary.
Corticosteroids
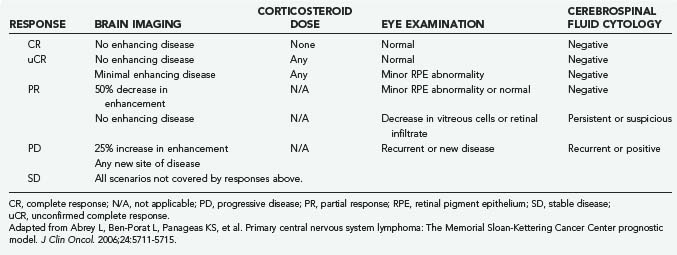
Corticosteroids may produce tumor regression on neuroimaging in 40% of patients, likely through direct lymphocytolysis and reduced tumor-associated edema.26 For this reason, corticosteroids should be withheld, if possible, before a diagnostic biopsy because these drugs may disrupt cellular morphology and lead to diagnostic inaccuracy at the time of microscopic analysis. Despite an initial response to corticosteroids, patients quickly relapse and require alternate treatment strategies. Nevertheless, initial radiographic response to corticosteroids in newly diagnosed PCNSL patients is a favorable prognostic marker, with survival of 117 months in responders compared with 5.5 months in nonresponders (Table 129-2).27
Radiation
Whole-brain radiation therapy (WBRT) was historically the modality of choice to treat PCNSL given the multifocal and infiltrative nature of the tumor. However, WBRT alone is inadequate therapy for PCNSL patients, particularly those with CSF dissemination of their tumor. Initial radiographic response to WBRT is observed in 90% of PCNSL patients, but relapse usually occurs within a few months.28 In patients receiving WBRT alone without chemotherapy, median survival varies from 12 to 18 months and 5-year survival rates from 18% to 35%.29,30 A radiation dose-response relationship exists for PCNSL because dose reduction from 45 Gy to 30 Gy increased relapse risk in one nonrandomized study.31 Although WBRT is effective for initial control of disease, it produces delayed neurotoxicity, especially in patients older than 60 years. For this reason, WBRT is often deferred in newly diagnosed PCNSL patients older than 60 years.
Combined-Modality Therapy
Given the disappointing outcomes with surgery or radiation alone, chemotherapy was added to WBRT in an attempt to improve survival (combined-modality therapy). Numerous studies with various chemotherapy drugs have been reported in PCNSL, and there is no compelling evidence for the superiority of any one regimen. The blood-brain barrier (BBB) is an obstacle for chemotherapeutic drugs that are hydrophilic or for those with a high molecular weight and may limit the efficacy of such drugs in CNS tumors, including PCNSL. MTX, a folate antagonist that interferes with DNA synthesis, is the most commonly used agent for PCNSL but has limited penetration into the CNS because of a high degree of ionization at physiologic pH. Using microdialysis catheters, Olson and colleagues measured the penetration of MTX (12 g/m2) into high-grade brain tumor tissue.32 The ratio of brain extracellular fluid MTX to plasma MTX was only 0.13. This low penetration is an important reason why high doses of systemic MTX are necessary to achieve cytotoxic intratumoral concentrations. Shorter infusion duration also appears to be important because a 3-hour infusion of 100 mg/kg of MTX resulted in greater tumor shrinkage than a 6-hour infusion in one small study.33
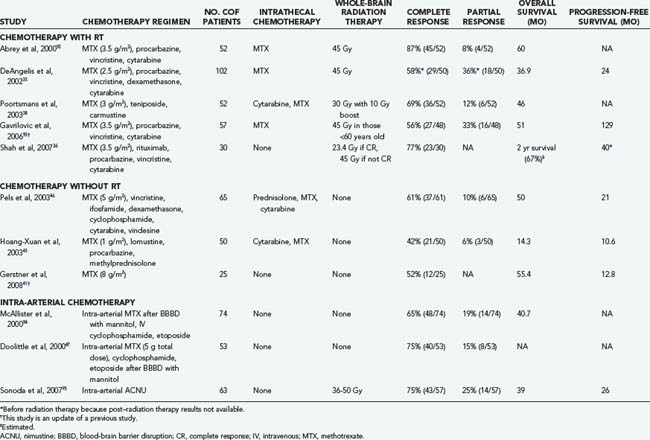
The addition of chemotherapy to WBRT improves survival over WBRT alone in historical comparisons. Combination regimens that include MTX and WBRT are associated with a radiographic response in more than 50% of patients and with a 2-year survival rate of 43% to 73%.26 Omission of MTX is associated with worse survival, so most combined-modality regimens include MTX.34 Most MTX-based regimens are associated with similar survival rates, but the toxicity varies depending on the regimen. One commonly used combination regimen is MTX, vincristine, and procarbazine (MVP) followed by WBRT and cytarabine in the postirradiation setting. This regimen is associated with an overall response rate of 91%, a progression-free survival of 24 months, and an overall survival of 36.9 months.35 Toxicity associated with this regimen was notable and included 8 patient deaths and 12 cases of clinically significant neurotoxicity in the 98 patients studied. A subsequent study tested rituximab with MVP followed by lower dose WBRT (23.4 Gy) if the patient achieved a complete response to chemotherapy or 45 Gy of WBRT if a complete response was not achieved.36 Overall response rate was 93%, and 2-year median progression-free survival was 57%. At a median follow-up of 37 months, none of the patients had experienced treatment-related neurotoxicity, but most patients required growth factor support. Other combined-modality regimens are listed in Table 129-3.37–39 Although the overall response rate reported in these studies is encouraging, the high frequency of treatment-related toxicity is a significant concern.
A common observation from these trials is that patients who respond to initial chemotherapy have improved outcomes. In one study, patients who could not tolerate MTX or failed to achieve a complete response had a median survival of 1.5 months, compared with 56 months in patients who did respond.40 Forty percent of patients in this study did not complete chemotherapy because of toxicity or disease progression, so more tolerable and effective agents are needed in this patient population.
Chemotherapy
In a phase II, multicenter study of 25 patients using intravenous MTX (8 g/m2) alone, the outcomes included a complete response rate of 52%, a median progression-free survival of 12.8 months, and a median overall survival of 55.4 months, but median disease-specific survival had not been reached at 72.3 months.41,42 In this study, 5 of the 25 patients treated with MTX alone achieved a complete response and have not relapsed after a median follow-up of 6.8 years. Duration of maintenance MTX therapy after a patient achieves a complete response remains unclear. Ng and coworkers43 retrospectively reviewed 10 cases of elderly patients treated with MTX alone (8 g/m2 followed by 3.5 g/m2) and found an overall response rate of 90% with a median overall survival of 36 months.43 Toxicity in these studies was modest and manageable, demonstrating that MTX can be both safe and effective in an elderly patient population if used without adjuvant radiation. MTX combined with temozolomide may also be beneficial in elderly patients.44
There have been a large number of phase II trials involving MTX-based, multiagent chemotherapy regimens without WBRT. In patients older than 60 years, a regimen consisting of MTX, lomustine (CCNU), procarbazine, methylprednisolone, intrathecal MTX, and intrathecal cytarabine was associated with a median overall survival of 14.3 months and a decreased risk for neurotoxicity.45 Another regimen including MTX, cytarabine, vincristine, ifosfamide, cyclophosphamide and intrathecal MTX, cytarabine, and prednisolone was associated with a 71% overall response rate and a median overall survival of 50 months. Despite these promising results, however, 6 patients died from treatment-related complications, and 12 patients had Ommaya reservoir infections.46
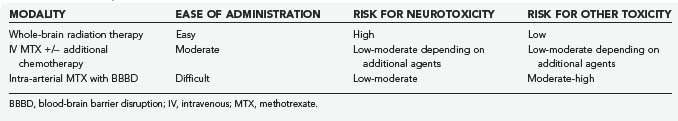
Blood-brain barrier disruption (BBBD) is a strategy aimed at circumventing the BBB to deliver higher concentrations of chemotherapeutics directly to the CNS. Doolittle and associates47 reported complete radiographic responses in 40 of 53 patients with PCNSL treated with intra-arterial MTX (total dose, 5 g), intravenous cyclophosphamide, and etoposide following BBBD with intra-arterial mannitol. Moreover, long-term follow-up of the subjects who achieved complete response with this therapy demonstrated maintenance of cognitive function (patients did not receive WBRT). However, BBBD is technically complex and should be performed only in centers with expertise and experience in the technique (Table 129-4).
Intrathecal Chemotherapy
A controversial issue in the management of PCNSL is the role of intrathecal chemotherapy. Historical comparisons have determined that there appears to be no difference in overall survival when intrathecal MTX is added to regimens that already include high doses of intravenous MTX.48 By administering MTX systemically, the risk for Ommaya placement, extra-CSF drug delivery, chemical meningitis, and infection can be avoided. As mentioned previously, the intravenous dose must be high enough, though, and administered over a rapid enough time interval for MTX to penetrate into the CSF and tumor. For patients with concurrent brain and leptomeningeal lymphoma, intrathecal chemotherapy is often recommended. Ommaya reservoir placement is the most efficient and safest way to deliver intrathecal chemotherapy. Repeated lumbar punctures are uncomfortable for patients and may result in inconsistent delivery of chemotherapy into the subarachnoid space. If a patient requires a ventricular-peritoneal shunt (VPS) and intrathecal chemotherapy, a VPS with an on-off valve is an option. Although not systematically studied, these shunts theoretically allow the physician to temporarily halt CSF drainage into the peritoneum after instillation of chemotherapy.
High-Dose Chemotherapy with Stem Cell Rescue
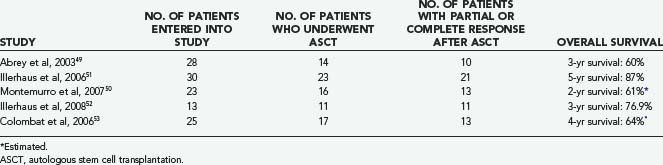
Initial studies of high-dose chemotherapy followed by autologous stem cell transplantation (ASCT) for patients with newly diagnosed PCNSL have involved limited numbers of patients and have yielded mixed results (Table 129-5).49–53 The heterogeneous therapies used and variable outcome measures reported make comparison among trials difficult. However, results are encouraging, and it is likely that high-dose chemotherapy with ASCT will assume an increasingly important role in younger patients with PCNSL in the newly diagnosed and relapsed setting. ASCT may be effective in patients with poor prognostic features as well.54 Neurotoxicity was low in patients younger than 60 years in these studies but aggressive induction chemotherapy appears to be necessary.
Salvage Therapy
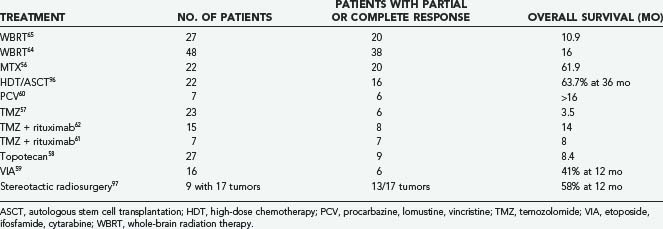
Despite aggressive treatment, most patients with PCNSL progress or relapse and require salvage therapy. Optimal management of relapsed or refractory PCNSL has yet to be determined and has only been studied in small patient series or case reports using heterogeneous therapies. In general, prognosis for patients with relapsed or progressive PCNSL is poor with a median survival of about 4.5 months.55 For patients who initially responded to a chemotherapy regimen that included MTX, retreatment with MTX alone may be effective.56 Temozolomide; topotecan; etoposide (VP-16), ifosfamide, and cytarabine (Ara-C) (VIA); high-dose chemotherapy followed by ASCT; and procarbazine, CCNU, and vincristine (PCV) have all been studied in patients with relapsed or refractory PCNSL with varying results, as summarized in Table 129-6.57–60
Rituximab, a monoclonal antibody to CD20, has been administered in combination with temozolomide in two studies of relapsed or progressive PCNSL, yielding a median survival of 8 and 14 months.61,62 Intraventricular rituximab (10 to 25 mg) was determined to be feasible in a phase I study of patients with relapsed or refractory lymphomatous meningitis, but further testing is needed.63 An unexpected observation in this study was a radiographic response of brain parenchymal lymphoma in one case and two patients with intraocular lymphoma who experienced disease resolution or clinical improvement in vision. Further studies are planned with rituximab including patients with brain parenchymal lymphoma.
Radiation as a salvage therapy has also been explored. Following WBRT, 74% to 79% of patients with relapsed or refractory PCNSL can achieve a radiographic response.64,65 Median survival after radiation treatment is 10.9 to 16 months, with patients younger than 60 years faring better. These results with WBRT as salvage therapy are comparable to the results when WBRT alone is used in the newly diagnosed PCNSL setting.
Neurotoxicity
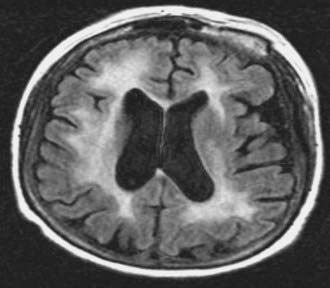
Neurotoxicity is a well-known, delayed consequence of treatment of PCNSL with chemoradiation (WBRT + chemotherapy) or WBRT alone. It is more common in patients older than 60 years and typically presents as a subcortical dementia with gait ataxia and incontinence. MRI changes associated with neurotoxicity include periventricular white matter abnormalities, cortical atrophy, and ventricular enlargement (Fig. 129-4). These changes may appear 6 to 12 months after WBRT, but it is important to note that radiographic changes do not always correlate with clinical symptoms that may appear earlier. Pathologic studies have demonstrated demyelination, neuronal loss, gliosis, and rarefaction of the white matter.66 Large vessel atherosclerosis has been observed as well, implicating vascular injury and resultant tissue ischemia as one possible mechanism for neurotoxicity. Although the pathophysiology of treatment-related neurotoxicity is multifactorial, toxicity to neural progenitor cells is likely to play a pivotal role.67
Studies examining neurotoxicity have several methodologic limitations including lack of baseline evaluations, different definitions of cognitive impairment, and small patient sample sizes.68 In one study of PCNSL patients, the 5-year cumulative incidence of neurotoxicity was 24%, and the use of WBRT was the only significant predictor of development of neurotoxicity on multivariate analysis.69 This is in contrast to chemotherapy alone in which less decline in cognitive function is observed despite evidence of white matter changes on MRI.68,70,71 The cognitive domains typically affected in patients with neurotoxicity are attention, executive function, memory (particularly verbal), and psychomotor speed. Unfortunately, there is no effective treatment for neurotoxicity, and patients often die from complications of neurotoxicity without evidence of recurrent lymphoma. To better assess cognitive function as an end point in this patient population, the IPCG has proposed a battery of psychometric tests for inclusion in all prospective PCNSL clinical trials.68
Treatment of Human Immunodeficiency Virus–Related Primary Central Nervous System Lymphoma
PCNSL is the second most common mass lesion in the brain of HIV-infected persons after toxoplasmosis. Therefore, the typical approach to an HIV-infected patient with an enhancing brain mass is to initially treat the patient with antitoxoplasmosis drugs for 2 weeks. If the patient fails to improve after 2 weeks, a biopsy is performed to determine if the patient has PCNSL. This approach highlights the importance of withholding steroids for as long as possible because steroids may render a biopsy for PCNSL nondiagnostic because of tumor cell lysis. EBV is closely associated with HIV-related PCNSL, leading to interest in a diagnostic test for EBV infection as an alternative to brain biopsy. CSF PCR for EBV is less invasive than a brain biopsy, but the positive predictive value of CSF PCR for EBV is only 10% to 50%, and the specificity is 66% to 90%.72,73 Thus, brain biopsy is recommended for definitive diagnosis.
After the adoption of highly active antiretroviral therapy (HAART), the proportion of HIV-infected patients with PCNSL dropped from 28% to 17% in one study.74 This is likely because fewer patients are progressing to such a severe immunocompromised state, reducing the likelihood of latent EBV reactivation in the CNS. Before the introduction of HAART in 1996, median survival of HIV-infected patients with PCNSL was only 2.1 to 2.6 months with radiation alone.7,75 Adding two or more antiretroviral agents to WBRT can prolong survival.10 Skiest and Crosby76 showed that six of the seven PCNSL patients who received HAART were still alive at a median follow-up of 22 months. In this study, WBRT prolonged survival but not as much as adding WBRT to HAART. A survival benefit was observed in patients whose viral load dropped and in those whose CD4 count increased to more than 50 cells/mm3 with treatment. A significant problem in these studies is the potential for selection bias. Patients who are treated are usually stable enough to undergo WBRT or tolerate HAART and may represent a healthier, more compliant subset of patients.
Chemotherapy has been used in HIV-infected patients as well, despite a concern that it will exacerbate immunosuppression. In 10 AIDS patients with histologically confirmed PCNSL treated with MTX (3 g/m2), the median survival was 9.7 months.77 In another study, thiotepa and procarbazine were added to MTX, and this regimen was associated with a median survival of 3.5 months in all patients and 7 months in patients who completed the entire course of treatment.78
Considering the association between EBV and HIV-related PCNSL, there has been interest in the application of EBV-directed therapies in this patient population. Ganciclovir, a nucleoside analogue with efficacy against EBV, reduced EBV DNA load in CSF and prolonged survival in HIV-positive patients with PCNSL.79 Another report of four patients showed promising results with intravenous zidovudine, ganciclovir, and interleukin-2 followed by oral ganciclovir, patient-specific HAART, and subcutaneous interleukin-2. After 4 years of follow-up, one patient was still in complete response.80 There is a critical need to conduct prospective studies of novel therapeutics to improve survival in patients with HIV-related PCNSL.
Secondary Central Nervous System Lymphoma
CNS dissemination of lymphoma is much more common in NHL than in Hodgkin’s lymphoma. Specific groups of patients with NHL who are at higher risk for CNS dissemination have been identified and include those with extranodal lymphoma such as testicular lymphoma or Burkitt’s lymphoma. The role of prophylactic intrathecal chemotherapy for these high-risk cases remains controversial but is recommended by some authorities.81
More than 60% of secondary NHL cases involve the leptomeninges whereas isolated brain parenchymal lesions are rare.82 Median time to isolated brain relapse was reported to be 1.8 years in patients who achieved complete remission in the body to systemic therapy for NHL and most cases (67%) relapsed within 3 years of the diagnosis of systemic NHL.82 Lymphoma cells may reach the leptomeninges by direct extension from local metastases, by hematologic dissemination, or by perineural tracking along cranial or peripheral nerves. When cancer cells gain access to the CSF, there is dissemination throughout the CSF axis by bulk flow. Tumor cells typically settle in the basal cisterns, posterior fossa, and cauda equina because of slower CSF flow in these areas. These deposits may then become sources for continuous shedding of malignant cells into the CSF.
Treatment
The prognosis for patients with LM or secondary parenchymal CNS lymphoma is poor, with a survival of less than 5 months if the patient presents with relapsed disease. Consequently, treatment is often palliative, although some patients do respond and can achieve a durable remission with therapy, especially those younger than 60 years and those who receive MTX-based chemotherapy.82 Radiation has a limited, palliative role in LM with minimal impact on overall survival but is often indicated if there is bulky or symptomatic disease visible by MRI. Intrathecal chemotherapy by Ommaya reservoir with MTX, thiotepa, or cytarabine is considered standard treatment for LM. The liposomal formulation of cytarabine extends the half-life of the drug from 3 hours to 141 hours, allowing for treatment twice a month compared with twice a week with MTX, thiotepa, or nonliposomal cytarabine. In a small randomized study of 28 patients with LM, liposomal cytarabine was associated with an improved cytologic response rate when compared with standard cytarabine.83 Intrathecal rituximab is under investigation as a potential treatment for LM in NHL patients.63 Alternatively, systemic chemotherapies that incorporate intravenous high-dose MTX therapy may result in cytologic responses.84,85 High-dose MTX-based chemotherapy or WBRT, or both, are typically used in patients with brain parenchymal relapses. Myeloablative therapies with stem cell transplantation have been studied in small trials and show some promise with complete response rates ranging from 23% to 61%.86,87
Central Nervous System Posttransplantation Lymphoproliferative Disorder
Posttransplantation lymphoproliferative disorder (PTLD) of the CNS (CNS-PTLD) arises in the setting of immunosuppression and is similar to PCNSL in that it is typically an aggressive, angiocentric B-cell tumor. More often than not, the tumor is associated with EBV infection. CNS-PTLD occurs in less than 7% of transplant recipients and may develop months to years after transplantation.88 Neuroimaging typically reveals multifocal, heterogeneously enhancing brain masses with extensive tumor-associated edema. Treatment consists of reduced immunosuppression, radiation, chemotherapy (e.g., MTX), antivirals, or rituximab.89 Survival is poor in patients who do not respond to reduction of immunosuppression or are older than 40 years. Some patients with isolated CNS involvement may respond to rituximab, radiation, or MTX-based chemotherapy.89,90
Neurolymphomatosis
Although, strictly speaking, neurolymphomatosis does not involve the CNS, the evaluation and treatment are similar to those for PCNSL. In neurolymphomatosis, lymphoma cells infiltrate peripheral or cranial nerves or the cervical or lumbar plexuses. The disease is rare, and symptoms may be present for months to years before a diagnosis is achieved. Unfortunately, many patients are not diagnosed until after death.91 Patients can present with painful or painless neuropathies, and MRI may show enlarged, enhancing nerves often visualized on coronal, fat-suppressed, spinal images. Not all cases enhance after contrast (only 40% in the largest series reported), and the changes can be hard to distinguish from inflammatory diseases. Treatment involves corticosteroids, MTX, focal radiation, or potentially rituximab.
Abrey L, Batchelor T, Ferreri A, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23:5034-5043.
Abrey L, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24:1281-1288.
Baehring JM, Damek D, Martin EC, et al. Neurolymphomatosis. Neuro Oncol. 2003;5:104-115.
DeAngelis L, Seiferheld W, Schold S, et al. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol. 2002;20:4643-4648.
Donahue BR, Sullivan JW, Cooper JS. Additional experience with empiric radiotherapy for presumed human immunodeficiency virus-associated primary central nervous system lymphoma. Cancer. 1995;76:328-332.
Enting R, Demopoulos A, DeAngelis L, Abrey L. Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide. Neurology. 2004;63:901-903.
Ferreri A, Abrey L, Blay J, et al. Summary statement on primary central nervous system lymphomas from the Eighth International Conference on Malignant Lymphoma, Lugano, Switzerland, June 12 to 15, 2002. J Clin Oncol. 2003;21:2407-2414.
Ferreri A, Blay J, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21:266-272.
Gerstner ER, Carson K, Grossman S, Batchelor TT. Long-term outcome in PCNSL patients treated with high-dose methotrexate and deferred radiation. Neurology. 2008;70:401-402.
Hoang-Xuan K, Taillandier L, Chinot O, et al. Chemotherapy alone as initial treatment for primary CNS lymphoma in patients older than 60 years: a multicenter phase II study (26952) of the European Organization for Research and Treatment of Cancer Brain Tumor Group. J Clin Oncol. 2003;21:2726-2731.
Hodson D, Bowles K, Cooke L, et al. Primary central nervous system lymphoma: a single-centre experience of 55 unselected cases. Clin Oncol (R Coll Radiol). 2005;17:185-191.
Hollender A, Kvaloy S, Nome O, Skovlund E, et al. Central nervous system involvement following diagnosis of non-Hodgkin’s lymphoma: a risk model. Ann Oncol. 2002;13:1099-1107.
Ivers L, Kim A, Sax P. Predictive value of polymerase chain reaction of cerebrospinal fluid for detection of Epstein-Barr virus to establish the diagnosis of HIV-related primary central nervous system lymphoma. Clin Infect Dis. 2004;38:1629-1632.
Jahnke K, Hummel M, Korfel A, et al. Detection of subclinical systemic disease in primary CNS lymphoma by polymerase chain reaction of the rearranged immunoglobulin heavy-chain genes. J Clin Oncol. 2006;24:4754-4757.
Jahnke K, Thiel E, Martus P, et al. Relapse of primary central nervous system lymphoma: clinical features, outcome and prognostic factors. J Neurooncol. 2006;80:159-165.
Monje M, Mizumatsu S, Fike J, Palmer T. Irradiation induces neural precursor cell dysfunction. Nat Med. 2002;8:955-962.
Rubenstein J, Fridlyand J, Shen A, et al. Gene expression and angiotropism in primary CNS lymphoma. Blood. 2006;107:3716-3723.
Shenkier TN, Voss N, Chhanabhai M, et al. The treatment of primary central nervous system lymphoma in 122 immunocompetent patients: a population-based study of successively treated cohorts from the British Colombia Cancer Agency. Cancer. 2005;103:1008-1017.
Tun HW, Personett D, Baskerville KA, et al. Pathway analysis of primary central nervous system lymphoma. Blood. 2008;111:3200-3210.
Yamanaka R, Morii K, Shinbo Y, et al. Modified ProMACE-MOPP hybrid regimen with moderate-dose methotrexate for patients with primary CNS lymphoma. Ann Hematol. 2005;84:447-455.
1 Kadan-Lottick N, Sklusarek M, Gurney J. Decreasing incidence rates of primary central nervous system lymphoma. Cancer. 2002;95:193-202.
2 Panageas KS, Elkin EB, DeAngelis LM, et al. Trends in survival from primary central nervous system lymphoma, 1975-1999: a population-based analysis. Cancer. 2005;104:2466-2472.
3 Miller DC, Hochberg FH, Harris NL, et al. Pathology with clinical correlations of primary central nervous system non-Hodgkin’s lymphoma. The Massachusetts General Hospital experience 1958-1989. Cancer. 1994;74:1383-1397.
4 Camilleri-Broët S, Crinière E, Broët P, et al. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood. 2006;107:190-196.
5 Rubenstein J, Fridlyand J, Shen A, et al. Gene expression and angiotropism in primary CNS lymphoma. Blood. 2006;107:3716-3723.
6 Tun HW, Personett D, Baskerville KA, et al. Pathway analysis of primary central nervous system lymphoma. Blood. 2008;111:3200-3210.
7 Fine HA, Mayer RJ. Primary central nervous system lymphoma. Ann Intern Med. 1993;119:1093-1104.
8 Meeker TC, Shiramizu B, Kaplan L, et al. Evidence for molecular subtypes of HIV-associated lymphoma: division into peripheral monoclonal, polyclonal and central nervous system lymphoma. AIDS. 1991;5:669-674.
9 Bataille B, Delwail V, Menet E, et al. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg. 2000;92:261-266.
10 Newell M, Hoy J, Cooper S, et al. Human immunodeficiency virus-related primary central nervous system lymphoma: factors influencing survival in 111 patients. Cancer. 2004;100:2627-2634.
11 Abrey L, Batchelor T, Ferreri A, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23:5034-5043.
12 Jahnke K, Hummel M, Korfel A, et al. Detection of subclinical systemic disease in primary CNS lymphoma by polymerase chain reaction of the rearranged immunoglobulin heavy-chain genes. J Clin Oncol. 2006;24:4754-4757.
13 Mohile NA, Deangelis LM, Abrey LE. The utility of body FDG PET in staging primary central nervous system lymphoma. Neuro Oncol. 2008;10:223-228.
14 Kuker W, Nagele T, Korfel A, et al. Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J Neurooncol. 2005;72:169-177.
15 Ferreri A, Blay J, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21:266-272.
16 Abrey L, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24:5711-5715.
17 Braaten K, Betensky R, de Leval L, et al. BCL-6 expression predicts improved survival in patients with primary central nervous system lymphoma. Clin Cancer Res. 2003;9:1063-1069.
18 Larocca LM, Capello D, Rinelli A, et al. The molecular and phenotypic profile of primary central nervous system lymphoma identifies distinct categories of the disease and is consistent with histogenetic derivation from germinal center-related B cells. Blood. 1998;92:1011-1019.
19 Takeshita M, Iwashita A, Kurihara K, et al. Histologic and immunohistologic findings and prognosis of 40 cases of gastric large B-cell lymphoma. Am J Surg Pathol. 2000;24:1641-1669.
20 Levy O, Deangelis LM, Filippa DA, et al. Bcl-6 predicts improved prognosis in primary central nervous system lymphoma. Cancer. 2007;112:151-156.
21 Lin C, Kuo K, Chuang S, et al. Comparison of the expression and prognostic significance of differentiation markers between diffuse large B-cell lymphoma of the central nervous system origin and peripheral nodal origin. Clin Cancer Res. 2006;12:1152-1156.
22 Rosenwald A, Wright G, Chan W. Lymphoma/Leukemia Molecular Profiling Project. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937-1947.
23 Lossos I, Jones C, Warnke R, et al. Expression of a single gene, BCL-6, strongly predicts survival in patients with diffuse large B-cell lymphoma. Blood. 2001;98:945-951.
24 Bellinzona M, Roser F, Ostertag H, et al. Surgical removal of primary central nervous system lymphomas (PCNSL) presenting as space occupying lesions: a series of 33 cases. Eur J Surg Oncol. 2005;31:100-105.
25 Batchelor T, Loeffler J. Primary CNS lymphoma. J Clin Oncol. 2006;24:1281-1288.
26 Ferreri A, Abrey L, Blay J, et al. Summary statement on primary central nervous system lymphomas from the Eighth International Conference on Malignant Lymphoma, Lugano, Switzerland, June 12 to 15, 2002. J Clin Oncol. 2003;21:2407-2414.
27 Beela MS, Carson K, Grossman SA. Initial response to glucocorticoids: a potentially important prognostic factor in patients with primary CNS lymphoma. Cancer. 2006:383-387.
28 Nelson D, Martz K, Bonner H, et al. Non-Hodgkin’s lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys. 1992;23:9-17.
29 Shibamoto Y, Ogino H, Hasegawa M, et al. Results of radiation monotherapy for primary central nervous system lymphoma in the 1990s. Int J Radiat Oncol Biol Phys. 2005;62:809-813.
30 Ishikawa H, Hasegawa M, Tamaki Y, et al. Comparable outcomes of radiation therapy without high-dose methotrexate for patients with primary central nervous system lymphoma. Jap J Clin Oncol. 2003;33:443-449.
31 Bessell E, Lopez-Guillermo A, Villa S, et al. Importance of radiotherapy in the outcome of patients with primary CNS lymphoma: an analysis of the CHOD/BVAM regimen followed by two different radiotherapy treatments. J Clin Oncol. 2002;20:231-236.
32 Olson J, Blakeley J, Grossman S, et al. Differences in the distribution of methotrexate into high grade gliomas following intraveneous administration as monitored by microdialysis, are associated with blood-brain-barrier integrity. ASCO Abstract. 2006;24:548.
33 Hiraga S, Arita N, Ohnishi T, et al. Rapid infusion of high-dose methotrexate resulting in enhanced penetration into cerebrospinal fluid and intensified tumor response in primary central nervous system lymphomas. J Neurosurg. 1999;91:221-230.
34 Shenkier TN, Voss N, Chhanabhai M, et al. The treatment of primary central nervous system lymphoma in 122 immunocompetent patients: a population-based study of successively treated cohorts from the British Colombia Cancer Agency. Cancer. 2005;103:1008-1017.
35 DeAngelis L, Seiferheld W, Schold S, et al. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol. 2002;20:4643-4648.
36 Shah G, Yahalom J, Correa D, et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2007;25:4730-4735.
37 Yamanaka R, Morii K, Shinbo Y, et al. Modified ProMACE-MOPP hybrid regimen with moderate-dose methotrexate for patients with primary CNS lymphoma. Ann Hematol. 2005;84:447-455.
38 Poortmans P, Kluin-Nelemans H, Haaxma-Reiche H, et al. High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J Clin Oncol. 2003;21:4483-4488.
39 Dabaja BS, McLaughlin P, Ha CS, et al. Primary central nervous system lymphoma: phase I evaluation of infusional bromodeoxyuridine with whole brain accelerated fractionation radiation therapy after chemotherapy. Cancer. 2003;98:1021-1028.
40 Hodson D, Bowles K, Cooke L, et al. Primary central nervous system lymphoma: a single-centre experience of 55 unselected cases. Clin Oncol (R Coll Radiol). 2005;17:185-191.
41 Gerstner ER, Carson KA, Grossman SA, TT Batchelor. Long-term outcome in PCNSL patients treated with high-dose methotrexate and deferred radiation. Neurology. 2008;70:401-402.
42 Batchelor T, Carson K, O’Neill A, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiation therapy: a report of the NABTT 96-07. J Clin Oncol. 2003;21:1044-1049.
43 Ng S, Rosenthal M, Ashley D, Cher L. High-dose methotrexate for primary CNS lymphoma in the elderly. Neuro Oncol. 2000;2:40-44.
44 Omuro A, Taillandier L, Chinot O, et al. Temozolomide and methotrexate for primary central nervous system lymphoma in the elderly. J Neurooncol. 2007;85:207-211.
45 Hoang-Xuan K, Taillandier L, Chinot O, et al. Chemotherapy alone as initial treatment for primary CNS lymphoma in patients older than 60 years: A multicenter phase II study (26952) of the European Organization for Research and Treatment of Cancer Brain Tumor Group. J Clin Oncol. 2003;21:2726-2731.
46 Pels H, Schmidt-Wolf, IG, Glasmacher, A, et al. Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol. 2003;21:4489-4495.
47 Doolittle N, Miner M, Hall W, et al. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer. 2000;88:637-647.
48 Khan R, Shi W, Thaler H, et al. Is intrathecal methotrexate necessary in the treatment of primary CNS lymphoma? J Neurooncol. 2002;58:175-178.
49 Abrey L, Moskowitz C, Mason W, et al. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. J Clin Oncol. 2003;21:4151-4156.
50 Montemurro M, Kiefer T, Schüler F, et al. Primary central nervous system lymphoma treated with high-dose methotrexate, high-dose busulfan/thiotepa, autologous stem-cell transplantation and response-adapted whole-brain radiotherapy: results of the multicenter Ostdeutsche Studiengruppe Hamato-Onkologie OSHO-53 phase II study. Ann Oncol. 2007;18:665-671.
51 Illerhaus G, Marks R, Ihorst G, et al. High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol. 2006;24:3865-3870.
52 Illerhaus G, Müller F, Feuerhake F, et al. High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system. Haematologica. 2008;93:147-148.
53 Colombat P, Lemevel A, Bertrand P, et al. High-dose chemotherapy with autologous stem cell transplantation as first-line therapy for primary CNS lymphoma in patients younger than 60 years: a multicenter phase II study of the GOELAMS group. Bone Marrow Transplant. 2006;38:417-420.
54 Cheng T, Forsyth P, Chaudhry A, et al. High-dose thiotepa, busulfan, cyclophosphamide and ASCT without whole-brain radiotherapy for poor prognosis primary CNS lymphoma. Bone Marrow Transplant. 2003;31:679-685.
55 Jahnke K, Thiel E, Martus P, et al. Relapse of primary central nervous system lymphoma: clinical features, outcome and prognostic factors. J Neurooncol. 2006;80:159-165.
56 Plotkin S, Betensky R, Hochberg F, et al. Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res. 2004;10:5643-5646.
57 Reni M, Mason W, Zaja F, et al. Salvage chemotherapy with temozolomide in primary CNS lymphomas: preliminary results of a phase II trial. Eur J Cancer. 2004;40:1682-1688.
58 Fischer L, Thiel E, Klasen H, et al. Prospective trial on topotecan salvage therapy in primary CNS lymphoma. Ann Oncol. 2006;17:1141-1145.
59 Arellano-Rodrigo E, Lopez-Guillermo A, Bessell EM, et al. Salvage treatment with etoposide (VP-16), ifosfamide and cytarabine (Ara-C) for patients with recurrent primary central nervous system lymphoma. Eur J Haematol. 2003;70:219-224.
60 Herrlinger U, Brugger W, Bamberg M. PCV salvage chemotherapy for recurrent primary CNS lymphoma. Neurology. 2000;54:1707-1708.
61 Wong ET, Tishler R, Barron L, Wu JK. Immunochemotherapy with rituximab and temozolomide for central nervous system lymphomas. Cancer. 2004;101:139-145.
62 Enting R, Demopoulos A, DeAngelis L, Abrey L. Salvage therapy for primary CNS lymphoma with a combination of rituximab and temozolomide. Neurology. 2004;63:901-903.
63 Rubenstein JL, Fridlyand J, Abrey L, et al. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol. 2007;25:1350-1356.
64 Hottinger AF, DeAngelis LM, Yahalom J, et al. Salvage whole brain radiotherapy for recurrent or refractory primary CNS lymphoma. Neurology. 2007;11:1178-1182.
65 Nguyen P, Chakravarti A, Finkelstein D, et al. Results of whole-brain radiation as salvage of methotrexate failure for immunocompetent patients with primary CNS lymphoma. J Clin Oncol. 2005;23:1507-1513.
66 Lai RA, Lauren E, Rosenblum MK, DeAngelis LM. Treatment-induced leukoencephalopathy in primary CNS lymphoma: a clinical and autopsy study. Neurology. 2004;62:451-456.
67 Monje M, Mizumatsu S, Fike J, Palmer T. Irradiation induces neural precursor cell dysfunction. Nat Med. 2002;8:955-962.
68 Correa D, Maron L, Harder H, et al. Cognitive functions in primary central nervous system lymphoma: literature review and assessment guidelines. Ann Oncol. 2007;18:1145-1151.
69 Omuro A, Ben-Porat L, Panageas K, et al. Delayed neurotoxicity in primary central nervous system lymphoma. Arch Neurol. 2005;62:1595-1600.
70 Correa D, Anderson N, Glass A, et al. Cognitive functions in primary central nervous system lymphoma patients treated with chemotherapy and stem cell transplantation: preliminary findings. Clin Adv Hematol Oncol. 2003;1:490.
71 Fliessbach K, Helmstaedter C, Urbach H, et al. Neuropsychological outcome after chemotherapy for primary CNS lymphoma: a prospective study. Neurology. 2005:647.
72 Ivers L, Kim A, Sax P. Predictive value of polymerase chain reaction of cerebrospinal fluid for detection of Epstein-Barr virus to establish the diagnosis of HIV-related primary central nervous system lymphoma. Clin Infect Dis. 2004;38:1629-1632.
73 Corcoran C, Rebe K, van der Plas H, et al. The predictive value of cerebrospinal fluid Epstein-Barr viral load as a marker of primary central nervous system lymphoma in HIV-infected persons. J Clin Virol. 2008:42.
74 Diamond C, Taylor TH, Aboumrad T, Anton-Culver H. Changes in acquired immunodeficiency syndrome-related non-Hodgkin lymphoma in the era of highly active antiretroviral therapy: incidence, presentation, treatment, and survival. Cancer. 2006;106:128-135.
75 Donahue BR, Sullivan JW, Cooper JS. Additional experience with empiric radiotherapy for presumed human immunodeficiency virus-associated primary central nervous system lymphoma. Cancer. 1995;76:328-332.
76 Skiest D, Crosby C. Survival is prolonged by highly active antiretroviral therapy in AIDS patients with primary central nervous system lymphoma. AIDS. 2003;17:1781-1793.
77 Jacomet C, Girard P-M, Lebrette M-G, et al. Intravenous methotrexate for primary central nervous system non-Hodgkin’s lymphoma in AIDS. AIDS. 1997;11:1725-1730.
78 Forsyth P, Yahalom J, DeAngelis L. Combined-modality therapy in the treatment of primary central nervous system lymphoma in AIDS. Neurology. 1994;44:1473-1479.
79 Bossolasco S, Falk KI, Ponzoni M, et al. Ganciclovir is associated with low or undetectable Epstein-Barr virus DNA load in cerebrospinal fluid of patients with HIV-related primary central nervous system lymphoma. Clin Infect Dis. 2006;42:21-25.
80 Aboulafia D, Ratner L, Miles S, Harrington WJ. Antiviral and Immunomodulatory Treatment for AIDS-Related Primary Central Nervous System Lymphoma: AIDS Malignancies Consortium Pilot Study 019. Clin Lymphoma Myeloma. 2006;6:399-402.
81 Hollender A, Kvaloy S, Nome O, Skovlund E, et al. Central nervous system involvement following diagnosis of non-Hodgkin’s lymphoma: a risk model. Ann Oncol. 2002;13:1099-1107.
82 Doolittle ND, Abrey LE, Shenkier TN, et al. Brain parenchyma involvement as isolated central nervous system relapse of systemic non-Hodgkin lymphoma: an International Primary CNS Lymphoma Collaborative Group report. Blood. 2008;111:1085-1093.
83 Glantz MJ, Jaeckle KA, Chamberlain MC, et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5:3394-3402.
84 Bokstein F, Lossos A, Lossos IS, Siegal T. Central nervous system relapse of systemic non-Hodgkin’s lymphoma: results of treatment based on high-dose methotrexate combination chemotherapy. Leuk Lymphoma. 2002;43:587-593.
85 Glantz MJ, Cole BF, Recht L, et al. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J Clin Oncol. 1998;16:1561-1567.
86 Gleissner B, Chamberlain M. Treatment of CNS dissemination in systemic lymphoma. J Neurooncol. 2007;84:107-117.
87 Kasamon YL, Jones RJ, Piantadosi S, et al. High-dose therapy and blood or marrow transplantation for non-Hodgkin lymphoma with central nervous system involvement. Biol Blood Marrow Transplant. 2005;11:93-100.
88 Castellano-Sanchez AA, Li S, Qian J, et al. Primary central nervous system posttransplant lymphoproliferative disorders. Am J Clin Pathol. 2004;121:246-253.
89 Schiff D, O’Neill B, Plotkin S, et al. Isolated central nervous system post-transplant lymphoproliferative disorder (CNS-PTLD): The International Primary CNS Lymphoma Collaborative Group experience. EANO Abstract 022, 2006.
90 Buell JF, Gross TG, Hanaway MJ, et al. Posttransplant lymphoproliferative disorder: significance of central nervous system involvement. Transplant Proc. 2005;37:954-955.
91 Baehring JM, Damek D, Martin EC, et al. Neurolymphomatosis. Neuro Oncol. 2003;5:104-115.
92 Abrey LE, Yahalom J, DeAngelis LM. Treatment for primary CNS lymphoma: the next step. J Clin Oncol. 2000;18:3144-3150.
93 Gavrilovic IT, Hormigo A, Yahalom J, et al. Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2006;24:4570-4574.
94 McAllister LD, Doolittle ND, Guastadisegni PE, et al. Cognitive outcomes and long-term follow-up results after enhanced chemotherapy delivery for primary central nervous system lymphoma. Neurosurgery. 2000;46:51-60.
95 Sonoda Y, Matsumoto K, Kakuto Y, et al. Primary CNS lymphoma treated with combined intra-arterial ACNU and radiotherapy. Acta Neurochir (Wien). 2007;149:1183-1189.
96 Soussain C, Suzan F, Hoang-Xuan K, et al. Results of intensive chemotherapy followed by hematopoietic stem-cell rescue in 22 patients with refractory or recurrent primary CNS lymphoma or intraocular lymphoma. J Clin Oncol. 2001;19:742-749.
97 Sakamoto M, Oya N, Mizowaki T, et al. Initial experiences of palliative stereotactic radiosurgery for recurrent brain lymphomas. J Neurooncol. 2005;71:53-58.