Chapter 16 Cell Biology of the Retinal Pigment Epithelium
![]() For additional online content visit http://www.expertconsult.com
For additional online content visit http://www.expertconsult.com
Embryology
At day 27 postconception, the optic vesicles invaginate to form the optic cup and the neuroepithelium undergoes early differentiation and thickening. By day 30, the basic cellular relationship of the outer retina is established with the prospective RPE layer found closely apposed to the prospective neural retina. Several transcription factors have been found to play a critical role in the determination and specification of RPE, among which are microphthalmia-associated transcription factor (Mitf), orthodenticle homeobox (Otx)1/2 and paired box (Pax) 6.1 The Sonic hedgehog (Shh) signaling pathway is also critical; the distinct expression pattern of its members in central and peripheral RPE suggests multiple regulatory roles in the process of RPE differentiation.2 By day 35, melanin pigment granules are identified in the presumptive RPE; this is the earliest site of pigmentation in the body. By the 6th week of gestation, RPE cells elaborate basement membrane material that participates in the formation of the first recognizable Bruch’s membrane. Between 4 weeks and 6 months of gestation, RPE cells exhibit a high rate of proliferation that peaks at 4 months of gestation.3 Meanwhile, most of the RPE cells have developed polarized characteristics with apical microvillous projections that extend into the subretinal space.4 The RPE’s consequent interaction with neurosensory retina coordinates the differentiation of primordial photoreceptors. While RPE fate may be reversible for several days following the initial activation of differentiation,5 recent studies suggest that, the Shh pathway,6 retinoic acid,7 bone morphogenetic protein (BMP) signaling,8 Notch9 and Wnt/β-catenin10 pathways all play a role in the maintenance of RPE differentiation. In addition, ezrin, a member of the ezrin/radixin/moesin (ERM) family, is important for morphogenesis of apical microvilli and basal infoldings in RPE cells.11 Polarization of the differentiated RPE monolayer is required for the normal development and function of the photoreceptors and choroid. A role for RPE in the induction and modulation of scleral development has also been described.12 Readers are referred to Chapter 13, Development of the retina, for a more comprehensive review of the development of the RPE and retina.
Anatomy and histology
There are approximately 3.5 × 106 RPE in the adult human eye, and this population remains relatively stable during young adult life in the absence of diseases in which RPE are induced to undergo proliferation or cell death.13 Studies evaluating cell cycle and cell proliferation markers show that a very low level of mature RPE are retained in the cell cycle and proliferate in vivo in both rats and an aged human sample.14 The RPE layer extends from the optic nerve to the ora serrata and continues as the pigment epithelium of the ciliary body. The foveal area contains the highest density of RPE cells, while the cell density exhibits in-gradient decrease to the periphery. These cobblestone-like cells with highly polarized structure form a monolayer between the neurosensory retina and the choroid. The apical microvilli of the RPE cells interdigitate with the OS of the photoreceptors, while the RPE basal side is attached firmly to the underlying Bruch’s membrane; the basement membrane of the RPE represents the innermost layer of Bruch’s membrane. Readers are referred to Chapter 20, Structure and function of Bruch’s membrane, for a detailed description of Bruch’s membrane physiology and interaction with the RPE.
The brown color of the RPE is imparted by its melanin granules and the typical patterned appearance of the fundus results from variations in the pigmentation of the RPE layer. In a healthy retina, the highest concentration of pigment is found in the peripheral retina, the lowest in the macular area.15 With age, RPE gradually lose melanin granules, possibly related to effects of photooxidation.16 The retina and the RPE are separated by a potential space known as the subretinal space. Although the retina conforms to the shape of the adjacent pigment epithelium and underlying sclera, it is not firmly attached to the pigment epithelium except at the optic disc and ora serrata; attachments elsewhere are weak and can be disrupted by relatively weak forces. Readers are referred to Chapter 19, Retinal adhesion and fluid physiology of the subretinal space, for a complete discussion of retinal adhesion.
Heterogeneity and polarity of the RPE
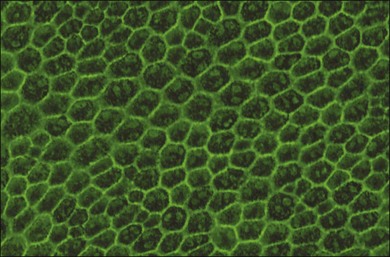
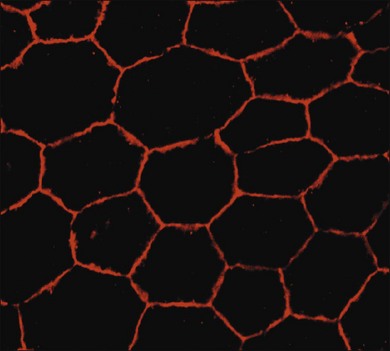
RPE cells exhibit regional heterogeneity in morphology and function.17 The cuboidal RPE cells that form the RPE monolayer appear polygonal in shape when viewed en face, a pattern that is maintained during early passage in culture when grown to confluence (Fig. 16.1). In situ, cell shape varies throughout the fundus; in the macular area the cells are tall and narrow, whereas in the periphery they are flatter, more spread out, and may be binucleated.13 Perhaps due to regional differences in requirements for RPE function, RPE cells vary regionally in growth potential,18 level of expression of vimentin and phosphotyrosine,17 distribution of Na/K adenosine triphosphatase (ATPase) pumps,19 and kinetics of rod outer-segment binding and ingestion.20 Regionally heterogeneous age-related alterations in expression of lysosomal enzymes, Mn-superoxide dismutase, and accumulation of lipofuscin have been identified in RPE,21–23 suggesting that this heterogeneity is probably involved in pathological changes in RPE. Quantification of RPE phenotypic variation can be achieved using laser scanning cytometry.22
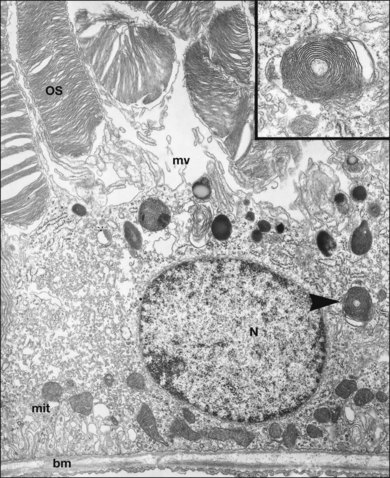
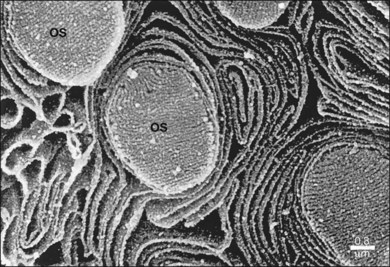
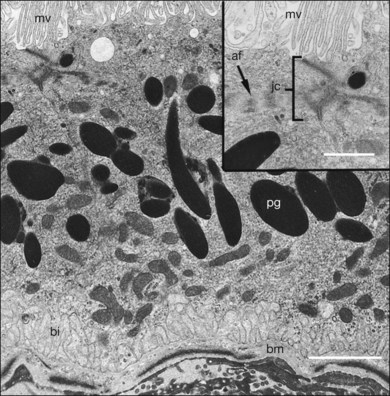
The polarity of RPE in the monolayer is characterized by distinct ultrastructural features and specialized functions in the apical and basolateral domains (Fig. 16.2). The apical cell membrane elaborates numerous microvilli (3–7 µm in length) that interdigitate with and ensheath the OS of the retinal photoreceptors.24 (Figs 16.3 and 16.4). These interdigitations, in conjunction with the extracellular matrix (ECM), and neural cell adhesion molecule (N-CAM) expressed on the RPE apical surface, allow some degree of adhesion between the retina and the RPE. Between 30 and 45 photoreceptors are in contact with each RPE cell.25 RPE cells engulf and degrade shed rod OS each day such that one human RPE cell will ingest and degrade hundreds of millions of discs during its lifetime.26
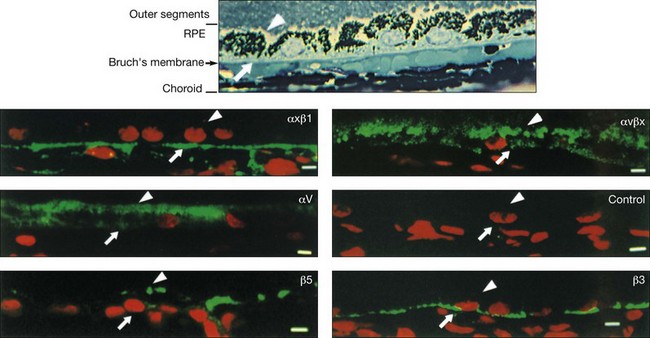
The functional polarity of RPE cells is expressed in the differential distribution of membrane proteins along the apical–basal axis (Table 16.1). Sharing many common characteristics with other transporting epithelia, RPE exhibit “reversed polarity” of certain proteins, such as the Na/K-ATPase pump, ECM metalloproteinase inducer (EMMPRIN), and N-CAM. In RPE these proteins are found at the apical surface, rather than at the basolateral surface where they are found in other epithelia.27 In a proteomic study of RPE apical microvilla,27 283 proteins were identified, which could be divided into different functional categories, including retinoid-metabolizing, cytoskeletal, enzymes, ECM components, membrane proteins, and transporters. αvβ5 integrin (Fig. 16.5), mannose receptors, and CD36 are localized to the apical membrane domain, specifically in the microvilli, where they play critical roles in rod outer-segment phagocytosis.28–30 Na/K-ATPase is mainly expressed at the apical RPE cell membrane where it is critical for transepithelial ion transport in the RPE.31 A newly identified membrane protein chloride intracellular channel 4 (CLIC4) has been shown to be enriched in apical RPE microvilli, possibly for modulating the activity of cell surface channels/transporters.32 The RPE basal membrane domain is characterized by infoldings that are approximately 1 µm in length (Fig. 16.6). The basal membrane expresses α3β1, α6β1, and αvβ3 integrins and mediates attachment to Bruch’s membrane (Fig. 16.5). The basal infolding also express Bestrophin-1, which is a chloride anion channel that regulates voltage-dependent L-type Ca2+ channels by interacting with the beta subunits of the Ca2+ channels.33 Ezrin is found in both apical and basolateral membranes where it promotes morphogenesis of apical microvilli and basal infoldings.34 A number of transporters work together in apical and basal domains. For example, glucose transport from the choroid to the photoreceptors is mediated by glucose transporter (GLUT) 1 located both basally and apically.35 In contrast, elimination of lactic acid from the subretinal space is mediated by different monocarboxylate transporters in the apical (MCT1) and basolateral (MCT3) membranes.36,37 The lateral membrane domain of the RPE cell demonstrates specialized junctions important for cell–cell attachment and communication, which will be discussed below.
Table 16.1 Polarized cell surface molecule expression in retinal pigment epithelium
| Location | Protein | Function |
|---|---|---|
| Apical membrane | Na/K-ATPase | Na flux |
| N-CAM | Adhesion to retina, phagocytosis | |
| αvβ5 Integrin | Phagocytosis | |
| CD36 | Phagocytosis | |
| Ezrin | Apical microvilli | |
| CLIC4 | Channels/transporters | |
| GLUT1 | Glucose transport | |
| MCT1 | Monocarboxylate transporter | |
| Lateral membrane | Occludin | Tight junction |
| Cadherin | Adherens junction | |
| Connexin | Gap junction | |
| Basolateral membrane | α3β1, α6β1, ανβ3 integrin | Attachment to Bruch’s membrane |
| Ezrin | Basal infoldings | |
| GLUT1 | Glucose transport | |
| MCT3 | Monocarboxylate transporter | |
| Bestrophin-1 | Chloride anion channel |
Na/K-ATPase, Na/K adenosine triphosphatase; N-CAM, neural cell adhesion molecule; CLIC4, chloride intracellular channel 4; GLUT1, glucose transporter 1; MCT1, monocarboxylate transporter 1.
The intracellular distribution of cell organelles in RPE also exhibits heterogeneity and polarization (Figs 16.2 and 16.6).38 Melanin granules, which are ovoid or spherical in shape, 2–3 µm in length, and 1 µm in diameter, are localized at the apical region of the cell, as is the endoplasmic reticulum, the site of protein synthesis. At the ora serrata, the RPE contain many dense round melanin granules throughout the cell; at the equator and macula, melanin granules are more apical, less frequent, and often elongated. The nucleus, with a diameter of 8–12 µm, is located in the basal part of the cell. The localization of mitochondria in the basal half of the RPE results from the oxygen pressure which is highest in this cell region and is most apparent at the macula.39 Other cytoplasmic elements, such as microperoxisomes, lysosomes, and phagosomes, do not appear to have a distinct distribution. However, inhibition of lysosomal degradation in RPE cells could induce exocytosis of phagocytic residual material at the basolateral plasma membrane.40
Cellular junctions
The outer blood–retinal barrier is formed by the RPE monolayer, in which the lateral domains of adjacent RPE cells connected by apical zonulae occludens (tight junctions) and adjacent zonulae adherentes (adherens junctions) enable the epithelium to form a barrier by joining neighboring cells together and regulating transepithelial diffusion through the paracellular spaces (Fig. 16.6).41 These junctions seal off the subretinal space where the exchange of macromolecules with the choriocapillaris takes place, and form the so-called Verhoeff’s membrane. Two major groups of proteins of zonulae occludens are the claudins and the occludins. The interaction between the extracellular domains of adjacent occludin molecules leads to high transepithelial resistance and an intact blood–retinal barrier. They are responsible for maintaining the polarity of cells by preventing the lateral diffusion of integral membrane proteins between the apical and lateral/basal surfaces, allowing the specialized functions (such as molecule transport ) of each to be preserved.42 The cytoplasmic domain of occludin interacts with several other proteins, including zonula occludens (ZO)-1 and ZO-2, to form a complex that interacts with the actin cytoskeleton and components of various signal transduction pathways (Fig. 16.7). ZO-1 regulates cell proliferation and gene expression by inhibiting the activity of the Y-box transcription factor ZONAB in cultured epithelial cells, indicating they are critical for the differentiation and homeostasis of the RPE monolayer.43 Early expression of the assembly proteins, including junctional adhesion molecule-A (JAM-A), AF-6, PAR-3, and PAR-6, corresponds to the initial establishment of the adherens and tight junctions,44 whereas diffusible factors secreted by the neural retina act synergistically with basolateral stimulation to regulate the structure and function of RPE tight junctions.45 Claudin 19 has been shown to be dominantly expressed in human fetal RPE cells.46 JAM-C localizes specifically in the tight junctions of human fetal RPE and adult native RPE, and functions to regulate the recruitment of N-cadherin and ZO-1 to the cell–cell contacts.47 In pathological conditions, oxidative stress,48 inhibition of Na/K-ATPase,49 matrix metalloproteinase (MMP)-9, interferon (IFN)-γ, tumor necrosis factor,50,51 and amyloid-beta(1–42)52 could decrease tight junction protein expression, resulting in the disruption of the integrity of the outer blood–retina barrier; in contrast, nitric oxide is involved in the maintenance of blood–retina barrier integrity.51
The zonulae adherentes (adherens junction) form a junction with a separation of 200 Å and are associated with circumferential microfilament bundles.53–55 The transmembrane cadherin molecules of the adherens junction require calcium for ensuring that cells within tissues are bound together. Their cytoplasmic domains interact with catenins, which in turn form a complex with proteins such as α-actinin and vinculin. The adherens junctions play a role in maintenance of the polygonal shape of the RPE cell and in the organization of the actin cytoskeleton.56 Gap junctions, which are also present in the lateral cell membranes, are associated with the expression of connexin and are important for the exchange of ions and metabolites between cells. The gap junction protein connexin 43-mediated communication between the retina and RPE is essential for the correct pacing of retinal organogenesis.57 In addition, ATP released from RPE hemichannels via connexin 43 speeds both cell division and proliferation in the neural retina.58 The basal cell membrane expresses various integrins and shows focal adhesion points with the ECM.59 The presence of desmosomes between RPE cells varies among species; they are often difficult to detect, and they do not appear to be necessary for establishment of a polar, functioning RPE layer.60 Occasional desmosomal–mitochondrial complexes have been described between RPE cells in human specimens, similar in appearance to those observed in nonpigmented ciliary epithelia.61 While the desmosome-associated protein desmoplakin is found in cultured bovine RPE, it has not been identified in human RPE cultures.
Cytoskeleton
Apart from the general role of the cytoskeleton in all nucleated cells, the RPE cytoskeleton is highly associated with its distinct functions such as melanosome transport and phagocytosis.62,63 These functions are disrupted in some diseases like PVR, caused by RPE cell proliferation and myofibroblastic transdifferentiation, where there are prominent rearrangements of the cytoskeleton.64 The cytoskeleton is composed of three major elements: the actin microfilaments (diameter 7 nm), microtubules (diameter 25 nm), and intermediate filaments (diameter 10 nm). Microfilaments and microtubules are dynamic structures that undergo polymerization and depolymerization and are critical for intracellular transport. Microtubules play a role in mitosis, and the movement of subcellular organelles and pigment granules. Actin microfilaments are located in the microvilli and throughout the cytoplasm where they are arranged in loose arrays or bundles. They play an important role in the generation and maintenance of cellular shape and cell migration.65 In fish RPE, actin is involved in melanosome transport within apical projections.66 Myosins are cytoskeletal motors critical for generating the forces necessary for establishing cell structure and mediating actin-dependent cell motility. The expression of multiple myosin family members has been shown in fish RPE.67 In addition, myosins are proposed to play a role in the movement and biogenesis of the melanosomes in RPE.68 Intermediate filaments provide a structural framework for the cell linking the nucleus with the cell membrane and to microfilaments and microtubules. In human RPE cells, intermediate filaments of type I (acidic keratins), type II (basic/neutral keratins), and type V (lamins) have been identified. In RPE, the pattern of cytokeratin filament expression varies with cellular differentiation, changes in cellular polarity, intercellular association, and conditions of cell culture.69,70 Human RPE cells in vivo express keratins 8 and 18.71 Under conditions such as cell culture, keratins 7 and 19 are also coexpressed. The presence of keratin 18/19 has been correlated with migration in cultured RPE cells.71
Role of RPE in Bruch’s membrane synthesis and remodeling
The basal surface of the RPE monolayer has elaborate basal infoldings that attach to Bruch’s basement membrane, an acellular layer separating the RPE from the choriocapillaris.72 Bruch’s membrane is a complex structure that not only serves as the attachment site for the RPE cells, but also serves as a selective conduit for nutrients transported to the retina from the choroidal vasculature and for metabolic wastes transported from the retina to the circulation. Bruch’s membrane consists of five distinct layers.73 From the RPE to the choroid the following layers can be distinguished: the basement membrane of the RPE, the inner collagenous layer, the elastic layer, the outer collagenous layer, and the choriocapillaris endothelium basement membrane. In normal mice the RPE basement membrane and the choroid basement membrane are synthesized first, followed by the deposition of a collagenous layer between the two basement membranes. Later the elastic layer is synthesized and deposited within the collagenous layer, eventually separating it into an inner and outer collagenous layer.74
The basement membrane of the RPE and choroid (1.4–1.5 µm thick) are similar to other basement membranes in composition; they contain collagens, laminin, fibronectin, and sulfated polysaccharides75–77 and serve the same functions, including anchoring subjacent cells, acting as a barrier and a filter, and stabilizing the structure of the tissue.78 The choriocapillaris basement membrane also contains collagen type VI, which is not present in the RPE basement membrane, and may be involved in capillary endothelial cell stabilization. The inner collagenous (1.4 µm thick) and outer collagenous (0.7 µm thick) layers are composed of collagens type I, II, and V organized in a lattice-like network embedded in an amorphous collection of glycosaminoglycans.79
Distributed throughout all the layers of Bruch’s membrane is collagen XVIII,80 which gives rise to the endostatin, an inhibitor of choroidal neovascularization.81 Extensive gene expression studies by Booij et al.82,83 have shown that both the RPE and choroid express most of the genes necessary for the formation and maintenance of Bruch’s membrane. Since both the RPE and choroid are able to synthesize the major components of Bruch’s membrane, it appears that the basal lamina of each RPE would be maintained by RPE cells, the choroid basement membrane by choroidal cells, whereas the inner, outer collagenous layer and the elastic layer would be maintained cooperatively by both tissues. Age-related biochemical alterations in the composition and three-dimensional organization of ECM molecules in Bruch’s membrane may affect this function and are described in detail in Chapter 20, Structure and function of Bruch’s membrane.
The apical domain of the RPE cells is embedded in the interphotoreceptor matrix (IPM), which is produced by the RPE and the inner segments of the photoreceptors. Major protein components of IPM that are involved in retinoid transport between the photoreceptors and the RPE include the interphotoreceptor-binding protein (IRBP), retinol-binding protein (RBP), and transthyretin (TTR).84–87 Retinal adhesion to the RPE is mediated in part by RPE transport of water and ions from the IPM toward the choriocapillaris. It has been identified that RPE predominantly secrete the neurotrophic pigment epithelial-derived factor (PEDF) from the apical side into the IPM, which is about 1000-fold greater than that for vascular endothelial growth factor (VEGF), which is mainly from the basolateral side.88 In addition, proteomic analysis of the porcine IPM indicated that a set of potentially neuroprotective proteins could be extracted, including phosphoprotein enriched in astrocytes (PEA)-15, peroxiredoxin 5, αB crystallin, macrophage migration inhibitory factor, 78-kDa glucose-regulated protein (GRP78), protein disulfide isomerase (PDI), and PEP-19.89 Notably, αB crystallin has been shown to be secreted by RPE and plays an important role in maintaining and facilitating a neuroprotective outer retinal microenvironment.90
Degradation of ECM is regulated, in part, by both MMPs and the urokinase-type plasminogen activator (uPA) cascade,91 which are activated by multiple types of cells including leukocytes, endothelial cells, and RPE. MMPs are a family of zinc-binding, Ca+-dependent endopeptidases that can degrade both collagen and proteoglycans in ECM during physiological and pathological ECM remodeling. The MMPs are tightly regulated by its tissue inhibitors of metalloproteinases (TIMPs), whose impairment may lead to progressive alterations in Bruch’s membrane.92 Normal RPE express the membrane-bound type 1 (MT1 MMP) as well as type 2 (MMP-2) metalloproteinase,93 as well as the metalloproteinase inhibitors TIMP-1 and TIMP-3.94 Degradation of the ECM is also promoted by uPA, a serine protease that catalyzes conversion of inactive plasminogen to active plasmin.95 Plasmin is a broad-spectrum protease that degrades fibrin, fibronectin, laminin, and other ECM components. uPA activity can be blocked by two endogenous inhibitors, plasminogen activator inhibitor-1 and -2 (PAI-1 and PAI-2),96 which belong to the serpin protease superfamily. Cultured human RPE cells produce immunoreactive uPA and a protein similar to PAI-1.97
Cell culture models of RPE
Cell culture models of RPE play a critical role in gaining basic knowledge about native tissue, evaluating polarized RPE function, facilitating drug discovery and toxicity, and offering potential to researchers working in the field of tissue engineering and cell transplantation. Culture of well-differentiated and characterized RPE cells from various sources may be a future cellular therapy for several retinal diseases.98 Methods to generate RPE with varying degrees of polarization have been reported for immortalized human RPE cell lines, such as ARPE19 and D407, and fetal human RPE99,100 (reviewed by Sonoda et al.101 and Maminishkis and Miller102). Polarized RPE monolayers derived from human fetal RPE develop high transepithelial resistance, and show polarized membrane specialization, including prominent apical microvilli and apical expression of Na/Ka-ATPase.101 More recently, cells from the ciliary margin progenitor zone,103 retinal stem cells,104 human embryonic stem cells,105,106 and human induced pluripotent stem cells107 have also been shown to have the potential to be directed into RPE cells. The reader is referred to Chapters 35, Stem cells and cellular therapy, and 125, Transplantation frontiers, for an indepth discussion of RPE cellular therapies.
specialized functions of the RPE
Absorption of light
The first step in the visual process is absorption of light by the opsins of rod and cone photoreceptors and by the melanopsin of retina ganglion cells. Melanopsin is involved in the regulation of circadian rhythms, pupillary light reflex, and other nonvisual responses to light,108 although recently it has been shown that melanopsin in ganglion cells may contribute directly to pattern vision.109 The light-absorbing chromophore of opsins is 11-cis retinaldehyde (11-cis-RAL), which is delivered to the rod photoreceptors by the RPE cells. The RPE cells possess the enzymatic mechanism to convert vitamin A to 11-cis-RAL as well as the mechanism to deliver it to the photoreceptors. An important function of the RPE is the absorption of light that passes beyond the OS of the photoreceptors. The melanin pigment within the pigment granules (Fig. 16.6) of the RPE absorbs stray photons of light which minimizes light scatter within the retina and protects from excess light.15
Phagocytosis of rod outer segments
In all vertebrates examined, nocturnal and diurnal, cold-blooded and warm-blooded, rod OS shedding occurs maximally at or shortly after light onset, a daily rhythm which is established early after birth, irrespective of the lighting conditions during development. In fact, in rats the daily rod OS shedding rhythm is established by 2 weeks postnatally and it is maintained even after optic nerve section, indicating that this rhythmic process of OS shedding is controlled by intrinsic signals within the eye.110,111The temporal pattern of cone OS shedding is much more variable since in some species it occurs at night,112,113 whereas in others cones and rod OS are shed just after light onset.114,115
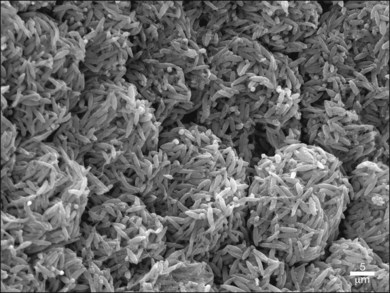
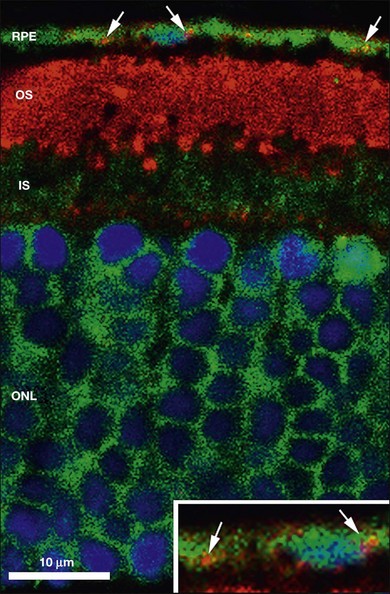
The disposal of the continuously shed OS is accomplished by phagocytosis of the OS at the expense of elevated metabolic activity and energy expenditure.116,117 It has been estimated that, during an 80-year span, one RPE cell has internalized and degraded about 200 million discs.118 The phagocytosis of ROS disc material can be visualized in normal rat eyes using confocal microscopy to visualize rhodopsin+ phagolysosomes by enucleating the eye 2 hours after onset of light (Fig. 16.8). The mechanisms involved in rod OS phagocytosis have recently been reviewed.119 Rod OS phagocytosis is a highly specialized receptor-mediated, multistep process that comprises recognition, attachment (receptor–ligand interactions), internalization (transmembrane signaling and contractile proteins), and degradation of the ingested OS.119 The first step in the phagocytic pathway is recognition. It appears the recognition is a receptor-mediated event that requires soluble molecules to bridge the receptor on the RPE cell and some functional molecules on the rod OS. Studies have shown that the receptor on the RPE cell is αvβ5 and the molecule on the rod OS is phosphatidylserine, which has been described on the OS plasma membranes.120 Although αvβ3 and αvβ5 have been demonstrated to be instrumental for substrate binding, they do not bind substrate directly. Rather they bind an opsonin that recognizes the “eat-me” signal on their phagocytic targets. A number of soluble molecules have been shown to be possible bridging elements, such as Gas6, protein S, and milk fat globule epidermal growth factor (MFG)-E8.121 Recently Tubby and tubby-like protein (Tulp) 1, which are secreted by photoreceptors, have been identified as playing a role in RPE phagocytosis. Tubby and Tulp1 are bridging molecules that bind to MerTk (a member of the TAM receptor tyrosine kinase subfamily) at their N-terminal region and likely to the rod OS at their C-terminal region.122
Binding of the rod OS is followed by invagination of the plasma membrane around the OS fragment, leading to its ingestion into a phagosome. A reorganization of cytoskeletal elements, particularly of microfilaments in the microvilli, appears to be involved in the initial stages of ingestion. An actin feltwork forms at the sites of attachment that extend into the pseudopods which surround and engulf the fragments to form the phagosome.123 This process includes an undefined, presumably G-protein-coupled transmembrane signal that stimulates the assembly of an appropriate contractile apparatus that, in turn, provides the motive force for internalization of the attached particle.124 Internalization appears to be mediated by the receptor tyrosine kinase, c-mer, and its ligand Gas6.125 Once inside the cytoplasm, phagosomes are transported basally. In the presence of colchicine, the phagosomes remain in the apical domain of the RPE, suggesting that microtubules are involved.126,127 Transport may also be partially mediated by myosin VIIa, an unconventional myosin that is mutated in patients with Usher syndrome.128 Cytokines, which play an important role in development, differentiation, and survival of retinal cells, are also involved in the phagocytic process. In vitro studies of RPE cells from normal rats and rats with defective phagocytosis have shown that transforming growth factor (TGF)-β1 and basic fibroblast growth factor (FGF) regulate rod OS phagocytosis.129
Basally transported phagosomes then fuse with lysosomes and are degraded. The lysosome–phagosome interaction occurs in two steps. First, smaller lysosomes fuse with phagosomes. Subsequently, larger lysosomes appear to interact with phagosomes via pore-like structures. Following fusion, lysosomal enzymes hydrolyze the sequestered OS into small molecules that diffuse out of the RPE cell or are reused within the cell. Of the wide array of lysosomal enzymes capable to hydrolyze photoreceptor OS, cathepsin D is the most important and plays a dominant role in the degradation of rhodopsin, the major glycosylated protein present in the OS.130–132 Another enzyme involved in degradation of rod OS is the cysteine protease, cathepsin S.133 With age and pathologic changes degradation of OS materials within the phagolysosomes leads to the formation of lipofuscin granules composed of rod OS residue.134,135
Role in visual cycle
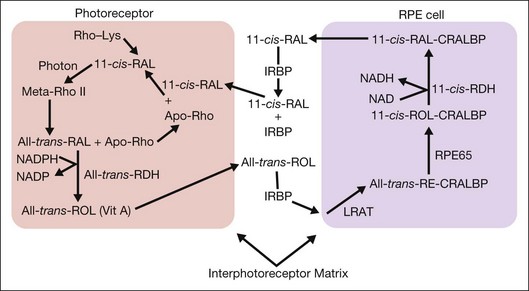
Light perception in the retina is initiated by the reaction of photons with light-sensitive pigments that are part of the membranes of photoreceptor OS. Cooperation between the photoreceptors and the RPE cells allows these pigments to be recycled through a complex series of oxidation–reduction reactions and transport mechanisms that are referred as the “visual cycle” (Fig. 16.9).
The visual cycle is initiated by a photon reacting with rhodopsin, which comprises a G-coupled receptor protein, opsin (rods) or cone-opsin (cones), and the chromophore 11-cis-RAL. Reaction with light changes 11-cis-RAL into all-trans-RAL, which is released from the opsin and reduced to all-trans-retinol (all-trans-ROL). ABCA4 (a member of the A subfamily of ATP-binding cassette proteins) supports the processing of highly reactive all-trans-RAL and prevents the buildup of A2E, an insoluble toxic compound that is shed from the photoreceptors, phagocytosed by RPE, and accumulates in lipofuscin.136 All-trans-ROL passes into the IPM, where it binds to the IRBP and is transported into the adjacent RPE cells. IRBP is a 140-kDa glycoprotein secreted by the photoreceptors, which enhances the translocation of 11-cis-RAL from the RPE to the photoreceptors137 and the translocation of all-trans-ROL from the photoreceptors to the RPE.138 IRBP is essential to the normal functioning of the visual cycle. In fact, in knockout mice (irbp−/−) that lack IRBP there is significant photoreceptor degeneration.139
Protection from oxidative stress
The RPE layer resides in a perilous environment and is subjected to oxidative stress (in part due to the continuous flux of polyunsaturated fatty acids), to high-energy radicals (as a result of the high oxygen consumption of the retina), and to exposure to radiant radiation.140 Confronted with such a risky environment, RPE cells produce neurotrophin 1,141 a potent neuroprotective docosahexaenoic acid-derived lipid that inhibits the expression of proinflammatory genes and enhances the expression of antiapoptotic molecules of the Bcl-2 family, thus promoting survival.142
The photoreceptors and neural retina depend on the RPE to provide metabolic balance for the maintenance of normal functions. RPE cell degeneration leads to secondary photoreceptor degeneration and neural dysfunction. PEDF is a critical factor produced by RPE that provides cytoprotection. PEDF is an endogenous regulator of proliferation, an inhibitor of neovascularization, and acts to protect neural cells from various environmental insults, such as glutamate stress, oxidative stress, and ischemic conditions.143–147 RPE cells also synthesize and secrete FGF1 and FGF2, which act as survival factors by preventing apoptosis and retinal degeneration.148,149
A variety of antioxidants and their enzymes play a critical role in cell protection and are abundantly expressed in RPE. The major ones among them are glutathione (GSH),150 thioredoxins (Trx), and glutaredoxins (Grx),151,152 methionine sulfoxide reductases (Msrs),153 catalase,154 superoxide dismutases,155 and glutathione peroxidase.156 The antioxidants as well as the enzymes have been shown to be either upregulated or downregulated depending on the severity of oxidative stress stimuli,154 which can include H2O2, 4-hydroxy-2-nonenal, hypoxia/hyperoxia, inflammation, and toxic drugs, as well as lipid signaling agents such as ceramide.157
Depletion of GSH causes apoptosis in RPE cells with an accompanying compensatory upregulation of glutathione S-transferase, gamma glutamylcysteine synthetase, and glutathione peroxidase-1 through a signaling pathway that involves the transcription factor nuclear factor-erythroid 2 p45-related factor 2 (NRF2) and phase 2 enzyme induction.158,159 Since mitochondria are directly involved in the generation of reactive oxygen species, the maintenance of the mitochondrial GSH pool is critical during oxidative injury. Regulation of superoxide dismutase has been shown to be effective in protection from retinal injury.156,160 Among the Trx and Grx family, overexpression of Trx1 protected RPE cells from oxidative injury.152 The intravitreous injection of Trx effectively attenuated N-methyl-d-aspartate (NMDA)-induced retinal cell damage, and suppression of oxidative stress and inhibition of apoptotic signaling pathways were involved in this neuroprotection.161 Similarly, the protective function of Grx in oxidant-induced apoptosis is also known.162,163 In addition to the enzymes of GSH metabolism, the key reductases of methionine oxidation, namely Msrs, play an important role in RPE protection.164 Suppression of MsrA augments H2O2-induced apoptosis while overexpression prevented cell death.153 Recently, the antiapoptotic action of small heat shock proteins in the retina has received attention.90,165–167 The antiapototic function of alpha crystallins may be related to their chaperone activity as well as to the metabolism of GSH metabolism in RPE.168,169
Role in maintaining avascular outer retina
The avascularity of the subretinal space is dependent on the antiangiogenic activity of PEDF, a factor that is expressed by many cells, including RPE cells, and the antiangiogenic activity of endostatin, the 20-kDa C-terminal fragment derived from type XVIII collagen by proteolysis. PEDF is synthesized and secreted by RPE cells into the IPM,143,144 where it acts as a neuroprotective factor for photoreceptors and neural retina ganglion cells. PEDF is also a most potent and selective antiangiogenic factor that inhibits the growth and mediates regression of newly formed blood vessels without affecting pre-existing vessels and in the retina prevents the growth of blood vessels into the subretinal space.170–172 The importance of PEDF in maintaining the avascularity of the outer retina is apparent from the distribution of PEDF in the retina. The expression of PEDF is highest in the developing fovea of midgestation human retinas and in the fovea of monkeys aged between fetal day 55 and 11 years of age.173 In addition, in normal adult eyes PEDF concentration is 10-fold higher than VEGF in the macular region but not in the peripheral region of the retina, strongly suggesting that PEDF is responsible for macular avascularity.174
In addition to PEDF, avascularity of the subretinal space is dependent on endostatin. Endostatin is cleaved from collagen XVIII in Bruch’s membrane by the action of RPE-derived proteases, such as MMP-9 and cathepsin L. Laser-induced choroidal neovascular lesions in Col18a1–/– mice, which lack endostatin, formed large confluent areas of neovascularization and led to increased vascular permeability; in contrast, choroidal neovascular lesions in control mice remained small and clearly circumscribed. Administration of recombinant endostatin to Col18a1–/– mice decreased the lesion size to the sizes observed in control mice.175
Immune privilege
Immune privilege refers to the fact that foreign-tissue grafts placed in the immune-privileged site are tolerated and survive for prolonged, often indefinite, intervals, while placement of such grafts at conventional body sites leads to acute irreversible immune rejection. Ocular immune privilege was demonstrated by Sir Peter Medawar in 1948 when skin allografts survived when implanted into the anterior chamber of the eye.176 Only in the past 20 years has evidence accumulated that the subretinal space was also an immune-privileged space.177,178 Subsequently, the RPE has been shown to be a major source of immune privilege in the subretinal space. Immune privilege has evolved from a concept of passive physical barriers (tight junctions between RPE; lack of lymphatic drainage from subretinal space; low levels of major histocompatibility antigen expression) to more of an active process. The RPE express both soluble (TGF-β, PEDF) and cell surface molecules (TGF-β, CD95 (FAS) ligand, CD59, CD46) that promote immune privilege. TGF-β has been identified as a major factor in the inhibition of T-cell proliferation and IFN-γ production, and the generation of T-regulatory cells (Tregs) in ocular immune-privileged sites.178 Recently, immune privilege in the aqueous humor was shown to require a role for both TGF-β and retinoic acid in the generation of Tregs; however, whether this is also true for the subretinal space is currently unknown.179 A full discussion of the blood–retinal barrier and immune privilege is found in Chapter 27, Blood–retinal barrier, immune privilege, and autoimmunity.
Transport of nutrients, ions, and water
Of the nutrients that RPE cells must deliver, glucose is one of the most important. RPE cells express very high levels of the glucose transporters, GLUT1 and GLUT3.180,181 GLUT1 is responsible for transport of glucose that is dependent on metabolic demand and its expression is regulated by the level of glucose: reduced glucose levels increase expression whereas increased glucose levels decrease expression.182 GLUT3 is a high-affinity glucose transporter that is responsible for the basal transport of glucose to maintain resting-level activity. It is interesting that GLUT1 also mediates the uptake of vitamin C. In RPE cells this may be important since the high energy requirements of the retina will produce high-energy radicals whose harmful effects may be moderated by the high levels of vitamin C.183
Of the many substances that are essential to vision, vitamin A occupies a prominent place. Vitamin A is transported from the blood to the RPE bound to a complex of RBP and TTR. Upon reaching the basal membrane of RPE cells, retinol is released to the membrane retinol receptor STRA6 (stimulated by retinoic acid 6), which transfers vitamin A into the cells, where it is converted to the active chromophore 11-cis-retinal.184
Ions are transported in and out of RPE cells via selective channels. Intracellular calcium, for example, which is essential to many functions of RPE cells, including growth factor secretion, phagocytosis, ion exchange, and water transport, is mediated by a number of channels; these include L-type and T-type voltage-gated calcium channels,31,185,186 calcium channels of the transient receptor potential canonical (TRPC), specifically TRPC1 and TRPC4.187,188 Recently, two calcium transport channels, TRPV5 and TRPV6, with a calcium–sodium selectivity of more than 100 : 1 for calcium versus sodium, have also been identified in RPE cells.189
RPE cells are responsible for removing water, ions, and catabolites from the retina to the choriocapillaries; however, RPE cell tight junctions establish a barrier between the subretinal space and the choriocapillaris such that water, ions or other molecules cannot leave via the paracellular route, but must be actively transported transcellularly. Na+,K+-ATPase, which is localized at the apical surface of RPE cells, provides the energy for transepithelial transport, and controls the flux of sodium and potassium ions across the plasma membrane, thereby maintaining the proper balance of these ions in the IPM and establishing membrane potentials. Water is eliminated from the subretinal space by active transport by the RPE and is mediated by the transepithelial transport of chloride ion from the subretinal space, across the RPE to the choroid.190 Water movement across the RPE is also facilitated by Aquaporin-1 channels.191,192 It is critical that water formed in the retina as a result of high metabolic activity is removed, since excess water in the retina will result in macular edema, RPE and photoreceptor degeneration, and eventually blindness. Elimination of lactic acid from the subretinal space is mediated by different monocarboxylate transporters in the apical (MCT1) and basolateral (MCT3) membranes.36,37
Secretion of cytokines and growth factors
RPE cells secrete many cytokines and growth factors that regulate many of the cellular pathways necessary for function, survival, and response to injury. The best known of these factors are VEGF and PEDF. VEGF, which is constitutively secreted from the basal surface of the RPE towards the choriocapillaris, has two main functions: (1) to provide prosurvival signals to the choroidal vascular endothelial cells; and (2) to maintain the fenestrations of the choriocapillaris endothelium.193 PEDF is predominantly secreted from the apical surface where it promotes an antiangiogenic and neuroprotective environment for the photoreceptors.88 Many of these factors show dysregulated expression in retinal diseases such as AMD, diabetic retinopathy, and PVR. The most common of these factors are listed in Table 16.2.
Table 16.2 Growth factors produced by retinal pigment epithelium cells
| Factor | Function | References |
|---|---|---|
| Pigment epithelial-derived factor (PEDF) | Neuroprotection, neurogenesis, antiangiogenesis | 88,143–147 |
| Vascular endothelial growth factor (VEGF) | Angiogenesis, endothelial cell survival factor, maintenance of choriocapillaris fenestrations | 88,174,193 |
| Nerve growth factor (NGF) | Survival and maintenance of sympathetic and sensory neurons. Ocular immune response | 194,195 |
| Brain-derived neurotrophic factor (BDNF) | Supports the survival of existing neurons, and fosters the growth and differentiation of new neurons and synapses | 195,196 |
| Neurotrophin-3 (NT-3) | Stimulation and control of neurogenesis | 196 |
| Insulin-like growth factor (IGF-1) | Regulation of neurogenesis, myelination, synaptogenesis, and dendritic branching and neuroprotection after neuronal damage | 197–199 |
| Neuroprotectin 1 (NPD1) | Protects neural tissues from injury caused by free radicals and other oxidative stress | 200–202 |
| Transforming growth factor-β (TGFβ) | Regulation of many cellular processes in the adult and developing embryo, including cell growth and differentiation and cellular homeostasis | 203–206 |
| Granulocyte–macrophage colony-stimulating factor (GM-CSF) | Stimulation of stem cells to differentiate into granulocytes and monocytes | 207–209 |
| Monocyte chemotactic protein-1 (MCP-1) | Monocyte chemoattractant | 210,211 |
| Hepatocyte growth factor (HGF) | Hepatocyte growth factor regulates cell growth, cell adhesion, cell motility, and morphogenesis | 212–214 |
| Erythropoietin (EPO) | Regulates red blood cell production. EPO protects retina from physiologic and pathologic light-induced oxidative injury | 215–217 |
| Platelet-activating factor (PAF) | Phospholipid activator and mediator of platelet aggregation, inflammation, and anaphylaxis | 218 |
| Melanoma growth-stimulatory activity/growth-regulated protein (MGSA/GRO) | Enhances chemotaxis of neutrophils and enhances the secretion of elastase and other matrix-degrading enzymes | 219,220 |
| Endothelin 1 | Vasoconstriction | 221,222 |
| Fibroblast growth factor | Mitogenic and cell survival activities. Involved in embryonic development, cell growth, morphogenesis, tissue repair, tumor growth, and invasion | 223–226 |
| Bone morphogenetic protein | Regulation of differentiation, senescence, and apoptosis | 227,228 |
| Interleukin-6 | Pleiotropic cytokine with a role in inflammation, hematopoiesis, angiogenesis, cell differentiation, and neuronal survival | 229,230 |
| Interleukin-8 | Interleukin-8 attracts and activates neutrophils | 231,232 |
| Connective tissue growth factor | Intraocular fibrosis | 233 |
![]() For online acknowledgments visit http://www.expertconsult.com
For online acknowledgments visit http://www.expertconsult.com
1 Martinez-Morales JR, Rodrigo I, Bovolenta P. Eye development: a view from the retina pigmented epithelium. Bioessays. 2004;26:766–777.
2 Perron M, Boy S, Amato MA, et al. A novel function for Hedgehog signalling in retinal pigment epithelium differentiation. Development. 2003;130:1565–1577.
3 Stroeva OG, Mitashov VI. Retinal pigment epithelium: proliferation and differentiation during development and regeneration. Int Rev Cytol. 1983;83:221–293.
4 Marmorstein AD. The polarity of the retinal pigment epithelium. Traffic. 2001;2:867–872.
5 Fuhrmann S. Eye morphogenesis and patterning of the optic vesicle. Curr Top Dev Biol. 2010;93:61–84.
6 Zhang XM, Yang XJ. Temporal and spatial effects of Sonic hedgehog signaling in chick eye morphogenesis. Dev Biol. 2001;233:271–290.
7 Matt N, Ghyselinck NB, Pellerin I, et al. Impairing retinoic acid signalling in the neural crest cells is sufficient to alter entire eye morphogenesis. Dev Biol. 2008;320:140–148.
8 Adler R, Belecky-Adams TL. The role of bone morphogenetic proteins in the differentiation of the ventral optic cup. Development. 2002;129:3161–3171.
9 Schouwey K, Aydin IT, Radtke F, et al. RBP-Jkappa-dependent Notch signaling enhances retinal pigment epithelial cell proliferation in transgenic mice. Oncogene. 2011;30:313–322.
10 Burke JM. Epithelial phenotype and the RPE: is the answer blowing in the Wnt? Prog Retin Eye Res. 2008;27:579–595.
11 Bonilha VL, Rayborn ME, Saotome I, et al. Microvilli defects in retinas of ezrin knockout mice. Exp Eye Res. 2006;82:720–729.
12 Lawrence MS, Azar DT. Myopia and models and mechanisms of refractive error control. Ophthalmol Clin North Am. 2002;15:127–133.
13 Panda-Jonas S, Jonas JB, Jakobczyk-Zmija M. Retinal pigment epithelial cell count, distribution, and correlations in normal human eyes. Am J Ophthalmol. 1996;121:181–189.
14 Al-Hussaini H, Kam JH, Vugler A, et al. Mature retinal pigment epithelium cells are retained in the cell cycle and proliferate in vivo. Mol Vision. 2008;14:1784–1791.
15 Schmidt SY, Peisch RD. Melanin concentration in normal human retinal pigment epithelium. Regional variation and age-related reduction. Invest Ophthalmol Vis Sci. 1986;27:1063–1067.
16 Sarna T, Burke JM, Korytowski W, et al. Loss of melanin from human RPE with aging: possible role of melanin photooxidation. Exp Eye Res. 2003;76:89–98.
17 Burke JM, Skumatz CM, Irving PE, et al. Phenotypic heterogeneity of retinal pigment epithelial cells in vitro and in situ. Exp Eye Res. 1996;62:63–73.
18 Burke JM, Soref C. Topographical variation in growth in cultured bovine retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1988;29:1784–1788.
19 Burke JM, McKay BS, Jaffe GJ. Retinal pigment epithelial cells of the posterior pole have fewer Na/K adenosine triphosphatase pumps than peripheral cells. Invest Ophthalmol Vis Sci. 1991;32:2042–2046.
20 McLaren MJ. Kinetics of rod outer segment phagocytosis by cultured retinal pigment epithelial cells. Relationship to cell morphology. Invest Ophthalmol Vis Sci. 1996;37:1213–1224.
21 Cabral L, Unger W, Boulton M, et al. Regional distribution of lysosomal enzymes in the canine retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1990;31:670–676.
22 Hjelmeland LM, Fujikawa A, Oltjen SL, et al. Quantification of retinal pigment epithelial phenotypic variation using laser scanning cytometry. Mol Vision. 2010;16:1108–1121.
23 Gulcan HG, Alvarez RA, Maude MB, et al. Lipids of human retina, retinal pigment epithelium, and Bruch’s membrane/choroid: comparison of macular and peripheral regions. Invest Ophthalmol Vis Sci. 1993;34:3187–3193.
24 Bonilha VL, Rayborn ME, Bhattacharya SK, et al. The retinal pigment epithelium apical microvilli and retinal function. Adv Exp Med Biol. 2006;572:519–524.
25 Young RW. The renewal of rod and cone outer segments in the rhesus monkey. J Cell Biol. 1971;49:303–318.
26 Bonnel S, Mohand-Said S, Sahel JA. The aging of the retina. Exp Gerontol. 2003;38:825–831.
27 Bonilha VL, Bhattacharya SK, West KA, et al. Proteomic characterization of isolated retinal pigment epithelium microvilli. Mol Cell Proteomics. 2004;3:1119–1127.
28 Finnemann SC, Bonilha VL, Marmorstein AD, et al. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc Natl Acad Sci U S A. 1997;94:12932–12937.
29 Tarnowski BI, Shepherd VL, McLaughlin BJ. Mannose 6-phosphate receptors on the plasma membrane on rat retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1988;29:291–297.
30 Ryeom SW, Sparrow JR, Silverstein RL. CD36 participates in the phagocytosis of rod outer segments by retinal pigment epithelium. J Cell Sci. 1996;109:387–395.
31 Wimmers S, Karl MO, Strauss O. Ion channels in the RPE. Prog Retin Eye Res. 2007;26:263–301.
32 Chuang JZ, Chou SY, Sung CH. Chloride intracellular channel 4 is critical for the epithelial morphogenesis of RPE cells and retinal attachment. Mol Biol Cell. 2010;21:3017–3028.
33 Milenkovic VM, Krejcova S, Reichhart N, et al. Interaction of bestrophin-1 and Ca2+ channels beta-subunits: identification of new binding domains on the bestrophic-1 C-terminus. PLoS ONE. 2011;6:e19364.
34 Bonilha VL, Finnemann SC, Rodriguez-Bouan E. Ezrin promotes morphogenesis of apical microvilli and basal infoldings in retinal pigment epithelium. J Cell Biol. 1999;147:1533–1547.
35 Senanayake P, Calabro A, Hu JG, et al. Glucose utilization by the retinal pigment epithelium: evidence for rapid uptake and storage in glycogen, followed by glycogen utilization. Exp Eye Res. 2006;83:235–246.
36 Philp NJ, Wang D, Yoon H, et al. Polarized expression of monocarboxylate transporters in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44:1716–1721.
37 Gallagher-Colombo S, Maminishkis A, Tate S, et al. Modulation of MCT3 expression during wound healing of the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2010;51:5343–5350.
38 Korte GE, Perlman JI, Pollack A. Regeneration of mammalian retinal pigment epithelium. Int Rev Cytol. 1994;152:223–263.
39 Gouras P, Ivert L, Neuringer M, et al. Topographic and age-related changes of the retinal epithelium and Bruch’s membrane of rhesus monkeys. Graefes Arch Clin Exp Ophthalmol. 2010;248:973–984.
40 Peters S, Reinthal E, Blitgen-Heinecke P, et al. Inhibition of lysosomal degradation in retinal pigment epithelium cells induces exocytosis of phagocytic residual material at the basolateral plasma membrane. Ophthalmic Res. 2006;38:83–88.
41 Fine BS. Limiting membranes of the sensory retina and pigment epithelium. An electron microscopic study. Arch Ophthalmol. 1961;66:847–860.
42 Rodriguez-Boulan E. Genesis of polarity in renal tubular cells. Miner Electrolyte Metab. 1986;12:20–24.
43 Georgiadis A, Tschernutter M, Bainbridge JW, et al. The tight junction associated signalling proteins ZO-1 and ZONAB regulate retinal pigment epithelium homeostasis in mice. PLoS ONE. 2010;5:e15730.
44 Luo Y, Fukuhara M, Weitzman M, et al. Expression of JAM-A, AF-6, PAR-3 and PAR-6 during the assembly and remodeling of RPE tight junctions. Brain Res. 2006;1110:55–63.
45 Peng S, Rahner C, Rizzolo LJ. Apical and basal regulation of the permeability of the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44:808–817.
46 Peng S, Rao VS, Adelman RA, et al. Claudin-19 and the barrier properties of the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011;52:1392–1403.
47 Economopoulou M, Hammer J, Wang F, et al. Expression, localization, and function of junctional adhesion molecule-C (JAM-C) in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2009;50:1454–1463.
48 Bailey TA, Kanuga N, Romero IA, et al. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:675–684.
49 Rajasekaran SA, Hu J, Gopal J, et al. Na,K-ATPase inhibition alters tight junction structure and permeability in human retinal pigment epithelial cells. Am J Physiol Cell Physiol. 2003;284:C1497–C1507.
50 Zhou J, He S, Zhang N, et al. Neutrophils compromise retinal pigment epithelial barrier integrity. J Biomed Biotechnol. 2010:289–360.
51 Zech JC, Pouvreau I, Cotinet A, et al. Effect of cytokines and nitric oxide on tight junctions in cultured rat retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1998;39:1600–1608.
52 Bruban J, Glotin AL, Dinet V, et al. Amyloid-beta(1–42) alters structure and function of retinal pigmented epithelial cells. Aging Cell. 2009;8:162–177.
53 Crawford BJ. Development of the junctional complex during differentiation of chick pigmented epithelial cells in clonal culture. Invest Ophthalmol Vis Sci. 1980;19:223–237.
54 Hudspeth AJ, Yee AG. The intercellular junctional complexes of retinal pigment epithelia. Invest Ophthalmol. 1973;12:354–365.
55 Kalnins VI, Sandig M, Hergott GJ, et al. Microfilament organization and wound repair in retinal pigment epithelium. Biochem Cell Biol. 1995;73:709–722.
56 Sandig M, Kalnins VI. Subunits in zonulae adhaerentes and striations in the associated circumferential microfilament bundles in chicken retinal pigment epithelial cells in situ. Exp Cell Res. 1988;175:1–14.
57 Tibber MS, Becker D, Jeffery G. Levels of transient gap junctions between the retinal pigment epithelium and the neuroblastic retina are influenced by catecholamines and correlate with patterns of cell production. J Comp Neurol. 2007;503:128–134.
58 Pearson RA, Dale N, Llaudet E, et al. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46:731–744.
59 Opas M, Kalnins VI. Light-microscopical analysis of focal adhesions of retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci. 1986;27:1622–1633.
60 Owaribe K, Kartenbeck J, Rungger-Brandle E, et al. Cytoskeletons of retinal pigment epithelial cells: interspecies differences of expression patterns indicate independence of cell function from the specific complement of cytoskeletal proteins. Cell Tissue Res. 1988;254:301–315.
61 Schlotzer-Schrehardt U, Muller HG, Wirtz PM, et al. Desmosomal–mitochondrial complexes in human nonpigmented ciliary and retinal pigment epithelia. Invest Ophthalmol Vis Sci. 1990;31:664–669.
62 Hunt RC, Davis AA. Altered expression of keratin and vimentin in human retinal pigment epithelial cells in vivo and in vitro. J Cell Physiol. 1990;145:187–199.
63 Grisanti S, Guidry C. Transdifferentiation of retinal pigment epithelial cells from epithelial to mesenchymal phenotype. Invest Ophthalmol Vis Sci. 1995;36:391–405.
64 Lee J, Ko M, Joo CK. Rho plays a key role in TGF-beta1-induced cytoskeletal rearrangement in human retinal pigment epithelium. J Cell Physiol. 2008;216:520–526.
65 Burnside B, Adler R, O’Connor P. Retinomotor pigment migration in the teleost retinal pigment epithelium. I. Roles for actin and microtubules in pigment granule transport and cone movement. Invest Ophthalmol Vis Sci. 1983;24:1–15.
66 McNeil EL, Tacelosky D, Basciano P, et al. Actin-dependent motility of melanosomes from fish retinal pigment epithelial (RPE) cells investigated using in vitro motility assays. Cell Motil Cytoskeleton. 2004;58:71–82.
67 Lin-Jones J, Sohlberg L, Dose A, et al. Identification and localization of myosin superfamily members in fish retina and retinal pigmented epithelium. J Comp Neurol. 2009;513:209–223.
68 Coudrier E. Myosins in melanocytes: to move or not to move? Pigment Cell Res. 2007;20:153–160.
69 Turksen K, Opas M, Aubin JE, et al. Microtubules, microfilaments and adhesion patterns in differentiating chick retinal pigment epithelial (RPE) cells in vitro. Exp Cell Res. 1983;147:379–391.
70 Korte GE. New ultrastructure of rat RPE cells: basal intracytoplasmic tubules. Exp Eye Res. 1984;38:399–409.
71 Robey HL, Hiscott PS, Grierson I. Cytokeratins and retinal epithelial cell behaviour. J Cell Sci. 1992;102:329–340.
72 Bok D. The retinal pigment epithelium: a versatile partner in vision. J Cell Sci Suppl. 1993;17:189–195.
73 Hogan MJ. Ultrastructure of the choroid. Its role in the pathogenesis of chorioretinal disease. Transactions of the Pacific Coast Oto-Ophthalmological Society Annual Meeting. 1961;42:61–87.
74 Hirabayashi Y, Fujimori O, Shimizu S. Bruch’s membrane of the brachymorphic mouse. Med Electron Microsc. 2003;36:139–146.
75 Timpl R. Structure and biological activity of basement membrane proteins. Eur J Biochem. 1989;180:487–502.
76 Marshall GE, Konstas AG, Lee WR. Collagens in ocular tissues. Br J Ophthalmol. 1993;77:515–524.
77 Aisenbrey S, Zhang M, Bacher D, et al. Retinal pigment epithelial cells synthesize laminins, including laminin 5, and adhere to them through alpha3- and alpha6-containing integrins. Invest Ophthalmol Vis Sci. 2006;47:5537–5544.
78 Libby RT, Hunter DD, Brunken WJ. The roles of the extracellular matrix in retinal development and maintenance. In: Fini E, ed. Vertebrate eye development. Berlin: Springer-Verlag; 2000:115–140.
79 Hewitt AT, Nakazawa K, Newsome DA. Analysis of newly synthesized Bruch’s membrane proteoglycans. Invest Ophthalmol Vis Sci. 1989;30:478–486.
80 Bhutto IA, Kim SY, McLeod DS, et al. Localization of collagen XVIII and the endostatin portion of collagen XVIII in aged human control eyes and eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45:1544–1552.
81 Ma DH, Yao JY, Kuo MT, et al. Generation of endostatin by matrix metalloproteinase and cathepsin from human limbocorneal epithelial cells cultivated on amniotic membrane. Invest Ophthalmol Vis Sci. 2007;48:644–651.
82 Booij JC, Baas DC, Beisekeeva J, et al. The dynamic nature of Bruch’s membrane. Prog Retin Eye Res. 2010;29:1–18.
83 Booij JC, Jacoline B, ten Brink JB, et al. A new strategy to identify and annotate human RPE-specific gene expression. PLoS ONE. 2010;5:e9341.
84 Chader GJ. Interphotoreceptor retinoid-binding protein (IRBP): a model protein for molecular biological and clinically relevant studies. Friedenwald lecture. Invest Ophthalmol Vis Sci. 1989;30:7–22.
85 Adler AJ, Evans CD. Proteins of the bovine interphotoreceptor matrix: retinoid binding and other functions. Prog Clin Biol Res. 1985;190:65–88.
86 Semenova EM, Converse CA. Comparison between oleic acid and docosahexaenoic acid binding to interphotoreceptor retinoid-binding protein. Vision Res. 2003;43:3063–3067.
87 Cunningham LL, Gonzalez-Fernandez F. Internalization of interphotoreceptor retinoid-binding protein by the Xenopus retinal pigment epithelium. J Comp Neurol. 2003;466:331–342.
88 Sonoda S, Sreekumar PG, Kase S, et al. Attainment of polarity promotes growth factor secretion by retinal pigment epithelial cells: relevance to age-related macular degeneration. Aging. 2009;2:28–42.
89 Hauck SM, Schoeffmann S, Deeg CA, et al. Proteomic analysis of the porcine interphotoreceptor matrix. Proteomics. 2005;5:3623–3636.
90 Sreekumar PG, Kannan R, Kitamura M, et al. alphaB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS ONE. 2010;5:e12578.
91 Elner SG, Elner VM, Kindzelskii AL, et al. Human RPE cell lysis of extracellular matrix: functional urokinase plasminogen activator receptor (uPAR), collagenase and elastase. Exp Eye Res. 2003;76:585–595.
92 Alexander JP, Bradley JM, Gabourel JD, et al. Expression of matrix metalloproteinases and inhibitor by human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1990;31:2520–2528.
93 Smine A, Plantner JJ. Membrane type-1 matrix metalloproteinase in human ocular tissues. Curr Eye Res. 1997;16:925–929.
94 Jomary C, Neal MJ, Iwata K, et al. Localization of tissue inhibitor of metalloproteinases-3 in neurodegenerative retinal disease. Neuroreport. 1997;8:2169–2172.
95 Siren V, Immonen I. uPA, tPA and PAI-1 mRNA expression in periretinal membranes. Curr Eye Res. 2003;27:261–267.
96 Andreasen PA, Kjoller L, Christensen L, et al. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer. 1997;72:1–22.
97 Siren V, Stephens RW, Salonen EM, et al. Retinal pigment epithelial cells secrete urokinase-type plasminogen activator and its inhibitor PAI-1. Ophthalmic Res. 1992;24:203–212.
98 Singh MS, MacLaren RE. Stem cells as a therapeutic tool for the blind: biology and future prospects. Proc R Soc B. 2011;278:3009–3016.
99 Hu J, Bok D. A cell culture medium that supports the differentiation of human retinal pigment epithelium into functionally polarized monolayers. Mol Vis. 2001;7:14–19.
100 Maminishkis A, Chen S, Jalickee S, et al. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–3624.
101 Sonoda S, Spee C, Barron E, et al. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat Protoc. 2009;4:662–673.
102 Maminishkis A, Miller SS. Experimental models for study of retinal pigment epithelial physiology and pathophysiology. J Vis Exp. 2010. doi: 10.3791/2032
103 Vossmerbaeumer U, Kuehl S, Kern S, et al. Induction of retinal pigment epithelium properties in ciliary margin progenitor cells. Clin Exp Ophthalmol. 2008;36:358–366.
104 Aruta C, Giordano F, De Marzo A, et al. In vitro differentiation of retinal pigment epithelium from adult retinal stem cells. Pigment Cell. Melanoma Res. 2011;24:233–240.
105 Idelson M, Alper R, Obolensky A, et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. 2009;5:396–408.
106 Zhu D, Deng X, Spee C, et al. Polarized secretion of PEDF from human embryonic stem cell-derived RPE promotes retinal progenitor cell survival. Invest Ophthalmol Vis Sci. 2011;52:1573–1585.
107 Buchholz DE, Hikita ST, Rowland TJ, et al. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009;27:2427–2434.
108 Hattar S, Liao HW, Takao M, et al. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070.
109 Allen AE, Cameron MA, Brown TM, et al. Visual responses in mice lacking critical components of all known retinal phototransduction cascades. PLoS ONE. 2010;5:e15063.
110 Tamai M, Chader GJ. The early appearance of disc shedding in the rat retina. Invest Ophthalmol Vis Sci. 1979;18:913–917.
111 Tamai M, Teirstein P, Goldman A, et al. The pineal gland does not control rod outer segment shedding and phagocytosis in the rat retina and pigment epithelium. Invest Ophthalmol Vis Sci. 1978;17:558–562.
112 Young RW. The daily rhythm of shedding and degradation of cone outer segment membranes in the lizard retina. J Ultrastruct Res. 1977;61:172–185.
113 Anderson DH, Fisher SK. Disc shedding in rod like and cone like photoreceptors of tree squirrels. Science. 1975;187:953–955.
114 Anderson DH, Fisher SK, Erickson PA, et al. Rod and cone disc shedding in the rhesus monkey retina: a quantitative study. Exp Eye Res. 1980;30:559–574.
115 Fisher SK, Pfeffer BA, Anderson DH. Both rod and cone disc shedding are related to light onset in the cat. Invest Ophthalmol Vis Sci. 1983;24:844–856.
116 Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72.
117 Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392–403.
118 Young RW. Biological renewal; applications to the eye. Trans Ophthalmol Soc UK. 1982;102:42–75.
119 Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology. 2010;25:8–15.
120 Boesze-Battaglia K, Albert AD. Phospholipid distribution among bovine rod outer segment plasma membrane and disk membranes. Exp Eye Res. 1992;54:821–823.
121 Nandrot EF, Anand M, Dena Almeida D, et al. Essential role for MFG-E8 as ligand for αvβ5 integrin in diurnal retinal phagocytosis. Proc Natl Acad Sci U S A. 2007;104:12005–12010.
122 Caberoy NB, Maiguel D, Kim Y, et al. Identification of tubby and tubby-like protein 1 as eat-me signals by phage display. Exp Cell Res. 2010;316:245–257.
123 Chaitin MH, Hall MO. The distribution of actin in cultured normal and dystrophic rat pigment epithelial cells during the phagocytosis of rod outer segments. Invest Ophthalmol Vis Sci. 1983;24:821–831.
124 Hall MO, Abrams T, Mittag TW. ROS ingestion by RPE cells is turned off by increased protein kinase C activity and by increased calcium. Exp Eye Res. 1991;52:591–598.
125 Hall MO, Obin MS, Prieto AL, et al. Gas6 binding to photoreceptor outer segments requires gamma-carboxyglutamic acid (Gla) and Ca2+ and is required for OS phagocytosis by RPE cells in vitro. Exp Eye Res. 2002;75:391–400.
126 Besharse JC, Dunis DA. Rod photoreceptor disc shedding in vitro: inhibition by cytochalasins and activation by colchicine. In: Hollyfield JG, ed. The structure of the eye. New York: Elsevier Biomedical, 1982.
127 Herman KG, Steinberg RH. Phagosome movement and the diurnal pattern of phagocytosis in the tapetal retinal pigment epithelium of the opossum. Invest Ophthalmol Vis Sci. 1982;23:277–290.
128 Gibbs D, Kitamoto J, Williams DS. Abnormal phagocytosis by retinal pigmented epithelium that lack myosin VIIa, the Usher syndrome 1B protein. Proc Natl Acad Sci U S A. 2003;100:6481–64860.
129 Hayashi A, Nakae K, Naka H, et al. Cytokine effects on phagocytosis of rod outer segments by retinal pigment epithelial cells of normal and dystrophic rats. Curr Eye Res. 1996;15:487–499.
130 Rakoczy PE, Baines M, Kennedy CJ, et al. Correlation between autofluorescent debris accumulation and the presence of partially processed forms of cathepsin D in cultured retinal pigment epithelial cells challenged with rod outer segments. Exp Eye Res. 1996;63:159–167.
131 Regan CM, De Grip WJ, Daemen FJM, et al. Degradation of rhodopsin by a lysosomal fraction of retinal pigment epithelium: biochemical aspects of the visual process, XLI. Exp Eye Res. 1980;30:183–191.
132 Zimmerman WF, Godchaux W, III., Belkin M. The relative proportions of lysosomal enzyme activities in bovine retinal pigment epithelium. Exp Eye Res. 1983;36:151–158.
133 Rakoczy PE, Mann K, Cavaney DM, et al. Detection and possible functions of a cysteine protease involved in digestion of rod outer segments by retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1994;35:4100–4108.
134 Feeney L, Mixon RL. An in vitro model of phagocytosis in bovine and human retinal pigment epithelium. Exp Eye Res. 1976;22:533–548.
135 Feeney-Burns L, Hilderbrand ES, Eldridge S. Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest Ophthalmol Vis Sci. 1984;25:195–200.
136 Pollock NL, Callaghan R. The lipid translocase, ABCA4: seeing is believing. FEBS J. 2011;278:3204–3214.
137 Edwards RB, Adler AJ. IRBP enhances removal of 11-cis-retinaldehyde from isolated RPE membranes. Exp Eye Res. 2000;70:235–245.
138 Wu Q, Blakeley LR, Cornwall MC, et al. Interphotoreceptor retinoid binding protein is the physiologically relevant carrier that removes retinol from rod photoreceptor outer segments. Biochemistry. 2007;46:8669–8679.
139 Jin M, Li S, Nusinowitz S, et al. The role of interphotoreceptor retinoid-binding protein on the translocation of visual retinoids and function of cone photoreceptors. J Neurosci. 2009;29:1486–1495.
140 Cai J, Nelson KC, Wu M, et al. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–221.
141 Mukherjee PK, Marcheselli VL, Serhan CN, et al. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A 2004;101:8491–6.
142 Lukiw WJ, Cui JG, Marcheselli VL, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783.
143 Wu YQ, Notario V, Chader GJ, et al. Identification of pigment epithelium-derived factor in the interphotoreceptor matrix of bovine eyes. Protein Expr Purif. 1995;6:447–456.
144 Perez-Mediavilla LA, Chew C, Campochiaro PA, et al. Sequence and expression analysis of bovine pigment epithelium-derived factor. Biochim Biophys Acta. 1998;1398:203–214.
145 Bilak MM, Corse AM, Bilak SR, et al. Pigment epithelium-derived factor (PEDF) protects motor neurons from chronic glutamate-mediated neurodegeneration. J Neuropathol Exp Neurol. 1999;58:719–728.
146 Duh EJ, Yang HS, Suzuma I, et al. Pigment epithelium-derived factor suppresses ischemia-induced retinal neovascularization and VEGF-induced migration and growth. Invest Ophthalmol Vis Sci. 2002;43:821–829.
147 Ogata N, Wang L, Jo N, et al. Pigment epithelium derived factor as a neuroprotective agent against ischemic retinal injury. Curr Eye Res. 2001;22:245–252.
148 Faktorovich E, Steinberg R, Yasumara D, et al. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature. 1990;347:83–86.
149 Campochiaro PA, Chang M, Ohsato M, et al. Retinal degeneration in transgenic mice with photoreceptor-specific expression of a dominant-negative fibroblast growth factor receptor. J Neurosci. 1996;16:1679–1688.
150 Marí M, Morales A, Colell A, et al. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal. 2009;11:2685–2700.
151 Holmgren A, Lu J. Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem Biophys Res Commun. 2010;396:120–124.
152 Sreekumar PG, Ding Y, Ryan SJ, et al. Regulation of thioredoxin by ceramide in retinal pigment epithelial cells. Exp Eye Res. 2009;88:410–417.
153 Sreekumar PG, Hinton DR, Kannan R. Methionine sulfoxide reductase A: Structure, function and role in ocular pathology. World J Biol Chem. 2011;2:184–192.
154 Kannan R, Jin M, Gamulescu M-A, et al. Ceramide-induced apoptosis: role of catalase and hepatocyte growth factor. Free Radic Biol Med. 2004;37:166–175.
155 Dong A, Shen J, Krause M, et al. Superoxide dismutase 1 protects retinal cells from oxidative damage. J Cell Physiol. 2006;208:516–526.
156 Usui S, Oveson BC, Iwase T, et al. Overexpression of SOD in retina: need for increase in H2O2-detoxifying enzyme in same cellular compartment. Free Radic Biol Med. 2011;51:1347–1354.
157 Zhu D, Sreekumar PG, Hinton DR, et al. Expression and regulation of enzymes in the ceramide metabolic pathway in human retinal pigment epithelial cells and their relevance to retinal degeneration. Vision Res. 2010;50:643–651.
158 Jin M, Yaung J, Kannan R, et al. Hepatocyte growth factor protects RPE cells from apoptosis induced by glutathione depletion. Invest Ophthalmol Vis Sci. 2005;46:4311–4319.
159 Johnson J, Maher P, Hanneken A. The flavonoid, eriodictyol, induces long-term protection in ARPE-19 cells through its effects on Nrf2 activation and phase 2 gene expression. Invest Ophthalmol Vis Sci. 2009;50:2398–2406.
160 Kowluru RA, Kowluru V, Xiong Y, et al. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Radic Biol Med. 2006;41:1191–1196.
161 Inomata Y, Nakamura H, Tanito M, et al. Thioredoxin inhibits NMDA-induced neurotoxicity in the rat retina. J Neurochem. 2006;98:372–385.
162 Wu H, Lin L, Giblin F, et al. Glutaredoxin 2 knockout increases sensitivity to oxidative stress in mouse lens epithelial cells. Free Radic Biol Med. 2011;51:2108–2117.
163 Wu H, Xing K, Lou MF. Glutaredoxin 2 prevents H(2)O(2)-induced cell apoptosis by protecting complex I activity in the mitochondria. Biochim Biophys Acta. 2010;1797:1705–1715.
164 Sreekumar PG, Kannan R, Yaung J, et al. Protection from oxidative stress by methionine sulfoxide reductases in RPE cells. Biochem Biophys Res Commun. 2005;334:245–253.
165 Mao YW, Liu JP, Xiang H, et al. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11:512–526.
166 Yaung J, Jin M, Barron E, et al. alpha-Crystallin distribution in retinal pigment epithelium and effect of gene knockouts on sensitivity to oxidative stress. Mol Vis. 2007;13:566–577.
167 Yaung J, Kannan R, Wawrousek EF, et al. Exacerbation of retinal degeneration in the absence of alpha crystallins in an in vivo model of chemically induced hypoxia. Exp Eye Res. 2008;86:355–365.
168 Pasupuleti N, Matsuyama S, Voss O, et al. The anti-apoptotic function of human αA-crystallin is directly related to its chaperone activity. Cell Death Dis. 2010;1:e31.
169 Kannan R, Sreekumar PG, Hinton DR. Glutathione metabolism and its contribution to antiapoptotic properties of alpha crystallins. In: Stratton RD, Hauswirth WW, Gardner TW. Studies on retinal and choroidal disorders. New York, NY: Springer, 2012. Chapter IX
170 Filleur S, Volz K, Nelius T, et al. Two functional epitopes of pigment epithelial-derived factor block angiogenesis and induce differentiation in prostate cancer. Cancer Res. 2005;65:5144–5152.
171 Bouck N. PEDF: anti-angiogenic guardian of ocular function. Trends Mol Med. 2002;8:330–334.
172 Bhutto IA, Mcleod DS, Hasegawa T, et al. Pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroid and eyes with age-related macular degeneration. Exp Eye Res. 2006;82:99–110.
173 Kozulin P, Natoli R, Bumsted O’Brien KM, et al. The cellular expression of antiangiogenic factors in fetal primate macula. Invest Ophthalmol Vis Sci. 2010;51:4298–4306.
174 Kociok N, Joussen AM. Varied expression of functionally important genes of RPE and choroid in the macula and in the periphery of normal human eyes. Graefes Arch Clin Exp Ophthalmol. 2007;245:101–113.
175 Marneros AG, She H, Zambarakji H, et al. Endogenous endostatin inhibits choroidal neovascularization. FASEB J. 2007;21:3809–3818.
176 Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58–69.
177 Streilein JW, Ma N, Wenkel H, et al. Immunobiology and privilege of neuronal retina and pigment epithelium transplants. Vis Res. 2002;42:487–495.
178 Masli S, Vega JL. Ocular immune privileged sites. Methods Mol Biol. 2011;677:449–458.
179 Zhou R, Horai R, Mattapallil MJ, et al. A new look at immune privilege of the eye: dual role for the vision-related molecule retinoic acid. J Immunol. 2011;187:4170–4177.
180 Ban Y, Rizzolo LJ. Regulation of glucose transporters during development of the retinal pigment epithelium. Dev Brain Res. 2000;121:89–95.
181 Bergersen L, Jóhannsson E, Veruki ML, et al. Cellular and subcellular expression of monocarboxylate transporters in the pigment epithelium and retina of the rat. Neuroscience. 1999;90:319–331.
182 Kim D-I, Lim S-K, Park M-J, et al. The involvement of phosphatidylinositol 3-kinase/Akt signaling in high glucose-induced downregulation of GLUT-1 expression in ARPE cells. Life Sci. 2007;80:626–632.
183 Montel-Hagen A, Kinet S, Manel N, et al. Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell. 2008;132:1039–1048.
184 Bridges CD, Alvarez RA, Fong SL, et al. Visual cycle in the mammalian eye. Retinoid-binding proteins and the distribution of 11-cis retinoids. Vision Res. 1984;24:1581–1594.
185 Wimmers S, Coeppicus L, Rosenthal R, et al. Expression profile of voltage-dependent Ca2+ channel subunits in the human retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol. 2008;246:685–692.
186 Wimmers S, Halsband C, Seyler S, et al. Voltage-dependent Ca2+ channels, not ryanodine receptors, activate Ca2+-dependent BK potassium channels in human retinal pigment epithelial cells. Mol Vis. 2008;14:2340–2348.
187 Bollimuntha S, Cornatzer E, Singh BB. Plasma membrane localization and function of TRPC1 is dependent on its interaction with beta-tubulin in retinal epithelium cells. Vis Neurosci. 2005;22:163–170.
188 Wimmers S, Strauss O. Basal calcium entry in retinal pigment epithelial cells is mediated by TRPC channels. Invest Ophthalmol Vis Sci. 2007;48:5767–5772.
189 Kennedy BG, Torabi AJ, Kurzawa R, et al. Expression of transient receptor potential vanilloid channels in retinal pigment epithelium. Mol Vis. 2010;16:665–675.
190 Moseley H, Foulds WS, Allan D, et al. Routes of clearance of radioactive water from the rabbit vitreous. Br J Ophthalmol. 1984;68:145–151.
191 Stamer WD, Bok D, Hu J, et al. Aquaporin-1 channels in human retinal pigment epithelium: role in transepithelial water movement. Invest Ophthalmol Vis Sci. 2003;44:2803–2808.
192 Motulsky E, Philippe Koch P, Sarah Janssens S, et al. Aquaporin expression in blood–retinal barrier cells during experimental autoimmune uveitis. Mol Vision. 2010;16:602–610.
193 Blaauwgeers HG, Holtkamp GM, Rutten H, et al. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol. 1999;155:421–428.
194 Dicou E, Nerriere V, Naud MC, et al. NGF involvement in ocular inflammation: secretion by rat resident retinal cells. Neuroreport. 1994;6:26–28.
195 Ishida K, Yoshimura N, Yoshida M, et al. Expression of neurotrophic factors in cultured human retinal pigment epithelial cells. Curr Eye Res. 1997;16:96–101.
196 Hackett SF, Friedman Z, Freund J, et al. A splice variant of trkB and brain-derived neurotrophic factor are co-expressed in retinal pigmented epithelial cells and promote differentiated characteristics. Brain Res. 1998;789:201–212.
197 Rosenthal R, Wohlleben H, Malek G, et al. Insulin-like growth factor-1 contributes to neovascularization in age-related macular degeneration. Biochem Biophys Res Commun. 2004;323:1203–1208.
198 Lambooij AC, van Wely KH, Lindenbergh-Kortleve DJ, et al. Insulin-like growth factor-I and its receptor in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2003;44:2192–2198.
199 Weng CY, Kothary PC, Verkade AJ, et al. MAP kinase pathway is involved in IGF-1-stimulated proliferation of human retinal pigment epithelial cells (hRPE). Curr Eye Res. 2009;34:867–876.
200 Halapin NA, Bazan NG. PD1 induction of retinal pigment epithelial cell survival involves PI3K/Akt phosphorylation signaling. Neurochem Res. 2010;35:1944–1947.
201 Calandria JM, Bazan NG. Neuroprotectin d1 modulates the induction of pro-inflammatory signaling and promotes retinal pigment epithelial cell survival during oxidative stress. Adv Exp Med Biol. 2010;664:663–670.
202 Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29:263–271.
203 Tan J, Deng ZH, Liu SZ, et al. TGF-beta2 in human retinal pigment epithelial cells: expression and secretion regulated by cholinergic signals in vitro. Curr Eye Res. 2010;35:37–44.
204 Yu AL, Fuchshofer R, Kook D, et al. Subtoxic oxidative stress induces senescence in retinal pigment epithelial cells via TGF-beta release. Invest Ophthalmol Vis Sci. 2009;50:926–935.
205 Uchida H, Kuroki M, Shitama T, et al. Activation of TGF-beta1 through up-regulation of TSP-1 by retinoic acid in retinal pigment epithelial cells. Curr Eye Res. 2008;33:199–203.
206 Nagineni CN, Cherukuri KS, Kutty V, et al. Interferon-gamma differentially regulates TGF-beta1 and TGF-beta2 expression in human retinal pigment epithelial cells through JAK-STAT pathway. J Cell Physiol. 2007;210:192–200.
207 Crane IJ, Kuppner MC, McKillop-Smith S, et al. Cytokine regulation of granulocyte–macrophage colony-stimulating factor (GM-CSF) production by human retinal pigment epithelial cells. Clin Exp Immunol. 1999;115:288–289.
208 Crane IJ, Wallace CA, Forrester JV. Regulation of granulocyte–macrophage colony-stimulating factor in human retinal pigment epithelial cells by IL-1beta and IFN-gamma. Cell Immunol. 2001;209:132–139.
209 Fukuoka Y, Strainic M, Medof ME. Differential cytokine expression of human retinal pigment epithelial cells in response to stimulation by C5a. Clin Exp Immunol. 2003;131:248–253.
210 Yang D, Elner SG, Chen X, et al. MCP-1-activated monocytes induce apoptosis in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011;52:6026–6034.
211 Pons M, Marin-Castaño ME. Cigarette smoke-related hydroquinone dysregulates MCP-1, VEGF and PEDF expression in retinal pigment epithelium in vitro and in vivo. PLoS ONE. 2011;6:e16722.
212 Chu R, Zheng X, Chen D, et al. Blue light irradiation inhibits the production of HGF by human retinal pigment epithelium cells in vitro. Photochem Photobiol. 2006;82:1247–1250.
213 Ohtaka K, Machida S, Ohzeki T, et al. Protective effect of hepatocyte growth factor against degeneration of the retinal pigment epithelium and photoreceptor in sodium iodate-injected rats. Curr Eye Res. 2006;31:347–355.
214 Jin M, Chen Y, He S, et al. Hepatocyte growth factor and its role in the pathogenesis of retinal detachment. Invest Ophthalmol Vis Sci. 2004;45:323–329.
215 Gawad AE, Schlichting L, Strauss O, et al. Antiapoptotic properties of erythropoietin: novel strategies for protection of retinal pigment epithelial cells. Eye. 2009;23:2245–2250.
216 Wang ZY, Shen LJ, Tu L, et al. Erythropoietin protects retinal pigment epithelial cells from oxidative damage. Free Radic Biol Med. 2009;46:1032–1041.
217 Xie Z, Wu X, Qiu Q, et al. Expression pattern of erythropoietin and erythropoietin receptor in experimental model of retinal detachment. Curr Eye Res. 2007;32:757–764.
218 He YG, Wang H, Zhao B, et al. Functional analysis of platelet-activating factor in the retinal pigment epithelial cells and choroidal endothelial cells. Curr Eye Res. 2009;34:957–965.
219 Shattuck RL, Wood LD, Jaffe GJ, et al. MGSA/GRO transcription is differentially regulated in normal retinal pigment epithelial and melanoma cells. Mol Cell Biol. 1994;14:791–802.
220 Jaffe GJ, Richmond A, Van Le L, et al. Expression of three forms of melanoma growth stimulating activity (MGSA)/gro in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1993;34:2776–2785.
221 Narayan S, Brun AM, Yorio T. Endothelin-1 distribution and basolateral secretion in the retinal pigment epithelium. Exp Eye Res. 2004;79:11–19.
222 Narayan S, Prasanna G, Tchedre K, et al. Thrombin-induced endothelin-1 synthesis and secretion in retinal pigment epithelial cells is rho kinase dependent. J Ocul Pharmacol Ther. 2010;26:389–397.
223 Mascarelli F, Hecquet C, Guillonneau X, et al. Control of the intracellular signaling induced by fibroblast growth factors (FGF) over the proliferation and survival of retinal pigment epithelium cells: example of the signaling regulation of growth factors endogenous to the retina. J Soc Biol. 2001;195:101–106.
224 Bryckaert M, Guillonneau X, Hecquet C, et al. Regulation of proliferation-survival decisions is controlled by FGF1 secretion in retinal pigmented epithelial cells. Oncogene. 2000;19:4917–4929.
225 Bryckaert M, Guillonneau X, Hecquet C, et al. Both FGF1 and bcl-x synthesis are necessary for the reduction of apoptosis in retinal pigmented epithelial cells by FGF2: role of the extracellular signal-regulated kinase 2. Oncogene. 1999;18:7584–7593.
226 Seidler DG, Schwegler JS, Liesenhoff H. Expression of fibroblast growth factors 1 and 2 in a human retinal pigment epithelium cell line (K1034). Ophthalmic Res. 1999;31:280–286.
227 Zhu D, Wu J, Spee C, et al. BMP4 mediates oxidative stress-induced retinal pigment epithelial cell senescence and is overexpressed in age-related macular degeneration. J Biol Chem. 2009;284:9529–9539.
228 Zhu D, Deng X, Xu J, et al. What determines the switch between atrophic and neovascular forms of age related macular degeneration? The role of BMP4 induced senescence. Aging. 2009;1:740–745.
229 Benson MT, Shepherd L, Rees RC, et al. Production of interleukin-6 by human retinal pigment epithelium in vitro and its regulation by other cytokines. Curr Eye Res. 1992;11:173–179.
230 Chong DY, Boehlke CS, Zheng QD, et al. Interleukin-6 as a photoreceptor neuroprotectant in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2008;49:3193–3200.
231 Bian ZM, Elner SG, Yoshida A, et al. Differential involvement of phosphoinositide 3-kinase/Akt in human RPE MCP-1 and IL-8 expression. Invest Ophthalmol Vis Sci. 2004;45:1887–1896.
232 Relvas LJ, Bouffioux C, Marcet B, et al. Extracellular nucleotides and interleukin-8 production by ARPE cells: potential role of danger signals in blood–retinal barrier activation. Invest Ophthalmol Vis Sci. 2009;50:1241–1246.
233 He S, Chen Y, Khankan R, et al. Connective tissue growth factor as a mediator of intraocular fibrosis. Invest Ophthalmol Vis Sci. 2008;49:4078–4088.