Cardiac Arrest and Cardiopulmonary Resuscitation
Epidemiology and General Principles
Sudden cardiac arrest is defined as the cessation of effective cardiac mechanical activity as confirmed by the absence of signs of circulation. Sudden cardiac arrest is the most common fatal manifestation of cardiovascular disease and a leading cause of death worldwide. In North America alone, approximately 350,000 persons annually undergo resuscitation for sudden cardiac arrest. Approximately 25% of sudden cardiac arrest events are due to pulseless ventricular arrhythmias (i.e., ventricular fibrillation [VF] or pulseless ventricular tachycardia [VT]), whereas the rest can be attributed to other cardiac rhythms (i.e., asystole or pulseless electrical activity [PEA]).1 Patients who suffer cardiac arrest due to VF or VT have a much higher chance of surviving the event compared with patients who present with PEA/asystole.2 Patients with ventricular arrhythmias have a better prognosis because (1) ventricular arrhythmias are potentially treatable with defibrillation (i.e., “shockable” initial rhythm) to restore circulation, whereas the other initial rhythms are not, and (2) ventricular arrhythmias are typically a manifestation of a cardiac cause of cardiac arrest (e.g., acute myocardial infarction), whereas the other initial rhythms are more likely to be related to a noncardiac cause and perhaps an underlying condition that is less treatable. The success with cardiopulmonary resuscitation (CPR) for VF as compared to other rhythms across varying levels of rescuer intervention is displayed in Table 1.1. The basic principles of resuscitation are an integral part of training for many health care providers (HCPs). Because timely interventions for cardiac arrest victims have the potential to be truly lifesaving, it is especially important for critical care practitioners to have a sound understanding of the evaluation and management of cardiac arrest.
Table 1.1
Estimates of Success of Cardiopulmonary Resuscitation Based on 31 Published Reports
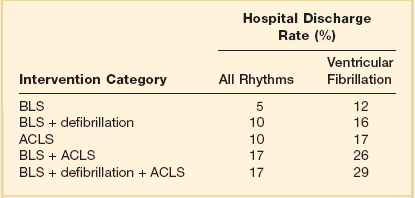
ACLS, advanced cardiac life support; BLS, basic life support.
Adapted from Cummins RO, Ornato JP, Thies WH, et al: Improving survival from sudden cardiac arrest: The “chain of survival” concept. Circulation 1991;83:1832-1847.
A number of critical actions (chain of survival) must occur in response to a cardiac arrest event. The chain of survival paradigm (Fig. 1.1) for the treatment of cardiac arrest has five separate and distinct elements: (1) immediate recognition that cardiac arrest has occurred and activation of the emergency response system; (2) application of effective CPR; (3) early defibrillation (if applicable); (4) advanced cardiac life support; and (5) initiation of postresuscitation care (e.g., therapeutic hypothermia).3
Cardiopulmonary Resuscitation and Advanced Cardiac Life Support
For CPR to be effective in restoring spontaneous circulation, it must be applied immediately at the time of cardiac arrest. Therefore, immediate recognition that a cardiac arrest has occurred and activation of the emergency response system is essential. Patients become unresponsive at the time of cardiac arrest. Agonal gasps may be observed in the early moments after a cardiac arrest event, although normal breathing ceases. Pulse checks (i.e., palpation of femoral or carotid arteries for detection of a pulse) are often unreliable, even when performed by experienced HCPs.4 Because delays in initiating CPR are associated with worse outcome, and prolonged attempts to detect a pulse may result in a delay in initiating CPR, prolonged pulse checks are to be avoided. CPR should be started immediately if the patient is unresponsive and either has agonal gasps or is not breathing.3
Chest Compressions
In CPR, chest compressions are used to circulate blood to the heart and brain until a pulse can be restored. The mechanism by which chest compressions generate cardiac output is through an increase in intrathoracic pressure plus direct compression of the heart. With the patient lying in the supine position, the rescuer applies compressions to the patient’s sternum. The heel of one hand is placed over the lower half of the sternum and the heel of the other hand on top in an overlapping and parallel fashion. The recommended compression depth in adults is 2 inches. The recommended rate of compression is 100 or more per minute. “Push hard, push fast” is now the American Heart Association (AHA) mantra for CPR instruction. This underscores the importance of vigorous chest compressions in achieving return of spontaneous circulation (ROSC).5 In addition, incomplete recoil of the chest impairs the cardiac output that is generated, and thus the chest wall should be allowed to recoil completely between compressions. Owing to rescuer fatigue, the quality of chest compressions predictably decreases as the time providing chest compressions increases, and the persons providing chest compressions (even experienced HCPs) may not perceive fatigue or a decrease in the quality of their compressions.6 Therefore, it is recommended that rescuers performing chest compressions rotate every 2 minutes.
The quality of CPR is a critical determinant of surviving a cardiac arrest event.7 Minimization of interruptions in chest compressions is imperative. Interruptions in chest compressions during CPR have been quite common historically, and the “hands off” time has been shown to take up a substantial amount of the total resuscitation time.7 Potential reasons for “hands off” time include pulse checks, rhythm analysis, switching compressors, procedures (e.g., airway placement), and pauses before defibrillation (“preshock pause”). All of these potential reasons for interruptions must be minimized. Pauses related to rotating compressors or pulse checks should take no longer than a few seconds.5 Eliminating (or minimizing) preshock pauses has been associated with higher likelihood of ROSC and improved clinical outcome.8
Defibrillation
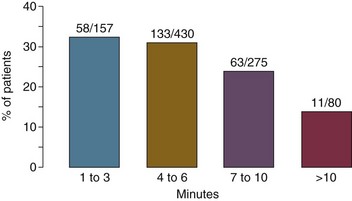
The next critically important action in the resuscitation of patients with cardiac arrest due to pulseless ventricular arrhythmias (i.e., VF or pulseless VT) is rapid defibrillation. Delays in defibrillation are clearly deleterious, with a sharp decrease in survival as the time to defibrillation increases.9 With the advent of automatic external defibrillators (AEDs) and their dissemination into public places, both elements of effective CPR (both effective chest compressions and rapid defibrillation) can be performed by lay rescuers in the field for patients with out-of-hospital cardiac arrest. Figure 1.2 shows the importance of rapid defibrillation, with decreasing success of resuscitation with increasing time to defibrillation.
Rescue Breathing
The most recent AHA recommendations regarding ventilation during CPR depends on who the rescuer is (i.e., trained HCPs versus lay person).5 For trained HCPs, the recommended ventilation strategy is a cycle of 30 chest compressions to two breaths until an endotracheal tube is placed, and then continuous chest compressions with one breath every 6 to 8 seconds after the endotracheal tube is placed. Excessive ventilations can be deleterious from a hemodynamic perspective due to increased intrathoracic pressure and reduction in the cardiac output generated by CPR and thus should be avoided during resuscitation. Excessive ventilation could also potentially result in alkalemia.
For lay persons who are attempting CPR in the field for a victim of out-of-hospital cardiac arrest, rescue breathing is no longer recommended. Rather, the recommended strategy is compression-only (or “hands-only”) CPR.5 The rationale is that compression-only CPR can increase the number of effective chest compressions that are delivered to the patient (i.e., minimizes interruptions for rescue breaths), and does not require mouth-to-mouth contact. Mouth-to-mouth contact is one of the perceived barriers to CPR in the field. By removing this element, the hope is that an increase in attempts at bystander CPR will result. Hands-only CPR has been found to be not inferior to conventional CPR including rescue breaths for victims of out-of-hospital cardiac arrest,10–12 and thus hands-only CPR has become the preferred technique to teach lay rescuers.
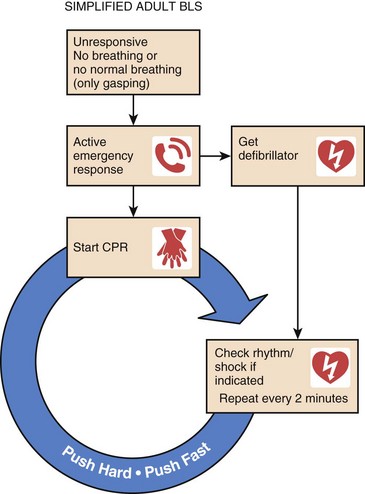
Figure 1.3 displays the AHA algorithm for adult basic life support.
Advanced Cardiac Life Support
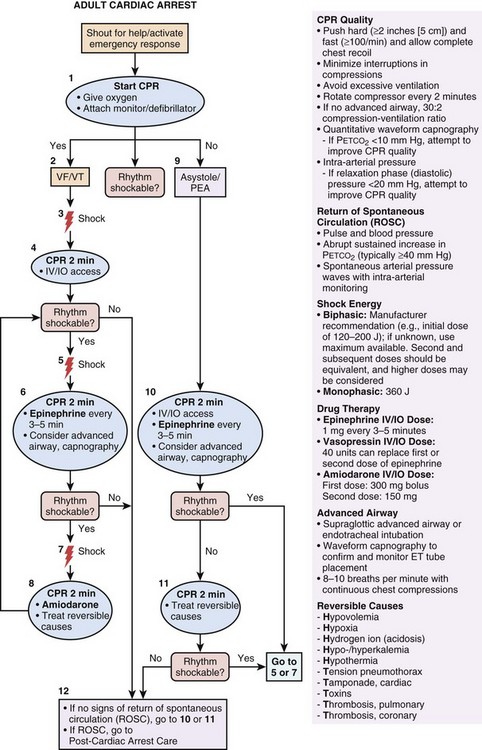
There are several additional elements of resuscitation that are intended specifically for trained HCPs (e.g., advanced cardiac life support [ACLS]), and these elements include pharmacologic therapy. Figure 1.4 displays the AHA algorithm for ACLS.13
The primary goal of pharmacologic interventions is to assist the achievement and maintenance of spontaneous circulation. The mainstay of pharmacologic interventions is vasopressor drugs. Epinephrine (1 mg) is administered by intravenous (IV) or intraosseous (IO) route every 3 to 5 minutes during CPR until ROSC is achieved.13 If IV/IO access cannot be established, epinephrine could be administered via endotracheal tube, but at a higher dose (2-2.5 mg). Vasopressin (40 mg IV/IO) can be substituted for the first or second dose of epinephrine. Amiodarone is the preferred antiarrhythmic agent. In patients with VF/VT not responding to CPR, defibrillation, and vasopressor therapy, amiodarone is recommended (300 mg IV/IO for the first dose, 150 mg IV/IO for the second dose).13 Recently, the use of atropine for PEA/asystole was removed from the ACLS algorithm. Along these lines, there is also insufficient evidence to recommend routine administration of sodium bicarbonate during CPR.
It is notable that the impact of recommended ACLS therapies on outcome from cardiac arrest remains a matter of debate. Some studies have shown that ACLS interventions did not improve clinical outcomes when compared to basic life support alone.14
Postresuscitation Care
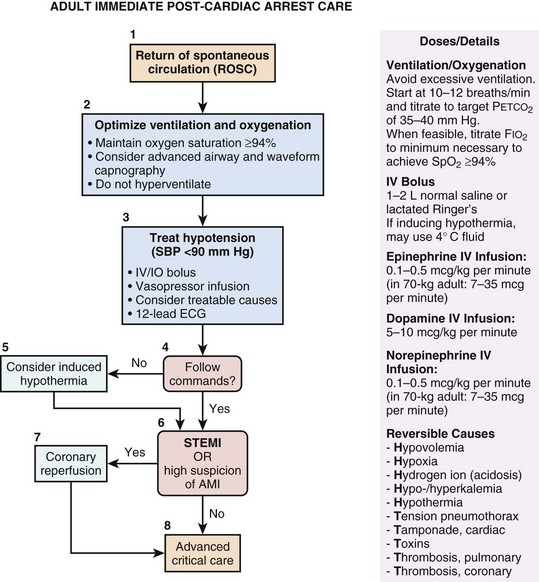
Even if ROSC is achieved with CPR and defibrillation, cardiac arrest victims are at extremely high risk of dying in the hospital, and many who survive sustain permanent crippling neurologic sequelae. Approximately 50% to 60% of patients successfully resuscitated from out-of-hospital cardiac arrest do not survive. After ROSC, global ischemia/reperfusion (I/R) injury results in potentially devastating neurologic disability. The primary cause of death among postresuscitation patients is brain injury. However, clinical trials have shown that mild therapeutic hypothermia after ROSC can improve outcomes. These landmark clinical trials have dramatically transformed the classical thinking about anoxic brain injury after cardiac arrest; this condition is in fact treatable. Early therapeutic interventions such as hypothermia initiated in the post-ROSC period can improve the trajectory of the long-term disease course. Accordingly, the postresuscitation care is now considered to be a crucial fifth link in the chain of survival paradigm (see Fig. 1.1).15
General Approach
Patients resuscitated from cardiac arrest should be admitted to a critical care unit with the following capabilities:16
• Critical care support to optimize cardiovascular indices and vital organ perfusion, and prevent repeat cardiac arrest (or provide rapid treatment of rearrest if it occurs)
• Interventional cardiac catheterization for possible percutaneous coronary intervention (PCI) if needed
• Mild therapeutic hypothermia (33°-34° C) for 12 to 24 hours in attempts to prevent permanent neurologic injury
• Systematic application of an evidence-based approach to neurologic prognostication to refrain from inappropriately early final determinations of poor neurologic prognosis (i.e., prevent inappropriately early withdrawal of life support before the neurologic outcome can be known with certainty)
Critical Care Support
I/R triggers profound systemic inflammation. In clinical studies, ROSC has been associated with sharp increases in circulating cytokines and other markers of the inflammatory response. Accordingly, some investigators have referred to the post–cardiac arrest syndrome as a “sepsis-like” state. The clinical manifestations of the systemic inflammatory response may include marked hemodynamic derangements such as sustained arterial hypotension similar to septic shock. Hemodynamic instability occurs in approximately 50% of patients who survive to intensive care unit admission after ROSC, and thus the need for aggressive hemodynamic support (e.g., continuous infusion of vasoactive agents and perhaps advanced hemodynamic monitoring) should be anticipated.17
Although there are no data on whether specific blood pressure or other hemodynamic goals are beneficial,18 expert opinion (and clinical intuition) suggests that hemodynamics and organ perfusion should be optimized.15 Rapidly raising the blood pressure in patients who remain markedly hypotensive after ROSC is prudent, because postresuscitation arterial hypotension has been associated with sharply lower survival rate.17 Whether or not postresuscitation hypotension has a cause-and-effect relationship with worse neurologic injury or is simply a marker of the severity of the I/R injury that has occurred remains unclear.
Regarding respiratory system support, exposure to hyperoxia (excessively high partial pressure of arterial oxygen [PaO2]) has been associated with poor clinical outcome among adult patients resuscitated from cardiac arrest and admitted to an intensive care unit.19 These data corroborate the findings of numerous laboratory studies in animal models in which hyperoxia exposure after ROSC worsens brain histopathologic changes and neurologic function.20–24 A paradox may exist regarding oxygen delivery to the injured brain, where inadequate oxygen delivery can exacerbate cerebral I/R injury, but excessive oxygen delivery can accelerate formation of oxygen free radical and subsequent reperfusion injury. Although clinical data on this topic are few at the present time (and specifically no interventional studies have been performed), expert opinion advocates limiting unnecessary exposure to an excessively high postresuscitation PaO2 by titrating the fraction of inspired oxygen down after ROSC as much as possible to maintain an arterial oxygen saturation of 94% or more.15
Therapeutic Hypothermia
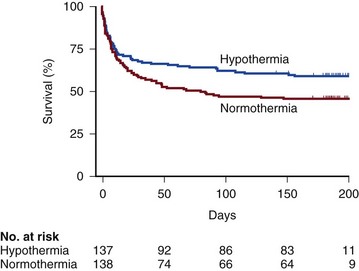
Therapeutic hypothermia (TH), also called mild therapeutic hypothermia or targeted temperature management, is a treatment strategy of rapidly reducing the patient’s body temperature after ROSC for the purposes of protection from neurologic injury. The body temperature is typically reduced to 33° to 34° C for 12 to 24 hours. After ROSC the severity of the reperfusion injury can be mitigated, despite the fact that the initial ischemic injury has already occurred. Reperfusion injury refers to tissue and organ system injury that occurs when circulation is restored to tissues after a period of ischemia, and is characterized by inflammatory changes and oxidative damage that are in large part a consequence of oxidative stress. Neuronal cell death after I/R injury is not instantaneous, but rather a dynamic process. In animal models of cardiac arrest, brain histopathologic changes may not be found until 24 to 72 hours after cardiac arrest.15 This indicates that a distinct therapeutic window of opportunity exists. In theory, TH may protect the brain by attenuating or reversing all of the following pathophysiologic processes: disruption of cerebral energy metabolism, mitochondrial dysfunction, loss of calcium ion homeostasis, cellular excitotoxicity, oxygen free radical generation, and apoptosis. Two clinical trials of TH were published in 2002.25,26 These trials showed improved outcomes with TH for comatose survivors of witnessed out-of-hospital cardiac arrest with VF as the initial rhythm. The survival data for the largest of these clinical trials (i.e., the Hypothermia After Cardiac Arrest Study Group) appear in Table 1.2 and Figure 1.5.
Table 1.2
Therapeutic Hypothermia Versus Normothermia for Management of Comatose Survivors of Cardiac Arrest*

*Neurologic outcome and mortality rate at 6 months in a randomized trial of therapeutic hypothermia versus normothermia in comatose survivors of out-of-hospital cardiac arrest due to ventricular fibrillation or pulseless ventricular tachycardia.
†The risk ratio was calculated as the rate of a favorable neurologic outcome or the rate of death in the hypothermia group divided by the rate in the normothermia group. CI, confidence interval.
‡Two-sided P values are based on Pearson’s chi square tests.
§A favorable neurologic outcome was defined as a cerebral performance category of 1 (good recovery) or 2 (moderate disability). One patient in the normothermia group and one in the hypothermia group were lost to neurologic follow-up.
Reprinted with permission from Hypothermia After Cardiac Arrest Study Group: Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346(8):549-556.
The current AHA guidelines for CPR and emergency cardiovascular care recommend 12 to 24 hours of TH for comatose survivors of out-of-hospital cardiac arrest due to VF or pulseless VT.16 TH may also be considered for victims of in-hospital cardiac arrest and other arrest rhythms. Figure 1.6 displays the AHA algorithm for post–cardiac arrest care including TH.16
Appropriate selection of candidates for TH is clearly important. If a patient does not follow verbal commands after ROSC is achieved, this indicates that the patient is at risk for brain injury and TH should be strongly considered. If a patient is clearly following commands immediately after ROSC, then significant brain injury is less likely and it is probably reasonable to withhold TH. There are multiple potential methods for inducing TH including specialized external or intravascular cooling devices for targeted temperature management, or a combination of conventional cooling methods such as ice packs, cooling blankets, and cold (4° C) IV saline infusion. Compared to the use of specialized cooling devices, overshoot (body temperature < 31° C) is a not uncommon occurrence with use of ice packs, cooling blankets, and cold saline.27 Regardless of what method is used, effective achievement of target temperature may be aided by the use of a uniform physician order set for TH induction.28 The current recommendation is to maintain TH for 12 to 24 hours.16 Whether or not a longer duration of therapy could be beneficial is currently unknown.
Neurologic Prognostication
Neurologic prognostication is often extremely difficult in the first few days after resuscitation from cardiac arrest.29 Although some neurologic examination findings may suggest poor prognosis, few of these signs on examination are sufficiently reliable upon which to base treatment decisions (e.g., support withdrawal). These critically important decisions should hinge on neurologic examination findings in which the false-positive rate (FPR) (i.e., the rate of predicting a poor outcome that ultimately proves not to be poor) approaches zero. An abundance of clinical data exists on neurologic assessment after cardiac arrest, and few examination findings have a sufficiently low false-positive rate in the first 72 hours after ROSC to form the basis of a limitation of support decision.15,30 In particular, neurologic prognostication immediately (e.g., first 24 hours) after resuscitation from cardiac arrest is especially unreliable. Among patients who are initially comatose after ROSC, one quarter to one half of patients could potentially have a favorable outcome, especially if TH is employed. In general, the recommended approach is to wait a minimum of 72 hours after ROSC before neurologic prognostication.30 However, it is important to recognize that the vast majority of data on neurologic prognostication was generated before the era of TH, that is, before an effective therapy existed. In the population of patients treated with TH, the optimal time course for making neurologic prognostication may be significantly different, not only because the therapy could modulate the degree of brain injury, but also because low body temperature decreases the metabolism of sedative agents that are typically used during TH, and it may take much longer for the effects of the sedation to be eliminated. Recently published data have shown that good neurologic outcome can potentially occur even with unfavorable neurologic findings at 72 hours, suggesting that the optimal time interval to wait before attempting neurologic prognostication in the population treated with TH may be longer than 72 hours.31 Our general approach is to not make any final neurologic prognostication until 72 hours after ROSC. In the population treated with TH, we perform daily neurologic assessments beyond the 72-hour mark and we do not make final neurologic prognostication as long as the patient continues to improve. If there are zero signs of neurologic improvement over 2 or more consecutive days, we typically deem neurologic prognostication to be reliable at that time.
References
1. Nichol, G, Thomas, E, Callaway, CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008; 300(12):1423–1431.
2. Nadkarni, VM, Larkin, GL, Peberdy, MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006; 295(1):50–57.
3. Travers, AH, Rea, TD, Bobrow, BJ, et al. Part 4: CPR overview: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010; 122(18 Suppl 3):S676–S684.
4. Field, JM, Hazinski, MF, Sayre, MR, et al. Part 1: Executive summary: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010; 122(18 Suppl 3):S640–S656.
5. Berg, RA, Hemphill, R, Abella, BS, et al. Part 5: Adult basic life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010; 122(18 Suppl 3):S685–S705.
6. Manders, S, Geijsel, FE. Alternating providers during continuous chest compressions for cardiac arrest: Every minute or every two minutes? Resuscitation. 2009; 80(9):1015–1018.
7. Abella, BS, Alvarado, JP, Myklebust, H, et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005; 293(3):305–310.
8. Edelson, DP, Abella, BS, Kramer-Johansen, J, et al. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation. 2006; 71(2):137–145.
9. Kitamura, T, Iwami, T, Kawamura, T, et al. Conventional and chest-compression-only cardiopulmonary resuscitation by bystanders for children who have out-of-hospital cardiac arrests: A prospective, nationwide, population-based cohort study. Lancet. 2010; 375(9723):1347–1354.
10. Hupfl, M, Selig, HF, Nagele, P. Chest-compression-only versus standard cardiopulmonary resuscitation: A meta-analysis. Lancet. 2010; 376(9752):1552–1557.
11. Rea, TD, Fahrenbruch, C, Culley, L, et al. CPR with chest compression alone or with rescue breathing. N Engl J Med. 2010; 363(5):423–433.
12. Svensson, L, Bohm, K, Castren, M, et al. Compression-only CPR or standard CPR in out-of-hospital cardiac arrest. N Engl J Med. 2010; 363(5):434–442.
13. Neumar, RW, Otto, CW, Link, MS, et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010; 122(18 Suppl 3):S729–S767.
14. Stiell, IG, Wells, GA, Field, B, et al. Advanced cardiac life support in out-of-hospital cardiac arrest. N Engl J Med. 2004; 351(7):647–656.
15. Neumar, RW, Nolan, JP, Adrie, C, et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008; 118(23):2452–2483.
16. Peberdy, MA, Callaway, CW, Neumar, RW, et al. Part 9: Post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010; 122(18 Suppl 3):S768–S786.
17. Trzeciak, S, Jones, AE, Kilgannon, JH, et al. Significance of arterial hypotension after resuscitation from cardiac arrest. Crit Care Med. 2009; 37(11):2895–2903.
18. Jones, AE, Shapiro, NI, Kilgannon, JH, Trzeciak, S. Goal-directed hemodynamic optimization in the post-cardiac arrest syndrome: A systematic review. Resuscitation. 2008; 77(1):26–29.
19. Kilgannon, JH, Jones, AE, Shapiro, NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010; 303(21):2165–2171.
20. Danilov, CA, Fiskum, G. Hyperoxia promotes astrocyte cell death after oxygen and glucose deprivation. GLIA. 2008; 56(7):801–808.
21. Fiskum, G, Danilov, CA, Mehrabian, Z, et al. Postischemic oxidative stress promotes mitochondrial metabolic failure in neurons and astrocytes. Ann N Y Acad Sci. 2008; 1147:129–138.
22. Richards, EM, Fiskum, G, Rosenthal, RE, et al. Hyperoxic reperfusion after global ischemia decreases hippocampal energy metabolism. Stroke. 2007; 38(5):1578–1584.
23. Richards, EM, Rosenthal, RE, Kristian, T, Fiskum, G. Postischemic hyperoxia reduces hippocampal pyruvate dehydrogenase activity. Free Radic Biol Med. 2006; 40(11):1960–1970.
24. Vereczki, V, Martin, E, Rosenthal, RE, et al. Normoxic resuscitation after cardiac arrest protects against hippocampal oxidative stress, metabolic dysfunction, and neuronal death. J Cereb Blood Flow Metab. 2006; 26(6):821–835.
25. Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002; 346(8):549–556.
26. Bernard, SA, Gray, TW, Buist, MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002; 346(8):557–563.
27. Merchant, RM, Abella, BS, Peberdy, MA, et al. Therapeutic hypothermia after cardiac arrest: Unintentional overcooling is common using ice packs and conventional cooling blankets. Crit Care Med. 2006; 34(12 Suppl):S490–S494.
28. Kilgannon, JH, Roberts, BW, Stauss, M, et al. Use of a standardized order set for achieving target temperature in the implementation of therapeutic hypothermia after cardiac arrest: A feasibility study. Acad Emerg Med. 2008; 15(6):499–505.
29. Young, GB. Clinical practice. Neurologic prognosis after cardiac arrest. N Engl J Med. 2009; 361(6):605–611.
30. Wijdicks, EF, Hijdra, A, Young, GB, et al. Practice parameter: Prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006; 67(2):203–210.
31. Al Thenayan, E, Savard, M, Sharpe, M, et al. Predictors of poor neurologic outcome after induced mild hypothermia following cardiac arrest. Neurology. 2008; 71(19):1535–1537.