Chapter 49 Cancer of the gallbladder
Overview
Gallbladder cancer is rare and traditionally has been considered an incurable disease with an extremely poor prognosis. The pessimism and nihilism that historically have been associated with gallbladder cancer stem from its commonly late presentation, often with disseminated disease, its overall dismal prognosis, and lack of effective therapy. The quote that sums up this sentiment best comes from Blalock, who stated in 1924, “In malignancy of the gallbladder, when a diagnosis can be made without exploration, no operation should be performed, inasmuch as it only shortens the patient’s life.” Although it is clear that gallbladder cancer has a tendency to spread early via lymphatic, hematogenous, and peritoneal metastases, it also has the unique ability to implant along biopsy tracts and wounds. Gallbladder cancer is often discovered incidentally after cholecystectomy performed for presumed benign disease; unfortunately, this operation is incomplete except for the earliest stage of disease.
Pessimistic attitudes about gallbladder cancer have persisted from its original description in 1778 (Kato et al, 1994) to more recent times and are supported by reports of overall 5-year survival of 5% and median survivals of less than 6 months. For advanced, untreated gallbladder cancer, the median survival is generally 2 to 5 months, and long-term survival is exceedingly rare (Perpetuo et al, 1978; Piehler & Crichlow, 1978). Systemic chemotherapy remains limited in its effectiveness, but with major improvements in systemic chemotherapy in other diseases, these benefits may begin to extend to patients with gallbladder cancer. The role of definitive resection, however, has now been shown to be effective in properly selected patients. Complete surgical removal of gallbladder cancer is the only potentially curative therapy. With improvements in imaging, staging, and hepatic and biliary resection, there is now hope for patients with nonmetastatic gallbladder cancer. Effective adjuvant therapy is lacking, however, and this represents a significant limitation of current treatment options.
Epidemiology
The incidence of gallbladder cancer varies by racial group, and it has been reported that internationally, incidence varies 20-fold based on geographic region (Randi et al, 2006). The highest incidence of gallbladder cancer is found in women living in India (21.5 cases per 100,000 population annually), Pakistan (13.8 per 100,000), and Ecuador (12.9 per 100,000). Approximately 9760 cases of gallbladder and other biliary cancers are diagnosed annually in the United States, with 3320 deaths annually (Jemal et al, 2010). In North America, high incidence rates are found among Native Americans and Hispanic women (Barakat et al, 2006). The annual incidence of gallbladder cancer in the United States is approximately 2 per 100,000 women and 1 per 100,000 men. In North America and Western Europe, urban areas and areas of lower socioeconomic status harbor higher rates of gallbladder cancer (Diehl, 1980; Zatonski et al, 1993). In these areas, limited access to health care delays and decreases the rate of cholecystectomy for gallstone disease (see Chapter 30) may lead to higher rates of gallbladder cancer (Serra et al, 1996). In the United States overall, gallbladder cancer is the most common cancer of the biliary tract and the fifth most common gastrointestinal cancer (Carriaga & Henson, 1995).
Across all populations that have been studied, women are approximately three times more likely to develop gallbladder cancer than are men (Lazcano-Ponce et al, 2001). This ratio is as high as 5 : 1 in countries such as Pakistan and Colombia (Randi et al, 2006). Although rare, reports of children (<21 years old) developing gallbladder cancer do exist (Rudolph & Cohen, 1972; De Aretxabala et al, 1994); it is generally a disease of advancing age, with the incidence steadily increasing to a plateau after age 60 years (Nakayama, 1991). Rare reports of familial gallbladder cancer exist but probably account for only a small percentage of cases (Trajber et al, 1982; Fernandez et al, 1994). Throughout the world, no consistent trend in the incidence of gallbladder cancer has been found, with decreases and increases reported in different countries. From 1978 to 1997, the incidence generally stabilized or declined in North America and Western Europe, but in Japan and South America, the incidence increased (Randi et al, 2006).
Obesity is now associated with increased death rates from many cancers. In a prospective cohort study of 900,000 U.S. adults, the relative risk of death from gallbladder cancer in women with a body mass index (BMI) of 30 to 34.9 was 2.13 compared with women with a BMI of 18.5 to 24.9. In men, the relative risk of death from gallbladder cancer with an elevated BMI was also significant at 1.76 (Calle et al, 2003). Other rare associations with gallbladder cancer include inflammatory bowel disease, primary sclerosing cholangitis (Lewis et al, 2007), and polyposis coli (Willson et al, 1987).
Etiology
The most consistently implicated etiologic factor in the development of gallbladder cancer is cholelithiasis and chronic inflammation. Of gallbladder cancer cases, 75% to 90% occur in the setting of cholelithiasis (Lazcano-Ponce et al, 2001; Serra & Diehl, 2002; Wanebo & Vezeridis, 1994). In one case-control study, the relative risk of gallbladder cancer was 10.1 in patients with stones larger than 3 cm (Diehl, 1983). Similar results were reported in another case-control study that compared gallstones in patients with gallbladder cancer with gallstones in patients with benign gallbladder disease (Roa et al, 2006). There were significantly more stones, heavier stones, and increased stone volume in the patients who had gallbladder cancer. The epidemiology of gallstones often parallels that of gallbladder cancer (Zatonski et al, 1997; Shrikhande et al, 2010); however, most patients with gallstones never have cancer develop, and a definitive cause-and-effect relationship has not been established. It is possible that stones and cancer share similar risk factors (see Chapter 8B), or stones may simply prompt a radiologic workup or cholecystectomy, increasing recognition in this group of patients.
There appears to be an association between cholesterol metabolism gene polymorphisms and a combined risk of gallbladder cancer and stones (Xu et al, 2010). Although nearly 90% of gallbladder cancer specimens contain stones, the incidence of gallbladder cancer in the population of patients with stones is 0.3% to 3%, which is low when considering this as the only risk factor. The other epidemiologic associations such as biliary-enteric fistulae, typhoid infection, and pancreaticobiliary maljunction also represent conditions in which the mucosa of the gallbladder is exposed to the effects of chronic inflammation. Although a true cause-and-effect relationship has never been proven, most epidemiologic data point to a significant relationship between chronic inflammation of the gallbladder and the development of neoplasia.
The presence of calcification in the wall of the gallbladder, otherwise known as porcelain gallbladder, is also a condition associated with a higher risk of gallbladder cancer. The calcification is probably the result of long-standing inflammation. The risk of malignancy within a porcelain gallbladder was previously reported to be extremely high (10% to 50%); modern series, however, have shown a much lower incidence (<10%) (Berk et al, 1973; Kim et al, 2009; Kwon et al, 2004; Stephen & Berger, 2001). The type of calcification seems to be associated with the degree of risk, with stippled calcification of the mucosa apparently representing a higher risk than diffuse intramural calcification.
Chronic inflammatory conditions of the gallbladder, such as cholecystoenteric fistula and chronic infection with typhoid bacillus, also have been associated with a risk for gallbladder cancer (Welton et al, 1979). Because bacterial colonization often accompanies chronic cholecystitis, bacteria has been proposed to play an important role in carcinogenesis. The argument against this etiology is that gallbladder cancer occurs in the setting of bacterial infection without stones and commonly occurs in the setting of stones without infection. The presence of an anomalous pancreaticobiliary junction with a long common channel between the pancreatic and bile duct also has been associated independently with gallbladder cancer risk and may be related to chronic inflammation (Chijiiwa et al, 1993). It has been difficult in experimental models, however, to induce gallbladder carcinoma with chronic inflammation. Fortner and Randall (1961) placed gallstones from patients with and without gallbladder carcinoma into the gallbladders of 126 cats. After 4 to 5 years, they found carcinoma in three cats, one of whose stones came from a patient without carcinoma. Despite these data, it is likely that chronic inflammation, regardless of cause, at the least predisposes to gallbladder carcinoma.
In patients with chronic inflammation as a predisposing factor, one hypothesis is that one or more carcinogenic exposures are required for carcinoma to develop. In numerous animal experiments, rates of gallbladder cancer were dramatically higher when inflammation was combined with known carcinogenic agents compared with exposure to the carcinogen alone (Enomoto et al, 1974; Kowalewski & Todd, 1971; Piehler & Crichlow, 1978). The composition of bile in patients with gallbladder cancer has been studied in attempts to identify carcinogenic agents. Higher concentrations of free radical oxidation products (Shukla et al, 1994) and secondary bile acids (Shukla et al, 1993) were found in gallbladders harboring cancer compared with gallbladders with gallstones alone. Some chemicals have been implicated in gallbladder carcinogenesis, including methyldopa (Broden & Bengtsson, 1980), oral contraceptives (Broden & Bengtsson, 1980), isoniazid (Lowenfels & Norman, 1978), and occupational exposure in the rubber industry (Mancuso & Brennan, 1970). None of these associations has been definitively proved (Pandey, 2006).
There is some suggestion of an adenoma-carcinoma progression in the development of gallbladder cancer. Severe dysplasia and carcinoma in situ are often adjacent to gallbladder carcinomas (Lazcano-Ponce et al, 2001). Unlike colorectal cancer, however, the association of polypoid gallbladder tumors and gallbladder cancer is rare. In fact, in patients with multiple gallbladder polyps, no increased risk of malignancy was found (Ito et al, 2009; Zielinski et al, 2009). Kubota and colleagues (1995) found that 11% of cholecystectomy specimens with gallbladder polyps had malignancies, and 88% of these polyps were larger than 1 cm. Yang and colleagues (1992) reported similar results with an 8% incidence of gallbladder cancer in gallbladder specimens with polyps, all more than 1 cm in size. Based on these studies, current practice recommendations are to perform cholecystectomy for gallbladder polyps larger than 1 cm.
Anatomic Considerations
It is important to understand the anatomy of the gallbladder, biliary tree, liver, and hepatic hilum to manage tumors of the gallbladder properly. The anatomy of the gallbladder is reviewed in Chapter 1A, Chapter 1B , but a few specific comments are worthy of review. The gallbladder is a partially intraperitoneal structure that lies attached to the undersurface of the liver on segments IVb and V. On the side of the gallbladder that is attached to the liver, there is no peritoneal covering; a fibrous lining known as the cystic plate occupies this space. When a simple cholecystectomy is performed, the plane between the muscularis of the gallbladder and the cystic plate is dissected, which is an inadequate resection for a malignancy involving this portion of the gallbladder. This fact makes a simple cholecystectomy inadequate for most gallbladder cancers. Because the body and fundus of the gallbladder generally lie at a distance from the major inflow structures to the liver, a limited segmental resection (segment IVb/V) is adequate to resect most tumors arising from this area of the gallbladder. The infundibulum and cystic duct encroach into the porta hepatis, however, and tumors of this area often involve the porta. The surgeon must be prepared to perform bile duct resections or major hepatic resections for tumors of the lower part of the gallbladder because major inflow structures are commonly involved.
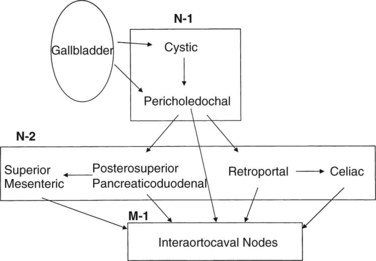
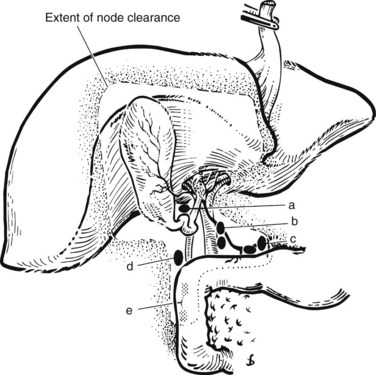
The lymphatic drainage of the gallbladder has been extensively studied (Shirai et al, 1992c); it is crucial to understand this drainage when radiologically or surgically staging patients with gallbladder cancer. The principal lymphatic drainage of the gallbladder as studied by dye injections is shown in Figure 49.1. When injected into the lymphatics of the gallbladder, the dye never ascends into the proximal porta hepatis lymphatics. The channels lead to the cystic and choledochal nodes first. From these nodes, the primary drainage areas are the retroportal and posterior-superior pancreaticoduodenal nodes. Progressing from these lower portal areas, the lymphatics course to the celiac, superior mesenteric, and interaortocaval nodes. An important finding was that some lymphatic material seemed to drain directly from the pericholedochal nodes to the interaortocaval nodes in the retropancreatic space. These connections may offer an explanation for the often advanced nature of gallbladder cancer at diagnosis and highlight the importance of analyzing the retropancreatic area radiologically and with a full Kocher maneuver at operation to stage patients with gallbladder cancer properly.
Pathology
Preneoplastic Lesions
Similar to many gastrointestinal malignancies, progression from dysplasia to carcinoma in situ to frank invasive carcinoma is seen in gallbladder epithelium (Albores-Saavedra et al, 1986; Kozuka et al, 1982). When surrounding mucosa is sectioned in cases of gallbladder cancer, more than 90% of the cases harbor high-grade dysplasia or carcinoma in situ. Although chronic inflammation of the gallbladder can cause cellular atypia, it is generally easy to differentiate this from dysplasia and carcinoma in situ (Fig. 49.2). Carcinoma in situ may appear in the Rokitansky-Aschoff sinuses and may be mistaken for invasive carcinoma. Although it is impossible to know definitively the time course between dysplastic changes and invasive carcinoma, estimations have been made. In one series, the rate of progression of precursor lesions to invasive carcinoma was estimated to be approximately 15 years based on the mean ages of patients at each stage (Roa et al, 1996). Another study found a 5-year difference between the mean ages of patients with dysplasia and those with carcinoma in situ, and a 10-year difference was found between the mean ages of patients with carcinoma in situ and those with invasive carcinoma (Albores-Saavedra et al, 1986).
The precancerous nature of gallbladder polyps is more controversial; however, it provides some evidence that an adenoma-adenocarcinoma progression exists. Gallbladder polyps have been noted in 3% to 6% of patients undergoing ultrasonography (US). Most are cholesterol polyps with no malignant potential (Choi et al, 2008; Zielinski et al, 2009). The incidence of carcinoma in nonadenomatous polyps—cholesterol polyps, inflammatory polyps, and hyperplastic polyps—is close to zero (Canturk et al, 2007; Choi et al, 2008; Ito et al, 2009). Approximately 1% of cholecystectomy specimens contain adenomatous polyps (Aldridge & Bismuth, 1990; Fong & Malhotra, 2001). In another series of 123 gallbladder polyps, 21 (17%) were found to be adenomatous, and seven (6%) were found to harbor malignancy. Risk of malignancy was associated with increasing age, size greater than 1 cm, and the presence of a single polyp (Yeh et al, 2001). Ito and colleagues (2009) reviewed 417 patients with gallbladder polyps found on US. Only 7% of patients had polyps larger than 1 cm. Among the 80 patients who underwent cholecystectomy, neoplastic (adenomatous) polyps were found in 10%, and one patient had a carcinoma in situ (in a 14-mm polyp). No patient was found to harbor an invasive malignancy.
Kozuka and colleagues (1982) described seven adenomas showing histologic progression into malignancy and showed that 19% of invasive carcinomas had adenomatous components. In a series of 182 resected gallbladder polyps, 13 (7%) were found to harbor a malignancy, which was related to the size of the polyp (Yang et al, 1992). One report described a higher incidence of malignancy in gallbladder polyps arising in the face of primary sclerosing cholangitis (Buckles et al, 2002).
Gross Morphology
Early-stage gallbladder cancers are difficult to distinguish from the findings typical of chronic cholecystitis because they both often present as a thickened gallbladder wall in the face of inflammation. This similar presentation accounts for the frequent discovery of gallbladder cancer incidentally in a cholecystectomy specimen. The overall incidence of gallbladder cancer discovered during cholecystectomy for presumed benign disease is less than 1% (Bazoua et al, 2007). As gallbladder tumors progress, they can present in numerous ways. The gallbladder may become distended with tumor, or it may become contracted and collapsed. Approximately 60% of tumors originate from the fundus, 30% from the body, and 10% from the neck of the gallbladder (Albores-Saavedra et al, 1986; Daines et al, 2004; Rajagopalan et al, 2004). Tumors in the lower end of the gallbladder may obstruct the neck or cystic duct, leading to hydrops. Advanced tumors of the neck, infundibulum, or cystic duct may infiltrate the porta hepatis and result in major vascular invasion, jaundice, and even hepatic atrophy. This presentation is often indistinguishable from a hilar cholangiocarcinoma.
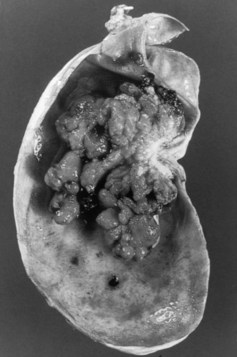
Gross descriptions of gallbladder cancer have been grouped into infiltrative, nodular, combined nodular-infiltrative, papillary, and combined papillary-infiltrative forms (Albores-Saavedra et al, 2005; Sumiyoshi et al, 1991). Most tumors have an infiltrative pattern as part of their presentation, which causes thickening and induration of the gallbladder wall (Fig 49.3). These types of tumors seem to spread in a subserosal plane and can invade the whole gallbladder wall and even invade into the porta hepatis, causing jaundice from diffuse biliary involvement. These types of tumor can also diffusely invade the liver. Nodular types of tumors tend to grow as a more circumscribed mass. Despite invasion into the liver, these lesions are more easily resected because they are less diffuse in their invasive pattern. Papillary tumors of the gallbladder are polypoid lesions with frondlike extensions that give a cauliflower-like appearance (Fig. 49.4). Despite the fact that papillary tumors can grow quite large, they tend to have a better prognosis than the other gross types; this is likely related to the less invasive nature of these tumors. Even at large sizes, papillary gallbladder tumors may have minimal invasion into the gallbladder wall (Albores-Saavedra et al, 2005).
Histology (See Chapter 47)
Table 49.1 shows the classification of malignant gallbladder tumors, and Table 49.2 summarizes the relative incidence of the various histologic types of gallbladder cancer. Over a 10-year period at Memorial Sloan-Kettering Cancer Center (MSKCC), 391 (90%) of the 435 patients with gallbladder cancer had adenocarcinomas. Additional diagnoses were squamous/adenosquamous carcinoma (4%), neuroendocrine carcinoma (3%), sarcoma/adenosarcoma (1.6%), unspecified carcinoma (1.1%), and melanoma (<1%) (Duffy et al, 2008). Most gallbladder tumors can be characterized histologically, but more than one histologic pattern is commonly found in each tumor. The only histologic subtype that seems to have prognostic significance is the papillary tumor. Papillary adenocarcinomas of the gallbladder tend to have a better prognosis than the other subtypes (Albores-Saavedra et al, 2005; Carriaga & Henson, 1995). This improved prognosis is likely related to the tendency of this tumor to be noninvasive or minimally invasive. When this tumor becomes invasive, however, it can metastasize and have a prognosis typical of the pathologic stage (Albores-Saavedra et al, 2005).
Table 49.1 Classification of Malignant Tumors of the Gallbladder
| Epithelial Tumors |
Modfied from Albores-Saavedra J, Henson DE, 1986: Tumors of the gallbladder and extrahepatic bile ducts. In Atlas of Tumor Pathology, Second Series. Bethesda, MD, Armed Forces Institute of Pathology.
Table 49.2 Relative Incidence of Gallbladder Cancer by Histologic Type
| RELATIVE INCIDENCE (%) | ||
|---|---|---|
| Histologic Type | Carriaga & Henson, 1995 | Duffy et al, 2008 |
| Carcinoma | 99 | 94 |
| Adenocarcinoma | 89.4 | 90 |
| Papillary | 5.7 | — |
| Mucinous and mucin producing | 5.3 | — |
| Squamous cell | 1.8 | 4 |
| Other and unspecified | 7.8 | 1.4 |
| Sarcoma | 0.2 | 1.6 |
| Neuroendocrine | — | 3 |
From Carriaga MT, Henson DE, 1995: Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer 75(Suppl 1):171-190; and Duffy A, et al, 2008: Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Center (MSKCC). J Surg Oncol 98(7):485-489.
Throughout the medical literature are many case reports and small case series of other rare gallbladder cancers. Primary sarcomas of the gallbladder—including embryonal rhabdomyosarcoma, leiomyosarcoma, malignant fibrous histiocytoma, angiosarcoma, and Kaposi sarcoma—have been reported and are extremely rare. Other reported uncommon gallbladder cancers include carcinosarcoma, carcinoid, lymphoma, melanoma, and metastatic tumors to the gallbladder. Although reports have suggested a poorer outcome for oat-cell carcinomas (Henson et al, 1992) and adenosquamous carcinomas (Yamaguchi & Enjoji, 1988), these conclusions are based on small numbers, and comparisons of such rare tumors are impossible.
Gallbladder cancers also have been grouped into metaplastic and nonmetaplastic types based on metaplastic changes in the tumors; this is similar to the classification of gastric cancers into intestinal and diffuse types. The two predominant types of metaplasia that are precursors to malignancy in the gallbladder mucosa are pseudopyloric and intestinal (Duarte et al, 1993). The intestinal type has a higher rate of carcinoma. The progression from metaplasia to carcinoma in situ to carcinoma is evident in that patients with invasive cancer are 15 and 5 years older than patients with dysplasia and carcinoma in situ, respectively (Roa et al, 1996).
Histologically, gallbladder cancers are graded into four categories that range from well differentiated to poorly differentiated. This differentiation does not have a significant impact on prognosis except for the observation that most papillary tumors are well differentiated, which may account for the better prognosis seen in these types of tumors. Most gallbladder cancers are poorly differentiated at presentation. DNA ploidy has been studied in gallbladder tumors and corresponds to the histologic grade but does not correlate with prognosis (Baretton et al, 1994). Although it is clear that gallbladder cancers can be differentiated based on histologic type and grade, the most important prognostic factor is the stage at presentation, making grade and type prognostically unimportant.
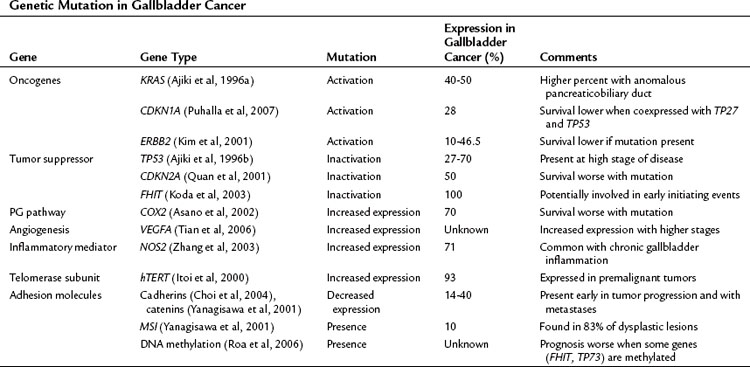
Molecular Biology (See Chapter 8B)
The precise pathway linking different genetic mutations responsible for gallbladder cancer is not clear; however, multiple genetic defects are known to be associated with gallbladder cancer (Lazcano-Ponce et al, 2001; Rashid, 2002; Sasatomi et al, 2000). Tumor suppressor genes, oncogenes, microsatellite instability, and DNA repair genes all participate in the initiation of gallbladder cancer (Table 49.3) (Goldin & Roa, 2009). Several of these genetic mutations have been identified in case-control studies and have been combined into risk scores. For example, Srivastava and colleagues (2010) compared genetic variations in three DNA repair genes—ERCC2, MSH2, and OGG1—among 230 patients with gallbladder cancer and 230 control subjects. They found that patients with more than six of the variant alleles that were studied had a fourfold increased risk for developing gallbladder cancer.
Serologic biomarkers can potentially be useful to identify groups of patients at increased risk for gallbladder cancer. When used together, polymorphisms CA242 and CA125 have been associated with high sensitivity and specificity for differentiating between cholelithiasis and gallbladder cancer (Shukla et al, 2006). Several other serum markers may identify patients at high risk for developing gallbladder cancer, regardless of the presence of cholelithiasis. These markers include the NAT2 slow acetylator phenotype, the X(+), D haplotype of apolipoprotein B, and the D allele of lipoprotein receptor–associated protein (LRPAP1) insertion/deletion polymorphism (Pandey, 2006; Pandey et al, 2007a, 2007b).
Pattern of Spread
An autopsy study showed a 94% incidence of lymphatic metastases and a 65% incidence of hematogenous dissemination (Kimura et al, 1989); however, autopsy studies represent the end stage of disease, with sufficient time for extensive metastases to develop. It has been postulated that hematogenous metastases originate from small veins extending directly from the gallbladder into the portal venous system of the gallbladder fossa. These connections can lead to segments IV and V of the liver or via larger veins to the portal venous branches of segments V and VIII (Boerma, 1994). A review of the incidence of regional invasion and metastases at the time of diagnosis and treatment is summarized in Table 49.4.
Table 49.4 Incidence of Regional Invasion and Metastasis at the Time of Diagnosis and Treatment Based on a Literature Review
| Pathologic Finding | Relative Incidence (%) |
|---|---|
| Confined to gallbladder wall | 10 |
| Liver invasion | 59 |
| Common bile duct infiltration | 35 |
| Lymphatic invasion and regional lymphatic metastases | 45 |
| Gallbladder vein infiltration | 39 |
| Portal vein or hepatic artery invasion | 15 |
| Adjacent organ invasion (excluding liver) | 40 |
| Perineural invasion | 42 |
| Liver metastasis | 34 |
| Distant metastasis (excluding liver) | 20 |
Data from Boerma EJ, 1994: Towards an oncological resection of gall bladder cancer. Eur J Surg Oncol 20(5):537-544.
It is also important to understand the patterns of spread after complete resection because these facts can help guide approaches to adjuvant therapy. Autopsy studies have shown widely disseminated disease, including liver metastases, in greater than 90%; abdominal lymph node metastases in greater than 80%; and peritoneal metastases in 60% of patients (Perpetuo et al, 1978). The only common extraabdominal site of distant metastases is the lung, but lung metastases are rarely seen in the absence of advanced intraabdominal disease. In an attempt to define sites of first recurrence after complete resection, Jarnagin and colleagues (2003) found that only 15% of patients had locoregional recurrence as the only site of recurrence, and most (85%) had recurrence involving a distant site. This finding shows the minimal potential utility of adjuvant locoregional strategies and underscores the importance of effective adjuvant systemic therapies.
Clinical Presentation
Three clinical scenarios are common for gallbladder cancer: 1) final pathology after routine cholecystectomy identifies gallbladder cancer; 2) gallbladder cancer is discovered intraoperatively; and 3) gallbladder cancer is suspected before surgery (Miller & Jarnagin, 2008). The clinical presentation varies depending on geographic location, rates of gallbladder cancer, and referral patterns. In a study of 435 patients with gallbladder cancer treated at MSKCC during a 10-year period, 47% had cancers discovered incidentally at the time of routine laparoscopic cholecystectomy (Duffy et al, 2008), and 53% were initially seen with advanced (16%) or disseminated (37%) disease. Given the high percentage of patients diagnosed with gallbladder cancer after routine cholecystectomy for presumed benign gallbladder disease, it is appropriate for the surgeon to inspect the gallbladder mucosa at the completion of the resection if there are any concerns. Frozen-section analysis should be used to examine any suspicious areas.
Gallbladder cancer is notorious for being asymptomatic in its early stages. When symptoms do occur, however, gallbladder cancer tends to present in a similar manner to biliary colic or chronic cholecystitis. Careful history taking often shows a history of constant right upper quadrant pain rather than the typical episodic crampy pain of biliary colic. The diagnosis of gallbladder cancer should be considered in an elderly patient with constant right upper quadrant pain with weight loss, anorexia, or both because weight loss, anorexia, and jaundice in particular are signs of advanced disease (Duffy et al, 2008). The presence of a palpable mass is also an ominous finding that predicts a high rate of unresectability and advanced disease (Thorbjarnarson & Glenn, 1959). The presence of jaundice is an especially ominous finding. In a report by Hawkins and colleagues (2004), 82 (34%) of 240 patients presented with jaundice. Of these 82 patients, only six (7%) were resectable, and all had recurrence or died of disease within 2 years. The median survival in jaundiced patients was 6 months compared with 16 months in patients presenting without jaundice.
The incidence of symptoms and signs at presentation is summarized in Table 49.5. A report from New York Hospital reviewed their experience with gallbladder cancer from 1915 through 2000 (Grobmyer et al, 2004). Throughout the years, the presentation was remarkably similar in that most patients had advanced disease at presentation. Over time, however, a decrease was reported in the percentage of patients who presented in the more recent era with weight loss (95% vs. 42%), palpable mass (50% vs. 9%), and nausea/vomiting (97% vs. 70%). The percentage of patients presenting with abdominal pain and jaundice was similar (approximate 85% and 50%, respectively).
Laboratory examination generally is not helpful except to identify the typical signs of advanced disease, such as anemia, hypoalbuminemia, leukocytosis, and elevated alkaline phosphatase or bilirubin levels (Grobmyer et al, 2004; Thorbjarnarson & Glenn, 1959). The only tumor markers studied that are of any potential value are carcinoembryonic antigen (CEA) and carbonin anhydrase 19-9 (CA19-9). An elevated CEA tends to be specific for gallbladder cancer (90%), but it lacks sensitivity (50%) when used as a screening test in cancer patients compared with patients who have benign gallbladder diseases (Strom et al, 1990). CA19-9 is more consistent as a marker for gallbladder cancer, with sensitivities and specificities of approximately 75% at a level greater than 20 U/mL (Ritts et al, 1994). Overall, serum tumor markers are of minimal clinical value compared with clinical awareness, a heightened level of suspicion in appropriate cases, and good-quality imaging studies. As is typical of the utility of serum tumor markers, they can be helpful in following a patient for recurrence, if they are elevated before treatment and normalize after treatment.
Radiologic Investigation
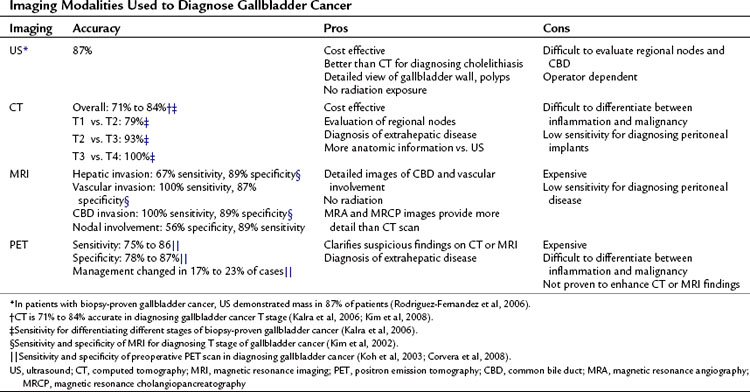
In the era before the availability of routine real-time US and computed tomography (CT), gallbladder cancer was rarely diagnosed preoperatively. With the development of rapid and more widely available imaging modalities, patients who have suspected or incidentally diagnosed gallbladder cancer should have high-resolution cross-sectional imaging for adequate staging. Because most patients present with advanced disease, it is important to try to establish the diagnosis and extent of disease with imaging to minimize the number of patients who have to undergo a nontherapeutic surgical exploration. Except for the earliest stage of disease, this should now be possible in most patients. In addition to the modalities available for examining the gallbladder and liver, chest radiographs or CT scan should be obtained during the complete staging workup to rule out pulmonary metastases. It is rare, however, to find pulmonary metastases without locally advanced or intraabdominal metastatic disease (Lee et al, 2010).
US is an excellent imaging modality for the gallbladder. Findings such as discontinuous mucosa, echogenic mucosa, and submucosal echolucency are more common in early malignancy compared with benign gallbladder disease. Doppler assessment of blood flow through areas of mucosal abnormalities can help to differentiate early malignancy from benign disease, and newer contrast-enhanced US techniques may improve detection confidence even further (Sato et al, 2001). In one study, a polypoid mass was present in 27% of cases, and a gallbladder-replacing or invasive mass was present 50% of the time (Wibbenmeyer et al, 1995). US is a good modality for evaluating the direct extension of gallbladder cancer. One retrospective study reported that in 203 patients with gallbladder cancer, a mass was identified in 177 patients (87%) on preoperative US (Pandey et al, 2000). US was limited, however, in identifying lymph node metastases in pericholedocal and peripancreatic nodes. Because most cases are advanced, the most typical findings in gallbladder cancer are an inhomogeneous mass replacing all or part of the gallbladder (Bach et al, 1998; Franquet et al, 1991). Diffuse thickening of the gallbladder wall also is a common finding on cross-sectional imaging and on US, but it can be difficult to differentiate from benign inflammatory changes.
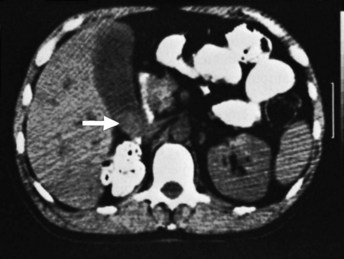
Cross-sectional imaging with CT or magnetic resonance imaging (MRI) is an important part of the preoperative assessment of gallbladder cancer (see Chapters 16 and 17). These techniques provide crucial information about the local extent of disease and show whether distant metastases are present. The most common finding on CT is a mass involving all or part of the gallbladder. Extension into local organs, particularly the liver, usually can be discerned. In one study of patients with gallbladder masses, asymmetric wall thickening was found in 45% of patients, a mass replacing the gallbladder was found in 35%, and an intraluminal mass was found in 20% (Fig. 49.5) (Kalra et al, 2006). Assessment of regional and distant lymph nodes is important and can be done with CT. With criteria of size greater than 1 cm and a ringlike heterogenous enhancement, accuracy rates greater than 80% have been reported (Ohtani et al, 1993). Although large lymph nodes replaced with tumor are relatively easy to identify with CT, false-negative examination results continue to be a problem because many involved lymph nodes can be small, with minimal tumor. CT is 71% to 84% accurate in staging gallbladder cancer. In one study of 118 patients with gallbladder cancer, CT was 79% accurate for differentiating T1 versus T2 tumors, 93% accurate for differentiating T2 versus T3 tumors, and 100% accurate for differentiating T3 versus T4 tumors (Kim et al, 2008). The overall accuracy improved from 72% to 85% when multiplanar reconstructions were added to conventional axial imaging.
The utility of MRI for evaluating patients with gallbladder cancer has increased in recent years. Improvements in MRI technology over the last 2 decades has been dramatic, with wider availability of MRI cholangiography and angiography. Invasive diagnostic cholangiography has largely been replaced by MRI cholangiography in most high-volume centers (Schwartz et al, 2002). Likewise, the use of diagnostic angiography has been replaced by CT/MRI angiography, and such modern equipment can provide detailed imaging of the related vessels at the hepatic hilum. Analyses of MRI for the assessment of gallbladder cancer have shown sensitivities of 70% to 100% for hepatic invasion and 60% to 75% for lymph node metastases (Kim et al, 2002; Schwartz et al, 2002). It is unclear whether MRI adds to the results obtained from CT scan. In one study of 25 patients with gallbladder cancer, MRI/MRCP did not change the preoperative stage as determined by CT scan (Rao et al, 2005).
The development and use of fluorodeoxyglucose (FDG) positron-emission tomography (PET) has resulted in superior staging and diagnosis of many tumors. Most gallbladder cancers are visible on PET scan; theoretically, PET imaging could help differentiate between benign and malignant tumors and can help diagnose extrahepatic spread (Petrowsky et al, 2006). PET, however, is limited in differentiating between benign inflammatory states, such as postcholecystectomy, and malignancy (Corvera et al, 2008). PET appears to be more accurate in diagnosing metastatic disease than is CT scan. In a study of 61 patients with biliary tract malignancies, PET/CT had a sensitivity of 100% compared with 25% for CT alone (P < .001), and PET alone changed surgical management in 17% of cases (Petrowsky et al, 2006). PET is more useful for evaluating primary gallbladder cancer than it is for evaluating recurrent disease. In 41 patients with gallbladder cancer at MSKCC, PET results altered surgical management in 23% during preoperative staging (for either the initial operation or reresection after an incidental finding of cancer after cholecystectomy) compared with 10% in patients evaluated for recurrent disease (Corvera et al, 2008). In one study from Japan, 16 patients with mass lesions of the gallbladder were evaluated with PET. Eight of the tumors were histologically proven to be malignant, and PET had a sensitivity and specificity of 75% and 88%, respectively. The false-positive case was in a patient with xanthogranulomatous cholecystitis (Koh et al, 2003).
The usual first test for gallbladder symptoms is US, and it is crucial always to consider the diagnosis of gallbladder cancer when abnormalities are found in the wall of the gallbladder. Duplex US adds information in terms of vascular tumors and helps assess local hepatic vasculature. When gallbladder carcinoma is proven or suspected preoperatively, good-quality cross-sectional imaging with CT or MRI should provide adequate data on local extent of tumor to help assess for metastases. PET can also be a valuable adjunct in searching for metastatic disease or when CT or MRI provides limited information about the primary tumor (Table 49.6).
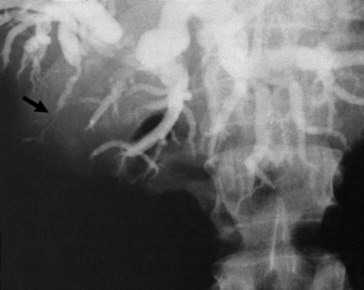
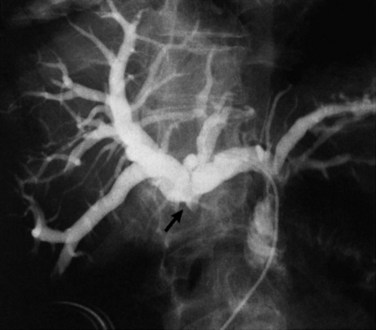
Gallbladder cancer presenting with jaundice is relatively common; this diagnosis must be in the differential diagnosis for any malignant-appearing, mid–bile duct stricture. Gallbladder cancer must also be considered when the diagnosis of Mirizzi syndrome has been made (Redaelli et al, 1997). To help plan therapeutic procedures, the level of obstruction can be assessed by CT or MRI cholangiography with reasonable accuracy. Stricturing, distortion, or nonfilling of the segment V or VI bile ducts can be caused by gallbladder tumors without effect on the rest of the biliary tree (Fig. 49.6) (Collier et al, 1984). Invasive cholangiography is indicated only when a therapeutic intervention, such as stenting, is anticipated. According to the level of obstruction, a judgment can be made regarding the best approach for biliary stenting (endoscopic retrograde vs. transhepatic cholangiography). As discussed later in this chapter, this can be a crucial part of the palliation of advanced gallbladder cancer.
Preoperative Pathologic Diagnosis
If gallbladder cancer is suspected on clinical and radiologic grounds, preoperative histologic diagnosis is unnecessary in patients who are potentially resectable. Gallbladder cancer has a tendency to seed the peritoneum, biopsy tracts, and surgical wounds (Fong et al, 1993; Hu et al, 2008; Merz et al, 1993), and unnecessary biopsies simply increase this risk. Although a positive biopsy result may make the surgeon feel more justified in the operative approach, a negative biopsy result cannot be trusted because of significant false-negative rates. If the diagnosis is suspected, the surgeon and patient must be prepared for a definitive operation. If concern for gallbladder cancer is significant, it is unwise to perform a cholecystectomy to make the diagnosis because the same risk of tumor spillage applies. The surgeon and patient also must be prepared for the possibility of performing a liver resection for benign disease. In experienced hands, a limited liver resection should be safe, and the risk of this procedure should be less than the risk of multiple biopsies or noncurative operations. For a patient with unresectable or metastatic disease, a percutaneous biopsy has an accuracy of nearly 90%, and the false-positive rate is negligible (Akosa et al, 1995).
Bile cytology has been proposed as a less risky way of making the diagnosis of gallbladder cancer without risking peritoneal seeding. Although it is reasonable to check bile cytology if the patient needs to undergo endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiography (PTC), for the reasons stated earlier, a deliberate attempt to make the diagnosis this way is unwarranted. Sensitivity of bile cytology has been reported to be approximately 75% (Akosa et al, 1995; Arora et al, 2005; Mohandas et al, 1994; Naito et al, 2009).
Staging
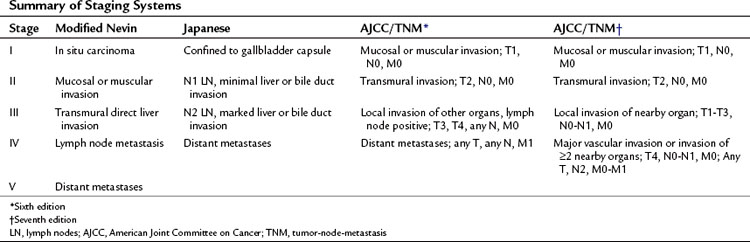
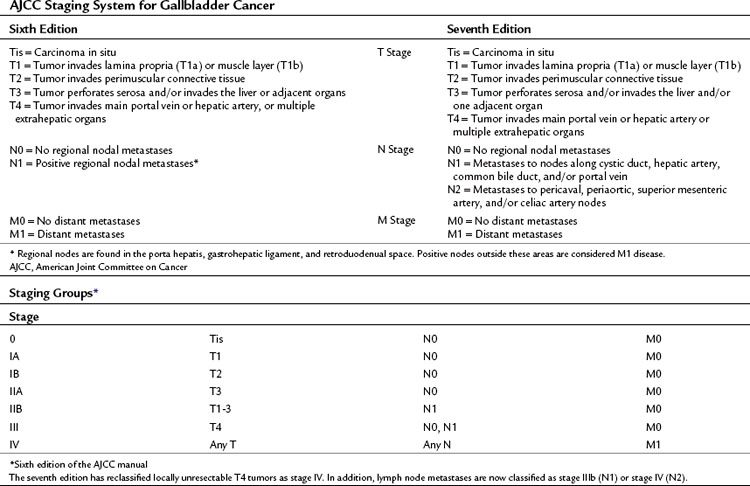
Over the years, a variety of staging systems for gallbladder cancer have been proposed based on prognostic clinical and pathologic factors. Historically, the American Joint Committee on Cancer (AJCC) system, the Japanese Biliary Surgical Society system (Onoyama et al, 1995), the Nevin system (Nevin et al, 1976), and the modified Nevin systems (Donohue et al, 1990) were all used. These staging systems are summarized in Table 49.7. The AJCC tumor-node-metastasis (TNM) staging of gallbladder cancer was adopted in 2002 as the standard reporting mechanism for gallbladder cancer studies in the literature, and it is the preferred staging system clinically (Table 49.8).
Surgical Management
Benign Polyps
Benign polypoid lesions of the gallbladder are common and are best assessed by US, although definitive diagnosis is often difficult. The only polypoid lesions that have malignant potential and are associated with a significant rate of harboring malignancy are adenomatous polyps. Other benign lesions—fibromas, lipomas, hemangiomas, cholesterol polyps, inflammatory polyps, and adenomyomas—are not known to be associated with malignant potential. Adenomyomatosis, defined as extension of Rokitansky-Aschoff sinuses through the muscular wall, is common and often diagnosed by US criteria (Stunell et al, 2008). Cholesterol polyps are the most common gallbladder polyp, but they are not easily differentiated from other lesions of the gallbladder wall without pathologic analysis. The relevant clinical question is which lesions mandate a cholecystectomy in an asymptomatic patient.
Numerous clinical reviews have identified factors associated with malignancy in gallbladder polyps. The most consistent predictors are single polyps, size greater than 1 cm, and age older than 50 years (Shinkai et al, 1998; Yeh et al, 2001). Although some clinicians have recommended cholecystectomy for any patient with fewer than three polyps (Shinkai et al, 1998), we generally recommend cholecystectomy for any polyp greater than 1 cm because the risk of malignancy for smaller polyps, regardless of their number, is exceedingly low. In a study from MSKCC of 417 patients with polypoid lesions of the gallbladder discovered on US, 10% had neoplastic/adenomatous polyps (Ito et al, 2009). Polyp growth was found in only 6% of patients on follow-up. Eighty patients underwent cholecystectomy, and a single patient had carcinoma in situ with a 13-mm gallbladder polyp. The exception to this recommendation of resection only for polyps larger than 1 cm is for those arising in the setting of primary sclerosing cholangitis. These polyps are more likely to be neoplastic, and the threshold for cholecystectomy probably should be lower in this patient population (Buckles et al, 2002).
US is the diagnostic modality of choice to measure and characterize gallbladder polyps (see Chapter 13), but if there is any suspicion of malignancy, cross-sectional imaging with contrast-enhanced CT or MRI is essential. For gallbladder polyps found in the context of abdominal symptoms, another cause of the pain should be sought. Most polyps are asymptomatic, and if no other cause is found to explain the abdominal symptoms, a cholecystectomy should be performed. Patients with polyps smaller than 1 cm who are asymptomatic should be followed up with serial US to rule out the possibility of a growing adenomatous polyp. Long-term follow-up of stable polyps does not seem warranted according to a multiinstitutional registry of approximately 70,000 patients who had gallbladder US. A prevalence of gallbladder cancer with polypoid lesions of the gallbadder equal to 0.08% was reported in white patients (Aldouri et al, 2009). Conversely, Kubota and colleagues (1995) reported that on periodic US 4 to 12 months after the original US, polyps grew 1.4 to 4 times larger. Thus our recommendation is for US follow-up at 6- to 12-month intervals for 2 years; if polyp size is stable, routine survelliance can be stopped. Additional studies should only be performed in the setting of new clinical findings or symptoms.
Incidentally Discovered Gallbladder Carcinoma During or After Routine Cholecystectomy
Gallbladder cancer is found in 0.27% to 2.1% of all laparoscopic cholecystectomies (Darmas et al, 2007; Frauenschuh et al, 2000; Kwon et al, 2008). If the diagnosis of gallbladder cancer is made by frozen-section analysis in the operating room, the operating surgeon should prepare for a curative resection with possible liver and bile duct resection. If the surgeon is not comfortable with this extent of surgical resection, no further dissection should be performed and the patient should be transferred to an experienced hepatobiliary surgeon. This strategy does not appear to affect outcomes; a retrospective study at MSKCC reported that outcomes after prior noncurative resection were similar to outcomes from primary curative resections (Fong et al, 2000). However, these findings are subject to selection bias because the majority of patients diagnosed with gallbladder cancer after routine cholecystectomy have early-stage tumors. Ouchi and colleagues (2002) reported on 498 patients diagnosed with gallbladder cancer after laparoscopic cholecystectomy. They found that 34% had T1a, 14% had T1b, 41% had T2, 8% had T3, and 2% had T4 tumors.
Once the diagnosis is made, all patients should undergo a complete staging workup, as previously described. If patients have resectable disease with no contraindications to additional surgery, a second operation should be considered. With T1a tumors, if margins are negative, standard cholecystectomy cures 85% to 100% of patients (Shirai et al, 1992; Yamaguchi & Tsuneyoshi, 1992); thus no additional resection or treatment is indicated. For tumors staged as T2 and above, additional resection is indicated. Controversy surrounds treatment of T1b tumors. Principe and colleagues (2006) demonstrated a 50% 1-year survival in patients with T1b gallbladder cancers after simple cholecystectomy (Shirai et al, 1992a). Other series, however, report cure rates for T1b tumors that are 90% to 100% at 5 years (Kang et al, 2007b). A multiinstitutional study of 115 patients reported residual disease at any site in the abdomen during re-resection for 38% of T1, 57% of T2, and 77% of T3 tumors (Pawlik et al, 2007). Residual invasion of the liver parenchyma in the gallbladder bed was found in 0% of T1, 10% of T2, and 36% of T3 tumors.
Given the results discussed above, re-resection is recommended for all patients who are medically fit with T1b or greater level of invasion. Although the role of re-resection in patients with T1b tumors has been the subject of some controversy, data from several centers now support reoperation and definitive resection in this setting. If the cystic duct stump is positive or the margin is a specific concern, a bile duct resection and reconstruction are generally required. In highly selected cases, a re-resection of the cystic duct stump with frozen-section assessment can be considered. Routine resection of the bile duct has not been globally associated with improvements in survival in all patients, but it is generally required for a complete resection in cases with a positive cystic duct stump margin (Pawlik et al, 2007; Sakamoto et al, 2006).
Staging Laparoscopy
Staging laparoscopy is an important consideration when operating on intraabdominal malignancy. Because most gallbladder cancer patients do not require palliative operations, and because incidence of occult metastatic disease is high, staging laparoscopy makes sense for this disease. The benefits of discovering occult metastases at laparoscopy compared with laparotomy are obvious and include less pain, less morbidity, quicker hospital discharge, more rapid resumption of normal activity, and earlier start of other therapies. The yield for staging laparoscopy in gallbladder cancer is high, and this examination should be routinely used. Of 44 cases in which staging laparoscopy was used, Weber and colleagues (2002) reported a 48% yield, and these patients all were spared a laparotomy. Despite this high yield, the examination still missed an additional 15 malignancies, suggesting room for further improvement. Even in patients who have had a prior noncurative cholecystectomy, the yield is still generally approximately 20% and is justified in this patient group.
Extent of Resection by Stage
An early description of a rational approach to the surgical treatment of gallbladder cancer was provided by Glenn and Hays in 1954. They recommended a wedge resection of the gallbladder bed and regional lymphadenectomy of the hepatoduodenal ligament. The late presentation and generally poor prognosis, compounded by the rarity of the disease, have resulted in significant controversy and confusion about the best surgical approach to gallbladder cancer since that time. For the same stage of disease, surgeons have recommended varying operations, from a simple cholecystectomy to combined hepatectomy, bile duct resection, and pancreatoduodenectomy. A survey of gastrointestinal surgeons from the early 1990s showed that 49% recommended lymph node dissection and 64% recommended some form of liver resection for stage T2 through T4 disease (Gagner & Rossi, 1991). No such consensus was reached on the optimal extent of resection. In recent years, however, much has been learned and consistent radical approaches have been met with some success. Despite the lack of definitive evidence, a rational approach to gallbladder cancer can be devised and should depend on the stage of the disease, location of the tumor, margin status (if previous cholecystectomy), and whether a prior, noncurative cholecystectomy has been performed. When considering extensive operations, the morbidity must be weighed carefully against the long-term outcome data.
Despite the confusion about the extent of resection for gallbladder cancer, strong evidence supports the use of liver resection and regional lymphadenectomy for T2 and T3 tumors without distant metastases. The modest improvements in survival over time probably reflect the better surgical resections applied to this overall minority of patients (Grobmyer et al, 2004). In a study from Toronto, two separate periods were analyzed and showed improved survival in completely resected patients; median survival increased from 9 to 17 months. In the second period, liver resection and regional lymphadenectomy were used more often, and the authors attributed the improved survival to this strategy (Dixon et al, 2005). In general, we recommend the following principles based on the literature and our own data. T1a tumors can be treated with a simple cholecystectomy. T1b tumors, although associated with good long-term survival after simple cholecystectomy, are associated with a higher locoregional recurrence rate and, in most patients, liver resection and lymph node dissection should be performed. A negative cystic duct margin must be ensured, and a bile duct resection to obtain a negative margin for a T1 tumor is recommended. In general, for T2 and T3 tumors, a complete resection of the tumor en bloc with segments IVb and V of the liver should be carried out to ensure complete clearance. If the tumor extent is such that invasion of hepatic inflow vascular structures is documented or suspected, an extended right hepatectomy may be necessary to clear all tumor; this is appropriate in a healthy patient with no evidence of distant disease. A negative margin at the cystic duct should be assessed and, if necessary, a bile duct resection should be performed. A lymph node dissection of the hepatoduodenal ligament also should be carried out. It is important to stage the patient carefully at operation, and major resections should be abandoned if distant nodal (retropancreatic or celiac) or other metastases are found. A diagrammatic representation of the extent of operation in a typical extended cholecystectomy is shown in Figure 49.7.
Liver Resection (See Chapter 90B)
If a segment IVb/V resection would result in an inadequate margin, an extended right hepatectomy may be required. This may be the case for large tumors invading the right portal pedicle or tumors of the lower end of the gallbladder encroaching on the porta hepatis. In the latter situation, a bile duct resection is usually required. Isolated invasion of local organs (stomach, duodenum, colon) in the absence of distant metastases requires a local resection, which is reasonable to perform to ensure tumor clearance. In patients who have had a prior noncurative cholecystectomy, the preoperative US should be reviewed to find the tumor; this can guide the resection. The cystic duct margin also should be reanalyzed. Typically, a segment IVb/V resection is adequate if no obvious tumor is present and the tumor was present in the body or fundus. It often is impossible to differentiate scar from tumor in the porta hepatis at the time of operation, forcing the surgeon to perform a bile duct resection and extended right hepatectomy; this is appropriate in well-selected patients. Reddy and colleagues (2007) described 11 patients with stage I and II gallbladder cancer who underwent extended hepatectomy for either large tumors, positive cystic duct margins, preoperative jaundice and biliary obstruction, known node-positive disease, or significant inflammation in the triangle of Calot. Morbidity and survival were similar when compared with historic controls treated with extended cholecystectomy (Reddy et al, 2007). In a series of 104 patients from MSKCC, however, morbidity and mortality increased with more extensive resections without any improvement in survival (D’Angelica et al, 2009). In this series, 20% of patients had direct invasion of other organs and concurrent en bloc resections. The principal finding of this analysis was that the stage of the tumor, rather than the extent of resection (given a margin-negative resection), was the overwhelming determinant of long-term survival. Thus current practice is to perform only the extent of hepatic resection necessary to achieve tumor clearance.
Lymph Node Dissection
Studies of the lymphatic drainage of the gallbladder have been performed and were reviewed earlier in this chapter (Shirai et al, 1992c). As in the extent of liver resection, there has been wide variability in the recommendation for the extent of lymphadenectomy, ranging from excision of the cystic duct node to a complete portal clearance combined with pancreatoduodenectomy (Matsumoto et al, 1992). Groups from Japan have reported some success for the treatment of extensive gallbladder cancer with hepatopancreatoduodenectomy even in the face of extensive nodal metastases (Sasaki et al, 2002). The recommendation for these extensive procedures is based on the fact that early lymph node metastases to the retropancreatic and interaortocaval nodes are common, and a pancreatic resection presumably improves the ability to clear these nodes. The more important question is whether the increased risk of such an approach is justified by the outcome. In general, the outcome for patients with nodal disease, in particular in patients with distant nodal disease, is so poor that this approach is not generally recommended. Given the importance of identifying lymph node metastases outside the “extended cholecystectomy” field, the first maneuver in the operating room is mobilization of the duodenum to assess the aortocaval and retropancreatic lymph nodes. The celiac lymph nodes should also be assessed early on; suspicious nodes are sent for frozen-section analysis and, if positive, the procedure is terminated.
Whether lymph node dissection improves outcome is unknown; given the rarity of this disease, this question will probably never be tested in a randomized trial. Lymph node dissection does provide important diagnostic information, however, and it may provide further regional control. Regional lymphadenectomy for gallbladder cancer includes removal of nodes in the porta hepatis, gastrohepatic ligament, and retroduodenal space (all N1 nodes). In patients with T1b or greater tumors, regional lymphadenectomy is associated with improved survival in retrospective studies when compared with extended cholecystectomy alone (Frauenschuh et al, 2000; Kwon et al, 2008). The rate of positive nodes with T1b to T3 tumors in these studies ranges from 16% to 30%. Despite these data, a study that used the epidemiology and Surveillance and Epidemiology End Results (SEER) database to track gallbladder treatment trends found that an adequate lymphadenectomy (three or more nodes) was only reported in 5.3% of patients treated surgically for gallbladder cancer (Coburn et al, 2008). The year of resection (after 1988, hazard ratio [HR] = 1.20; 95% confidence interval [CI] 1.14 to 1.26) and T1 stage (compared with T2, HR = 0.48; 95% CI, 0.27 to 0.83) were associated with adequate lymphadenectomy. Only 6% of patients with T2 or T3 tumors underwent lymphadenectomy. In addition, the majority of T3 tumors (75%) were treated with inadequate lymphadenectomies (up to two nodes). Although this study suggests that the majority of patients with gallbladder cancer did not receive appropriate surgical treatment, the limitations of the SEER database may be responsible for overestimating this trend.
Controversy surrounds the question of whether a routine bile duct resection is necessary for an adequate lymph node dissection. Although there is no question that excising the extrahepatic bile duct can make a lymph node dissection easier, it also increases the morbidity of the operation (Bartlett et al, 1996). However, no differences are reported in the numbers of lymph nodes harvested in regional lymphadenectomy with or without a bile duct resection (Sakamoto et al, 2006; Pawlik et al, 2007; D’Angelica et al, 2009). In general, a bile duct resection should not be necessary unless there is suspicion of infiltration into the porta hepatis. One study from Japan reported on patients with T2 or T3 tumors who underwent routine bile duct resection (Shimizu et al, 2004). Histologic spread of tumor in 30 of 50 specimens into the hepatoduodenal ligament was documented; some represented direct spread of tumor, and others represented lymph node metastases. It is unknown whether this resection would have any impact on outcome, especially in patients with lymph node metastases. In a study from MSKCC of 109 patients undergoing surgical resection for gallbladder cancer (D’Angelica et al, 2009), 68 patients (65%) had common bile duct resections, with 36 the result of clinical involvement and 32 with empirical resections. Bile duct resection was not associated with clinical or pathologic variables. The 5-year survival of patients without bile duct involvement was 49%, which was significantly higher than for those with bile duct involvement (20%). This study, combined with the data from Sakamoto and others, reinforces the point that stage of disease, not extent of surgery (assuming an R0 resection), determines survival in patients who undergo resection of gallbladder cancer.
Bile duct resection combined with regional lymphadenectomy requires a Kocher maneuver, division of the bile duct at the level of the duodenum, and complete dissection of all the associated soft tissue. This tissue should be swept superiorly, skeletonizing the porta hepatis vasculature. Reconstruction is with a Roux-en-Y hepaticojejunostomy (see Chapter 50B). Malignant invasion of the bile duct with jaundice would necessitate a bile duct resection, but the surgeon must consider the overwhelmingly poor prognosis in these patients and the low likelihood of a complete resection (Hawkins et al, 2004); a palliative approach may be best in this patient population.
Port Site Recurrences
There is a theoretical risk of port site seeding after laparoscopic cholecystectomy for what is eventually diagnosed as gallbladder cancer. This problem may be exacerbated by spillage of bile or stones inside the peritoneal cavity (Winston et al, 1999). One study looked at 409 patients who underwent laparoscopic cholecystectomy for presumed benign gallbladder disease but were diagnosed with gallbladder cancer on final pathology (Paolucci et al, 1999). Seventeen percent of patients at a median of 180 days were diagnosed with laparoscopic port site recurrences. As a result of this high percentage, some surgeons recommend port site excision during reoperation for gallbladder cancer. It is important to note, however, that it is rare for port site recurrences to occur as the sole site of disease (Povoski et al, 2004; Shoup & Fong, 2002). Given that it is more a marker of aggressive disease than a single site of resectable disease, our general practice does not include empirically resecting prior port sites during reexploration for gallbladder cancer.
Results
The overall picture of gallbladder cancer is slowly improving but is still a dismal one. Historically, more than two thirds of patients are initially seen with disease beyond the scope of surgery; even including patients who are lucky enough to make it to resection, the overall survival is poor. The overall 5-year survival is consistently less than 5%, with a median survival of 5 to 8 months. Older large reviews (Perpetuo et al, 1978; Piehler & Crichlow, 1978) documented a 5-year survival of 4% to 5% and a median survival of 5 months. One-year survival was 12%. Approximately 25% of the patients were resected for cure, and in this select group, 5-year survival was only 17%. Studies published in Europe and Australia in the 1990s report overall median survivals of approximately 3 months and 5-year survivals of approximately 5% (Carty & Johnson, 1991; Cubertafond et al, 1994; Wilkinson, 1995). Survival results from MSKCC for patients treated from 1995 to 2005 show some improvements compared with older studies (Duffy et al, 2008). Of the 435 patients studied, 36.6% presented with AJCC stage IV disease. Median overall survival for the entire patient cohort was 10.3 months (95% CI, 8.8 to 11.8 months) with a median follow-up of 26.6 months. For patients with stage Ia through stage III disease, median survival was 12.9 months (95% CI, 11.7 to 15.8 months) compared with 5.8 months (95% CI, 4.5 to 6.7 months) for those with stage IV disease. Median survival for patients who had an incidental diagnosis of gallbladder carcinoma after laparoscopic cholecystectomy was 15.7 months (95% CI, 12.4 to 18.4 months). Of this subset of patients, those who were reexplored had a median survival of 72 months (95% CI, 34/not reached); for patients with no pathologic evidence of residual disease, median survival was 19 months (95% CI, 12.9 to 28 months) if an R0 resection was done and 12.7 months (95% CI, 8.2 to 15.7 months) for an R1 or R2 resection (P < .0001). Of these patients, 104 were analyzed in a separate study (D’Angelica et al, 2009) that showed T and N stage to be strong independent predictors of outcome along with histologic differentiation and bile duct involvement.
T1 Tumors: Confined to the Muscular Coat
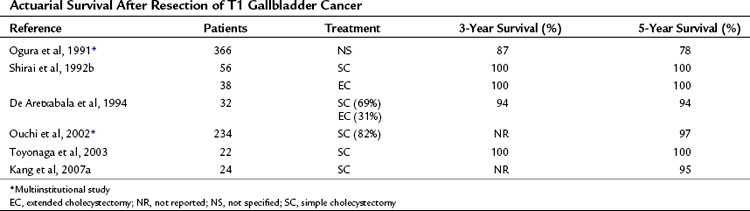
T1 tumors are typically diagnosed incidentally in cholecystectomy specimens because they are usually not obvious on imaging studies. By definition, they do not penetrate the muscular layer, and because a simple cholecystectomy dissects the perimuscular layer, this operation theoretically should be curative. These tumors are rarely associated with lymph node metastases. In the uncommon circumstance that a T1 tumor is detected intraoperatively, the surgeon should palpate lymph nodes and sample any that are suspicious. It is crucial to obtain negative margins, and the cystic duct margin always should be reviewed to ensure this. It is sometimes necessary to resect the common bile duct to obtain this negative margin. Overall, the cure rate is 85% to 100% with a simple cholecystectomy (Table 49.9). Of the small number of patients with T1 gallbladder cancer that recurs, they are commonly found to have submucosal spread involving the cystic duct margin (Shirai et al, 1992b). T1b tumors also seem to be at a higher risk of recurrence, as previously described; because of this, an extended cholecystectomy is recommended in patients with T1b tumors who can tolerate the procedure.
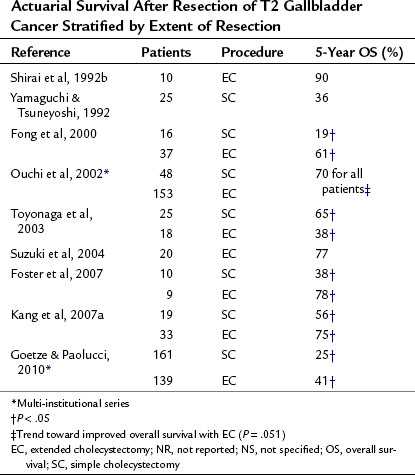
T2 Tumors: Invading into the Subserosal Layer
T2 tumors are most likely to benefit from an extended resection of the lymph nodes near the liver and porta hepatis. Results from modern series are listed in Table 49.10. Because these tumors are difficult to diagnose preoperatively, they also are commonly diagnosed incidentally at cholecystectomy. The dissection plane of a simple cholecystectomy in the subserosal plane is often involved with tumor, resulting in a positive margin for many T2 tumors (Yamaguchi et al, 1992). In addition, approximately one third of patients with T2 tumors have nodal metastases, reinforcing the need for a regional lymphadenectomy for diagnostic and potentially therapeutic purposes (Chijiiwa et al, 2001). Although based on multiple retrospective reviews, the data indicate that survival is significantly improved in patients undergoing an extended cholecystectomy compared with patients undergoing a simple cholecystectomy. What is striking from these series is that long-term survival generally is achieved in 60% to 100% of patients undergoing an extended resection, depending on the stage, compared with less than 50% with simple cholecystectomy.
T3 and T4 Tumors: Locally Advanced
The most controversial aspect of the surgical treatment of gallbladder cancer involves patients with nonmetastatic locally advanced tumors (T3 and T4 tumors). Table 49.11 summarizes representative studies in this patient group. Historically, these patients were documented to have extremely poor survival, and surgical extirpation was thought to be futile. Since the 1990s, however, numerous small series have documented that with varying levels of extended resections, long-term survival is possible in highly selected patients. In a series from MSKCC, 15% to 20% of patients with locally advanced disease were long-term survivors (Fig. 49.8) (D’Angelica et al, 2009).
Table 49.11 Actuarial Survival After Extended Resection for T3 and T4 Nonmetastatic Gallbladder Cancer
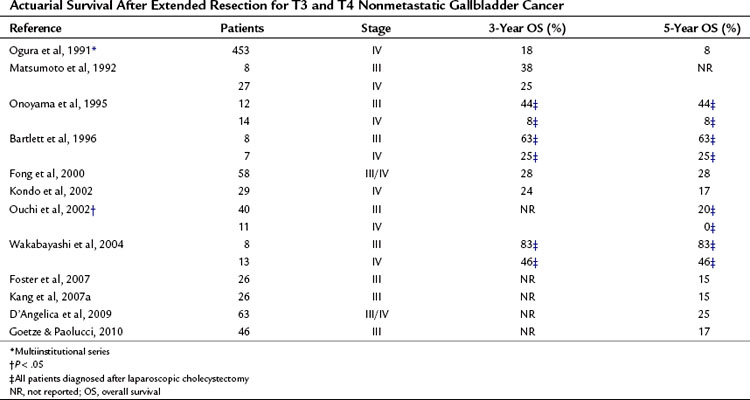
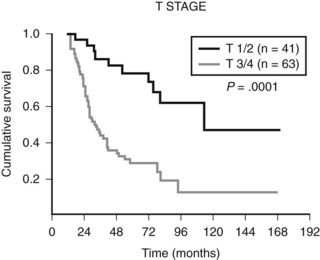
FIGURE 49.8 Disease-specific survival for 104 patients with gallbladder cancer stratified by T stage.
(From D’Angelica M, et al, 2009: Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol 16[4]:806-816.)
Numerous series from Japan, in particular, have shown the possibility of long-term survival in these patients. Japanese surgeons liberally use extensive surgical procedures, including vascular resection, bile duct resection, extended hepatectomy, and hepatopancreatoduodenectomy (Nakamura et al, 1989; Onoyama et al, 1995; Shirai et al, 1992b). In general, extensive lymph node dissections are routinely performed, and survival results may be a reflection of stage migration and better staging. Western series also have begun to show similar results with an aggressive surgical approach (Gall et al, 1991). These data must be interpreted in the proper context, however; overall, most patients with locally advanced disease have metastatic disease, and resection is often impossible. The most important associated prognostic factor in every series is the status of lymph nodes. Long-term survival rarely accompanies distant lymph node disease, and it is uncommon with hepatoduodenal lymph node metastases. The uncommon patient with a locally advanced tumor and no lymph node metastases probably benefits the most from an extensive operation. Patients with locally advanced gallbladder tumors should be carefully evaluated to rule out distant metastases. In general, patients with lymph node metastases outside the hepatoduodenal ligament should not undergo resection. In carefully selected patients, however, extended radical resection provides the only chance for long-term survival.
Regional Lymph Node Positivity
Nodal status was found to be an independent predictor of outcome in multiple studies. Although the number and location of positive nodes can affect survival, the presence of any nodal disease is associated with poor survival. In a study of 104 patients with gallbladder cancer at MSKCC, for example, the 34 patients with N1 disease had a significantly worse 5-year survival (17%) compared with patients with N0 disease (51%; P = .0002) (D’Angelica et al, 2009). Endo and colleagues (2006) reported a similar difference in survival, with 5-year survival rates of 77% with node-negative disease and 33% with one positive lymph node. These findings also apply to patients who had gallbladder cancer discovered incidentally after cholecystectomy. Pawlik and colleagues (2007) examined nodal status in patients diagnosed after surgical resection and found a 5-year survival of 73% with node-negative disease compared with a 5-year survival of 27% in patients with node-positive disease (Endo et al, 2006). The number of positive nodes has also been described as a predictor of outcome. Patients with a single lymph node metastasis had a higher 5-year survival rate (33%) compared with patients who had two or more lymph node metastases (0%; P < .05) (Endo et al, 2006).
Gallbladder Cancer Discovered Incidentally After Laparoscopic Cholecystectomy
The surgical approaches to patients who have undergone a prior noncurative cholecystectomy have been previously outlined. When a patient is seen after a noncurative cholecystectomy with no evidence of disseminated disease, a repeat resection is warranted in most cases. For patients who undergo definitive operation, the outcome seems to be similar to that of patients who undergo primary resection; this seems to be true stage for stage (Fong et al, 2000).
Significance of Jaundice on Presentation
The presence of jaundice is a troubling sign; it suggests porta hepatis involvement with tumor. In 240 patients at MSKCC who presented with gallbladder cancer, 82 (34%) were jaundiced and were more likely to have advanced stage disease; only six patients underwent resection with curative intent, and only four had an R0 resection (Hawkins et al, 2004). The median disease-specific survival of patients with jaundice was 6 months, significantly worse than those without jaundice (16-month disease-specific survival; P < .0001), and none from the jaundiced group survived 2 years. A retrospective study by Varma and colleagues (2009) reported that although jaundice was associated with a higher stage at presentation, it did not preclude resection. Half of those patients who presented with jaundice underwent R0 resections. Survival differences, however, were not reported between jaundiced and nonjaundiced patients. Thus curative resection can be attempted in select patients with gallbladder cancer and jaundice, but the expectations of the surgical team and patient should be tempered.
Adjuvant Therapy
The rarity of gallbladder cancer has traditionally precluded large randomized trials, and the literature abounds with small case series and retrospective comparisons that attempt to address whether adjuvant chemotherapy and/or radiation therapy are beneficial after complete resection. In a study from MSKCC, 85% of recurrences included distant sites as part of the recurrence pattern (Jarnagin et al, 2003), highlighting the importance of systemic therapies. Reports of small series with fewer than 10 patients from the 1970s and 1980s suggested a small benefit to adjuvant chemotherapy compared with historic controls (Morrow et al, 1983; Oswalt & Cruz, 1977). Chao and Greager (1991) reported on 15 patients who received some form of chemotherapy or radiation or both after resection and found no difference compared with patients who had not received adjuvant therapy. Similarly, studies of adjuvant radiation therapy over the last few decades have suggested modest benefits (Bosset et al, 1989; Hanna & Rider, 1978; Todoroki et al, 1991).
A recent retrospective review of 123 patients who underwent resection of gallbladder cancer with adjuvant therapy (n = 24; 66% of patients with chemoradiation, 34% of patients with chemotherapy alone) or without (n = 99) showed no difference in overall survival between the groups; however, patients treated with adjuvant therapy had a higher rate of incomplete resections, higher stage of disease, and more positive lymph nodes (Duffy et al, 2008). A recent SEER database analysis of 4000 patients with gallbladder cancer found a survival benefit with adjuvant radiotherapy in patients with positive nodal disease or T2 or greater tumors (15 months vs. 8 months). This difference was significant on multivariate analysis (HR = 1.46; 95% CI, 1.07 to 1.98; P = .014) (Wang et al, 2008).
Three randomized, controlled trials have included patients with gallbladder cancer. Takada and colleagues (2002) reported a phase III multiinstitutional trial of adjuvant chemotherapy performed in Japan. This trial included 508 patients with biliary and pancreatic cancers. On subset analysis, this study included 140 gallbladder cancer patients who were randomized to receive surgical resection alone or resection plus adjuvant mitomycin C and 5-fluorouracil (5-FU). When considering the gallbladder cancer patients only, the actuarial 5-year disease-free survival favored the adjuvant chemotherapy group (20.3%) compared with the surgery-alone group (11.6%, P = .02). Data regarding the extent of surgical resection and histologic staging were lacking, making the results difficult to interpret. More recently, gemcitabine-based regimens, often combined with a platinum agent, have become the drug of choice for treating gallbladder cancer. Much of the data for the adjuvant setting has been extrapolated from the metastatic setting. A recently published randomized, phase III study of palliative gemcitabine compared with gemcitabine and cisplatin for metastatic biliary cancers offers information that may be useful in the adjuvant setting (Valle et al, 2010). With overall survival as the primary end point, 410 patients with locally advanced or metastatic gallbladder cancer, cholangiocarcinoma, or ampullary cancers were randomized; 149 patients had gallbladder cancer. After a median follow-up of 8.2 months for all patients in the trial, a significant difference in overall survival was reported, with an HR of 0.61 (CI, 0.42 to 0.89) favoring the gemcitabine-cisplatin combination. There were, however, more neutropenic events in the gemcitabine-cisplatin group. It remains to be seen whether this new trial will change clinical practice in the adjuvant setting, but it continues to reinforce gemcitabine-based regimens as the standard chemotherapy for gallbladder cancer. Another smaller randomized, controlled trial of gemcitabine combined with oxaliplatin (GEMOX) showed a similar benefit when the gemcitibine regimen was compared with 5-FU or best supportive care in patients with unresectable or metastatic gallbladder cancer (Sharma et al, 2010). Median overall survival was 9.5 months in the GEMOX group (n = 27), compared with 4.6 months in the 5-FU group (n = 28; P = .039).
Palliative Management
Most treatment options in patients with gallbladder cancer are palliative because most patients are unresectable by the time they come to medical attention. The most common symptoms to palliate include pain, jaundice, and bowel obstruction. Palliation of jaundice in gallbladder cancer can be complex and depends on the location and extent of biliary obstruction. The outcome for these patients is poor, and chemotherapy often offers little in terms of prolongation of life. In the past, operative approaches provided the most effective relief of obstruction in well-selected patients. Operations such as the segment III bypass (Fig. 49.9) (Bismuth & Corlette, 1975; Kapoor et al, 1996) and the Longmire operation (Longmire et al, 1973) have become less common with the development of interventional percutaneous stenting techniques. In patients who have symptoms from obstructive jaundice, endoscopic or percutaneous interventions are the preferred approach for palliation and minimalization of morbidity (Lung et al, 2009; Naitoh et al, 2009; Togawa et al, 2008).
In general, patients in need of palliative procedures for symptomatic gallbladder cancer have high complication rates after such procedures. A prospective study from MSKCC included 109 patients undergoing percutaneous biliary drainage for malignant biliary obstruction. A 58% rate of major complication and two procedure-related deaths were reported, and the 8-week postprocedure mortality rate was 28%. These palliative procedures were successful in alleviating pruritus, but no improvement was reported in measures of quality of life (Robson et al, 2010). The high risk of morbidity and mortality precludes recommending these treatments in the absence of symptoms or a specific reason to treat jaundice, such as to allow the administration of chemotherapy. Saluja and colleagues (2008) randomized 44 patients with gallbladder cancer and obstructive jaundice to percutaneous or endoscopic stenting. They found that successful stenting occurred in 89% of patients in the percutaneous group compared with 41% in the endoscopic group (P < .001). Cholangitis was also significantly higher (48%) in the endoscopic stent group compared with the percutaneous group (11%; P = .002). The mortality rates were similar between the two groups, but percutaneous stenting in this trial offered better palliation than endoscopic stenting. The choice between endoscopic and percutaneous interventions often depends on local resources and personnel. Intestinal bypass can also be performed in patients with symptomatic bowel obstructions. Percutaneous feeding tubes or drains and endoscopic stents are favored over surgical bypass because of the extremely poor prognosis.
Chemotherapy has been used to palliate unresectable disease but often offers little benefit. Historically, regimens have included 5-FU, leucovorin, mitomycin C, doxorubicin, and methotrexate. Response rates in these studies generally range from 10% to 20% (Hejna & Zielinski, 2001). More recently, however, phase II trials with gemcitabine and oxaliplatin have showed improved response rates ranging from 40% to 50% (Andre et al, 2008; Harder et al, 2006; Verderame et al, 2006). Radiation therapy may be an effective palliative therapy for locally advanced disease; it is generally well tolerated, may have an impact on local symptoms, and is usually combined with chemotherapy (Lin et al, 2005; Valle et al, 2010).
Summary
Although the overall picture of gallbladder cancer is a dismal one, we have come a long way since Alfred Blalock’s quote in 1924, which summarized the nihilism of the day. Localized gallbladder cancer can be cured with an extended resection, and although most patients who are cured had early-stage disease, there is some hope for patients with locally advanced tumors. Perhaps the most important lesson is to recognize abnormalities of the gallbladder wall as possibly malignant at an early stage because primary resection remains the best chance of a good outcome. General surgeons who treat gallbladder disease should critically review the results of preoperative imaging in patients with presumed benign gallstone disease, particularly the US findings, and the possibility of a malignant process must always be considered when abnormalities of the gallbladder wall are reported.
Cancers incidentally discovered at cholecystectomy are common, and this is sufficient treatment for most T1 cancers (Table 49.12). Exceptions are patients with positive cystic duct margins, who should undergo a definitive complete resection, and patients with T1b tumors, who should also be considered for reoperation and definitive resection. For T2 through T4 tumors, patients should undergo reexcision as described throughout this chapter. In general, patients with distant nodal metastases are not able to be cured and probably do not benefit from reoperation. Preoperative imaging and surgical staging are crucial because of the high incidence of metastatic disease. Tumors recognized before cholecystectomy should be resected with an en bloc liver resection and portal lymphadenectomy as described. Even large, locally advanced tumors are potentially curable, albeit the likelihood of such is low. In well-selected, otherwise healthy patients, an aggressive approach is warranted in the absence of distant metastases, including distant nodal disease. For the rare patient with a locally advanced, node-negative tumor, complete resection offers a significant chance for long-term survival. This is probably also true for patients with limited nodal disease in the hepatoduodenal ligament.
Table 49.12 Treatment Recommendations for Gallbladder Cancer Diagnosed After Cholecystectomy
| T Stage | Recommended Surgical Procedure | Notes |
|---|---|---|
| Ia | Simple cholecystectomy | 85% to 100% of patients cured |
| Ib, II | Extended cholecystectomy with resection of segments IVb and V with en bloc regional lymph node dissection Bile duct resection if bile duct margin positive |
|
| III | Hepatic resection (segments IVb, V) with major hepatectomy as necessary | More commonly involves bile duct and right-sided vascular structures, requiring major liver resection |
| IV | Same as stage III if resectable | Often unresectable because of hilar vascular invasion |
Ajiki T, et al. K-ras gene mutation in gall bladder carcinomas and dysplasia. Gut. 1996;38(3):426-429.
Akosa AB, et al. Cytologic diagnosis in the management of gallbladder carcinoma. Acta Cytol. 1995;39(3):494-498.
Albores-Saavedra J, et al. Intestinal-type adenocarcinoma of the gallbladder: a clinicopathologic study of seven cases. Am J Surg Pathol. 1986;10(1):19-25.
Albores-Saavedra J, et al. Papillary carcinomas of the gallbladder: analysis of noninvasive and invasive types. Arch Pathol Lab Med. 2005;129(7):905-909.
Aldouri AQ, et al. The risk of gallbladder cancer from polyps in a large multiethnic series. Eur J Surg Oncol. 2009;35(1):48-51.
Aldridge MC, Bismuth H. Gallbladder cancer: the polyp-cancer sequence. Br J Surg. 1990;77:363-364.
Andre T, et al. Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br J Cancer. 2008;99(6):862-867.
Arora VK, et al. Intraoperative bile cytology of the dysplasia–carcinoma in situ sequence of gallbladder carcinoma. Cancer. 2005;105(5):277-281.
Asano T, et al. Expressions of cyclooxygenase-2 and prostaglandin E-receptors in carcinoma of the gallbladder: crucial role of arachidonate metabolism in tumor growth and progression. Clin Cancer Res. 2002;8(4):1157-1167.
Bach AM, et al. Gallbladder cancer: can ultrasonography evaluate extent of disease? J Ultrasound Med. 1998;17(5):303-309.
Barakat J, et al. Changing patterns of gallbladder carcinoma in New Mexico. Cancer. 2006;106(2):434-440.
Baretton G, et al. DNA ploidy in carcinoma of the gallbladder: prognostic significance and comparison of flow and image cytometry on archival tumor material. Pathol Res Pract. 1994;190(6):584-592.
Bartlett DL, et al. Long-term results after resection for gallbladder cancer: implications for staging and management. Ann Surg. 1996;224(5):639-646.
Bazoua G, et al. Do we need histology for a normal-looking gallbladder? J Hepatobiliary Pancreat Surg. 2007;14(6):564-568.
Berk RN, et al. Carcinoma in the porcelain gallbladder. Radiology. 1973;106(1):29-31.
Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140(2):170-178.
Blalock A. A statistical study of 888 cases of biliary tract disease. Johns Hopkins Hosp Bull. 1924;35:391-409.
Boerma EJ. Towards an oncological resection of gall bladder cancer. Eur J Surg Oncol. 1994;20(5):537-544.
Bosset JF, et al. Primary carcinoma of the gallbladder: adjuvant postoperative external irradiation. Cancer. 1989;64(9):1843-1847.
Broden G, Bengtsson L. Carcinoma of the gallbladder: its relation to cholelithiasis and to the concept of prophylactic cholecystectomy. Acta Chir Scand Suppl. 1980;500:15-18.
Buckles DC, et al. In primary sclerosing cholangitis, gallbladder polyps are frequently malignant. Am J Gastroenterol. 2002;97(5):1138-1142.
Calle EE, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625-1638.
Canturk Z, et al. Prevalence and risk factors for gall bladder polyps. East Afr Med J. 2007;84(7):336-341.
Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75(Suppl 1):171-190.
Carty NJ, Johnson CD. Carcinoma of the gallbladder: a survey of cases in Wessex 1982-1989. J R Coll Surg Edinb. 1991;36(4):238-241.
Chao TC, Greager JA. Primary carcinoma of the gallbladder. J Surg Oncol. 1991;46(4):215-221.
Chijiiwa K, et al. Adenocarcinoma of the gallbladder associated with anomalous pancreaticobiliary ductal junction. Am Surg. 1993;59(7):430-434.
Chijiiwa K, et al. Surgical treatment of patients with T2 gallbladder carcinoma invading the subserosal layer. J Am Coll Surg. 2001;192(5):600-607.
Choi JH, et al. Pre-operative predictive factors for gallbladder cholesterol polyps using conventional diagnostic imaging. World J Gastroenterol. 2008;14(44):6831-6834.
Choi YL, et al. An immunohistochemical study of the expression of adhesion molecules in gallbladder lesions. J Histochem Cytochem. 2004;52(5):591-601.
Coburn NG, et al. Surgery for gallbladder cancer: a population-based analysis. J Am Coll Surg. 2008;207(3):371-382.
Collier NA, et al. Preoperative diagnosis and its effect on the treatment of carcinoma of the gallbladder. Surg Gynecol Obstet. 1984;159(5):465-470.
Corvera CU, et al. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg. 2008;206(1):57-65.
Cubertafond P, et al. Surgical treatment of 724 carcinomas of the gallbladder: results of the French Surgical Association Survey. Ann Surg. 1994;219(3):275-280.
Daines WP, et al. Gallbladder and biliary tract carcinoma: a comprehensive update, Part 2. Oncology (Williston Park). 2004;18(8):1049-1059. discussion 1060, 1065-1046, 1068
D’Angelica M, et al. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol. 2009;16(4):806-816.
Darmas B, et al. Is there any justification for the routine histological examination of straightforward cholecystectomy specimens? Ann R Coll Surg Engl. 2007;89(3):238-241.
De Aretxabala X, et al. Gallbladder cancer in patients less than 40 years old. Br J Surg. 1994;81(1):111.
Diehl AK. Epidemiology of gallbladder cancer: a synthesis of recent data. J Natl Cancer Inst. 1980;65(6):1209-1214.
Diehl AK. Gallstone size and the risk of gallbladder cancer. JAMA. 1983;250(17):2323-2326.
Dixon E, et al. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American Center. Ann Surg. 2005;241(3):385-394.
Donohue JH, et al. Carcinoma of the gallbladder: does radical resection improve outcome? Arch Surg. 1990;125(2):237-241.
Duarte I, et al. Metaplasia and precursor lesions of gallbladder carcinoma: frequency, distribution, and probability of detection in routine histologic samples. Cancer. 1993;72(6):1878-1884.
Duffy A, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan–Kettering Cancer Center (MSKCC). J Surg Oncol. 2008;98(7):485-489.
Endo I, et al. Prognostic significance of the number of positive lymph nodes in gallbladder cancer. J Gastrointest Surg. 2006;10(7):999-1007.
Enomoto M, et al. Carcinogenesis in extrahepatic bile duct and gallbladder: carcinogenic effect of N-hydroxy-2-acetamidofluorene in mice fed a “gallstone-inducing” diet. Jpn J Exp Med. 1974;44(1):37-54.
Fernandez E, et al. Family history and the risk of liver, gallbladder, and pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 1994;3(3):209-212.
Fong Y, et al. Gallbladder cancer discovered during laparoscopic surgery: potential for iatrogenic tumor dissemination. Arch Surg. 1993;128(9):1054-1056.
Fong Y, et al. Evidence-based gallbladder cancer staging: changing cancer staging by analysis of data from the National Cancer Database. Ann Surg. 2006;243:767-771.
Fong Y, et al. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232(4):557-569.
Fong Y, Malhotra S. Gallbladder cancer: recent advances and current guidelines for surgical therapy. Adv Surg. 2001;35:1-20.
Fortner JG, Randall HT. On the carcinogenicity of human gallstones. Surg Forum. 1961;12:155-157.
Foster JM, et al. Gallbladder cancer: defining the indications for primary radical resection and radical re-resection. Ann Surg Oncol. 2007;14(2):833-840.
Franquet T, et al. Primary gallbladder carcinoma: imaging findings in 50 patients with pathologic correlation. Gastrointest Radiol. 1991;16(2):143-148.
Frauenschuh D, et al. How to proceed in patients with carcinoma detected after laparoscopic cholecystectomy. Langenbecks Arch Surg. 2000;385(8):495-500.
Gagner M, Rossi RL. Radical operations for carcinoma of the gallbladder: present status in North America. World J Surg. 1991;15(3):344-347.
Gall FP, et al. Radical operations for carcinoma of the gallbladder: present status in Germany. World J Surg. 1991;15(3):328-336.
Glenn F, Hays DM. The scope of radical surgery in the treatment of malignant tumors of the extrahepatic biliary tract. Surg Gynecol Obstet. 1954;99(5):529-541.
Goetze TO, Paolucci V. Adequate extent in radical re-resection of incidental gallbladder carcinoma: analysis of the German Registry. Surg Endosc. 2010;24(9):2156-2164.
Goldin RD, Roa JC. Gallbladder cancer: a morphological and molecular update. Histopathology. 2009;55(2):218-229.
Grobmyer SR, et al. Gallbladder cancer in the twentieth century: single institution’s experience. World J Surg. 2004;28(1):47-49.
Hanna SS, Rider WD. Carcinoma of the gallbladder or extrahepatic bile ducts: the role of radiotherapy. Can Med Assoc J. 1978;118(1):59-61.
Harder J, et al. Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br J Cancer. 2006;95(7):848-852.
Hawkins WG, et al. Jaundice predicts advanced disease and early mortality in patients with gallbladder cancer. Ann Surg Oncol. 2004;11(3):310-315.
Hejna M, Zielinski CC. Nonsurgical management of gallbladder cancer: cytotoxic treatment and radiotherapy. Expert Rev Anticancer Ther. 2001;1(2):291-300.
Henson DE, et al. Carcinoma of the gallbladder: histologic types, stage of disease, grade, and survival rates. Cancer. 1992;70(6):1493-1497.
Hu JB, et al. Port site and distant metastases of gallbladder cancer after laparoscopic cholecystectomy diagnosed by positron emission tomography. World J Gastroenterol. 2008;14(41):6428-6431.
Ito H, et al. Polypoid lesions of the gallbladder: diagnosis and follow up. J Am Coll Surg. 2009;208(4):570-575.
Itoi T, et al. Detection of telomerase activity in biopsy specimens for diagnosis of biliary tract cancers. Gastrointest Endosc. 2000;52(3):380-386.
Jarnagin WR, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98(8):1689-1700.
Jemal A, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277-300.
Kalra N, et al. MDCT in the staging of gallbladder carcinoma. Am J Roentgenol. 2006;186(3):758-762.
Kang CM, et al. Laparoscopic cholecystectomy only could be an appropriate treatment for selected clinical R0 gallbladder carcinoma. Surg Endosc. 2007;21(9):1582-1587.
Kang CM, et al. Does “clinical” R0 have validity in the choice of simple cholecystectomy for gallbladder carcinoma? J Gastrointest Surg. 2007;11(10):1309-1316.
Kapoor VK, et al. Intrahepatic segment III cholangiojejunostomy in advanced carcinoma of the gallbladder. Br J Surg. 1996;83(12):1709-1711.
Kato S, et al. Septum formation of the common hepatic duct associated with an anomalous junction of the pancreaticobiliary ductal system and gallbladder cancer: report of a case. Surg Today. 1994;24(6):534-537.
Kim JH, et al. Preoperative evaluation of gallbladder carcinoma: efficacy of combined use of MR imaging, MR cholangiography, and contrast-enhanced dual-phase three-dimensional MR angiography. J Magn Reson Imaging. 2002;16(6):676-684.
Kim JH, et al. Should we perform surgical management in all patients with suspected porcelain gallbladder? Hepatogastroenterology. 2009;56(93):943-945.
Kim SJ, et al. Accuracy of preoperative T-staging of gallbladder carcinoma using MDCT. Am J Roentgenol. 2008;190(1):74-80.
Kim YW, et al. Expression of the c-erb-B2 and p53 protein in gallbladder carcinomas. Oncol Rep. 2001;8(5):1127-1132.
Kimura W, et al. Clinicopathologic study of asymptomatic gallbladder carcinoma found at autopsy. Cancer. 1989;64:98-103.
Koda M, et al. Expression of Fhit, Mlh1, and P53 protein in human gallbladder carcinoma. Cancer Lett. 2003;199(2):131-138.
Koh T, et al. Differential diagnosis of gallbladder cancer using positron emission tomography with fluorine-18-labeled fluoro-deoxyglucose (FDG-PET). J Surg Oncol. 2003;84(2):74-81.
Kondo S, et al. Extensive surgery for carcinoma of the gallbladder. Br J Surg. 2002;89(2):179-184.
Kowalewski K, Todd EF. Carcinoma of the gallbladder induced in hamsters by insertion of cholesterol pellets and feeding dimethylnitrosamine. Proc Soc Exp Biol Med. 1971;136(2):482-486.
Kozuka S, et al. Relation of adenoma to carcinoma in the gallbladder. Cancer. 1982;50(10):2226-2234.
Kubota K, et al. How should polypoid lesions of the gallbladder be treated in the era of laparoscopic cholecystectomy? Surgery. 1995;117(5):481-487.
Kwon AH, et al. Laparoscopic cholecystectomy in patients with porcelain gallbladder based on the preoperative ultrasound findings. Hepatogastroenterology. 2004;51(58):950-953.
Kwon AH, et al. Unsuspected gallbladder cancer diagnosed during or after laparoscopic cholecystectomy. J Surg Oncol. 2008;97(3):241-245.
Lazcano-Ponce EC, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51(6):349-364.
Lee JH, et al. Clinical usefulness of preoperative and postoperative chest computed tomography for colorectal cancer. J Korean Soc Coloproctol. 2010;26(5):359-364.
Lewis JT, et al. Prevalence and risk factors for gallbladder neoplasia in patients with primary sclerosing cholangitis: evidence for a metaplasia–dysplasia–carcinoma sequence. Am J Surg Pathol. 2007;31(6):907-913.
Lin LL, et al. A phase II study of alternating cycles of split course radiation therapy and gemcitabine chemotherapy for inoperable pancreatic or biliary tract carcinoma. Am J Clin Oncol. 2005;28(3):234-241.
Longmire WP, et al. Carcinoma of the extrahepatic biliary tract. Ann Surg. 1973;178(3):333-345.
Lowenfels AB, Norman J. Isoniazid and bile duct cancer. JAMA. 1978;240(5):434-435.
Lung PF, et al. Novel deployment of a covered duodenal stent in open surgery to facilitate closure of a malignant duodenal perforation. World J Surg Oncol. 2009;7:79.
Mancuso TF, Brennan MJ. Epidemiological considerations of cancer of the gallbladder, bile ducts and salivary glands in the rubber industry. J Occup Med. 1970;12(9):333-341.
Matsumoto Y, et al. Surgical treatment of primary carcinoma of the gallbladder based on the histologic analysis of 48 surgical specimens. Am J Surg. 1992;163(2):239-245.
Merz BJ, et al. Implant metastasis of gallbladder carcinoma in situ in a cholecystectomy scar: a case report. Surgery. 1993;114(1):120-124.
Miller G, Jarnagin WR. Gallbladder carcinoma. Eur J Surg Oncol. 2008;34(3):306-312.
Mohandas KM, et al. Diagnosis of malignant obstructive jaundice by bile cytology: results improved by dilating the bile duct strictures. Gastrointest Endosc. 1994;40(2 Pt 1):150-154.
Morrow CE, et al. Primary gallbladder carcinoma: significance of subserosal lesions and results of aggressive surgical treatment and adjuvant chemotherapy. Surgery. 1983;94(4):709-714.
Naito Y, et al. Usefulness of lavage cytology during endoscopic transpapillary catheterization into the gallbladder in the cytological diagnosis of gallbladder disease. Diagn Cytopathol. 2009;37(6):402-406.
Naitoh I, et al. Unilateral versus bilateral endoscopic metal stenting for malignant hilar biliary obstruction. J Gastroenterol Hepatol. 2009;24(4):552-557.
Nakamura S, et al. Aggressive surgery for carcinoma of the gallbladder. Surgery. 1989;106(3):467-473.
Nakayama F. Recent progress in the diagnosis and treatment of carcinoma of the gallbladder—introduction. World J Surg. 1991;15(3):313-314.
Nevin JE, et al. Carcinoma of the gallbladder: staging, treatment, and prognosis. Cancer. 1976;37(1):141-148.
North JHJr, et al. Prognostic factors for adenocarcinoma of the gallbladder: an analysis of 162 cases. Am Surg. 1998;64(5):437-440.
Ogura Y, et al. Radical operations for carcinoma of the gallbladder: present status in Japan. World J Surg. 1991;15(3):337-343.
Ohtani T, et al. Carcinoma of the gallbladder: CT evaluation of lymphatic spread. Radiology. 1993;189:875-880.
Onoyama H, et al. Extended cholecystectomy for carcinoma of the gallbladder. World J Surg. 1995;19(5):758-763.
Oswalt CE, Cruz ABJr. Effectiveness of chemotherapy in addition to surgery in treating carcinoma of the gallbladder. Rev Surg. 1977;34(6):436-438.
Ouchi K, et al. Laparoscopic cholecystectomy for gallbladder carcinoma: results of a Japanese survey of 498 patients. J Hepatobiliary Pancreat Surg. 2002;9(2):256-260.
Pandey M. Environmental pollutants in gallbladder carcinogenesis. J Surg Oncol. 2006;93(8):640-643.
Pandey M, et al. Carcinoma of the gallbladder: role of sonography in diagnosis and staging. J Clin Ultrasound. 2000;28(5):227-232.
Pandey SN, et al. Slow acetylator genotype of N-acetyl transferase 2 (NAT2) is associated with increased susceptibility to gallbladder cancer: the cancer risk not modulated by gallstone disease. Cancer Biol Ther. 2007;6(1):91-96.
Pandey SN, et al. Haplotype analysis of signal peptide (insertion/deletion) and XbaI polymorphisms of the APOB gene in gallbladder cancer. Liver Int. 2007;27(7):1008-1015.
Paolucci V, et al. Tumor seeding following laparoscopy: international survey. World J Surg. 1999;23(10):989-995. discussion 996-987
Pawlik TM, et al. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg. 2007;11(11):1478-1486. discussion 1486-1477
Perpetuo MD, et al. Natural history study of gallbladder cancer: a review of 36 years experience at M. D. Anderson Hospital and Tumor Institute. Cancer. 1978;42(1):330-335.
Petrowsky H, et al. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006;45(1):43-50.
Piehler JM, Crichlow RW. Primary carcinoma of the gallbladder. Surg Gynecol Obstet. 1978;147(6):929-942.
Povoski SP, et al. Axillary lymph node metastasis following resection of abdominal wall laparoscopic port site recurrence of gallbladder cancer. J Hepatobiliary Pancreat Surg. 2004;11(3):197-202.
Principe A, et al. Radical surgery for gallbladder carcinoma: possibilities of survival. Hepatogastroenterology. 2006;53(71):660-664.
Puhalla H, et al. Expression of p21(Wafl/Cip1), p57(Kip2) and HER2/neu in patients with gallbladder cancer. Anticancer Res. 2007;27(3B):1679-1684.
Quan ZW, et al. Association of p53, p16, and vascular endothelial growth factor protein expressions with the prognosis and metastasis of gallbladder cancer. J Am Coll Surg. 2001;193(4):380-383.
Rajagopalan V, et al. Gallbladder and biliary tract carcinoma: a comprehensive update, Part 1. Oncology (Williston Park). 2004;18(7):889-896.
Randi G, et al. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118(7):1591-1602.
Rao ND, et al. Three-dimensional helical computed tomography cholangiography with minimum intensity projection in gallbladder carcinoma patients with obstructive jaundice: comparison with magnetic resonance cholangiography and percutaneous transhepatic cholangiography. J Gastroenterol Hepatol. 2005;20(2):304-308.
Rashid A. Cellular and molecular biology of biliary tract cancers. Surg Oncol Clin N Am. 2002;11(4):995-1009.
Redaelli CA, et al. High coincidence of Mirizzi syndrome and gallbladder carcinoma. Surgery. 1997;121(1):58-63.
Reddy SK, et al. Extended hepatic resection for gallbladder cancer. Am J Surg. 2007;194(3):355-361.
Ritts REJr, et al. Comparison of preoperative serum CA19-9 levels with results of diagnostic imaging modalities in patients undergoing laparotomy for suspected pancreatic or gallbladder disease. Pancreas. 1994;9(6):707-716.
Roa I, et al. Preneoplastic lesions and gallbladder cancer: an estimate of the period required for progression. Gastroenterology. 1996;111(1):232-236.
Roa JC, et al. Promoter methylation profile in gallbladder cancer. J Gastroenterol. 2006;41(3):269-275.
Robson PC, et al. Prospective study of outcomes after percutaneous biliary drainage for malignant biliary obstruction. Ann Surg Oncol. 2010;17(9):2303-2311.
Rodriguez-Fernandez A, et al. Application of modern imaging methods in diagnosis of gallbladder cancer. J Surg Oncol. 2006;93(8):650-664.
Rudolph R, Cohen JJ. Cancer of the gallbladder in an 11-yr-old Navajo girl. J Pediatr Surg. 1972;7(1):66-67.
Sakamoto Y, et al. Clinical significance of extrahepatic bile duct resection for advanced gallbladder cancer. J Surg Oncol. 2006;94(4):298-306.
Saluja SS, et al. Endoscopic or percutaneous biliary drainage for gallbladder cancer: a randomized trial and quality of life assessment. Clin Gastroenterol Hepatol. 2008;6(8):944-950. e943
Sasaki R, et al. Hepatopancreatoduodenectomy with wide lymph node dissection for locally advanced carcinoma of the gallbladder—long-term results. Hepatogastroenterology. 2002;49:912-915.
Sasatomi E, et al. Precancerous conditions of gallbladder carcinoma: overview of histopathologic characteristics and molecular genetic findings. J Hepatobiliary Pancreat Surg. 2000;7(6):556-567.
Sato M, et al. Localized gallbladder carcinoma: sonographic findings. Abdom Imaging. 2001;26(6):619-622.
Schwartz LH, et al. Gallbladder carcinoma: findings at MR imaging with MR cholangiopancreatography. J Comput Assist Tomogr. 2002;26(3):405-410.
Serra I, Diehl AK. Number and size of stones in patients with asymptomatic and symptomatic gallstones and gallbladder carcinoma. J Gastrointest Surg. 2002;6(2):272-273. author reply 273
Serra I, et al. Risk factors for gallbladder cancer: an international collaborative case-control study. Cancer. 1996;78(7):1515-1517.
Sharma A, et al. A phase II study of gemcitabine and oxaliplatin (Oxigem) in unresectable gall bladder cancer. Cancer Chemother Pharmacol. 2010;65(3):497-502.
Shimizu Y, et al. Should the extrahepatic bile duct be resected for locally advanced gallbladder cancer? Surgery. 2004;136(5):1012-1017. discussion 1018
Shinkai H, et al. Surgical indications for small polypoid lesions of the gallbladder. Am J Surg. 1998;175(2):114-117.
Shirai Y, et al. Inapparent carcinoma of the gallbladder: an appraisal of a radical second operation after simple cholecystectomy. Ann Surg. 1992;215(4):326-331.
Shirai Y, et al. Radical surgery for gallbladder carcinoma: long-term results. Ann Surg. 1992;216(5):565-568.
Shirai Y, et al. Identification of the regional lymphatic system of the gallbladder by vital staining. Br J Surg. 1992;79(7):659-662.
Shoup M, Fong Y. Surgical indications and extent of resection in gallbladder cancer. Surg Oncol Clin N Am. 2002;11(4):985-994.
Shrikhande SV, et al. Cholelithiasis in gallbladder cancer: coincidence, cofactor, or cause? Eur J Surg Oncol. 2010;36(6):514-519.
Shukla VK, et al. Biliary bile acids in cholelithiasis and carcinoma of the gall bladder. Eur J Cancer Prev. 1993;2(2):155-160.
Shukla VK, et al. Lipid peroxidation product in bile from patients with carcinoma of the gallbladder: a preliminary study. J Surg Oncol. 1994;56(4):258-262.
Shukla VK, et al. Diagnostic value of serum CA242, CA 19-9, CA 15-3 and CA 125 in patients with carcinoma of the gallbladder. Trop Gastroenterol. 2006;27(4):160-165.
Srivastava K, et al. Polymorphisms in ERCC2, MSH2, and OGG1 DNA repair genes and gallbladder cancer risk in a population of Northern India. Cancer. 2010;116(13):3160-3169.
Stephen AE, Berger DL. Carcinoma in the porcelain gallbladder: a relationship revisited. Surgery. 2001;129(6):699-703.
Strom BL, et al. Serum CEA and CA 19-9: potential future diagnostic or screening tests for gallbladder cancer? Int J Cancer. 1990;45(5):821-824.
Stunell H, et al. Imaging of adenomyomatosis of the gall bladder. J Med Imaging Radiat Oncol. 2008;52(2):109-117.
Sumiyoshi K, et al. Pathology of carcinoma of the gallbladder. World J Surg. 1991;15(3):315-321.
Suzuki S, et al. Appraisal of surgical treatment for pT2 gallbladder carcinomas. World J Surg. 2004;28(2):160-165.
Takada T, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95(8):1685-1695.
Thorbjarnarson B, Glenn F. Carcinoma of the gallbladder. Cancer. 1959;12:1009-1015.
Tian Y, et al. Analysis of p53 and vascular endothelial growth factor expression in human gallbladder carcinoma for the determination of tumor vascularity. World J Gastroenterol. 2006;12(3):415-419.
Todoroki T, et al. Resection combined with intraoperative radiation therapy (IORT) for stage IV (TNM) gallbladder carcinoma. World J Surg. 1991;15(3):357-366.
Togawa O, et al. Management of occluded uncovered metallic stents in patients with malignant distal biliary obstructions using covered metallic stents. J Clin Gastroenterol. 2008;42(5):546-549.
Toyonaga T, et al. Completion radical surgery after cholecystectomy for accidentally undiagnosed gallbladder carcinoma. World J Surg. 2003;27(3):266-271.
Trajber HJ, et al. Adenocarcinoma of the gallbladder in two siblings. Cancer. 1982;50(6):1200-1203.
Valle J, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273-1281.
Varma V, et al. Does the presence of jaundice and/or a lump in a patient with gallbladder cancer mean that the lesion is not resectable? Dig Surg. 2009;26(4):306-311.
Verderame F, et al. Gemcitabine and oxaliplatin combination chemotherapy in advanced biliary tract cancers. Ann Oncol. 2006;17(Suppl 7):vii68-72.
Wakabayashi H, et al. Analysis of prognostic factors after surgery for stage III and IV gallbladder cancer. Eur J Surg Oncol. 2004;30(8):842-846.
Wanebo HJ, Vezeridis MP. Treatment of gallbladder cancer. Cancer Treat Res. 1994;69:97-109.
Wang SJ, et al. Prediction model for estimating the survival benefit of adjuvant radiotherapy for gallbladder cancer. J Clin Oncol. 2008;26(13):2112-2117.
Weber SM, et al. Staging laparoscopy in patients with extrahepatic biliary carcinoma: analysis of 100 patients. Ann Surg. 2002;235(3):392-399.
Welton JC, et al. Association between hepatobiliary cancer and typhoid carrier state. Lancet. 1979;1(8120):791-794.
Wibbenmeyer LA, et al. Sonographic diagnosis of unsuspected gallbladder cancer: imaging findings in comparison with benign gallbladder conditions. Am J Roentgenol. 1995;165(5):1169-1174.
Wilkinson DS. Carcinoma of the gallbladder: an experience and review of the literature. Aust N Z J Surg. 1995;65(10):724-727.
Willson SA, et al. Gallbladder carcinoma in association with polyposis coli. Br J Radiol. 1987;60(716):771-773.
Winston CB, et al. Recurrent gallbladder carcinoma along laparoscopic cholecystectomy port tracks: CT demonstration. Radiology. 1999;212(2):439-444.
Xu HL, et al. Cholesterol metabolism gene polymorphisms and the risk of biliary tract cancers and stones: a population-based case-control study in Shanghai. China. Carcinogenesis. 2011;33(1):58-62.
Yamaguchi K, Enjoji M. Carcinoma of the gallbladder: a clinicopathology of 103 patients and a newly proposed staging. Cancer. 1988;62(7):425-1432.
Yamaguchi K, Tsuneyoshi M. Subclinical gallbladder carcinoma. Am J Surg. 1992;163(4):382-386.
Yamaguchi A, et al. Carcinoma in situ of the gallbladder with superficial extension into the Rokitansky-Aschoff sinuses and mucous glands. Gastroenterol Jpn. 1992;27(6):765-772.
Yanagisawa N, et al. More frequent beta-catenin exon 3 mutations in gallbladder adenomas than in carcinomas indicate different lineages. Cancer Res. 2001;61(1):19-22.
Yang HL, et al. Polypoid lesions of the gallbladder: diagnosis and indications for surgery. Br J Surg. 1992;79:227-229.
Yeh CN, et al. Laparoscopic cholecystectomy for polypoid lesions of the gallbladder: a clinicopathologic study. Surg Laparosc Endosc Percutan Tech. 2001;11(3):176-181.
Zatonski W, et al. Descriptive epidemiology of gallbladder cancer in Europe. J Cancer Res Clin Oncol. 1993;119(3):165-171.
Zatonski WA, et al. Epidemiologic aspects of gallbladder cancer: a case-control study of the SEARCH Program of the International Agency for Research on Cancer. J Natl Cancer Inst. 1997;89(15):1132-1138.
Zhang M, et al. Correlated expression of inducible nitric oxide synthase and P53, Bax in benign and malignant diseased gallbladder. Ann Anat. 2003;185(6):549-554.
Zielinski MD, et al. Comparison of surgically resected polypoid lesions of the gallbladder to their pre-operative ultrasound characteristics. J Gastrointest Surg. 2009;13(1):19-25.