Chapter 50C Cancer of the bile ducts
Perihilar cholangiocarcinoma with emphasis on presurgical management
Overview
Perihilar cholangiocarcinoma is often diagnosed as advanced disease at initial presentation and is difficult to treat (de Groen et al, 1999). Hepatobiliary resection represents the definitive surgery and most effective treatment for this disease (Bismuth et al, 1992; Blumgart et al, 1984; Burke et al, 1998; Hemming et al, 2005; Kawasaki et al, 2003). Combined portal vein resection (Ebata et al, 2003; Nimura et al, 1991a), combined hepatic artery resection, or hepatopancreatoduodenectomy (Ebata et al, 2007; Nimura et al, 1991b) have also been aggressively used at leading hepatobiliary centers in Japan, Korea, Europe, and the United States (Igami et al, 2010; Lee et al, 2010; Miyazaki et al, 2010; Rocha et al, 2010; Unno et al, 2010; Young et al, 2010). However, hepatobiliary resection for perihilar cholangiocarcinoma is well known to result in higher morbidity or mortality rates than those for simple hepatectomy because of impaired liver function with cholestasis or cholangitis (Belghiti et al, 2000; Nagino et al, 1993, 2001). To eradicate the involvement of the hilar duct, extensive hepatectomy should be designed based on tumor extension and individual liver functional reserve. It is of particular importance that the presurgical management is optimized. Perioperative management and surgical techniques have evolved over the past 30 years, which has succeeded in reducing postoperative complications. Current methods and recommendations for the presurgical management of perihilar cholangiocarcinoma are outlined in this section.
Basic Anatomic Pathology Dictates Surgical Strategy (See Chapter 47)
According to the Johns Hopkins classification for cholangiocarcinomas (Nakeeb et al, 1996), perihilar cholangiocarcinomas are defined as cholangiocarcinomas involving or requiring resection of the hepatic duct conference. Because the boundary between the intrahepatic and extrahepatic bile duct is unclear, perihilar tumors are potentially composed of two types of tumors: extrahepatic hilar cholangiocarcinomas, which arise from the large hilar bile duct, and intrahepatic hilar cholangiocarcinomas, which have an intrahepatic component and involve the hepatic hilum. These two tumors exhibit the same clinical presentation, demonstrate similar radiologic features, require an identical surgical intervention, and have comparable histologic characteristics (Ebata et al, 2009). In addition, they can be well classified by the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging for extrahepatic bile duct cancer (Greene et al, 2002), providing well-stratified survival (Ebata et al, 2009). Thus these two tumor categories overlap considerably, as already suggested by Klatskin (1965); therefore both tumors should be treated under the banner of perihilar cholangiocarcinoma (Ebata et al, 2009; Nakeeb et al, 1996).
Cholangiocarcinoma microscopically infiltrates the surrounding tissue beyond the macroscopic tumor extent (main tumor) (Ebata et al, 2002; Sakamoto et al, 1998). The lateral extension of invasive cancer is limited within 1 cm in both directions beyond the main mass in most tumors, and noninvasive cancer extension is limited within 2 cm in 90% of tumors (Ebata et al, 2002). These observations indicate that a 1-cm margin and a 2-cm margin of the duct are recommended for eradication of invasive and noninvasive cancer cells, respectively.
However, in perihilar cholangiocarcinomas, it is often difficult to obtain a satisfactory margin length that meets the above requirements because of the anatomy of the liver, especially in the proximal direction. Therefore a proximal resection line is set at the farthest proximal point as technically possible, and pathologic assessment with frozen sections of the ductal stump is widely used to confirm a negative ductal margin. Flaws in the results of intraoperative frozen section have been reported, including 1) the diagnostic difficulty of differentiation between intraepithelial neoplasm and reactive/regenerative epithelial changes, 2) the absence of a standard histologic classification (Konishi et al, 2008), 3) difficulty in obtaining sufficient material for diagnosis because of crushing and exfoliation resulting from manipulation (Konishi et al, 2008), and 4) relatively low diagnostic accuracy, ranging from 25% to 57% (Okazaki et al, 2002; Yamaguchi et al, 2005). These limitations suggest an inherent limitation of frozen-section examination. When the ductal margin is positive by intraoperative frozen-section diagnosis, several factors must be taken into account. First, prognostic indicators after surgery are nodal metastasis, poor histologic grade, portal vein invasion, and positive surgical margins (Ebata et al, 2003, 2009). Of these predictors, nodal involvement is observed most frequently, in more than 50% of patients (Ebata et al, 2003; Igami et al, 2009; Kitagawa et al, 2001). Second, a positive ductal margin with noninvasive cancer, unlike invasive cancer, may not have a negative impact on survival (Igami et al, 2009; Nakanishi et al, 2010; Sasaki et al, 2007; Wakai et al, 2005). It has been noted that residual in situ carcinoma does not result in early fatal recurrence; however, over time, it steadily and insidiously progresses to an anastomotic tumor mass (Nakanishi et al, 2010). Third, additional resection of more than 5 mm of the proximal duct is difficult, practically, after maximal or near-maximal resection of the duct. Such limited resection of a margin-positive proximal duct does not improve survival, even when a negative margin can be achieved after additional resection (Shingu et al, 2010). Overall, the clinical value of additional resection of the proximal duct in perihilar cholangiocarcinoma is limited.
A right hemihepatectomy or trisectionectomy with en bloc resection of the caudate lobe is generally recommended for perihilar cholangiocarcinomas (Kawasaki et al, 1994; Neuhaus et al, 1999). This right-sided resection satisfies the nontouch technique, en bloc resection, and wide tumor-free margins and consequently leads to good local control and a favorable prognosis (Jonas et al, 2008). However, the optimal procedure (extensive vs. limited surgery) for Bismuth type I and II tumors is still under debate. Tumor configuration is classified into papillary, nodular, or diffusely infiltrating tumors (Henson et al, 2000). Papillary tumors display less invasive behavior and, accordingly, they have a more favorable survival than other tumor types (Albores-Saavedra et al, 2000; Jarnagin et al, 2005). Recently, the surgical strategy for Bismuth type I and II tumors were reappraised based on gross tumor type (Ikeyama et al, 2007). When optimizing by the gross tumor configuration, right-sided hepatectomy is suitable for nodular or flat tumors that display an infiltrating nature in the advancing margin; whereas a limited surgery—including resection of S1, S1+4, or bile duct resection alone—is acceptable for papillary tumors, provided the surgical margin is negative (Ikeyama et al, 2007).
In addition, there is a specific tumor type called superficial spreading type cholangiocarcinoma (Igami et al, 2009; Nakanishi et al, 2008; Sakamoto et al, 1998), which is defined as cholangiocarcinoma with superficial spread; that is, a noninvasive cancer extension longer than 2 cm. The mean length of the superficial spread is 54 mm, suggesting that this tumor often requires extensive resection to obtain a negative ductal margin. In contrast, superficial spreading-type cholangiocarcinomas are also characterized by less advanced pT and pN classifications and a more differentiated histologic grade, which leads to a favorable prognosis (Igami et al, 2009; Nakanishi et al, 2008). In such patients, complete eradication of the superficial spreading lesion (in situ carcinoma) is necessary. Taken together, these findings suggest that a patient with this specific tumor may benefit from extensive surgery, such as hepatopancreatoduodenectomy (Ebata et al, 2007; Nimura et al, 1991b).
Hepatobiliary surgeons should keep the possibility of multiple synchronous cancers in mind. Multiple tumors are more common than previously thought: the incidence ranges from 5% to 9% (Gertsch et al, 1990; Kozuka et al, 1984; Kurosaki et al, 1997). Thus, multiplicity is a feature of biliary neoplasia that may be clinically relevant in some patients. Gertsch and colleagues (1990) proposed three criteria for multiplicity: 1) no direct continuity between the two tumors, 2) a growth pattern typical of a primary tumor, and 3) clear histologic differences between the two tumors. However, a true synchronous tumor and metastatic tumor originating from a biliary site elsewhere cannot always be clinically distinguished. Interestingly, a second tumor may not be identified by the preoperative radiologic workup but rather by pathologic assessment. Most second biliary tumors are an incidental early stage gallbladder cancer (Gertsch et al, 1990; Kurosaki et al, 1997). This indicates a need for intraoperative surveillance, meticulous inspection of the specimen immediately after resection, and appropriate sampling of the tissue for pathology.
The concept of field cancerization potentially explains the multiplicity of biliary tumors. Recently, pathoepidemiologic evidence for multiple biliary tumors has been reported (Henson et al, 2009). The age-specific incidence of biliary tract and gallbladder tumors in the United States demonstrates a parallel incline pattern, indicating that the rate of cancer development is identical and that the sequential molecular/cellular events toward the carcinogenesis among multiple isolated lesions share common pathways.
Biliary Drainage In The Preoperative Setting (See Chapter 7, Chapter 18 )
It is well known that chronic obstruction of the bile duct causes hepatic fibrosis, secondary biliary cirrhosis, and portal hypertension. When biliary obstruction is appropriately relieved, the fibrosis and portal hypertension can be ameliorated (Hammel et al, 2001). Furthermore, prolonged obstruction often induces acute obstructive suppurative cholangitis (Glenn & Moody, 1961), which can only be treated by biliary drainage (Berliner & Burson, 1973). The purposes of biliary drainage are summarized as follows: 1) treatment of biliary sepsis (Kanai et al, 1996), 2) relieving jaundice and recovery of functional capacity, 3) diagnosis of lateral tumor extension (Nimura et al, 1989), 4) liver function assessment using bile samples (Higuchi et al, 2005; Kurumiya et al, 2003; Maeda et al, 1999; Takeuchi et al, 1997; Uesaka et al, 1996), and 5) improvement of poor food intake (Padillo et al, 2001).
Obstructive jaundice associated with perihilar cholangiocarcinoma differs from jaundice associated with distal tumors. In the latter, a single catheter is enough for biliary decompression of the entire liver; in the former, multiple drainage catheters are necessary because perihilar cholangiocarcinomas frequently isolate the intrahepatic biliary tree into several subunits (Klatskin, 1965). Under such difficult conditions, it remains controversial which method is better: an endoscopic approach versus a percutaneous approach or unilateral versus bilateral drainage. Several authors (Gholson & Burton, 1991; Polydorou et al, 1991; Speer et al, 1987) have stressed the advantages of endoscopic retrograde biliary drainage (ERBD) because it is associated with low procedure-related morbidity and mortality rates, a shorter hospital stay, and good quality of life (external tube free). However, ERBD is associated with a high incidence of cholangitis because of catheter occlusion and/or inability to perform multiple drainages (Andersen et al, 1989; Chang et al, 1998; Liu et al, 1998; Rerknimitr et al, 2004). Hence, percutaneous transhepatic biliary drainage (PTBD), rather than ERBD, may be indicated for perihilar cholangiocarcinomas (Nomura et al, 1997).
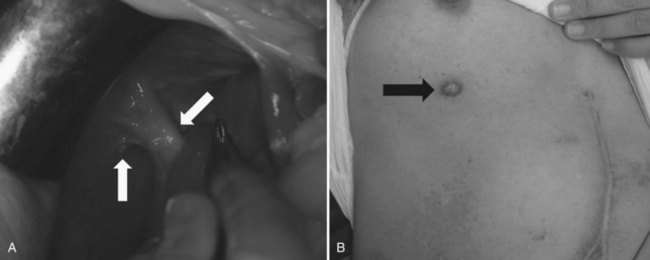
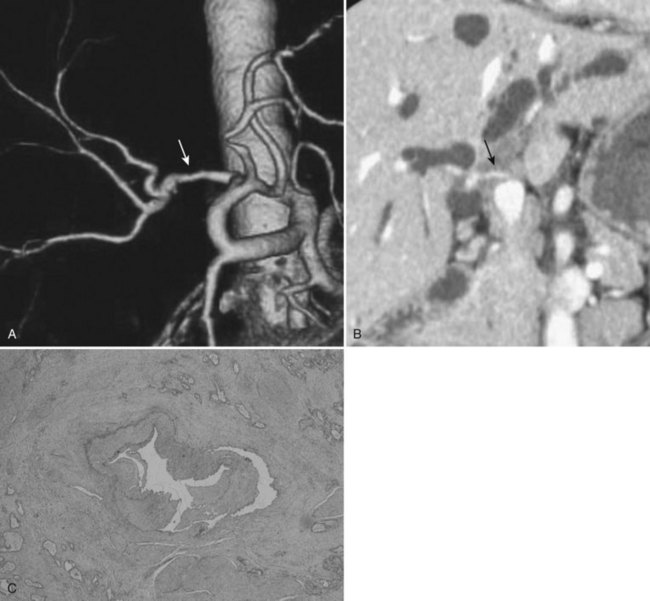
Recently, endoscopic nasobiliary drainage (ENBD) has been recommended as a preoperative drainage procedure for perihilar cholangiocarcinomas (Arakura et al, 2009; Maguchi et al, 2007). Arakura and colleagues (2009) reported that a single ENBD was enough to relieve jaundice in 46 of 62 patients (74%) with perihilar cholangiocarcinoma, and additional PTBD was necessary in only eight patients. Early complications, such as acute cholangitis, dislodgement, and hemobilia, occur 14.5%, 0%, and 0% of the time, respectively, indicating the low morbidity associated with ENBD. Furthermore, ENBD carries no risk of seeding metastases along the catheter tract, which could compromise the results of curative resection (Fig. 50C.1). In our experience, seeding metastases are encountered in up to 5% of patients with cholangiocarcinoma who underwent resection after PTBD.
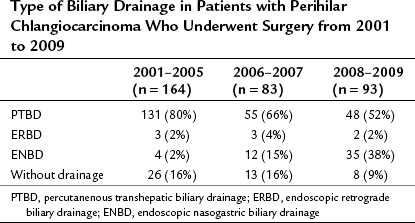
Obstructive jaundice appears when more than 75% of the liver is involved (Abraham et al, 2002), and based on the experience of palliative biliary drainage, it is usually relieved by drainage of a single liver segment (Abraham et al, 2002; Ballinger et al, 1994; Van Laethem et al, 2003). In addition, unilateral drainage promotes hypertrophy of the future liver remnant (FLR), which leads to a good functional reserve (Ishizawa et al, 2007). Thus the current strategy of preoperative biliary drainage for perihilar cholangiocarcinoma is summarized as follows: first, all ducts in the FLR should be drained; second, ENBD is superior to PTBD. Previously, we preferred PTBD (Nagino et al, 1992; Nimura, 1993; Nimura et al, 1995, 1998) but have not changed the policy for preoperative drainage since 2007. The first-line treatment is currently a single ENBD placed in the FLR alone, irrespective of the Bismuth tumor type. PTBD is performed as a second-line procedure only when 1) multiple, separated ducts are present in the FLR; 2) total liver drainage is necessary for prolonged cholestasis or segmental cholangitis of the undrained lobe; or 3) the ENBD tube is dislodged or kinked. As a result, the proportion of patients treated with ENBD has recently increased, but PTBD has decreased, although it is still needed for approximately half of the patients (Table 50C.1).
Table 50C.1 Type of Biliary Drainage in Patients with Perihilar Chlangiocarcinoma Who Underwent Surgery from 2001 to 2009
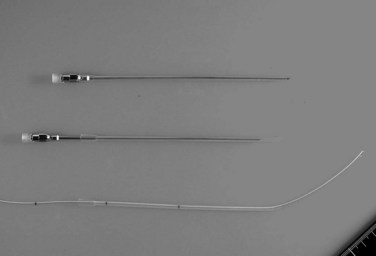
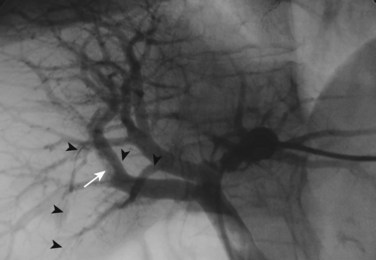
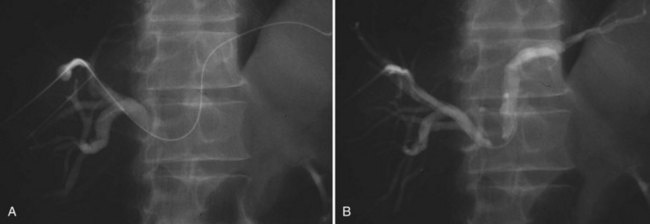
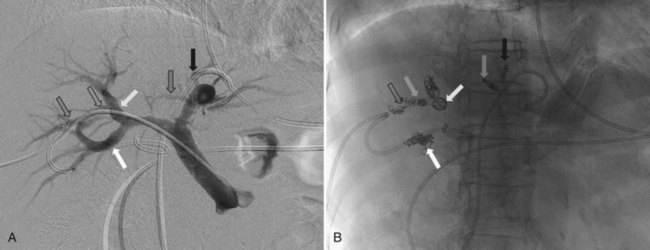
The current role of PTBD is mainly to salvage the procedure if ENBD fails. In this difficult scenario, multiple PTBD is frequently required to decompress the whole liver, except for the caudate lobe (Nagino et al, 1992; Nimura et al, 1995). Our favorite method for PTBD is Takada’s direct anterior approach (Takada et al, 1976), using a Takada needle, approximately 3 mm in diameter, which is composed of an internal metallic needle and an outer Teflon sheath (Fig. 50C.2). First, the target duct is opacified with a fine needle; next, the needle is advanced parallel to the radiograph into the duct; and finally, a 6-Fr soft catheter is installed through the sheath. Previously, the intrahepatic bile duct of the FLR was directly punctured; however, vascular injury is possible with such an approach (Fig. 50C.3). A large series of PTBD between 1991 and 2006 (Dowsett, 1989; Fidelman et al, 2008) showed that the risk of procedure-related arterial injury is 2.2%, and the incidence has not changed since the 1970s and 1980s. To prevent accidental vascular injury, the ipsilateral approach, similar to portal vein embolization (Nagino et al, 1996), is recommended when it is possible. In this approach, the bile duct in the sector to be removed is punctured, and the straight single catheter is introduced into the duct in the FLR via the hilar duct, which is stenotic as a result of tumor. Although this procedure requires a meticulous searching technique using a flexible guidewire, it offers complete bilateral drainage with the least numbers of single catheters (Fig. 50C.4). PTBD with the ipsilateral approach should be considered when total liver drainage is necessary.
Although biliary drainage is the routine preoperative management strategy for jaundiced patients in Japan (Nagino et al, 2008), randomized control trials (RCTs) conducted in Western countries have raised questions about the effectiveness of preoperative biliary drainage (see Chapters 7 and 50B). The results of these trials showed no significant difference in postoperative morbidity and mortality among patients who received preoperative biliary drainage and those who did not. These RCTs concluded that, considering the potential risk related to PTBD, preoperative biliary drainage has no advantage (Hatfield et al, 1982; McPherson et al, 1984; Pitt et al, 1985). However, these studies involved patients undergoing bypass surgeries or palliative small resections. In addition, PTBD-related complications were very common. These flaws in the previous RCTs made it difficult to accept their conclusions. In Western centers, preoperative biliary drainage is performed in selected patients (Laurent et al, 2008; Kennedy et al, 2009). Over time, the bile is contaminated after biliary drainage (Nomura et al, 1999): the incidence of bacterobilia and fungobilia is 85% and 40%, respectively (Jethwa et al, 2007), leading to surgical site infection, cholangitis, and bacteremia after hepatectomy (Hochwald et al, 1999; Jethwa et al, 2007; Nomura et al, 1999; Shigeta et al, 2002). These observations may partly support the limited indications for biliary drainage; however, the mortality rate after extended hepatectomy for jaundiced patients is still high at near 10%, and the cause of death is mainly hepatic failure (Nagino et al, 1993b, 2001). Therefore, preoperative biliary drainage followed by portal vein embolization is recommended before extended hepatectomy, in spite of a lack of clear evidence based on previous RCTs. This is the Japanese standard of care (Henson et al, 2000; Kawasaki et al, 2003).
Cholangiography (See Chapter 18)
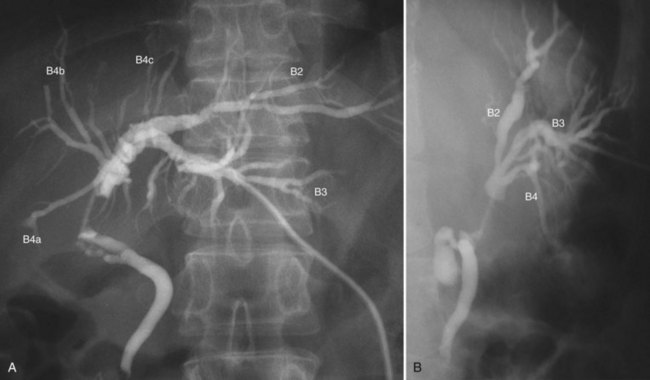
Cholangiograms precisely delineate the segmental anatomy of the intrahepatic bile duct (Ohkubo et al, 2004), the tumor configuration, and the extent of the tumor border. To avoid misinterpretation related to overlap of segmental ducts, cholangiograms should be taken in various projections (Fig. 50C.5). Most perihilar cholangiocarcinomas are infiltrating tumors, in which cancer cells are likely to extend predominantly in the submucosal layer rather than in the mucosal layer. Histologically, direct and lymphatic invasion are common routes of invasion to the ductal wall, evoking reactive fibrosis (Sakamoto et al, 1998; see Chapter 47). These changes cause rigidity, narrowing, tapering, and obstruction of the duct on the cholangiogram. The border of these findings on cholangiograms correspond to the leading edge of cancer infiltration (Sakamoto et al, 1998).
In hilar biliary obstruction, direct cholangiography frequently shows insufficient opacification of the proximal ductal system. However, MR cholangiography (MRC) delineates the whole biliary system, and its ability to diagnose the Bismuth classification is superior, with an accuracy of 81% (Yeh et al, 2000). Overestimation is a common pitfall of MRC, which may preclude patients from potentially curative surgery (Otto et al, 2004). The limitations of MRC include lower spatial resolution, longer acquisition time, and sensitivity to motion.
Cholangioscopy
Despite the advent of new diagnostic modalities, cholangioscopic examination with biopsy sampling plays an important role in the diagnosis of biliary malignancies (see Chapter 28). Differentiation between malignant and benign lesions, evaluation of proximal and distal cancer extent, and early detection of small lesions and multiple cancer foci can be accomplished by cholangioscopic observation with biopsy sampling (Nimura et al, 1989). Irregularly dilated and tortuous vessels are characteristic of malignancy. A fine granular/papillary mucosa extending continuously from a main lesion is a characteristic finding of superficial spread (Nimura & Kamiya, 1998). Papillary tumors are often associated with the superficial spread of carcinoma (Igami et al, 2009; Sakamoto et al, 1998), and mucosal extension is easily detectable by cholangioscopy. Therefore, cholangioscopy should be considered for patients with papillary tumors. Attention should be paid not only to the intrahepatic extension but also to the distal extension of the tumor.
Percutaneous transhepatic cholangioscopy (PTCS) is performed through the PTBD sinus tract. One week after PTBD, the drainage catheter is replaced by a 9- or 10-Fr PTCS catheter, and then the sinus tract is gradually dilated by replacing the preceding catheter with a larger catheter two or three times a week. A small-caliber cholangioscope (Olympus XP-20) can be used via the sinus tract of the 12-Fr catheter; a standard-type cholangioscope (Olympus, P-20) requires placement of a 15-Fr catheter. This preparation process for PTCS is time consuming and painful; therefore PTCS has recently been replaced by peroral cholangioscopy (POCS). With technical advances in endoscopy, POCS with biopsy gives a high diagnostic accuracy of the extent of the superficial spreading lesion (Kawakami et al, 2009).
Multidetector Row Computed Tomography
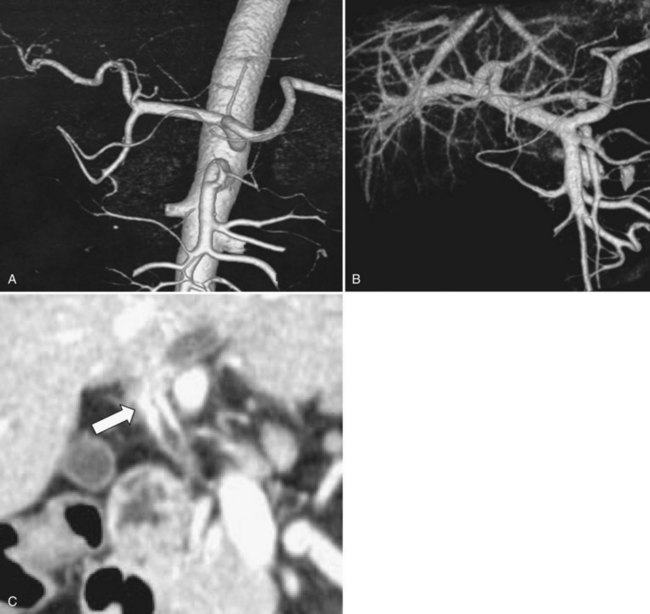
Recent advances in helical CT technology, such as multislice detectors and subsecond rotation, have dramatically reduced scanning times in comparison with single-slice CT and have provided unparalleled capability for fast data acquisition and narrow collimation. Thus, multidetector row computed tomography (MDCT) permits the acquisition of three distinct circulatory phases, consisting of the arterial, pancreatic, and portal venous phases in the pancreatobiliary region with 1-mm collimation (Itoh et al, 2003; see Chapter 16). These isotropic volume data allow multiplanar reformation (MPR), which can demonstrate complex anatomic relationships and three-dimensional volume rendering (3DVR) images, such as CT arteriography and CT portography, in a single scanning session (Fig. 50C.6). These postprocessing techniques enhance the diagnostic capability of CT (Choi et al, 2007). In patients with perihilar cholangiocarcinoma, preoperative MDCT is used to diagnose 1) distant metastasis, including dissemination, liver metastasis, and nodal metastasis; 2) tumor lateral extension or Bismuth classification; 3) vascular invasion with or without lobar atrophy; 4) anatomic variation of the vascular system; and 5) the liver volume of each sector. MDCT is now a pivotal element of the preoperative staging of perihilar cholangiocarcinoma, and it provides definitive information about resectability and design of resectional procedure. Previously, we used PTCS, celiac angiography, and percutaneous transhepatic portography (PTP) for staging (Nimura, 1993; Nimura et al, 1995, 1998; Nishio et al, 1999). However, these procedures are invasive in nature and have been replaced by MDCT.
With MDCT, cholangiocarcinoma can be correctly identified in nearly 100% of patients, and the accuracy in prediction of resectability has improved to 75% to 100% (Chen et al, 2006; Lee et al, 2006). MDCT with or without enhancement prior to biliary drainage often displays the tumor as a ductal thickness. To evaluate the value of MDCT on longitudinal extension in one study, the authors analyzed 73 patients with cholangiocarcinoma who underwent MDCT prior to biliary drainage (Senda et al, 2009). Of the 73 patients, 40 showed a hyperattenuated tumor, and the remaining 33 had a hypoattenuated tumor; in the latter group, it was difficult to identify the border between normal and thickened duct. Hence, the extent of lateral extension could be assessed in only those 40 patients (55%) with a hyperattenuated tumor. The diagnostic accuracy of the extent of ductal cancer infiltration was 76% and 82% in the proximal and distal direction, respectively. The extent of cancer was underestimated by 24% and 15% at the proximal and distal border, respectively, because of superficial spread, minimal invasive cancer extension, or both. Thus the ability of MDCT to diagnose lateral extension is limited, and cholangiography is still the standard tool.
A belief has persisted that involvement of the main portal trunk denotes irresectability of the cholangiocarcinoma (Blumgart et al, 1984; Lee et al, 2006). Hoevels and Ihse (1979) emphasized that a portography showing portal vein invasion suggests irresectability. Launois and colleagues (1999) also reported that the main reason for irresectability of hilar cholangiocarcinomas is invasion of the portal bifurcation; however, several surgeons have recently reported portal vein resection for advanced biliary cancer (Kondo et al, 2004; Miyazaki et al, 2007; Nimura et al, 1991a; Sakaguchi & Nakamura, 1986). We have carried out combined portal vein and hepatobiliary resection in nearly 200 patients with advanced perihilar cholangiocarcinoma and have demonstrated significantly better survival in patients who underwent portal vein resection than in those with unresectable disease (Ebata et al, 2003; Igami 2010; Nimura et al, 1991a). Currently, portal vein resection and reconstruction are more or less routine in leading centers. Some surgeons have reported the clinical significance of prophylactic portal vein resection for all patients, irrespective of invasion of the portal vein, based on the nontouch technique (Kondo et al, 2004; Neuhaus et al, 1999). We do not think that this strategy improves survival, and they emphasize that portal vein resection should be performed only in patients with gross invasion of the portal vein.
We assessed the value of MDCT on portal vein invasion, defined as absence of a visible hypoattenuated layer between the portal bifurcation and the adjacent tumor on an MPR image. The diagnostic accuracy for macroscopic portal vein invasion (i.e., the requirement for portal vein resection) was 98%, showing the high sensitivity of MPR images for macroscopic portal vein invasion. Meanwhile, 3D portography clearly demonstrates the portal branch anatomy and the site and length of the portal vein invasion. Three-dimensional portography facilitates the design of the resection and the reconstruction procedure: wedge versus segmental resection and direct end-to-end anastomosis versus autologous vein grafting. In practical terms, the presence or absence of portal vein invasion should be first assessed by using MPR images; 3DVR images should then be used to plan the surgical procedure. When the right portal vein is markedly involved, if contralateral involvement does not reach the umbilical portion of the left portal vein, curative resection is possible by using a right-sided hepatectomy with portal vein resection and reconstruction (Fig. 50C.7). In contrast, when the left portal vein is considerably involved, if contralateral involvement is limited within the right portal trunk, a left hepatectomy with portal vein resection and reconstruction is indicated (Fig. 50C.8). If the bifurcation of the right anterior and posterior portal veins is involved, a left trisectionectomy with portal vein resection and reconstruction may be required.
Three-dimensional understanding of the hepatic artery distribution expedites skeletonization resection of the hepatoduodenal ligament; 3D understanding of the intrahepatic artery branching is helpful when the segmental or subsegmental bile ducts are divided as the final step of hepatobiliary resection. Hepatobiliary surgeons must appreciate two variations of the hepatic artery distribution that may be associated with potential injury (Fig. 50C.9): one is the left hepatic artery (LHA) running right to the umbilical portion of the left portal vein; and the other is a posterior branch of the right hepatic artery (RHA) running cranially to the right portal trunk. Careful attention should be paid to the former variant in right hepatic trisectionectomy (extended right hepatectomy) with caudate lobectomy (Nagino et al, 2006a) and to the latter variant in left hepatic trisectionectomy (extended left hepatectomy) with caudate lobectomy. These variations can be clearly identified on a CT arteriography prior to surgery.
In general, unilateral involvement of the hepatic artery is compatible with resection. Because the RHA is usually just behind the common hepatic duct, it is likely to be involved by a cancer of the hepatic bifurcation. In cases of a right-sided tumor, bilateral involvement signifies local irresectability, because the LHA runs along the left border of the hepatoduodenal ligament. However, when the tumor is located on the left side, a left-sided hepatectomy with resection and reconstruction of the RHA can be performed in selected patients (see Fig. 50C.8; Fig. 50C.10). Hepatic artery reconstruction using microscopy is now well established and safely performed by experienced cosmetic or vascular surgeons; however, it remains unclear whether combined hepatic artery and liver resection offers a better chance of long-term survival. From 2001 to 2008, Igami and colleagues (2010) performed combined hepatic artery resection and reconstruction in 53 patients. Despite the risky nature of this procedure, only one of the 53 patients died of complications, and the 5-year survival rate was 33%. This satisfactory result may encourage hepatobiliary surgeons to attempt this aggressive surgery.
Portal Vein Embolization
Portal vein embolization (PVE), introduced by Makuuchi and Kinoshita in the 1980s (Kinoshita et al, 1986; Makuuchi et al, 1990), can induce hypertrophy of the nonembolized lobe, has the potential to enhance the safety of extended hepatectomy, and has been considered the standard of care in preoperative management (see Chapter 93A, Chapter 93B ). Some authors have reported that PVE can be safely performed without serious complications, and it can minimize postoperative liver dysfunction. A recent meta-analysis involving 37 highly selected reports from the literature also supports the utility of PVE (Abulkhir et al, 2008). However, because no RCT has been conducted, the indication for PVE still remains undefined. The choice of device, technique, or embolic agent is generally based on the operator’s preference.
Belghiti (2004) summarized the indication for PVE as follows: 1) a percentage of FLR of 25% or less in normal liver; 2) scheduled concomitant extrahepatic surgery or complex hepatectomy; and 3) any major hepatectomy in patients with underlying chronic diseases or with an injured liver (chemotherapy, major steatosis, or cholestasis). In this context, most patients with perihilar cholangiocarcinoma may be candidates for preoperative PVE because they require extensive hepatectomy, frequent vascular resection, and have a cholestatic liver requiring biliary drainage. In our department, PVE is performed when the estimated FLR is below 40% (Nagino et al, 2006b).
The two most common techniques for PVE are transileocolic portal vein embolization (TIPE) (Makuuchi et al, 1990) and percutaneous transhepatic portal vein embolization (PTPE) (Kinoshita et al, 1986). Many surgeons prefer TIPE to PTPE (Imamura et al, 1999; Makuuchi et al, 1990) for three main reasons: First, surgeons can perform a minilaparotomy. Second, catheterization is easier because of the straightforward ramification of the portal branches. Third, a diagnostic laparotomy and intraoperative ultrasound provide further accurate staging of the disease (Imamura et al, 1999). However, TIPE has some disadvantages; it requires general anesthesia, and it comes with a theoretical higher risk of postoperative complications such as ileus (Imamura et al, 1999). In contrast, PTPE can be performed in the radiologic suite with the patient under local anesthesia and conscious sedation. Because general anesthesia is avoided, cost is reduced, and recovery time is short. Overall, PTPE is superior to TIPE and, accordingly, TIPE should be used only when interventional radiologic facilities are not available.
As an alternative to the conventional transhepatic contralateral approach, in which the umbilical portion of the left portal vein is punctured for right-sided PTPE access, we developed a different approach for PTPE, termed the ipsilateral approach (Nagino et al, 1996). In this approach, the portal branch of the sector to be resected is punctured, thereby minimizing the risk of vascular injury in the FLR. This is the main advantage of the transhepatic ipsilateral approach. Furthermore, there is no risk of portal bleeding after removal of the catheter sheath. As PTPE is indicated in patients who are to undergo right hemihepatectomy or right or left trisectionectomy, the right anterior sector will always be resected. Hence, the right anterior portal branch is recommended for puncture using any type of PVE. Importantly, to confirm the individual anatomy of the portal system, a portogram is obtained with at least three different projections: 1) supine position, 2) right anterior oblique projection, and 3) right anterior oblique projection tilting caudally 20 degrees (Nishio et al, 2003). The third projection provides the best visualization of the right anterior/posterior portal vein and the left portal vein. Selective catheterization of the right portal branches is also important for complete occlusion without migration of the embolic material. In the case of a right PVE, a proximal segment at least 1 cm long must remain unembolized to facilitate the division and closure of the right portal vein during surgery.
We have developed an extensive PTPE procedure called trisegment portal vein embolization (Madoff et al, 2005; Nagino et al, 1995, 2000a). Right or left trisectionectomy is the most extensive hepatectomy performed and is often associated with major sacrifice of parenchyma (Nagino et al, 1995). The ipsilateral approach through the right anterior portal vein makes trisegment PVE possible (Fig. 50C.11). It allows catheterization of the segment IV branch (P4) in the case of right trisegment PVE (P4 plus right anterior and posterior portal vein) and of the left portal vein in the case of left trisegment PVE (left and right anterior portal vein). The use of a coaxial catheter system or a curved catheter adjusted for the angulation helps to advance the catheter into P4, which often has two to three branches (Takayasu et al, 1985). Although additive P4 embolization prior to right trisectionectomy still remains controversial, the volume gain of the left lateral segment is larger in patients with right trisegment PVE than in those with conventional right PVE (Madoff et al, 2005; Nagino et al, 2000a). On the other hand, left trisectionectomy is a technically demanding and challenging procedure with higher morbidity than other anatomic hepatectomies (Nishio et al, 2005). With left trisegment PVE, a demarcation between the right anterior and posterior sector appears on the liver surface just after temporary clamping of the RHA. This is simple and useful in patients with locally advanced perihilar cholangiocarcinoma requiring combined hepatic artery and portal vein resection, in which hilar dissection cannot be completed before liver resection. Therefore, we recommend use of left trisegment PVE before this complex surgery.
From 1991 to 2005, we performed PTPE in 240 patients with biliary cancer, which included 150 perihilar cholangiocarcinomas and 90 gallbladder cancers involving the hepatic hilus (Nagino et al, 2006b). All patients were scheduled to undergo extended major hepatectomy, and all procedures were performed using the ipsilateral approach. The PTPE was done 2 to 4 weeks before surgery, when the total serum bilirubin levels were lower than 5 mg/dL. Details of the PTPEs performed are listed in Table 50C.2. From 1991 to 2000, fibrin glue mixed with iodized oil was injected thorough two different types of triple-lumen balloon catheters (Nagino et al, 1993a). Recently, absolute ethanol injection followed by placement of steel coil has been used. Because absolute ethanol is not radiopaque, and it permeates into the peripheral portal system and induces endothelial injury/thrombosis, it must be carefully and slowly administered in 0.5- to 2.0-mL increments, meeting the extent of the liver volume fed by the target portal branch. After ethanol injection, steel coils are placed at the second- or third-order portal branch for permanent and complete occlusion. Although the rate of recanalization was slightly higher with fibrin glue (8.3%) than with ethanol and coils (5.1%), no significant difference was reported in either the hypertrophy ratio of the nonembolized lobe or the atrophy ratio of the embolized lobe. No procedure-related serious complications requiring a blood transfusion or interventions occurred. In addition, unexpected tumor growth after PVE was not observed.
Table 50C.2 Type of Planned Hepatectomy and Embolized Portal Vein
| Planned Hepatectomy | Embolized Portal Vein | No. Patients |
|---|---|---|
| Right trisectionectomy | Right and left medial | 23 |
| Right | 4 | |
| Left trisectionectomy | Left and right anterior | 26 |
| Left | 5 | |
| Right anterior | 8 | |
| Right hepatectomy | Right | 160 |
| Right anterior | 9 | |
| Right posterior | 5 | |
| Total | 240 |
Our strategy for unsatisfactory volume gain of the nonembolized liver after PVE is straightforward. In the case of revascularization, repeat PVE should be performed. When sufficient hypertrophy cannot be achieved despite complete PVE, second-line interventions should be considered for further hypertrophy. Sequential hepatic artery embolization of the ipsilateral liver or hepatic vein embolization may be useful to boost liver regeneration (Hwang et al, 2009; Nagino et al, 2000b). Selective intrahepatic biliary ablation with absolute ethanol can also be applied for this purpose, when the biliary duct in the embolized lobe is completely separated from the duct of the FLR (Kyokane et al, 2001). Because all of these optional interventions are empiric, technically demanding, and still experimental, the indication should be strictly selective.
Other Specific Managements
Internal Drainage and Bile Replacement
The superiority of internal biliary drainage is shown in experimental and clinical studies that have demonstrated that liver regeneration after partial hepatectomy is suppressed under external drainage but not under internal drainage (Clements et al, 1993; Saiki et al, 1999; Suzuki et al, 1994). Ogata and colleagues (2003) investigated bacterial translocation in bile duct–ligated rats and found that bile replacement is essential to maintain intestinal barrier function. Recently, we studied the effect of bile replacement on intestinal permeability, integrity, and microflora in 25 biliary cancer patients who underwent PTBD as a part of presurgical management (Kamiya et al, 2004). Impaired intestinal barrier function did not recover using PTBD without bile replacement, but bile replacement did restore intestinal barrier function. Our results strongly support that bile replacement during external biliary drainage is beneficial in preoperative patients with perihilar cholangiocarcinoma, because this type of hepatectomy is complicated and still a high-risk procedure. We ask all patients to drink their own externally drained bile juice.
Inchinkoto and Ursodeoxycholic Acid
Plants contain abundant bioactive materials. In Japan, oriental herbal medicines are now manufactured under modern scientific quality controls and have been empirically administered to many kinds of patients for more than 30 years. Inchinkoto, one such medicine, has been traditionally recognized as a “magic bullet” for jaundice. In experimental rat models, inchinkoto attenuates gene expression for inflammatory cytokines and inducible nitric oxide synthase and also upregulates antioxidants genes (Kawai et al, 2010). We confirmed the choleretic effect of inchinkoto in a prospective controlled trial involving 27 patients with cholangiocarcinoma (Watanabe et al, 2009). The biliary concentration of total bilirubin and total bile acids were significantly increased after administration of inchinkoto, and the protein levels of MRP2, a bilirubin transporter expressed in the canalicular membrane of hepatocytes, was also significantly higher in patients treated with inchinkoto. Thus, inchinkoto has a therapeutic potential in obstructive cholestasis associated with cholangiocarcinoma.
Ursodeoxycholic acid (UDCA) is a major primary bile acid in some species of bear (Hagey et al, 1993). For centuries, dried bear bile has been used in traditional Chinese medicine as a remedy for liver disorders. In humans, UDCA is considered a minor secondary bile acid that is formed in the gut by intestinal bacteria and accounts for less than 3% of the whole bile acid pool (Hofmann, 1994). Pathophysiologically, UDCA has several effects on hepatocytes: protection against oxidative stress, inhibition of apoptosis, stimulation of bile flow, detoxification of cholephilic compounds, and immunomodulation (Perez & Briz, 2009). Therefore, UDCA is widely used for cholestatic liver disease. In our department, UDCA and Inchinkoto are routinely used in jaundiced patients undergoing biliary drainage.
Synbiotic Administration
Infections in immunocompromised hosts often arise from their intestinal microflora (Tancrede & Andremont, 1985; Wang et al, 1993). Indigenous enteric gram-negative bacteria, such as the Enterobacteriaceae, are among the leading causes of infections. Lilly and Stillwell first introduced the term probiotics for bacteria that benefit the host by improving the intestinal microbial balance. Lactobacilli and Bifidobacteria are widely used as probiotics (Fuller, 1991). In addition, prebiotics are nondigestive food constituents that selectively alter the growth and/or activity of a limited number of beneficial bacteria in the colon, thus potentially improving the health of the host (Collins & Gibson, 1999; Fuller, 1991; Gibson et al, 1995). Several types of ingredients, such as fructooligosaccharides, galactooligosaccharides, and inulin, are used as prebiotics. The combined use of probiotics and prebiotics is called synbiotics (Collins & Gibson, 1999). Synbiotic combinations have beneficial effects on human health, but their clinical value in surgical patients remains unclear because, to date, only a few clinical studies have been conducted on synbiotics (Rayes et al, 2002a, 2002b). We investigated the effect of postoperative synbiotics, combined with early enteral nutrition, in biliary cancer patients undergoing hepatectomy (Kanazawa et al, 2005). The primary result of this RCT was that the incidence of infection was 19% in the synbiotics group and 53% in the control group (P < .05), demonstrating that synbiotics can reduce postoperative infections. This beneficial effect encouraged us to schedule the next RCT, which investigated the benefit of perioperative use of synbiotics compared with postoperative use only (Sugawara et al, 2006). The incidence of postoperative infection was 30% in the former group and 12% in the latter group (P < .05), showing that perioperative synbiotics can decrease postoperative infections. Our latest protocol for perioperative supplementation is presented in Table 50C.3.
Table 50C.3 Supplemental Protocol for Preoperative Patients Who Are to Undergo Hepatobiliary Resection in Nagoya University Hospital
(14 Days to 1 Day Before Surgery)Oligomate, galactooligosaccharides; Yakult 400, Lactobacillus casei strain Shirota 400 × 108; Bifile, Bifidobacterium breve strain Yakult 100 × 108 plus galactooligosaccharides 0.6 g
Abraham NS, Barkun JS, Barkun AN. Palliation of malignant biliary obstruction: a prospective trial examining impact on quality of life. Gastrointest Endosc. 2002;56(6):835-841.
Abulkhir A, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247(1):49-57.
Albores-Saavedra J, et al. Noninvasive and minimally invasive papillary carcinomas of the extrahepatic bile ducts. Cancer. 2000;89(3):508-515.
Andersen JR, et al. Randomised trial of endoscopic endoprosthesis versus operative bypass in malignant obstructive jaundice. Gut. 1989;30(8):1132-1135.
Arakura N, et al. Efficacy of preoperative endoscopic nasobiliary drainage for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2009;16(4):473-477.
Ballinger AB, et al. Symptom relief and quality of life after stenting for malignant bile duct obstruction. Gut. 1994;35(4):467-470.
Belghiti J. Arguments for a selective approach of preoperative portal vein embolization before major hepatic resection. J Hepatobiliary Pancreat Surg. 2004;11(1):21-24.
Belghiti J, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191(1):38-46.
Berliner SD, Burson LC. Acute obstructive suppurative cholangitis: report of a case treated by internal drainage. Am J Gastroenterol. 1973;59(5):423-426.
Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215(1):31-38.
Blumgart LH, et al. Surgical approaches to cholangiocarcinoma at confluence of hepatic ducts. Lancet. 1984;1(8368):66-70.
Burke EC, et al. Hilar cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. 1998;228(3):385-394.
Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest Endosc. 1998;47(5):354-362.
Chen HW, et al. Preoperative evaluation of resectability of Klatskin tumor with 16-MDCT angiography and cholangiography. Am J Roentgenol. 2006;186(6):1580-1586.
Choi JY, et al. Assessment of hilar and extrahepatic bile duct cancer using multidetector CT: value of adding multiplanar reformations to standard axial images. Eur Radiol. 2007;17(12):3130-3138.
Clements WD, et al. Biliary drainage in obstructive jaundice: experimental and clinical aspects. Br J Surg. 1993;80(7):834-842.
Collins MD, Gibson GR. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999;69(5):1052S-1057S.
de Groen PC, et al. Biliary tract cancers. N Engl J Med. 1999;341(18):1368-1378.
Dowsett JF, et al. Endoscopic biliary therapy using the combined percutaneous and endoscopic technique. Gastroenterology. 1989;96(4):1180-1186.
Ebata T, et al. Pathological appraisal of lines of resection for bile duct carcinoma. Br J Surg. 2002;89(10):1260-1267.
Ebata T, et al. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Ann Surg. 2003;238(5):720-727.
Ebata T, et al. Right hepatopancreatoduodenectomy: improvements over 23 years to attain acceptability. J Hepatobiliary Pancreat Surg. 2007;14(2):131-135.
Ebata T, et al. The concept of perihilar cholangiocarcinoma is valid. Br J Surg. 2009;96(8):926-934.
Fidelman N, et al. Hepatic arterial injuries after percutaneous biliary interventions in the era of laparoscopic surgery and liver transplantation: experience with 930 patients. Radiology. 2008;247(3):880-886.
Fuller R. Probiotics in human medicine. Gut. 1991;32(4):439-442.
Gertsch P, et al. Multiple tumors of the biliary tract. Am J Surg. 1990;159(4):386-388.
Gholson CF, Burton FR. Obstructive jaundice: nonsurgical options for ‘surgical’ jaundice. Postgrad Med. 1991;90(8):107-110. 113-114, 116
Gibson GR, et al. Selective stimulation of Bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108(4):975-982.
Glenn F, Moody FG. Acute obstructive suppurative cholangitis. Surg Gynecol Obstet. 1961;113:265-273.
Greene, FL, et al. AJCC Cancer Staging Manual, 6th ed, New York: Springer-Verlag, 2002.
Hagey LR, et al. Ursodeoxycholic acid in the Ursidae: biliary bile acids of bears, pandas, and related carnivores. J Lipid Res. 1993;34(11):1911-1917.
Hammel P, et al. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N Engl J Med. 2001;344(6):418-423.
Hatfield AR, et al. Preoperative external biliary drainage in obstructive jaundice: a prospective controlled clinical trial. Lancet. 1982;2(8304):896-899.
Hemming AW, et al. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005;241(5):693-699. discussion 699-702
Henson DE, Albores-Saavedra J, Compton CC. Protocol for the examination of specimens from patients with carcinomas of the extrahepatic bile ducts, exclusive of sarcomas and carcinoid tumors: a basis for checklists. Cancer Committee of the College of American Pathologists. Arch Pathol Lab Med. 2000;124(1):26-29.
Henson DE, et al. Carcinomas of the pancreas, gallbladder, extrahepatic bile ducts, and ampulla of Vater share a field for carcinogenesis: a population-based study. Arch Pathol Lab Med. 2009;133(1):67-71.
Higuchi R, Otsubo T, Takasaki K. Evaluation of functional liver reserve in patients with obstructive jaundice undergoing hepatectomy. Hepatogastroenterology. 2005;52(62):537-540.
Hochwald SN, et al. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch Surg. 1999;134(3):261-266.
Hoevels J, Ihse I. Percutaneous transhepatic portography in bile duct carcinoma: correlation with percutaneous transhepatic cholangiography and angiography. Rofo. 1979;131(2):140-150.
Hofmann AF. Pharmacology of ursodeoxycholic acid, an enterohepatic drug. Scand J Gastroenterol Suppl. 1994;204:1-15.
Hwang S, et al. Sequential preoperative ipsilateral hepatic vein embolization after portal vein embolization to induce further liver regeneration in patients with hepatobiliary malignancy. Ann Surg. 2009;249(4):608-616.
Igami T, et al. Surgical treatment of hilar cholangiocarcinoma in the “new era”: the Nagoya University experience. J Hepatobiliary Pancreat Surg. 2010;17(4):449-454.
Igami T, et al. Clinicopathologic study of cholangiocarcinoma with superficial spread. Ann Surg. 2009;249(2):296-302.
Ikeyama T, et al. Surgical approach to Bismuth type I and II hilar cholangiocarcinomas: audit of 54 consecutive cases. Ann Surg. 2007;246(6):1052-1057.
Imamura H, et al. Preoperative portal vein embolization: an audit of 84 patients. Hepatology. 1999;29(4):1099-1105.
Ishizawa T, et al. Selective versus total biliary drainage for obstructive jaundice caused by a hepatobiliary malignancy. Am J Surg. 2007;193(2):149-154.
Itoh S, et al. Assessment of the pancreatic and intrapancreatic bile ducts using 0.5 mm collimation and multiplanar reformatted images in multislice CT. Eur Radiol. 2003;13(2):277-285.
Jarnagin WR, et al. Papillary phenotype confers improved survival after resection of hilar cholangiocarcinoma. Ann Surg. 2005;241(5):703-712. discussion 712-714
Jethwa P, et al. The microbiological impact of pre-operative biliary drainage on patients undergoing hepato-biliary-pancreatic (HPB) surgery. Aliment Pharmacol Ther. 2007;25(10):1175-1180.
Jonas S, et al. Radical surgery for hilar cholangiocarcinoma. Eur J Surg Oncol. 2008;34(3):263-271.
Kamiya S, et al. The value of bile replacement during external biliary drainage: an analysis of intestinal permeability, integrity, and microflora. Ann Surg. 2004;239(4):510-517.
Kanai M, et al. Preoperative intrahepatic segmental cholangitis in patients with advanced carcinoma involving the hepatic hilus. Surgery. 1996;119(5):498-504.
Kanazawa H, et al. Synbiotics reduce postoperative infectious complications: a randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbecks Arch Surg. 2005;390(2):104-113.
Kawai K, et al. Inchinkoto, an herbal medicine, exerts beneficial effects in the rat liver under stress with hepatic ischemia-reperfusion and subsequent hepatectomy. Ann Surg. 2010. (in press)
Kawakami H, et al. Endoscopic retrograde cholangiography versus peroral cholangioscopy to evaluate intraepithelial tumor spread in biliary cancer. Endoscopy. 2009;41(11):959-964.
Kawasaki S, et al. Radical operation after portal embolization for tumor of hilar bile duct. J Am Coll Surg. 1994;178(5):480-486.
Kawasaki S, et al. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238(1):84-92.
Kennedy TJ, et al. Role of preoperative biliary drainage of liver remnant prior to extended liver resection for hilar cholangiocarcinoma. HPB (Oxford). 2009;11(5):445-451.
Kinoshita H, et al. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986;10(5):803-808.
Kitagawa Y, et al. Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg. 2001;233(3):385-392.
Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis: an unusual tumor with distinctive clinical and pathological features. Am J Med. 1965;38:241-256.
Kondo S, et al. Forty consecutive resections of hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins: results of a prospective study. Ann Surg. 2004;240(1):95-101.
Konishi M, et al. A new histological classification for intra-operative histological examination of the ductal resection margin in cholangiocarcinoma. Cancer Sci. 2008;100(2):255-260.
Kozuka S, Tsubone M, Hachisuka K. Evolution of carcinoma in the extrahepatic bile ducts. Cancer. 1984;54(1):65-72.
Kurosaki I, et al. Synchronous primary tumors of the extrahepatic bile duct and gallbladder. J Surg Oncol. 1997;65(4):258-262.
Kurumiya Y, et al. Biliary bile acid concentration is a simple and reliable indicator for liver function after hepatobiliary resection for biliary cancer. Surgery. 2003;133(5):512-520.
Kyokane T, et al. An experimental study of selective intrahepatic biliary ablation with ethanol. J Surg Res. 2001;96(2):188-196.
Launois B, et al. Proximal bile duct cancer: high resectability rate and 5-year survival. Ann Surg. 1999;230(2):266-275.
Laurent A, Tayar C, Cherqui D. Cholangiocarcinoma: preoperative biliary drainage. HPB (Oxford). 2008;10(2):126-129.
Lee HY, et al. Preoperative assessment of resectability of hepatic hilar cholangiocarcinoma: combined CT and cholangiography with revised criteria. Radiology. 2006;239(1):113-121.
Lee SG, et al. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asian experience. J Hepatobiliary Pancreat Surg. 2010;17(4):476-489.
Lilly DM, Stillwell RH. Probiotics: growth-promoting factors produced by microorganisms. Science. 1965;147(3659):747-748.
Liu CL, et al. Endoscopic retrograde cholangiopancreatography and endoscopic endoprosthesis insertion in patients with Klatskin tumors. Arch Surg. 1998;133(3):293-296.
Madoff DC, et al. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol. 2005;16(2 Pt 1):215-225.
Maeda A, et al. Interleukin 6 in bile as an indicator of liver function after hepatectomy in patients with biliary tract carcinoma. Br J Surg. 1999;86(4):458-464.
Maguchi H, et al. Preoperative biliary drainage for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2007;14(5):441-446.
Makuuchi M, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107(5):521-527.
McPherson GA, et al. Pre-operative percutaneous transhepatic biliary drainage: the results of a controlled trial. Br J Surg. 1984;71(5):371-375.
Miyazaki M, et al. Combined vascular resection in operative resection for hilar cholangiocarcinoma: does it work or not? Surgery. 2007;141(5):581-588.
Miyazaki M, et al. One hundred seven consecutive surgical resections for hilar cholangiocarcinoma of Bismuth types II, III, IV between 2001 and 2008. J Hepatobiliary Pancreat Surg. 2010;17(4):470-475.
Nagino M, Nimura Y, Hayakawa N. Percutaneous transhepatic portal embolization using newly devised catheters: preliminary report. World J Surg. 1993;17(4):520-524.
Nagino M, et al. Percutaneous transhepatic biliary drainage in patients with malignant biliary obstruction of the hepatic confluence. Hepatogastroenterology. 1992;39(4):296-300.
Nagino M, et al. Logistic regression and discriminant analyses of hepatic failure after liver resection for carcinoma of the biliary tract. World J Surg. 1993;17(2):250-255.
Nagino M, et al. Right or left trisegment portal vein embolization before hepatic trisegmentectomy for hilar bile duct carcinoma. Surgery. 1995;117(6):677-681.
Nagino M, et al. Selective percutaneous transhepatic embolization of the portal vein in preparation for extensive liver resection: the ipsilateral approach. Radiology. 1996;200(2):559-563.
Nagino M, et al. Right trisegment portal vein embolization for biliary tract carcinoma: technique and clinical utility. Surgery. 2000;127(2):155-160.
Nagino M, et al. Portal and arterial embolization before extensive liver resection in patients with markedly poor functional reserve. J Vasc Interv Radiol. 2000;11(8):1063-1068.
Nagino M, et al. Complications of hepatectomy for hilar cholangiocarcinoma. World J Surg. 2001;25(10):1277-1283.
Nagino M, et al. “Anatomic” right hepatic trisectionectomy (extended right hepatectomy) with caudate lobectomy for hilar cholangiocarcinoma. Ann Surg. 2006;243(1):28-32.
Nagino M, et al. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243(3):364-372.
Nagino M, et al. Preoperative biliary drainage for biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg. 2008;15(1):25-30.
Nakanishi Y, et al. Extrahepatic bile duct carcinoma with extensive intraepithelial spread: a clinicopathological study of 21 cases. Mod Pathol. 2008;21(7):807-816.
Nakanishi Y, et al. Impact of residual in situ carcinoma on postoperative survival in 125 patients with extrahepatic bile duct carcinoma. J Hepatobiliary Pancreat Surg. 2010;17(2):166-173.
Nakeeb A, et al. Cholangiocarcinoma: a spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224(4):463-473. discussion 473-475
Neuhaus P, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230(6):808-818. discussion 819
Nimura Y. Staging of biliary carcinoma: cholangiography and cholangioscopy. Endoscopy. 1993;25(1):76-80.
Nimura Y, Kamiya J. Cholangioscopy. Endoscopy. 1998;30(2):182-188.
Nimura Y, et al. Cholangioscopic differentiation of biliary strictures and polyps. Endoscopy. 1989;21(Suppl 1):351-356.
Nimura Y, et al. Combined portal vein and liver resection for carcinoma of the biliary tract. Br J Surg. 1991;78(6):727-731.
Nimura Y, et al. Hepatopancreatoduodenectomy for advanced carcinoma of the biliary tract. Hepatogastroenterology. 1991;38(2):170-175.
Nimura Y, et al. Technique of inserting multiple biliary drains and management. Hepatogastroenterology. 1995;42(4):323-331.
Nimura Y, et al. Aggressive surgical treatment of hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 1998;5(1):52-61.
Nishio H, et al. Value of percutaneous transhepatic portography before hepatectomy for hilar cholangiocarcinoma. Br J Surg. 1999;86(11):1415-1421.
Nishio H, et al. Most informative projection for portography: quantitative analysis of 47 percutaneous transhepatic portograms. World J Surg. 2003;27(4):433-436.
Nishio H, et al. Left hepatic trisectionectomy for hepatobiliary malignancy: results and an appraisal of its current role. Ann Surg. 2005;242(2):267-275.
Nomura T, Shirai Y, Hatakeyama K. Cholangitis after endoscopic biliary drainage for hilar lesions. Hepatogastroenterology. 1997;44(17):1267-1270.
Nomura T, Shirai Y, Hatakeyama K. Bacteribilia and cholangitis after percutaneous transhepatic biliary drainage for malignant biliary obstruction. Dig Dis Sci. 1999;44(3):542-546.
Ogata Y, et al. Role of bile in intestinal barrier function and its inhibitory effect on bacterial translocation in obstructive jaundice in rats. J Surg Res. 2003;115(1):18-23.
Ohkubo M, et al. Surgical anatomy of the bile ducts at the hepatic hilum as applied to living donor liver transplantation. Ann Surg. 2004;239(1):82-86.
Okazaki Y, et al. Study of the intrahepatic surgical margin of hilar bile duct carcinoma. Hepatogastroenterology. 2002;49(45):625-627.
Otto G, et al. Preoperative imaging of hilar cholangiocarcinoma: surgical evaluation of standard practises. Z Gastroenterol. 2004;42(1):9-14.
Padillo FJ, et al. Anorexia and the effect of internal biliary drainage on food intake in patients with obstructive jaundice. J Am Coll Surg. 2001;192(5):584-590.
Perez MJ, Briz O. Bile-acid–induced cell injury and protection. World J Gastroenterol. 2009;15(14):1677-1689.
Pitt HA, et al. Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost? Ann Surg. 1985;201(5):545-553.
Polydorou AA, et al. Palliation of proximal malignant biliary obstruction by endoscopic endoprosthesis insertion. Gut. 1991;32(6):685-689.
Rayes N, et al. Early enteral supply of Lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation. 2002;74(1):123-127.
Rayes N, et al. Early enteral supply of fiber and lactobacilli versus conventional nutrition: a controlled trial in patients with major abdominal surgery. Nutrition. 2002;18(7-8):609-615.
Rerknimitr R, et al. Result of endoscopic biliary drainage in hilar cholangiocarcinoma. J Clin Gastroenterol. 2004;38(6):518-523.
Rocha FG, et al. Hilar cholangiocarcinoma: the Memorial Sloan–Kettering Cancer Center experience. J Hepatobiliary Pancreat Surg. 2010;17(4):490-496.
Saiki S, et al. Preoperative internal biliary drainage is superior to external biliary drainage in liver regeneration and function after hepatectomy in obstructive jaundiced rats. Ann Surg. 1999;230(5):655-662.
Sakaguchi S, Nakamura S. Surgery of the portal vein in resection of cancer of the hepatic hilus. Surgery. 1986;99(3):344-349.
Sakamoto E, et al. The pattern of infiltration at the proximal border of hilar bile duct carcinoma: a histologic analysis of 62 resected cases. Ann Surg. 1998;227(3):405-411.
Sasaki R, et al. Significance of ductal margin status in patients undergoing surgical resection for extrahepatic cholangiocarcinoma. World J Surg. 2007;31(9):1788-1796.
Senda Y, et al. Value of multidetector row CT in the assessment of longitudinal extension of cholangiocarcinoma: correlation between MDCT and microscopic findings. World J Surg. 2009;33(7):1459-1467.
Shigeta H, et al. Bacteremia after hepatectomy: an analysis of a single-center, 10-year experience with 407 patients. Langenbecks Arch Surg. 2002;387(3-4):117-124.
Shingu Y, et al. Clinical value of additional resection of a margin-positive proximal bile duct in hilar cholangiocarcinoma. Surgery. 2010;147(1):49-56.
Speer AG, et al. Randomised trial of endoscopic versus percutaneous stent insertion in malignant obstructive jaundice. Lancet. 1987;2(8550):57-62.
Sugawara G, et al. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: a randomized controlled trial. Ann Surg. 2006;244(5):706-714.
Suzuki H, et al. Internal biliary drainage, unlike external drainage, does not suppress the regeneration of cholestatic rat liver after partial hepatectomy. Hepatology. 1994;20(5):1318-1322.
Takada T, et al. Percutaneous transhepatic cholangial drainage: direct approach under fluoroscopic control. J Surg Oncol. 1976;8(1):83-97.
Takayasu K, et al. Intrahepatic portal vein branches studied by percutaneous transhepatic portography. Radiology. 1985;154(1):31-36.
Takeuchi E, et al. Human hepatocyte growth factor in bile: an indicator of posthepatectomy liver function in patients with biliary tract carcinoma. Hepatology. 1997;26(5):1092-1099.
Tancrede CH, Andremont AO. Bacterial translocation and Gram-negative bacteremia in patients with hematological malignancies. J Infect Dis. 1985;152(1):99-103.
Uesaka K, Nimura Y, Nagino M. Changes in hepatic lobar function after right portal vein embolization: an appraisal by biliary indocyanine green excretion. Ann Surg. 1996;223(1):77-83.
Unno M, et al. Major hepatectomy for perihilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2010;17(4):463-469.
Van Laethem JL, et al. Clinical impact of biliary drainage and jaundice resolution in patients with obstructive metastases at the hilum. Am J Gastroenterol. 2003;98(6):1271-1277.
Wakai T, et al. Impact of ductal resection margin status on long-term survival in patients undergoing resection for extrahepatic cholangiocarcinoma. Cancer. 2005;103(6):1210-1216.
Wang XD, et al. Bacterial translocation in acute liver failure induced by 90 per cent hepatectomy in the rat. Br J Surg. 1993;80(1):66-71.
Watanabe S, et al. Choleretic effect of inchinkoto, a herbal medicine, on livers of patients with biliary obstruction due to bile duct carcinoma. Hepatol Res. 2009;39(3):247-255.
Yamaguchi K, et al. Frozen section and permanent diagnoses of the bile duct margin in gallbladder and bile duct cancer. HPB (Oxford). 2005;7(2):135-138.
Yeh TS, et al. Malignant perihilar biliary obstruction: magnetic resonance cholangiopancreatographic findings. Am J Gastroenterol. 2000;95(2):432-440.
Young AL, et al. Surgical treatment of hilar cholangiocarcinoma in a new era: comparison among leading Eastern and Western centers, Leeds. J Hepatobiliary Pancreat Surg. 2010;17(4):497-504.