Chapter 50A Cancer of the bile ducts
Intrahepatic cholangiocarcinoma
Overview
Intrahepatic cholangiocarcinoma is also known as peripheral cholangiocarcinoma, cholangiolar cancer, or cholangiocellular carcinoma, terms that have previously been used interchangeably. Cholangiocellular carcinoma was first used by Steiner and Higginson (1959) to describe a subtype of cholangiocarcinoma in which the glands are small and regular and resemble proliferating cholangioles with inconspicuous lumens. Current nomenclature uses the term intrahepatic cholangiocarcinoma (IHCC) to define tumors arising from biliary epithelium in intrahepatic bile ducts above the level of the left main and right main ducts (Liver Cancer Study Group of Japan, 1990). These tumors constitute 10% of primary hepatic malignancies, and although much is known about extrahepatic cholangiocarcinoma, IHCC is less well understood.
Hepatic resection for IHCC was infrequently described until recently. Foster and Berman (1977) describe only 13 cases in their summary of hepatic surgery in the United States, although they present 112 resections for hepatocellular carcinoma (HCC) and 47 cases of hepatoblastoma. This low number of resections may represent the frequency with which advanced disease was diagnosed at presentation and also reflects that recognition of IHCCs as a discrete primary liver tumor was slow to occur and that many were historically diagnosed as metastatic adenocarcinoma from unknown primary sites.
Epidemiology and Demographics
The incidence of cholangiocarcinoma is rising worldwide and is now the second most common primary cancer of the liver behind HCC (Olnes & Erlich, 2004). Approximately 5000 new cases are diagnosed annually in the United States (Vauthey & Blumgart, 1994), and more than 1000 cases are diagnosed annually in the United Kingdom (Khan et al, 2008). However, overall, cholangiocarcinoma is rare and makes up only 3% of all GI tract cancers (Lazaridis & Gores, 2005). This means that it is infrequently seen by general surgeons or gastroenterologists, and its rarity has frustrated attempts to design therapeutic trials to treat it.
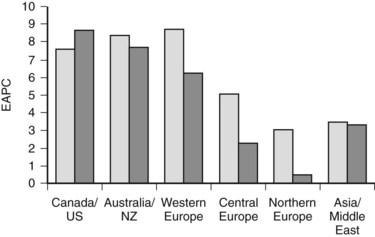
More detailed analysis of epidemiologic data shows that the incidence and mortality rates of IHCC are increasing and those of extrahepatic cholangiocarcinoma are decreasing (see Chapter 50B; Endo et al, 2008; Khan et al, 2002; Patel, 2001, 2002), suggesting that the tumors may have different etiologic factors but similar microscopic morphology. These findings must be interpreted with caution. IHCC may occur as solitary or multiple liver lesions and, in the past, many were probably diagnosed as metastatic tumors and not further investigated. In addition, analysis of the Surveillance, Epidemiology, and End Results (SEER) database has shown that more than 90% of hilar tumors were wrongly classified as IHCCs, and extrahepatic lesions were often classified as gallbladder cancers (Shaib & El-Serag, 2004). Indeed, even when these classification issues are addressed, the mortality rate of IHCC is still increasing worldwide (Fig. 50A.1; Shaib et al, 2005).
IHCC currently has an incidence of 0.85 per 100,000 population in the United States (Shaib et al, 2004; Shaib & El-Serag, 2004); the highest incidence in the world is recorded in northeast Thailand (96 per 100,000) (Khan et al, 2008). Patients most commonly are seen in the seventh decade, and IHCCs are more common in men (Khan et al, 2002; Shaib et al, 2005).
Etiology and Risk Factors
Primary Sclerosing Cholangitis
Primary sclerosing cholangitis (PSC) (see Chapter 41) is the most common risk factor for cholangiocarcinoma in the West (Farrant et al, 1991). The cumulative annual risk of cholangiocarcinoma in patients with PSC is 1.5% per year (Farrant et al, 1991), and the prevalence of cholangiocarcinoma in these patients is between 8% and 40% (Pitt et al, 1995; Rosen et al, 1991). The risk of developing cholangiocarcinoma is increased in those with associated inflammatory bowel disease, in whom the 10- and 20-year rates for cholangiocarcinoma are 14% and 31% versus 2% and 2% in patients without inflammatory bowel disease, respectively (Claessen et al, 2009).
Cholangiocarcinoma often develops 2 to 3 decades earlier in patients with PSC than in those with sporadic tumors (30 to 50 years vs. 60 to 70 years of age, respectively) (Bergquist & Broome, 2001; Farrant et al, 1991). In addition, PSC-associated tumors often present later with advanced-stage disease because of difficulties in detecting malignant change in inflammatory strictures. Surgical treatment can be difficult in the presence of chronic liver disease, and such patients are often ineligible for orthotropic liver transplantation. Consequently. they have a poor prognosis (Kaya et al, 2001). Factors suggestive of cholangiocarcinoma in patients with PSC are the sudden development of jaundice, weight loss, marked biliary dilation proximal to a dominant stricture, a sudden rise in carbohydrate antigen 19-9 (CA19-9), the presence of a hypovascular mass with late contrast enhancement on radiologic imaging, and cytologic evidence of dysplasia or malignancy obtained on brushings of the bile ducts (Harewood, 2008).
Parasitic Infections (See Chapter 45)
Chronic infection with liver flukes (Opisthorchis viverrini and Clinorchis sinensis) is closely related to increased risk of developing cholangiocarcinoma, especially in Southeast Asia (Hasweel-Elkins et al, 2008; Jang et al, 2008; Sripa & Pairojkul, 2008; Watanapa, 1996; Watanapa & Watanapa, 2002). The mechanism of carcinogenesis is unclear; however, mechanical irritation, excreted metabolic products, and the actions of proinflammatory cytokines, particularly those that stimulate the release of nitric oxide from activated white cells, may all play a role (Sripa et al, 2007).
A further parasitic hepatic infection is caused by the trematodes Fasciola hepatica and F. gigantica. These parasites are widely spread throughout Asia, Africa, the Americas, and Oceania. They migrate into the liver from the duodenum and cause hepatic fibrosis, but no evidence is available to support the theory that fascioliasis increases the risk of cholangiocarcinoma. In addition, the fibrotic pathologic changes accompanying an infection can be difficult to distinguish from carcinoma (Kim et al, 2005; Marcos et al, 2008).
Hepatolithiasis (See Chapters 39 and 44)
Recurrent pyogenic cholangiohepatitis (RPC) is characterized by recurrent episodes of ascending cholangitis, hepatolithiasis, biliary stricturing, and dilation. The syndrome is present in one fifth of the population of Southeast Asia, and up to 10% of these patients develop IHCC (Chen et al, 1993; Kubo et al, 1995; Lesurtel et al, 2002; Su et al, 1997). Patients are seen with recurrent episodes of cholangitis and, on investigation, have significant hepatolithiasis and associated inflammatory biliary strictures (Chu et al, 1997). Infection with liver flukes may also be present, but RPC appears to be a separate condition and can develop in the absence of parasitic infection (Kim et al, 2003).
Congenital Biliary Cystic Disease (See Chapter 46)
Untreated choledochal cysts carry an increased risk of developing malignant change. The incidence of cholangiocarcinoma is estimated at between 10% and 20% if the cyst is not resected by the age of 20 years (Lipsett et al, 1994; Ohtsuka et al, 2001). Correspondingly, patients who have had their cysts resected have a very low incidence of cholangiocarcinoma (Hewitt et al, 1995), although the subsequent development of cholangiocarcinoma has been recorded after cyst excision (Goto et al, 2001). The mechanism of malignant transformation is not well understood, but many patients with choledochal disease have an abnormally high union of the pancreatic and bile ducts, suggesting that biliary stasis and chronic reflux of pancreatic secretions may contribute to the development of chronic inflammation of biliary epithelium (Chapman, 1999).
Hepatic Cirrhosis and Viral Infections (See Chapter 64)
The risk of developing cholangiocarcinoma is increased in patients with cirrhosis (10.7% vs. 0.7% in the general population) (Shaib & El-Serag, 2004; Sorensen et al, 1998). In addition, the incidence of cholangiocarcinoma is increased in patients with hepatitis C viral infection (0.8% vs. 0.2% in the general population) (Donato et al, 2001; Shaib et al, 2005) and in patients with chronic hepatitis B viral infection (11.5% vs. 5.5% in the general population) (Donato et al, 2001). It has been suggested the increasing incidence of cholangiocarcinoma in Western populations is related to the increasing prevalence of chronic liver disease and chronic viral infection (Shaib et al, 2005; Shaib & El-Serag, 2004).
Human immunodeficiency virus (HIV) does not cause cirrhosis, but cholangiocarcinomas have been found in up to 0.5% of patients infected with the virus compared with 0.1% in control subjects, suggesting that HIV is also associated with an increased risk of biliary carcinogenesis (Shaib et al, 2005).
Benign Biliary Tumors (See Chapter 79B)
The development of biliary cystadenocarcinomas from biliary cystadenomas is rare; in general, it occurs if a cystadenoma is untreated for many years. Patients are first seen with cystadenocarcinomas in the sixth or seventh decades of life, although cystadenomas present at an earlier age (Buetow et al, 1995). Intrahepatic cholangiocarcinoma has also been reported to develop in patients with biliary papilliomas (Cox et al, 2005; Galluoglu et al, 2007).
Chemical Agents
Thorotrast (thorium dioxide) was used as a radiologic contrast agent between 1928 and 1950. It is an α-emitter with a biologic half-life of 400 years. It accumulates in the reticuloendothelial cells in the liver and spleen and increases the risk of developing cholangiocarcinoma by 300 times compared with the general population (Lipshutz et al, 2002; Rubel & Ishak, 1982). It is now no longer in use, although the latency period of 16 to 45 years means that patients who received this agent during childhood radiologic examinations will still occasionally come to medical attention (Massachusetts General Hospital, 1981).
A number of other agents have been implicated in the development of cholangiocarcinoma. Associations have been shown for asbestos (Szendroi et al, 1983), vinyl chloride (Wong et al, 1991), nitrosamines (Mitacek et al, 1999), the antituberculosis agent isoniazid (Lowenfels & Norman, 1978), and first-generation oral contraceptives (Yen et al, 1987).
General Risk Factors
Diabetes and obesity are associated with an increased risk of cholangiocarcinoma (Malhi & Gores, 2006; Oh et al, 2005). Similarly, surgical biliary-enteric bypass and surgical sphincteroplasty may also increase the risk (Hakamada et al, 1997). Smoking tobacco is a significant risk factor for the development of cholangiocarcinoma in patients with PSC (Bergquist et al, 1998), although the relationship is less marked in the general population (Shaib et al, 2005).
Pathogenesis
Cholangiocarcinoma develops from the malignant transformation of cholangiocytes. These cells line the intrahepatic bile ducts and canaliculi. Their physiologic functions center around the modification of bile at the canalicular surface and the detoxification of xenobiotics (Alpini et al, 2001). Normal growth and renewal of cholangiocytes is important in the maintenance of biliary mass, as well as hepatic secretory and detoxification functions, and is achieved by careful regulation of proliferation and apoptosis; however, when this process becomes uncontrolled, cholangiocarcinogenesis can occur (Wise et al, 2008).
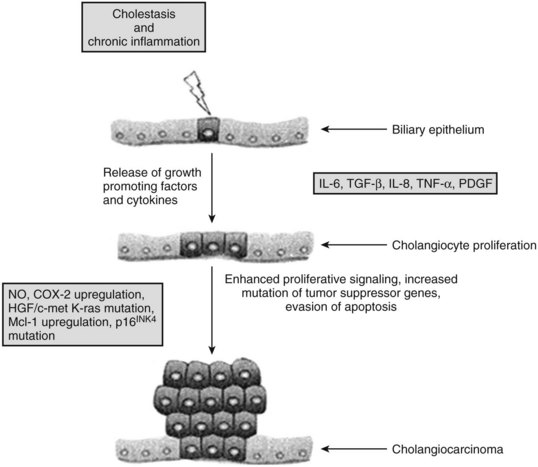
Chronic inflammation is the most common and important feature of many of the risk factors associated with malignant transformation of cholangiocytes (see Chapter 6). Chronic inflammation can result in biliary obstruction, injury to the biliary epithelium, and increased cholangiocyte turnover (Jaiswal et al, 2000). Chronic inflammation also causes DNA damage, activates local tissue repair, stimulates cellular proliferation, and results in a local environment that promotes neoplastic transformation (Fig. 50A.2) (Schottenfeld & Beebe-Dimmer, 2004). Cholangiocytes exert significant paracrine and autocrine effects and stimulate secretion of cytokines, such as interleukin-6 (IL-6), IL-8, transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), and platelet-derived growth factor (PDGF) (see Chapter 10; Berthiaume & Wands, 2004).
These cytokines activate nitric oxide synthase in cholangiocytes and result in the generation of nitric oxide, which reacts with other active oxygen species such as superoxide, all of which can damage DNA and downregulate DNA repair mechanisms (Jaiswal et al, 2000). This damage can lead to expression of oncogenes and reduced expression of tumor suppressor genes. Overexpression of the epidermal growth-factor receptor (EGFR), erb-2, K-ras, BRAF, and hepatocyte growth factor c-Met have been reported in cholangiocarcinoma (Endo et al, 2002; Lai et al, 2005). The protooncogene Erbb2 is activated in cholangiocarcinoma, and tumor suppressor genes CDKN2A, P53, APC, and SMAD4 are underexpressed (Rashid, 2002; Taniai et al, 2002). Cytokines may also assist cholangiocytes in evading apoptosis. IL-6 is secreted by cholangiocytes and activates the prosurvival p38 mitogen-activated protein kinase (see Chapter 8B; Ishimura et al, 2004; Park et al, 1999).
Pathologic Subtypes and Mode of Spread
Macroscopically, IHCCs are firm, white, sclerotic tumors often with associated satellite lesions nearby. However, the variability in gross morphologic appearance has led to a number of pathologic subclassifications. Nakanuma and colleagues (1985) initially classified IHCCs into two types: mass forming and periductal. Mass-forming tumors were described as tumors with clear borders between malignant and nonmalignant tissues; periductal tumors were described as more infiltrative and extended along peri–bile duct tissues without forming a discrete nodular mass. This classification was widely adopted in Japan (Fujita, 1990) and was further modified by Ohashi and colleagues (1994), who added a specula-forming lesion present when a nodular tumor has poorly defined and irregular borders. Yamamoto and colleagues (1998) emphasized that all three morphologic types appear to have different proliferative activity and different biologic behavior. According to their research, the distinctive feature of the mass-forming type is its tendency to develop intrahepatic metastases as a result of localized vascular invasion, and the infiltrative type is distinguished by infiltrative spread via the Glisson capsule and hilar lymph node metastases. On the basis of these observations, these researchers recommended hepatectomy as the procedure of choice for the mass-forming subtype; hepatectomy with extrahepatic ductal resection and hilar lymphadenectomy is the procedure of choice for the infiltrating subtype. They also added a fourth subtype, the intraductal variant, characterized by papillary or granular growth within the lumen. They also were able to show that the frequency of lymph node metastases was higher in the mass-forming and periductal types compared with the intraductal subtype. A single report of a Western hepatobiliary unit used this classification and was unable to show any difference in overall survival or the frequency of lymph node metastases (Weber et al, 2001), although the intraductal subtype may carry a more favorable prognosis, as it does in the extrahepatic bile duct (Jarnagin et al, 2005). Although the subclassification of IHCC is intriguing and may have implications for surgical therapy, one significant weakness of this approach is that many tumors exhibit features of a number of the described subtypes (Shirabe et al, 2002).
Other less common histologic variants of IHCC have been reported, including mucin-hypersecreting lesions, which are similar to intraductal papillary mucinous neoplasms of the pancreas and are characterized by large mucin-filled cystic spaces (see Chapter 57; Chow et al, 1997; Kim et al, 2000; Suh et al, 2000) and intraductal oncocytic papillary carcinoma. These lesions have a distinctive appearance defined by the presence of oncocytes but appear to behave in a favorable manner, similar to the papillary type (Sudo et al, 2001; Wolf et al, 1992).
Intraabdominal lymph nodes are the most common site of metastatic spread for IHCCs and are present in up to 75% of cases at presentation (Shirabe et al, 2002). In addition, up to two thirds of patients may have evidence of remote organ metastases, most commonly lung and bone, at presentation (Endo et al, 2008; Shirabe et al, 2002). The common sites of lymphatic metastases are at the hepatic hilus, around the pancreas, in the retroperitoneum, around the aorta, and in the mediastinum (Nakajima et al, 1988). Nozaki and colleagues (1998) showed significant differences in lymphatic spread between left lobar and right lobar tumors. Patients with right lobar tumors always had lymph node metastases in the hepatoduodenal ligament, whereas in patients with left lobar tumors, 50% of nodal metastases were found distant from the hepatoduodenal ligament in the cardia and around the lesser curvature of the stomach. Furthermore, no lymph node metastases were present in the hepatoduodenal ligament in these patients.
Clinical Presentation
In contrast to extrahepatic cholangiocarcinoma, which usually presents with jaundice and symptoms of biliary obstruction, IHCC often presents as asymptomatic hepatic masses detected during physical examination or on cross-sectional imaging. In patients with symptoms, abdominal pain is most common (Martin & Jarnagin, 2003). A significant proportion of patients may present with nonspecific constitutional symptoms, such as weight loss and decreased energy and appetite (Weber et al, 2001). Jaundice can be present in centrally placed lesions that compress or invade the biliary confluence, although extensive replacement of hepatic parenchyma by tumor, along with portal vein compromise, intrabiliary tumor invasion, or mucobilia, can also cause symptoms of biliary obstruction. An increase in liver enzymes, most commonly alkaline phosphatase or γ-glutamyl transferase, may be the only presenting feature to prompt further investigations, including physical examination and cross-sectional imaging. Roayaie and colleagues (1998) have shown that jaundice as a presenting symptom is predictive of unresectable disease because of significant involvement of inflow structures bilaterally or because of massive parenchymal replacement.
Diagnosis and Evaluation
In clinical practice, the diagnosis of IHCC is established with the typical finding of a hypovascular mass present on cross-sectional imaging (see Chapters 16 and 17). GI metastases are excluded by performing upper and lower GI endoscopies without the finding of a primary tumor and in the absence of other primary malignancies (pancreas, kidney, lung) on a staging computed tomography (CT) scan of the chest, abdomen, and pelvis. Women should have had a recent mammogram and gynecologic examination. Routine tumor biopsy is often unnecessary, particularly in patients who will undergo resection, and it is not recommended because of the small risk of tumor dissemination (Metcalfe et al, 2004; Ohlsson et al, 1994). In general, biopsy is indicated only to establish the presence of irresectable disease. Final pathologic confirmation of cholangiocarcinoma is defined by a specimen showing an adenocarcinoma that stains positively for CK-7 and CK8/18 but is negative for CK-20 (Fig. 50A.3). Staging laparoscopy is also useful in the evaluation of IHCC to exclude peritoneal disease, nodal disease, or abdominal wall invasion, which may preclude an attempt at resection (D’Angelica et al, 2003).
Serum Markers for Cholangiocarcinoma
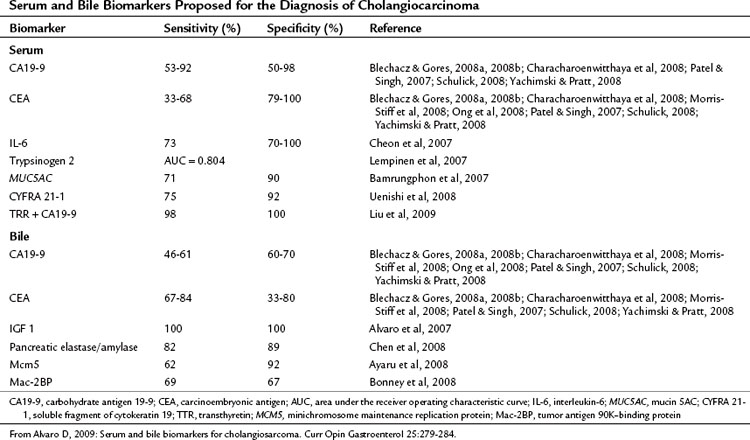
Serum tumor markers are an attractive method for diagnosing and monitoring treatment response in cholangiocarcinoma because of the ease of obtaining samples and their relatively low cost. To be effective a marker must be accurate in detecting the presence of malignancy (sensitivity) and in defining the presence of benign disease (specificity). Most of the research on tumor markers in cholangiocarcinoma has been directed at diagnosis of extrahepatic tumors, particularly in the context of PSC; however, much of this is applicable to peripheral cholangiocarcinoma. The data on tumor markers that have been applied to cholangiocarcinoma are summarized in Table 50A.1.
Carcinoembryonic antigen (CEA) is widely used because of its availability but is elevated in only one third of patients with cholangiocarcinoma (Blechacz & Gores, 2008a, 2008b; Patel & Singh, 2007; Yachimski & Pratt, 2008). CA19-9 is also widely used in the diagnosis of cancers of the upper GI tract and is elevated in gastric cancer, pancreatic cancer, biliary, and gallbladder cancers as well as in smokers, during cholangitis, and in conditions causing cholestasis. In addition, it is not present in 7% of the population who are Lewisa-antigen negative (Alvaro, 2009). In a retrospective investigation of patients with suspected biliary malignancies, of whom 10% were later found to have benign disease, CA19-9 was elevated in all patients and was decreased after biliary decompression. Elevated bilirubin was identified as the most important predictor of raised CA19-9 (Ong et al, 2008).
Other biomarkers investigated have included IL-6 either alone or in conjunction with CA19-9. Serum levels of IL-6 correlate with tumor burden in cholangiocarcinoma, but this marker is also elevated in hepatocellular carcinoma, metastatic disease, and benign biliary lesions (Cheon et al, 2007). Smith and colleagues (2008) recently proposed that an elevated preoperative platelet/lymphocyte ratio (PLR) may be a prognostic marker for resected ampullary cancer, but this ratio is also elevated in conditions associated with systemic inflammation. Serum trypsinogen-2 has also been evaluated in patients with cholangiocarcinoma and was found to be accurate in differentiating between patients with PSC and benign stricture and those who had cholangiocarcinoma develop (Lempinen et al, 2007). In addition, no correlation between serum trypsinogen-2 and bilirubin levels was found, indicating that this marker is unaffected by the presence of biliary obstruction or cholangitis (Lempinen et al, 2007). Abnormal expression of mucin 5AC (MUC5AC) is observed in mucin-producing adenocarcinomas, such as cholangiocarcinoma, and is detectable in 70% of patients with cholangiocarcinoma. Its presence is associated with poorer prognosis (Bamrungphon et al, 2007).
Bile Markers for Cholangiocarcinoma
Bile markers of cholangiocarcinoma have been investigated for their use in the diagnosis of biliary obstruction, and they may play a significant role in the evaluation of extrahepatic biliary obstruction because they can be easily obtained during ERCP. It is much less common for IHCC to be seen initially with biliary obstruction, however, and the use of bile markers in this tumor is limited. However, in the future they may play a role in the diagnosis of central lesions related to the hilus or intrahepatic lesions developing in patients with PSC. Bile markers that have been suggested for use in the diagnosis and management of cholangiocarcinoma are summarized in Table 50A.1.
Imaging
Transabdominal Ultrasound (See Chapter 13)
Ultrasound is often used as a screening examination by primary health care practitioners investigating patients with right upper quadrant pain, a palpable mass, or unexplained jaundice. IHCC has a nonspecific appearance as a hypoechoic hepatic mass (Bloom et al, 1999; Slattery & Sahani, 2006). Satellite lesions and capsular retraction may be seen, and the tumors are hypovascular and usually have minimal Doppler evidence of internal blood flow. Ultrasound is useful for defining associated biliary dilatation, portal venous invasion, hepatic venous invasion and, rarely, portal lymphadenopathy (Bach et al, 1996; Hann et al, 1997).
Computed Tomography
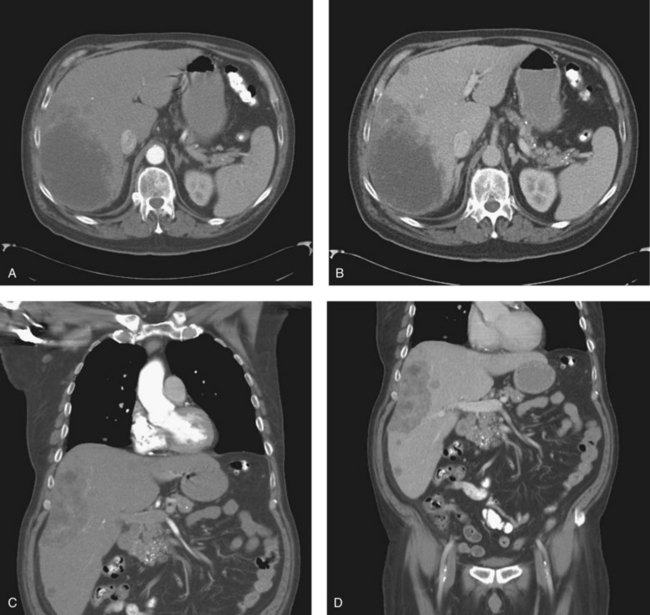
Triple-phase CT scan (see Chapter 16) is widely available and is the single most effective investigation in diagnosing and staging IHCC, which is seen as hypodense lesions with irregular, infiltrative margins and a variable degree of delayed enhancement in the portal venous phase (Fig. 50A.4) (Bach et al, 1996). CT scan will also demonstrate the presence of biliary dilatation, portal or hepatic venous involvement (Asayama et al, 2006), lobar atrophy as a result of long-standing biliary obstruction, or portal venous involvement (Kim et al, 2002). CT scan is also useful in detecting metastatic disease affecting regional lymph nodes, peritoneum, or lung fields. Digital Imaging and Communications in Medicine data from newer, multiphase, fast-acquisition scanners can also be used to construct three-dimensional representations of hepatic and tumor anatomy for use in the planning of resections and in obtaining accurate measurement of hepatic volumetry, particularly in relation to remnant volume and risk of postoperative liver failure (see Chapter 93A). This is an emerging area of hepatic imaging but is particularly relevant in the management of large central tumors (Wigmore et al, 2001).
Magnetic Resonance Imaging
IHCC appears as hypointense on T1-weighted images and hyperintense on T2-weighted images with pooling of contrast within the lesions on delayed images (6 to 8 minutes after contrast injection) (Manfredi et al, 2004). Magnetic resonance imaging (MRI) is also useful in defining venous and arterial involvement by tumor, and cholangiopancreatography is a noninvasive method of obtaining cholangiograms.
Positron Emission Tomography
Positron emission tomography (PET) scanning is now a commonly used modality for staging GI malignancy, and the integration of PET and CT now provides the opportunity to obtain anatomic and functional information in a single scan (Iglehart, 2006; Slattery & Sahani, 2006). IHCCs are present as glucose-avid lesions within the liver. PET CT is also useful in detecting intraabdominal lymph node metastases (sensitivity, 42%; specificity, 80%) and distant metastases (sensitivity, 56%; specificity, 88%) (Iglehart, 2006). However, PET is limited by false-positive results in patients with biliary inflammation (Petrowsky et al, 2006), and patients with mucinous tumors can have false-negative scan findings (Fritscher-Ravens et al, 2001).
Staging
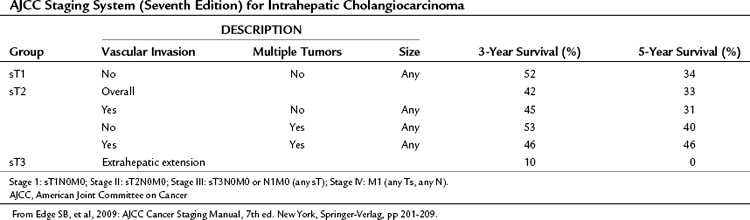
IHCCs are classified as primary cancers of the liver by the American Joint Committee on Cancer (AJCC). Western hepatobiliary centers commonly use the AJCC systems to stage tumors. The sixth edition of the AJCC staging manual proposes a single staging system for primary cancers of the liver and applies this to both HCCs and IHCCs (Greene et al, 2002). The seventh edition of the staging manual (Edge et al, 2009), which entered clinical use in January 2010, includes a number of changes (Table 50A.2) and incorporates the staging system of Nathan and colleagues (2009), who used data from the SEER database to develop a new staging system for IHCC. They found that the sixth edition of the AJCC staging system failed to stratify T2 and T3 cohorts into distinct groups. Survival in the T3 group exceeded that of the T2 group (43% vs. 31%). In addition, these investigators found that the presence of multiple tumors at presentation and vascular invasion had an adverse effect on outcome, but tumor size at presentation was not predictive of outcome. Lymph node metastases and extrahepatic extension were also associated with poorer outcome.
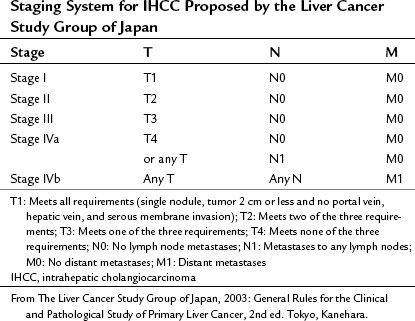
Two other staging systems have been proposed from Japan, one by Okabayashi and colleagues (2001) and one by Yamasaki (2003), with that of Yamasaki being adopted by the Liver Cancer Study Group of Japan as its preferred staging system for IHCC (Table 50A.3).
Treatment
Because of its rarity, treatment protocols for IHCC are in their infancy compared with other intrahepatic tumors such as HCC. Nevertheless, there is a clear role for hepatic resection when feasible and in patients with disease confined to the liver. Orthotopic liver transplantation has been studied in some detail but is currently not used routinely for IHCC (see Chapter 97E). The roles of neoadjuvant and adjuvant chemotherapy, both systemic and regional; conformal radiation therapy; and ablative therapies have yet to be fully clarified.
Surgical
Hepatic Resection
Surgical therapy for intrahepatic cholangiocarcinoma is based on the principles applied to resections for HCC and metastatic tumors. Criteria for irresectability of IHCC are 1) locally advanced solitary tumors involving either inflow or outflow bilaterally, 2) multiple intrahepatic tumors, and 3) metastatic disease to distant sites. Regional lymph node metastases are an indicator of poor outcome but are not necessarily an absolute contraindication to surgery (Endo et al, 2008). An R0 resection must be performed for a potential survival benefit to be achieved. Hepatic resection is undertaken with the aim of obtaining a clear margin of 1 cm or greater, leaving a well-vascularized remnant with adequate venous and biliary drainage. Resection of up to 80% of hepatic volume can be contemplated, but resections of this magnitude often need to be preceded by portal vein embolization.
Untreated patients with IHCC have a median survival of less than 12 months (Chu & Fan, 1999; Kim et al, 1999). Resection with positive margins or residual macroscopic disease is associated with median survivals of 1.8 months to 3 months (Chu & Fan, 1999; Kim et al, 1999), indicating that a cytoreductive approach is ineffective at prolonging survival.
In contrast, 5-year survival rates after complete resection range between 13% and 42%. The principal reason for the variability in survival appears to be the presence of lymph node metastases. Lieser and colleagues (1998) reported a 5-year survival of 42%, but only 13% of patients presented with lymph node metastases. In the series of Chu and Fan (1999), 50% of patients presented with nodal metastases, and none survived 5 years. Recently presented data from a number of centers in the United States, Asia, and Europe show reasonable survival after curative resection (Table 50A.4). Cherqui and colleagues (1995) report 100% survival 2 years after resection, Nakeeb and collegues (1996) report a 5-year survival of 44%, Lieser and colleagues (1998) report a 60% survival at 3 years, and Shirabe and colleagues (2002) report a 5-year survival of 32%. All these clinical series emphasize the importance of obtaining an R0 resection in achieving long-term survival.
Table 50A.4 Summary of Contemporary Results for Surgical Resection of Intrahepatic Cholangiocarcinoma
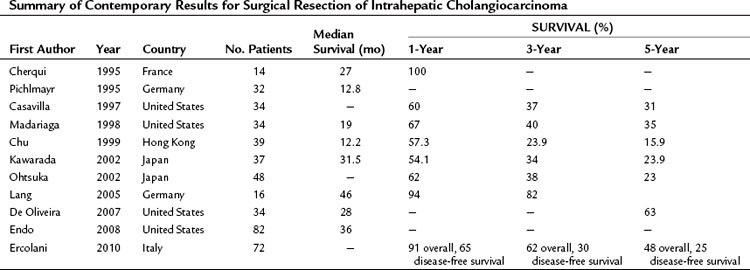
This point was further emphasized by Lang and colleagues (2005), who reported a single-center experience with extended hepatectomy for IHCC. Twenty-seven patients underwent extended hepatectomies (19 extended right, 8 extended left), and 16 patients had en bloc resections of other structures. The median survival in this series was 46 months with a 3-year survival rate of 82%. Cherqui and others (1995) have achieved similar results with an aggressive surgical policy.
Although the importance of achieving an R0 resection is clear, the role of routine lymph node dissection is debated. The presence and extent of nodal metastatic disease is an important prognostic factor. Chu and Fan (1999) dissected portal lymph nodes in their series, and all patients with portal lymph node metastases died within 10 months of resection. A further study from Japan (Shirabe et al, 2002) confirmed that no survival benefit was evident for patients undergoing hepatectomy with portal lymphadenectomy versus hepatectomy alone. However, Nozaki and colleagues (1998) have recommended routine dissection of cardia and lesser curvature nodes for left-sided tumors and dissection of the hepatoduodenal ligament for right-sided tumors, although using this approach did not improve survival, and extended surgery was associated with a higher mortality rate (Yamamoto et al, 1999).
Most surgical series confirm that the presence of lymph node metastases is the most important prognostic factor. Endo and colleagues (2008) documented a recurrence rate of 93% in node-positive patients undergoing R0 resection versus 47% in node-negative patients. These investigators also showed that a tumor greater than 5 cm in diameter and the presence of multiple intrahepatic tumors were significant adverse prognostic factors. Other investigators have also defined lymphatic permeation, vascular invasion, and intrahepatic satellite lesions as predictors of poor survival, and two Japanese groups (Isaji et al, 1999; Shirabe et al, 2002) have also shown that the survival is higher in patients with the mass-forming tumor type than in those with the periductal infiltrating type.
After resection, the most common site of recurrence is in the liver, followed by intraabdominal sites (lymph nodes, peritoneum), lungs, and bone (Jan et al, 2005). Salvage surgery for intrahepatic recurrence or metastasectomy is usually not indicated.
Transplantation
The first significant report regarding liver transplantation for IHCC is that of Pichlmayr and colleagues (1995), who reported a median survival of 5 months in 18 patients treated with liver transplantation, with a 1-year survival rate of 13.9%. Several studies confirmed these findings (Ismail et al, 1990; O’Grady et al, 1988), although Cherqui and colleagues (1995) reported two long-term survivors and concluded that an intrahepatic tumor with no extrahepatic spread that cannot be resected for anatomic reasons may be a candidate for liver transplantation.
More recent experience has resulted in improved survival with disease-free survivals at 5 and 10 years of 27% and 23%, respectively, reported in 23 patients with IHCC treated with orthotopic liver transplantation (Robles et al, 2004). Most of these patients were deemed irresectable but showed no evidence of extrahepatic spread, and the authors concluded that transplantation could be considered in this group because the results achieved are better than palliative treatment. However, the role of transplantation in peripheral cholangiocarcinoma will continue to be limited by the lack of an effective adjuvant systemic treatment regimen. This is in contrast to the emerging protocol of neoadjuvant chemoradiation before transplantation for hilar cholangiocarcinoma (Schwartz et al, 2009).
Tumor Ablation
The role of ablative therapies in peripheral cholangiocarcinoma is currently limited but subject to significant investigation. Cryotherapy and radiofrequency ablation (RFA) are widely used in the treatment of metastatic colorectal cancer, either in addition to hepatectomy or as a primary therapy. Unfortunately, in IHCC neither of these approaches can be used; the primary tumors are hard, and percutaneous or operative ablation can be difficult to achieve. Because many of the lesions are large at presentation, their size often precludes effective ablation, and smaller lesions can be resected. Likewise, the use of ablation to treat intrahepatic metastases is ill advised because these are indicative of vascular invasion. However, Rai and colleagues (2005) reported a case of recurrent tumor after transplantation that was treated with RFA and controlled for 12 months, and others have used this technique to manage small cholangiocarcinomas that are unsuitable for resection (Zgodzinzki & Espat, 2005).
Both cryotherapy (Cuschieri et al, 1995; Sheen et al, 2002) and selective internal radiation therapy (Dubel & Soares, 2008) have been used in heterogeneous series of hepatic tumors treated with ablative therapies. Both treatments are reported to be safe, although no long-term follow-up data are available.
A number of publications have reported on the use of transarterial chemoembolization (TACE) of unresectable IHCC (Gusani et al, 2008; Herber et al, 2007; Kim et al, 2008) with gemcitabine in combination with cisplatin, oxaliplatin, or mitomycin C. Repeated treatments are usually undertaken at 8-week intervals, with median survivals reported between 12 (Kim et al, 2008) and 21 months (Herber et al, 2007). Overall combination therapy appears to be more effective than single-agent therapy (Gusani et al, 2008), with large tumor size, tumor hypovascularity, and Child-Pugh class B being adverse prognostic factors (Kim et al, 2008).
Chemotherapy
Systemic
Unfortunately, no effective adjuvant chemotherapy protocols are currently available for IHCC. This reflects the aggressive nature of the tumor as well as its rarity. Much of the information on the effectiveness of chemotherapy in this tumor has been derived from Phase II trials rather than Phase III trials. In addition, most of these trials have been carried out in groups of patients with tumors of biliary epithelia and include patients with intrahepatic and extrahepatic cholangiocarcinoma, gallbladder cancer, and pancreatic cancer. Many postoperative therapy regimens have been based on 5-fluorouracil (5-FU) and have included irinotecan, mitomycin C, and gemcitabine. None has been effective, and routine use of adjuvant chemotherapy after an R0 resection is not current practice. A recent randomized trial established the superiority of combined gemcitabine and cisplatin compared with gemcitabine alone in patients with locally advanced or metastatic biliary cancer (Valle et al, 2010); however, this study included the full range of biliary tract adenocarcinoma, including ampullary cancers, and it remains to be seen whether this therapy has any impact in the adjuvant setting. Ercolani and colleagues (2010) retrospectively analyzed long-term results of resection in 72 patients with IHCC and demonstrated improved survival in those who received adjuvant gemcitabine-based chemotherapy (5-year survival 65% after chemotherapy vs. 40% with resection alone), although this observation has not been confirmed prospectively (Ercolani et al, 2010).
Systemic chemotherapy for unresectable disease is associated with partial responses in between 10% and 20% of patients and uses either 5-FU or contemporary combination therapy with gemcitabine and cisplatin or oxaliplatin. The responses are modest, but the use of newer, targeted therapies continues to stimulate investigative trials in this area (Hezel & Zhu, 2008). More recent Phase I and II investigations have used the biologic agents sunitinib (Sweeney et al, 2010), cetuximab (Chang et al, 2010), or bevacizumab and erlotinib (Lubner et al, 2010) in the treatment of patients with advanced-stage disease. Endo and colleagues (2008) described five patients with initially unresectable tumors treated with either regional fluorodeoxyuridine in combination with systemic camptothecin-11 or systemic gemcitabine-based therapy whose tumors were downstaged sufficiently to undergo resection, indicating that effective neoadjuvant therapy is a realistic future goal for this tumor.
Regional
Regionally administered chemotherapy has not been extensively investigated in peripheral cholangiocarcinoma, although sporadic reports of its effectiveness have surfaced. Shirabe and colleagues (2002) report a single case treated with intraarterial cisplatin and 5-FU achieving a complete response for 12 months. Regional chemotherapy is an attractive means to attempt to downstage large hepatic tumors for subsequent resection, although adequate pretherapy staging would need to be undertaken to exclude extrahepatic disease, and patients with underlying cirrhosis could not be treated because of the risk of treatment-related complications (Jarnagin et al, 2009).
Radiation Therapy
Cholangiocarcinoma is a radiosensitive tumor, although the doses required (60 Gy) are toxic to normal liver and adjacent structures, including the stomach, duodenum, and kidney. However, when accurately administered, radiation therapy does have an emerging role in the management of IHCC. Postoperative radiation therapy is ineffective in improving survival after R0 resections, but several studies have shown a potential benefit in postoperative radiation therapy in patients with microscopically positive margins after resection (Stein et al, 2005), suggesting that radiation in this setting may be an effective salvage therapy. Lawrence and colleagues (Ben-Josef et al, 2005; Robertson et al, 1993, 1997) have reported on the use of conformal radiation combined with intraarterial infusional chemotherapy in patients with unresectable IHCC. This treatment resulted in a median survival of 13.3 months, which was significantly better than historical controls (Ben-Josef et al, 2005), although 30% of patients developed grade 3 or 4 toxicity, and there was one treatment-related death.
Palliation
Up to 90% of patients presenting with IHCC may not be candidates for surgical resection because of patient comorbidity or extent of disease, and these will be managed palliatively (Endo et al, 2008). Unlike extrahepatic cholangiocarcinoma, jaundice is unusual except in patients with centrally placed tumors compressing the hilus; these can be treated by placement of biliary endostents radiologically or endoscopically. Other priorities for these patients are management of capsular pain and ascites and maintenance of adequate nutritional intake.
Alpini G, Prall RT, LaRusso NF. The pathobiology of biliary epithelia. In: Arias, IM, et al. The Liver Biology and Pathobiology. 4th ed. Philadelphia: Williams & Wilkins; 2001:421-425.
Alvaro D. Serum and bile biomarkers for cholangiocarcinoma. Curr Opin Gastroenterol. 2009;25:279-284.
Alvaro D, et al. Serum and biliary insulin-like growth factor 1 and vascular endothelial growth factor in determining the cause of obstructive cholestasis. Ann Int Med. 2007;2:451-459.
Asayama Y, et al. Delayed-phase dynamic CT enhancement as a prognostic factor for mass-forming intrahepatic cholangiocarcinoma. Radiology. 2006;238:150-155.
Ayaru L, et al. Diagnosis of pancreatobiliary malignancy by detection of minichromosome maintenance protein 5 in bile aspirates. Brit J Cancer. 2008;6:1548-1554.
Bach AM, et al. Portal vein evaluation with US: comparison to angiography combined with CT arterial portography. Radiology. 1996;201:149-154.
Bamrungphon W, et al. A new mucin antibody/enzyme-linked lectin-sandwich assay of serum MUC5AC mucin for the diagnosis of cholangiocarcinoma. Cancer Lett. 2007;247:301-308.
Ben-Josef E, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005;23:8739-8747.
Bergquist A, Broome U. Hepatobiliary and extra-hepatic malignancies in primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2001;15:643-656.
Bergquist A, et al. Risk factors and clinical presentation of hepatobiliary carcinoma in patients with primary sclerosing cholangitis. Hepatology. 1998;27:311-316.
Berthiaume EP, Wands J. The molecular pathogenesis of cholangiocarcinoma. Semin Liver Dis. 2004;24:127-137.
Blechacz B, Gores GJ. Cholangiocarcinoma. Clin Liver Dis. 2008;12:131-150.
Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis and treatment. Hepatology. 2008;48:308-321.
Bloom CM, Langer B, Wilson SR. Role of US in the detection, characterization and staging of cholangiocarcinoma. Radiographics. 1999;19:1199-1218.
Bonney GK, et al. Circulating markers of biliary malignancy: opportunities in proteomics? Lancet Oncol. 2008;9:149-158.
Buetow PC, et al. Biliary cystadenoma and cystadenocarcinoma: clinical–imaging–pathologic correlation with emphasis on the importance of ovarian stroma. Radiology. 1995;196:805-810.
Casavilla FA, et al. Hepatic resection and transplantation for peripheral cholangiocarcinoma. J Am Coll Surg. 1997;185:429-436.
Chang PY, et al. Preliminary experience of cetuximab in the treatment of advanced-stage biliary tract cancer. Onkologie. 2010;33:45-47.
Chapman RW. Risk factors for biliary tract carcinogenesis. Ann Oncol. 1999;10(Suppl 4):308-311.
Characharoenwitthaya P, et al. Utility of serum tumour markers, imaging and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48:1106-1117.
Chen CY, et al. Diagnostic role of biliary pancreatic elastase for cholangiocarcinoma in patients with cholestasis. Clin Chim Acta. 2008;390:82-89.
Chen MF, et al. A reappraisal of cholangiocarcinoma in patient with hepatolithiasis. Cancer. 1993;71:2461-2465.
Cheon YK, et al. Diagnostic utility of interleukin-6 (IL-6) for primary bile duct cancer and changes in serum IL-6 levels following photodynamic therapy. Am J Gastroenterol. 2007;102:2164-2170.
Cherqui D, et al. Intrahepatic cholangiocarcinoma: results of aggressive surgical treatment. Arch Surg. 1995;130:1073-1078.
Chow LT, et al. Mucinous cholangiocarcinoma: an unusual complication of hepatolithiasis and recurrent pyogenic cholangitis. Histopathology. 1997;30:491-494.
Chu KM, Fan ST. Intrahepatic cholangiocarcinoma in Hong Kong. J Hepatobilary Pancreat Surg. 1999;6:149-153.
Chu KM, et al. Malignancy associated with hepatolithiasis. Hepatogastroenterology. 1997;44:352-357.
Claessen MM, et al. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009;50:158-164.
Cox H, et al. Well-differentiated intrahepatic cholangiocarcinoma in the setting of biliary papillomatosis: a case report and review of the literature. Canadian J Gastroenterol. 2005;19:731-733.
Cuschieri A, et al. Hepatic cryotherapy for liver tumors: development and clinical evaluation of a high-efficiency insulated multineedle probe system for open and laparoscopic use. Surg Endosc. 1995;9:483-489.
D’Angelica M, et al. The role of staging laparoscopy in hepatobiliary malignancy: prospective analysis of 401 cases. Ann Surg Oncol. 2003;10:183-189.
De Oliveira ML, et al. Cholangiocarcinoma: thirty-one-year experience with 564 cases at a single institution. Ann Surg. 2007;245:755-762.
Donato F, et al. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case control study in Italy. Cancer Causes Control. 2001;12:959-964.
Dubel GJ, Soares GM. Regional infusion–radioembolization. Surg Oncol Clin North Am. 2008;17:957-985.
Edge SB, et al. AJCC Cancer Staging Manual, 7th ed. New York: Springer-Verlag; 2009.
Endo I, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84-96.
Endo K, et al. ERBB-2 overexpression and cyclooxygenase-2 up-regulation in human cholangiocarcinoma and risk conditions. Hepatology. 2002;36:439-450.
Ercolani G, et al. Intrahepatic cholangiocarcinoma: primary liver resection and aggressive multimodal treatment of recurrence significantly prolongs survival. Ann Surg. 2010;252:107-114.
Farrant JM, et al. Natural history and prognostic variables in primary sclerosing cholangitis. Gastroenterology. 1991;100:1710-1717.
Foster JH, Berman MM. Solid liver tumors. Major Probl Clin Surg. 1977;22:1-342.
Fritscher-Ravens A, et al. FDG PET in the diagnosis of hilar cholangiocarcinoma. Nucl Med Commun. 2001;22:1277-1285.
Fujita T. Clinicopathological studies of the resected intahepatic bile duct carcinoma. Jpn J Gastroenterol Hepatol. 1990;23:36-46.
Galluoglu MG, et al. Intraductal growth-type mucin-producing peripheral cholangiocarcinoma associated with biliary papillomatosis. Ann Diagn Pathol. 2007;11:34-38.
Goto N, et al. Intrahepatic cholangiocarcinoma arising 10 years after the excision of congenital extrahepatic biliary dilatation. J Gastroenterol. 2001;36:856-862.
Greene FL, Page DL, Fleming ID, the American Joint Committee on Cancer. Cancer Staging Manual, 6th ed. New York: Springer-Verlag; 2002.
Gusani NJ, et al. Treatment of unresectable cholangiocarcinoma with gemcitabine-based transcatheter arterial chemoembolization (TACE): a single institution experience. J Gastrointestinal Surg. 2008;12:129-137.
Hakamada K, et al. Late development of bile duct cancer after sphincteroplasty: a ten- to twenty-two-year follow-up study. Surgery. 1997;121:488-492.
Hann LE, et al. Cholangiocarcinoma at the hepatic hilus: sonographic findings. Am J Roentgenol. 1997;168:985-989.
Harewood G. Endoscopic tissue diagnosis of cholangiocarcinoma. Curr Opin Gastroenterol. 2008;24:627-630.
Hasweel-Elkins MR, et al. Cross-sectional study of Opisthorchis viverrini infection and cholangiocarcinoma in communities within a high-risk area in northeast Thailand. Int J Cancer. 2008;59:505-509.
Herber S, et al. Transarterial chemoembolization (TACE) for inoperable intrahepatic cholangiocarcinoma. Cardiovasc Intervent Radiol. 2007;30:1156-1165.
Hewitt PM, et al. Choledochal cysts in adults. Brit J Surg. 1995;82:382-385.
Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008;13:415-423.
Iglehart JK. The new era of medical imaging-progress and pitfalls. N Engl J Med. 2006;354:2822-2828.
Isaji S, et al. Clinicopathological features and outcome of hepatic resection for intrahepatic cholangiocarcinoma in Japan. J Hepatobilary Pancreat Surg. 1999;6:108-116.
Ishimura N, Bronk SF, Gores GJ. Inducible nitric oxide synthase upregulates cyclooxygenase-2 in mouse cholangiocytes promoting cell growth. Am J Physiol Gastrointest Liver Physiol. 2004;287:G88-G95.
Ismail T, et al. Primary hepatic malignancy: the role of liver transplantation. Brit J Surg. 1990;77:983-988.
Jaiswal M, et al. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60:184-190.
Jan YY, et al. Clinicopathological factors predicting long-term overall survival after hepatectomy for peripheral cholangiocarcinoma. World J Surg. 2005;29:894-898.
Jang KT, et al. Intraductal papillary neoplasm of the bile duct associated with Clinorchis senesis infection. Virchows Arch. 2008;453:589-598.
Jarnagin WR, et al. Papillary phenotype confers improved survival after resection of hilar cholangiocarcinoma. Ann Surg. 2005;241:703-712.
Jarnagin WR, et al. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol. 2009;20:1589-1595.
Kawarada Y, Yamagiwa K, Das PC. Analysis of the relationship between clinicopathological factors and survival time in intrahepatic cholangiocarcinoma. Am J Surg. 2002;183:679-685.
Kaya M, et al. Treatment of cholangiocarcinoma complicating primary sclerosing cholangitis: the Mayo Clinic experience. Am J Gastroenterol. 2001;96:1164-1169.
Khan SA, Toledano MB, Taylor-Robinson S. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB. 2008;10:77-82.
Khan SA, et al. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806-813.
Kim HJ, et al. Intrahepatic cholangiocarcinoma in Korea. J Hepatobilary Pancreat Surg. 1999;6:142-148.
Kim HJ, et al. Mucin-hypersecreting bile duct tumor characterized by striking homology with an intraductal papillary mucinous tumor (IPMT) of the pancreas. Endoscopy. 2000;32:389-393.
Kim JH, et al. Transcatheter arterial chemoembolization or chemoinfusion for unresectable intrahepatic cholangiocarcinoma: clinical efficacy and factors influencing outcomes. Cancer. 2008;113:1614-1622.
Kim TK, et al. Peripheral cholangiocarcinoma of the liver: two-phase CT findings. Radiology. 2002;204:539-543.
Kim YH, Kang KJ, Kwon JH. Four cases of hepatic fascioliasis mimicking cholangiocarcinoma. Korean J Hepatol. 2005;11:169-175.
Kim YT, et al. Factors predicting concurrent cholangiocarcinomas associated with hepatolithiasis. Hepatogastroenterology. 2003;50:8-12.
Kubo S, et al. Hepatolithiasis associated with cholangiocarcinoma. World J Surg. 1995;19:637-641.
Lai GH, et al. erbB-2/neu transformed rat cholangiocytes recapitulate key cellular and molecular features of human bile duct cancer. Gastroenterology. 2005;123:2047-2057.
Lang H, et al. Extended hepatectomy for intrahepatic cholagiocellular carcinoma (ICC). When is it worthwhile? Single center experience with 27 resections in 50 patients over a 5-year period. Ann Surg. 2005;241:134-143.
Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655-1667.
Lempinen M, et al. Enhanced detection of cholangiocarcinoma with serum trypsinogen-2 in patients with severe bile duct strictures. J Hepatol. 2007;47:677-683.
Lesurtel M, et al. Intrahepatic cholangiocarcinoma and hepatolithiasis: an unusual association in Western countries. Eur J Gastrenterol Hepatol. 2002;14:1025-1027.
Lieser MJ, et al. Surgical management of intrahepatic cholangiocarcinoma. J Hepatobilary Pancreat Surg. 1998;5:41-47.
Lipsett PA, et al. Choledochal cyst disease: a changing pattern of presentation. Ann Surg. 1994;220:644-652.
Lipshutz GS, Brennan TV, Warren RS. Thorotrast-induced liver neoplasia: a collective review. J Am Coll Surg. 2002;195:713-718.
Liu L, et al. Serum levels of variants of transthyretin downregulation in cholangiocarcinoma. J Cell Biochem. 2009;104:745-755.
Liver Cancer Study Group of Japan. Primary liver cancer in Japan: clinicopathologic features and results of surgical treatment. Ann Surg. 1990;211:277-287.
Liver Cancer Study Group of Japan. General rules for the clinical and pathological study of primary liver cancer, 2nd ed. Tokyo: Kanehara; 2003.
Lowenfels AB, Norman J. Isoniazid and bile duct cancer. JAMA. 1978;240:434-435.
Lubner SJ, et al. Report of a multicenter phase II trial testing a combination of biweekly bevicizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II consortium study. J Clin Oncol. 2010;28:3491-3497.
Madariaga JR, et al. Liver resection for hilar and peripheral cholangiocarcinoma. Ann Surg. 1998;227:70-79.
Malhi H, Gores GJ. Review article: the modern diagnosis and therapy of cholangiocarcinoma. Aliment Pharmacol Ther. 2006;23:1287-1296.
Manfredi R, et al. Magnetic resonance imaging of cholangiocarcinoma. Semin Liver Dis. 2004;24:155-164.
Marcos L, Terashima A, Gotuzzo E. Update on hepatobiliary flukes: fascioliasis, opisthorchiasis and clinorchiasis. Curr Opin Infect Dis. 2008;21:523-530.
Martin RC, Jarnagin WR. Peripheral/intrahepatic cholangiocarcinoma: current management. Minerva Chir. 2003;58:469-478.
Massachusetts General Hospital. Case records of the Massachusetts General Hospital. N Engl J Med. 1981;304:893-899.
Metcalfe MS, et al. Useless and dangerous: fine needle aspiration of hepatic colorectal metastases. Br Med J. 2004;328:507-508.
Mitacek EJ, et al. Volatile nitrosamines and tobacco-specific nitrosamines in the smoke of Thai cigarettes: a risk factor for lung cancer and a suspected risk factor for liver cancer. Carcinogenesis. 1999;20:133-137.
Morris-Stiff G, et al. Cholangiocarcinoma complicating primary sclerosing cholangitis: a 24-year experience. Dig Surg. 2008;25:126-132.
Nakajima T, et al. A histopathologic study of 102 cases of intrahepatic cholangiocarcinoma. Human Pathol. 1988;19:112-134.
Nakanuma Y, et al. Are hepatolithiasis and cholangiocarcinoma aetiologically related? A morphological study of 12 cases of hepatolithiasis associated with cholangiocarcinoma. Virchows Arch A Pathol Anat Histopathol. 1985;406:45-58.
Nakeeb A, et al. Cholangiocarcinoma: a spectrum of intahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463-475.
Nathan H, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16:14-22.
Nozaki Y, et al. Reconsideration of the lymph node metastases pattern (N factor) from intrahepatic cholangiocarcinoma using the International Union Against Cancer TNM staging system for primary liver cancer. Cancer. 1998;83:1923-1929.
O’Grady JG, et al. Liver transplantation for malignant disease. Ann Surg. 1988;207:373-376.
Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005;23:4742-4754.
Ohashi K, et al. Clinical characteristics and proliferating activity of intrahepatic cholangiocarcinoma. J Gastroenterol Hepatol. 1994;9:442-446.
Ohlsson B, et al. Percutaneous fine-needle aspiration cytology in the diagnosis and management of liver tumours. Brit J Surg. 1994;89:757-762.
Ohtsuka M, et al. Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Brit J Surg. 2002;89:1525-1531.
Ohtsuka T, et al. Carcinoma arising in a choledochoele. Endoscopy. 2001;33:614-619.
Okabayashi T, et al. A new staging system for mass-forming intrahepatic cholangiocarcinoma: analysis of preoperative and postoperative variables. Cancer. 2001;92:2374-2383.
Olnes MJ, Erlich RA. review and update on cholangiocarcinoma. Oncology. 2004;66:167-179.
Ong SL, et al. Elevation of carbohydrate antigen 19.9 in benign hepatobiliary conditions and its correlation with serum bilirubin. Dig Dis Sci. 2008;53:3213-3217.
Park J, et al. Inhibition of interleukin 6–mediated mitogen-activated protein kinase activation attenuates growth of cholangiocarcinoma cell line. Hepatology. 1999;30:1128-1133.
Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353-1357.
Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:1-5.
Patel T, Singh P. Cholangiocarcinoma: emerging approaches to a challenging cancer. Curr Opin Gastroenterol. 2007;23:317-323.
Petrowsky H, et al. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006;45:43-50.
Pichlmayr R, et al. Surgical treatment of cholangiocellular carcinoma. World J Surg. 1995;19:83-88.
Pitt HA, et al. Malignancies of the biliary tree. Current Probl Surg. 1995;32:1-90.
Rai R, Manas D, Rose J. Radiofrequency ablation of recurrent cholangiocarcinoma after orthotopic liver transplantation: a case report. World J Gastroenterol. 2005;11:612-613.
Rashid A. Cellular and molecular biology of biliary tract cancers. Surg Clin North Am. 2002;11:995-1009.
Roayaie S, Guarrera JV, Ye MQ. Aggressive surgical treatment of intrahepatic cholangiocarcinoma: predictors of outcomes. J Am Coll Surg. 1998;187:365-372.
Robertson JM, et al. Treatment of primary hepatobiliary cancers with conformal radiation therapy and regional chemotherapy. J Clin Oncol. 1993;11:1286-1293.
Robertson JM, et al. A phase I trial of hepatic arterial bromodeoxyuridine and conformal radiation therapy for patients with primary hepatobiliary cancers or colorectal liver metastases. Int J Radiat Oncol Biol Phys. 1997;39:1087-1092.
Robles R, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg. 2004;239:265-271.
Rosen CB, et al. Cholangiocarcinoma complicating primary sclerosing cholangitis. Ann Surg. 1991;213:21-25.
Rubel LR, Ishak KG. Thorotrast-associated cholangiocarcinoma: an epidemiologic and clinicopathologic study. Cancer. 1982;50:1408-1415.
Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin. 2004;56:69-83.
Schulick RD. Primary sclerosing cholangitis: detection of cancers in strictures. J Gastrointestinal Surg. 2008;12:420-422.
Schwartz J, et al. Liver transplantation for cholangiocarcinoma. Transplantation. 2009;88:295-298.
Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115-125.
Shaib Y, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472-477.
Shaib Y, et al. Risk factors for intrahepatic cholangiocarcinoma in the United States: a case control study. Gastroenterology. 2005;128:620-626.
Sheen AJ, Poston GJ, Sherlock DJ. Cryotherapeutic ablation of liver tumours. Brit J Surg. 2002;89:1396-1401.
Shirabe K, et al. Intrahepatic cholangiocarcinoma: its mode of spreading and therapeutic modalities. Surgery. 2002;131(1 Pt 2):S159-S164.
Slattery JM, Sahani DV. What is the current state-of-the-art imaging for detection and staging of cholangiocarcinoma? Oncologist. 2006;11:913-922.
Smith RA, et al. Prognosis of resected ampullary adenocarcinoma by preoperative serum CA19-9 levels and platelet-lymphocyte ratio. J Gastrointestinal Surg. 2008;12:1422-1428.
Sorensen HT, et al. Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology. 1998;28:921-925.
Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol. 2008;24:349-356.
Sripa B, et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:1148-1155.
Stein DE, et al. Positive microscopic margins alter outcome in lymph node negative cholangiocarcinoma when resection is combined with adjuvant radiotherapy. Am J Clin Oncol. 2005;28:21-23.
Steiner PE, Higginson J. Cholangiocellular carcinoma of the liver. Cancer. 1959;12:753-759.
Su CH, et al. Hepatolithiasis associated with cholangiocarcinoma. Brit J Surg. 1997;84:969-973.
Sudo Y, et al. Oncocytic biliary cystadenocarcinomais a form of intraductal oncocytic papillary neoplasm of the liver. Mod Pathol. 2001;14:1304-1309.
Suh KS, et al. Clinicopathologic features of the intraductal growth type of peripheral cholangiocarcinoma. Hepatology. 2000;31:12-17.
Sweeney CJ, et al. A phase I study of sunitinib plus capecitabine in patients with advanced solid tumors. J Clin Oncol. 2010;28:4513-4520.
Szendroi M, Nemeth L, Vajta G. Asbestos bodies in a bile duct cancer after occupational exposure. Environ Res. 1983;30:270-280.
Taniai M, et al. p16INK4a promotor mutations are frequent in primary sclerosing cholangitis (PSC) and PSC-associated cholangiocarcinoma. Gastroenterology. 2002;123:1090-1098.
Uenishi T, et al. Serum cytokeratin 19 fragment (CYFRA21-1) as a prognostic factor in intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2008;15:583-589.
Valle JW, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281.
Vauthey JN, Blumgart LH. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis. 1994;14:109-114.
Watanapa P. Cholangiocarcinoma in patients with opisthorchiasis. Brit J Surg. 1996;83:1062-1064.
Watanapa P, Watanapa WB. Liver fluke-associated cholangiocarcinoma. Brit J Surg. 2002;89:962-970.
Weber SM, et al. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193:384-391.
Wigmore S, et al. Virtual hepatic resection using three-dimensional reconstruction of helical computed tomography angioportograms. Ann Surg. 2001;233:221-226.
Wise C, et al. Mechanisms of biliary carcinogenesis and growth. World J Gastroenterol. 2008;14:2986-2989.
Wolf H, Garcia JA, Bossen EA. Oncocytic differentiation in intrahepatic biliary cystadenocarcinoma. Mod Pathol. 1992;5:665-668.
Wong O, et al. An industry-wide epidemiologic study of vinyl chloride workers. Am J Ind Med. 1991;20:317-334.
Yachimski P, Pratt DS. Cholangiocarcinoma: natural history, treatment and strategies for surveillance in high-risk patients. J Clin Gastroenterol. 2008;42:178-190.
Yamamoto M, Takasaki K, Yoshikawa T. Extended resection for intrahepatic cholangiocarcinoma in Japan. J Hepatobilary Pancreat Surg. 1999;6:117-121.
Yamamoto M, et al. Does gross appearance indicate prognosis in intrahepatic cholangiocarcinoma? J Surg Oncol. 1998;69:162-167.
Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobilary Pancreat Surg. 2003;10:288-291.
Yen S, Hsieh CC, MacMahon B. Extrahepatic bile duct cancer and smoking, beverage consumption, past medical history and oral contraceptive use. Cancer. 1987;59:2112-2116.
Zgodzinzki W, Espat NJ. Radiofrequency ablation for incidentally identified primary intrahepatic cholangiocarcinoma. World J Gastroenterol. 2005;11:5239-5240.