Chapter 50D Cancer of the bile ducts
Interventional techniques in hilar and intrahepatic biliary structures
Overview
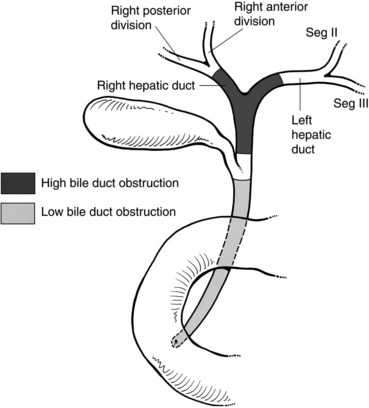
Malignant disease resulting in high bile duct obstruction (Fig. 50D.1)—that is, obstruction above the junction with the cystic duct—is not uncommon. Although frequently related to primary tumors of the biliary tree (see Chapters 49, 50A, and 50B), hilar obstruction, and even intraductal tumor, can be seen with other common malignancies such as breast, pancreatic, and colorectal cancers. Significant technical progress has occurred in both endoscopic and percutaneous biliary drainage, allowing safer palliative treatment of patients with such obstructions. Because these patients are often asymptomatic at presentation, the goals of treatment should be clearly defined prior to committing the patient to even a minimally invasive procedure. First and foremost, whether the patient is a surgical candidate should be determined. With the exception of patients who are clearly in a palliative situation, we prefer to discuss these patients in a multidisciplinary group with hepatobiliary surgeons, interventional radiologists, oncologists, and gastroenterologists to outline a plan of treatment. A thorough understanding of this plan and of the patient’s prognosis facilitates the development of a strategy for drainage. Accepted indications for palliative biliary drainage in these patients include intractable pruritus, cholangitis, the need to restore liver function to allow for administration of chemotherapeutic agents with biliary metabolism/excretion, access for intraluminal brachytherapy or choledochoscopy, and diversion for bile leak. Given the availability of high-quality magnetic resonance cholangiopancreatography (MRCP), direct cholangiography as a diagnostic tool is rarely warranted (see Chapters 17 and 18; Choi et al, 2008; Park et al, 2008).
Indications For Biliary Drainage
Neither hyperbilirubinemia alone nor the computed tomographic (CT) or ultrasound finding of dilated bile ducts is an indication for biliary drainage. Pruritus, cholangitis, and the need to lower the bilirubin to administer certain chemotherapeutic agents, on the other hand, are all accepted indications for biliary drainage. Patients who have undergone biliary-enteric bypass as part of curative resection for a benign or malignant lesion may develop postoperative bile leaks that require drainage for diversion. In some cases, access to the biliary tree may be undertaken as a method of delivering local treatment for primary bile duct cancer, such as brachytherapy or photodynamic therapy. Many physicians have the impression that patients feel better and have improved performance status after relief of jaundice; however, this has not been confirmed in clinical studies. Indeed, in a prospective trial, we have shown that in patients with malignant biliary obstruction, percutaneous drainage does not significantly improve quality of life (QOL), except for improving pruritus (Robson et al, 2007). Controversy remains in regard to the role of biliary drainage prior to surgery (Johnson & Ahrendt, 2006; Mezhir et al, 2009; Wang et al, 2008).
Endoscopic Versus Percutaneous Drainage (See Chapter 18, Chapter 27, Chapter 28 )
Patients with low bile duct obstruction are typically treated endoscopically. Patients with high bile duct obstruction, particularly when the obstruction extends above the hilus, have traditionally been treated percutaneously. This is because the success rate of percutaneous drainage has been higher, and the complication rate lower, when compared with endoscopic methods (Rerknimitr et al, 2004). This perspective is evolving as endoscope technology becomes more advanced, and endoscopists become better trained and more experienced in wire-guided procedures and have better guidewires and stents with which to work.
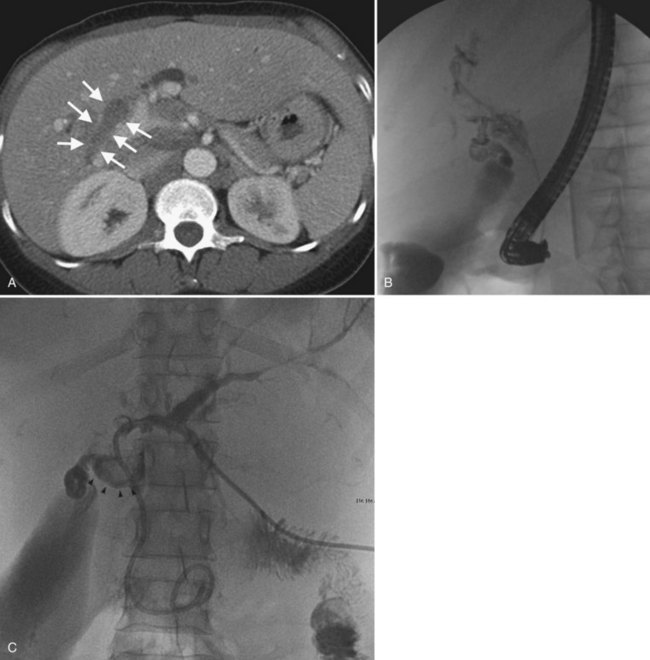
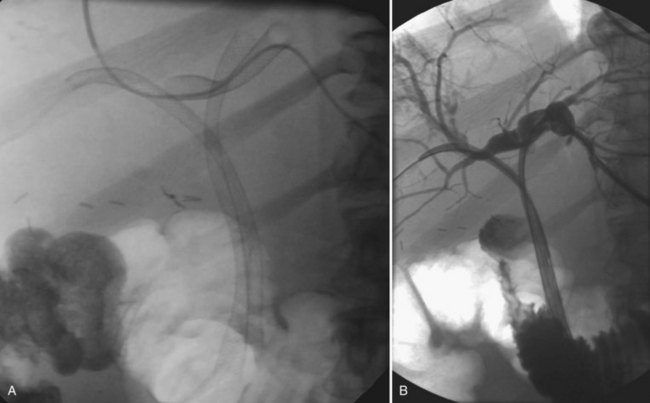
The two biggest drawbacks to endoscopic drainage for high bile duct obstruction have been the lack of ability to reliably target a specific area of the liver for drainage and the risk of contamination, by retrograde injection of contrast, of parts of the biliary tree that will not be drained. It is not uncommon to see a patient who is thought to have undergone successful endoscopic stenting of the right and left liver only to find, upon repeat imaging, that is not the case at all (Fig. 50D.2). Despite this, endoscopic management is indicated in some cases of high bile duct obstruction. This is particularly so when the risk of the patient having a permanent exteriorized catheter is high or when a patient has a higher risk of contaminating the entire biliary tree if approached percutaneously.
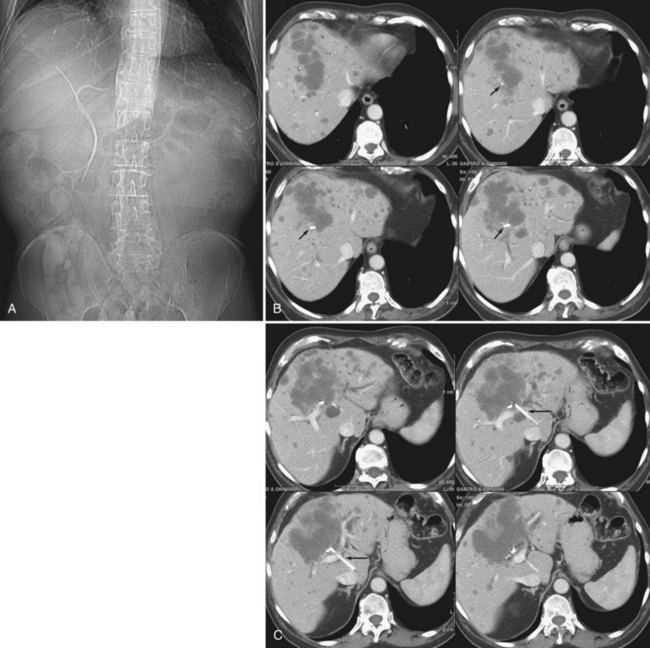
One exciting new endoscopic approach is the placement of a transgastric segment III stent using endoscopic ultrasound. This procedure is considered when a patient is best treated by draining the left lateral segment, and the percutaneous approach is technically difficult, or where the risk of contaminating the right-sided bile ducts is thought to be high with or without being able to cross the obstruction. This is particulary indicated when draining the right liver would be of no clinical benefit, either because of replacement of right liver by tumor or occlusion of the right portal vein (Fig. 50D.3). When technically feasible, this procedure can be gratifying for the patient, with relief of pruritus and lowering of bilirubin without the added burden of a percutaneous catheter. Endoscopic drainage is almost never indicated in patients with papillary intraductal tumor because it can easily grow into a metallic stent and result in early stent occlusion. Intraductal tumor can be seen from cancers other than papillary cholangiocarcinoma. When intraductal tumor occurs in the setting of metastatic colorectal cancer, it is usually the direct extension of a parenchymal metastasis into the duct. Patients with intraductal tumor often require permanent indwelling catheter drainage. The indwelling multiple sidehole catheter allows bile to drain around the intraductal tumor, and it can be changed every 10 to 12 weeks to maintain catheter patency (Fig. 50D.4). Having a realistic idea of what is feasible endoscopically and percutaneously is very important when making the initial decision regarding who will treat the patient. This depends on a thorough understanding of the goal of treatment as well as knowledge of the skill level of the interventional radiologists and endoscopists available to care for the patient.
Preprocedure Preparation
Imaging
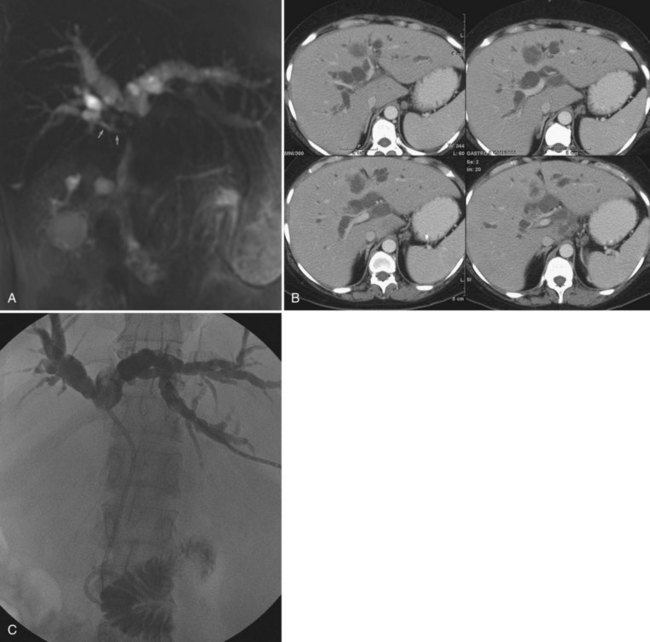
The importance of excellent preprocedure imaging in patients with bile duct obstruction cannot be overemphasized. This imaging should include at a minimum a contrast-enhanced CT scan of the abdomen. Ultrasound is often used to establish the presence of dilated bile ducts, identify the level of obstruction, evaluate portal vein patency, and demonstrate intraductal tumor, but ultrasound is not adequate for drainage planning. Although MRCP provides an excellent three-dimensional (3D) rendering of the obstructed bile ducts and is excellent at delineating intraductal abnormalities and depicting isolated bile duct (Fig. 50D.5), it excludes certain things that can serve as targeting aides at the time of drainage, such as surgical clips, dystrophic calcifications, and bone landmarks. CT scans performed on multislice scanners in a picture archiving and communication system (PACS) environment allow the development of a 3D understanding of the anatomy while identifying relevant landmarks that can be used for targeting, and it also demonstrates other structures that should be avoided, such as liver lesions and bowel. Of course, as with MRI, one may also identify ascites, hepatic atrophy, and portal vein occlusion or encasement. All of this information is important in making prognostic assessments and in determining the best approach for drainage.
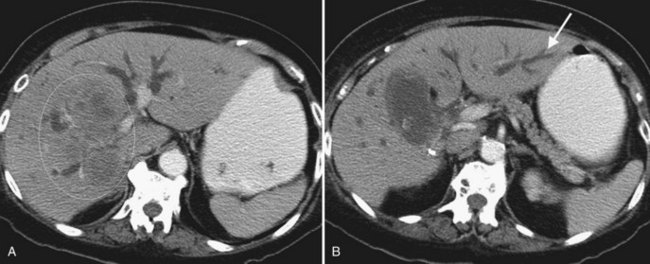
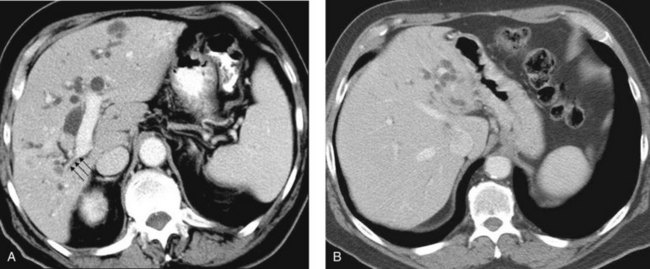
When planning drainage in patients with high bile duct obstruction, it is important to determine the amount of functional parenchyma that can be drained. Functional parenchyma is the part of the liver that is not replaced by tumor, and it has an intact portal venous supply. If 75% of the hepatic parenchyma is replaced by tumor, even if it were possible to drain the entire biliary tree, it is unlikely that normal hepatic function or normalization of serum bilirubin level would be restored. The portal vein provides the trophic blood supply to the liver, and occlusion results in atrophy of the affected segment or segments, particularly when the ipsilateral bile duct is also occluded (see Chapter 5; Hann et al, 1996). Atrophy may be recognized by the diminutive size of the involved part of the liver, accompanied by crowding of the bile ducts (Fig. 50D.6; see also Chapter 50B and Figs. 50B.6 and 50B.7). When portal vein occlusion causes atrophy, drainage of the atrophied liver does not improve liver function. In addition, it is frequently difficult to cross out of the obstructed/atrophied portion of the liver. The end result is an external drainage catheter that provides no clinical benefit to the patient but degrades QOL.
Central tumors frequently obstruct the right and left biliary tree, isolating them from one another. Obstruction may extend even higher, isolating the ductal system at the sectoral, segmental, or subsegmental level. When drainage is undertaken for relief of pruritus, draining even a segment of the liver may result in relief of symptoms (Abraham et al, 2002; Van Laethem et al, 2003). When isolation is present, and drainage is undertaken to lower the serum bilirubin, it is important to estimate how much functional parenchyma can be drained with one catheter. Functional liver parenchyma is defined as hepatic parenchyma with intact portal venous inflow and hepatic venous drainage. Analogous to a surgeon resecting no more than 75% of the liver to ensure an adequate liver remnant, it seems that at least 30% of functional hepatic parenchyma must be drained to improve liver function for chemotherapy, returning the serum bilirubin to normal or near-normal levels. In some cases, this goal may be impossible to achieve, unless more than one drainage catheter or stent is placed. Recognition of this allows for informing both the patient and referring physician about the potential need for more than one catheter or stent.
Cholangitis is rarely the primary indication for drainage in patients with malignant bile duct obstruction, except in patients who have undergone instrumentation and in those who have reason to have a contaminated biliary tree by virtue of previous biliary-enteric bypass, cross ampullary stenting, or sphincterotomy (Ozden et al, 2005). When draining a hemiliver or a segment, either percutaneously or endoscopically, it is possible to contaminate functionally “isolated” parts of the biliary tree. In this case, subsequent cholangitis may drive the placement of additional catheters. Widespread contamination of a severely isolated biliary tree with drainage of only part of the obstructed system can result in chronic recurrent cholangitis. Whether induced by endoscopic or percutaneous means, this situation can be difficult to manage and should be anticipated and avoided if possible.
Antibiotics
Preprocedure antibiotic prophylaxis is given to all patients undergoing biliary drainage, even if the patient does not have an elevated white blood cell count or fever, as bactibilia is present in 30% to 40% of patients without these signs (see Chapter 11; Brody et al, 1998). Many patients become bacteremic during the procedure, even in the absence of preintervention signs or symptoms of infection. Generally, patients receive broad coverage with an agent such as ticarcillin-clavulanate or ceftriaxone. When cholangitis is not present, administration of intravenous antibiotics beyond 24 hours is not warranted. Patients with a history of cholangitis or sepsis or those presumed to have a contaminated bilary tree related to transampullary stenting, biliary-enteric bypass, sphincterotomy, or recent instrumentation should receive prophylactic coverage with an agent such as piperacillin-tazobactam (Zosyn), which is excreted into the bile. Appropriate postprocedure coverage should be determined based on bile cultures obtained at the time of drainage.
Psychological Preparation
Even in the presence of segmental isolation, pruritus often resolves following biliary drainage. Unfortunately, it is not always possible to lower the serum bilirubin, particularly to levels that would allow for the administration of certain chemotherapeutic agents. In certain situations, two or more biliary drainage catheters may be placed without a significant decrease in bilirubin; sometimes, the bilirubin may even increase. It is possible that a single biliary drainage catheter may drain one portion of the liver well, while converting near-complete obstructions in other areas to complete obstructions (Fig. 50D.7). It also is conceivable that intervening cholangitis or progression of disease may play a role. Patients who have realistic expectations and some understanding of these issues before the initial biliary drainage procedure are less likely to become distressed if multiple procedures are required, or if the ultimate outcome is less than satisfactory.
Intraprocedural Issues
Approach
The direct approach from the right is sometimes complicated, when the site of puncture is the right posterior duct, and that duct enters the left hepatic duct. This anatomic variant is often evident on preprocedure imaging studies (see Chapter 1B); it occurs in 20% of patients and may add a 180-degree turn to the approach to the hepatic hilum, making it more difficult to traverse the obstruction. On the other hand, when the obstruction extends to the left-right confluence but no higher, approach from the posterior right duct will drain the right posterior sector as well as the entire left liver. The main disadvantages to a right-sided approach are the discomfort associated with intercostal catheters, leaking of ascites around a more gravity-dependent right-sided catheter, and the fact that tumors are more likely to extend to the secondary confluence on the right.
A left-sided approach to the biliary tree is preferred in several instances. When an obstructing tumor extends above the biliary confluence, it may isolate the right and left bile ducts and may extend toward a secondary confluence or higher. As alluded to above, the right hepatic duct is typically shorter than the left (see Chapter 1B); as a result, tumor is more likely to extend to and isolate the right anterior and posterior sectoral ducts, while the left medial and lateral sectors continue to communicate. In such cases it may be possible to drain more functional hepatic parenchyma by placing a single catheter or stent on the left. Patients with ascites are less likely to leak around a left anterior catheter, so a left approach is preferred when possible. When atrophy or compromise of the portal vein is present, the patient will always derive more benefit from drainage of the contralateral functional liver, as previously discussed.
Technical Aids
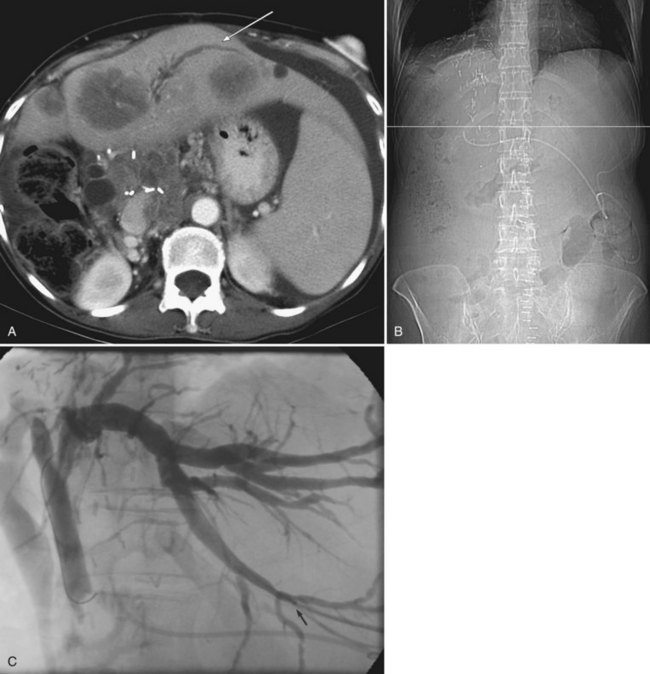
The most significant technical development to facilitate biliary drainage and other fluoroscopically guided procedures in recent years has been the advent of multidetector CT scans (MDCT), particularly when used within a PACS environment. The ability to scan the liver in a single breath hold and then cine through the images facilitates identification of the level of obstruction and the likelihood of isolation. It also enables the formulation of a three-dimensional mental image of the biliary tree much more easily than looking at hard copy scans, and understanding the biliary anatomy facilitates a successful, uncomplicated procedure. It is frequently possible to anticipate normal variants of bile duct anatomy, which can have a significant impact on preprocedure planning (see Chapter 1B). In addition, most PACS workstations allow cross-reference to the axial images (Fig. 50D.8A) with the “scout” image (Fig. 50D.8B), which is a simulated frontal radiograph used for programming the extent of the scan. In this way, targeting of a specific region, or even a specific duct, is made much easier—and the final catheter resides within the planned segment of the liver (Fig. 50D.8C). Although this degree of sophistication is unnecessary for obstruction below the hepatic hilum, MDCT facilitates drainage in patients with complex high bile duct obstruction such that it is sometimes worth performing MDCT, even when the patient has had a recent hard copy CT scan from the referring institution.
Drainage Catheter Versus Primary Stent Placement
The suitability of a patient for primary stent placement is determined at the time of biliary drainage based on the indication for the drainage, evidence of infected bile, presence of blood or tumor within the biliary tree, and cholangiographic findings. The goal of biliary drainage is to solve the patient’s clinical problem by placing as few catheters or stents as possible, intending that the bile should drain internally when the intervention is complete. Patients with obstruction below the common hepatic duct without cholangitis may be stented primarily, at the time of the initial drainage procedure, provided significant blood is not present within the biliary tree. Blood clot within the intrahepatic bile ducts impairs drainage and can cause stent obstruction that may result in bile leaking into the peritoneal cavity from the site of duct puncture. When hemobilia occurs (see Chapter 105), a drainage catheter is placed until the bile clears, at which time the patient can be easily stented. Patients undergoing drainage for cholangitis cannot be stented primarily in most cases. Catheter manipulation in this group of patients is kept to a minimum to diminish the risk of procedure-related sepsis. Once the biliary tree is drained and cholangitis resolves, a stent can be placed.
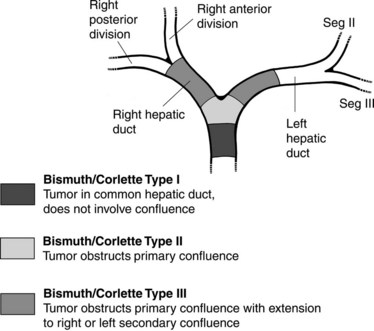
Having dispensed with the straightforward decisions regarding patients who clearly can or cannot be stented, the more difficult situations that occur in patients with high bile duct obstruction must be addressed. In 1975, Bismuth and Corlette (1975) classified obstruction of the hepatic confluence as types I through IV (Fig. 50D.9; see Chapter 42A). Predictions about the level of obstruction and degree of isolation of the biliary tree can be made based on the preprocedure imaging studies. These predictions are not always accurate, however, and it is sometimes necessary to modify the stenting approach based on cholangiographic findings; in rare instances, it may be necessary to abandon the procedure altogether. Patients with type I obstruction can have the entire liver drained with one catheter or stent, because right and left ducts communicate freely. Barring one of the previously mentioned contraindications, these patients have a primary stent placed.
Patients with type II obstruction cannot be completely drained with one catheter, although it is sometimes possible to opacify ducts in both sides of the liver. Some suggest that survival is better when both sides of the liver are drained (Chang et al, 1998), although even in Bismuth type II and III hilar obstructions, Inal and colleagues (2003a) saw no significant difference in clinical response to treatment or stent patency rate with unilobar versus bilobar drainage.
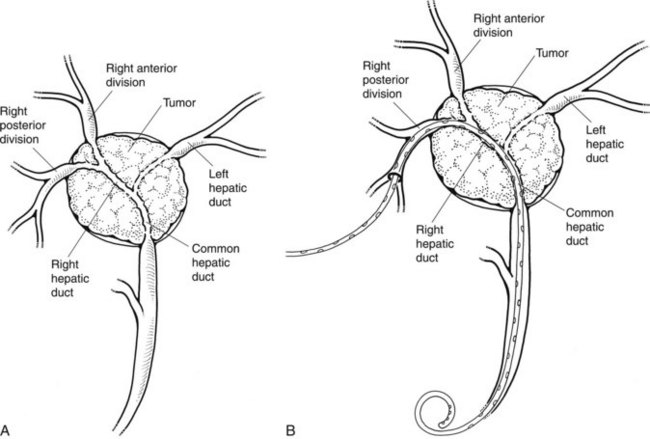
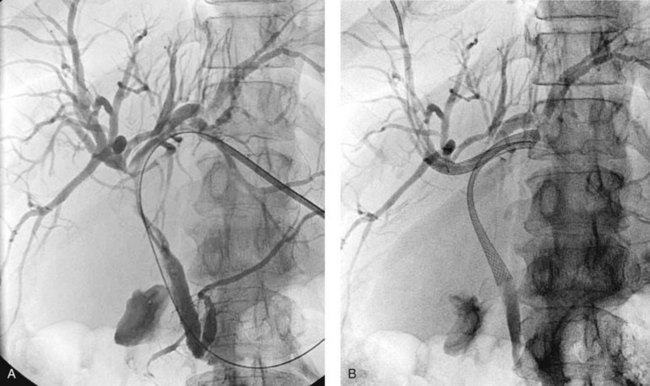
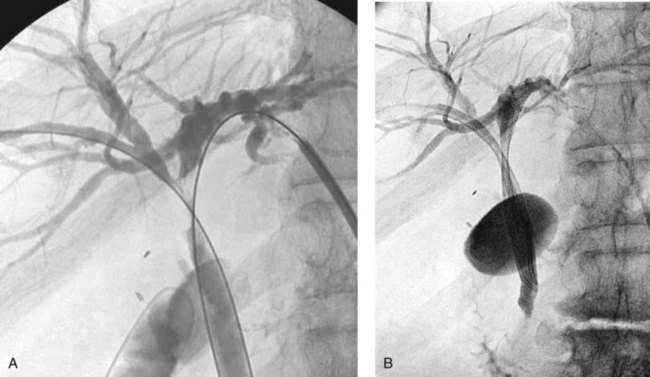
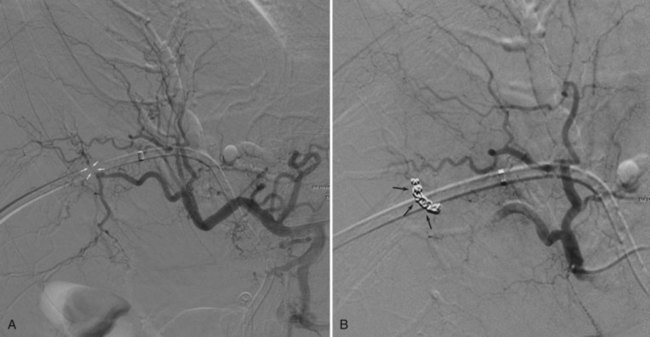
When contrast material enters the obstructed side opposite the puncture, it usually does not drain effectively, and that side is considered contaminated. These patients can be stented from the ipsilateral approach by inserting one stent from the ipsilateral side to the contralateral side and a second stent from the ipsilateral side into the common bile duct or duodenum (Fig. 50D.10), in a T-shaped configuration. The other option is to puncture the contralateral side and place side-by-side stents (Fig. 50D.11), in a Y-shaped configuration. In either case, we prefer to stent into the common bile duct and not into the duodenum if possible so as to preserve function of the sphincter of Oddi. Barring any contraindication, this could be done primarily at the time of initial drainage, as advocated by Inal and colleagues (2003b). These authors found that whether stents were placed primarily or at a second sitting, patients without cholangitis at the time of stenting did not develop cholangitis subsequently, and in all patients with a type I or II obstruction, the serum bilirubin decreased to 2 mg/dL or less. One advantage of the more anatomic Y-shaped configuration stent placement is that if stent occlusion occurs, both stents are approachable endoscopically. In addition, the patency rate of the Y-shaped configuration may be better in patients with type IV obstruction (Inal et al, 2003a). Specially constructed stents have been developed that allow placement of one stent through an opening in the midbody of the other (Kim et al, 2004).
Sometimes the contralateral biliary tree is not opacified, precluding knowledge of the type of obstruction, or the ipsilateral obstruction is a type III obstruction or worse. In this case, and in the absence of some other contraindication, a patient being drained for pruritus alone might have a primary stent placed, because only a small portion of the liver need be drained to alleviate pruritus. If the patient is being drained in an attempt to lower the bilirubin level for chemotherapy, a stent should be considered, if it is thought that 30% or more of the liver will be drained. We use this as a rule of thumb, despite the fact that in a series of 149 patients drained at Memorial Sloan-Kettering Cancer Center, we found only a marginally significant difference in the number of patients attaining a bilirubin less than 2 mg/dL based on the estimated volume of liver drained. In this analysis, 6 (29%) of 21 patients with less than a third of the liver drained attained a bilirubin level below 2 mg/dL, whereas this was achieved in 65 (51%) of 128 patients with more than one third of the liver drained (P = .06; Ulrich et al, 2009). Following stent placement, if the bilirubin fails to fall to the desired level, a second drainage procedure can be performed. Given the QOL issues for the patient, as well as the risk of contaminating an undrained part of the liver by having an externalized catheter in place, the slight inconvenience of working through a previously placed stent seems warranted.
When the initial drainage has been on the right side, and the tumor has extended up the right hepatic duct so as to isolate the anterior and posterior divisions from each other and from the left hepatic duct, side-by-side self-expanding metallic stents can be placed on the right to drain both the anterior and posterior ducts. Alternatively, when the left side of the liver is functional, a left drainage can be performed as the next step; then one stent can be placed from the left, and another can be placed from the right. If instead of a stent, a second catheter has been placed on the left, and the serum bilirubin still does not decrease to an appropriate level or cholangitis develops, stents can be placed from the right to the common bile duct, from the left to the common bile duct, and from the left to the residual undrained right system (Fig. 50D.12). Although a significant difference in patency is reported when more than one stent is placed in a noncoaxial fashion (Maybody et al, 2009), the mean patency is almost 6 months, which justifies stent placement. Even when one part of the liver is not functional, drainage may be necessary to eliminate a source of ongoing cholangitis.
The concepts of biliary drainage are simple, but when high bile duct obstruction is present, the planning is complex, and execution can be difficult. The patient must have enough of the liver drained to be free of cholangitis and pruritus and to effect a reduction in serum bilirubin to receive chemotherapy if indicated. Given the fact that no difference in stent patency is reported if the stent is inserted for proximal or distal obstruction, a significant difference in patency is seen when more than one stent is placed, and lower complication rates are reported when stents are placed primarily, primary stent placement should be considered whenever possible (Inal et al, 2003a, 2003c; Maybody et al, 2009). Additional stents can be placed later if necessary. Patients with Bismuth type I or II obstructions may be treated with a single stent, whereas patients with type III or IV obstructions may ultimately require placement of additional stents (Inal et al, 2003a).
Postprocedure Care
Patients are monitored carefully for the first 24 hours after drainage for signs of bleeding or sepsis. With proper technique, including peripheral bile duct puncture, serious bleeding complications are uncommon. Because the hepatic artery, portal vein, and bile duct travel side by side within portal triads, it is common for blood to enter the bile duct during catheter exchanges, resulting in hemobilia in the immediate postprocedure period (see Chapter 105). Hemobilia usually clears within 24 hours, and new or recurrent hemobilia within the first few days of drainage typically is related to catheter malposition. If the catheter is pulled out from its original position, it is possible for a catheter sidehole to become positioned adjacent to a portal vein branch; this problem can be corrected by simply repositioning the catheter, but the catheter is often upsized as well. Significant arterial bleeding during this period is rare. No matter where the initial puncture is performed to opacify the biliary tree, attempts are always made to puncture a peripheral bile duct for catheter placement, preferably a fourth-order or fifth-order branch. The more peripheral the bile duct punctured, the smaller the accompanying hepatic artery branch, and the lower the risk of arterial injury and postprocedure bleeding.
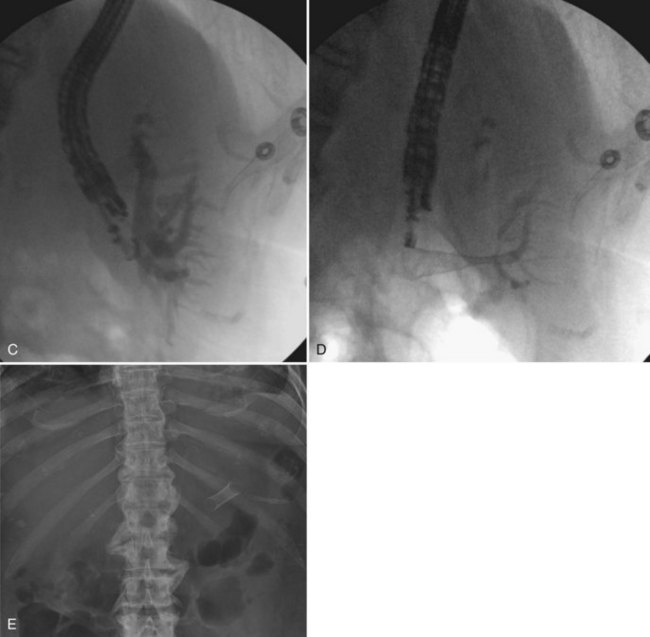
When bleeding occurs a week or more after biliary drainage—especially when the event is sudden in onset, and not only hemobilia but bleeding also occurs around the catheter entry site—arterial injury should be suspected, and the patient should be studied angiographically (see Chapter 19). Although a pseudoaneurysm or extravasation of contrast is sometimes seen, as in any other case of vascular trauma, any abnormality of a hepatic arterial branch adjacent to the biliary drainage catheter should be taken as presumptive evidence of injury to the branch, and the vessel should be selectively embolized (Fig. 50D.13). If the clinician is determined to demonstrate extravasation of contrast material angiographically, it is sometimes necessary to remove the biliary drainage catheter over a guidewire during the angiogram. In the correct clinical setting, empiric superselective embolization of the arterial branch corresponding to the bile duct punctured is acceptable. There is little down side to this approach, and the patient’s bleeding is stopped; the biliary catheter is exchanged at this point, because it is usually at least partially occluded by thrombus.
Despite prophylactic antibiotic coverage, sepsis may occur immediately after or within several hours of drainage and should be treated appropriately (Smith et al, 2004). This is most commonly manifested by the development of rigors with normal or low body temperature, but hypotension and fever may also occur. Sepsis is managed with continued administration of appropriate antibiotics, expansion of intravascular volume, and pressor support if necessary. Blood cultures should be drawn to identify organisms responsible for the bacteremia. Cultures of bile obtained at the time of drainage are routinely sent in for all patients. This is particularly important for those with preprocedure fever, biliary-enteric anastomosis or sphincterotomy, previous endoscopic retrograde cholangiopancreatography, or an indwelling stent or catheter. Although positive bile cultures are more common in patients with benign bile duct obstruction, cultures are positive in more than half of patients with malignant obstruction. Five percent of patients without fever, previous biliary surgery, or endoscopic or percutaneous intervention have positive bile cultures (Brody et al, 1998).
Abraham NS, Barkun JS, Barkun AN. Palliation of malignant biliary obstruction: a prospective trial examining impact on quality of life. Gastrointest Endosc. 2002;56(6):835-841.
Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140(2):170-178.
Brody LA, et al. Clinical factors associated with positive bile cultures during primary percutaneous biliary drainage. J Vasc Interv Radiol. 1998;9(4):572-578.
Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest Endosc. 1998;47(5):354-362.
Choi JY, et al. Navigator-triggered isotropic three-dimensional magnetic resonance cholangiopancreatography in the diagnosis of malignant biliary obstructions: comparison with direct cholangiography. J Magn Reson Imaging. 2008;27(1):94-101.
Hann LE, et al. Hepatic lobar atrophy: association with ipsilateral portal vein obstruction. Am J Roentgenol. 1996;167(4):1017-1021.
Inal M, et al. Percutaneous placement of biliary metallic stents in patients with malignant hilar obstruction: unilobar versus bilobar drainage. J Vasc Interv Radiol. 2003;14(11):1409-1416.
Inal M, et al. Percutaneous placement of metallic stents in malignant biliary obstruction: one-stage or two-stage procedure? Pre-dilate or not? Cardiovasc Intervent Radiol. 2003;26(1):40-45.
Inal M, et al. Percutaneous self-expandable uncovered metallic stents in malignant biliary obstruction: complications, follow-up and reintervention in 154 patients. Acta Radiol. 2003;44(2):139-146.
Johnson RC, Ahrendt SA. The case against preoperative biliary drainage with pancreatic resection. HPB (Oxford). 2006;8(6):426-431.
Kim CW, et al. T-configured dual stent placement in malignant biliary hilar duct obstructions with a newly designed stent. J Vasc Interv Radiol. 2004;15(7):713-717.
Maybody M, et al. Primary patency of Wallstents in malignant bile duct obstruction: single vs. two or more noncoaxial stents. Cardiovasc Intervent Radiol. 2009;32(4):707-713.
Mezhir JJ, et al. A matched case-control study of preoperative biliary drainage in patients with pancreatic adenocarcinoma: routine drainage is not justified. J Gastrointest Surg. 2009;13:2163-2169.
Ozden I, et al. Endoscopic and radiologic interventions as the leading causes of severe cholangitis in a tertiary referral center. Am J Surg. 2005;189(6):702-706.
Park HS, et al. Preoperative evaluation of bile duct cancer: MRI combined with MR cholangiopancreatography versus MDCT with direct cholangiography. AJR Am J Roentgenol. 2008;190(2):396-405.
Rerknimitr R, et al. Result of endoscopic biliary drainage in hilar cholangiocarcinoma. J Clin Gastroenterol. 2004;38(6):518-523.
Robson PC, et al, 2007: Prospective study on the effect of percutaneous biliary drainage for malignant obstruction on quality of life. Paper presented at the ASCO Annual Meeting, Chicago, 1997.
Smith TP, Ryan JM, Niklason LE. Sepsis in the interventional radiology patient. J Vasc Interv Radiol. 2004;15(4):317-325.
Ulrich R, et al. Outcomes of patients undergoing percutaneous biliary drainage to reduce bilirubin for administration of chemotherapy. New York: Memorial Sloan-Kettering Cancer Center & New York University School of Medicine; 2009.
Van Laethem JL, et al. Clinical impact of biliary drainage and jaundice resolution in patients with obstructive metastases at the hilum. Am J Gastroenterol. 2003;98(6):1271-1277.
Wang Q, et al, 2008: Preoperative biliary drainage for obstructive jaundice. Cochrane Database Syst Rev (3):CD005444.