Bronchoscopy and Lung Biopsy in Critically Ill Patients
Fiberoptic Bronchoscopy–Induced Physiologic Changes
General Considerations
The act of cannulation of the airway is not a physiologically neutral event.1–5 It stimulates both cardiovascular and respiratory responses. In the ICU, there are three common stimuli that provoke these reflexes: intubation, bronchoscopy, and suctioning. Even after the use of sedation and application of topical anesthesia, the passage of a bronchoscope through the larynx typically results in a 30% increase in mean arterial blood pressure and cardiac index, a 40% increase in heart rate, and an 86% increase in pulmonary arterial occlusion pressure.1 The response seems to represent a reflex to mechanical stimulation of the larynx. A significant hemodynamic response occurs even when patients are anesthetized and paralyzed before the passage of a bronchoscope through the larynx and into the trachea, with conflicting data as to whether there is a difference in hemodynamic response between the nasal and the oral routes.3,4 Topical anesthesia of the upper airway with lidocaine attenuates (but does not ablate) the cardiovascular responses.3,5
The significance of the cardiovascular changes associated with bronchoscopy is related primarily to their potential impact upon the heart and the brain. The increase in rate-pressure product has the potential to cause cardiac ischemia in patients with limited cardiac perfusion. In one small study, 3 of 10 monitored patients undergoing bronchoscopy, all of whom were 55 years old or older, developed electrocardiographic changes of ischemia.2 In another study designed specifically to look at older patients, the incidence of ischemia was 17%.6 Despite these findings, bronchoscopy has a low mortality rate from ischemia. In studies that have described complications from bronchoscopy (ranging from as few as 10 to as many as 48,000 procedures), the rate of death from ischemic events ranged from 0% to approximately 0.01%.6–11
A second potential cardiac complication of bronchoscopy is the triggering of arrhythmias. One study looking specifically for arrhythmias during bronchoscopy found an 11% incidence of “major arrhythmias.”12 As with ischemia, however, the reported mortality rate from arrhythmias has been very low; in two large studies of complications of bronchoscopy the mortality rate from arrhythmias ranged from 0% to 0.04%.10,11
With respect to the brain, both bronchoscopy and endotracheal suctioning cause significant increases in intracranial pressure.2,13 Because mean arterial pressure and intracerebral pressure increase roughly in parallel, there is little overall change in cerebral perfusion pressure.2,13 This may explain why no adverse intracerebral sequelae have been reported despite the observed increase in intracerebral pressure.13 Nevertheless, we believe that close attention to intracerebral pressure is warranted whenever possible in brain-injured individuals undergoing bronchoscopy.
Bronchoscopy also affects the work of breathing, respiratory pressures, and lung volumes. Transnasal cannulation of the trachea leads to a decrease in inspiratory and expiratory flow, a decrease in vital capacity, and an increase in functional residual capacity.14 Bronchoscopy through an endotracheal tube in a sedated but not ventilated patient leads to significant further decreases in flows and vital capacity and a marked increase in the work of breathing.14,15
Bronchoscopy in Mechanically Ventilated Patients
Mechanical ventilation adds additional complexity to the physiologic effects of bronchoscopy. The details were elegantly delineated in a study by Lindholm and colleagues.15 The first notable change is a dramatic increase in upper airway resistance. Airway narrowing related to endotracheal tube insertion by itself increases airway resistance. The subsequent introduction of a bronchoscope through the endotracheal tube greatly increases that resistance. The actual degree of increased airway resistance observed is related to the cross-sectional areas of both the endotracheal tube and the bronchoscope being inserted.15 The increased resistance has implications for ventilator gas delivery. The first is a potential decrease in delivered volume. With a volume-cycled mode, pressure increases as needed to deliver the specified volume, but the set volume may not be delivered owing to a pressure pop-off set in the alarm/safety parameters of the ventilator. (Although peak pressures of 60 to 80 cm H2O can be reached at the airway opening, note that the pressure gradient from the proximal obstruction limits the pressure delivered to the lung parenchyma.15) With a pressure-cycled mode of mechanical ventilation, the increase in upper airway resistance caused by bronchoscopic cannulation leads to an immediate decrease in delivered volume, the severity of which again depends upon the cross-sectional diameters of both the endotracheal tube and the bronchoscope. This effect can be countered by raising the applied pressure and the associated inspiratory airway pressure.
Whereas the driving pressure for inspiratory volumes depends upon external force (generated by the ventilator), exhalation is largely dependent upon the passive recoil of the lungs. The increase in airway resistance caused by introduction of the bronchoscope into the endotracheal tube thus has a disparate and more potentially dramatic effect upon expiratory volumes than upon inspiratory volumes. Sequential delivered volumes may be incompletely exhaled, causing a gradual buildup of intrathoracic volume and intrinsic positive end-expiratory pressure (auto-PEEP). Lindholm and colleagues15 documented several cases of auto-PEEP greater than 18 cm H2O. In one patient being bronchoscoped through an endotracheal tube with an internal diameter of 7 mm, auto-PEEP was recorded at 35 cm H2O.
Suctioning adds another layer of complexity. In addition to the cardiovascular changes stimulated by suctioning mentioned earlier, suctioning can cause profound changes in intrathoracic pressures and lung volumes. Not only can the auto-PEEP induced during bronchoscopy be eliminated, but with prolonged suctioning negative intrathoracic pressure can be created.15 This can lead to clinically relevant decreases in functional residual capacity, tidal volume, and minute ventilation, with potential for alveolar collapse, hypercarbia, and hypoxemia.
Hypoxemia
That bronchoscopy can cause hypoxemia is clear. The degree of hypoxemia ranges from mild to severe, and a variety of potential mechanisms are involved. The administration of benzodiazepines and narcotics can cause hypoxemia and respiratory depression in normal volunteers,16 and one study showed a 35% incidence of desaturation after sedation even before bronchoscopy was performed.17 The desaturation may not be completely due to central nervous system depression; Chhajed and coworkers18 showed that hypoxemia during bronchoscopy could be corrected in most of their lung transplant patients by the insertion of a nasopharyngeal tube, indicating that in their population upper airway collapse was a major cause of hypoxemia. Apart from the effects of sedation upon oxygenation, bronchoscopy itself can cause hypoxemia. Changes in airway resistance and the impact of suctioning upon functional residual capacity are probably major factors.19–22 Higher incidences of hypoxemia have been shown to correlate with the degree of prebronchoscopy pulmonary impairment, extent of suctioning, and performing a bronchoalveolar lavage (BAL).20–25 Furthermore, the hypoxemia induced by bronchoscopy often does not resolve immediately upon removal of the bronchoscope; it can persist for more than 2 hours.17,24,25 Hypoxemia represents another stress on top of the cardiovascular reflex changes cited earlier and may negatively impact organs at risk. Hypoxemia may play a significant role in cardiac ischemia and arrhythmias associated with bronchoscopy.1
Bronchoscopy through the endotracheal tube of a patient on a ventilator has been reported to cause an increase in PO2 with an increase in PCO2 and a decrease in PO2 with no change in PCO2.14,15 The seemingly contradictory differences in findings probably reflect individual differences in how much patients are suctioned and the degree to which suctioning impacts functional residual capacity.
Airway Evaluation and Management
Intubation and Endotracheal Tube Management
Bronchoscopy can be an asset in the insertion and subsequent management of endotracheal tubes.26,27 For patients with difficult airways, an endotracheal tube can be placed over a bronchoscope and slid into the trachea after direct visual intubation with the bronchoscope. A similar technique can be used for changing the endotracheal tube in a tenuous patient, especially if switching from nasal to oral or oral to nasal.27 Bronchoscopy is valuable in the placement of tubes for single-lung ventilation or placement of double-lumen tubes.27 Bronchoscopy also allows the bronchoscopist to position the tip of an endotracheal tube at a point approximately 2.5 cm above the carina under direct visualization or, when there is a process such as tumor compression of the trachea, to place the tube wherever needed for optimal ventilation.26
Percutaneous Dilational Tracheostomy
Percutaneous dilational tracheostomy (PDT) has emerged as a primary method of tracheostomy for ICU patients. It has a lower incidence of overall complications than does open surgical tracheostomy, and it can be performed at the bedside, avoiding the risks of transporting a critically ill patient.28–31 It also is more cost-effective.29 PDT can be performed not only by surgeons, but also by trained intensivists and pulmonologists;30 it has become a standard intensivist procedure in many institutions.32,33 Although its overall safety profile is better than for open surgical tracheostomy, PDT does have a higher risk of posterior tracheal wall injury and false passage.34 Bronchoscopy can be combined with PDT to allow direct endobronchial guidance for the procedure. Bronchoscopy has been suggested by several authors to be an important safety factor in PDT,31,34–40 although its use is not universally practiced.32,41 Direct visualization is a protection against inadvertent tracheal injury and a means of assessing an injury for intervention should it occur.34,36,38 The most logical approach to PDT is a team approach42 with at least two principal operators—one managing the bronchoscope and endotracheal tube and the second working at the neck to insert the tracheostomy tube.31,34,39 The risk of using bronchoscopy for PDT is that of increased hypoventilation because the patient is being ventilated through the endotracheal tube during the procedure.37 This can be obviated with the use of a pediatric bronchoscope for guidance; a large working channel is not needed for this application.
Airway Obstruction and Atelectasis
The upper airway can become obstructed via several mechanisms. Bronchoscopy is valuable in diagnosing all of them. When an endotracheal tube is inserted, it becomes part of the airway. Kinking of the endotracheal tube or luminal blockage by hardened, inspissated secretions causes the same physiologic problems as a primary tracheal process. Granulation tissue at the proximal end of a tracheostomy tube or at the distal end of a tracheostomy tube or endotracheal tube can cause obstruction and difficulty with inflation or deflation of the lungs. (This is much less common with endotracheal tubes than with tracheostomy tubes because of Murphy’s eye, the distal side port, on endotracheal tubes.) Benign or malignant processes can cause strictures or compression of the trachea. Finally, edema of the vocal cords and periglottic structures can lead to upper airway obstruction that is not evident with an endotracheal tube in place but that can cause recurrent respiratory failure after extubation; performing extubation over a bronchoscope allows immediate evaluation of the upper airway as the endotracheal tube is withdrawn with immediate diagnosis of this problem.27
Foreign bodies can cause obstruction at various points in the airways. Traditionally, rigid bronchoscopy was used for management of foreign bodies given its capacity for airway control and the availability of tools larger than those that can be passed through a flexible bronchoscope.43 Flexible bronchoscopes are the only choice, however, in an intubated patient, and the combination of larger channel scopes (2.8 mm and larger) for the passage of instruments and of different baskets and grasping tools makes flexible fiberoptic bronchoscopy a viable option in many cases.26,43,44 For nonintubated patients, a flexible bronchoscope through a rigid bronchoscope is sometimes an optimal approach.43
Atelectasis is a common ICU problem and has been one of the most common indications for bronchoscopy in ICUs.45,46 The argument is obvious: If standard “blind” suctioning is effective and useful, why not use suctioning under direct visualization for local airway obstruction due to secretions? One key study done by Marini and coworkers47 challenged the assumption that bronchoscopy is an optimal approach to atelectasis. In their study of patients with radiologically evident atelectasis, one group underwent a regimen of initial bronchoscopy followed by regular chest physiotherapy and a second group received regular chest physiotherapy alone followed by delayed bronchoscopy at 48 hours only if atelectasis persisted. The authors showed early bronchoscopy to be the inferior approach; there was no difference in rate or degree of improvement between the two groups, and bronchoscopy led to more significant decreases in oxygenation.47 The authors also looked at the presence or absence of air bronchograms, postulating that patients with air bronchograms had more distal consolidation (as opposed to proximal obstruction causing atelectasis) and would be more refractory to any attempts to re-expand consolidated lung. Their results strongly supported their postulate.47
These data are valuable and should lead to initial trials of chest physiotherapy in most cases of atelectasis, but they do not apply universally in clinical medicine. Some patients with chest trauma or spinal cord injury are not candidates for chest physiotherapy.48 Patients with chest trauma or spinal cord injury are usually not candidates for chest physiotherapy. Some patients have atelectasis and a severe refractory hypoxemia as a result of shunting of blood through the atelectatic lung. In these patients, a 24-hour wait may be inappropriate, and bronchoscopy sometimes leads to marked improvement. Judicious use of bronchoscopy for atelectasis is thus a clinical decision involving the art of medicine.48 Some patients with atelectasis have severe refractory hypoxemia as a result of shunting of blood through the atelectatic lung. In these patients, trying chest physiotherapy may be inappropriate because bronchoscopy often can lead to marked improvement almost immediately. Judicious use of bronchoscopy for atelectasis thus depends on the clinical situation. For patients who have a bronchoscope inserted for atelectasis, one technique that has been moderately successful is that of isolated segmental inflation; the bronchoscope or a ballooned catheter is wedged into the atelectatic segment, and pressure of at least 30 mm H2O is applied through the bronchoscope or catheter.45,48 This focal approach is more rational for focal atelectasis than are recruitment maneuvers applied to the whole chest because recruitment maneuvers are more likely to overdistend compliant alveoli than to open atelectatic alveoli.45
Trauma
The value of bronchoscopy for the evaluation of chest trauma was defined in a landmark paper by Hara and Prakash.49 The authors looked at 53 cases of blunt trauma to the chest, neck, or both in which bronchoscopy was performed within 3 days of injury. Bronchoscopy was believed to be of clinical value in 53% of the 53 cases. They documented injury and tears of the upper and lower airways, contusion or hemorrhage, aspirated material, and plugging or secretions. Only one of eight major tracheal injuries was not completely diagnosed. These data give compelling evidence for visual bronchoscopic evaluation of the bronchial tree in all cases of significant blunt chest trauma.
Smoke Inhalation
Inhalation injury is a unique form of trauma to the bronchial tree. Most inhalation injuries are due not to heat alone, but to the deposition in the bronchial tree of soot and gases, the products of combustion.50 Early ventilatory support and aggressive pulmonary toilet probably improve outcomes for inhalation injury,51,52 but the severity of inhalation injury may not become fully manifest for up to 5 days (usually 3 days).52 Bronchoscopy has been advocated in the evaluation of patients with possible inhalation injury.51–53 Some patients have mucosal injuries that are obvious to even an inexperienced bronchoscopist, but sometimes even when there is significant injury, the bronchoscopic appearance of the airways may be minimally abnormal. For patients who have relatively normal-appearing mucosae, biopsy specimens from segmental or subsegmental carinae can yield pathologic information that is predictive of the degree of inhalation injury (or lack thereof) and can be used to guide aggressiveness of management.51,52 Another tool for evaluation of inhalation injury is the ventilation-perfusion scan,50,53 but the objectivity and reproducibility of bronchoscopic endobronchial biopsy could lead one to argue that it should be the current standard of care for the diagnosis of possible inhalation injury.
Hemoptysis
Hemoptysis can run the gamut from scant to massive. There are several definitions of massive hemoptysis, with varying quantities of blood specified over different intervals. The best definition of massive hemoptysis is functional: Massive hemoptysis is bleeding in quantities that threaten to cause death by asphyxiation from filling of the bronchial tree with blood or by exsanguination.54,55 Patients with massive hemoptysis should always be admitted to the ICU.
Bronchoscopy plays two roles in massive hemoptysis—diagnostic and therapeutic. Most authors recommend bronchoscopy as soon as possible to localize the site of bleeding.54–56 Rigid bronchoscopy and flexible bronchoscopy each have their advocates. The argument for rigid bronchoscopy is that it allows better visualization of the major airways, more aggressive suctioning, removal of clots, and local tamponade with packing materials.56,57 The argument for flexible bronchoscopy is that it does not require the operating room or general anesthesia, it allows more thorough evaluation of the bronchial tree, and it allows directed intubation.54,56 Intubation may be with a regular endotracheal tube in the trachea or in the left lung or with a double-lumen tube. (The right upper lobe takeoff is too proximal in the right bronchial tree to allow selective right main bronchus intubation.) If bleeding is too great, flexible bronchoscopy is of limited value; small amounts of blood can obscure the relatively small lens of a bronchoscope.54 For massive hemoptysis, the goals of bronchoscopy are to identify the bleeding site and to stabilize the patient enough to allow arterial embolization, surgery, or a sequence of the two; embolization and surgery are usually the two most effective therapies. Several temporizing endobronchial therapies have been advocated: iced saline lavage, topical 1 : 20,000 epinephrine, endobronchial tamponade with packing or a Fogarty catheter, and endobronchial laser therapy.54–56
Diagnosis of Infection
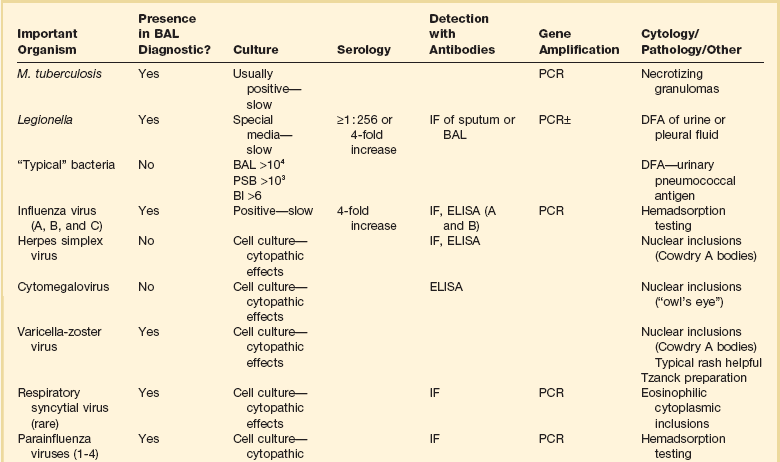
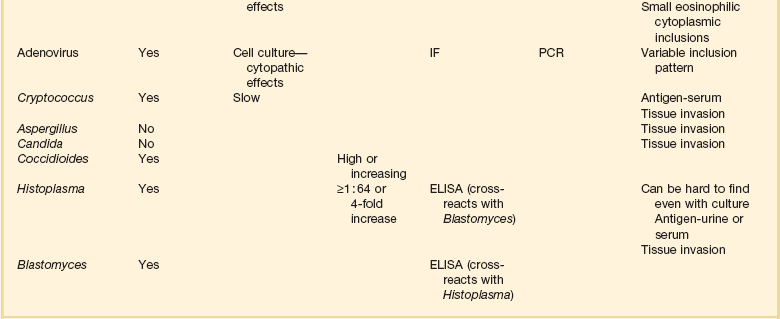
For BAL, the bronchoscope is wedged into a peripheral airway, and aliquots of sterile saline totaling at least 120 mL are instilled to ensure that alveolar spaces are reached. Box 12.1 summarizes the recommended method for BAL. Fluid retrieved via suctioning is sent for diagnostic studies. The BAL technique samples about 1 million alveoli, or 1% of the lung.58 For some infections, the presence of an organism in the lung is pathognomonic for active infection, whereas for others quantitative cultures are needed to distinguish lower respiratory tract infection from upper airway contamination.
M. tuberculosis is a cause of pneumonia that should not be forgotten in critical care medicine. BAL is a sensitive tool for the diagnosis of M. tuberculosis, one of the organisms whose presence always means active infection.59,60 BAL is more sensitive than gastric washings for the diagnosis of tuberculosis.61 BAL or protected specimen brushings are often positive in patients who have pulmonary tuberculosis but negative sputum smears,59,62,63 and BAL is also more sensitive than transbronchial biopsies for diagnosis of tuberculous pneumonia.64 (For miliary tuberculosis, biopsy is of great value.65) The addition of molecular techniques such as polymerase chain reaction (PCR) can further increase the sensitivity of BAL for M. tuberculosis.66
P. jiroveci is a pathogen in immunocompromised individuals. Its incidence increased dramatically in the 1980s with the emergence of the acquired immunodeficiency syndrome (AIDS). In the 1960s and 1970s, only about 100 cases were diagnosed per year in the United States.67 In the 1980s, thousands of cases were diagnosed.67 Because the organism cannot be cultured, its detection depends on identification in respiratory secretions. BAL greatly increases the yield for Pneumocystis pneumonia over bronchial washings and brushings and is the diagnostic procedure of choice.64,68,69 As with M. tuberculosis, P. jiroveci is an organism that can sometimes be difficult to see on smears, especially in patients with Pneumocystis pneumonia who do not have AIDS or who have AIDS but have received inhaled pentamidine.70,71 PCR can increase the sensitivity of fluid examination with the caveat that some patients can have Pneumocystis colonization without infection, and the PCR method is sensitive enough to detect those cases.72,73 In patients without AIDS or patients with AIDS receiving inhaled pentamidine, transbronchial biopsy increases the diagnostic yield.70,74
The diagnosis of fungal pulmonary infection is more problematic; some fungi can colonize the bronchial tree without being a cause of active infection. Fungal infection is a major issue in the immunocompromised host, but can occur in immunocompetent individuals and can be community-acquired.75 Candida species often colonize the respiratory tract in critically ill patients; their isolation from BAL fluid alone is not diagnostic of tissue invasion.75,76 Aspergillus runs the gamut of possibilities; it can colonize the upper airways without infection, and it can be difficult to detect sometimes when it is the cause of deep tissue infection. In the appropriate clinical and radiologic setting, a positive BAL for Aspergillus would be a reasonable criterion for starting therapy, but a negative BAL for Aspergillus is inadequate to rule out infection.77 PCR would seem promising to increase sensitivity for Aspergillus, but published results have been variable, and it is not a standard tool.78–80 Because of the possibility of colonization with Aspergillus and Candida species, lung biopsy showing tissue invasion is needed to confirm active infection by these fungi.76,80 In contrast, Cryptococcus neoformans, although ubiquitous, does not routinely colonize the respiratory tract; its detection in samples of lung fluid is strong presumptive evidence for an active role in pulmonary infection.81,82
The endemic fungi, Histoplasma capsulatum, Blastomyces dermatitidis, and Coccidioides immitis, are capable of causing fulminant infection and cannot be ignored as diagnostic possibilities for pneumonia in patients who live in or travel through endemic areas.81–86 Diagnosis is sometimes difficult, although the organism may be more plentiful and easier to identify in pulmonary secretions of patients who have fulminant disease. BAL fluid has a much higher yield than sputum.87 If organisms are not seen on smear, fungal cultures can take weeks, an unacceptable interval for seriously ill patients. For H. capsulatum, an antigen can be detected in bodily fluids (including BAL) and is of clinical value.86 The other two endemic fungi are more problematic when not demonstrable on staining of fluids or biopsy material, but promising techniques with antigen detection, DNA probes, and PCR are evolving and should become clinically available soon.83,85,88,89
Similar to fungi, viral pneumonias occur more frequently in immunocompromised hosts, but viruses play a role in community-acquired pneumonias as well.90–92 In a multinational study, viruses alone were identified in 9% of community-acquired pneumonias, and viruses as co-pathogens were identified in an additional 9% of community-acquired pneumonias.92 Viral pneumonia is relevant to the critical care setting; in the multinational study, 8% of patients with pure viral pneumonia were admitted to the ICU owing to severity of disease.92 In another study using the most sensitive available virus detection technique, reverse transcriptase PCR, viruses were present as pathogens or co-pathogens in 23% of patients hospitalized for community-acquired pneumonia.90 Analogous to the atypical pneumonias, no clinical characteristics reliably differentiate viral from bacterial pneumonia.90,92,93
The virology of viral pneumonia is different for pneumonia in immunocompetent and immunocompromised hosts. In immunocompetent individuals, the most common viruses are influenza A and B, parainfluenza, respiratory syncytial virus, and adenovirus.93 In immunocompromised patients, the herpesviruses—herpes simplex, varicella zoster, and cytomegalovirus—are most common, with adenovirus, respiratory syncytial virus, and measles also occurring.93,94 BAL is useful in the diagnosis of viral pneumonia; BAL fluid is an ideal substrate for most studies done to detect viral infection.95,96 For all but herpes simplex virus and cytomegalovirus, the presence of the virus is diagnostic of infection.95,97 Cytomegalovirus and herpes simplex virus can be present in BAL fluid in the absence of pneumonia; proof of tissue invasion is more specific for active infection.95,97 Viral pneumonias are often difficult to diagnose, and cultures can take days to weeks. Newer diagnostic techniques such as reverse transcriptase PCR offer a more sensitive diagnostic tool.98
BAL is of major utility in the diagnosis of bacterial pneumonias. It has long been recognized that patients in the ICU often have colonization of the upper airways by several potentially pathogenic organisms, and that culture of upper airway secretions cannot be used to diagnose infections of the lower airways and lung parenchyma. Quantitative culture of BAL specimens has been proved to best obviate the issue of upper airway colonization, and BAL has thus emerged as the best currently available diagnostic test for bacterial pneumonia.58,99 An alternative to BAL is the protected specimen brush (PSB). BAL allows the sampling of a much larger area of lung than does the PSB and logically would be a better diagnostic modality. Although the two modalities are equally accurate in some studies, for several reasons BAL has become the diagnostic procedure of choice for bacterial pneumonia (Box 12.2).58,99 A third technique, the blind passage of a protected brush into the airways without bronchoscopic guidance, has been described.100 This technique has a relatively lower sensitivity than BAL, which probably depends on which lobe is most involved with infection. For example the catheter cannot be passed into the upper lobes.58,100 For this reason and the reasons cited in Box 12.2, BAL has evolved as the procedure of choice.99
Two quantitative methods for bacterial growth of samples obtained by BAL have been described. The first is a simple log count; any bacteria present in greater than or equal to 104 colonies by quantitative methods is a pathogen unless proved otherwise.58 (If PSB is used, a smaller area of lung is sampled and a concentration of >103 colonies is considered diagnostic of infection.58) The second, called the bacterial index, was described by Johanson and colleagues.101 This method recognizes the fact that in careful studies numerous patients with pneumonia have more than one infecting organism.23,92,101,102 For the bacterial index, the log numbers of each cultured organism are summed (104 Staphylococcus aureus + 103 Pseudomonas aeruginosa = bacterial index of 7). With this system, a bacterial index greater than 6 is a sign of moderate to severe pneumonia.101 Although the bacterial index system seems more rational, single-organism quantification has become the de facto standard in current clinical practice.
Reinforcing the value of BAL for clinical management of bacterial infection is a study by Rodriguez and Fishman.74 The authors performed quantitative cultures on BAL fluid and PSB from 32 ventilated patients not suspected to have pneumonia. There were six “false-positives,” with quantitative cultures in the pneumonia range. Four of the six patients subsequently developed clinical pneumonia.
If a patient with suspected bacterial pneumonia has already been given antibiotics, the quantitative culture of distal airway secretions loses sensitivity.103,104 A decrease in recovered organisms becomes significant within 12 hours of initiation of antimicrobial therapy and reaches 50% between 24 and 48 hours.105 This is not surprising because even quantitative cultures of lung tissue have a dramatic loss of sensitivity and specificity for patients who have received prior antibiotic therapy.106 Despite this loss in sensitivity, BAL may make sense in the evaluation of critically ill patients with lung infiltrates who are receiving antibiotics as long as these limitations are recognized. BAL can detect organisms not being covered by the current antibiotic regimen, and BAL could help to define nonbacterial infectious agents or other noninfectious processes responsible for the clinical presentation.
When discussing the diagnostic utility of bronchoscopy for the diagnosis of severe lung infection, it is important to mention diagnoses that may be missed by the techniques discussed previously. The “atypical pneumonias”—principally Legionella pneumophila, Mycoplasma pneumoniae, and Chlamydia pneumoniae and C. psittaci—can be community-acquired. The myth that “atypical” and “typical” pneumonias can be distinguished clinically has been refuted. Legionella is particularly capable of causing severe disease, and the other organisms can be present as coinfectants in severe disease.107–109 Legionella is not typically visible on Gram stain, does not grow on standard bacterial culture, and requires about 1 week to grow on special media when it is present. BAL material in a patient being evaluated for severe community-acquired pneumonia should be sent for direct fluorescent antibody staining for Legionella and for culture.110 The direct fluorescent antibody staining is reasonably sensitive and very specific for Legionella,107 and direct fluorescent antibody staining of pulmonary secretions and of urine are the most rapid diagnostic tools available. The direct fluorescent antibody of pulmonary secretions remains positive for at least 48 hours after institution of appropriate therapy.110
There is an importance to negative BAL for quantitative bacterial culture. A prospective study comparing management based upon quantitative cultures from invasive diagnostic techniques (BAL or PSB) with management based upon clinical data without quantitative cultures showed that the patients whose therapy was started or withheld based on quantitative bacterial studies had less antibiotic use, lower mortality rate, and less sepsis-related organ failure.111 The importance of negative bacterial studies lies not only in the capacity to withhold or discontinue treatment for bacterial pneumonia, but also in the implication that other types of pulmonary infection or injury should be sought and that, in some cases, extrapulmonary sources of bacterial infection may be present.58,111 The implications of BAL for a critically ill patient thus extend beyond the lungs.
In summary, bronchoscopic techniques for the diagnosis of pulmonary infection are valuable tools in intensive care medicine. BAL is the most valuable single tool, with diagnostic implications dependent on the organism being evaluated. It is important to remember that a wide spectrum of organisms can cause pneumonia in critically ill patients; the intensivist needs to consider tuberculosis, Pneumocystis, viruses, fungi, and atypical organisms in addition to typical bacteria.112 When BAL fluid has been obtained, the diagnostic techniques of greatest value vary with the infecting organism. For some infecting organisms, detection of a causative organism can be difficult even in the presence of severe infection, and sensitive assays such as PCR can help in the rapid detection of the causative agent. In other cases, such as bacterial infection, organisms abound and it can be difficult to separate colonization from infection; in these cases, quantitative cultures are of greatest value. For other organisms, tissue samples are needed for definitive diagnosis. Table 12.1 summarizes diagnostic methods relevant to different lung infections. BAL fluid is the optimal substrate for almost every listed study with the exception of titers and biopsy material.
Lung Biopsy—Surgical and Transbronchial
The decision of whether or when to perform an open lung biopsy is difficult and a matter of debate. Open lung biopsy is the gold standard for the diagnosis of severe cryptogenic lung disease.113,114 When performed, it has a high diagnostic accuracy, although it is not 100% accurate; in one study with 25 patients who had biopsies and subsequent autopsies, the autopsy confirmed the open lung biopsy diagnosis in 76% of cases.115 Open lung biopsy is useful not only in the diagnosis of infection (particularly infections for which tissue invasion is essential to prove clinical relevance), but also in the diagnosis of primary lung involvement by diseases such as malignancy, nonspecific inflammatory lung disease, and lung-related toxicities of therapeutic interventions. Box 12.3 demonstrates the breadth of possible diagnoses that can be made with open lung biopsy after other diagnostic studies are negative.
Open lung biopsy leads to a change in therapy in 57% to 75% of cases in which it is performed.113,114,116–118 Open lung biopsy can be performed in patients with respiratory distress and patients on ventilators with a reasonable rate of perioperative complications; death attributed to the biopsy procedure itself is rare, and complications such as persistent air leak usually resolve over time.114,116,117 Complication rates have been reported to be the same with video-assisted thoracic surgery as with thoracotomy.118 Several recent studies have reported the frequent use of bedside open lung biopsy, which avoids transport of a critically ill patient and appears to have a morbidity rate similar to that of doing the biopsy in the operating room.118–122
Although open lung biopsy sounds promising, it has several drawbacks. First, authors who separated open lung biopsy results into “specific” (allowing focused therapy thought or known to have some efficacy) and “nonspecific” (a pattern of injury such as diffuse lung damage with no clear cause and no documented effective therapy) found that 38% to 46% of patients had nonspecific injury and no specific therapeutic benefit from the procedure.114,118 Second, mortality rate is extremely high in this group despite specific diagnoses and the previously mentioned changes in therapy, particularly if the patients have respiratory compromise; for those patients, the short-term mortality rate ranges from 52% to 70%.116–118 (Note that short-term mortality rate is believed by most authors to be related far more to the underlying disease than to the open lung biopsy procedure.116–118) Third, most patients already have received appropriate therapy; in most cases, the change in therapy would be discontinuation of unnecessary drugs or increased dosing of a drug already being given (usually steroids). In some, the benefit of open lung biopsy would be acknowledgment of end-stage disease and withdrawal of all therapy. The most important outcome of open lung biopsy would be a change in therapy that altered outcome from death to survival. Institution of a new potentially lifesaving therapeutic intervention as a result of open lung biopsy is uncommon.114,116,117 In 1988, Warner and colleagues117 discussed open lung biopsy in patients with respiratory compromise and said, “any complications of a procedure of unknown benefit is of concern.” Some authors hold this opinion to this day. Most patients have received anti-infective drugs or steroids or both before open lung biopsy is considered, and one could argue that most patients who could respond would have responded before open lung biopsy. Nevertheless, that the controversy is “alive and well” is illustrated by a quote from Chuang and associates:113 “It is not ethical to avoid open lung biopsy in patients with unclear diagnoses, as open lung biopsy is known to be the most sensitive and specific test available at this time.”
Another controversy with respect to open lung biopsy concerns when to perform it. Two groups have reported open lung biopsy relatively early in the course of respiratory failure with diffuse infiltrates and have withheld therapies until and unless biopsy results demonstrated a specific indication.121,122 Not unexpectedly, this approach led to a high number of new therapies, usually steroids or antiviral agents. Papazian and coworkers argued that their approach improved survival.122 The data provide valuable support for a counterargument—that all patients with idiopathic diffuse lung disease should be treated with both steroids and antiviral agents. If this had been done in these two series, the added benefit of open lung biopsy would have been very small.
Given the controversies and the available data, a few principles would seem appropriate in considering whether or not open lung biopsy should be performed. First, in most cases, BAL (and perhaps transbronchial biopsy; see later) should be performed before open lung biopsy is considered. Second, patients who have three or more organs failing (lung plus two more) probably should not undergo biopsy; the prognosis is too poor.114 Third, decisions about open lung biopsy should be based more upon the prior condition of the patient and the potential utility of the study than upon the degree of ventilatory distress or compromise of the patient; post-operative death related to the biopsy itself is rare. Open lung biopsy in a critically ill patient with undiagnosed pulmonary infiltrates remains a valuable tool that is uncommonly indicated but that will occasionally provide a diagnosis that results in lifesaving therapy.
Bronchoscopic transbronchial lung biopsy may fill the gap between “nontissue” procedures such as BAL and open lung biopsy with its large amount of tissue for pathologic examination and culture. Open lung biopsy by definition requires anesthesia and by definition causes a breach in pleural integrity and requires chest tube placement, with many patients having air leaks after the procedure.116–118 Transbronchial lung biopsy requires only conscious sedation and would require chest tube placement only if a complication occurred, not as a matter of routine. As noted earlier in the discussion of infection, some diagnoses, such as invasive aspergillosis, Candida pneumonia, and cytomegalovirus pneumonia require tissue samples showing tissue invasion. Tissue samples can increase the diagnostic yield for other organisms such as M. tuberculosis and P. jiroveci.123 Diagnoses such as cryptogenic organizing pneumonia and several others listed in Box 12.3 cannot be made without tissue. Transbronchial lung biopsy is reasonable with BAL or after a negative BAL in compromised patients with infiltrates of unknown cause.123–125 It was formerly thought that to perform transbronchial lung biopsy in a patient on a ventilator carried too high a risk of complications to be warranted.126 The available data challenge this concept. Two early studies127,128 and several more recent studies69,125,129,130 have evaluated the yield and complications of transbronchial lung biopsy in ventilated patients. All of these studies showed high yields similar to the yields for open lung biopsy. The pneumothorax rates varied from 0% to 24%. Bleeding was considered to be significant in 6% to 20%, with all cases self-limited. No fatalities occurred. When transbronchial lung biopsy in ventilated patients is compared with open lung biopsy, for which general anesthesia, incisions, violation of the visceral pleura, and a chest tube are requisites, it is apparent that transbronchial lung biopsy is underused in this population; it has a favorable risk-to-benefit ratio.
Special Situations
Immunocompromised Host
Immunocompromise is a recognized risk factor for respiratory infection. Years of elegant work have helped to define classic types of immunocompromise and patterns of infection that are more typical for the different types.131–133 Long-recognized diseases causing immunocompromise include solid tumors on chemotherapy, hematologic malignancies, organ transplantation, chronic diseases for which cytotoxic or steroid therapy is given, human immunodeficiency virus (HIV) infection, and other less common acquired or congenital diseases.126,133 Although immunocompromise definitely includes the preceding diseases, it has no clear boundaries. Patients with chronic insulin-dependent diabetes, alcoholics, patients with severe malnutrition, and patients on drugs such as infliximab, a tumor necrosis factor antagonist, almost certainly have degrees of immunocompromise that affect their susceptibility to infection.134–138 The stresses of critical illness and organ dysfunction coupled with the broaching of natural defense systems (skin by central lines, lungs by intubation, urinary tract by catheters) put critically ill patients at increased risk for infection.
Many chronic illnesses probably also carry an increased risk for infection. Shelhamer and colleagues133 recognized the difficulty with this issue and defined immunocompromise as “any condition, congenital or acquired, temporary or chronic, in which the response of the host to a foreign antigen is subnormal.” For patients with classic known forms of immunocompromise, special diagnostic attention has to be paid to the possibility of Pneumocystis pneumonia, invasive fungal infection, infection with the herpesviruses, and tuberculosis. Three factors make this area gray rather than black-and-white: (1) Lack of knowledge that a patient is immunocompromised does not rule out immunocompromise, (2) degrees of immunocompromise exist, and (3) most of the pathogens discussed are capable of causing disease in “normal” hosts. For these reasons, some specific issues related to immunocompromise have been mentioned throughout this chapter, but in the clinical approach to a patient with severe respiratory disease, it makes sense to assume that any patient might be immunocompromised; the diagnostic workup should, as mentioned, cast a wide net.
Thrombocytopenia
Thrombocytopenia is an obvious risk factor for bleeding from invasive procedures. BAL has been shown to be safe and clinically useful in thrombocytopenic patients.139 Bronchoscopic procedures and open lung biopsies have been performed safely in patients with thrombocytopenia.118,140 Historically, procedures have been limited to BAL, or platelets have been given before bronchoscopic or surgical procedures.118,140
Complications and Death
The true incidence of complications of bronchoscopy in the ICU is poorly defined. Most reviews of the topic27,40,141–144 cite several older studies of bronchoscopy in general,7–9,11,145 not specifically in critically ill patients. The two largest and most-cited reports are by Credle and colleagues7 and Suratt and coworkers11 and reviewed 24,521 (Credle) and “approximately 48,000” (Suratt) procedures. These were retrospective reports based on mailed questionnaires. Death rates were 0.01% and 0.03%.7,11
The next largest study was a retrospective single-institution study of 4273 bronchoscopies, which reported 0% mortality rate and a 0.5% frequency of major complications (pneumothorax, bleeding, respiratory failure requiring intubation).9 Three prospective studies reported on 205 to 1146 flexible bronchoscopies and reported mortality rates of 0%,9 0.1%,145 and 0.5%.8 The highest reported mortality rate came out of a study from a consortium of expert bronchoscopists studying autofluorescence.146 The study included the requisite of at least two endobronchial biopsies, and in a series of 300 cases the immediate postbronchoscopic mortality rate was 0.7%. Two retrospective studies46,147 and one prospective study69 reviewed bronchoscopies performed specifically on patients in the ICU. No procedure-related deaths were reported. Throughout these studies, only death rates are consistently noted; major and minor complications are variably defined.
Several principles emerge from the previously cited studies. The complication rates depend on the invasiveness of the procedure. The highest incidence of complications occurs with lung biopsy and then, in descending order, with brushings, BAL, and simple observation. For the patients expected to have the highest complication rate—patients on ventilators undergoing transbronchial biopsies—there is a pneumothorax rate of 24%, but no fatalities have been reported.69,125,127–130 None of those studies involved large numbers of patients; it is inevitable that fatalities would occur in this subset of high-risk cases. Nevertheless, many years of experience with flexible fiberoptic bronchoscopy have led to the conclusion that its benefits outweigh its risks. No bronchoscopy should be done on a critically ill patient without a reason, but when a reason is present, bronchoscopy is often the unique or the safest method known of obtaining data that may affect care.
References
1. Lundgren, R, Haggmark, S, Reiz, S. Hemodynamic effects of flexible fiberoptic bronchoscopy performed under topical anesthesia. Chest. 1982; 82:295–299.
2. Rudy, EB, Turner, BS, Baun, M, et al. Endotracheal suctioning in adults with head injury. Heart Lung. 1991; 20:667–674.
3. Kaplan, JD, Schuster, DP. Physiologic consequences of tracheal intubation. Clin Chest Med. 1991; 12:425–432.
4. Staender, S, Marsch, SC, Schumacher, P, et al. Haemodynamic response to fibreoptic versus laryngoscopic nasotracheal intubation under total intravenous anaesthesia. Eur J Anaesthesiol. 1994; 11:175–179.
5. Stoelting, RK. Circulatory changes during direct laryngoscopy and tracheal intubation: Influence of duration of laryngoscopy with or without prior lidocaine. Anesthesiology. 1977; 47:381–384.
6. Matot, I, Kramer, MR, Glantz, L, et al. Myocardial ischemia in sedated patients undergoing fiberoptic bronchoscopy. Chest. 1997; 112:1454–1458.
7. Credle, WF, Jr., Smiddy, JF, Elliott, RC. Complications of fiberoptic bronchoscopy. Am Rev Respir Dis. 1974; 109:67–72.
8. Dreisin, RB, Albert, RK, Talley, PA, et al. Flexible fiberoptic bronchoscopy in the teaching hospital. Yield and complications. Chest. 1978; 74:144–149.
9. Lukomsky, GI, Ovchinnikov, AA, Bilal, A. Complications of bronchoscopy: Comparison of rigid bronchoscopy under general anesthesia and flexible fiberoptic bronchoscopy under topical anesthesia. Chest. 1981; 79:316–321.
10. Pue, CA, Pacht, ER. Complications of fiberoptic bronchoscopy at a university hospital. Chest. 1995; 107:430–432.
11. Suratt, PM, Smiddy, JF, Gruber, B. Deaths and complications associated with fiberoptic bronchoscopy. Chest. 1976; 69:747–751.
12. Shrader, DL, Lakshminarayan, S. The effect of fiberoptic bronchoscopy on cardiac rhythm. Chest. 1978; 73:821–824.
13. Kerwin, AJ, Croce, MA, Timmons, SD, et al. Effects of fiberoptic bronchoscopy on intracranial pressure in patients with brain injury: A prospective clinical study. J Trauma. 2000; 48:878–882.
14. Matsushima, Y, Jones, RL, King, EG, et al. Alterations in pulmonary mechanics and gas exchange during routine fiberoptic bronchoscopy. Chest. 1984; 86:184–188.
15. Lindholm, CE, Ollman, B, Snyder, JV, et al. Cardiorespiratory effects of flexible fiberoptic bronchoscopy in critically ill patients. Chest. 1978; 74:362–368.
16. Bailey, PL, Pace, NL, Ashburn, MA, et al. Frequent hypoxemia and apnea after sedation with midazolam and fentanyl. Anesthesiology. 1990; 73:826–830.
17. Kristensen, MS, Milman, N, Jarnvig, IL. Pulse oximetry at fibre-optic bronchoscopy in local anaesthesia: Indication for postbronchoscopy oxygen supplementation? Respir Med. 1998; 92:432–437.
18. Chhajed, PN, Aboyoun, C, Malouf, MA, et al. Management of acute hypoxemia during flexible bronchoscopy with insertion of a nasopharyngeal tube in lung transplant recipients. Chest. 2002; 121:1350–1354.
19. Albertini, RE, Harrell, JH, 2nd., Kurihara, N, et al. Arterial hypoxemia induced by fiberoptic bronchoscopy. JAMA. 1974; 230:1666–1667.
20. Jones, AM, O’Driscoll, R. Do all patients require supplemental oxygen during flexible bronchoscopy? Chest. 2001; 119:1906–1909.
21. Petersen, GM, Pierson, DJ, Hunter, PM. Arterial oxygen saturation during nasotracheal suctioning. Chest. 1979; 76:283–287.
22. Shinagawa, N, Yamazaki, K, Kinoshita, I, et al. Susceptibility to oxygen desaturation during bronchoscopy in elderly patients with pulmonary fibrosis. Respiration. 2006; 73:90–94.
23. Guerra, LF, Baughman, RP. Use of bronchoalveolar lavage to diagnose bacterial pneumonia in mechanically ventilated patients. Crit Care Med. 1990; 18:169–173.
24. Montravers, P, Gauzit, R, Dombret, MC, et al. Cardiopulmonary effects of bronchoalveolar lavage in critically ill patients. Chest. 1993; 104:1541–1547.
25. Sharma, SK, Pande, JN, Sarkar, R. Effect of routine fiberoptic bronchoscopy and bronchoalveolar lavage on arterial blood gases. Indian J Chest Dis Allied Sci. 1993; 35:3–8.
26. Dellinger, RP. Fiberoptic bronchoscopy in adult airway management. Crit Care Med. 1990; 18:882–887.
27. Dellinger, RP, Bandi, V. Fiberoptic bronchoscopy in the intensive care unit. Crit Care Clin. 1992; 8:755–772.
28. Freeman, BD, Isabella, K, Cobb, JP, et al. A prospective, randomized study comparing percutaneous with surgical tracheostomy in critically ill patients. Crit Care Med. 2001; 29:926–930.
29. Freeman, BD, Isabella, K, Lin, N, et al. A meta-analysis of prospective trials comparing percutaneous and surgical tracheostomy in critically ill patients. Chest. 2000; 118:1412–1418.
30. Yarmus, L, Pandian, V, Gilbert, C, et al. Safety and efficiency of interventional pulmonologists performing percutaneous tracheostomy. Respiration. 2012; 84(2):123–127.
31. Melloni, G, Muttini, S, Gallioli, G, et al. Surgical tracheostomy versus percutaneous dilatational tracheostomy. A prospective-randomized study with long-term follow-up. J Cardiovasc Surg (Torino). 2002; 43:113–121.
32. Ernst, A, Silvestri, GA, Johnstone, D. Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest. 2003; 123:1693–1717.
33. Polderman, KH, Spijkstra, JJ, de Bree, R, et al. Percutaneous dilatational tracheostomy in the ICU: Optimal organization, low complication rates, and description of a new complication. Chest. 2003; 123:1595–1602.
34. Barba, CA, Angood, PB, Kauder, DR, et al. Bronchoscopic guidance makes percutaneous tracheostomy a safe, cost-effective, and easy-to-teach procedure. Surgery. 1995; 118:879–883.
35. McCague, A, Alianabi, H, Wong, DT. Safety analysis of percutaneous dilational tracheostomies with bronchoscopy in the obese patient. Laryngoscope. 2012; 122(5):1031–1034.
36. deBoisblanc, BP, Deblieux, P. Percutaneous dilational tracheostomy. Clin Pulm Med. 2002; 9:109–112.
37. Grundling, M, Pavlovic, D, Kuhn, SO, et al. Is the method of modified percutaneous tracheostomy without bronchoscopic guidance really simple and safe? Chest. 2005; 128:3774–3775.
38. Marx, WH, Ciaglia, P, Graniero, KD. Some important details in the technique of percutaneous dilatational tracheostomy via the modified Seldinger technique. Chest. 1996; 110:762–766.
39. Polderman, KH, Spijkstra, JJ, de Bree, R, et al. Percutaneous tracheostomy in the intensive care unit: Which safety precautions? Crit Care Med. 2001; 29:221–223.
40. Shennib, H, Baslaim, G. Bronchoscopy in the intensive care unit. Chest Surg Clin North Am. 1996; 6:349–361.
41. Kearney, PA, Griffen, MM, Ochoa, JB, et al. A single-center 8-year experience with percutaneous dilational tracheostomy. Ann Surg. 2000; 231:701–709.
42. Mirski, MA, Pandian, V, Bhatti, N, et al. Safety, efficiency and cost effectiveness of a mutlidisciplinary percutaneous tracheostomy program. Crit Care Med. 2012; 40(6):1827–1834.
43. Swanson, KL. Airway foreign bodies: What’s new? Semin Respir Crit Care Med. 2004; 25:405–411.
44. Cunanan, OS. The flexible fiberoptic bronchoscope in foreign body removal. Experience in 300 cases. Chest. 1978; 73:725–726.
45. Kreider, ME, Lipson, DA. Bronchoscopy for atelectasis in the ICU: A case report and review of the literature. Chest. 2003; 124:344–350.
46. Olopade, CO, Prakash, UB. Bronchoscopy in the critical-care unit. Mayo Clin Proc. 1989; 64:1255–1263.
47. Marini, JJ, Pierson, DJ, Hudson, LD. Acute lobar atelectasis: A prospective comparison of fiberoptic bronchoscopy and respiratory therapy. Am Rev Respir Dis. 1979; 119:971–978.
48. Tsao, TC, Tsai, YH, Lan, RS, et al. Treatment for collapsed lung in critically ill patients. Selective intrabronchial air insufflation using the fiberoptic bronchoscope. Chest. 1990; 97:435–438.
49. Hara, KS, Prakash, UB. Fiberoptic bronchoscopy in the evaluation of acute chest and upper airway trauma. Chest. 1989; 96:627–630.
50. Pruitt, BA, Jr., Cioffi, WG. Diagnosis and treatment of smoke inhalation. J Intensive Care Med. 1995; 10:117–127.
51. Chou, SH, Lin, SD, Chuang, HY, et al. Fiberoptic bronchoscopic classification of inhalation injury: Prediction of acute lung injury. Surg Endosc. 2004; 18:1377–1379.
52. Masanes, MJ, Legendre, C, Lioret, N, et al. Using bronchoscopy and biopsy to diagnose early inhalation injury. Macroscopic and histologic findings. Chest. 1995; 107:1365–1369.
53. American Burn Association. Inhalation injury: Diagnosis. J Am Coll Surg. 2003; 196:307–312.
54. Cahill, BC, Ingbar, DH. Massive hemoptysis. Assessment and management. Clin Chest Med. 1994; 15:147–167.
55. Lenner, R, Schilero, GJ, Lesser, M. Hemoptysis: Diagnosis and management. Compr Ther. 2002; 28:7–14.
56. Thompson, AB, Teschler, H, Rennard, SI. Pathogenesis, evaluation, and therapy for massive hemoptysis. Clin Chest Med. 1992; 13:69–82.
57. Knott-Craig, CJ, Oostuizen, JG, Rossouw, G, et al. Management and prognosis of massive hemoptysis. Recent experience with 120 patients. J Thorac Cardiovasc Surg. 1993; 105:394–397.
58. Chastre, J, Combes, A, Luyt, CE. The invasive (quantitative) diagnosis of ventilator-associated pneumonia. Respir Care. 2005; 50:797–807.
59. de Gracia, J, Curull, V, Vidal, R, et al. Diagnostic value of bronchoalveolar lavage in suspected pulmonary tuberculosis. Chest. 1988; 93:329–332.
60. Miro, AM, Gibilara, E, Powell, S, et al. The role of fiberoptic bronchoscopy for diagnosis of pulmonary tuberculosis in patients at risk for AIDS. Chest. 1992; 101:1211–1214.
61. Dickson, SJ, Brent, A, Davidson, RN, et al. Comparison of bronchoscopy and gastric washings in the investigation of smear-negative pulmonary tuberculosis. Clin Infect Dis. 2003; 37:1649–1653.
62. Jett, JR, Cortese, DA, Dines, DE. The value of bronchoscopy in the diagnosis of mycobacterial disease. A five-year experience. Chest. 1981; 80:575–578.
63. Willcox, PA, Benatar, SR, Potgieter, PD. Use of the flexible fibreoptic bronchoscope in diagnosis of sputum-negative pulmonary tuberculosis. Thorax. 1982; 37:598–601.
64. Stover, DE, Zaman, MB, Hajdu, SI, et al. Bronchoalveolar lavage in the diagnosis of diffuse pulmonary infiltrates in the immunosuppressed host. Ann Intern Med. 1984; 101:1–7.
65. Willcox, PA, Potgieter, PD, Bateman, ED, et al. Rapid diagnosis of sputum negative miliary tuberculosis using the flexible fibreoptic bronchoscope. Thorax. 1986; 41:681–684.
66. Tueller, C, Chhajed, PN, Buitrago-Tellez, C, et al. Value of smear and PCR in bronchoalveolar lavage fluid in culture positive pulmonary tuberculosis. Eur Respir J. 2005; 26:767–772.
67. Schliep, TC, Yarrish, RL. Pneumocystis carinii pneumonia. Semin Respir Infect. 1999; 14:333–343.
68. Roblot, F, Le Moal, G, Godet, C, et al. Pneumocystis carinii pneumonia in patients with hematologic malignancies: A descriptive study. J Infect. 2003; 47:19–27.
69. Turner, D, Schwarz, Y, Yust, I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: New data, new issues. Eur Respir J. 2003; 21:204–208.
70. Edelstein, H, McCabe, RE. Atypical presentations of Pneumocystis carinii pneumonia in patients receiving inhaled pentamidine prophylaxis. Chest. 1990; 98:1366–1369.
71. Zahar, JR, Robin, M, Azoulay, E, et al. Pneumocystis carinii pneumonia in critically ill patients with malignancy: A descriptive study. Clin Infect Dis. 2002; 35:929–934.
72. Elvin, K, Olsson, M, Lidman, C, et al. Detection of asymptomatic Pneumocystis carinii infection by polymerase chain reaction: Predictive for subsequent pneumonia. AIDS. 1996; 10:1296–1297.
73. Olsson, M, Elvin, K, Lofdahl, S, et al. Detection of Pneumocystis carinii DNA in sputum and bronchoalveolar lavage samples by polymerase chain reaction. J Clin Microbiol. 1996; 34:2052.
74. Rodriguez, M, Fishman, JA. Prevention of infection due to Pneumocystis spp. in human immunodeficiency virus-negative immunocompromised patients. Clin Microbiol Rev. 2004; 17:770–782.
75. Chen, KY, Ko, SC, Hsueh, PR, et al. Pulmonary fungal infection: Emphasis on microbiological spectra, patient outcome, and prognostic factors. Chest. 2001; 120:177–184.
76. el-Ebiary, M, Torres, A, Fabregas, N, et al. Significance of the isolation of Candida species from respiratory samples in critically ill, non-neutropenic patients. An immediate postmortem histologic study. Am J Respir Crit Care Med. 1997; 156:583–590.
77. Cordonnier, C, Escudier, E, Verra, F, et al. Bronchoalveolar lavage during neutropenic episodes: Diagnostic yield and cellular pattern. Eur Respir J. 1994; 7:114–120.
78. Bart-Delabesse, E, Marmorat-Khuong, A, Costa, JM, et al. Detection of Aspergillus DNA in bronchoalveolar lavage fluid of AIDS patients by the polymerase chain reaction. Eur J Clin Microbiol Infect Dis. 1997; 16:24–25.
79. Bretagne, S, Costa, JM, Marmorat-Khuong, A, et al. Detection of Aspergillus species DNA in bronchoalveolar lavage samples by competitive PCR. J Clin Microbiol. 1995; 33:1164–1168.
80. Reichenberger, F, Habicht, JM, Gratwohl, A, et al. Diagnosis and treatment of invasive pulmonary aspergillosis in neutropenic patients. Eur Respir J. 2002; 19:743–755.
81. Bottone, EJ, Sindone, M, Caraballo, V. Value of assessing cryptococcal antigen in bronchoalveolar lavage and sputum specimens from patients with AIDS. Mt Sinai J Med. 1998; 65:422–425.
82. Nadrous, HF, Antonios, VS, Terrell, CL, et al. Pulmonary cryptococcosis in nonimmunocompromised patients. Chest. 2003; 124:2143–2147.
83. Martynowicz, MA, Prakash, UB. Pulmonary blastomycosis: An appraisal of diagnostic techniques. Chest. 2002; 121:768–773.
84. McAdams, HP, Rosado-de-Christenson, ML, Lesar, M, et al. Thoracic mycoses from endemic fungi: Radiologic-pathologic correlation. Radiographics. 1995; 15:255–270.
85. Valdivia, L, Nix, D, Wright, M, et al. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg Infect Dis. 2006; 12:958–962.
86. Wheat, LJ, Kauffman, CA. Histoplasmosis. Infect Dis Clin North Am. 2003; 17:1–19.
87. Baughman, RP, Dohn, MN, Loudon, RG, et al. Bronchoscopy with bronchoalveolar lavage in tuberculosis and fungal infections. Chest. 1991; 99:92–97.
88. Bialek, R, Gonzalez, GM, Begerow, D, et al. Coccidioidomycosis and blastomycosis: Advances in molecular diagnosis. FEMS Immunol Med Microbiol. 2005; 45:355–360.
89. Hage, CA, Davis, TE, Egan, L, et al. Diagnosis of pulmonary histoplasmosis and blastomycosis by detection of antigen in bronchoalveolar lavage fluid using an improved second-generation enzyme-linked immunoassay. Respir Med. 2007; 101:43–47.
90. Angeles Marcos, M, Camps, M, Pumarola, T, et al. The role of viruses in the aetiology of community-acquired pneumonia in adults. Antiviral Ther. 2006; 11:351–359.
91. Bartlett, JG, Mundy, LM. Community-acquired pneumonia. N Engl J Med. 1995; 333:1618–1624.
92. de Roux, A, Marcos, MA, Garcia, E, et al. Viral community-acquired pneumonia in nonimmunocompromised adults. Chest. 2004; 125:1343–1351.
93. Greenberg, SB. Viral pneumonia. Infect Dis Clin North Am. 1991; 5:603–621.
94. Chien, JW, Johnson, JL. Viral pneumonias. Infection in the immunocompromised host. Postgrad Med. 2000; 107:67–74.
95. Connolly, MG, Jr., Baughman, RP, Dohn, MN, et al. Recovery of viruses other than cytomegalovirus from bronchoalveolar lavage fluid. Chest. 1994; 105:1775–1781.
96. Leland, DS, Emanuel, D. Laboratory diagnosis of viral infections of the lung. Semin Respir Infect. 1995; 10:189–198.
97. Ruutu, P, Ruutu, T, Volin, L, et al. Cytomegalovirus is frequently isolated in bronchoalveolar lavage fluid of bone marrow transplant recipients without pneumonia. Ann Intern Med. 1990; 112:913–916.
98. Osiowy, C. Direct detection of respiratory syncytial virus, parainfluenza virus, and adenovirus in clinical respiratory specimens by a multiplex reverse transcription-PCR assay. J Clin Microbiol. 1998; 36:3149–3154.
99. Fagon, JY. Diagnosis and treatment of ventilator-associated pneumonia: Fiberoptic bronchoscopy with bronchoalveolar lavage is essential. Semin Respir Crit Care Med. 2006; 27:34–44.
100. Casetta, M, Blot, F, Antoun, S, et al. Diagnosis of nosocomial pneumonia in cancer patients undergoing mechanical ventilation: A prospective comparison of the plugged telescoping catheter with the protected specimen brush. Chest. 1999; 115:1641–1645.
101. Johanson, WG, Jr., Seidenfeld, JJ, Gomez, P, et al. Bacteriologic diagnosis of nosocomial pneumonia following prolonged mechanical ventilation. Am Rev Respir Dis. 1988; 137:259–264.
102. Chastre, J, Viau, F, Brun, P, et al. Prospective evaluation of the protected specimen brush for the diagnosis of pulmonary infections in ventilated patients. Am Rev Respir Dis. 1984; 130:924–929.
103. Gracia, JD, Miravitlles, M, Mayordomo, C, et al. Empiric treatments impair the diagnostic yield of BAL in HIV-positive patients. Chest. 1997; 111:1180–1186.
104. Montravers, P, Fagon, JY, Chastre, J, et al. Follow-up protected specimen brushes to assess treatment in nosocomial pneumonia. Am Rev Respir Dis. 1993; 147:38–44.
105. Prats, E, Dorca, J, Pujol, M, et al. Effects of antibiotics on protected specimen brush sampling in ventilator-associated pneumonia. Eur Respir J. 2002; 19:944–951.
106. Torres, A, el-Ebiary, M, Padro, L, et al. Validation of different techniques for the diagnosis of ventilator-associated pneumonia. Comparison with immediate postmortem pulmonary biopsy. Am J Respir Crit Care Med. 1994; 149:324–331.
107. Falco, V, Fernandez de Sevilla, T, Alegre, J, et al. Legionella pneumophila. A cause of severe community-acquired pneumonia. Chest. 1991; 100:1007–1011.
108. Lieberman, D, Schlaeffer, F, Boldur, I, et al. Multiple pathogens in adult patients admitted with community-acquired pneumonia: A one year prospective study of 346 consecutive patients. Thorax. 1996; 51:179–184.
109. Stout, JE, Yu, VL. Legionellosis. N Engl J Med. 1997; 337:682–687.
110. Kohorst, WR, Schonfeld, SA, Macklin, JE, et al. Rapid diagnosis of Legionnaires’ disease by bronchoalveolar lavage. Chest. 1983; 84:186–190.
111. Fagon, JY, Chastre, J, Wolff, M, et al. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann Intern Med. 2000; 132:621–630.
112. Chien, JW, Johnson, JL. Viral pneumonias. Multifaceted approach to an elusive diagnosis. Postgrad Med. 2000; 107:67–72.
113. Chuang, ML, Lin, IF, Tsai, YH, et al. The utility of open lung biopsy in patients with diffuse pulmonary infiltrates as related to respiratory distress, its impact on decision making by urgent intervention, and the diagnostic accuracy based on the biopsy location. J Intensive Care Med. 2003; 18:21–28.
114. Flabouris, A, Myburgh, J. The utility of open lung biopsy in patients requiring mechanical ventilation. Chest. 1999; 115:811–817.
115. Canzian, M, Soeiro Ade, M, Taga, MF, et al. Correlation between surgical lung biopsy and autopsy findings and clinical data in patients with diffuse pulmonary infiltrates and acute respiratory failure. Clinics (Sao Paulo). 2006; 61:425–432.
116. Canver, CC, Mentzer, RMJr. The role of open lung biopsy in early and late survival of ventilator-dependent patients with diffuse idiopathic lung disease. J Cardiovasc Surg (Torino). 1994; 35:151–155.
117. Warner, DO, Warner, MA, Divertie, MB. Open lung biopsy in patients with diffuse pulmonary infiltrates and acute respiratory failure. Am Rev Respir Dis. 1988; 137:90–94.
118. White, DA, Wong, PW, Downey, R. The utility of open lung biopsy in patients with hematologic malignancies. Am J Respir Crit Care Med. 2000; 161:723–729.
119. Baumann, HJ, Kluge, S, Balke, L, et al. Yield and safety of bedside open lung biopsy in mechanically ventilated patients with acute lung injury or acute respiratory distress syndrome. Surgery. 2008; 143:426–433.
120. Charbonney, E, Robert, J, Pache, JC, et al. Impact of bedside open lung biopsies on the management of mechanically ventilated immunocompromised patients with acute respiratory distress syndrome of unknown etiology. J Crit Care. 2009; 24:122–128.
121. Kao, KC, Tsai, YH, Wu, YK, et al. Open lung biopsy in early-stage acute respiratory distress syndrome. Crit Care. 2006; 10:R106.
122. Papazian, L, Thomas, P, Bregeon, F, et al. Open-lung biopsy in patients with acute respiratory distress syndrome. Anesthesiology. 1998; 88:935–944.
123. Cazzadori, A, Di Perri, G, Todeschini, G, et al. Transbronchial biopsy in the diagnosis of pulmonary infiltrates in immunocompromised patients. Chest. 1995; 107:101–106.
124. Jain, P, Sandur, S, Meli, Y, et al. Role of flexible bronchoscopy in immunocompromised patients with lung infiltrates. Chest. 2004; 125:712–722.
125. Martin, C, Papazian, L, Payan, MJ, et al. Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients. Chest. 1995; 107:196–200.
126. Williams, D, Yungbluth, M, Adams, G, et al. The role of fiberoptic bronchoscopy in the evaluation of immunocompromised hosts with diffuse pulmonary infiltrates. Am Rev Respir Dis. 1985; 131:880–885.
127. Papin, TA, Grum, CM, Weg, JG. Transbronchial biopsy during mechanical ventilation. Chest. 1986; 89:168–170.
128. Pincus, PS, Kallenbach, JM, Hurwitz, MD, et al. Transbronchial biopsy during mechanical ventilation. Crit Care Med. 1987; 15:1136–1139.
129. Bulpa, PA, Dive, AM, Mertens, L, et al. Combined bronchoalveolar lavage and transbronchial lung biopsy: Safety and yield in ventilated patients. Eur Respir J. 2003; 21:489–494.
130. O’Brien, JD, Ettinger, NA, Shevlin, D, et al. Safety and yield of transbronchial biopsy in mechanically ventilated patients. Crit Care Med. 1997; 25:440–446.
131. Rolston, KV. The spectrum of pulmonary infections in cancer patients. Curr Opin Oncol. 2001; 13:218–223.
132. Sharma, S, Nadrous, HF, Peters, SG, et al. Pulmonary complications in adult blood and marrow transplant recipients: Autopsy findings. Chest. 2005; 128:1385–1392.
133. Shelhamer, JH, Toews, GB, Masur, H, et al. NIH conference. Respiratory disease in the immunosuppressed patient. Ann Intern Med. 1992; 117:415–431.
134. Hage, CA, Wood, KL, Winer-Muram, HT, et al. Pulmonary cryptococcosis after initiation of anti-tumor necrosis factor-alpha therapy. Chest. 2003; 124:2395–2397.
135. Happel, KI, Nelson, S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005; 2:428–432.
136. Imaizumi, K, Sugishita, M, Usui, M, et al. Pulmonary infectious complications associated with anti-TNF-alpha therapy (infliximab) for rheumatoid arthritis. Intern Med. 2006; 45:685–688.
137. Kristan, SS, Kern, I, Music, E. Invasive pulmonary aspergillosis. Respiration. 2002; 69:521–525.
138. Wood, KL, Hage, CA, Knox, KS, et al. Histoplasmosis after treatment with anti-tumor necrosis factor-alpha therapy. Am J Respir Crit Care Med. 2003; 167:1279–1282.
139. Weiss, SM, Hert, RC, Gianola, FJ, et al. Complications of fiberoptic bronchoscopy in thrombocytopenic patients. Chest. 1993; 104:1025–1028.
140. Cordasco, EM, Jr., Mehta, AC, Ahmad, M. Bronchoscopically induced bleeding. A summary of nine years’ Cleveland clinic experience and review of the literature. Chest. 1991; 100:1141–1147.
141. Anzueto, A, Levine, SM, Jenkinson, SG. The technique of fiberoptic bronchoscopy. Diagnostic and therapeutic uses in intubated, ventilated patients. J Crit Illness. 1992; 7:1657–1664.
142. Brandstetter, RD, Croce, SA, Schiaffino, E, et al. Flexible fiberoptic bronchoscopy in the elderly. N Y State J Med. 1984; 84:546–548.
143. Feldman, NT, Huber, GL. Fiberoptic bronchoscopy in the intensive care unit. Int Anesthesiol Clin. 1976; 14:31–42.
144. Jolliet, P, Chevrolet, JC. Bronchoscopy in the intensive care unit. Intensive Care Med. 1992; 18:160–169.
145. Pereira, W, Jr., Kovnat, DM, Snider, GL. A prospective cooperative study of complications following flexible fiberoptic bronchoscopy. Chest. 1978; 73:813–816.
146. Bechara, R, Beamis, J, Simoff, M. Practice and complications of flexible bronchoscopy with biopsy procedures. J Bronchol. 2005; 12:139–142.
147. Lindholm, CE, Ollman, B, Snyder, J, et al. Flexible fiberoptic bronchoscopy in critical care medicine. Diagnosis, therapy and complications. Crit Care Med. 1974; 2:250–261.
148. Baughman, RP, Dohn, MN, Shipley, R, et al. Increased Pneumocystis carinii recovery from the upper lobes in Pneumocystis pneumonia. The effect of aerosol pentamidine prophylaxis. Chest. 1993; 103:426–432.
149. Meduri, GU, Reddy, RC, Stanley, T, et al. Pneumonia in acute respiratory distress syndrome. A prospective evaluation of bilateral bronchoscopic sampling. Am J Respir Crit Care Med. 1998; 158:870–875.
150. Meduri, GU, Stover, DE, Greeno, RA, et al. Bilateral bronchoalveolar lavage in the diagnosis of opportunistic pulmonary infections. Chest. 1991; 100:1272–1276.