9 Breathing and chronic pelvic pain Connections and rehabilitation features
Pelvic girdle pain: Respiratory connections
Varieties of breathing pattern disorder
Breathing rehabilitation assessment and interventions
Research on breathing as a pain intervention
Connective tissue manipulation
Trigger point deactivation and slow stretching
Introduction
The lumbopelvic cylinder: Functional and structural connections
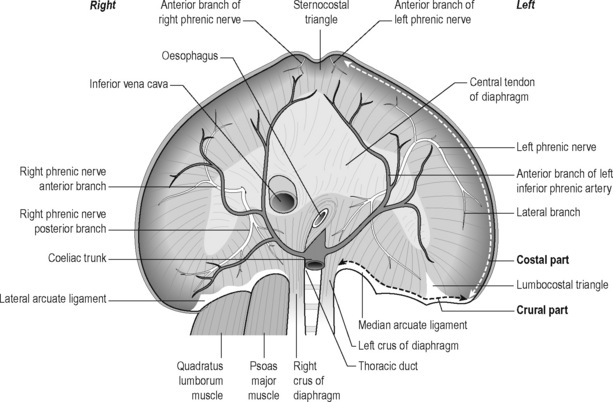
The pelvic floor and the respiratory diaphragm are structurally and functionally bound together by fascial and muscular connections (Figure 9.1). The abdominal canister has been described as a functional unit that involves the diaphragm, including its crura; psoas; obturator internus; deep abdominal wall and its associated fascial connections; deep fibres of multifidus; intercostals; quadratus lumborum; thoracolumbar vertebral column (T6–T12 and associated ribs, L1–L5) and osseus components of the pelvic girdle (Jones 2001, Gibbons 2001, Newell 2005, Lee et al. 2008). Gibbons (2001) has described the anatomical link between the diaphragm, psoas and the pelvic floor: ‘The diaphragm’s medial arcuate ligament is a tendinous arch in the fascia of the psoas major. Distally, the psoas fascia is continuous with the PF fascia, especially the pubococcygeus’. See Box 9.1 for detailed anatomy of the diaphragm.
Box 9.1 Anatomy of the diaphragm
The elliptical cylindroid-shaped diaphragm is a dome-shaped, musculotendinous structure with a non-contractile central tendon. Diaphragmatic fibres radiate peripherally to attach to all margins of the lower thorax, representing the inferior aspect of the pleural cavity, as well as a superior arch that covers the abdominal cavity (Pacia & Aldrich 1998). Its structures comprise both striated skeletal muscle and tendinous elements. When the diaphragm contracts, it increases the vertical, transverse and anteroposterior diameter of the internal thorax (Kapandji 1974).
The lumbar, costal and sternal muscular components (Schumpelick & Steinau 2000) (see Figures 9.1 and 9.2)
The muscular segments of the diaphragm originate from the entire circumference of the lower thoracic aperture: from the lumbar spine, ribs and sternum. There are three components, which are typically separated from each other by muscle-free gaps, the lumbar costal and sternal sections. These muscular diaphragmatic components insert at the central tendon, which is considered the central aponeurosis. In good health the diaphragm comprises type I, slow-twitch, fatigue-resistant muscle fibres as well as type II, fast-twitch, fatiguing muscle fibres. Fibre-type modifies in response to chronic obstructive pulmonary disease and to diaphragmatic inactivity (Anraku & Shargall 2009).
Lumbar (crural) part
1. Medial, which is tendinous in nature and lies in the fascia covering psoas major. Medially it is continuous with the corresponding medial crus and also attaches to the body of L1 or L2. Laterally it attaches to the transverse process of L1. The medial arcuate ligament is continuous medially with the lateral margin of the crus and is attached to the side of the body of the first or second lumbar vertebra. Laterally, it is fixed to the front of the transverse process of T12 and arches over the psoas muscle. Abnormal tensions in this ligament may irritate the psoas muscle, resulting in pain and spasm. Conversely psoas spasm may influence diaphragmatic mechanics (Burkill & Healy 2000, Carriero 2003, Carriere 2006).
3. The lateral arm, which is formed from a thick fascial covering that arches over the upper aspect of quadratus lumborum, to attach medially to the anterior aspect of the transverse process of L1, and laterally to the inferior margin of the 12th rib.
Carriero (2003) notes that the lateral arcuate ligament is a thickened band of fascia extending from the anterior aspect of the transverse process of the first lumbar vertebra to the lower margin of the 12th rib near its midpoint. It arches across the upper part of the quadratus lumborum muscle. Besides affecting respiratory excursion, dysfunction of the 12th rib may affect the lateral arcuate ligament, resulting in irritation of the iliohypogastric or ilioinguinal nerves that pass under it; ‘this may present as paresthesias or radiating pain over the anterior aspect of the thigh and groin with running activities’.
Costal part
Alternating with the dentations of the transverse abdominis muscle (Standring 2008), the costal part originates from the six caudal ribs, radiating into the central non-contractile tendon. In most cases, a triangle lacking muscle fibres, the lumbocostal triangle, exists between the lumbar and costal parts of the diaphragm, more commonly on the left side. In these weak areas, the gaps are usually closed only by means of pleura, peritoneum and fascia (i.e. fascia transversalis and fascia phrenicopleuralis).
Tendinous part
Schumpelick & Steinau (2000) note that the tendinous part (i.e. the central tendon) has ‘almost the shape of a cloverleaf (one anterior and two lateral leaves), with its largest expansion in the transverse plane’. The inferior vena cava, firmly anchored by connective tissue, passes through a foramen located to the right of the midline. The pericardium is also firmly attached to the cranial surface of the central tendon.
The left and right domes of the diaphragm arise lateral to the heart.
The right dome is commonly slightly higher than the left.
The location of the diaphragm is considered to be ‘variable’ (Schumpelick & Steinau 2000) depending on variables such as age, gender, posture and the extent of inhalation and exhalation, as well as on intestinal status. Any changes in the volume in the pleural or peritoneal cavity are likely to influence altered shape and position of the diaphragm.
Following full inhalation, the right dome of the diaphragm is situated close to the level of the cartilage–bone transition of the sixth rib, while the left dome is approximately one intercostal space lower (Tondury & Tillman 1998).
Newell (2005) has further detailed the relationship between psoas and quadratus lumborum, with the diaphragm and thoracic structures, observing that the posterior edge of the diaphragm crosses the psoas muscles medially, forming the medial arcuate ligaments, and the quadratus lumborum muscles laterally, forming the lateral arcuate ligaments.
• The skeletal attachments of the lateral arcuate ligaments are the first lumbar transverse process and the midpoint of the 12th rib. The costal origins include the lower six ribs and costal cartilages, the fibres of the diaphragm interdigitating with those of transversus abdominis.
• The medial arcuate ligament is continuous medially with the lateral margin of the crus, and is attached to the side of the body of the first or second lumbar vertebra. Laterally, it is fixed to the front of the transverse process of T12, and arches over the psoas muscle. Abnormal tensions in this ligament may irritate psoas, resulting in pain and spasm. Conversely psoas spasm may influence diaphragmatic mechanics (Burkill & Healy 2000, Carriere 2006).
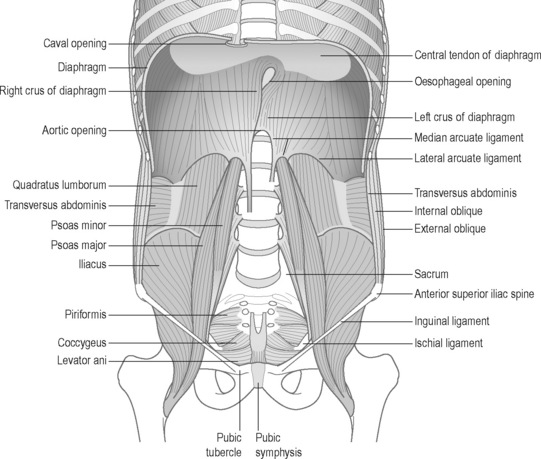
The retroperitoneal space
Lying between the posterior parietal peritoneum and the transversalis fascia is the retroperitoneal space, an anatomical region seldom discussed in relationship to CPP (Burkill & Healy 2000). This space houses (in whole or in part): the adrenal glands, kidneys, ureters, bladder, aorta, inferior vena cava, oesophagus (part), superior two-thirds of the rectum; as well as parts of the pancreas, duodenum and colon (Ryan et al. 2004). This area involves vital connections that intimately bind pelvic and thoracic structures. The anterior pararenal space extends superiorly to the dome of the diaphragm, and hence to the mediastinum. Inferiorly it communicates with the pelvis and below the inferior renal cone with the posterior pararenal space. The posterior pararenal opens inferiorly towards the pelvis but fuses superiorly with the posterior perirenal fascia the fascia of the quadratus lumborum (QL) and psoas muscles (Burkill & Healy 2000).
Grewar & McLean (2008) indicate that respiratory dysfunctions are commonly seen in patients with low back pain, pelvic floor dysfunction and poor posture. Additional evidence exists connecting diaphragmatic and breathing pattern disorders, with various forms of pelvic girdle dysfunction (including sacroiliac pain) (O’Sullivan et al. 2002, O’Sullivan & Beales 2007) as well as with CPP and associated symptoms, such as stress incontinence (Hodges et al. 2007). Similarly Carriere (2006) noted that disrupted function of either the diaphragm or the PFM may alter the normal mechanisms for regulating intra-abdominal pressure (IAP).
The presence of dysfunctional breathing patterns which influence pelvic function (McLaughlin 2009) and pelvic dysfunction which influences breathing patterns (Hodges et al. 2007) therefore suggests that rehabilitation of the thorax, pelvic girdle and pelvic floor will be enhanced by more normal physiological breathing patterns. This can be achieved through exercise, breathing retraining, postural reeducation, manual therapy and other means (Chaitow 2007, O’Sullivan & Beales 2007, McLaughlin 2009).
Interaction of CPP, pelvic girdle pain and breathing pattern disorders aetiological features
• Aetiologically, pregnancy or trauma may result in pelvic girdle problems and pain, via skeletal malalignment, such as separation of the symphysis pubis or sacroiliac dysfunction (Shuler & Gruen 1996).
• Additionally, the development of low back pain during pregnancy increases the odds of developing pelvic floor disorder complaints (Pool-Goudzwaard et al. 2004).
• The combined prevalence of lumbopelvic pain, incontinence and breathing disorders has suggest that pelvic floor dysfunction is related to altered breathing patterns or disorders of breathing (Smith et al 2006, 2007, O’Sullivan & Beales 2007).
• Hodges et al. (2007) observe that there is a clear connection between sacroiliac joint (SIJ) stability and respiratory and pelvic floor function, particularly in women. They suggest that if the PFM are dysfunctional, spinal support may be compromised, increasing obliquus externus activity, which in turn may alter PFM activity.
• It is suggested that any part of the structural unit, involving the respiratory diaphragm and the pelvic floor (‘pelvic diaphragm’), that fails to operate efficiently, will necessarily influence the function of other aspects of the complex.
Postural and breathing patterns as aetiological features
In a study involving 40 women with CPP, 20 received standard gynaecological attention, while the 20 women in the experimental group received the same attention, together with somatocognitive therapy, comprising postural, movement, gait and breathing assessment, re-education and rehabilitation. Haugstad et al. (2006a) observed that in the experimental group, women with CPP ‘typically’ displayed upper chest breathing patterns, with almost no movement of the thorax or the abdominal area. Haugstad et al. (2006a) were also able to confirm ‘a characteristic pattern of standing, sitting, and walking, as well as lack of coordination and irregular high costal respiration’. Of interest in relation to diaphragmatic function was their finding that: ‘the highest density, and the highest degree of elastic stiffness, [was] found in the iliopsoas muscles’.
Key (2010) suggests that clinicians should keep in mind: ‘the continuous, largely internal three dimensional myofascial web, providing a scaffold of tensile inner support and stability [ … ] contributing to a structural and functional bridge between the lower torso and legs’. Key also notes that: ‘This includes the obvious contractile elements for which there is accumulating evidence of deficient function in subjects with low back and/or pelvic pain – the transversus abdominis (Hodges & Richardson 1996, 1998, 1999), multifidus (Hides et al. 1996), the diaphragm and PFM’.
Impressions from clinical practice suggest attention should also be given to the obturators, iliacus, psoas, and all their related and interconnecting fascial sheaths. Sound activity within this myofascial ‘inner stocking’ sustains many functional roles: providing deep anterior support to the lower half of the spinal column; with the spinal intrinsic muscles it contributes to lumbopelvic control (Hodges 2004); while also contributing to the generation of IAP (Cresswell et al. 1994), continence and respiration (Figure 9.2).
The lack of normal diaphragmatic movement in individuals with breathing pattern disorders (BPD) deprives the viscera and abdominal cavity of rhythmic stimulation (internal ‘massage’) which may be important for maintaining normal pelvic circulation. Pelvic pain and congestion have been correlated with chronic muscle tension, chronic hypoxia, as well as accumulation of metabolites such as lactic acid and potassium (Kuligowska et al. 2005).
Jones (2001) has summarized the integrated structural and functional thoracopelvic unit as follows:
Pelvic girdle pain: Respiratory connections
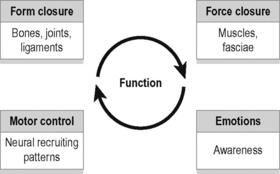
As discussed in Chapter 2, stabilization of the SIJs is enhanced by a combination of self-bracing and self-locking mechanisms, which have colloquially been described as ‘form closure’ (Vleeming et al. 1990a) and ‘force closure’ (Snijders et al. 1997, Hu et al. 2010) (Figure 9.3).
Cusi (2010) has suggested that shear is prevented by a combination of the specific anatomical features (form closure) and the compression generated by muscles and ligaments (force closure) that can accommodate to specific loading situations. Force closure has been defined as the effect of changing joint reaction forces generated by tension in ligaments, fasciae, and muscles and ground reaction force (Vleeming et al. 1990a, 1990b).
A significant part of this process involves increases in muscular, ligamentous and fascial stiffness, including that of the thoracolumbar fascia, and the multifidus and transversus abnominis, i.e. the major local stabilizers of the lumbar spine and the pelvis (Mens et al. 2001).
Additionally, and important to this discussion, using cadaveric studies the PFM have been shown to be capable of enhancing stiffness in the lumbar-pelvic region of women (Pool-Goudzwaard et al. 2004).
By performing biomechanical analysis of SIJ stability, Pel et al. (2008) have demonstrated that the training of transversus abdominis and the PFM helps to relieve SIJ related pelvic pain, via reduction of vertical shear forces. In rehabilitation of sacroiliac dysfunction, related to force closure, Cusi (2010) notes that a successful exercise programme needs to be specific, targeted and progressive. The initial demands of such a programme require the individual to develop the ability to recruit transversus abdominis, deep multifidus and the muscles of the pelvic floor.
Hodges et al. (2001) have demonstrated that, after approximately 60 seconds of over-breathing (hyperventilation), the postural (tonic) and phasic functions of both the diaphragm and transversus abdominis are reduced or absent, with major implications for spinal and sacroiliac stability. As major hip flexors the psoas muscles have the potential to influence pelvic girdle position and function. They should therefore attract therapeutic attention (along with the accessory breathing muscles) in any attempt to rehabilitate respiratory or pelvic function.
Gut connections to CPP and to respiration
Various studies of pelvic pain patients have shown irritable bowel syndrome (IBS) to be a common co-morbid condition (Zondervan et al. 1999, Whitehead et al. 2002). IBS, defined as pain more than once a month, associated with bloating and altered bowel habit (Moore & Kennedy 2000), is common in women with CPP. For example in one study, among 798 women referred to a gynaecology clinic, the incidence of IBS was 37%, compared to 28% among women attending ENT or dermatology clinics. Among those with chronic pain symptoms (including dyspareunia or dysmenorrhoea), the incidence was 50% (Prior et al. 1989).
Ford et al. (1995) have reported on the high incidence of increased colonic tone and dysfunction in hyperventilating individuals. Hypocapnic hyperventilation (low CO2 blood levels) produces an increase in colonic tone and phasic contractility in the transverse and sigmoid regions. These findings are consistent with either inhibition of sympathetic innervation to the colon, or the direct effects of hypocapnia on colonic smooth muscle contractility, or both.
It has also been observed – based on rectal and anal sphincter recordings – that during defecation the respiratory diaphragm and abdominal wall contract together, which results in an increase in IAP and rectal pressure (Olsen & Rao 2001).
Additionally pelvic floor contraction during exhalation allows for synergy between the pelvic and respiratory diaphragms (Prather et al. 2009), suggesting that when normal, respiratory function and the pelvic floor can be seen to synchronize intimately.
In the study by Prior et al. (1989), the authors did not seek to address whether IBS was the cause of the pelvic pain, but in a similar study, patients with symptoms of IBS were found to be less likely to receive a positive gynaecological diagnosis, and more likely to be still in pain, one year later, than patients without IBS symptoms (Whitehead et al. 2002). Rosenbaum & Owens (2008) note that gastroenterological conditions, such as coeliac disease and IBS, affect sexual function/comfort (Fass et al. 1998).
The anatomical location and innervation of both bladder and colon mean that they share similar vital functions, so that malfunction of one organ may result in a functional disturbance in the other. Furthermore the concepts of organ cross-talk, and organ cross-sensitization, between the bladder and the colon are important in the understanding of complex CPP syndromes (Watier 2009).
An integrated system
The concept of an integrated continence system (Grewar & McLean 2008) allows some coherence to be identified in apparently random presence of pain and dysfunction, in the pelvic region. Grewar & McLean suggest that the foundational mechanisms that support continence are relatively impervious to manual therapy when dysfunctional. However, there are also ‘external’ features that exert influence over these structural components – which are potentially modifiable.
• Motor control factors – including postural and movement dysfunction, BPD, pelvic floor dysfunction and low back and pelvic girdle dysfunction;
• Musculoskeletal features – including altered muscle strength, length and range of motion;
• Behavioural factors – such as physical inactivity, psychosocial issues, abnormal IAP and dysfunctional bowel and bladder habits.
There is evidence that respiration also has an influence on motor control (Butler 2000, Chaitow 2004) – emphasizing its importance amongst those factors to be considered in rehabilitation of continence dysfunction.
Varieties of breathing pattern disorder
Courtney et al. (2008) and Courtney & Greenwood (2009) suggest a distinction can be made between those BPD that appear to have a predominately biomechanical nature – where the patient may have a ‘perception of inappropriate, or restricted, breathing’, as distinguished from BPDs where a chemoreceptor aetiology may exist, for example linked to reported sensations such as there being a ‘lack of air’. Courtney et al. (2008) note that the sensory quality of ‘air hunger’ or ‘urge to breathe’ is most strongly linked to changes in blood gases, such as CO2, or changes in the respiratory drive deriving from central and peripheral afferent input. These sensations may be distinguishable from breathing sensations related to the effort of breathing, which are biomechanical in nature (Simon et al. 1989, Banzett et al. 1990, Lansing 2000, Chaitow et al. 2002).
Questionnaires exist for assessment of these BPD variations, with the Nijmegen Questionnaire (NQ) (van Dixhoorn & Duivenvoorden 1985) having greater relevance for hyperventilation, and the Self-Evaluation Breathing Questionnaire (SEBQ) (Courtney et al. 2009) discriminating between the chemoreceptor and the biomechanical variations of BPD (see Appendix).
Irrespective of the major aetiological features (see above and listed below in Box 9.2), chronic BPD results in altered function and, in time, structure of accessory and obligatory respiratory muscles. It is suggested that these should attract therapeutic attention in any attempt to normalize breathing, or the distant effects of BPD, on pelvic function (Chaitow 2004).
Box 9.2 Aetiological features in BPD
• Acidosis: Hyperventilation may represent a homeostatic response to acidosis. Chaulier et al. (2007) note that acidosis may result from iatrogenic sources, major hypoxaemia, cardiovascular collapse or sepsis.
• Atmosphere/altitude: ‘During expeditions … mountaineers have extremely low values of arterial oxygen saturation (SaO2), similar to those of patients with severe respiratory failure’. Hyperventilation would be the physiological response to this (Botella De Maglia et al. 2008). Altitude implications are not confined to mountaineers. Travellers to, for example, Johannesburg, Mexico City or Denver, would find themselves at altitude and potentially hyperventilating for some days, or weeks, before acclimatizing.
• Allergies/intolerances: Haahtela et al. (2009) report that airway inflammation commonly affects swimmers, ice hockey players, and cross-country skiers, which suggests multifactorial features in which both allergic and irritant mechanisms play a role in resultant over-breathing.
• Diabetic ketoacidosis (DKA): Patients with DKA generally present with classic clinical findings of hyperventilation, altered mental status, weakness, dehydration, vomiting and polyuria (Bernardon et al. 2009; see also Kitabchi et al. 2006).
• Hormonal – progesterone, oestradiol: Slatkovska et al. (2006) demonstrated that phasic menstrual cycle changes in PaCO2 may be partially due to stimulatory effects of progesterone and oestradiol on ventilatory drive. See also Damas-Mora et al. (1980).
• Pregnancy: Jensen et al. (2008) suggest that hyperventilation and attendant hypocapnia/alkalosis during pregnancy result from an interaction of pregnancy-induced changes in central chemoreflex drives to breathe and wakefulness, acid–base balance, metabolic rate and cerebral blood flow.
• Pseudo-asthma: A high proportion of individuals diagnosed as asthmatics have been shown to in fact be hyperventilators.
• Sleep disorders: There is an direct temporal, and possibly a etiological, connection, between sleep disorders and overbreathing, including sleep apnoea and cardiorespiratory fitness (Vanhecke et al. 2008).
Breathing pattern disorders – The postural connection
Carriere (2006) has reported that respiratory dysfunction is commonly observed in patients with low back pain and pelvic floor dysfunction.
Key et al. (2007) have observed and catalogued a number of variations within the patterns of compensation/adaptation associated with chronic postural realignment involved in crossed-syndromes commonly associated with pelvic deviation.
In Figure 9.4A the major features include:
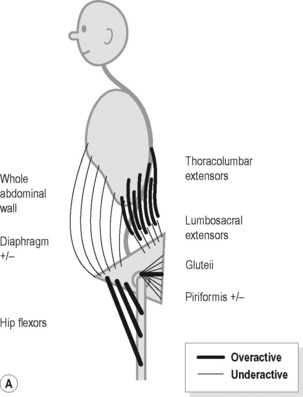
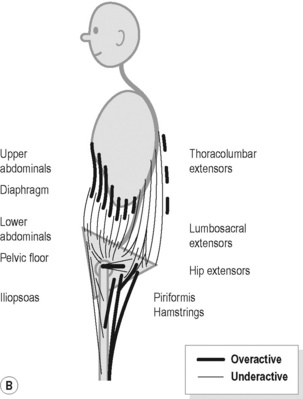
Figure 9.4 • Schematic views of (A) posterior (B) anterior pelvic crossed syndrome.
Reproduced from Key (2010) J. Bodyw. Mov. Ther. M. 14, 299–301.
In Figure 9.4B the major features include:
Examples
1. Haugstad et al. (2006a) evaluated 60 women with CPP, compared to healthy controls. They reported that in the standing posture, the area of support was minimal, with the feet being posed close together, the pelvic area pushed forward, and the shoulders and upper parts of the back pulled backwards. Compare this description with Figure 9.4B. In addition they identified a common pattern of high costal respiration with almost no movement in the thorax or in the abdominal area.
2. Psoas involvement has been identified in men with CPP. In a case-control series Hetrick et al (2003) noted that: ‘controls and patients with pain showed a significant difference in muscle spasm, increased muscle tone, pain with internal transrectal palpation of the pelvic muscles, and increased tension and pain with palpation of the levator ani and coccygeus muscles (P <0.001), as well as significantly greater pain and tension with palpation of the psoas muscles and groin’.
Repercussions of breathing pattern disorders
BPD has been shown to potentially have multiple, body-wide, influences which are summarized below.
Nixon & Andrews (1996) vividly summarize a common situation applying to the individual with BPD tendencies: ‘Muscular aching at low levels of effort; restlessness and heightened sympathetic activity; increased neuronal sensitivity; and, constriction of smooth muscle tubes (e.g. the vascular, respiratory and gastrointestinal) can accompany the basic symptom of inability to make and sustain normal levels of effort’.
Breathing pattern disorders (with hyperventilation as the extreme of this) may influence health by:
• Altering blood pH, creating respiratory alkalosis (Pryor & Prasad 2002, Celotto et al. 2008);
• Inducing increased sympathetic arousal, altering neuronal function – including motor control (Dempsey et al. 2002, Brotto et al. 2009);
• Encouraging a sense of apprehension, anxiety, affecting balance, muscle tone and motor control (Rhudy & Meagher 2000, Balaban & Thayer 2001, Van Dieën et al. 2003);
• Depleting Ca and Mg ions, enhancing sensitization, encouraging reduced pain threshold and the evolution of myofascial trigger points (Gardner 1996, Cimino et al. 2000, Schleifer et al. 2002, Simons et al. 1999);
• Triggering smooth muscle cell constriction, leading to vasoconstriction and/or spasm – including colon spasm (Ford et al. 1995, Yokoyama et al. 2008, Debreczeni et al. 2009) or pseudo-angina (Evans et al. 1980, Wilke et al. 1999);
• Reducing oxygen release to cells, tissues, brain (Bohr effect) so encouraging ischaemia, fatigue, pain and the evolution of myofascial trigger points (Freeman & Nixon 1985, Suwa 1995);
• Creating biomechanical overuse stresses and compromising core stability and posture (Lewit 1980, 1999, Haugstad et al. 2006b, Hodges et al. 2007).
BPD and hyperventilation: Physical features – Implications for rehabilitation
Deep and rapid breathing (hyperpnoea) results in progressive muscular fatigue and increasing sensations of distress, to the point of breathlessness. For example, Renggli et al. (2008) report that during normocapnic hyperpnoea (involving partial rebreathing of CO2), contractile fatigue of the diaphragm and abdominal muscles develops, long before task failure, triggering an increased recruitment of rib cage muscles. Since the diaphragm and abdominal muscles are key features of low back and pelvic stability, the implications for core instability of chronic, habitual, overbreathing – where normocapnic hyperpnoea would be unlikely – are clear. Respiratory alkalosis, and its numerous effects as described earlier in this chapter, would then accompany reduced pelvic and low back stability.
• The implication is that methods to help avoidance of hyperpnoea should be a feature of breathing retraining.
Hudson et al. (2007) observe that human scalenes are obligatory inspiratory muscles that have a greater mechanical advantage than sternocleidomastoid (SCM) muscles, which are accessory respiratory muscles. They found that irrespective of respiratory tasks these muscles are recruited in the order of their mechanical advantages – with scalenes starting to operate earlier than SCM, involving what they term to be an ‘efficient, fail-safe, system of neural control’.
• The implication in breathing rehabilitation is to ensure that these muscles receive focused attention as to their functionality.
Schleifer et al. (2002) recapitulate the known effects of overbreathing which they have identified as occurring in stress-related work settings:
• The implications suggest that these changes: ‘provide a unique rationale for coping with job stress and musculoskeletal discomfort through breathing training, light physical exercise, and rest breaks’.
Masubuchi et al. (2001) used fine-wire electrodes inserted into muscles, and high-resolution ultrasound, to identify the activity of three muscle groups, in response to various respiratory and postural manoeuvres. They concluded that the scalenes are the most active, and trapezius the least active, cervical accessory inspiratory muscles, while SCM is intermediate.
• This confirms what has long been suspected by observation and palpation – that the scalenes are the most important respiratory muscle group lying superior to the thorax.
Scalene dysfunction and the presence of trigger points (‘functional pathology’) were identified in excess of 50% of individuals, in a series of 46 hospitalized patients who demonstrated paradoxical patterns of respiration. A combination of Muscle Energy Technique (‘post-isometric relaxation’) and self-stretching of the scalenes, was used during rehabilitation (Pleidelová et al. 2002).
• The implication is that these key respiratory muscles require focused attention via palpation and appropriate therapeutic interventions, as part of breathing rehabilitation.
Renggli et al. (2008) showed (see above) that the progressive fatigue of the diaphragm and abdominal muscles, during overbreathing, results in recruitment of the muscles of the rib cage (intercostals).
Han et al. (1993) described the action, and interaction, of these rib cage muscles, during ventilation, noting that the parasternal intercostal muscles, act in concert with the scalenes to expand the upper rib cage, and/or to prevent it from being drawn inward by the action of the diaphragm, during quiet breathing. The respiratory activity of the external intercostals however appear to constitute a reserve system, only to be recruited when increased expansion of the rib cage is required.
• The implications of this information point to the need for attention to the often-neglected intercostal muscles, during breathing rehabilitation.
Earlier in this chapter the relationship between the psoas and quadratus lumborum muscles, and the retroperineal space, the pelvic floor, the pelvic girdle and respiratory function have been summarized (see Burkill & Healy 2000, Hetrick et al. 2003, Haugstad et al. 2006a, Key et al. 2007, Lee et al. 2008).
Viscerosomatic effects
Prather et al. (2009) expand on these relationships in a review of the anatomy, evaluation and treatment of musculoskeletal pelvic floor pain in women. They note that persistent muscle contraction of the pelvic floor, related to noxious visceral stimulation, such as that deriving from endometriosis or IBS, can lead to splinting and pain, with reduction of normal PFM function. Specifically, they report that viscerosomatic reflex activity may be responsible for increased resting tone of the pelvic floor with reduced ability to fully relax the muscle group as a whole. As a result, they suggest, adaptation occurs via recruitment of global muscles in the region – e.g. psoas and iliacus – leading to symptoms such as posterior pelvic and low back pain.
Tu et al. (2008) compared the biomechanical features of the pelvic girdle, as well as the associated muscles in 20 CPP patients and 20 normal controls. Among their findings – relevant to this chapter – are the following:
1. Several tests of pelvic girdle instability were more common in CPP cases than in controls (asymmetric iliac crest and pubic symphysis heights and positive posterior pelvic provocation testing).
2. In addition, patients with CPP were more tender on palpation of the left oblique, right and left rectus and right psoas (P > 0.05). Previous evidence, described above, suggests that such changes would be likely to impair respiration; however, this feature was not a part of Tu et al.’s study.
• Optimal rehabilitation of breathing function, requires appropriate attention to both psoas and QL status, as well as key accessory and obligatory respiratory muscles. Additionally, optimal rehabilitation of the pelvic floor requires attention to respiration and the multiple structures that influence it.
‘Biologically unsustainable patterns’ (Garland 1994)
He describes a series of changes including:
• Visceral stasis/pelvic floor weakness;
• Abdominal and erector spinae muscle imbalance;
• Fascial restrictions from the central tendon via the pericardial fascia to the basiocciput;
• Upper rib elevation with increased costal cartilage tension;
• Thoracic spine dysfunction and possible sympathetic disturbance;
• Accessory breathing muscle hypertonia and fibrosis;
• Evolution of myofascial trigger points in hypertonic and ischemic tissues;
• Promotion of rigidity in the cervical spine with possibility of fixed lordosis;
• Reduction in mobility of 2nd cervical segment and disturbance of vagal outflow.
Myofascial trigger points
Within the patterns of overuse and misuse that characterize postural and respiratory insults to the body as described above, the evolution of myofascial trigger points is a common feature (Lewit 1999, Travell & Simons 1999, Schleifer et al. 2002, Anderson et al. 2009, Key 2010). Greater detail regarding myofascial trigger points can be found in Chapter 14. One of the pioneers of manual approaches to CPP, Slocumb (1984) described how trigger points in the following areas can all produce virtually identical referred pelvic pain:
2. In tissue overlying the pubic bone;
4. Tissues lateral to the cervix;
5. Close to vaginal cuff scar tissue more than 3 months after hysterectomy;
In addition, Weiss (2001), Anderson et al. (2009), Fitzgerald et al. (2009) and many others have unequivocally demonstrated that trigger points are the cause of serious levels of pelvic pain and dysfunction.
What remains unclear is trigger point causation, and the potential functionality in some circumstances (e.g. ligamentous laxity) of the effects of myofascial trigger points. Recently, the European Association of Urology has published guidelines suggesting that trigger points should be considered in the diagnosis of CPP (Fall et al. 2010).
Breathing rehabilitation assessment and interventions (Chaitow et al. 2002)
For example, in relation to retraining, Mattsson et al. (2000) have reported altered patterns of posture, movement and respiration in a study of women with CPP. In the context of treating women with CPP who had a history of sexual abuse, using what is termed psychiatric physiotherapy to develop body awareness, Mattsson et al. (1997) note that ‘Focusing on breathing … mostly works indirectly by practicing to become more aware of one’s breathing in different situations’.
Breathing retraining appears to require a combination of elements for best results:
1. Understanding the processes – a cognitive, intellectual, awareness of the mechanisms and issues involved in BPDs;
2. Retraining exercises that include aspects that operate subcortically, allowing replacement of currently habituated patterns with more appropriate ones;
3. Biomechanical structural modifications that remove obstacles to desirable and necessary functional changes;
4. Time for these elements to merge and become incorporated into moment-to-moment use patterns.
Box 9.3 offers a summary of physical medicine approaches as utilized by one of this chapter’s authors (L.C.).
Box 9.3 Phases of breathing intervention (modified from McLaughlin 2009)
Assessment
• Identify symptoms related to poor breathing chemistry and biomechanics
• Observe breathing pattern, e.g. paradoxical pattern/upper chest (Courtney et al. 2008, 2009)
• Observe posture, particularly crossed patterns (Key et al. 2007)
• Assess spinal, rib mobility/restriction, form/force closure (active straight leg raise test), shortness and/or weakness of key muscles, as well as assessing for active trigger points (Lee & Lee 2004, Lee 2007, Lee et al. 2008)
• Identify BPD triggers (pain, stress, situations, thoughts, emotions, etc.)
• Identify faulty breathing behaviours (upper chest, no pause between breaths, etc.)
• Utilize questionnaires (Nijmegen, SEBQ; see Appendix)
• Utilize palpation assessments such as HiLo and manual assessment of breathing pattern (see text)
Education
Retraining
• Teach smooth and rhythmic breathing methods in which ratio of inhalation to exhalation is roughly 1:2 (i.e. exhale should take longer). A key to changing breathing behaviour is to focus on long, slow exhalation, informing patient that, if this is adequate, ‘inhalation takes care of itself’
• Consider home use of a capnograph designed for biofeedback can help skill acquisition
Behaviour modification
• Modify poor breathing in response to subtler and subtler cues through increased awareness of the symptoms and mechanics of both poor and good breathing
• Teach basic strategies to inhibit habitual overuse of accessory breathing muscles on inhalation (see below)
• Encourage daily practice, morning and evening, of breathing exercises, to reinforce new learning
Manual therapy
• If restrictions are identified in the articular or myofascial tissues of the trunk or cervical spine, use manual therapy to free the tightness and provide extensibility, particularly of key muscles: psoas, QL, scalenes, intercostals, diaphragm attachment region. See examples later in this chapter and in Chapter 14
• If pelvic girdle structures are restricted, these should be mobilized. See Chapter 16 for examples
• If poor motor control is identified in the trunk or cervical spine add an appropriate exercise programme
• Postural correction may be required to optimize ventilation mechanics
Functional examination: Identifying the locus of motion
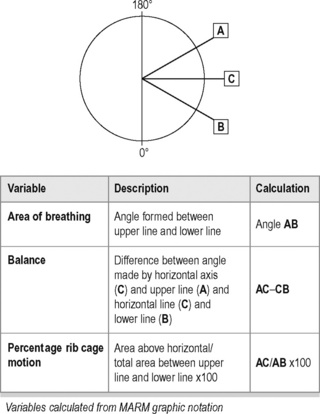
An initial assessment is required to determine whether the breathing pattern is paradoxical, upper chest or diaphragmatic/abdominal (Lewit 1980). Two validated methods are described below – the so-called HiLo test (Bradley 1998, Courtney et al. 2009), and the MARM (manual assessment of respiratory motion) method (Courtney 2008, Courtney & Greenwood 2009)![]() .
.
HiLo test
Courtney & Greenwood (2009) note that:![]() ‘The HiLo test can be used to assess the motion of the upper rib cage and lower rib cage/abdomen and determine aspects of breathing such as rate, rhythm, relative motion and phase relation of upper and lower breathing compartments’ (Figure 9.5).
‘The HiLo test can be used to assess the motion of the upper rib cage and lower rib cage/abdomen and determine aspects of breathing such as rate, rhythm, relative motion and phase relation of upper and lower breathing compartments’ (Figure 9.5).
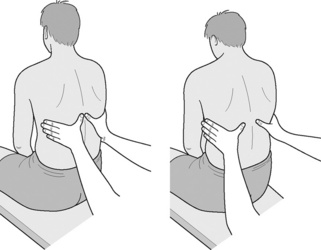
Manual assessment of respiratory motion (Figure 9.6)
The examiner sits behind the subject with hands resting lightly on the lower lateral rib cage, so as not to inhibit breathing motion. The hands should be placed so that the little fingers are oriented horizontally, with the thumbs more or less vertical. The lower fingers should be inferior to the lower ribs to allow assessment of abdominal expansion (Figure 9.6).
Charting is possible by means of two lines being drawn relative to a horizontal line representing the lumbodorsal junction (see line C on Figure 9.7). The upper line (A) represents the degree of vertical and upper thoracic motion, while the lower line (B) represents the degree of lower rib and abdominal motion. Calculations are made for thoracic diaphragm ‘balance’ and percentage of rib cage motion.
Current thoracic excursion
For the purpose of subsequent reassessment, a record of the current excursion at various levels of the thorax should be recorded. Pryor & Prasad (2002) report that normal total lateral rib excursion in the lower thorax is between 3 and 5 cm – ideally with an equal excursion bilaterally. Assessment is accurately achieved by means of a cloth tape measure![]() .
.
The reliability of measuring thoracic excursion has been established, ideally using a standard cloth tape measure (taken at 5th and 10th thoracic levels). It is suggested that upper thoracic excursion measurements should be taken at the level of the fifth thoracic spinous process and the third intercostal space at the mid-clavicular line. Lower thoracic excursion measurements should be taken at the level of the 10th thoracic spinous process and the xiphoid process. (Bockenhauer et al. 2007).
Breath-holding tests
There is no agreed ‘normal’ breath-holding time, but it can be a useful clinical point of reference. Gardner (1996) reports that patients with hyperventilation syndrome seldom breath hold beyond 10–12 seconds. In the Buteyko (1990) system, a control pause is practised regularly to encourage increased CO2 tolerance.
• Control pause: normal exhalation is held until ‘a need to breathe again’ is experienced. ‘Normal’ is between 25 and 30 seconds. Less than 15 seconds, it is suggested, represents low tolerance to CO2![]() .
.
Courtney et al. (2011) have suggested that two breath-holding tests may usefully be performed:
1. The participant exhales and holds the breath until experiencing a definite sensation of discomfort or recognizable difficulty in holding the breath (BHT-DD).
2. The time may be measured until the first involuntary movement of respiratory muscles (BHT-IRM).
Courtney et al. (2011) found that where MARM assessment demonstrated thoracic dominance, this correlated with diminished breath-holding time until first involuntary movement (BHT-IRM). They hypothesized that this may be because both measures reflect respiratory drive, with increased respiratory drive increasing the extent of thoracic breathing and decreasing breath-holding time.
Teaching control of breathing
Learning to modify and regulate breathing may at first seem unrelated to the problem of pelvic pain, but there are several ways that it can help to alleviate CPP, as well as pain in other sites. Breathing has special significance for the pelvic region, because it is a constantly occurring event that physically affects the pelvic muscles, tissues and circulatory systems. Various modes of biofeedback can be used to facilitate learning breathing control (see Chapter 13).
Advantages of controlled breathing include:
1. Promotes general calming, reduction of emotional arousal, and feeling of control.
2. Ensures that level of CO2 in bloodstream is optimal, so that pH stays in normal range, muscle tension and myofascial trigger points are not stimulated, smooth muscle is less likely to constrict, and cerebral circulation remains stable.
3. Abdominal circulation of both the blood and the lymph is stimulated by regular diaphragm action.
4. Pelvic floor muscles are ‘entrained’ by diaphragmatic action and participate in the rhythmic contraction and relaxation.
5. Heart rate variability is enhanced by breathing at a particular frequency. This also helps stabilize the autonomic nervous system (ANS).
6. The parasympathetic nervous system (PNS) becomes stronger relative to the sympathetic nervous system (SNS) because of prolonged exhalation. Imbalance of SNS relative to PNS is minimized.
Dinah Bradley, a New Zealand physiotherapist and co-author of Self-Help for Hyperventilation Syndrome (2001), recommends to patients the phrase ‘low and slow’ to encapsulate good breathing. This means breathing low down in the upper body, expanding the abdomen during inhalation, and reducing the breathing rate.
1. Practice slowing the breathing rate to 20–30% below whatever it is at that moment. Tidal volume should automatically enlarge to compensate for the reduced rate. If that goes well, try for even slower, down to six breaths per minute without straining or gasping.
2. Practice abdominal breathing, including lateral expansion of lower ribs. Stand before a mirror or use your hands to note where the body is expanding. This will usually help regain a natural breathing style and relieve overuse of chest and neck accessory breathing muscles.
3. Practice shifting between abdominal and ‘paradoxical breathing’ – meaning drawing in the abdomen and expanding the chest on the inhale. Alternating between one and the other, and comparing the two, accentuates the contrast between the two styles and strengthens the ability to shift into abdominal breathing.
4. Prolong the exhale to a maximum of twice as long as the inhale. This can be done either with diaphragm control or with pursed lips exhalation.
5. Imagine breathing as an internal downward expansion, feel the sensations of this action, and imagine the abdominal and pelvic organs being gently massaged by this breathing.
Each of the steps above can be expanded in sensory and motor detail and practised so that they are available when needed during pain flares. With practice, relaxed breathing becomes more the default, easier to access and easier to sustain when needed. See Boxes 9.4 and 9.5 for additional breathing rehabilitation methods.
Box 9.4 Example of breathing rehabilitation exercise (morning and evening, 30–40 cycles each session)![]()
Pursed lip breathing, combined with diaphragmatic breathing, enhances pulmonary efficiency (Tiep et al. 1986, Faling 1995). One study (Hochstetter et al. 2005) found that pursed lip breathing has the potential to help the individual control breathing and improve functional activity, during episodes of breathlessness associated with BPD as well as chronic obstructive lung disease.
In addition both anxiety and pain should reduce (Cappo & Holmes 1984, Grossman et al. 1985).
• The patient is seated or supine with dominant hand on the abdomen and the other hand on the chest.
• The patient is asked to breathe in through the nose, and out through the mouth, with pursed lips, ensuring diaphragmatic involvement by means of movement of the abdomen against the hand, on inhalation.
• Exhalation through the pursed lips is performed slowly, and has been shown to relieve dyspnoea, to slow the respiratory rate, increase tidal volume, and to help restore diaphragmatic function (Tiep et al, 1986, Faling 1995).
• The patient is asked to imagine blowing a thin stream of air at a candle flame about 6 inches from the mouth. Exhalation should be slow and continuous.
• After exhaling fully, without strain, a pause for a count of ‘one’ is introduced, followed by inhalation through the nose.
• Without pausing to hold the breath after inhalation, the patient is asked to again exhale slowly and fully, through pursed lips, blowing the air in a thin stream, and to then pause for a count of one.
• The inhalation and exhalation should be repeated for not less than 30 cycles.
• After some weeks of daily practice an inhalation phase which lasts 2–3 seconds, and an exhalation phase of 6–7 seconds should be achieved, without strain.
• The patient is asked to practise twice daily, and to repeat the exercise for a few minutes (6 cycles takes about a minute) every hour if anxious, or when stress levels increase.
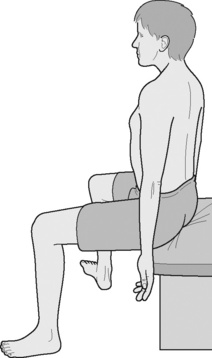
Box 9.5 Instructions to patient to minimize overuse of accessory breathing muscles during retraining, using Brugger’s Relief Position (Lewit 1999, Brugger 2000, Liebenson 2006) (see Figure 9.8)
1. Perch on chair edge, arms hanging down, feet below knees, slightly apart and turned outward.
2. Let arms hang loose, so that palms face forward.
3. Roll pelvis forward to produce slight low back arching.
4. Ease sternum slightly forward and up.
6. Practise slow, pursed lip breathing, turning arms further outward until thumbs face slightly back, on inhalation.
CO2 regulation study
McLaughlin & Goldsmith (2007) and McLaughlin (2009) described a case series of 24 patients with either chronic pelvic or lower back pain. They were included because they were either not improved from manual therapy and exercise, or their improvement had plateaued. These patients were first assessed with capnometry (Box 9.6) for level of baseline (resting) end-tidal CO2 and evidence of hyperventilation. Initially, all had lower than normal CO2 levels. They were then helped to improve their breathing pattern (more abdominal breathing, avoiding lower back bracing, nose rather than mouth breathing, lowering the breathing rate, practising breathing with capnometer feedback). Treatment was individualized to address individual aberrations from optimal breathing and to maximize the chance of generalization outside of the therapy context. The number of individual training sessions varied; the mean number was six.
Box 9.6 Assessment by capnography
The website capnography.com has basic and advanced information about monitoring CO2.
• Such widespread improvement from simply developing a more normal breathing pattern can be understood with reference to the effect of low CO2 (hypocapnia) on various body systems. Low CO2 alters the pH of the blood, making it more alkaline (respiratory alkalosis). Smooth muscle is stimulated to constrict, including blood vessels, viscera and bronchial airways. (Cardiologists use brief hyperventilation to test for susceptibility to vasospastic angina (Hirano et al. 2001).). Skeletal muscle becomes hypertonic, contraction thresholds drop, and muscle may twitch. The nervous system in general becomes hyperexcitable and oxygen delivery is reduced (haemoglobin retains O2 under alkaline conditions; see West (2008) or pulmonology texts for details).
• Mehling et al. (2005) also demonstrated the value of breathing rehabilitation in cases of chronic low back pain, when this approach was compared to standard physical therapy in a randomized, controlled study. At 6 months follow-up, patients in both groups maintained statistically significant improvements in the main outcome measures, pain reduction and functional ability.
The Mensendieck approach
This technique proceeds through three stages:
1. The ‘cognitive’ phase: attending more closely to body sensations – visual, tactile, kinesthetic and others – in essence providing a richer basis for change by maximizing attention to the feedback signalling system while focusing on more ideal sensation patterns;
2. The ‘associative’ phase: developing awareness of new or enhanced body sensations in comparison with the ideal patterns;
3. The ‘automatized’ phase – where the person uses more efficient or functional motor patterns with less or no conscious intention, and new motor and behavioural patterns are integrated into activities of daily life.
An important feature of the Mensendieck approach is its emphasis on improving poor body awareness. Practitioners have found that women with CPP are deficient in the ability to integrate proprioceptive and other interoceptive impressions with concurrent thoughts and feelings. They describe a pattern in many patients of loss of contact with parts of the body, in this case the pelvic region. This characteristic is compared to alexithymia (relative lack of ability to feel or express emotions); the parallel term ‘alexisomia’ was suggested to describe a type of dissociative process in which body awareness is suppressed. Nijenhuis (2004) surveyed the evidence and made the case for what is called ‘somatic dissociation’. This disconnection from normal somatic feedback leads to poor regulation of muscle tension, breathing, movement, and posture. Mensendieck practitioners note that CPP patients typically cannot contract their PFMs in isolation, and when asked to do so, may contract adjacent muscles instead.
In a systematic comparison of clinical characteristics of 60 women with CPP with 15 controls, several differences were apparent that may be implicated in the creation and maintenance of the pain (Haugstad et al. 2006a). The method of examination used was the Standardized Mensendieck Test, a set of prescribed movements to be observed in order to assess several features of posture, movement, gait, sitting posture and respiration. The protocol was individually administered, and movements were rated by how much they deviated from optimal performance.
In the main Mensendieck-specific study to date (Haugstad et al. 2006b) a group of 40 women with CPP were divided into two groups: both groups first received standard gynaecological treatment, including hormones, non-opioid analgesics, general education, dietary and sexual advice. Participants with major psychiatric problems were excluded. The experimental group also participated in the Mensendieck somatocognitive protocol, training in body awareness through ten individual sessions. Six months later, the symptomatic improvement in group 1 was near zero, and changes in the Mensendieck scores (posture, movement, breathing) were not evident upon retesting. The improvement in the experimental group included a near 50% reduction in pain scores, plus clear improvements in posture, movement and breathing. Breathing variables showed the most improvement.
Although the experimental design included a control group, it was not double-blinded and group 1 received less therapeutic attention than group 2. So conclusions about efficacy therefore must be tempered by these limits to the research design. However, the improvements held, and were stable at follow-up testing 1 year later. The improvements in experienced pain, psychological distress, natural movement and breathing all were maintained or increased beyond what was noted at the 90-day point (Haugstad et al. 2008) and the breathing pattern was especially improved. Natural diaphragmatic movement and abdominal expansion were generally restored.
Breathing pattern is not simply a mechanical function, but is influenced by psychological factors. For instance, Fry et al. (1997) studied social–psychological correlates of pelvic venous congestion in a series of women seeking help for CPP. Detailed interviews and questionnaires assessed social and psychological variables, present and past family background, illness history, hostility, parenting patterns and childhood sexual abuse. Compared with women having CPP but without venous congestion, those with congestion had more history of childhood sexual abuse and differences in parenting patterns. The father’s parenting style seemed influential, and presence of hostility in childhood seemed suspect as increasing the development of chronic congestion. Breathing patterns were not assessed in Fry’s study, but given the more stressful backgrounds in those with pelvic congestion, breathing may be the missing variable mediating between social stress, deficient pelvic circulation and pain. Myofascial trigger points could also emerge from the restricted movements of breathing.
Smith et al. (2006) studied reports of symptoms correlated with or predicting back pain in over 38 000 women, using data from the Australian Longitudinal Study on Women’s Health. Complaints of breathing difficulties and incontinence were consistently associated with back pain, while the more traditional factors of obesity and degree of physical activity were not as predictive. The authors speculate that the involvement of the diaphragm, transversus abdominis, and PFMs in both trunk stabilization and breathing make back pain more likely if PFMs or diaphragm are weak. This would be the case if poor breathing habits (shallow chest breathing) distort the usual interactions among these muscles. Hodges et al. (2007) provided evidence about the muscle–function interrelationships among breathing, continence and spinal stabilization.
Research on breathing as a pain intervention
Heart rate variability (HRV) is an emerging variable in the study of pain. It is a measure of cardiac activity sensitive to balance between sympathetic and parasympathetic influence, and can also be used as a biofeedback signal to help the patient regulate and balance the ANS by altering breathing. ANS imbalance is implicated in IBS (Mazur et al. 2007). There are no studies available for pelvic pain and HRV training, but a study by Appelhans & Luecken (2008), using an applied thermal pain stimulus and frequency-domain based spectral analysis with 59 normal subjects, found an inverse relationship between greater low-frequency HRV and pain intensity, including unpleasantness ratings. The low-frequency band (0.04–0.15 Hz) increases with both regular breathing and emotional calmness, and generally correlates with ANS balance and cardiovascular health.
An experimental pain stimulus such as heat or intramuscular hypertonic saline infusions can be adjusted and administered in order to measure pain thresholds. For example, (Chalaye et al. 2009) to study variability of pain tolerance and thresholds, the researchers applied thermal pain stimuli to subjects under two breathing conditions: distraction and feedback of heart rate (HRV). Compared to a 16/min breathing rate, slow deep breathing at a rate of 6/min resulted in better pain tolerance and higher pain thresholds. Increase in HRV correlates with increased vagal tone and general lowering of arousal.
Tan et al. (2009), using data from US war veterans suffering from chronic pain and other injuries, used a time-domain analysis of HRV. A –0.46 correlation was found between HRV (in this case SDNN, a time measure of variability) and presence of pain. So, in these two samples, a variable associated with breathing quality was also associated with presence of pain or sensitivity to pain. This is significant because HRV is a widely used biofeedback modality, and learning to raise low-frequency HRV by regulating breathing may have favourable effects on pain and homeostasis in general.
A study of experienced Zen meditators found that breathing pattern correlated with a significantly higher pain threshold to an applied heat stimulus. Better control over pain sensitivity was attributed to both attentional regulation and breathing regulation. The breathing pattern, being subject to disruptions in calmness and predictability, may be a good general index of peace of mind, which raises the threshold for pain of any sort. Zautra et al. (2010), comparing fibromyalgia patients to healthy controls, assigned slow breathing to volunteers subjected to controlled thermal stimuli. ‘Slow breathing’ was defined as breathing at one-half their normal rate. In general, slow breathing reduced pain intensity and unpleasantness more than normal breathing. The authors cited these results as support for Zen meditation and yogic breathing as a way to combat pain.
Pain may seem like a simple unitary sensation, but it has several facets, some mainly psychological. Using a brief intervention, Downey & Zun (2009) instructed patients in an emergency department to handle their pain by slow deep breathing. By self-report, no significant reduction in pain resulted, but the patients reported significant improvements in rapport with treating physicians, greater willingness to follow the medical recommendations, and conclusions that the intervention was useful.
Another study (Flink et al. 2009) of back pain patients showed that the effect of practising breathing exercises for 3 weeks was not so much on reducing pain levels as lowering catastrophizing and pain-related distress, along with greater acceptance of the pain condition.
Stress and breathing
Another aspect of the interaction between breathing and emotion is the location of breathing in the body. Optimal breathing most often involves the diaphragm flattening on inhalation and the lower rib cage expanding outward, with the abdomen also expanding forward and laterally. Chest breathing, by contrast, minimizes the diaphragm action and substitutes pectoral, scalene, trapezius, SCM and upper intercostal muscles. This latter type of breathing is more prevalent during emotional stress and preparation for action. Thoracic breathing actually produces increases in cardiac output and heart rate (Hurwitz 1981). During emergency action, this kind of breathing would provide an advantage. The diaphragm also contributes to spinal stabilization, so during action preparation it is likely to be diverted from breathing duties.
Conditioned breathing responses
In the case of pelvic pain, positive changes in breathing and emotion interact with pelvic physiology and can affect pain mechanisms in both general and specific ways (inhibiting pain through descending inhibitory tracts, raising endorphins and dopamine, interrupting cycles of worry and suffering, lowering CCK and adrenaline, reducing sympathetic output to trigger points, and resuming rhythmic stimulation of viscera and PFMs) (Scott et al. 2007, Wager et al. 2007, Zubieta & Stohler 2009).
Pelvic and abdominal pain from trigger points or other pain sources can be aggravated by full abdominal breathing, so downward expansion may be avoided, consciously or not. Shallow thoracic breathing is substituted, but this compounds the problem. The thoracic breathing is consistent with excessive emotional arousal, which may further the dysfunctional breathing pattern. Lewit (1999, described in Carrière 2006) describes how trigger points in either the diaphragm or the pelvic floor can make full abdominal breathing painful, and also that manually releasing trigger points in one region can relieve them in the other.
Psychology is very relevant to breathing pattern problems. The original source of thoracic breathing and chronic hyperventilation could be in early experience, trauma or chronic abuse, or an insecure environment fostering hypervigilance (Conway et al. 1988, Gilbert 1998). A transient breathing reaction can persist for years and become the default breathing style, even if the formative context has changed; breathing habits can be embedded through repetition. Lum (1975) observed that regardless of the source of hyperventilation, it could become habitual, persistent but amenable to reversal through breathing retraining with therapeutic guidance.
Manual treatment of selected key structures associated with respiration
Therapeutically, it is suggested that rehabilitation of the pelvic girdle and pelvic floor will be enhanced by more normal physiological breathing patterns, while enhancing these patterns will be aided by pelvic functionality, whether achieved through exercise, breathing retraining, manual therapy or other means (Chaitow 2007, Mehling et al. 2005, McLaughlin 2009) (see Box 9.7).
Box 9.7 Trigger points, BPD and pelvic pain
• Disturbances of ventilation mechanics
• Disturbances of the functional dynamics of the neck, shoulder girdle and lumbar spine
• Paradoxical respiration is a critical link in many such pathogenetic chain reactions (Travell & Simons 1999).
Anderson (2002) has described palpation and treatment protocols for locating myofascial trigger points (TrPs) associated with prostatitis symptoms.
Anderson et al. (2009) have identified the most common location of TrPs related to pelvic pain as follows:
Other relevant muscles in which TrPs also contribute to CPP are levator ani, iliopsoas, quadratus lumborum, gluteus maximus and thoracolumbar extensor muscle (Travell & Simons 1999, FitzGerald & Kotarinos 2003a, Chaitow 2007a, Montenegro et al. 2008, Anderson et al. 2009).
Newell (2005), Gibbons (2001), Prather et al. (2009), Pleidelová et al. (2002), Cox & Bakkum (2005), Lewit (1999), Janda et al. (2007), Fitzgerald et al. (2009), and many others, have implicated (in particular) psoas, iliacus, quadratus lumborum, piriformis, the adductors, rectus abdominis, abdominal obliques and scalenes intercostals.
• The primary inspirational muscles are the diaphragm, the more lateral external intercostals, parasternal internal intercostals, scalene group and the levator costarum, with the diaphragm providing 70–80% of the inhalation force (Simons et al. 1999).
• These muscles are supported by the accessory muscles during increased demand (or dysfunctional breathing patterns): SCM, upper trapezius, pectoralis major and minor, serratus anterior, latissimus dorsi, serratus posterior superior, iliocostalis thoracis, subclavius and omohyoid (Kapandji 1974, Simons et al. 1999) (see Box 9.7).
Connective tissue manipulation
In 2009, the Urological Pelvic Pain Collaborative Research Network (UPPCRN) concluded that somatic abnormalities, including myofascial trigger points and connective tissue restrictions, were found to be very common in women and men with IC (interstitial cystitis)/painful bladder syndrome and chronic prostatitis/CPP syndrome, respectively. (Fitzgerald et al 2009).
In a study to assess the value of combined connective tissue manipulation (CTM) and trigger point deactivation, in cases of urologic CPP, Fitzgerald et al. (2009) report:
Trigger point deactivation and slow stretching (Travell & Simons 1999, Cox 2005)
Travell & Simons described (1983, 1999) variations on their basic trigger point release approach:
• Ischaemic compression (1983): Pressure is applied to the point lying in a fully lengthened muscle. The pressure should be sufficient to maintain pain at a level of between 5 and 7 – where 10 is the maximum that can be tolerated, until pain eases by around 50–75% – or until 90 seconds have passed.
• Trigger point release (1999): In this version the muscle is partially lengthened and pressure is to the first perception of a tissue barrier, ideally with no sign of discomfort. Pressure is maintained until a sense of a release of the characteristic taut band is noted, or until 90 seconds have passed.
• Other versions exist including pulsed ![]() ischaemic compression (Chaitow 1994) in which a trigger point in a partially lengthened muscle received 5 seconds of compression, sufficient to induce pain at level 7 (numerical pain rating scale) –- followed by 2 seconds of no pressure – repeated for up to 90 seconds or until local or referred pain changes are reported or palpated.
ischaemic compression (Chaitow 1994) in which a trigger point in a partially lengthened muscle received 5 seconds of compression, sufficient to induce pain at level 7 (numerical pain rating scale) –- followed by 2 seconds of no pressure – repeated for up to 90 seconds or until local or referred pain changes are reported or palpated.
Diaphragm
NMT for diaphragm (Chaitow 2007, Chaitow & DeLany 2008)
• The patient is supine with the knees flexed and feet resting flat on the table. This position will relax the overlying abdominal fibres and allow better access to the diaphragm attachments.
• It is suggested that the upper rectus abdominis fibres should be treated before the diaphragm.
• This treatment of the diaphragm is contraindicated for patients with liver and gallbladder disease or if the area is significantly tender or swollen.
• The practitioner stands at the level of the abdomen contralateral to the side being treated.
• The fingers, thumbs or a combination of thumb of one hand and fingers of the other may be used to extremely gently insinuate contact beneath the lower border of the rib cage, directed partly cephalad and obliquely laterally, until a barrier is noted.
• As the patient exhales, the fingers penetrate further.
• As the patient inhales the diaphragm attachments press against the treating digit(s), forcing these caudally, unless this pressure is resisted – which it should be.
• When penetration appears to be as far as possible, the finger (thumb) tips are directed toward the inner surface of the ribs where static pressure or gentle friction is applied to the diaphragm’s attachment.
• The treatment may be applied on full exhalation or at half-breath and is repeated to as much of the internal costal margins as can be reached.
• While it is uncertain as to the degree to which diaphragm’s fibres can be reached by this exercise, the connective tissue associated with its costal attachment is probably influenced.
• Simons et al. (1999) describe a similar procedure, which ends in an anterior lifting of the rib cage (instead of friction or static pressure) to stretch the fibres of the diaphragm.
PRT for diaphragm
• The patient is supine and the practitioner stands at waist level facing cephalad and places the hands over the lower thoracic structures with the fingers along the lower rib shafts![]() .
.
• Treating the structure being palpated as a cylinder, the hands test the preference this cylinder has to rotate around its central axis, one way and then the other. ‘Does the lower thorax rotate more easily to the right or the left?’
• Once the rotational preference has been established, with the lower thorax held in its preferred rotation direction, the preference to sidebend one way or the other is evaluated. ‘Does the lower thorax sideflex more easily to the right or the left?’
• Once these two pieces of information have been established, the combined positions of ease (rotation and side-flexion), are introduced, and maintained for between 30 and 90 seconds, before slowly restoring the structures to neutral.
• Re-evaluation should demonstrate a marked change in previously restricted motion.
Intercostals
NMT for the intercostal muscles
• The intercostal areas are commonly extremely sensitive and care must be taken not to distress the patient by using inappropriate pressure.
• In most instances the intercostal spaces on the contralateral side will be treated using the finger stroke.
• The (well-trimmed) thumb tip or a finger tip should be run along both surfaces of the rib margins, as well as along the muscle tissue itself.
• In this way the fibres of the internal and external intercostal muscles will receive adequate assessment contacts.
• When there is over-approximation of the ribs, a simple stroke along the intercostal space may be all that is possible until a degree of rib and thoracic normalization has taken place, allowing greater access.
• The tip of a finger (supported by a neighbouring digit) is placed in one intercostal space at a time, close to the mid-axillary line (patient prone or supine), and gently but firmly brought around the curve of the trunk toward the midline, combing for signs of dysfunction.
• The probing digit feels for contracted or congested tissues, in which trigger points might be located.
• When an area of contraction is noted, firm pressure toward the centre of the body is applied to elicit a response from the patient (‘Does it hurt? Does it radiate or refer? If so, to where?’).
• Trigger points noted during the assessment may be treated using standard manual protocols.
• Caution: Dry needling or acupuncture to deactivate trigger points in the intercostal spaces is not recommended due to high risk of penetration of the lungs.
Psoas
Strategies for reducing excessive tone, and deactivating trigger points, in psoas are to be found in Chapter 14. There are a variety of alternative measures, and one, a muscle energy procedure, is described here.
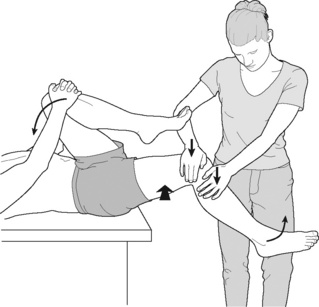
MET for psoas (Grieve 1994, Chaitow 2006)
• The patient is supine with the buttocks at the very end of the table, non-treated leg fully flexed at hip and knee, and either held in that state by the patient ![]() or by the practitioner.
or by the practitioner.
• The practitioner stands at the foot of the table and the leg on the affected side (shortened/hypertonic psoas) is placed so that the medioplantar aspect of the foot rests on the practitioner’s knee or shin (Figure 9.9).
• The leg should be placed so that the hip flexors, including psoas, are in a mid-range position, not at their barrier.
• The practitioner should request the patient to use a small degree of effort to externally rotate the leg and, at the same time, to flex the hip.
• The practitioner resists both efforts and an isometric contraction of the psoas and associated muscles therefore takes place.
• After a ~7-second isometric contraction, and complete relaxation of effort, the thigh should, on an exhalation, be taken without force into slight stretch. This stretch position is held there for 30 seconds.
Quadratus lumborum
Strategies for reducing excessive tone, and deactivating trigger points, in quadratus lumborum are to be found in Chapter 14.
A PRT procedure, is described here.
PRT for quadratus lumborum
• The patient is prone and the practitioner stands on the side contralateral to that being treated.
• The tender points for quadratus lie close to the transverse processes of L1–5. Medial pressure (toward the spine) is usually required to access the tender points, which should be pressed lightly as pain in the area is often exquisite.
• Once the most sensitive tender point has been identified this should be lightly compressed and the patient asked to register the discomfort as a ‘10’.
• While the practitioner maintains the monitoring contact on the tender point, the patient is asked to externally rotate, abduct and flex the hip on the side being treated to a position that reduces the ‘score’ significantly.
• The limb, flexed at hip and knee, should lie supported on the treatment table
• The patient turns his head ipsilaterally and slides his ipsilateral hand beneath the flexed thigh, easing the hand very slowly toward the foot of the treatment table, until a further reduction in the pain score is noted.
• This combination of hip flexion/abduction/rotation and arm movement effectively laterally flexes the lumbar spine, so slackening quadratus fibres.
• If further reduction is required in the pain score (i.e. if it is not already at ‘3’ or less), the practitioner’s caudad hand should apply gentle cephalad pressure from the ipsilateral ischial tuberosity.
• This final compressive force usually reduces the score to ‘0’. This position should be held for at least 30, and ideally up to 90, seconds before a slow return to the starting position.
Scalenes (and other upper fixators of the shoulder/accessory breathing muscles)
PRT for scalenes
• The patient lies supine and the practitioner sits at the head of the table, palpating a tender point with sufficient pressure to allow the discomfort to be ascribed a value of no more than 7/10 (where 10 is extreme pain on a VAS).
• The patient is then told, for the purpose of the technique, to change this value to ‘10’.
• For the anterior and medial scalene, the head and neck are flexed and side-flexed toward the affected side.
• For the posterior scalene a neutral position may be employed.
• The head and neck may be supported on a small cushion or rolled towel.
• The non-palpating hand engages the 2nd and 3rd ribs close to the axilla and eases them cephalad, until the reported discomfort reduces from ‘10’ to ‘3’ or less (Figure 9.10)
• This is held for 30–90 seconds, after which a slow release of the tissues being held is allowed.
Thoracic and costal mobilization
An effective MET procedure for mobilizing the thoracic spine is described below (Lenehan et al. 2003).
MET for thoracic spine
• The patient is seated on a treatment table, with arms folded, hands on shoulders, elbows forward.
• The practitioner stands behind and to the side, one hand cupping the patient’s elbows, and the other palpating or stabilizing the thoracic spine.
• The ability of the trunk to rotate left and right is assessed.
• It is then taken into rotation, to its easy end of range, in the direction of greatest restriction.
• In that position the patient is asked to attempt, using no more than 20% of available strength, to side flex (either direction) for 5 seconds while the practitioner prevents any movement.
• Following this isometric contraction the patient should be taken into a new easy end of range of rotation – commonly (Lenehan et al. 2003) around 10% further than previously.
• This process is repeated once more, in the same direction, and is then performed with the trunk rotated in the opposite direction.
Scar tissue release
• Kobesova et al. (2007) suggest that scars may develop adhesive properties that compromise tissue tensioning, altering proprioceptive input, behaving in much the same way as active myofascial trigger points. It is suggested that faulty afferent input can result in disturbed efferent output leading to, for example, protective postural patterns, increased neurovascular activity and pain syndromes. The term active scar is designated to describe the ongoing additional neural activity associated with adhesive scar formations.
• Lewit & Olsanska (2004) reported a series of 51 such cases in which postsurgical scar tissue was found to be the primary pain generator for a multitude of locomotor system pain syndromes. On palpation (light stretching) of dysfunctional tissues the patient commonly reports sensations of ‘burning, prickling, or lightning-like jabs of pain’.
• Valouchova & Lewit (2009) report that active scars in the abdomen and pelvis commonly restrict back flexion, which the patient feels as low back pain.
• Treatment methods are simple, involving ‘mini-myofascial release’ methods – where skin alongside scars is treated initially, with subsequent attention to deeper layers. Treatment involves ‘engaging the pathologic barrier and waiting; after a short delay, a release gradually occurs until the normal barrier is restored’.
Myofascial release (myofascial induction)
King (2010) notes that myofascial release (MFR) is ‘a system of diagnosis and treatment first described by A.T. Still, and his early students, which involves continual palpatory feedback to achieve release of myofascial tissues’.
• Direct MFR: a myofascial tissue restrictive barrier is engaged for the myofascial tissues and the tissue is loaded with constant force, until tissue release occurs![]() .
.
• Indirect MFR: the dysfunctional tissues are guided along the path of least resistance, until free movement is achieved (Educational Council on Osteopathic Principles 2009).
Myofascial induction is a simultaneous evaluation and treatment process using tri-dimensional movements of sustained pressures, applied to myofascial structures in order to release restrictions. The term ‘induction’ is preferred because clinicians do not passively stretch the system but only apply an initial tension or compression force and follow the facilitating movement. The aim of the process is the recovery of motion amplitude, force and coordination (Pilat 2009).
Conclusions
• ‘Structure’ would include temperament and enduring personality traits whether genetically based or formed early in life: chronic depression, anxiety, anger, tendency to take risks, or predisposition to ignore body warnings.
• ‘Function’ denotes the behaviour following from habits and personality traits: examples are the persistent slump of depression, the tense rigid body and clenched jaw of anger, and the hyperventilation of one prone to panic.
Chapter 12 examines the research and techniques of biofeedback as a way to improve function by providing precise information about body changes, allowing the person to expand self-influence.
Chapter 4 covers the area of psychophysiology and pelvic pain in some detail.
Anderson R. Management of chronic prostatitis – chronic pelvic pain syndrome. Urol. Clin. North Am.. 2002;29(1):235-239.
Anderson R.U., Sawyer T., Wise D., et al. Painful myofascial trigger points and pain sites in men with chronic prostatitis/chronic pelvic pain syndrome. J. Urol.. 2009;182(6):2753-2758.
Anraku M., Shargall Y. Surgical conditions of the diaphragm: anatomy and physiology. Thorac. Surg. Clin.. 2009;19:419-429.
Appelhans B.M., Luecken L.J. Heart rate variability and pain: associations of two interrelated homeostatic processes. Biol. Psychol.. 2008;77(2):174-182.
Balaban C., Thayer J. Neurological bases for balance–anxiety links. J. Anxiety Disord.. 2001;15(1–2):53-79.
Banzett R.B., Lanzing R.W., Brown R., Topulos G.P., Yagar D., Steel S.M. Air hunger’ from increased PCO2 persists after complete neuromuscular block in humans. Respir. Physiol.. 1990;81:1-17.
Beales D. I’ve got this pain …. Hum. Givens J.. 2004;11(4):16-18.
Bernardon M., Limone A., Businelli C. Diabetic ketoacidosis in pregnancy. Gazz. Med. Ital. Arch. Sci. Med.. 2009;168(1):45-49.
Bockenhauer S., Chen H., Julliard K., et al. Measuring thoracic excursion: reliability of the cloth tape measure technique. J. Am. Osteopath. Assoc.. 2007;107:191-196.
Botella De Maglia J., Real Soriano R., Compte Torrero L. Arterial oxygen saturation during ascent of a mountain higher than 8,000 meters. Med. Intensiva. 2008;32(6):277-281.
Bradley D. Hyperventilation Syndrome/Breathing Pattern Disorders. Auckland, NZ: Tandem Press; 1998.
Bradley D. Self-help for hyperventilation syndrome. Alameda, CA: Hunter House; 2001. p. 70
Brashear R. Hyperventilation syndrome. Lung. 1983;161(1):257-273.
Brotto L., Klein C., Gorzalka B. Laboratory induced hyperventilation differentiates female sexual arousal disorder subtypes. Arch. Sex. Behav.. 2009;38(4):463. 447
Brugger A. Lehrbuch der Funktionellen Storungen des Bewegungssystems. Zollikon Benglen: Brugger-Verlag; 2000.
Burkill G., Healy J. Anatomy of the retroperitoneum. Imaging. 2000;12(1):10-20.
Buteyko K. Buteyko Method : Experience of Application in Medical Practice. Moscow: Patriot; 1990.
Butler D. The Sensitive Nervous System. Adelaide: Noigroup Publications; 2000:89.
Cappo B., Holmes D. Utility of prolonged respiratory exhalation for reducing physiological and psychological arousal in non-threatening and threatening situations. J. Psychosom. Res.. 1984;28(4):265-273.
Carriere B. Interdependence of posture and the pelvic floor. In: Carriere B., Markel Feldt C., editors. The pelvic floor. New York: Thieme; 2006:68. 76
Carriero J. An osteopathic approach to children. Edinburgh: Churchill Livingstone; 2003.
Celotto A.C., Capellini V.K., Baldo C.F. Effects of acid-base imbalance on vascular reactivity. Braz. J. Med. Biol. Res.. 2008;41(6):439-445.
Chaitow L. INIT in treatment of pain and trigger points. Br. J. Osteopathy. 1994;XIII:17-21.
Chaitow L. Breathing pattern disorders, motor control, and low back pain. J. Osteopath. Med.. 2004;7(1):34-41.
Chaitow L. Muscle Energy Techniques, third ed. Edinburgh: Churchill Livingstone; 2006.
Chaitow L. Chronic pelvic pain: Pelvic floor problems, sacroiliac dysfunction and the trigger point connections. J. Bodyw. Mov. Ther.. 2007;11(4):327-339.
Chaitow L., DeLany J. Clinical Application of Neuromuscular Techniques. Churchill Livingstone, Edinburgh: The Upper Body; 2008. vol 1. second ed.
Chaitow L., Bradley D., Gilbert C. Multidisciplinary Approaches to Breathing Pattern Disorders. Edinburgh: Churchill Livingstone; 2002.
Chalaye P., Goffaux P., Lafrenaye S., Marchand S. Respiratory effects on experimental heat pain and cardiac activity. Pain Med.. 2009;10(8):1334-1340.
Chaulier K., Chalumeau S., Ber C.E. Metabolic acidosis in a context of acute severe asthma. Ann. Fr. Anesth. Reanim.. 2007;26(4):352-355.
Cimino R., Farella M., Michelotti A. Does the ovarian cycle influence the pressure-pain threshold of the masticatory muscles in symptom-free women? J. Orofac. Pain. 2000;14(2):105-111.
Conway A.V., Freeman L.J., Nixon P.G.F. Hypnotic examination of trigger factors in the hyperventilation syndrome. Am. J. Clin. Hypn.. 1988;30:296-304.
Courtney R., Greenwood K.M. Preliminary investigation of a measure of dysfunctional breathing symptoms: the Self Evaluation of Breathing Questionnaire (SEBQ). Int. J. Osteopath. Med.. 2009;12:121-127.
Courtney R., van Dixhoorn J., et al. Evaluation of breathing pattern: comparison of a manual assessment of respiratory motion (MARM) and respiratory induction plethysmography. Appl. Psychophysiol. Biofeedback. 2008;33:91-100.
Courtney R., Cohen M., Reece J. Comparison of the Manual Assessment of Respiratory Motion (MARM) and the Hi Lo Breathing Assessment in determining a simulated breathing pattern. Int. J. Osteopath. Med.. 2009;12(2009):86-91.
Courtney R., Greenwood K.M., Cohen M. Relationship between measures of breathing functionality and dimensions of dysfunctional breathing. J. Bodyw. Mov. Ther.. 2011;15(1):24-34.
Cox J., Bakkum B. Possible generators of retrotrochanteric gluteal and thigh pain: the gemelli-obturator internus complex. J. Manipulative Physiol. Ther.. 2005;28:534-538.
Cresswell A.G., Oddsson L., Thorstensson A. The influence of sudden perturbations on trunk muscle activity and intra-abdominal pressure while standing. Exp. Brain Res.. 1994;98:336-341.
Cusi M. Paradigm for assessment and treatment of SIJ mechanical dysfunction. J. Bodyw. Mov. Ther.. 2010;14(2):152-161.
Damas-Mora J., Davies L., Taylor W., et al. Menstrual respiratory changes and symptoms. Br. J. Psychiatry. 1980;136:492-497.
Debreczeni R., Amrein I., Kamondi A., et al. Hypocapnia induced by involuntary hyperventilation during mental arithmetic reduces cerebral blood flow velocity. Tohoku J. Exp. Med.. 2009;217(2):147-154.
Dempsey J., Sheel A., Croix C.St. Respiratory influences on sympathetic vasomotor outflow in humans. Respir. Physiol. Neurobiol.. 2002;130(1):3-20.
Downey L.V., Zun L.S. The effects of deep breathing training on pain management in the emergency department. South Med. J.. 2009;102(7):688-692.
Educational Council on Osteopathic Principles (ECOP). Glossary of Osteopathic Terminology. Washington, DC: American Association of Colleges of Osteopathic Medicine; 2009.
Evans D., Lum L., Dart A. Chest pain with normal coronary arteries. Lancet. 1980;315(8163):311.
Faling L. Controlled breathing techniques and chest physical therapy in chronic obstructive pulmonary disease. In: Casabur R., editor. Principles and Practices of Pulmonary Therapy. Philadelphia: WB Saunders, 1995.
Fall M., Baranowski A.P., Elneil S., et al. Guidelines on Chronic Pelvic Pain. European Association of Urology. Eur. Urol.. 2010;57:35-48.
Fass R., Fullerton S., Naliboff B., et al. Sexual dysfunction in patients with irritable bowel syndrome and non-ulcer dyspepsia. Digestion. 1998;59:79-85.
FitzGerald M.P., Kotarinos R. Rehabilitation of the short pelvic floor. II: Treatment of the patient with the short pelvic floor. Int. Urogynecol. J. Pelvic Floor Dysfunction. 2003;14(4):269-275.
Fitzgerald M.P., Anderson R.U., Potts J., et al. Randomised multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J. Urol.. 2009;182:570-580.
Flink I.K., Nicholas M.K., Boersma K., Linton S.J. Reducing the threat value of chronic pain: A preliminary replicated single-case study of interoceptive exposure versus distraction in six individuals with chronic back pain. Behav. Res. Ther.. 2009;47(8):721-728.
Ford M., Camilleri M., Hanson R. Hyperventilation, central autonomic control, and colonic tone in humans. Gut. 1995;37:499-504.
Freeman L.J., Nixon P. Chest pain and the hyperventilation syndrome – Some aetiological considerations. Postgrad. Med. J.. 1985;61(721):957-961.
Fry R.P., Beard R.W., Crisp A.H., McGuigan S. Sociopsychological factors in women with chronic pelvic pain with and without pelvic venous congestion. J. Psychosom. Res.. 1997;42(1):71-85.
Gardner W. The pathophysiology of hyperventilation disorders. Chest. 1996;109:516-534.
Garland W. Somatic changes in hyperventilating subject. Presentation at International Society for the Advancement of Respiratory Psychophysiology Congress,. 1994. Paris
Gibbons S.G.T. The model of psoas major stability function. Proceedings of 1st International Conference on Movement Dysfunction. 2001. Sept 21–23 Edinburgh, Scotland
Gilbert C. Emotional sources of dysfunctional breathing. J. Bodyw. Mov. Ther.. 1998;2:224-230.
Grewar H., McLean L. The integrated continence system: A manual therapy approach to the treatment of stress urinary incontinence. Man. Ther.. 2008;13:375-386.
Grieve G. The masqueraders. In Boyling J.D., Palastanga N., editors: Grieve?s modern manual therapy, second ed., Edinburgh: Churchill Livingstone, 1994.
Grossman P., De Swart J.C.G., Defares P.B. A controlled study of breathing therapy for treatment of hyperventilation syndrome. J. Psychosom. Res.. 1985;29(1):49-58.
Haahtela T., Tamminen K., Kava T., et al. Thirteen-year follow-up of early intervention with an inhaled corticosteroid in patients with asthma. J. Allergy Clin. Immunol.. 2009;124(6):1180.
Han J., Gayan-Ramirez G., Dekhuijzen R. Respiratory function of the rib cage muscles. Eur. Respir. J.. 1993;6(5):722-728.
Haugstad G.K., Haugstad T.S., Kirste U.M. Posture, movement patterns, and body awareness in women with chronic pelvic pain. J. Psychosom. Res.. 2006;61(5):637-644.
Haugstad G.K., Haugstad T.S., Kirste U.M., et al. Mensendieck somatocognitive therapy as treatment approach to chronic pelvic pain: results of a randomized controlled intervention study. Am. J. Obstet. Gynecol.. 2006;194:1303-1310.
Haugstad G.K., Haugstad T.S., Kirste U.M., et al. Continuing improvement of chronic pelvic pain in women after short-term Mensendieck somatocognitive therapy: results of a 1-year follow-up study. Am. J. Obstet. Gynecol.. 2008;199:615.e1-615.e8.
Hetrick D., Ciol M., Rothman I. Musculoskeletal dysfunction in men with chronic pelvic pain syndrome type III: A case-control study. J. Urol.. 2003;170(3):828-831.
Hides J.A., Richardson C.A., Jull G.A. Multifidus muscle recovery is not automatic following resolution of acute first episode low back pain. Spine. 1996;21:2763-2769.
Hirano Y., Ozasa Y., Yamamoto T., et al. Hyperventilation and cold-pressor stress echocardiography for noninvasive diagnosis of coronary artery spasm. J. Am. Soc. Echocardiogr.. 2001(6):626-633.
Hochstetter J., et al. An investigation into the immediate impact of breathlessness management on the breathless patient: randomised controlled trial. Physiotherapy. 2005;91:178-185.
Hodges P. Abdominal mechanism and support of the lumbar spine and pelvis. In Richardson C., Hodges P., Hides J., editors: Therapeutic exercise for lumbopelvic stabilisation: A motor control approach for the treatment and prevention of low back pain, second ed., Edinburgh: Churchill Livingstone, 2004.
Hodges P.W., Richardson C.A. Inefficient muscular stabilisation of the lumbar spine associated with low back pain: a motor control evaluation of transversus abdominis. Spine. 1996;21(22):2640-2650.
Hodges P.W., Richardson C.A. Delayed postural contraction of transversus abdominis in low back pain associated with movement of the lower limb. J. Spinal Disorders. 1998;11(1):46-56.
Hodges P.W., Richardson C.A. Altered trunk muscle recruitment in people with low back pain with upper limb movements at different speeds. Arch. Phys. Med. Rehabil.. 1999;80(9):1005-1012.
Hodges P., Sapsford R., Pengel L. Postural and respiratory functions of the PFMs. Neurourol. Urodyn.. 2007;26(3):362-371.
Hodges P.W., Heinjnen I., Gandevia S.C. Postural activity of the diaphragm is reduced in humans when respiratory demand increases. J. Physiol.. 2001;537(3):999.
Hu H., Meijer O., van Dieën J. Muscle activity during the active straight leg raise (ASLR), and the effects of a pelvic belt on the ASLR and on treadmill walking. J. Biomech.. 2010;43(3):532-539.
Hudson A., Gandevia S., Butler J. The effect of lung volume on the co-ordinated recruitment of scalene and sternomastoid muscles in humans. J. Physiol.. 2007;584(1):261-270.
Hurwitz B.E. The effect of inspiration and posture on cardiac rate and T-wave amplitude during apneic breathholding in man. Psychophysiology. 1981;18:179-180. (abstract)
Janda V., Frank C., Liebenson C. Evaluation of muscle imbalance. In Liebenson C., editor: Rehabilitation of the Spine: A Practitioner’s Manual, second ed., Baltimore: Lippincott/Williams & Wilkins, 2007.
Jensen D., Duffin J., Lam Y.M. Physiological mechanisms of hyperventilation during human pregnancy. Respir. Physiol. Neurobiol.. 2008;161(1):76-86.
Jones R. Pelvic floor muscle rehabilitation. Urol. News. 2001;5(5):2-4.
Kapandji I.A. The physiology of the joints, vol. III: The trunk and the vertebral column, second ed. Edinburgh: Churchill Livingstone; 1974.
Kapreli E., Vourazanis E., Strimpakos N. Neck pain causes respiratory dysfunction. Med. Hypotheses.. 2008;70(5):1009-1013.
Key J. The pelvic crossed syndromes: A reflection of imbalanced function in the myofascial envelope; a further exploration of Janda’s work. J. Bodyw. Mov. Ther.. 14(3), 2010.
Key J., et al. A model of movement dysfunction provides a classification system guiding diagnosis and therapeutic care in spinal pain and related musculo-skeletal syndromes: a paradigm shift. J. Bodyw. Mov. Ther.. 2007;12(2):105-120.
King H. Osteopathic manipulative therapies and fascia. In: Chaitow L., Findley, Huijing, Schleip. Fascia in Manual Therapy. Elsevier, 2010. In Press
Kitabchi A.E., Umpierrez G.E., Murphy M., et al. Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(12):2739-2748.
Kobesova A., et al. Twenty-year-old pathogenic “active” postsurgical scar: a case study of a patient with persistent right lower quadrant pain. J. Manipulative Physiol. Ther.. 2007;30(3):234-238. M
Kuligowska E., Deeds L.3rd, Lu K.3rd. Pelvic pain: overlooked and underdiagnosed gynecologic conditions. Radiographics. 2005;25(1):3-20.
Lansing R.W. The perception of respiratory work and effort can be independent of the perception of air hunger. Am. J. Respir. Crit. Care Med.. 2000;162:1690-1696.
Lee D. An integrated approach for the management of low back and pelvic girdle pain. In: Vleeming A., Mooney V., Stoekart R., editors. Movement stability & lumbopelvic pain. Edinburgh: Churchill Livingstone/Elsevier; 2007:593-620.
Lee D., Lee L. Stress urinary incontinence – a consequence of failed load transfer through the pelvis?. Presented at the 5th World Interdisciplinary Congress on Low Back and Pelvic Pain, Melbourne, November 2004. 2004.
Lee D., Lee L.J., McLaughlin L. Stability, continence and breathing: The role of fascia following pregnancy and delivery. J. Bodyw. Mov. Ther.. 2008;12:333-348.
Lee J., Doggweiler-Wiygul R., Kim S., et al. interstitial cystitis an allergic disorder? A case of interstitial cystitis treated successfully with anti-IgE. Int. J. Urol.. 2006;13(5):631-634.
Lenehan K., Fryer G., McLaughlin P. The effect of muscle energy technique on gross trunk range of motion. J. Osteopath. Med.. 2003;6(1):13-18.
Levitsky M.G. Pulmonary Physiology, sixth ed. Toronto, ON: McGraw-Hill; 2003.
Lewit K. Relationship of faulty respiration to posture, with clinical implications. J. Am. Osteopath. Assoc.. 1980;79:525-528.
Lewit K. Manipulative therapy in rehabilitation of the motor system, third ed. London: Butterworths; 1999.
Lewit K., Olšanská Š. Clinical importance of active scars as a cause of myofascial pain. J. Manipulative Physiol. Ther.. 2004;27(6):399-402.
Liebenson C. J. Bodyw. Mov. Ther.. 2006;10:65-70.
Lum L. Editorial: Hyperventilation and anxiety states. J. R. Soc. Med.. 1984(January):1-4.
Lum L. Treatment difficulties and failures: causes and clinical management. Biol. Psychol.. 1996;43(3):24.
Lum L.C. Hyperventilation: the tip and the iceberg. J. Psychosom. Res.. 1975;19(5-6):375-383.
Masubuchi Y., Abe T., Yokoba M. Relation between neck accessory inspiratory muscle electromyographic activity and lung volume. Journal Japanese Respiratory Society. 2001;39(4):244-249.
Mattsson M., Wikman M., Dahlgren L., et al. Body awareness therapy with sexually abused women Part 1: Description of a treatment modality. J. Bodyw. Mov. Ther.. 1997;1(5):280-288.
Mattsson M., Wikman M., Dahlgren L., et al. Physiotherapy as empowerment – treating women with chronic pelvic pain. Advanced Physiotherapy. 2000;2:125-143.
Mazur M., Furgała A., Jabłoński K., et al. Dysfunction of the autonomic nervous system activity is responsible for gastric myoelectric disturbances in the irritable bowel syndrome patients. J. Physiol. Pharmacol.. 2007;58(Suppl. 3):131-139.
McLaughlin L. Breathing evaluation and retraining in body work. J. Bodyw. Mov. Ther.. 2009;13(3):276-282.
McLaughlin L., Goldsmith C.H. Altered respiration in a case series of low back/pelvic pain patients. 6th Interdisciplinary World Congress on Low Back & Pelvic Pain. 2007. November 2007, Barcelona
Mehling W.E., Hamel K.A., et al. Randomized, controlled trial of breath therapy for patients with chronic low-back pain. Altern. Ther. Health Med.. 2005;11(4):44-52.
Mens J.M., Vleeming A., Snijders C.J. Reliability and validity of the active straight leg raise test in posterior pelvic pain since pregnancy. Spine. 2001;26(10):1167-1171.
Montenegro M.L., Vasconcelos E.C., Canidido Dos Reis F.J., et al. Physical therapy in the management of women with chronic pelvic pain. Int. J. Clin. Pract.. 2008;62(2):174-175.
Moore J., Kennedy S. Causes of chronic pelvic pain Baillieres Clin. Obstet. Gynaecol.. 2000;14(3):389-402.
Newell R. Anatomy of the post-laryngeal airways, lungs and diaphragm. Surgery. 2005;23(11):393-397.
Nijenhuis E.R.S. Somatoform dissociation: phenomena, measurement and theoretical issues. NewYork: WW Norton & Company; 2004.
Nishino T., Shimoyama N., Ide T., et al. Experimental pain augments experimental dyspnea, but not vice versa in human volunteers. Anesthesiology. 1999;91(6):1633-1638.
Nixon P., Andrews J. A study of anaerobic threshold in chronic fatigue syndrome (CFS). Biol. Psychol.. 1996;43(3):264.
O’Sullivan P., Beales D. Changes in pelvic floor and diaphragm kinematics and respiratory patterns in subjects with sacroiliac joint pain following a motor learning intervention: A case series. Man. Ther.. 2007;12:209-218.
O’Sullivan P., Beales D., Beetham J., et al. Altered motor control strategies in subjects with sacroiliac joint pain during the active straight-leg-raise test. Spine. 2002;27(1):E1-E8.
Olsen A., Rao S. Clinical neurophysiology and electrodiagnostic testing of the pelvic floor. Gastroenterol. Clin. North Am.. 2001;30:33-54. v–vi
Pacia E.B., Aldrich T.K. Assessment of diaphragm function. Chest Surg. Clin. North Am.. 1998;8(2):225-236.
Pel J., Spoor C., Pool-Goudzwaard A., et al. Biomechanical analysis of reducing sacroiliac joint shear load by optimization of pelvic muscle and ligament forces. Ann. Biomed. Eng.. 2008;36(3):415-424.
Perri M., Halford E. Pain and faulty breathing: a pilot study. J. Bodyw. Mov. Ther.. 2004;8(4):297-306.
Pilat A. Myofascial induction approaches for headache. In: Fernández-de-las- Peñas C., Arendt-Nielsen L., Gerwin R.D., editors. Tension Type and Cervicogenic Headache: pathophysiology, diagnosis and treatment. Boston: Jones & Bartlett Publishers, 2009.
Pleidelová J., Balá!iová M., Porubská V. Frequency of scalenal muscle disorders. Rehabilitacia. 2002;35(4):203-207.
Pool-Goudzwaard A., van Dijke G., van Gurp M. Contribution of PFMs to stiffness of the pelvic ring. Clin. Biomech.. 2004;19(6):564-571.
Prather H., Dugan S., Fitzgerald C., et al. Review of anatomy, evaluation, and treatment of musculoskeletal pelvic floor pain in women. Phy. Med. Rehabil.. 2009;1(4):346-358.
Prior A., Whorwell P., Faragher E. Irritable bowel syndrome in the gynaecological clinic. Survey of 798 new referrals. Dig. Dis. Sci.. 1989;34:1820-1824.
Pryor J.A., Prasad S.A. Physiotherapy for respiratory and cardiac problems. third ed. Edinburgh: Churchill Livingstone; 2002:81.
Renggli A., Verges S., Notter D. Development of respiratory muscle contractile fatigue in the course of hyperpnoea. Respir. Physiol. Neurobiol.. 2008;164:366-372.
Rhudy J., Meagher M. Fear and anxiety: divergent effects on human pain thresholds. Pain. 2000;84:65-75.
Rosenbaum T., Owens A. The role of pelvic floor physical therapy in the treatment of pelvic and genital pain-related sexual dysfunction. J. Sex. Med.. 2008;5:513-523.
Ryan S., McNicholas M., Eustace S. Anatomy for Diagnostic Imaging. Sydney: Saunders; 2004:191.
Schleifer L., Ley R., Spalding T. A hyperventilation theory of job stress and musculoskeletal disorders. Am. J. Ind. Med.. 2002;41(5):420-432.
Schumpelick V., Steinau G. Surgical embryology and anatomy of the diaphragm with surgical applications. Surg. Clin. North Am.. 2000;80(1):213-239.
Scott D.J., Stohler C.S., Egnatuk C.M., Wang H., Koeppe R.A., Zubieta J.K. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55(2):325-336.
Shuler T., Gruen G. Chronic postpartum pelvic pain treated by surgical stabilization. Orthopedics. 1996;19:687-689.
Simon P., Schwartzstein M., Weiss J., et al. Distinguishable sensations of breathlessness induced in normal volunteers. Am. Rev. Respir. Dis.. 1989;140:1021-1027.
Simons D., Travell J., Simons L. Myofascial pain and dysfunction: the trigger point manual. Upper Half of Body, vol. 1, second ed. Baltimore: Williams & Wilkins; 1999.
Slatkovska L., Jensen D., Davies G., et al. Phasic menstrual cycle effects on the control of breathing in healthy women. Respir. Physiol. Neurobiol.. 2006;154(3):379-388.
Slocumb J. Neurological factors in chronic pelvic pain: trigger points and the abdominal pelvic pain syndrome. Am. J. Obstet. Gynecol.. 1984;149(5):536-543.
Smith M., Russell A., Hodges P. Disorders of breathing and continence have a stronger association with back pain than obesity and physical activity. Aust. J. Physiother.. 2006;21(52):11-16.
Smith M., Russell A., Hodges P. Is there a relationship between parity, pregnancy, back pain and incontinence? Int. Urogynecol. J. Pelvic Floor Dysfunct.. 2007;19(2):205-211.
Snijders C., Vleeming A., Stoeckary R., et al. Biomechanics of the interface between spine and pelvis in different poistures. In: Vleeming A., Mooney V., Dorman T., Snijders C.H., Stoeckart R., editors. Movement, Stability &Low Back Pain. The essential role of the pelvis. Edinburgh: Churchill Livingstone, 1997.
Schumpelick V., Steinau G. Surgical embryology and anatomy of the diaphragm with surgical applications. Surg. Clin. North Am.. 2000;80(1):213-239.
Suwa K. Ischemia may be less detrimental than anemia for O2 transport because of CO2 transport: A model analysis. J. Anesth.. 1995;9(1):61-64.
Standring S., editor. Gray?s Anatomy. The Anatomical Basis of Clinical Practice, 40th ed. Section 8 Abdomen & Pelvis. Livingstone: Elsevier Churchill, 2008.
Tak L., Rosmalen J. Dysfunction of stress responsive systems as a risk factor for functional somatic syndromes. J. Psychosom. Res.. 2010;68(5):461-468.
Tan G., Fink B., Dao T.K., et al. Associations among pain, PTSD, mTBI, and heart rate variability in veterans of Operation Enduring and Iraqi Freedom: a pilot study. Pain Med.. 2009;10(7):1237-1245.
Ternesten-Hasséus E., Johansson E.L., Bende M. Dyspnea from exercise in cold air is not always asthma. J. Asthma. 2008;45(8):705-709.
Tiep B., Burns M., Kro D., et al. Pursed lip breathing using ear oximetry. Chest. 1986;90:218-221.
Tondury G., Tillmann B. Zwerchfell, Diaphragma. In: Leonhardt H., Tillmann B., Tondury G., et al, editors. Rauber/Kopsch: Anatomie des Menschen. second ed. New York: Thieme; 1998:303-307. vol. 1
Travell J., Simons D. Myofascial pain and dysfunction. vol 1. Baltimore: Williams & Wilkins; 1983.
Troosters T., Verstraete A., Ramon K. Physical performance of patients with numerous psychosomatic complaints suggestive of hyperventilation. Eur. Respir. J.. 1999;14(6):1314-1319.
Tu F., Holt J., Gonzales J., et al. Physical therapy evaluation of patients with chronic pelvic pain: a controlled study. Am. J. Obstet. Gynecol.. 2008;198:272e1-272e7.
Valouchová P., Lewit K. Surface electromyography of abdominal and back muscles in patients with active scars. J. Bodyw. Mov. Ther.. 2009;13:262-267.
Van Dieën J., Selen L., Cholewicki J. Trunk muscle activation in low-back pain patients, an analysis of the literature. J. Electromyogr. Kinesiol. 2003;13:333-351.
Van Dixhoorn J., Duivenvoorden H.J. Efficacy of Nijmegen Questionnaire in recognition of the hyperventilation syndrome. J. Psychosom. Res.. 1985;29(2):199-206.
Vanhecke T., Franklin B., Ajluni S., et al. Cardiorespiratory fitness and sleep-related breathing disorders. Expert Rev. Cardiovasc. Ther.. 2008;6(5):745-758.
Vleeming A., Volkers A.C.W., Snijders C., Stoeckart R. Relation between form and function in the sacroiliac joint. Part I: Clinical anatomical aspects. Spine. 1990;15:130-132.
Vleeming A., Volkers A.C.W., Snijders C., Stoeckart R. Relation between form and function in the sacroiliac joint. Part 2.Biomechanical aspects. Spine. 1990;15(2):133-136.
Wager T.D., Scott D.J., Zubieta J.K. Placebo effects on human mu-opioid activity during pain. Proc. Natl. Acad. Sci. U. S. A.. 2007;104(26):11056-11061.
Watier A. Irritable bowel syndrome and bladder-sphincter dysfunction. Pelvi-perineologie. 2009;4(2):136-141.
Weinberger M., Abu-Hasan M. Pseudo-asthma: When cough, wheezing, and dyspnea are not asthma. Pediatrics. 2007;120(4):855-864.
Weiss J. Pelvic floor myofascial trigger points: manual therapy for interstitial cystitis and the urgency-frequency syndrome. J. Urol.. 2001;166:2226-2231.
West J. Respiratory Physiology: The Essentials, eighth ed. Baltimore: Lippincott Williams & Wilkins; 2008. p. 78
Whitehead W., Palsson O., Jones K. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140-1156.
Wilke A., Noll B., Maisch B. Angina pectoris caused by extra-coronary diseases. Herz. 1999;24(2):132-139.
Yokoyama I., Inoue Y., Kinoshita T., et al. Heart and brain circulation and CO2 in healthy men. Acta Physiol.. 2008;193(3):202-330.
Zautra A.J., Fasman R., Davis M.C., Craig A.D. The effects of slow breathing on affective responses to pain stimuli: An experimental study. Pain (in press). 2010.
Zondervan K., Yudkin P., Vessey M., et al. Patterns of diagnosis and referral in women consuiting for chronic pelvic pain in U.K. primary care. Br. J. Obstet. Gynaecol.. 1999;106:1156-1161.
Zubieta J.K., Stohler C.S. Neurobiological mechanisms of placebo responses. Ann. N. Y. Acad. Sci.. 2009;1156:198-210.