2.3 Anatomy of the pelvic floor
There is increasing evidence that the pelvic floor muscles (PFM) perform multiple functions such as: continence and pelvic organ support (DeLancey 1990, Howard et al. 2000); sexual function (Baytur et al. 2005); respiration (Hodges et al. 2007); spinal stability; and containment of intra-abdominal pressure (IAP) (Hemborg et al. 1985, Pool-Goudzwaard et al. 2004, Smith et al. 2008). The physiological mechanisms by which they perform these roles are not clearly understood, predominately due to a lack of suitable instrumentation. The pelvic floor (PF) remains, particularly from a biomechanical perspective, an understudied region of the body (Ashton-Miller & DeLancey 2007). PF dysfunction for certain encompasses both urinary and faecal incontinence, pelvic organ prolapse (POP) and pelvic pain (Martins et al. 2007). It is a significant problem for both women and men and has been termed the hidden epidemic (DeLancey 2005), with estimates in the USA indicating that between 21% and 26% of American women have at least one PF disorder, with the greatest percentage experiencing urinary incontinence (Nygaard et al. 2008). PF dysfunction affects between 300 000 and 400 000 American women so severely that they require surgery, and 30% of those will require re-operations (Olsen et al. 1997, Boyles et al. 2003). Similar prevalence data of PF dysfunction are currently unavailable in the UK.
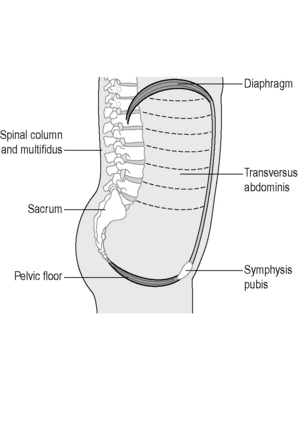
A combination of neuropathic changes, muscle, fascial or connective tissue damage is most likely responsible for the development of PF disorders yet the precise mechanisms remain controversial (Shafik et al. 2005, Ashton-Miller & DeLancey 2007, Petros 2007, Smith et al. 2007). The PF is an intricate, complicated structure, and by virtue of its anatomical position makes it a challenging area to study. The support mechanisms of the PF are responsible for the maintenance of continence and prevention of POP during rises of IAP (Ashton-Miller & DeLancey 2007), so functionally it not only has to allow the passage of urine and faeces at the appropriate time, it must also prevent incontinence. The PF is essential for sexual activity, conception, fertility and vaginal delivery (Herschorn 2004, Delancey 2005). More recently the PF has been implicated in contributing to spinal stability and respiration (Hodges et al. 2007). The lumbopelvic cylinder is closed inferiorly by the PF, the respiratory diaphragm forms the top, the transversus abdominis the sides and the spinal column runs through the middle, supported posteriorly by segmental attachments of lumbar multifidus, anteriorly by segmental attachments of psoas and the abdominal muscles (Figure 2.3.1).
The following sections describe the gross anatomy of the PF and organs. Further details and a practical PF anatomy guide for the palpating physician can be found in Chapter 13.
Pelvic floor muscles
The PFM is the collective name for the muscles of the PF; unfortunately throughout the literature, there still remains lack of consensus regarding their description and terminology. A recent review of the literature revealed over 16 different overlapping terms for different parts of the muscle, yet the anatomy was found to be very consistent amongst different studies (Kearney et al. 2004). Kearney et al. concluded that confusion could be limited by standardizing terminology based upon origin and insertion points of the muscle. Therefore, for the purposes of this book, wherever possible, nomenclature is based upon origin-insertion (attachment) points.
The deep PFM: Levator ani muscle
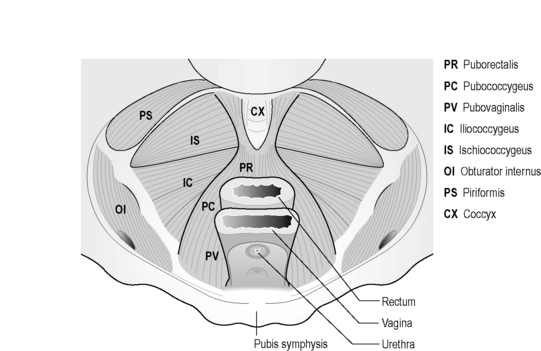
The pubovisceral muscle is a thick U-shaped muscle which arises from the pubic bones on either side of the midline, and a band of fascia, the arcus tendineus levator ani (ATLA), laterally (Figure 2.3.2).
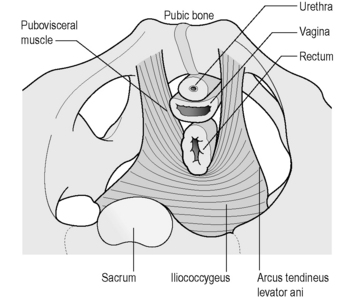
Figure 2.3.2 Levator ani muscle seen from above.
Adapted, with permission from Elsevier North-Holland, from Kearney et al. (2004). © DeLancey 2003).
It passes behind the rectum forming a sling-like arrangement, attaching to the walls of the vagina, or fascial sheath of the prostate and urethra in the male, the perineal body and anal sphincter. The pubovisceral muscle can then be divided into three main components based upon the anatomical attachments: puborectalis, pubovaginalis or levator prostate in the male and pubococcygeus (Figure 2.3.3). In the female, the puborectalis portion passes beside the vagina with some attachment to the lateral vaginal walls. Some other fibres insert into the rectum, and others pass behind the anorectal junction. The fibres of the pubovaginalis pass between the vagina and pubis, connecting to the fascia that supports the urethra. Pubococcygeus only comprises a small proportion of the levator complex, although clinicians have often referred to the entire pubovisceral muscle as pubococcygeus. Its fibres pass from the pubic bones to the coccyx, with some extending behind the rectum and to the anal canal.
The ischiococcygeus is not technically part of the PFM, but forms the remainder of the pelvic diaphragm posteriorly, attaching to the ischial spine, the coccyx and lower part of the sacrum (Figure 2.3.3).
The superficial PFM and perineal body
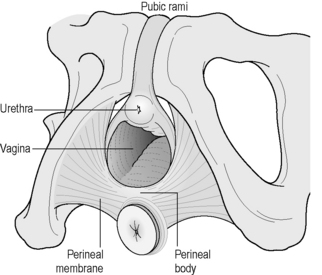
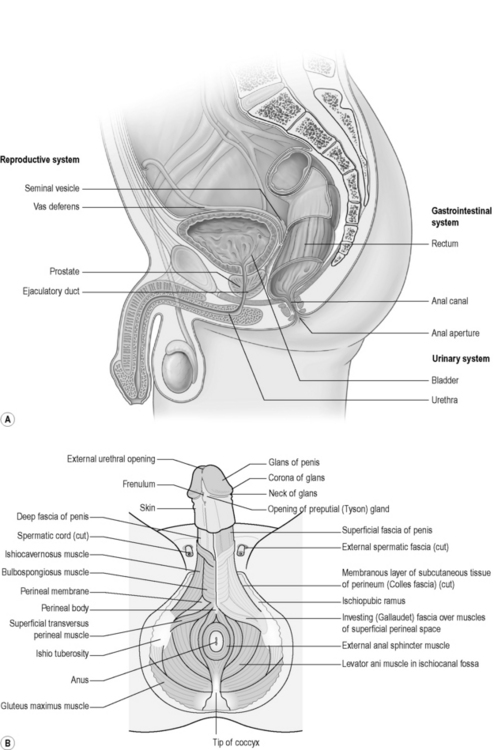
Along with the connective tissue membrane surrounding them, the superficial PFM is often called the perineal membrane or urogenital diaphragm (Figures 2.3.4, 2.3.5). In the female, the perineal membrane lies level with the hymenal ring and attaches the urethra, vagina and perineal body to the ischiopubic rami.
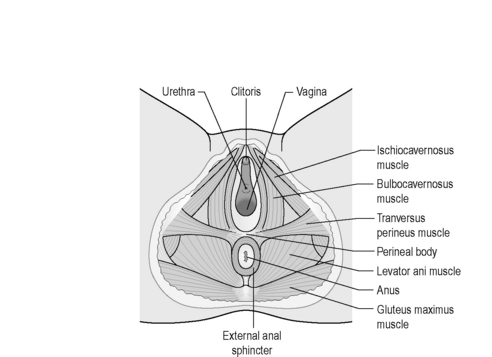
Figure 2.3.5 Superficial muscles of the pelvic floor.
Reproduced from Drake (2009) Gray’s Anatomy Student Edition, Churchill Livingston.
The perineal body is a fibromuscular structure containing smooth muscle, elastic fibres and nerve endings in the middle of the perineum, between the urogenital and anal hiatuses (Figures 2.3.4, 2.3.5). Fibres of the rectum and anal sphincter, superficial PFM and levator ani also extend into the perineal body (Herschorn 2004). There is no consensus over the anatomical configuration of these muscles and fascia (Oelrich 1983, DeLancey 1999) yet the striated muscle fibres are generally thought of as the transverse perinei superficialis, urogenital sphincters (compressor urethrae, urethrovaginal) and external anal sphincters (Figures 2.3.4, 2.3.5, 2.3.6). The bulbocavernous and ischiocavernosus are the most superficial layer of the PF and have a mainly sexual function (Peschers & DeLancey 2008).
Endopelvic fascia
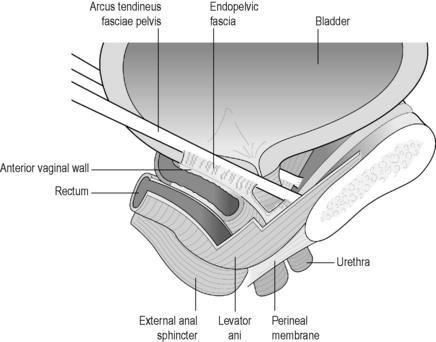
The endopelvic fascia is the collective name given to the connective tissue that attaches the bladder, urethra, vagina and uterus to the pelvic walls (Figure 2.3.6). The pelvic sidewall attachment of the endopelvic fascia is called the arcus tendineus fascia pelvis (ATFP) which is attached to the pubic bone ventrally and to the ischial spine dorsally (Ashton-Miller et al. 2001).
The ATFP is well-defined anteriorly, and lies approximately 3 cm below the levator ani muscle. Posteriorly it is more like a broad sheet of fascia, blending with the endopelvic fascia, and merges with the levator ani. Near the spine, the ATFP fuses with the ATLA which is the band of fascia that the levator ani arises from (Figure 2.3.6).
Pelvic viscera
Bladder and urethra
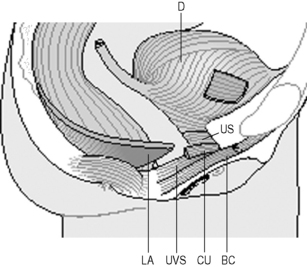
The lower urinary tract can be divided into the bladder (or detrusor) and urethra, with the junction of these two continuous structures termed the bladder (vesical) neck or urethrovesical junction (Figure 2.3.7). The bladder is attached by ligaments and fascia to the side walls of the pelvis and pubic bones. Anteriorly in males the bladder neck is attached to the posterior surface of the pubic symphysis by the puboprostatic ligament, and in females the pubovesical ligament.
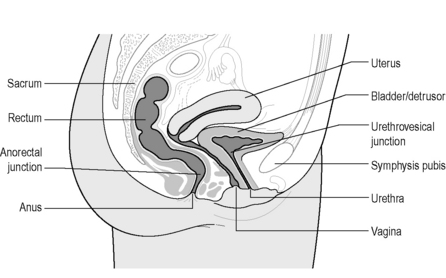
Figure 2.3.7 Sagittal cross-section of female pelvis indicating main pelvic viscera and bony landmarks.
Copyright and courtesy of Maeve Whelan, Specialist Womens Health Physiotherapist, Dublin, Ireland
The urethra is a highly vascular multilayered tube approximately 0.6 cm in diameter and 3–4 cm in length in females (Strohbehn et al. 1996) and longer in the male (18–22 cm). In the female, the outer layer of the urethra is formed by the muscle of the striated urogenital sphincter whose fibres lie predominately in a circular orientation in the upper two-thirds of the urethra. The striated urogenital sphincter muscle region of the ventral urethral wall has three component parts: the sphincter urethrae, the compressor urethrae and the urethrovaginal sphincter (Figure 2.3.8).
The smooth muscle of the urethra is present in the upper four-fifths of the urethra, lies inside the striated urogenital sphincter and is contiguous with the bladder. The orientations of the inner fibres are longitudinal whilst the outer layers are circular (Strohbehn et al. 1996) (Figure 2.3.9).
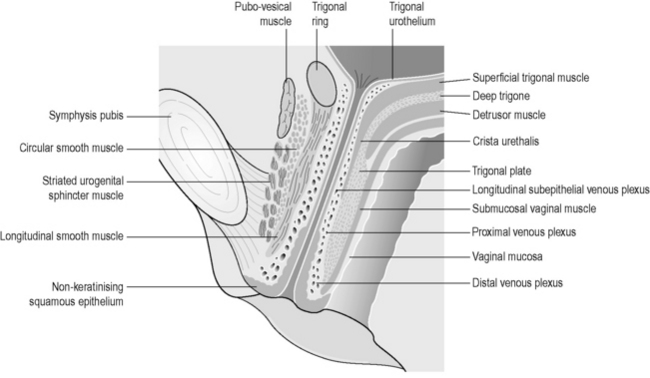
Figure 2.3.9 Anatomy of the urethra shown in longitudinal section.
Reproduced, with permission, from Strohbehn et al. (1996). Magnetic resonance imaging anatomy of the female urethra: a direct histologic comparison. Obstet. Gynecol. 88(5), 750–756.
In the female urethra, closure pressure is known to be developed principally by contraction of the smooth and striated muscle (Perucchini et al. 2002). All elements of the striated urogenital sphincter muscle are found in the ventral urethral wall, whereas the dorsal wall contains only the sphincter urethrae because the urethrovaginal sphincter and the compressor urethrae diverge from the urethral wall to pass laterally toward the ischiopubic ramus and the vaginal wall (Oelrich 1983).
Prostate
The prostate is a firm, partly glandular and partly muscular body, the base of which lies at the bladder neck, and the apex at the urogenital diaphragm. A thin filmy layer of connective tissue separates the prostate and seminal vesicles from the rectum posteriorly. Skeletal muscle fibres from the urogenital diaphragm extend into the prostate at the apex and up to the midprostate anteriorly (Hammerich et al. 2009). Further details of the prostate can be found in Chapter 12.
Vagina and uterus
The vagina is a fibromuscular structure, resting on the rectum, lying posterior to the urethrovesical system (see Figure 2.3.7). It is attached laterally to the vaginal walls by combination of fascia and connective tissue containing smooth muscle, elastic fibres nerves and vessels. The distal third of the vagina is fused with the urethra anteriorly, the perineal body posteriorly, and the perineal membrane and PFM laterally (Peschers & DeLancey 2008). The uterus lies behind and above the bladder, in front of the rectum. The neck of the uterus is the cervix, which is directly attached to the vaginal wall.
Penis, scrotum and testes
The penis is anatomically divided into two continuous areas: the external body and the root (Figure 2.3.10). The root comprises a right and a left crus, which lies medial to the corresponding ischiopubic ramus; between the crura lies the bulb of the penis attached to the perineal membrane. Forming the body of the penis are three cylindrical masses of erectile tissue: the right and left corpus cavernosa, continuing from the crura, and the corpus spongiosum, continuing from the bulb of the penis. Each mass is covered by a layer of fascia, the tunica albuginea, and external to this is the deep fascia of the penis, Buck’s fascia, which binds the erectile masses together. The scrotum and scrotal tissue overlie the base of the penis. Deep to the skin is the superficial (dartos) fascia of the scrotum. The septum of the scrotum formed by the dartos fascia extends up to the deep Buck’s fascia of the penis creating the sac separating right from left. Deep to the dartos fascia each side is the cremaster muscle and fascia and deeper again is the testis. Extending upwards from the testis are the spermatic tissue, arteries and veins (see Figure 2.3.10).
Rectum and anal canal
The anterior wall of the rectum lies directly behind the posterior vaginal wall of the vagina in the female (Figure 2.3.2) and the base of the bladder in men. The rectum begins at the level of the third sacral vertebrae, is the lowest part of the gastrointestinal tract and is continuous above with the sigmoid colon and below with the anal canal and anorectal junction, which is at the level of the pelvic diaphragm. The rectum and its contents are supported by the pelvic floor muscles. The rectum is composed of a circular and longitudinal layer of smooth muscle. At its distal end, the circular layer thickens to form the internal anal sphincter. The external anal sphincter is placed outside the internal sphincter, between which are fused longitudinal fibres of the intestinal wall and PFM (Figures 2.3.5, 2.3.6, 2.3.7) (Peschers et al. 1997).
Innervation of the pelvic organs and PFM
Neural control of pelvic organs is affected by a unique coordination of somatic and autonomic motor nervous systems; sensory information and feedback is supplied by both visceral and somatic sensory fibre systems (Enck & Vodusek 2006).
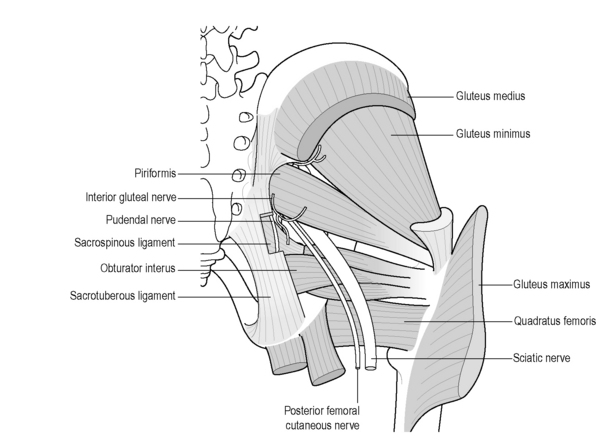
The sciatic (L4–S3) and pudendal nerves (S2–S4) (described in detail in Chapter 13) are the two main nerves arising from the sacral plexus, although the posterior femoral cutaneous nerve (S1–S3/4) gives rise to the inferior cluneal and perineal branches and has been implicated in chronic pelvic pain (CPP) (Darnis et al. 2008, Tubbs et al. 2009) (Figure 2.3.11).The pudendal nerve carries motor, sensory and autonomic fibres; consequently both afferent and efferent pathways can be affected by its injury (Gray et al. 1995). The coccygeal plexus overlies the coccygeus muscle and comprises small nerves from S4 and S5 and the coccygeal nerves. It is thought to supply the coccygeus muscle, part of the levator ani muscle, the sacrococcygeal joint and a small region of skin between the coccyx and anus (Pattern & Hughes 2008).
The levator ani muscles have constant myoelectric activity and are composed of smooth and striated muscle fibres (Shafik et al. 2002), approximately two-thirds of which are type I (Gosling et al. 1981) reflecting their predominately support function (Shafik et al. 2003). Although there are differences of opinion regarding the exact innervation of the PFM, there is consensus that the nerve supply is from the pudendal nerve with direct branches from the sacral nerves S3–S4 (Shafik 2000, Guaderrama et al. 2005, Grigorescu et al. 2008). It is thought that the PFM predominantly contract or relax en masse (Shafik 1998). Yet due to the separate, though identical innervation of each individual muscle, there may also exist the capacity for voluntary selective activity by which an individual muscle might behave independently from the others (Shafik 1998, Kenton & Brubaker 2002).
The autonomic nerves help control micturition, defecation and sexual intercourse. The sympathetic nerves arise from the lumbar splanchnic nerves and parasympathetic nerves from the pelvic splanchnic nerves. The superior and inferior hypogastric plexuses form the major sympathetic supply to the pelvic viscera. The superior hypogastric plexus receives contributions from the lumbar splanchnic nerves (L3–L4) and divides into the left and right hypogastric nerve on the anterior surface of the sacrum (Drake 2005). They are then joined by the pelvic splanchnic nerves and are therefore a mixed plexus of sympathetic, parasympathetic fibres and visceral afferents (Pattern & Hughes 2008). In general it is thought that the pelvic splanchnic nerves are a combination of efferent fibres from S2–S4 and visceral afferent fibres, supplying the pelvic viscera, descending and sigmoid colon. The pelvic sympathetic innervation produces vasomotor effects, inhibits peristaltic contraction of the rectum and stimulates contraction of the internal genitals during orgasm, producing ejaculation in the male (Pattern & Hughes 2008). Parasympathetic innervation causes contraction of the bladder and rectum for micturition and defecation and clitoral or penile erection (Pattern & Hughes 2008). The pelvic visceral afferents travel with the parasympathetic fibres to the spinal ganglia (S2–S4). Nociceptive visceral afferents (NVAF) from the prostate, seminal vesicles, vagina, cervix, distal sigmoid colon and rectum follow the parasympathetic fibres to the spinal ganglia. NVAF from the bladder, ovaries and uterus travel with the sympathetic fibres to the inferior thoracic and superior lumbar spinal ganglia (Pattern & Hughes 2008). However, in clinical practice with paraplegic patients, sometimes the picture appears more complex.
Ashton-Miller J.A., DeLancey J.O.L. Functional anatomy of the female pelvic floor. Reproductive Biomechanics Annals of the New York Academy of Sciences. 2007;1101:266-296.
Ashton-Miller J.A., Howard D., DeLancey J.O.L. The functional anatomy of the female pelvic floor and stress continence control system. Scand. J. Urol. Nephrol. Suppl.. 2001;35(207):1-7.
Baytur Y.B., Deveci A., Uyar Y., Ozcakir H.T., Kizilkaya S., Caglar H. Mode of delivery and pelvic floor muscle strength and sexual function after childbirth. Int. J. Gynaecol. Obstet.. 2005;88(3):276-280.
Boyles S.H., Weber A.M., Meyn L. Procedures for urinary incontinence in the United States, 1979–1997. Am. J. Obstet. Gynecol.. 2003;189(1):70-75.
Darnis B., Robert R., Labat J.J., Riant T., Gaudin C., Hamel A., et al. Perineal pain and inferior cluneal nerves: Anatomy and surgery. Surg. Radiol. Anat.. 2008;30(3):177-183.
DeLancey J.O.L. Anatomy and physiology of urinary continence. Clin. Obstet. Gynecol.. 1990;33(2):298-307.
DeLancey J.O.L. Structural anatomy of the posterior pelvic compartment as it relates to rectocele. Am. J. Obstet. Gynecol.. 1999;180(4):815-823.
DeLancey J.O. The hidden epidemic of pelvic floor dysfunction: achievable goals for improved prevention and treatment. [Review] [9 refs]. Am. J. Obstet. Gynecol.. 2005;192;(5):1488-1495.
Drake R. Gray’s Anatomy. London: Churchill Livingstone; 2005.
Enck P., Vodusek D.B. Electromyography of pelvic floor muscles. J. Electromyogr. Kines.. 2006;16(6):568-577.
Gray H., Williams P.L., Bannister L.H. Gray’s anatomy: the anatomical basis of medicine and surgery, thirty-eighth ed. NewYork: Churchill Livingstone; 1995.
Gosling J.A., Dixon J.S., Critchley H.O., Thompson S.A. A comparative study of the human external sphincter and periurethral levator ani muscles. Br. J. Urol.. 1981;53(1):35-41.
Grigorescu B.A., Lazarou G., Olson T.R., Downie S.A., Powers K., Greston W.M., et al. Innervation of the levator ani muscles: description of the nerve branches to the pubococcygeus, iliococcygeus, and puborectalis muscles. Int. Urogynecol. J.. 2008;19(1):107-116.
Guaderrama N.M., Liu J., Nager C.W., Pretorius D.H., Sheean G., Kassab G., et al. Evidence for the innervation of pelvic floor muscles by the pudendal nerve. Obstet. Gynecol.. 2005;106(4):774-781.
Hammerich K., Ayala G., Wheeler. Anatomy of the prostate gland and surgical pathology of prostate cancer. In: Hedvig H., Scardino P., editors. Prostate Cancer. Cambridge University Press, 2009.
Hemborg B., Moritz U., Lowing H. Intra-abdominal pressure and trunk muscle activity during lifting. IV. The causal factors of the intra-abdominal pressure rise. Scand. J. Rehabil. Med.. 1985;17(1):25-38.
Herschorn S. Female pelvic floor anatomy: the pelvic floor, supporting structures, and pelvic organs. Reviews in Urology. 2004;6(Suppl.):S10.
Hodges P.W., Sapsford R., Pengel L.H. Postural and respiratory functions of the pelvic floor muscles. Neurourol. Urodyn.. 2007;26(3):362-371.
Howard D., Miller J.M., DeLancey J.O., Ashton-Miller J.A. Differential effects of cough, valsalva, and continence status on vesical neck movement. Obstet. Gynecol.. 2000;95(4):535-540.
Kearney R., Sawhney R., DeLancey J.O.L. Levator ani muscle anatomy evaluated by origin-insertion pairs. Obstet. Gynecol.. 2004;104(1):168-173.
Kenton K., Brubaker L. Relationship between levator ani contraction and motor unit activation in the urethral sphincter. Am. J. Obstet. Gynecol.. 2002;187(2):403-406.
Martins J.A.C., Pato M.P.M., Pires E.B., Natal Jorge R.M., Parente M., Mascarenhas T. Finite element studies of the deformation of the pelvic floor. Reproductive Biomechanics Annals of the New York Academy of Sciences. 2007;1101:316-334.
Nygaard I., Barber M.D., Burgio K.L., Kenton K., Meikle S., Schaffer J., et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311-1316.
Oelrich T.M. The striated urogenital sphincter muscle in the female. Anat. Rec.. 1983;205(2):223-232.
Olsen A.L., Smith V.J., Bergstrom J.O., Colling J.C., Clark A.L. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet. Gynecol.. 1997;89(4):501-506.
Pattern D., Hughes J. Anatomy of the Urogenital Pain Systems. In: Urogenital Pain in Clinical Practice. Informa Healthcare; 2008:23-43.
Perucchini D., DeLancey J.O.L., Ashton-Miller J.A., Peschers U., Kataria T. Age effects on urethral striated muscle: I. Changes in number and diameter of striated muscle fibers in the ventral urethra. Am. J. Obstet. Gynecol.. 2002;186(3):351-355.
Peschers U.M., DeLancey J.O.L. Anatomy. In: Haslam J., Laycock J., editors. Therapeutic Management of Incontinence and Pelvic Pain. second ed. Springer; 2008:9-20.
Peschers U.M., DeLancey J.O., Fritsch H., Quint L.E., Prince M.R. Cross-sectional imaging anatomy of the anal sphincters. Obstet. Gynecol.. 1997;90(5):839-844.
Petros P.E. The anatomy and dynamics of pelvic floor function and dysfunction. The Female Pelvic Floor Function dysfunction and management according to the integral theory. second ed. Heidelberg: Springer; 2007.
Pool-Goudzwaard A., van Dijke G.H., van G.M., Mulder P., Snijders C., Stoeckart R. Contribution of pelvic floor muscles to stiffness of the pelvic ring. Clin. Biomech.. 2004;19(6):564-571.
Shafik A. A new concept of the anatomy of the anal sphincter mechanism and the physiology of defecation: Mass contraction of the pelvic floor muscles. Int. Urogynecol. J. Pelvic Floor Dysfunct.. 1998;9(1):28-32.
Shafik A. Neuronal innervation of urethral and anal sphincters: surgical anatomy and clinical implications. [Review] [88 refs]. Curr. Opin. Obstet. Gynecol.. 2000;12;(5):387-398.
Shafik A., Asaad S., Doss S. The histomorphologic structure of the levator ani muscle and its functional significance. Int. Urogynecol. J.. 2002;13(2):116-124.
Shafik A., Doss S., Asaad S. Etiology of the resting myoelectric activity of the levator ani muscle: Physioanatomic study with a new theory. World J. Surg.. 2003;27(3):309-314.
Shafik A., Ahmed I., Shafik A.A., El-Ghamrawy T.A., El-Sibai O. Surgical anatomy of the perineal muscles and their role in perineal disorders. Anat. Sci. Int.. 2005;80(3):167-171.
Smith M.D., Coppieters M.W., Hodges P.W. Postural response of the pelvic floor and abdominal muscles in women with and without incontinence. Neurourol. Urodyn.. 2007;26(3):377-385.
Smith M.D., Russell A., Hodges P.W. Is there a relationship between parity, pregnancy, back pain and incontinence? Int. Urogynecol. J.. 2008;19(2):205-211.
Strohbehn K., Quint L.E., Prince M.R., Wojno K.J., DeLancey J.O. Magnetic resonance imaging anatomy of the female urethra: a direct histologic comparison. Obstet. Gynecol.. 1996;88(5):750-756.
Tubbs R., Miller J., Loukas M., Shoja M., Shokouhi G., Cohen-Gadol A. Surgical and anatomical landmarks for the perineal branch of the posterior femoral cutaneous nerve: implications in perineal pain syndromes. J. Neurosurg.. 2009;111:332-335.