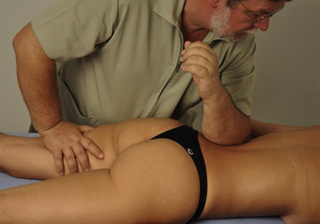
11.1 Soft tissue manipulation approaches to chronic pelvic pain (external)
Introduction
There is increasing evidence demonstrating the importance of treating muscle and connective tissue in patients with chronic pelvic pain (CPP). Eighty-five percent of patients with CPP present with dysfunction or impairments in the musculoskeletal system, including poor posture and pelvic floor muscle (PFM) imbalances (Baker 1993, Hetrick et al. 2003, Prendergast & Weiss 2003, Tu et al. 2006). Shoskes et al. (2008) found that 51% of men with chronic CPP reported tenderness to palpation of the PFM, and Tu et al. (2008) demonstrated that women with CPP had a greater prevalence of musculoskeletal disorders compared with women without CPP. Furthermore, tenderness to palpation of the PFM was related to a decreased ability to relax these muscles (Tu et al. 2008).
It is apparent that proper functioning of the pelvic region is directly related to appropriate integration of the connective tissue and muscles of the lower quadrant. The presence of musculoskeletal dysfunctions may contribute to improper functioning of the pelvic region from both a biomechanical and neurophysiological perspective; for example, increasing tension and/or shortening of the PFM (Haugstad et al. 2006), and initiation or maintenance of a neurogenic inflammation (Wesselmann 2001). In the animal model, Miranda et al. (2004) found that irritation of pelvic musculoskeletal structures promoted antidromic transmission of nociceptive inputs to bladder sensory neurons, promoting a state of neurogenic inflammation.
The diversity of clinical symptoms and physical findings found in patients with CPP emphasizes the necessity of multimodal approaches for the management of this patient population (FitzGerald & Kotarinos 2003a, Fox 2009), as outlined Chapters 8.1 and 8.2. Additionally, there is clinical and scientific evidence for suggesting that CPP can become a chronic syndrome (Bajaj et al. 2003), and hence treatment should be directed at both biomechanical and neurophysiological issues (Samraj et al. 2005). This multimodal approach is also based on the clinical relationship between CPP and pelvic girdle pain (PGP), as many patients diagnosed with PGP also suffer from CPP (Vleeming et al. 2008).
Local muscle dysfunction: Muscle trigger points
Trigger points and chronic pelvic pain
The association between CPP and myofascial pain syndrome was identified several years ago (Slocumb 1984, 1990, Schmidt 1991). Myofascial pain is also related to urogenital pain (Doggweiler-Wiygul 2004). Jarrell (2004) found that abdominal trigger points (TrPs) predicted evidence of visceral disease in 90% of a sample of 55 patients with CPP. Montenegro et al. (2009) recently proposed that abdominal myofascial syndrome should be considered in the differential diagnosis of CPP. In 2009 the European Association of Urology published guidelines suggesting that TrPs should be considered in the diagnosis of CPP (Fall et al. 2010). In fact, Anderson et al. (2009) found that TrPs in the abdominal muscles were the most prevalent in male with CPP.
Myofascial pain syndrome can be associated with both TrPs and restrictions of the fascial tissue. The most commonly accepted definition for TrP is: ‘a hyperirritable spot in a taut band of a skeletal muscle that is painful on compression, stretch, overload or contraction which responds with a referred pain that is perceived distant from the spot’ (Simons et al. 1999). From a clinical viewpoint, we distinguish active and latent TrPs. Active TrPs are those in which local and referred pain reproduce symptoms reported by the patient, with the pain being recognized by the patient as a ‘familiar’ pain (Simons et al. 1999). For instance, in patients with CPP, active TrPs will reproduce perineal or pelvic pain. Latent TrPs are those where local and referred pain do not reproduce pain symptoms, or where elicited pain is not familiar to the patient (Simons et al. 1999). For instance, in a patient with neuropathic pelvic pain, referred pain can be elicited but does not reproduce the patient’s symptoms. Furthermore, a relevant feature of both active and latent TrPs is that each can induce muscle imbalances or altered motor recruitment (Lucas et al. 2004).
There are several studies demonstrating a relationship between CPP and muscle TrPs. Weiss (2001) reported the successful amelioration of symptoms in patients with interstitial cystitis using myofascial TrP release. Doggweiler-Wiygul & Wiygul (2002) found that inactivation of TrPs in PFM, gluteus and piriformis muscles improved or resolved the pain in four patients with severe CPP, interstitial cystitis and irritative voiding symptoms. Anderson et al. (2005) showed that incorporation of TrP inactivation into a multimodal approach for CPP in men resulted in an effective therapeutic approach, by providing a reduction in pain and urinary symptoms superior to that of traditional therapy. This study included voluntary isometric contractions and relaxation, to induce post-isometric relaxation and reciprocal inhibition, together with deep soft tissue mobilization (stripping, strumming, skin rolling and effleurage) as interventions directed at TrPs (Anderson et al. 2005). Anderson et al. (2006, 2009) also found that TrP inactivation was associated with significant improvement in urinary symptoms, libido, ejaculatory and erectile pain, and ejaculatory dysfunction in men with CPP. See Chapter 12 for more detail of Anderson’s studies. Langford et al. (2007) demonstrated the effectiveness of TrPs inactivation of the levator ani muscle for the management of some patients with CPP. In this study 13 of 18 women improved with the first TrP injection resulting in a success rate of 72%, whereas 6 of 18 (33%) were completely pain-free. FitzGerald et al. (2009) demonstrated a better response rate (57%) in CPP patients treated with TrP therapy as compared to the response rate (21%) in those patients receiving global therapeutic massage. In a review of prostatitis and CPP, Anderson (2002) described palpation and treatment protocols for locating muscle TrPs associated with prostatitis symptoms. In a subsequent later study, Anderson et al. (2009) confirmed a relationship between muscle TrPs and CPP in men, identifying the most common location of TrPs: pubococcygeus or puborectalis (90%), external oblique (80%), rectus abdominis (75%), adductors (19%) and gluteus medius (18%) muscles. Other relevant muscles in which TrPs also contribute to CPP are levator ani, iliopsoas, quadratus lumborum, gluteus maximus and the thoracolumbar extensor muscles (Simons et al. 1999, Carter 2000, Liebenson 2000, FitzGerald & Kotarinos 2003a, Chaitow 2007a, Montenegro et al. 2008, Anderson et al. 2009).
Why is inactivation of trigger points in chronic pelvic pain important?
The role of neurogenic inflammation has been emphasized as contributing to the pathophysiology of CPP (Wesselmann 2001). It is well accepted that noxious (nociceptive) stimuli can increase the production of pain-promoting substances at the nerve-free endings of the primary afferent nociceptors. When a sensitive nerve fibre is stimulated the impulse runs towards the spinal cord (orthodromic flow) and towards the periphery (antidromic). When the antidromic stimulus reaches the periphery, there is a release of several neuropeptides (e.g. nitric oxide, substance P, calcitonin gene-related protein) promoting neurogenic inflammation, characterized by vasodilatation, oedema and hyperalgesia (Wesselmann 2001). Clinicians should be aware of the neurophysiological theories for inactivating muscle TrPs in CPP.
1. Trigger points are a focus of peripheral nociception. Muscle pain is associated with the activation of nociceptors by a variety of endogenous substances, e.g. bradykinin or serotonin (Babenko et al. 1999a), substance P (Babenko et al. 1999b) and glutamate (Svensson et al. 2003). Microdialysis studies have found that concentrations of bradykinin, calcitonin gene-related peptide, substance P, tumour necrosis factor-α, interleukin-1β, serotonin or norepinephrine were significantly higher in active TrPs as compared to latent TrP or non-TrP tissues (Shah et al. 2005, 2008). Another study has demonstrated the existence of nociceptive hypersensitivity (hyperalgesia) and non-nociceptive hypersensitivity (allodynia) at the sites of muscle TrPs (Li et al. 2009). These studies support the proposal that TrPs constitute a focus of sensitization of both nociceptive and non-nociceptive nerve endings.
2. Trigger point nociception induces central sensitization.When muscle tissue is sensitized, nociceptors are more readily activated and respond inappropriately to normal innocuous or weak stimuli, e.g. light pressure or movement. The presence of multiple TrPs in different muscles (spatial summation), or the presence of TrPs for prolonged periods of time (temporal summation), can sensitize the spinal cord and supraspinal structures by means of a continued nociceptive afferent barrage into the central nervous system (Mense 1994). Kuan et al. (2007) demonstrated that spinal cord connections of muscle TrPs were effective in inducing neuroplastic changes in the dorsal horn neurons. Niddam et al. (2007) demonstrated that pain associated with TrPs is at least partially processed at supraspinal levels, particularly the peri-aqueductal grey matter. Readers are referred to Chapter 3 for a review of neurophysiology.
3. Trigger points and the sympathetic nervous system. There is evidence of an association between TrPs and the sympathetic nervous system (McNulty et al. 1994, Chen et al. 1998, Chung et al. 2004). Ge et al. (2006) found increased referred pain intensity and tenderness with sympathetic hyperactivity at muscle TrPs, suggesting a sympathetic contribution to the mechanisms responsible for the generation of referred pain. A study by Zhang et al. (2009) demonstrated an attenuated skin blood flow response after painful stimulation of latent TrPs, as compared with control non-TrPs, suggesting increased sympathetic vasoconstriction activity at latent TrPs.
Best evidence of soft tissue interventions for muscle trigger points
In this section we review the evidence for soft tissue interventions targeted at inactivating muscle TrPs. However, clinicians should consider that current evidence is based on the application of single treatments applied to TrPs, when multimodal approaches are usually practised by clinicians. Further, it is clear that management of myofascial dysfunction in patients with CPP requires a multidisciplinary approach (Srinivasan et al. 2007; see also Chapters 8.1 and 8.2). The inclusion of these techniques into a multimodal approach for patients with CPP has been found to be effective (Weiss 2001, Doggweiler-Wiygul & Wiygul 2002, Anderson et al. 2005, 2006).
Among the different interventions directed at inactivating TrPs, manual therapy is the first treatment option (Dommerholt et al. 2006). Different soft tissue interventions have been suggested, including: static compression (Hong et al. 1993, Simons et al. 1999, Fryer & Hodgson 2005, Fernández-de-las-Peñas et al. 2006, Gemmell et al. 2008, Dommerholt & McEvoy 2010), massage (Simons et al. 1999), stretching (Hong et al. 1993, Simons et al. 1999, Hanten et al. 2000), muscle energy techniques (Lewit 1999, Chaitow 2006, Rodríguez-Blanco et al. 2006), strain–counterstrain (Ibáñez-Garcia et al. 2009, Lewit 1999), neuromuscular techniques (Chaitow & Delany 2008, Ibáñez-García et al. 2009, Palomeque-del-Cerro & Fernández-de-las- Peñas 2009), positional release techniques (Chaitow 2007b) and manipulative interventions (Ruiz-Sáez et al. 2007, Fernández-de-las-Peñas 2009).
Systematic reviews have investigated the effectiveness of soft tissue manual intervention for inactivating TrPs (Fernández-de-las-Peñas et al. 2005, Rickards 2006, Vernon & Schneider 2009). These reviews found moderate to strong evidence supporting the use of static compression for immediate pain relief of muscle TrPs but limited evidence for long-term pain relief. Additionally, there is preliminary evidence demonstrating changes in muscle sensitivity after spinal manipulations (Ruíz-Sáez et al. 2006, Fernández-de-las-Peñas 2009), although further studies are required.
Application of soft tissue interventions for trigger points
In this section soft tissue interventions applied to TrPs in those muscles in which referred pain can contribute to CPP are described. Clinicians are encouraged to develop their own techniques based on a clinical reasoning process (see Chapter 7). Selection of any technique should include consideration of TrP irritability and the degree of sensitization of the central nervous system of the patient with CPP.
Compression interventions
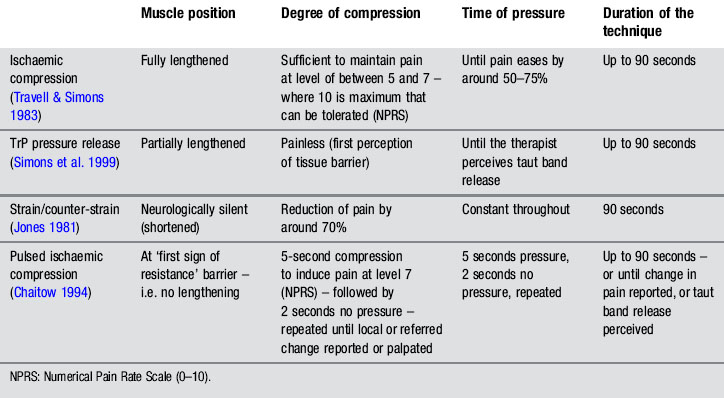
Different compression techniques, depending on the amount of pressure applied, presence/absence of pain (Lewit 1999, Simons et al. 1999), duration of application (Hou et al. 2002), or position of the tissue (shortened or lengthened), have been described. In our clinical practice, the pressure level, duration of application, and position of the muscle, depend on sensitization mechanisms of the patient, and degree of irritability of the TrP. Table 11.1.1 summarizes clinical application of four different forms of compression: ischaemic compression (Travell & Simons 1983), TrP pressure release (Lewit 1999, Simons et al. 1999), strain/counter-strain (Jones 1981) or positional release therapies (Chaitow 2007b), and intermittent compression (Chaitow 1994).
Simons (2002) proposed that compressing the sarcomeres by direct pressure in a vertical and perpendicular manner may equalize the length of the muscle sarcomeres in the involved TrP and decrease pain. Hou et al. (2002) suggested that pain relief may result from reactive hyperaemia within the TrP or a spinal reflex mechanism for the relief of muscle tension.
Under the next heading, we describe different forms of compression interventions applied to pelvic muscle TrPs. Clinicians can apply ischaemic compression (Travell & Simons 1983), pressure release (Lewit 1999, Simons et al. 1999) or positional release therapy (Chaitow 2007b) principles depending on the patient’s characteristics.
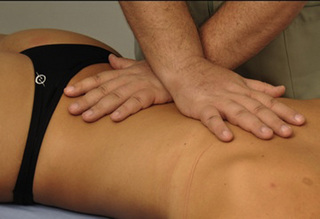
Static compression of piriformis/external obturator muscle trigger points 
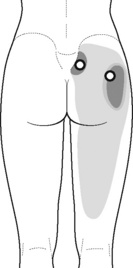
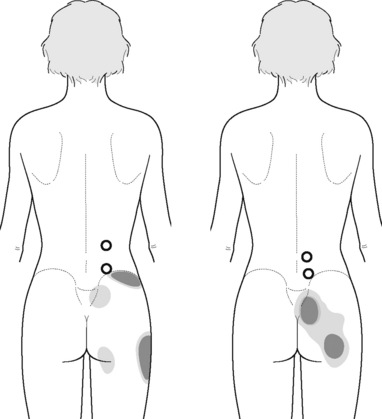
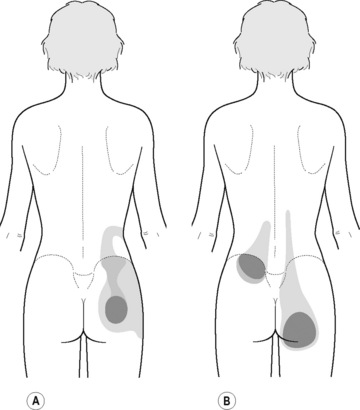
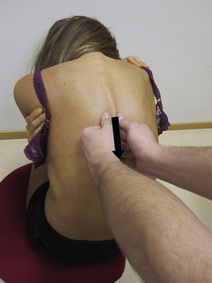
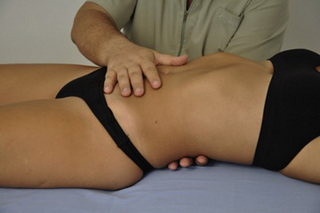
Typical piriformis TrP referred pain is shown in Figure 11.1.1 (Simons et al. 1999). TrPs in this muscle may contribute to pain in the lower back, buttock, hip, posterior thigh and leg, but also to pain into the groin, perineum and sometimes in the rectum during defecation. For this technique, the patient is prone with the therapist standing to the side. The therapist localizes the TrP (it can be located just lateral to the sacrum or the muscle belly), and applies a static compression directly over it. Clinicians are encouraged to use both hands during the technique (Figure 11.1.2). A similar technique may be applied over the external obturator muscle belly which has a similar pattern of referral to that of the piriformis (Cox & Bakkum 2005).
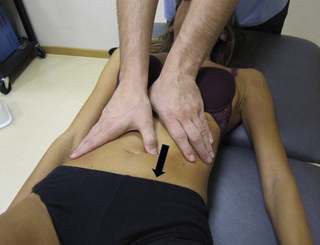
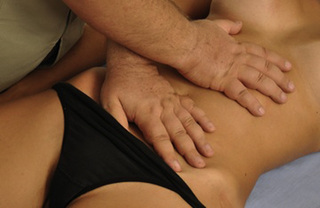
Static compression of pectineus muscle trigger points
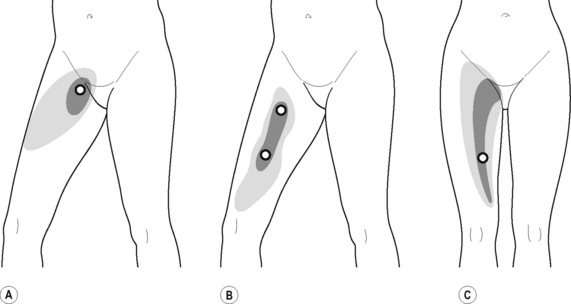
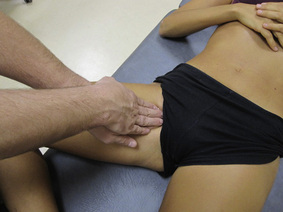
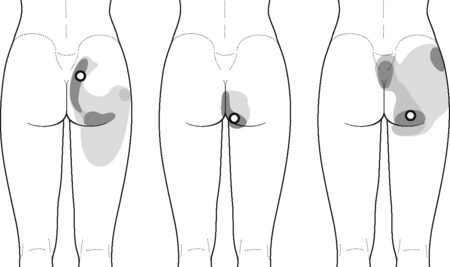
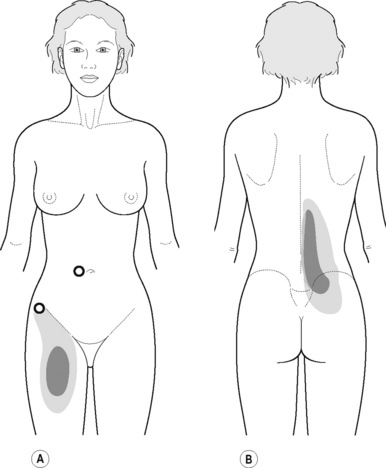
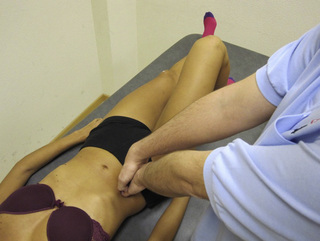
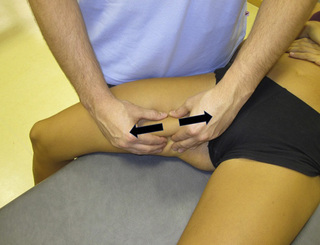
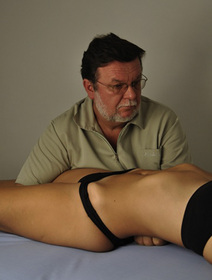
The pectineus muscle is an important adductor muscle in relation to CPP since TrP referred pain is commonly perceived as a deep dull pain in the lateral groin area (Figure 11.1.3A). For the technique, the patient lies supine with the therapist standing to the side. The therapist localizes the TrP which is usually located in the muscle belly (just lateral to the tendon of the adductor longus muscle at the pubic bone), and applies a static compression directly over it. Clinicians are encouraged to use both hands during the compression, although the thumb is also frequently employed (Figure 11.1.4).
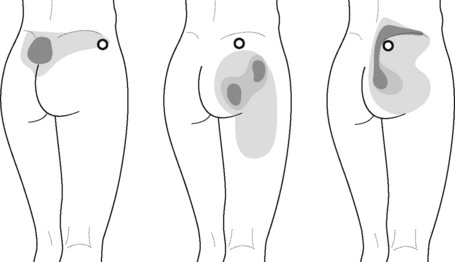
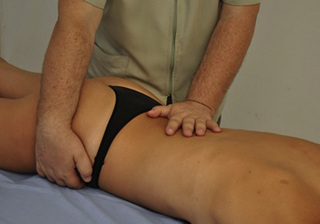
Intermittent compression of pelvic floor muscle trigger points
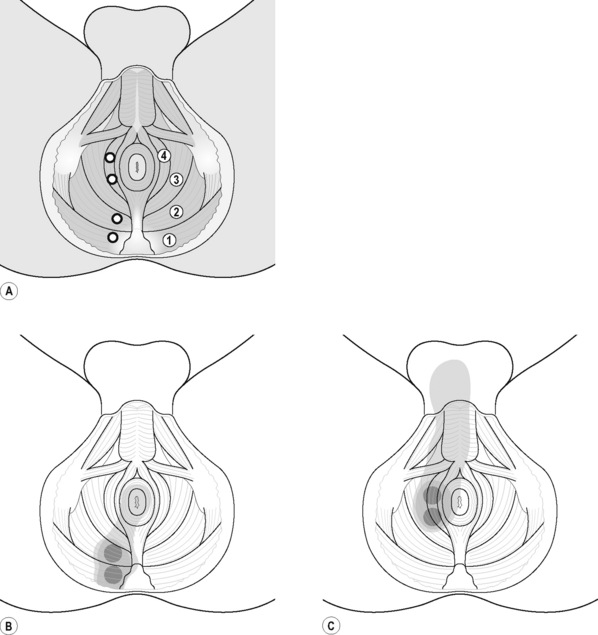
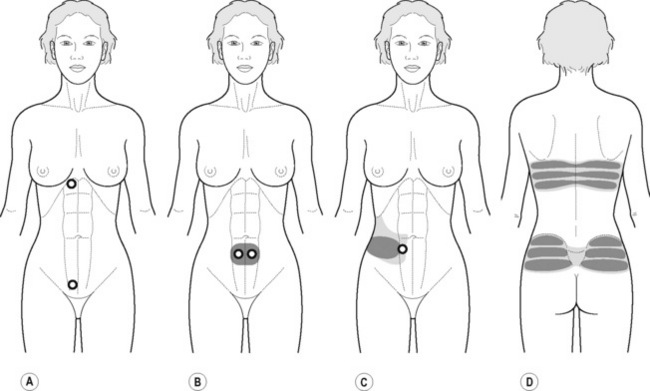
PFM TrPs can mimic symptoms of painful coccydynia or levator ani syndrome (Simons et al. 1999, Chaitow 2007a). In general, pelvic floor muscle TrPs refer pain toward the perineum, vagina, penile base, and give a sensation of fullness into the rectum and an urgency to urinate (Figure 11.1.5A–C). Nevertheless, it seems that TrPs in some PFM are more prevalent in CPP (Anderson et al. 2009). For instance, referred pain from pubococcygeus and puborectalis muscles spreads to the perineum and adjacent urogenital structures. Lewit & Horacek (2004) demonstrated that inactivation of pubococcygeus muscle TrPs induced secondary inactivation of erector spine TrPs. Levator ani and coccygeus TrPs refer pain to the sacrococcygeal region and also to the vagina or penis. TrPs in the internal obturator refer pain to the anococcygeal region and to the vagina. It has also been observed clinically that in some patients with CPP, pelvic floor muscle TrPs can refer pain to the sacrum.
In our clinical experience, PFM respond very well to TrP pressure release. For this technique the patient lies supine or side-lying. The therapist localizes the TrP which is usually located in a specific PFM (particularly located in the ischiorectal fossa) and applies an intermittent digital (finger or thumb) compression to it (Figure 11.1.6). Further details regarding PFM trigger point techniques can be found in Chapter 13.
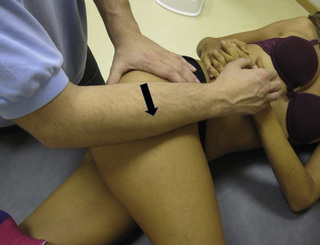
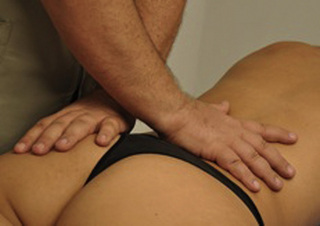
Compression and contraction of gluteus maximus muscle trigger points
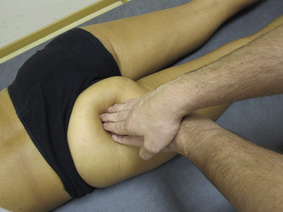
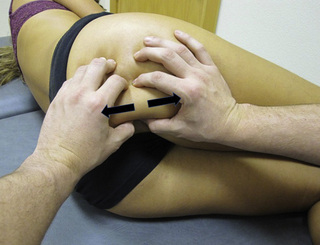
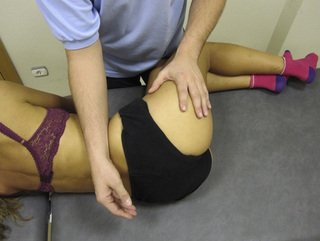
TrP-referred pain from gluteus maximus muscle is perceived as deep and burning pain located in the sacroiliac, coccyx and buttock areas (Figure 11.1.7). This technique consists of applying a TrP compression combined with an isometric contraction of the compressed muscle (Gröbli & Dejung 2003). For that purpose, the patient is side-lying with the therapist behind. TrPs within the gluteus maximus muscle are best palpated and compressed by pincer palpation. Once the therapist locates a TrP (it can placed in any part of the muscle belly) a pincer compression is applied using one or both hands (as illustrated in Figure 11.1.8). When the therapist perceives a slight relaxation of the TrP, the patient is asked to contract the muscle by squeezing both buttocks for 5 seconds. The therapist should maintain the compression during the contraction. If two hands are employed, stretching of the tissues housing the trigger point should follow the contraction, as illustrated. In the authors’ clinical experience, a total of ten repetitions is usually sufficient to achieve inactivation of gluteal muscle TrPs.
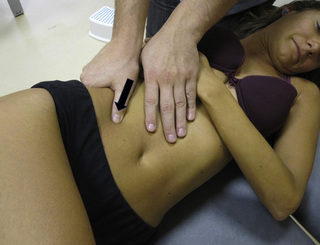
Stretching compression of iliopsoas muscle trigger points 
TrPs in the iliopsoas muscle refer pain to the groin area, superior part of the thigh and to the back (Figure 11.1.9). This is an important muscle since it is anatomically related to several urogenital structures and the lumbar plexus (Stepnik et al. 2006).
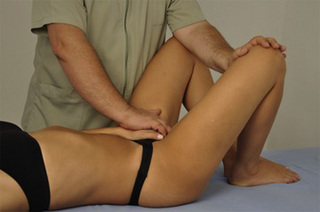
A stretching compression technique combines a compression intervention with passive or active stretching of the TrP taut band. For this purpose the patient is supine with the knee and hip flexed, and the foot on the table. The therapist compresses the TrP (usually located within the muscle belly reached through overlying abdominal muscles) with the tips of the fingers of one or both hands. At the time that the therapist perceives a slightly relaxation of the TrP taut band, the patient is asked to straighten the knee and the hip, either passively or actively, to increase the tension in the taut band (Figure 11.1.10). The aim of this technique is for the patient to achieve pain-free extension of the hip and knee, at the same time that the therapist maintains the compression.
Massage
Massage has multiple clinical applications with positive effects, but often lacks scientific evidence. This may be related to the fact that there are so many different forms of massage that still remain under-researched. The application of massage for inactivating muscle TrPs was discussed by Simons (2002) and Hong et al. (1993), who proposed that massage may exert a lengthening effect, similar to compression interventions. Massage can be performed along the TrP taut band (stretching longitudinal massage) or across the taut band (transverse massage). Hence, transverse massage offers transverse mobilization to the TrP taut band, whereas a longitudinal massage offers longitudinal mobilization to the taut band. In those muscles where clinicians can use pincer palpation, fingers can grasp the taut band from both sides of the TrP. Strokes centrifugally away from the TrP can lengthen the tissues (Simons 2002).
Transverse massage of quadratus lumborum muscle trigger points 
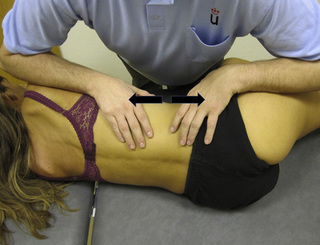
Referred pain from quadratus lumborum TrPs spreads along the crest of the iliac bone, to the outer upper aspect of the groin, the greater trochanter, the sacroiliac joint and the lower buttock (Figure 11.1.11). In some patients, additional referred pain to the anterior thigh, testicle and scrotum has been described (Simons et al. 1999).
For this technique the patient is side-lying with the therapist standing in front of the patient. The ulnar aspect of the therapist’s forearm should be placed over the muscle belly of the quadratus lumborum (where TrPs are usually located). The technique consists of applying a smooth and slow transverse massage over the TrP taut band (Figure 11.1.12). It is important to note that there is no established guideline (number of repetitions, time of application, or amount of pressure) for this technique. In the authors’ experience, the transverse massage should be painless, and applied until the therapist feels the tissues relax.
 Stretching longitudinal massage of adductor muscle trigger points
Stretching longitudinal massage of adductor muscle trigger points
TrPs within the adductor (brevis, longus and magnus) muscles refer pain to the medial aspect of the thigh and to the groin area (Figure 11.1.3B,C). In some patients, the adductor magnus TrPs also elicit an intrapelvic referred pain (Simons et al, 1999). For this technique the patient is supine or side-lying. Once the therapist locates a TrP (it can located in any of the adductor muscles), a pincer palpation of the TrP taut band is applied. The fingers of the therapist grasp the taut band from both sides, and stroke centrifugally away from the TrP (Figure 11.1.13).
Muscle energy interventions
There are several stretching applications targeted at inactivating TrPs: passive stretching (where the therapist passively stretches the muscle without participation of the patient), active stretching (where the patient actively stretches the muscle without participation of the therapist), spray and stretch involving a vapo-coolant spray applied during stretch (Hong et al. 1993, Simons et al. 1999), or muscle energy techniques (Fryer & Fossum 2009).
Muscle energy techniques comprise a system of manual procedures that utilize isometric and isotonic muscle contraction efforts from the patient, usually against a controlled matching counterforce from the therapist. Although there are different approaches for application of muscle energy techniques, the ‘contract–relax–release’, is the most commonly utilized technique. This involves the accurate localization of an isometric contraction (3–7 seconds) at a barrier defined as ‘the first sign of resistance’. An unyielding counterforce is supplied by the therapist. After the patient releases the contraction effort a new barrier is engaged, or stretching is introduced, past the previous barrier, actively or passively. The force and duration of isometric contraction can be varied, depending on the objective of the technique and the tissues involved. In fact, different durations of contraction have been proposed: 2–3 seconds (Mitchell & Mitchell 1995), 3–5 seconds (Greenman 2003), or 5–7 seconds (Chaitow 2006).
Several studies have demonstrated that muscle energy techniques increase muscle extensibility (Feland et al. 2001, Ferber et al. 2002, Ballantyne et al. 2003) and range of motion (Fryer & Ruszkowski 2004, Burns & Wells 2006) in healthy subjects. For a review of scientific evidence of muscle energy techniques, readers are referred to another text (Fryer 2006). The physiological therapeutic mechanisms by which muscle energy techniques exert their effect are speculative and controversial. Of the three most studied mechanisms that have been proposed, i.e. reflex relaxation, viscoelastic or muscle property changes (Fryer 2000), and increased tolerance to stretch, it is the latter that is most supported by the scientific literature (Fryer 2006).
Increased stretch tolerance may result from a decrease in pain perception (hypoalgesia) through the activation of muscle and joint mechanoreceptors, peripheral and central (activation of descending inhibitory pain systems) mechanisms (Fryer & Fossum 2009), and/or reduced concentrations of pro-inflammatory cytokines, and reduced sensitivity of peripheral nociceptors. Enhanced release of endocannabinoids may be one of the mechanisms of osteopathic manipulative treatment (McPartland et al. 2005), parallel to the effects of manipulative treatment upon serum endorphin levels (Vernon et al. 1986).
Muscle energy technique of quadratus lumborum muscle trigger points
The patient is side-lying with the superior leg in front of the other leg. A pillow can be placed under the waist to increase the lateral convexity of the lumbar spine. The therapist’s caudal hand stabilizes the iliac bone and the cranial hand, the rib cage. The rib cage is stretched away from the iliac bone until tension is perceived (Figure 11.1.14). In that position, the patient contracts the muscle, by lifting the leg for 4–8 seconds, and then releases. When the therapist feels that the muscle is relaxed, an increase in muscle tension to the point of stretch is introduced. The patient can be asked to be actively involved by gently lengthening the leg at the start of the stretch.
Neuromuscular technique connective tissue approaches for chronic pelvic pain
TrPs represent a local muscle dysfunction; however, clinicians should consider that dysfunctional connective soft tissue is also involved in CPP. In fact, Han (2009) has hypothesized that the afferent signals from loose connective tissue may be capable of transmitting noxious stimuli from superficial (skin) to deep (muscle) tissue (see Chapter 3 for further information on this topic). According to this theory, muscle TrP-referred pain would also be related to soft tissue dysfunction. However, this theory requires further investigation.
Neuromuscular connective tissue approaches aim to release stressful tension in fascial connective tissue (Chaitow & Delany 2008). It has been suggested that the application of a mechanical stimulus to soft connective tissue induces a piezoelectric effect, which modifies the ‘gel’ state of tissues to a more solute state (Barnes 1997). This effect can also be obtained with myofascial induction (release) approaches described in the following section.
Longitudinal stroke of abdominal wall muscle trigger points
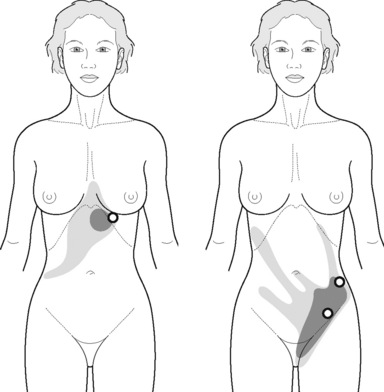
TrPs in rectus abdominis or external oblique muscles may confuse the diagnosis by mimicking visceral pathology (Simons et al. 1999, Maloney & Newman 2005). The referred pain elicited by the external oblique muscle TrPs is perceived as burning pain over the anterior chest wall, spreading to the lower quadrant abdominal and groin area (Figure 11.1.15) The rectus abdominis muscle TrP (Figure 11.1.16A) refers pain to the lower quadrant abdominal simulating nausea and vomiting symptoms (Figure 11.1.16B,C) and also producing pain bilaterally across the upper and lower back (Figure 11.1.16D). One common mechanism of TrP activation is soft tissue scars after surgery (Simons et al. 1999). Readers are referred to treatment of scars after surgery to the connective tissue manipulation section of this chapter.
Longitudinal strokes can be performed with the thumb over rectus abdominis muscle TrPs, from a cranial to caudal direction, with the patient supine (Figure 11.1.17). The degree of pressure applied is determined by the feedback reported by the patient or the tension felt within the patient’s tissue. For external oblique muscle TrPs, the patient is side-lying, with the trunk in contralateral rotation. The strokes can be performed with the thumb or with pincer palpation (Figure 11.1.18).
Stretching stroke of gluteus medius muscle trigger points
The referred pain from gluteus medius muscle TrPs is perceived as deep pain into the lower pelvic quadrant and sacroiliac joint area (Figure 11.1.19). A stretching stroke consists of a longitudinal stroke applied over a muscle placed in a stretched position. With the patient side-lying, gluteus medius can be stretched by adducting the leg with the knee flexed. In this stretched position, longitudinal strokes can be performed with the ulnar aspect of the therapist’s forearm, from a posterior to anterior direction (Figure 11.1.20).
 Dynamic longitudinal stroke of thoracolumbar extensor muscle trigger points
Dynamic longitudinal stroke of thoracolumbar extensor muscle trigger points
Thoracolumbar extensor muscle (iliocostalis lumborum and longissimus muscle) TrPs refer pain to the lower pelvic quadrant and to the buttock area (Figure 11.1.21). The proposed treatment technique combines longitudinal strokes while the patient moves the trunk into flexion (Gröbli & Dejung 2003). The patient is seated with the therapist standing behind the patient. The therapist applies longitudinal strokes over TrP taut bands with the knuckles from a cranial (neck) to caudal (lumbar spine) direction, at the time that the patient flexes the trunk (Figure 11.1.22). It is suggested that, for optimal results, the stroke should be synchronized with the motion of the patient’s trunk into flexion.
Myofascial induction interventions
Introduction to fascial tissue
The fascia is a connective tissue that forms a continual network between the different components of the body (Pilat 2003, Langevin 2006, Vanacore et al. 2009). Its fibrous construction allows the fascial tissue to accommodate to intrinsic and extrinsic compressive and tensional body requirements (Pilat 2009). The different characteristic of fascial structures (e.g. density, distribution) allows these to act as a synergistic functional structure which absorbs and distributes local tensional and compressive forces throughout the body. This inherent synergy of the fascia may play a relevant role in functional tasks, e.g. maintenance of body posture against gravity (Langevin 2006).
Further, it is hypothesized that fascia could also integrate sensory stimuli (i.e. mechanical, thermal or chemical) from the central nervous system (Pilat & Testa 2009, Pilat 2009). Sensory information integrated into the fascia may interact with inputs originating in the central nervous system at three different levels:
1. Physical (mechanical–anatomical) links. Observations on fresh cadavers show a mechanical continuity of fascial tissues where muscles that attach to the fascia act synergistically creating myofascial kinetic links which act at both macroscopic (Stecco et al. 2006, Pilat 2009) and microscopic levels. These are associated with the contraction of myofibroblasts, which are the contractile fascia cells (Maniotis et al. 1997, Vleeming et al. 1997, Hu et al. 2003, Myers 2003, Ingber 2006, Gabbiani 2007, Stecco et al. 2006, Wang et al. 2009).
2. Functional link. Fascial tissue is considered a mechano-sensitive structure that constitutes a particular network of mechanoreceptors, mostly interstitial ones. The mechanical modifications are created primarily in the extracellular matrix that is characterized by piezoelectric and semiconducting properties (Langevin 2006, Vaticón 2009).
3. Chemical link. Ingber (2006) identified the mediating structures for the mechanochemical integration process in the fascial tissue based on mechanotransduction activities. Vanacore et al. (2009) have identified networks that provide structural integrity to the tissues. These serve as ligands for integrin cell-surface receptors related to collagen IV, the major structural component of glomerular basement membranes. It is suggested that these networks can mediate cell adhesion, migration, growth and differentiation (Wang 2009).
Some theories have suggested that the three-dimensional fascial tissue may be involved in pain transmission processes (Liptan 2010). For instance, pain experienced in the pelvic area is usually a referred pain, i.e. perceived in remote areas of the site of noxious stimulation, which does not usually follow neuropathic patterns (Travell & Bigelow 1946). The central hyperexcitability theory explains the mechanisms of pain from deep structures (Mense 1994) but does not clarify the presence of non-segmental patterns of the superficial musculature. Han (2009) has proposed an hypothesis (a connective tissue theory) that the signalling present in the loose connective tissue may be capable of transmitting noxious stimuli from the surface to muscles or other deep structures through the cells of the vascular and neural systems. According to this theory, some peripheral pain may have a direct origin in the connective tissue.
Fascial continuity model
The continuity of the fascial system and its links to the pelvic bones facilitate the interaction with the aponeurosis of the PFM and associated neurovascular structures. For instance, the hypogastric plexus is overlaid by the endopelvic fascia forming the complex fascial skeleton which controls the uterine, vaginal, bladder and urethral vessels. Thus, the myofascial tissue joins the viscerofascial system creating a more complex functional unit (Santos et al. 2009). Therefore, the altered load transfer through the pelvis may affect musculoskeletal dynamics, and may be associated with multiple impairments such as low back/pelvic girdle pain, pelvic adhesions, intestinal and urologic disorders, endometriosis, prolapses, orgasm difficulties, dyspareunia or nerve injuries (Delancey 1993, Snijders et al. 1993a, 1993b, Hodges & Richardson 1996, Vleeming et al. 1996, Lee & Vleeming 1998, Mens et al. 1999, Occelli 2001, Hungerford et al. 2003, Lee & Lee 2004a, Lee & Vleeming 2004, Peters & Carrico 2006, Wurn et al. 2004). Pool-Goudzwaard et al. (2003) reported that 52% of patients studied developed a combination of PGP and pelvic floor disorders during pregnancy, including voiding difficulties, urinary incontinence, sexual dysfunction and/or constipation. Of these patients, 82% stated that their symptoms began with either low or pelvic girdle pain (Pool-Goudzwaard 2003). This interaction in the manifestations of pain and/or dysfunction in the pelvic girdle make diagnosis and clinical decision-making difficult (see Chapter 9).
Which functional model may link all these requirements?
Ingber proposed the intercommunication system theory based on tensegrity principles (Ingber 1998, Pilat & Testa 2009). The tensegrity theory describes a system of shared tensions in the distribution of the mechanical forces at multiple body levels. This model attempts to explain global fascial responses to mechanical stimuli (Chicurel et al. 1998, Khalsa et al. 2000). Different studies have shown that the cell dynamics and active responses of the cytoskeleton induce a tissue remodelling at cellular and subcellular levels when the tissue absorbs mechanical forces from the extracellular matrix (Ingber 1998, 2003, 2006, Parker & Ingber et al. 2007, Stamenovic et al. 2007, Wang et al. 2009). Considering that the construction of the body follows the principles of hierarchical assembly (demonstrated at cellular and subcellular levels) this process is not limited to cells, but also involves tissues, organs and the whole body (Huang & Ingber 1999, 2000).
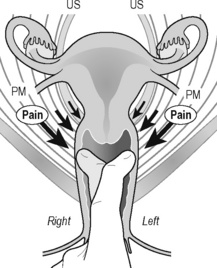
Jarrell (2004) reported an increasing interest in therapeutic measures that incorporate the principles of myofascial dysfunction in CPP syndromes. Lukban et al. (2001) reported a 94% improvement associated with urination in patients with chronic interstitial cystitis after the application of myofascial release interventions, muscle energy and stretching exercises. Santos et al. (2009) established the ‘tensegrity connection’ between the changes in pelvic girdle myofascial function and the endopelvic fascia. They described the Santos sign, which allows diagnosis of damage to pelvic ligamentous support by simple compression with the clinician’s finger (Figure 11.1.23). This fascial dysfunction may be associated with urinary incontinence, coital dysfunction, orgasm dysfunctions, low back pain, and impairments in postural alignment, e.g. hyperlordosis (Santos et al. 2009).
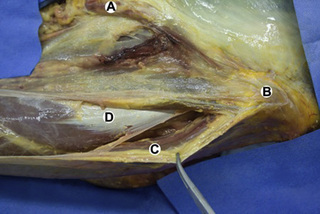
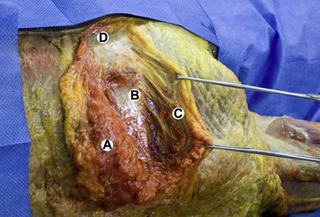
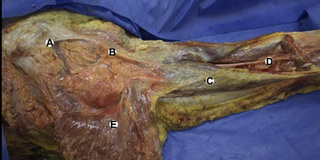
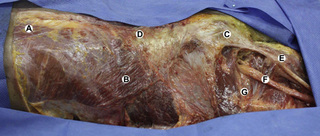
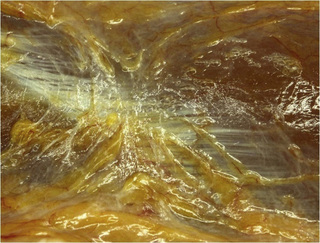
Observations of fresh cadaver dissections confirm the hypothesis of anatomical fascial continuity (Pilat 2009). At the superficial level, just beneath the skin, superficial fascia that contains a considerable amount of fat is located, which differs depending on the anatomical area (Figures 11.1.24–11.1.28). Superficial fascia is characterized by great elasticity, while deep fascia is continuous and represents a more fibrous and dense structure (Figures 11.1.26–11.1.31). At the intermuscular level fascial envelopment (Figure 11.1.32) and tendon–ligament–fascial connections can be observed (Figures 11.1.33, 11.1.34).
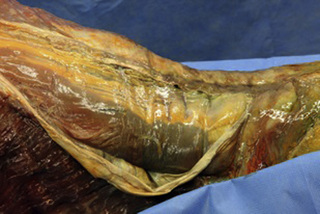
Figure 11.1.30 • Deep fascia in the superficial layer of the paraspinal muscles from a fresh cadaver dissection
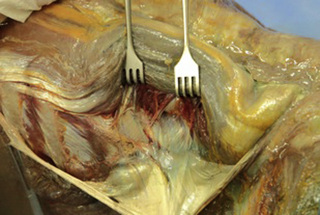
Figure 11.1.31 • Deep fascia in the deep layer of the paraspinal muscles from a fresh cadaver dissection
Theoretical aspects for the treatment of myofascial dysfunction syndrome
Mechanics of the myofascial dysfunction syndrome
Appropriate dynamics of the fascial tissue is necessary for an optimal functioning of the body. For instance, a reduction in the mobility of the fascial tissue may alter circulation (Bhattacharya 2005, Kubo et al. 2009a, 2009b), contributing to the development of ischaemia. An excessive stimulation of collagen production can alter the quality of movement, potentially encouraging facilitating reduced physiological motion (Pilat 2009).
Fascial restrictions (Figure 11.1.35) can promote the formation of compensatory movement patterns that may lead to musculoskeletal dysfunction. These changes impact the loose connective tissue structure resulting in remodelling of the specialized structures (dense regular and irregular connective tissue) leading to fibre reorientation. Short-term tissue changes will affect local function, but long-term changes could create global dysfunctional patterns (Pilat 2003). Further research is needed to support these hypotheses.
Neurophysiological mechanisms for releasing the restrictions of the fascial tissue
1. Piezoelectricity. Since collagen tissue (a basic connective tissue component) is considered a semiconductor structure (O’Connell & Judith 2003), this tissue may be capable of forming an integrated information network enabling the interconnection of fascial components (Szent-Gyorgi 1994, Cope 1975, Bouligard 1978, Oschman 2003).
2. Dynamics of the myofibroblasts. The muscle is a contractile tissue that enables the body to move. Fascial tissue should be considered as an intramuscular connective tissue that forms a functional unit with muscle fibres. The fascial system is highly innervated by mechanoreceptors (Schleip et al. 2005, Langevin 2006, Stecco et al. 2008). Mechanical input (pressure or traction) received by the mechanoreceptors can create a broad range of responses in the fascial system that may result in changes at both macro- and microscopic levels related to the function of the myofibroblasts (Staubesand & Li 1997, Schleip et al. 2005, 2007). Various studies that have focused on the Dupuytren contracture, plantar fasciitis, frozen shoulder and fibromyalgia syndrome support this reasoning (Fidzianska & Jablonska 2000, Gabbiani 2003, 2007, Satish et al. 2008). Chaudhry et al. (2008) using a 3D mathematical model for deformation of human fascia suggested that mechanical forces applied during manual therapy can create mechanical changes in the loose connective tissue (i.e. superficial nasal fascia). Schleip et al. (2007) suggested that the possible changes in resting tone of skeletal muscle fibres can transmit their tension force to the respective fascial tissues.
3. Viscoelasticity. These are the phenomena related to the remodeling process of the extracellular matrix hydration according to the long-term behaviour of the material. The viscoelastic properties of fascia have been observed in numerous studies which have analysed different structures: thoracolumbar fascia (Yahia et al. 1993), fascia lata (Wright & Rennels 1964), subcutaneous fascia of rats (Iatridies et al. 2003), plantar and nasal fascia (Chaudhry 2007). The clinical utilization of the viscoelastic properties of fascia has been described by various authors: Rolf (1977), Barnes (1990), Threlkeld (1992), Cantu & Grodin (2001), Barnes (1997), Pilat (2003), Schleip et al. (2005), Pilat (2009). Recent theories hypothesized that different chemical mediators may be involved in this process (Vaticón 2009), although further research is clearly needed.
Therapeutic strategies applied to the myofascial induction process
General observations related to the therapeutic process
There are different clinical approaches that target the management of dysfunctional fascial structures (Barnes 1990, Rolf 1977, Manheim 1998, Paoletti 1998, Cantu & Grodin 2001, Chaitow & Delany 2002, Pilat 2003). There is, however, a need for unification and validation of the clinical procedures through research (Remving 2007).
The applications suggested in the current chapter are based on clinical experiences of the authors (Pilat 2003) and are based on the theoretical framework previously described. It is important to note that the myofascial induction process may be applied as an exclusive treatment procedure or combined with other manual therapy strategies.
Bases for clinical applications
General observations (Pilat 2003, 2010)
• The evaluation of fascial dysfunction should be included in clinical reasoning processes. We suggest that clinicians follow the common physical explorations for movement dysfunctions of each region.
• Biomechanically, the myofascial system responds to compression and traction forces. These two mechanical strategies can be used when applying myofascial induction techniques.
• Restrictions may occur in various directions and planes. They may even occur in different directions in the same plane, in the same direction in various planes, or in different planes in various directions.
• The direction of the releasing movement is towards facilitation. The therapist should refrain from performing movements in arbitrary directions.
• There is no need for active muscle contraction performed by the patient. The patient may be asked to maintain a state of active passiveness.
Clinical procedure principles (Pilat 2003, 2007a, 2009, 2010)
• The therapist should apply a 3D compression or traction causing the tissue to become tense. This is referred to as the first restriction barrier.
• The applied pressure is constant during the first 60–90 seconds, which is the time required for releasing the first restriction barrier (Pilat 2003, Chaudhry et al. 2007).
• During the first phase of the technique, the therapist barely causes the tissue to move.
• Upon overcoming the first restriction barrier, the therapist accompanies the movement in the direction of the facilitation pausing at each next barrier.
• In each technique, the therapist is advised to overcome three to six consecutive barriers and a minimum time of application is usually 3–5 minutes.
• The tension applied to the tissue should be constant, but the pressure applied by the therapist may be modified after overcoming the first barrier. Pressure should be reduced if there is an increase in pain and/or abundant movement activity.
Examples of clinical applications
Transverse plane induction of the pelvic region (Figure 11.1.36)
This is a common myofascial induction procedure for the pelvic region (Barnes 1990, Upledger 1997, Pilat, 2003). The patient is supine and the therapist is seated on a chair. The therapist places the non-dominant hand under the patient’s back and the dominant one over the abdominal wall, just below the navel. Both hands are placed transversely in relation to the spine. The therapist applies 3D pressure (i.e. each hand engages restriction barriers in the contacted tissues, on all planes: medial-lateral, inferior-superior, clockwise-anticlockwise rotation) and the principles of myofascial induction are followed.
Lumbosacral induction (Figure 11.1.37)
The patient is supine and the therapist is seated. The therapist places the non-dominant hand at the L5 lumbar vertebra level. The fist of the hand is closed in order to get the L5 spinous process to sit snugly in the channel formed by the fingers. The dominant hand of the therapist is placed beneath the sacrum of the patient. With the dominant hand the therapist applies a very gentle caudal traction over the sacrum. The principles of induction are followed (Barnes 1990, Upledger 1997, Pilat, 2003).
Induction of the pubic region (Figures 11.1.38, 11.1.39)
This technique consists of two phases (Pilat 2003). For phase 1, the patient is supine and the therapist is standing at the level of the patient’s hip. The therapist flexes the patient’s ipsilateral hip to 90–100° leaving the contralateral leg flat on the table. Using a cross-handed contact, the therapist then places one hand on the back of the flexed (ipsilateral) thigh, and the other hand on the front of the unflexed contralateral thigh. The process involves the therapist simultaneously applying pressure in a cranial direction with the ipsilateral hand and caudad with the other contralateral hand. The therapist should avoid increasing the flexion of the thigh. The force lines should cross over the pubis, and the principles of induction should then be followed. The technique is applied bilaterally (Figure 11.1.38). For the second phase the patient lies supine with both knees flexed. The therapist stands at the level of the patient’s pelvis. The therapist’s caudal forearm is placed between the inner faces of both patient knees. The patient places one hand on the pubis and the therapist places the cranial hand over the patient’s hand. The patient exerts a slight pressure with the knees ‘squeezing’ the therapist’s forearm, while the therapist exerts a slight pressure on the pubis in an external direction following the movement of facilitation (Figure 11.1.39).
Cross-hand induction of the lumbar spine (Figure 11.1.40)
With the patient prone, the therapist is standing at the level of the patient’s back. The therapist places the crossed hands on the patient’s back and applies a slight pressure force towards the table in a craniocaudal direction (Pilat 2003). The principles of induction are then followed.
Cross-hand induction of the abdominal fascia (Figure 11.1.41)
With the patient supine, the therapist is standing at the level of the patient’s waist. The therapist places the crossed hands over the abdominal fascia. The cranial hand is placed at the level of the xiphoid process of the sternum and the caudal hand is placed at the level of the pubis (Pilat 2003). The principles of induction are then followed.
Lower induction of the thoracolumbar fascia (Figure 11.1.42)
With the patient prone, the therapist is standing at the level of the patient’s pelvis. The therapist places the cranial hand on the patient’s lumbar region, pressing toward the table. With the caudal hand, the therapist contacts the contralateral side at the anterior-superior iliac spine level applying traction in the direction to the ceiling. The principles of induction are then followed (Pilat 2003).
Cross-hand induction of the thoracolumbar and gluteal fascia (Figure 11.1.43)
The patient is prone and the therapist is standing at the level of the patient’s pelvis. The therapist places the cranial hand at the lumbar region of the contralateral patient’s side and the caudal hand over the ipsilateral gluteal fascia. The principles of induction are then followed (Pilat 2003).
Quadratus lumborum fascia induction (Figure 11.1.44)
With the patient prone, the therapist is standing at the level of the pelvis facing the patient’s head. The therapist places an elbow over the ipsilateral lumbar region between the last rib, the iliac crest, and laterally to the paravertebral muscles. In this position, the therapist presses with the elbow towards the table. The other hand should be placed over the patient’s thigh. With this hand, the therapist pushes in a cranial direction. This manoeuvre shortens the quadratus lumborum muscle and allows easier access to its fascia. The therapist should use body weight. This position is held for approximately 3–5 minutes and the principles of induction are followed (Pilat 2003).
Paravertebral muscles fascia induction (Figure 11.1.45)
The patient is lying on his side, with the upper thorax in the prone position. The therapist leans over the patient in order to place the elbow over the lumbar paraspinal mass. Subsequently, the therapist strokes longitudinally with this elbow from L4 to T10 level. Simultaneously, the patient attempts to flex both the hip and knee while the therapist partially resists this effort. The rate of flexion of the hip and knee should take place at the same speed as the elbow movement. The active contraction of the patient’s hip muscles inhibits defensive paraspinal muscle tension and thus enables deeper myofascial induction (Pilat 2003).
Anderson R. Management of chronic prostatitis: chronic pelvic pain syndrome. Urol. Clin. North Am.. 2002;29(1):235-239.
Anderson R.U., Wise D., Sawyer T., Chan C. Integration of myofascial trigger point release and paradoxical relaxation training treatment of chronic pelvic pain in men. J. Urol.. 2005;174(1):155-160.
Anderson R.U., Wise D., Sawyer T., Chan C. Sexual dysfunction in men with chronic protatitis/chronic pelvic pain syndrome: Improvement after trigger point release and paradoxical relaxation training. J. Urol.. 2006;176(4):1534-1539.
Anderson R.U., Sawyer T., Wise D., Morey A., Nathanson B. Painful myofascial trigger points and pain sites in men with chronic prostatitis/chronic pelvic pain syndrome. J. Urol.. 2009;182(12):2753-2758.
Babenko V., Graven-Nielsen T., Svensson P., et al. Experimental human muscle pain and muscular hyperalgesia induced by combinations of serotonin and bradykinin. Pain. 1999;82(1):1-8.
Babenko V., Graven-Nielsen T., Svensson P., et al. Experimental human muscle pain induced by intra-muscular injections of bradykinin, serotonin, and substance P. Eur. J. Pain. 1999;3(2):93-102.
Bajaj P., Madsen H., Arendt-Nielsen L. Endometriosis is associated with central sensitization: a psychophysical controlled study. J. Pain. 2003;4(7):372-380.
Baker P.K. Musculoskeletal origins of chronic pelvic pain: Diagnosis and treatment. Obstet. Gynecol. Clin. North Am.. 1993;20(4):719-742.
Ballantyne F., Fryer G., McLaughlin P. The effect of muscle energy technique on hamstring extensibility: the mechanism of altered flexibility. J. Osteopath. Med.. 2003;6(1):59-63.
Barnes J. Myofascial Release. Paoli: MFR Seminars, 1990.
Barnes M. The basic science of myofascial release. J. Bodyw. Mov. Ther.. 1997;1(4):231-238.
Bhattacharya V. Live Demonstration of microcirculation in the deep fascia and its implication. Plast. Reconstr. Surg.. 2005;115(2):458-463.
Bouligard Y. Liquid crystals and their analogs in biological systems. Solid State Phys.. 1978;14:259-294.
Burns D.K., Wells M.R. Gross range of motion in the cervical spine: the effects of osteopathic muscle energy technique in asymptomatic subjects. J. Am. Osteopath. Assoc.. 2006;106(3):137-142.
Cantu T.I., Grodin A.J. Myofascial manipulation: Theory and clinical application. Maryland: Aspen Publishers, 2001.
Carter J.E. Abdominal wall and pelvic myofascial trigger points. In: Howard F.M., editor. Pelvic Pain. Philadelphia: Lippincott, Williams & Wilkins; 2000:314-358.
Chaitow L. Integrated neuromuscular inhibition technique. British Journal of Osteopathy. 1994;13(1):17-20.
Chaitow L. Muscle Energy Technique, third ed. Edinburgh: Churchill Livingstone, 2006.
Chaitow L. Chronic pelvic pain: Pelvic floor problems, sacroiliac dysfunction and the trigger point connections. J. Bodyw. Mov. Ther.. 2007;11(4):327-339.
Chaitow L. Positional release techniques. Edinburgh: Churchill Livingstone, 2007.
Chaitow L., Delany J. Clinical application of neuromuscular techniques. vol. 2: The Lower Body. Edinburgh: Churchill Livingstone, 2002.
Chaitow L., Delany J. Clinical application of neuromuscular techniques. vol. 1: The upper body. Churchill Livingstone: Edinburgh, 2008.
Chaudhry H. Viscoelastic behavior of human fasciae under extension in manual therapy. Journal of Bodywork and Movement Therapies. 2007;11(3):159-167.
Chaudhry H., Schleip R., Zhiming J.I., et al. Three-dimensional mathematical model for deformation of human fasciae in manual therapy. J. Am. Osteopath. Assoc.. 2008;108(8):379-390.
Chen J.T., Chen S.M., Kuan T.S., et al. Phentolamine effect on the spontaneous electrical activity of active loci in a myofascial trigger spot of rabbit skeletal muscle. Arch. Phys. Med. Rehabil.. 1998;79(7):790-794.
Chicurel M., Chen C., Ingber D. Cellular control lies in the balance of forces. Curr. Opin. Cell Biol.. 1998;10(2):232-239.
Chung J.W., Ohrbach R., McCall W.D.Jr. Effect of increased sympathetic activity on electrical activity from myofascial painful areas. Am. J. Phys. Med. Rehabil.. 2004;83(11):842-850.
Cope F.W. A review of the applications of solid state physics concepts to biological systems. J. Biol. Phys.. 1975;3(1):1-41.
Cox J.M., Bakkum B.W. Possible generator of retrotrochanteric gluteal and thigh pain: The gemelli-obturator internus complex. J. Manipulative Physiol. Ther.. 2005;28(7):534-538.
Delancey J. Anatomy and biomechanics of genital prolapse. Clin. Obstet. Gynecol.. 1993;36(4):897-909.
Doggweiler-Wiygul R. Urologic myofascial pain syndromes. Curr. Pain Headache Rep.. 2004;8(6):445-451.
Doggweiler-Wiygul R., Wiygul J.P. Interstitial cystitis, pelvic pain, and the relationship to myofascial pain and dysfunction: a report on four patients. World J. Urol.. 2002;20(5):310-314.
Dommerholt J., McEvoy J. Myofascial trigger point release approach. In: Wise C.H., editor. Orthopaedic manual physical therapy: from art to evidence. Philadelphia: FA Davis, 2010.
Dommerholt J., Bron C., Franssen J.L.M. Myofascial trigger points: an evidence informed review. J. Man. Manip. Ther.. 2006;14(4):203-221.
Fall M., Baranowski A., Elneil S., et al. EAU guidelines on chronic pelvic pain. Eur. Urol.. 2010;57(1):35-48.
Feland J.B., Myrer J.W., Schulthies S.S., Fellingham G.W., Measom G.W. The effect of duration of stretching of the hamstring muscle group for increasing range of motion in people aged 65 years or older. Phys. Ther.. 2001;81(5):1100-1117.
Ferber R., Osternig L.R., Gravelle D.C. Effect of PNF stretch techniques on knee flexor muscle EMG activity in older adults. J. Electromyogr. Kinesiol.. 2002;12(5):391-397.
Fernández-de-las-Peñas C. Interaction between trigger points and joint hypo-mobility: A clinical perspective. J. Man. Manip. Ther.. 2009;17(2):74-77.
Fernández-de-las-Peñas C., Sohrbeck-Campo M., Fernández J., Miangolarra- Page J.C. Manual therapies in the myofascial trigger point treatment: a systematic review. J. Bodyw. Mov. Ther.. 2005;9(1):27-34.
Fernández-de-las-Peñas C., Alonso-Blanco C., Fernández J., Miangolarra-Page J.C. The immediate effect of ischemic compression technique and transverse friction massage on tenderness of active and latent myofascial triggers points: a pilot study. J. Bodyw. Mov. Ther.. 2006;10(1):3-9.
Fidzianska A., Jablonska S. Congenital fascial dystrophy: abnormal composition of the fascia. J. Am. Acad. Dermatol.. 2000;43(Pt 1):797-802.
FitzGerald M.P., Kotarinos R. Rehabilitation of the short pelvic floor. I: Background and patients evaluation. Int. Urogynecol. J. Pelvic Floor Dysfunct.. 2003;14(4):261-268.
FitzGerald M.P., Anderson R.U., Potts J., et al. Randomized multicenter feasibility trial of myofascial physical therapy for the treatment of urological chronic pelvic pain syndromes. J. Urol.. 2009;82(2):570-580.
Fox W.B. Physical therapy for pelvis floor dysfunction. Medical Health. 2009;92(1):10-11.
Fryer G. Muscle energy concepts: a need for change. J. Osteopath. Med.. 2000;3(1):54-59.
Fryer G. Muscle energy technique: Efficacy and research. In Chaitow L., editor: Muscle Energy Technique, third ed, Edinburgh: Churchill Livingstone, 2006.
Fryer G., Fossum C. Muscle energy techniques. In: Fernández-de-las-Peñas C., Arendt-Nielsen L., Gerwin R.D., editors. Tension-type and cervicogenic headache: Pathophysiology, diagnosis, and management. Boston: Jones & Bartlett Publishers, 2009.
Fryer G., Hodgson L. The effect of manual pressure release on myofascial trigger points in the upper trapezius muscle. J. Bodyw. Mov. Ther.. 2005;9(4):248-255.
Fryer G., Ruszkowski W. The influence of contraction duration in muscle energy technique applied to the atlanto-axial joint. J. Osteopath. Med.. 2004;7(1):79-84.
Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol.. 2003;200(4):500-503.
Gabbiani G. Evolution and clinical implications of the myofibroblast concept. In: Findley T.W., Schleip R., editors. Fascia Research. Basic Science and Implications for Conventional and Complementary Health Care. Munich: Urban and Fischer, 2007.
Ge H.Y., Fernández-de-las-Penas C., Arendt-Nielsen L. Sympathetic facilitation of hyperalgesia evoked from myofascial tender and trigger points in patients with unilateral shoulder pain. Clin. Neurophysiol.. 2006;117(7):1545-1550.
Gemmell H., Miller P., Nordstrom H. Immediate effect of ischaemic compression and trigger point pressure release on neck pain and upper trapezius trigger points: A randomized controlled trial. Clinical Chiropractics. 2008;11(1):30-36.
Greenman P.E. Principles of Manual Medicine, third ed. Philadelphia: Lippincott William & Wilkins, 2003.
Gröbli C., Dejung B. Nichtmedikamentöse Therapie myofaszialer Schmerze. Schmerz. 2003;17:475-480.
Han D.G. The other mechanism of muscular referred pain: The ‘‘connective tissue” theory. Med. Hypotheses. 2009;73(3):292-295.
Hanten W.P., Olson S.L., Butts N.L., Nowicki A.L. Effectiveness of a home program of ischemic pressure followed by sustained stretch for treatment of myofascial trigger points. Phys. Ther.. 2000;80(10):997-1003.
Haugstad G.K., Haugstad T.S., Kirste U.M., et al. Posture, movement patterns, and body awareness in women with chronic pelvic pain. J. Psychosom. Res.. 2006;61(5):637-644.
Hetrick D.C., Ciol M.A., Rothman I., et al. Musculoskeletal dysfunction in men with chronic pelvic pain syndrome type III: a case-control study. J. Urol.. 2003;170(3):828-831.
Hodges P.W., Richardson C.A. Inefficient muscular stabilization of the lumbar spine associated with low back pain: a motor control evaluation of transversus abdominis. Spine. 1996;21(22):2640-2650.
Hong C.Z., Chen Y.C., Pon C.H., Yu J. Immediate effects of various physical medicine modalities on pain threshold of an active myofascial trigger point. J. Musculoskel. Pain. 1993;1(1):37-53.
Hou C.R., Tsai L.C., Cheng K.F., et al. Immediate effects of various physical therapeutic modalities on cervical myofascial pain and trigger-point sensitivity. Arch. Phys. Med. Rehabil.. 2002;83(10):1406-1414.
Huang S., Ingber D. The structural and mechanical complexity of cell growth control. Nat. Cell Biol.. 1999;1(5):E131-E138.
Huang S., Ingber D. Shape-dependent control of cell growth, differentiation, and apoptosis: switching between attractors in cell regulatory networks. Exp. Cell Res.. 2000;261(1):91-103.
Hungerford B., Gilleard W., Hodges P. Evidence of altered lumbo-pelvic muscle recruitment in the presence of sacroiliac joint pain. Spine. 2003;28(14):1593-1600.
Hu S., Chen J., Fabry B., et al. Intracellular stress tomography reveals stress focusing and structural anisotropy in cytoskeleton of living cells. Am. J. Cell Physiol.. 2003;285(5):C1082-C1090.
Iatridies J., Wu J., Yandow J., Langevin H. Subcutaneous tissue mechanical behavior is linear and viscoelastic under uni-axial tension. Connect. Tissue Res.. 2003;44(5):208-217.
Ibáñez-García J., Alburquerque-Sendín F., Rodríguez-Blanco C., et al. Changes in masseter muscle trigger points following strain-counter/ strain or neuro-muscular technique. J. Bodyw. Mov. Ther.. 2009;13(1):2-10.
Ingber D. Tensegrity I. Cell structure and hierarchical systems biology. J. Cell Sci.. 2003;116(10):1157-1173.
Ingber D.E. Cellular mechano-transduction: putting all the pieces together again. FASEB J.. 2006;20(7):811-827.
Ingber D. The architecture of life. Sci. Am.. 1998;278(1):48-57.
Jarrell J. Myofascial dysfunction in the pelvis. Curr. Pain Headache Rep.. 2004;8(6):452-456.
Jones L.N. Strain and counter-strain. Newark, OH: American Academy of Osteopathy, 1981.
Khalsa P., Zhang C., Sommerfeldt D. Expression of integrin alpha2beta1 in axons and receptive endings of neurons in rat, hairy skin. Neurosci. Lett.. 2000;293(1):13-16.
Kuan T.S., Hong C.Z., Chen J.T., et al. The spinal cord connections of the myofascial trigger spots. Eur. J. Pain. 2007;11(6):624-634.
Kubo K., Ikebukuro T., Yaeshima K., Kanehisa H., Yata H., Tsunoda H. Effects of static and dynamic training on the stiffness and blood volume of tendon in vivo. Eur. J. Appl. Physiol.. 2009;106(2):412-417.
Kubo K., Ikebukuro T., Yaeshima K., Kanehisa H. Effects of different duration contractions on elasticity, blood volume, and oxygen saturation of human tendon in vivo. Eur. J. Appl. Physiol.. 2009;106(3):445-455.
Langevin H.M. Connective tissue: a body-wide signaling network? Med. Hypotheses. 2006;66(6):1074-1077.
Langford C.F., Udvari Nagy S., Ghoniem G.M. Levator ani trigger point injections: An underutilized treatment for chronic pelvic pain. Neurourol. Urodyn.. 2007;26(1):59-62.
Lee D., Lee L.J. Stress urinary incontinence: A consequence of failed load transfer through the pelvis?. Proceedings from the 5th interdisciplinary world congress on low back and pelvic pain. Australia: Melbourne; 2004.
Lee D.G., Vleeming A. Impaired load transfer through the pelvic girdle – a new model of altered neutral zone function. Proceedings from the 3rd interdisciplinary world congress on low back and pelvic pain. 1998. Vienna, Austria
Lee D.G., Vleeming A. The management of pelvic joint pain and dysfunction. In Boyling J., Jull G., editors: Grieve’s modern manual therapy of the vertebral column, third ed., Elsevier, 2004.
Lewit K. Manipulative therapy in rehabilitation of the locomotor system, third ed. Oxford: Butterworth Heinemann, 1999.
Lewit K., Horacek O. A case of selective paresis of the deep stabilization system due to borreliosis. Man. Ther.. 2004;9(3):173-175.
Liebenson C. The pelvic floor muscles and the silverstolpe phenomena. J. Bodyw. Mov. Ther.. 2000;4(3):195.
Li L.T., Ge H.Y., Yue S.W., Arendt-Nielsen L. Nociceptive and non-nociceptive hypersensitivity at latent myofascial trigger points. Clin. J. Pain. 2009;25(2):132-137.
Liptan L. Fascia: A missing link in our understanding of the pathology of fibromialgia. J. Bodyw. Mov. Ther.. 2010;14(1):3-12.
Lucas K.R., Polus B.I., Rich P.A. Latent myofascial trigger points: their effects on muscle activation and movement efficiency. J. Bodyw. Mov. Ther.. 2004;8(2):160-166.
Lukban J., Whitmore K., Kellog-Spadt S., et al. The effect of manual physical therapy in patients diagnosed with interstitial cystitis, high-tone pelvic floor dysfunction, and sacroiliac dysfunction. Urology. 2001;57(6, Suppl. 1):121-122.
Maloney M.L., Newman J.M. Abdominal pain of myofascial origin. In: Ferguson L., Gerwin R., editors. Clinical mastery in the treatment of myofascial pain. Philadelphia: Lippincott Williams & Wilkins; 2005:303-326.
Manheim C. The Myofascial Release Manual. US: Slack Inc.; 1998.
Maniotis A., Chen C., Ingber D. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. U. S. A.. 1997;94(3):849-854.
McNulty W.H., Gevirtz R., Hubbard D., Berkoff G. Needle electromyographic evaluation of trigger point response to a psychological stressor. Psychophysiology. 1994;31(3):313-316.
McPartland J.M., Giuffrida A., King J., Skinner E., Scotter J., Musty R.E. Cannabimimetic effects of osteopathic manipulative treatment. J. Am. Osteopath. Assoc.. 2005;105(4):283-291.
Mense S. Referral of muscle pain. Am. Pain Soc. J. 1994;3(1):1-9.
Mens J.M., Vleeming A., Snijders C.J., Stam H.J., Ginai A.Z. The active straight leg raising test and mobility of the pelvic joints. Eur. Spine J.. 1999;8(6):468-473.
Myers T. Anatomy Trains. London: Elsevier, 2003.
Miranda A., Peles S., Rudolph C., et al. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126(4):1082-1089.
Mitchell F.L., Mitchell P.K.G. The Muscle Energy Manual. Michigan: MET Press, 1995. vol. 1
Montenegro M.L.L.S., Mateus-Vasconcelos, Candido-dos-Reis F.J., Nogueira A.A., Poli-Nieto O.B. Physical therapy in the management of women with chronic pelvis pain. Int. J. Clin. Pract.. 2008;62(2):263-269.
Montenegro M.L.L.S., Gomide L.B., Mateus-Vasconcelos E.L.M., et al. Abdominal myofascial pain syndrome must be considered in the differential diagnosis of chronic pelvic pain. Eur. J. Obstet. Gynecol. Reprod. Biol.. 2009;147(1):21-24.
Niddam D.M., Chan R.C., Lee S.H., et al. Central modulation of pain evoked from myofascial trigger point. Clin. J. Pain. 2007;23(5):440-448.
Occelli B. Anatomic study of arcus tendineus fasciae pelvis. Eur. J. Obstet. Gynecol. Reprod. Biol.. 2001;97(2):213-219.
O’Connell J.A., Judith A. Bioelectric responsiveness of fascia. Tech. Orthopaed.. 2003;18(1):67-73.
Oschman J. Energy medicine in therapeutics and human performance. New Hampshire: Nature’s own research Association Dover, 2003.
Palomeque-del-Cerro L., Fernández-de-las-Peñas C. Neuromuscular approaches. In: Fernández-de-las-Peñas C., Arendt-Nielsen L., Gerwin R., editors. Tension Type and Cervicogenic Headache: pathophysiology, diagnosis and treatment. Boston: Jones & Bartlett Publishers; 2009:327-338.
Paoletti S. Les fascias: role des tissus dans la mécanique humaine. 1998. Sully
Parker K.K., Ingber D.E. Extracellular matrix, mechanotransduction and structural hierarchies in heart tissue engineering. Philos Trans R Soc Lond B Biol Sci. 2007;362(1484):1267-1279.
Peters K., Carrico D. Frequency, urgency, and pelvic pain: Treating the pelvic floor versus the epithelium. Curr. Urol. Rep.. 2006;7(6):450-455.
Pilat A. Inducción Miofascial. Madrid: McGraw-Hill, 2003.
Pilat A. El lenguaje del dolor (el proceso de interpretación del dolor en fisioterapia), Libro de Ponencias XV Jornadas de Fisioterapia. Madrid: EUF ONCE, 2007.
Pilat A. Myofascial induction approaches for headache. In: Fernández-de-las- Peñas C., Arendt-Nielsen L., Gerwin R.D., editors. Tension Type and Cervicogenic Headache: pathophysiology, diagnosis and treatment. Boston: Jones & Bartlett Publishers, 2009.
Pilat A., Testa M. Tensegridad: El Sistema Craneosacro como la unidad biodinámica, Libro de Ponencias XIX Jornadas de Fisioterapia. Madrid: EUF ONCE; 2009:95-111.
Pool-Goudzwaard A., Hoek Van Dijke G., Mulder P., et al. The iliolumbar ligament: its influence on stability of the sacroiliac joint. Clinical Biomechnics. 2003;18(2):99-105.
Prendergast S.A., Weiss J.M. Screening for musculoskeletal causes of pelvic pain. Clin. Obstet. Gynecol.. 2003;46(4):773-782.
Remving L. Fascia Research. Myofascial release: 5.4.5–140: An evidence based treatment concept. Elsevier Urban & Fischer, 2007.
Rickards L.D. The effectiveness of non-invasive treatments for active myofascial trigger point pain: A systematic review of the literature. Int. J. Osteopath. Med.. 2006;9(2):120-136.
Rodríguez-Blanco C., Fernández-de-las-Peñas C., Hernández-Xumet J.E., et al. Changes in active mouth opening following a single treatment of latent myofascial trigger points in the masseter muscle involving post-isometric relaxation or strain/counter-strain. J. Bodyw. Mov. Ther.. 2006;10(3):197-205.
Rolf I. La integración de las estructuras del cuerpo humano. Barcelona: Ediciones Urano, 1977.
Ruiz-Sáez M., Fernández-de-las-Peñas C., Rodríguez-Blanco C., et al. Changes in pressure pain sensitivity in latent myofascial trigger points in the upper trapezius muscle following a cervical spine manipulation in pain-free subjects. J. Manipulative Physiol. Ther.. 2007;30(8):578-583.
Samraj G.P., Kuritzky L., Curry R.W. Chronic pelvic pain in women: Evaluation and management in primary care. Complementary Therapy. 2005;31(1):28-39.
Santos G., Gonzalez L., Hernandez B., Lorenzo J. Avances diagnósticos en uroginecología. Maniobra de Santos en incontinencia urinaria y prolapso. 2009. Poster No. 26 XVIII Congreso Latinoamericano de Cirugía F.E.L.A.C. Caracas Venezuela
Satish L., Laframboise W.A., O’Gorman D.B., et al. Identification of differentially expressed genes in fibroblasts derived from patients with Dupuytren’s contracture. Biomedicine Central Medical Genomics. 2008;1(1):1-10.
Schleip R., Klingler W., Lehmann-Horn F. Active fascial contractility: fascia may be able to contract in a smooth muscle-like manner and thereby influence musculoskeletal dynamics. Med. Hypotheses. 2005;65(2):273-277.
Schleip R., Kingler W., Lehmann-Horn F. Fascia is able to contract in a smooth muscle-like manner and thereby influence musculoskeletal mechanics. In: Findley T.W., Schleip R., editors. Fascia Research. Basic Science and Implications for Conventional and Complementary Health Care. Munich: Urban and Fischer; 2007:76-77.
Schmidt R. Pelvic floor behaviour and interstitial cystitis. Semin. Urol.. 1991;9(2):154-159.
Shah J.P., Phillips T.M., Danoff J.V., Gerber L.H. An in vitro microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J. Appl. Physiol.. 2005;99(5):1977-1984.
Shah J.P., Danoff J.V., Desai M.J., et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch. Phys. Med. Rehabil.. 2008;89(1):16-23.
Shoskes D.A., Berger R., Elmi A., et al. Muscle tenderness in men with chronic prostatitis/chronic pelvic pain syndrome: The chronic prostatitis cohort study. J. Urol.. 2008;179(2):556-560.
Simons D.G. Understanding effective treatments of myofascial trigger points. J. Bodyw. Mov. Ther.. 2002;6(1):81-88.
Simons D.G., Travell J.G., Simons L.S. Travell & Simons’ myofascial pain and dysfunction: the trigger point manual. second ed. Baltimore: Lippincott William & Wilkins; 1999:278-307. vol. 1
Slocumb J.C. Neurological factors in chronic pelvic pain: trigger points and the abdominal pelvic pain syndrome. Am. J. Obstet. Gynecol.. 1984;149(5):536-543.
Slocumb J.C. Chronic somatic, myofascial, and neurogenic abdominal pelvic pain. Clin. Obstet. Gynecol.. 1990;33(1):145-153.
Snijders C.J., Vleeming A., Stoeckart R. Transfer of lumbo-sacral load to iliac bones and legs. 1: Biomechanics of self-bracing of the sacroiliac joints and its significance for treatment and exercise. Clin. Biomech.. 1993;8(6):285-294.
Snijders C.J., Vleeming A., Stoeckart R. Transfer of lumbo-sacral load to iliac bones and legs. 2: Loading of the sacroiliac joints when lifting in a stooped posture. Clin. Biomech.. 1993;8(6):295-301.
Srinivasan A.K., Kaye J.D., Moldwin R. Myofascial dysfunction associated with chronic pelvic floor pain: management strategies. Curr. Pain Headache Rep.. 2007;11(5):359-364.
Stamenovic D., Rosenblatt N., Montoya-Zavala M., et al. Rheological behavior of living cells is timescale dependent. J. Biophys.. 2007;93(1):39-41.
Staubesand J., Li Y. Begriff und Substrat der Faziensklerose bei chronisch-venöser Insuffizienz. Phlebologie. 1997;26(1):72-77.
Stecco C., Porzionato A., Macchi V., et al. A histological study of the deep fascia of the upper limb. Ital J Anat Embryol. 2006;111(2):105-110.
Stecco C., Porzionato A., Macchi V., et al. The expansions of the pectoral girdle muscles onto the brachial fascia: morphological aspects and spatial disposition. Cells Tissues Organs. 2008;188(3):320-329.
Stepnik M.W., Olby N., Thompson R.R., Marcellin-Little D.J. Femoral neuropathy in a dog with iliopsoas muscle injury. Vet. Surg.. 2006;35(2):186-190.
Svensson P., Cairns B.E., Wang K., Arendt-Nielsen L. Glutamate-evoked pain and mechanical allodynia in the human masseter muscle. Pain. 2003;101(3):221-227.
Szent-Gyorgyi A. The study of energy-levels in biochemistry. Nature. 1994;148(6469):157-159.
Threlkeld A.J. The effects of manual therapy on connective tissues. Phys. Ther.. 1992;72(12):893-902.
Travell J., Bigelow N.H. Referred somatic pain does not follow a simple ‘‘segmental” pattern. Fed. Proc.. 1946;5:106.
Travell J.G., Simons D.G. Myofascial Pain and Dysfunction: The Trigger Point Manual. Baltimore: Williams & Wilkins, 1983. vol. 1
Tu F.F., As-Sanie S., Steege J.F. Prevalence of pelvic musculoskeletal disorders in a female chronic pelvic pain clinic. J. Reprod. Med.. 2006;51(3):185-189.
Tu F.F., Holt J., Gonzales J., Fitzgerald C.M. Physical therapy evaluation of patients with chronic pelvic pain: a controlled study. Am. J. Obstet. Gynecol.. 2008;198(3):272.e1-272.e7.
Upledger J. Craniosacral therapy I: Study guide. UI Publishing, 1997.
Vanacore R., Ham A., Voehler M., et al. Sulfilimine bond identified in collagen IV. Science. 2009;325(5945):1230-1234.
Vernon H., Schneider M. Chiropractic management of myofascial trigger points and myofascial pain syndrome: A systematic review of the literature. J. Manipulative Physiol. Ther.. 2009;32(1):14-24.
Vernon H.T., Dhami M.S., Howley T.P., Annett R. Spinal manipulation and beta-endorphin: A controlled study of the effect of a spinal manipulation on plasma beta-endorphin levels in normal males. J. Manipulative Physiol. Ther.. 1986;9(2):115-123.
Vleeming A., Pool-Goudzwaard A.L., Hammudoghlu D., Stoeckart R., Snijders C.J., Mens J.M.A. The function of the long dorsal sacroiliac ligament: its implication for understanding low back pain. Spine. 1996;21(5):556-562.
Vleeming A., Albert H.G., Ostgaard H.C., Sturesson B., Stuge B. European guidelines for the diagnosis and treatment of pelvic girdle pain. Eur. Spine J.. 2008;17(6):794-819.
Wang N., Tytell J., Ingber D. Mechano-transduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Science. 2009;10(1):75-81.
Weiss J.M. Pelvic floor myofascial trigger points: manual therapy for interstitial cystitis and the urgency-frequency syndrome. J. Urol.. 2001;166(6):2226-2231.
Wesselmann. Neurogenic inflammation and chronic pelvic pain. World J. Urol.. 2001;19(3):180-185.
Wright D.G., Rennels D.C. A study of the elastic properties of plantar fascia. J. Bone Joint Surg. Am.. 1964;46:482-492.
Wurn L., Wurn B., King C.R., et al. Increasing orgasm and decreasing dyspareunia by a manual physical therapy technique. MedGenMed.. 2004;6(4):47.
Yahia L.H., Pigeon P., DesRosiers E.A. Viscoelastic properties of the human lumbo-dorsal fascia. J. Biomed. Eng.. 1993;15(5):425-429.
Zhang Y., Ge H.Y., Yue S.W., et al. Attenuated skin blood flow response to nociceptive stimulation of latent myofascial trigger points. Arch. Phys. Med. Rehabil.. 2009;90(2):325-332.