Chapter 88 Blood transfusion
Blood component therapy has a central therapeutic role in clinical medicine, but blood banking and transfusion medicine has tended to focus on the blood component supply rather than the demand/patient perspective. The clinical focus should naturally be on ‘what is best for the patient?’ not, ‘what is best for the blood supply?’
Traditionally, transfusion has been regarded as the ‘default’ decision when there is clinical uncertainty. The benefits of transfusion have been assumed with little or no evidence to support the assumption and patients are thereby unnecessarily exposed to potential morbidity or even mortality. Given that the decision-making process for using blood component therapy can be difficult, that indications may be controversial or when there is no evidence for potential benefit, there are good common sense and scientifically evidence-based reasons to adopt a non-transfusion default position.1,2 If allogeneic blood component therapy can be avoided, the potential hazards cease to be an issue.
In considering the use of allogeneic blood transfusion the following questions need to be addressed:
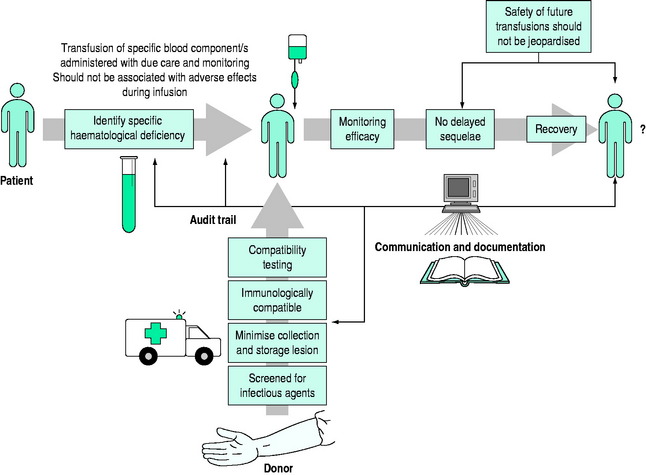
Safe and effective transfusion requires attention to the following details (Figure 88.1):
BLOOD STORAGE AND THE STORAGE LESIONS
Blood is altered from the moment of collection and the ‘lesions’ of collection – anticoagulation, separation, cooling, preservation and storage – compound and progressively increase until the date of expiry.3 The extent of these changes is determined by collection technique, the specific blood component, the preservative medium, the container, storage time and storage conditions. The threshold storage time for blood components has generally been arbitrarily determined by in vitro studies and assessment of in vivo survival. In the case of red cell concentrates, greater than 75% of transfused cells should survive post transfusion.
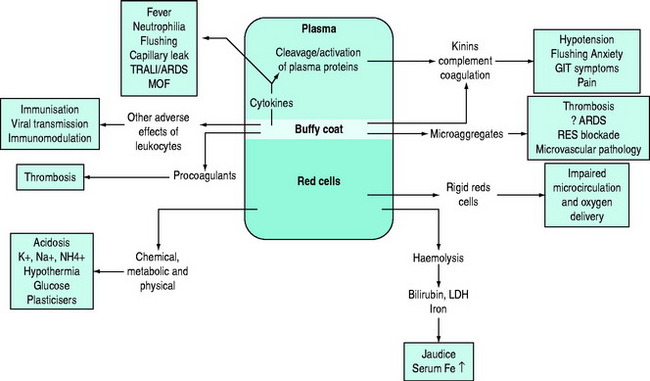
Storage results in quantitative and/or qualitative deficiencies in blood components, which may reduce the immediate efficacy of a transfusion. In parallel with these storage changes is an accumulation of degenerate material (e.g. microaggregates and procoagulant material), release of vasoactive agents, cytokine generation and haemolysis.4 Many of the changes occurring during storage are related to the presence of leukocytes and can be minimised by pre-storage leukoreduction.5 Red cells undergo a change from their biconcave disc shape to spiky spherocytes (echinocytes) and in so doing lose their flexibility. There are also changes in the red cell membrane resulting in an increased tendency to adhere to endothelial cell surfaces in the microcirculation, especially if there is activation of endothelial cells, for example in the presence of the systemic inflammatory response (e.g. with shock or sepsis).6 There is evidence that the immediate post-transfusion function of stored red cells and haemoglobin in delivering oxygen to the microcirculation and unloading is questionable, and several hours are required for red cell oxygen carriage and delivery to return to normal.7,8 It is important to differentiate between the storage lesion being responsible for failure to achieve clinical/laboratory end-points due to reduced survival and/or qualitative defects in cellular function and the ‘toxic’ effects of blood storage (Figure 88.2).
The use of blood filters has been an acknowledgement of the existence of the blood storage lesion and its possible clinical significance. The 170 μm blood-giving filters were first introduced into transfusion medicine to stop the occlusion of blood-giving sets. Ironically, there was little concern that the fibrin clots may harm the patient but fortunately the lung is one of nature’s remarkable filters. Adult respiratory distress syndrome (ARDS) and the Vietnam War increased interest in unfiltered microaggregates accumulating during storage. Both logic and animal data suggested their implication in ARDS and that microfilters to remove microparticles 20–40 μm in size may be protective. This proved difficult to confirm but microaggregate filters do not adequately address the problem of the storage lesion and its clinical significance. Using pre-storage leukodepletion filters, and preventing the development of the storage lesion in both blood and platelets from its inception, is more logical and scientific. Universal pre-storage leukoreduction is now standard practice in many countries, although it was primarily introduced as a precautionary measure against the possible transmission of variant Creutzfeldt–Jakob disease (vCJD) and not to address the numerous other indications for the removal of leukocytes.9
The clinical significance of blood storage lesions is still controversial. Further studies are needed to assess their relevance in conditions such as ARDS, multiorgan failure (MOF), vasoactive reactions and alterations in laboratory parameters.7,10,11 It is assumed that blood components have been appropriately collected, processed, stored, transported and transfused, but despite much greater attention to standard operating procedures and regulation generally, the quality of the final product cannot be guaranteed.12 The ‘assumed’ quality of labile cellular blood products is based on research data and monitoring of standard operating procedures. There is rarely detailed individual product assessment prior to transfusion.8 It is accepted that the adverse effects of storage increase with time and an arbitrary ‘cut off’ is mandated on the basis of research studies.
THE ROLE OF LEUKOCYTES AS A ‘CONTAMINANT’ IN LABILE STORED BLOOD AND THE ROLE OF PRE-STORAGE LEUKODEPLETION
There are several difficulties assessing potential adverse effects of leukocytes as they may be responsible for a wide range of blood component quality and safety issues.9 The presence of leukocytes has been shown to be responsible for specific adverse outcomes in some patients (e.g. non-haemolytic febrile transfusion reaction, platelet refractoriness and transfusion-associated graft-versus-host disease, TAGVHD), but this is the minority. In the larger context the overall available evidence, in the absence of adequate large randomised clinical trials, suggests that universal pre-storage leukodepletion may reduce transfusion-related morbidity and mortality as well as generating cost savings.13 Leukodepletion of red cell and platelet concentrates minimises the clinical consequences of the immunomodulatory effects of allogeneic transfusion. Hence it may decrease the incidence of recurrence of some cancers, of postoperative infections, of blood stream infections and reduce ICU and hospital length of stay. In many patients transfusion-related acute lung injury (TRALI) is a multifactorial disorder and in ‘at risk’ patients non-leukodepleted blood may be a risk factor. Patients in whom there is activation of the systemic inflammatory response syndrome (SIRS) are at risk of developing the multiorgan failure syndrome.5,14 Patients at particular risk include those with trauma, burns, critical bleeding, shock, sepsis and those undergoing cardiopulmonary bypass.15–19 The quality and function of pre-storage leukodepleted red cell concentrates is better maintained on storage, ensuring better post-transfusion efficacy and survival.6,20
CLINICAL GUIDELINES FOR BLOOD COMPONENT THERAPY
RED CELL TRANSFUSIONS
What constitutes appropriate use of red cell transfusions in acute medicine is contentious because of the difficulties in identifying the benefits of red cell transfusion in many circumstances.21 Pursuit of the lowest safe haematocrit continues to receive considerable attention, but pushing any aspect of a system to its limits risks ‘sailing too close to the wind’ which may be appropriate in some situations, but potentially hazardous in others.22,23
PLATELET TRANSFUSIONS
Platelet transfusion therapy may benefit patients with platelet deficiency or dysfunction. The following are the indications for platelet transfusions:24
PROPHYLAXIS
BLEEDING PATIENT
The transfusion of platelet concentrates is not generally considered appropriate:
FRESH FROZEN PLASMA
There are few specific indications for fresh frozen plasma, but its use may be appropriate:25
The use of fresh frozen plasma is generally not considered appropriate in cases of:
IMMUNOGLOBULIN
Normal human immunoglobulin is available in intramuscular and intravenous forms for the treatment or prevention of infection in patients with proven hypogammaglobulinaemia.29 Intravenous immunoglobulin G has been recommended as adjunctive therapy in patients with fulminant sepsis syndrome, especially those with toxic shock syndrome caused by group A streptococci.30
Intravenous immunoglobulin therapy also has a role in therapy of some autoimmune disorders, such as idiopathic thrombocytopenic purpura, autoimmune polyneuropathy and others (see Chapter 90).
FACTOR CONCENTRATES
There is an increasing number of plasma protein concentrates available for clinical use. Some are prepared from donor plasma and some by recombinant technology. Factor VIII and factor IX concentrates have an established role in the management of haemophilia, but others are in the process of establishing their clinical efficacy and indications. Antithrombin III (ATIII) concentrates are available for thrombophilia due to ATIII deficiency and are increasingly recommended in other disorders where ATIII may be depleted (e.g. DIC, MOF).31 Recombinant human activated protein C has antithrombotic, anti-inflammatory and profibrinolytic properties but its role in the treatment of patients with severe sepsis remains controversial.32
Of recent interest is the use of the recombinant activated haemostatic proteins and inhibitors. Recombinant activated factor VII (rFVIIa) was developed for the management of haemophilic patients with coagulation factor inhibitors. rFVIIa is now being widely used ‘off label’ for controlling haemorrhage in the non-haemophilic setting and there is considerable controversy as to its benefits and risks.33 There are an increasing number of case reports and series reporting on the use of rFVIIa in critical life-threatening haemorrhage. Patients with uncontrolled critical bleeding and coagulopathy, despite large transfusions and surgical intervention, have significant mortality rates and rFVIIa in these clinical situations is used as salvage therapy. rFVIIa is now being studied in controlled trials where there is critical bleeding in various clinical settings. rFVIIa initiates the extrinsic coagulation pathway when complexed to tissue factors at sites of injury and on activated platelet surfaces. It potentially has a role in a wide range of haemostatic disorders (e.g. massive blood transfusion, liver disease, uraemia, severe thrombocytopenia and platelet disorders).34
POTENTIAL ADVERSE EFFECTS OF ALLOGENEIC TRANSFUSION
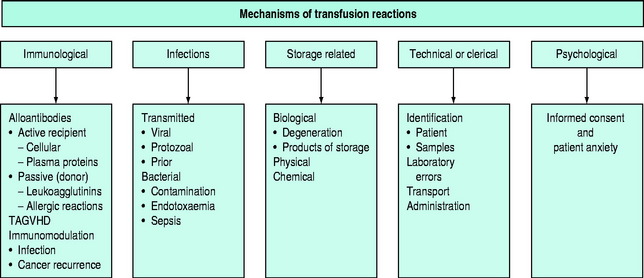
The pathophysiology of blood transfusion reactions can broadly be divided into five categories (Figure 88.3):
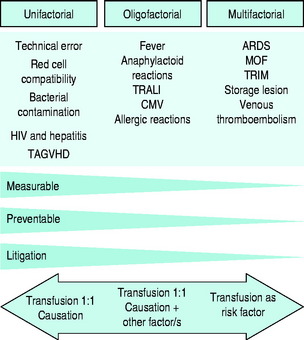
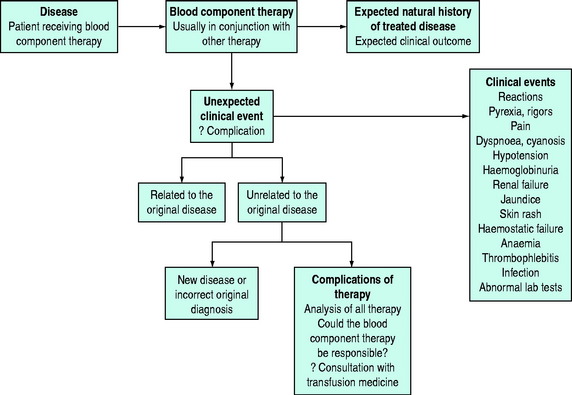
In terms of causation of an adverse clinical event, the possible role of blood transfusion can be classified into three categories on the basis of probability (Figure 88.4):
Adverse reactions to blood component therapy may present in a wide range of ‘guises’ and transfusion must always be considered in the differential diagnosis of any unexpected clinical presentation or indeed as a contributory factor to a clinical picture that may have several contributory factors (Figure 88.5).
OTHER TRANSFUSION-TRANSMISSIBLE INFECTIONS
There is an increasing range of agents that have the potential to be transmitted by blood transfusion, the most recent one of concern being vCJD. The reader is referred to reviews for further information.36,37
BACTERIAL CONTAMINATION
Bacterial contamination of stored blood has always been recognised as a potential cause of fulminant endotoxic shock. Although a rare complication, continuing reports of its occurrence, especially in relation to platelet concentrate transfusion, have increased awareness.38,39 The clinical features of transfusion-related bacterial sepsis in the non-anaesthetised patient include rigors, fever, tachycardia and vascular collapse, with prominent nausea, vomiting and diarrhoea. Anaesthetised patients may have a delayed onset of symptoms (fever, tachycardia, hypotension and cyanosis), followed by DIC, renal failure and sometimes ARDS. The requirement to store platelet concentrates at room temperature increases the risks of bacterial contamination. Pre-release bacterial testing is being more widely advocated as a measure to minimise the problem.
HAEMOLYTIC TRANSFUSION REACTIONS
HAEMOSTATIC FAILURE
Haemorrhagic diathesis due to DIC may be a feature, resulting in severe generalised haemostatic failure, with haemorrhage and oozing from multiple sites.40 As the responsible transfusion is likely to have been administered for haemorrhage, increasing severity of local bleeding may be the first clue to an incompatible transfusion, especially if the patient is unconscious or anaesthetised in the operating room.
ALLERGIC AND ANAPHYLACTOID REACTIONS
Non-cellular blood (plasma and plasma derivatives) components are rarely considered to be a major cause for adverse reactions to transfusion therapy. However, the complexity of plasma and its various components and the effects from component processing results in a broader spectrum of potential adverse effects than frequently recognised. The antigenic heterogeneity of plasma proteins and the presence of antibodies make the non-cellular components of blood responsible for a range of adverse effects, many of which remain poorly understood and commonly pass unrecognised in clinical practice.41
The clinical syndromes of immediate reactions have been classified as follows:
PLASMA AND PLASMA COMPONENTS MAY CAUSE ADVERSE EFFECTS BY SEVERAL PATHOPHYSIOLOGICAL MECHANISMS
Immunological reactions to normal components of plasma may occur in two ways:
TRANSFUSION-ASSOCIATED GRAFT-VERSUS-HOST DISEASE (TAGVHD)
Graft-versus-host disease, classically observed in relationship to allogeneic bone marrow transplantation, may occur following a blood transfusion due to the infusion of immunocompetent lymphocytes precipitating an immunological reaction against the host tissues of the recipient.42 It is most commonly observed in immunocompromised patients, but may also be seen in recipients of directed blood donation from first-degree relatives, and occasionally where donor and recipient are not related, due to homozygosity for HLA haplotypes for which the recipient is heterozygous. The syndrome usually occurs 3–30 days post allogeneic transfusion with fever, liver function test abnormalities, profuse watery diarrhoea, erythematous skin rash and progressive pancytopenia.
TRANSFUSION-RELATED ACUTE LUNG INJURY (TRALI)
TRALI is receiving a great deal of attention as a potentially serious complication of blood transfusion.43–46 In the classic plasma neutrophil antibody-mediated form of the disease symptoms usually arise within hours of a blood transfusion.47 In contrast to most patients with ARDS, recovery usually occurs within 48 hours. The underlying pathophysiology of ‘classic’ TRALI is due to the presence of leukoagglutinins in donor plasma. When complement is activated, C5a promotes neutrophil aggregation and sequestration in the microcirculation of the lung, causing endothelial damage leading to an interstitial oedema and acute respiratory failure. This classic form of transfusion-related acute lung injury has been recognised for over five decades, but was thought to be a rare complication of allogeneic plasma transfusion. As leukoagglutinins occur typically in plasma from multiparous females there is a move to using male donor only fresh frozen plasma.
It is now accepted that there has been underrecognition and underdiagnosis of TRALI, partly due to a lack of clinical awareness, but also to a broader understanding of mechanisms by which blood transfusion may cause or be a contributory factor to lung injury.48,49 The term TRALI is now being expanded to include cases in which transfusion is identified as an independent risk factor predisposing patients to lung injury.50,51 The reader is referred to recent reviews addressing the expanding area of concern in which transfusion, per se, is being identified as an independent risk factor for poor clinical poor outcomes, of which TRALI is only one.
TRANSFUSION-RELATED IMMUNOMODULATION (TRIM)
There is evidence that allogeneic blood transfusion is immunosuppressive in the recipient that has implications in relation to resistance to infection and the likelihood of cancer recurrence.52–54 Donor leukocytes probably play the most important role in this immunomodulation. TRIM, along with the clinical effects of the blood storage lesion, is probably the mechanism by which allogeneic blood transfusion is responsible for poorer clinical outcomes discussed in the next section.
ALLOGENEIC TRANSFUSION AS AN INDEPENDENT RISK FACTOR FOR POORER CLINICAL OUTCOMES
Over the last decade experimental and clinical studies have identified blood transfusion as an independent risk factor for morbidity and mortality as well as increased admission rates to ICUs, increased length of hospital stay and additional costs.18,19,55–57 Blood transfusion being implicated as part of the problem rather than optimal therapy has been a surprise to many clinicians, health administrators and patients, as it has always been assumed that blood transfusion can only be of benefit to the bleeding or anaemic patient. This long-standing belief is now being effectively challenged and currently the debate on the management of the acutely haemorrhaging or anaemic patient has moved significantly from a paradigm with a focus on transfusion to one in which the urgent control of critical bleeding is the paramount priority, with avoidance and/or minimisation of blood transfusion. There is thus increasing evidence that transfusion-related immunomodulation (TRIM) and the transfusion effects of storage lesions are responsible for poorer clinical outcomes in a range of clinical settings.18,19,55,58–63
However, it is not possible to categorically conclude causation and that pre-storage leukoreduction will eliminate or minimise the risk. While the case for causation is strengthened, it is important to be aware that there is minimal evidence for the efficacy or appropriateness of many transfusions, and there is evidence that restrictive red cell transfusion policies do not jeopardise clinical outcomes; indeed, the reverse may be the case.64,65 Thus, until these issues are resolved, a precautionary approach should be adopted, with the modus operandi being to avoid or minimise allogeneic transfusion and use appropriate patient blood conservation techniques.66 An additional association with transfusion is the higher incidence of venous thromboembolism if patients are transfused.67
CRITICAL HAEMORRHAGE AND MASSIVE BLOOD TRANSFUSION
There is a reappraisal of clinical practice guidelines for the use of blood components in the bleeding patient.21,68 The guidelines are no longer for the management of massive blood transfusion, but rather for the management of critical bleeding and avoiding getting into the massive transfusion coagulopathy quagmire in which the patient can spiral down into the ‘triad of death’ – coagulopathy, acidosis and hypothermia.
This reappraisal of management of patients with acute haemorrhage is resulting in the challenging of long-standing dogmas. There is greater tolerance of hypotension until haemorrhage is controlled, and lower haemoglobin levels are tolerated with closer attention to the clinical context of the anaemia and its impact on systemic and local oxygen delivery, especially if there are any compromises in cardiorespiratory function. More attention and research is being directed toward the role of clear fluids and the importance of plasma viscosity, colloid oncotic pressure and functional capillary density.69 The search for safe and effective haemoglobin-based oxygen carriers continues.70–72
If blood loss has been massive or there are defects in the haemostatic system, specific component therapy may be indicated. Advances in patient retrieval, resuscitation protocols, techniques for rapid and real-time diagnosis, trauma teams and early ‘damage control’ surgery have improved the management of acutely haemorrhaging patients. Patients are now surviving with larger volumes of blood transfusion, but sepsis, acute lung injury and multiorgan failure remain major challenges, and blood transfusion is increasingly being recognised as a two-edged sword and probably a contributory factor to these complications in which microcirculatory dysfunction is recognised as central in the pathophysiology. As already discussed, the evidence that the immediate post-transfusion ability of stored red cells and haemoglobin to deliver and unload oxygen to microcirculation may be impaired is resulting in stored red cell concentrates not being automatically the first therapy for acutely enhancing oxygen delivery. Indeed, recent animal data indicate that the immediate clinical benefit of transfused red cells in treating hypovolaemic shock relates to reconstitution of the macrocirculation, with adverse effects on the functional capillary density in the microcirculation.73
In previously healthy patients who have suffered an acute blood loss of less than 25% of their blood volume, restoring volume is more important than replacing oxygen-carrying capacity. Plasma volume expanders may preclude the necessity for allogeneic transfusion, especially if bleeding can be controlled. Clear fluids also allow time for transfusion compatibility testing. In the context of acute bleeding and hypovolaemic shock, the haemoglobin level is not the primary indicator for determining the need for allogeneic red cell transfusion. A normal human may survive a 30% deficit in blood volume without fluid replacement, in contrast to an 80% loss of red cell mass if normovolaemia is maintained. Minimisation of allogeneic blood transfusion is important and haemodilution with tolerance of anaemia is now accepted clinical practice. However, there are limits to haemodilution, not only from the oxygen-carrying capacity perspective. Marked reduction in blood viscosity is counterproductive as reactive peripheral vasoconstriction to maintain total peripheral resistance results in reduction in functional capillary density in the microcirculation. The role of clear fluids and the importance of plasma viscosity, colloid oncotic pressure, electrolyte composition and functional capillary density is the subject of current research.74
A protocol approach to blood component therapy is generally not recommended, as each patient should be treated individually. However, this remains a controversial issue with advocates for ‘blind’, up-front component therapy with red cell and haemostatic components, especially fresh frozen plasma and cryoprecipitate or fibrinogen concentrates. With better understanding of coagulopathy in the critical haemorrhage setting and the importance of hypofibrinogenaemia and hyperfibrinolysis there is a reanalysis of the approach to blood component therapy.75 In an elective situation in which normovolaemia is generally maintained from the outset, defects can be predicted and appropriate blood components prescribed, a protocol approach may be justified. This should be done in close consultation with the haematologist and hospital transfusion service.
Failure of haemostasis is common in critically bleeding patients and may be complex and multifactorial in pathogenesis.76,77 The importance of avoiding acidosis and hypothermia cannot be overemphasised as the triad of coagulopathy, acidosis and hypothermia has an extremely poor prognosis. Coagulopathy associated with critical haemorrhage is related to other aspects of the pathophysiology of the clinical setting. The assumption that there is a ‘generic coagulopathy’ vaguely referred to as DIC is incorrect and can result in inappropriate therapeutic decisions. The reader is referred to an expanding literature on this topic where it will be seen that there can be no standard approach to therapy.77 This author is increasingly convinced that the pathophysiology of coagulopathy, when occurring in the context of critical haemorrhage, should be viewed as related to the primary insult/initiating event, and that a ‘secondary’ coagulopathy (e.g. massive stored blood transfusion, haemodilution, hypothermia, continuing tissue hypoxia, etc.) may compound the problem in the resuscitated patient. The primary mechanisms of coagulopathy relating to the initiating event may relate to trauma, hypoxia, pregnancy, microbial infection, envenomation or iatrogenic (antithrombotic) agents. In all circumstances there is activation or inhibition of some aspect of the haemostatic system, and therapy is better informed if the varied mechanisms are understood. Frequently, complex tests are required for definitive diagnosis, but the urgency of the situation cannot always await the results, and therapy may be initiated on clinical evidence with minimal laboratory information.
Many trauma patients have coagulopathy at presentation that is more related to hypovolaemic shock and not consumption or dilution. Recent evidence indicates that excessive activation of the protein C system and hypofibrinogenaemia secondary to secondary hyperfibrinolysis are important.78 Except for severe abnormalities, haemostatic laboratory parameters correlate poorly with clinical evidence of haemostatic failure. In the massively transfused patient thrombocytopenia and impaired platelet function are the most consistent significant haematological abnormalities, correction of which may be associated with control of microvascular bleeding. Coagulation deficiency from massive blood loss is initially confined to factors V and VIII. Activated partial thromboplastin time (APTT), prothrombin time (PT) and fibrinogen assay should be performed, but the urgency of the situation does not usually allow for other specific factor assays.
A problem with the standard screen tests of coagulation function is that they do not give information about the actual formation of the haemostatic plug, its size, structure or stability. For this reason there is increasing interest in global tests of haemostatic plug formation and stability such as thromboelastography, thrombin generation tests and clot waveform analysis in which changes in light transmission in routine APTT are measured.79 Fresh frozen plasma should be infused if the test results are abnormal, including cryoprecipitate or fibrinogen concentrates if the fibrinogen level is < 1 g/l. A case can be made for prophylactic fresh frozen plasma in infusions in patients with massive blood loss which has been replaced with red cell concentrates and plasma substitutes.
With ongoing bleeding with associated microvascular oozing various approaches may be taken. Having ensured that all identifiable haemostatic defects have been corrected, questions arise as to the role of fresh blood and, more recently, recombinant activated factor VII (rFVIIa).80 The use of freshly collected whole blood remains controversial and is an ‘emotive’ subject needing some comment. The provision of fresh whole blood can present logistic, ethical and safety problems needing consideration. Many transfusion medicine specialists categorically state that there is never an indication for fresh whole blood. Such dogma can be difficult to defend in the clinical setting of the massive haemorrhage and transfusion syndrome where pathophysiology remains poorly understood and specific blood component therapy may be ineffective.
The following statements reasonably summarise the current status of fresh blood transfusion:
SPECIFIC HAZARDS OF MASSIVE BLOOD TRANSFUSION
Massive blood transfusion may be defined in several ways:
ACID–BASE AND ELECTROLYTE DISTURBANCES
Acid–base
Stored bank blood contains an appreciable acid load and is often used in a situation of pre-existing or continuing metabolic acidosis. The acidity of stored blood is mainly due to the citric acid of the anticoagulant and the lactic acid generated during storage. Their intermediary metabolites are rapidly metabolised with adequate tissue perfusion, resulting in a metabolic alkalosis. Hence the routine use of sodium bicarbonate is usually unnecessary and is generally contraindicated. Alkali further shifts the oxygen dissociation curve to the left, provides a large additional sodium load, and depresses the return of ionised calcium to normal following citrate infusion. Acid–base estimations should be performed and corrected in the context of the clinical situation. With continuing hypoperfusion, however, metabolism of citrate and lactate will be depressed, lactic acid production will continue, and there may be an indication for i.v. bicarbonate and calcium to correct acidosis and low ionised calcium.
BASIC IMMUNOHAEMATOLOGY
REGULAR AND IRREGULAR (ATYPICAL) ANTIBODIES
The regular alloantibodies (isoagglutinins) of the ABO system are naturally occurring agglutinins present in all ABO types (except AB), depending on the ABO group. Group O people have anti-A and anti-B isoagglutinins, group A have anti-B and Group B have anti-A. Group A cells are the cause of the commonest and most dangerous ABO incompatible haemolytic reactions. Atypical antibodies are not normally present in the plasma, but may be found in some people as naturally occurring antibodies or as immune antibodies. Immune antibodies result from previous exposure due to blood transfusion or pregnancy. Naturally occurring antibodies more frequently react by saline agglutination and, although they may be stimulated by transfusion, are usually of minimal clinical significance. In contrast, many of the immune atypical antibodies are of major clinical significance and their recognition is the raison d’étre for pretransfusion compatibility testing and antenatal antibody screening. Most of the clinically significant immune atypical antibodies are detected by the IAT.
1 Isbister JP. Decision making in perioperative transfusion. Transfus Apher Sci. 2002;27:19-28.
2 Isbister JP. Clinicians as gatekeepers: what is the best route to optimal blood use? Dev Biol (Basel). 2007;127:9-14.
3 Scott KL, Lecak J, Acker JP. Biopreservation of red blood cells: past, present, and future. Transfus Med Rev. 2005;19:127-142.
4 Anniss AM, Glenister KM, Killian JJ, et al. Proteomic analysis of supernatants of stored red blood cell products. Transfusion. 2005;45:1426-1433.
5 Sparrow RL, Patton KA. Supernatant from stored red blood cell primes inflammatory cells: influence of prestorage white cell reduction. Transfusion. 2004;44:722-730.
6 Anniss AM, Sparrow RL. Storage duration and white blood cell content of red blood cell (RBC) products increases adhesion of stored RBCs to endothelium under flow conditions. Transfusion. 2006;46:1561-1567.
7 Tinmouth A, Fergusson D, Yee IC, et al. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014-2027.
8 Elfath MD. Is it time to focus on preserving the functionality of red blood cells during storage? Transfusion. 2006;46:1469-1470.
9 Blajchman MA. The clinical benefits of the leukoreduction of blood products. J Trauma. 2006;60(6 Suppl):S83-90.
10 Basran S, Frumento RJ, Cohen A, et al. The association between duration of storage of transfused red blood cells and morbidity and mortality after reoperative cardiac surgery. Anesth Analg. 2006;103:15-20.
11 Ho J, Sibbald WJ, Chin-Yee IH. Effects of storage on efficacy of red cell transfusion: when is it not safe? Crit Care Med. 31(12 Suppl), 2003.
12 Hogman CF, Meryman HT. Red blood cells intended for transfusion: quality criteria revisited. Transfusion. 2006;46:137-142.
13 van Hilten JA, van de Watering LM, van Bockel JH, et al. Effects of transfusion with red cells filtered to remove leucocytes: randomised controlled trial in patients undergoing major surgery. BMJ. 2004;328:1281.
14 Despotis GJ, Zhang L, Lublin DM. Transfusion risks and transfusion-related pro-inflammatory responses. Hematol Oncol Clin North Am. 2007;21:147-161.
15 Jeschke MG, Herndon DN. Blood transfusion in burns: benefit or risk? Crit Care Med. 2006;34:1822-1823.
16 Blumberg N, Fine L, Gettings KF, et al. Decreased sepsis related to indwelling venous access devices coincident with implementation of universal leukoreduction of blood transfusions. Transfusion. 2005;45:1632-1639.
17 Hebert PC, Fergusson D, Blajchman MA, et al. Clinical outcomes following institution of the Canadian universal leukoreduction program for red blood cell transfusions. JAMA. 2003;289:1941-1949.
18 Palmieri TL, Caruso DM, Foster KN, et al. Effect of blood transfusion on outcome after major burn injury: a multicenter study. Crit Care Med. 2006;34:1602-1607.
19 Malone DL, Dunne J, Tracy JK, et al. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J Trauma. 2003;54:898-905. discussion 907
20 Sparrow RL, Healey G, Patton KA, et al. Red blood cell age determines the impact of storage and leukocyte burden on cell adhesion molecules, glycophorin A and the release of annexin V. Transfus Apher Sci. 2006;34:15-23.
21 Spiess BD. Red cell transfusions and guidelines: a work in progress. Hematol Oncol Clin North Am. 2007;21:185-200.
22 Carson JL, Ferreira G. Transfusion triggers: how low can we go? Vox Sang. 2004;87(Suppl 2):218-221.
23 Vallet B, Adamczyk S, Barreau O, et al. Physiologic transfusion triggers. Best Pract Res Clin Anaesthesiol. 2007;21:173-181.
24 Guidelines for the use of platelet transfusions. Br J Haematol. 2003;122:10-23.
25 Stanworth SJ, Brunskill SJ, Hyde CJ, et al. Appraisal of the evidence for the clinical use of FFP and plasma fractions. Best Pract Res Clin Haematol. 2006;19:67-82.
26 Lankiewicz MW, Hays J, Friedman KD, et al. Urgent reversal of warfarin with prothrombin complex concentrate. J Thromb Haemost. 2006;4:967-970.
27 Baker RI, Coughlin PB, Gallus AS, et al. Warfarin reversal: consensus guidelines, on behalf of the Australasian Society of Thrombosis and Haemostasis. Med J Aust. 2004;181:492-497.
28 Schulman S, Bijsterveld NR. Anticoagulants and their reversal. Transfus Med Rev. 2007;21:37-48.
29 Looney RJ, Huggins J. Use of intravenous immunoglobulin G (IVIG). Best Pract Res Clin Haematol. 2006;19:3-25.
30 Norrby-Teglund A, Muller MP, McGeer A, et al. Successful management of severe group A streptococcal soft tissue infections using an aggressive medical regimen including intravenous polyspecific immunoglobulin together with a conservative surgical approach. Scand J Infect Dis. 2005;37:166-172.
31 Wiedermann CJ, Hoffmann JN, Juers M, et al. High-dose antithrombin III in the treatment of severe sepsis in patients with a high risk of death: efficacy and safety. Crit Care Med. 2006;34:285-292.
32 Gardlund B. Activated protein C (Xigris) treatment in sepsis: a drug in trouble. Acta Anaesthesiol Scand. 2006;50:907-910.
33 Mittal S, Watson HG. A critical appraisal of the use of recombinant factor VIIa in acquired bleeding conditions. Br J Haematol. 2006;133:355-363.
34 Mathew P, Simon TL, Hunt KE, et al. How we manage requests for recombinant factor VIIa (NovoSeven). Transfusion. 2007;47:8-14.
35 Brand A. Immunological aspects of blood transfusions. Transpl Immunol. 2002;10:183-190.
36 Stramer SL. Current risks of transfusion-transmitted agents: a review. Arch Pathol Lab Med. 2007;131:702-707.
37 Alter HJ, Stramer SL, Dodd RY. Emerging infectious diseases that threaten the blood supply. Semin Hematol. 2007;44:32-41.
38 Rao PL, Strausbaugh LJ, Liedtke LA, et al. Bacterial infections associated with blood transfusion: experience and perspective of infectious diseases consultants. Transfusion. 2007;47:1206-1211.
39 Palavecino E, Yomtovian R. Risk and prevention of transfusion-related sepsis. Curr Opin Hematol. 2003;10:434-439.
40 Davenport RD. Pathophysiology of hemolytic transfusion reactions. Semin Hematol. 2005;42:165-168.
41 Gilstad CW. Anaphylactic transfusion reactions. Curr Opin Hematol. 2003;10:419-423.
42 Oto OA, Paydas S, Baslamisli F, et al. Transfusion-associated graft-versus-host disease. Eur J Intern Med. 2006;17:151-156.
43 Swanson K, Dwyre DM, Krochmal J, et al. Transfusion-related acute lung injury (TRALI): current clinical and pathophysiologic considerations. Lung. 2006;184:177-185.
44 Popovsky MA. Pulmonary consequences of transfusion: TRALI and TACO. Transfus Apher Sci. 2006;34:243-244.
45 Goldman M, Webert KE, Arnold DM, et al. Proceedings of a consensus conference: towards an understanding of TRALI. Transfus Med Rev. 2005;19:2-31.
46 Nathens AB. Massive transfusion as a risk factor for acute lung injury: association or causation? Crit Care Med. 2006;34(5 Suppl):S144-150.
47 Curtis BR, McFarland JG. Mechanisms of transfusion-related acute lung injury (TRALI): anti-leukocyte antibodies. Crit Care Med. 2006;34(5 Suppl):S118-123.
48 Silliman CC, McLaughlin NJ. Transfusion-related acute lung injury. Blood Rev. 2006;20:139-159.
49 Silverboard H, Aisiku I, Martin GS, et al. The role of acute blood transfusion in the development of acute respiratory distress syndrome in patients with severe trauma. J Trauma. 2005;59:717-723.
50 Croce MA, Tolley EA, Claridge JA, et al. Transfusions result in pulmonary morbidity and death after a moderate degree of injury. J Trauma. 2005;59:19-23. discussion 24
51 Gong MN, Thompson BT, Williams P, et al. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33:1191-1198.
52 Blajchman MA. Transfusion immunomodulation or TRIM: what does it mean clinically? Hematology. 2005;10(Suppl 1):208-214.
53 Blumberg N. Deleterious clinical effects of transfusion immunomodulation: proven beyond a reasonable doubt. Transfusion. 2005;45(Suppl 2):S33-40.
54 Raghavan M, Marik PE. Anemia, allogenic blood transfusion, and immunomodulation in the critically ill. Chest. 2005;127:295-307.
55 Chang H, Hall GA, Geerts WH, et al. Allogeneic red blood cell transfusion is an independent risk factor for the development of postoperative bacterial infection. Vox Sang. 2000;78:13-18.
56 Strumper-Groves D. Perioperative blood transfusion and outcome. Curr Opin Anaesthesiol. 2006;19:198-206.
57 Dutton RP, Lefering R, Lynn M. Database predictors of transfusion and mortality. J Trauma. 2006;60(6 Suppl):S70-77.
58 Robinson WP3rd, Ahn J, Stiffler A, et al. Blood transfusion is an independent predictor of increased mortality in nonoperatively managed blunt hepatic and splenic injuries. J Trauma. 2005;58:437-445.
59 Miller PR, Croce MA, Kilgo PD, et al. Acute respiratory distress syndrome in blunt trauma: identification of independent risk factors. Am Surg. 2002;68:845-850. discussion 850–1
60 Moore FA, Moore EE, Sauaia A. Blood transfusion. An independent risk factor for postinjury multiple organ failure. Arch Surg. 1997;132:620-624. discussion 624–5
61 Taylor RW, O’Brien J, Trottier SJ, et al. Red blood cell transfusions and nosocomial infections in critically ill patients. Crit Care Med. 2006;34:2302-2308.
62 Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608-1616.
63 Koch CG, Li L, Duncan AI, et al. Transfusion in coronary artery bypass grafting is associated with reduced long-term survival. Ann Thorac Surg. 2006;81:1650-1657.
64 Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-417.
65 Kwan P, Gomez M, Cartotto R. Safe and successful restriction of transfusion in burn patients. J Burn Care Res. 2006;27:826-834.
66 McIntyre L, Hebert PC, Wells G, et al. Is a restrictive transfusion strategy safe for resuscitated and critically ill trauma patients? J Trauma. 2004;57:563-568. discussion 568
67 Nilsson KR, Berenholtz SM, Garrett-Mayer E, et al. Association between venous thromboembolism and perioperative allogeneic transfusion. Arch Surg. 2007;142:126-132. discussion 133
68 Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198-208.
69 Tsai AG, Cabrales P, Intaglietta M. Blood viscosity: a factor in tissue survival? Crit Care Med. 2005;33:1662-1663.
70 Thyes C, Spahn DR. Current status of artificial O2 carriers. Anesthesiol Clin North Am. 2005;23:373-389. viii
71 Winslow RM. Targeted O2 delivery by low-p50 hemoglobin: a new basis for hemoglobin-based oxygen carriers. Artif Cells Blood Substit Immobil Biotechnol. 2005;33:1-12.
72 Tsai AG, Cabrales P, Intaglietta M. Oxygen-carrying blood substitutes: a microvascular perspective. Expert Opin Biol Ther. 2004;4:1147-1157.
73 Tsai AG, Cabrales P, Intaglietta M. Microvascular perfusion upon exchange transfusion with stored red blood cells in normovolemic anemic conditions. Transfusion. 2004;44:1626-1634.
74 Wettstein R, Erni D, Intaglietta M, et al. Rapid restoration of microcirculatory blood flow with hyperviscous and hyperoncotic solutions lowers the transfusion trigger in resuscitation from hemorrhagic shock. Shock. 2006;25:641-646.
75 Fries D, Haas T, Klingler A, et al. Efficacy of fibrinogen and prothrombin complex concentrate used to reverse dilutional coagulopathy – a porcine model. Br J Anaesth. 2006;97:460-467.
76 Tieu BH, Holcomb JB, Schreiber MA. Coagulopathy: its pathophysiology and treatment in the injured patient. World J Surg. 2007;31:1055-1065.
77 Levy JH. Massive transfusion coagulopathy. Semin Hematol. 2006;43(1 Suppl 1):S59-63.
78 Brohi K, Cohen MJ, Ganter MT, et al. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245:812-818.
79 Zwaginga JJ, Sakariassen KS, Nash G, et al. Flow-based assays for global assessment of hemostasis. Part 2: current methods and considerations for the future. J Thromb Haemost. 2006;4:2716-2717.
80 Hedner U. Mechanism of action of factor VIIa in the treatment of coagulopathies. Semin Thromb Hemost. 2006;32(Suppl 1):77-85.