10 Biofeedback in the diagnosis and treatment of chronic essential pelvic pain disorders
Pain, relaxation and biofeedback
Research applications to pelvic pain problems
Developing evidence-based biofeedback applications for pelvic pain
Pain, relaxation and biofeedback
Changes in emotion and expectations have clear effects on level of pain. These underlying connections have been explored recently as part of research into the mechanism of the placebo effect (Wager et al. 2007, Zubieta & Stohler 2009). The conclusions go beyond mental constructs like distraction and endurance; pain intensity turns out to be modulated by specific chemical and neurological changes in a way resembling the gain control on an amplifier. Changes in synapses, in descending excitatory and inhibitory tracts, in specific brain site excitation and inhibition, in opioid receptor sensitivity, and positive and negative expectations about pain, all interact to enlarge or diminish the experience of pain. Evolution has apparently fine-tuned this set of mechanisms to maximize the chance of survival.
In the field of clinical biofeedback, chronic pain has been a frequent symptom of interest because it is common, distressing, and often seems unnecessary when the signal has little value for warning of body damage. Chronic pain seems to become detached from its origin, or spreads out so much (allodynia, spreading cortical representation; Flor 2002) that the mechanistically minded allopathic physician is without answers as to why something hurts so much. Medical imaging may offer little explanation as to pain sources, and blood tests may show nothing that would explain high pain levels not related or only loosely related to body use. Exercise sometimes improves and sometimes aggravates the pain. Decreased tolerance of the pain may be attributed to deconditioning, even though the original advent of the pain may have discouraged activity and thus led to deconditioning.
Biofeedback depends on providing continuous feedback of a signal to the person it is coming from. With pain, however, there is hardly any way to detect and feed back an actual ‘pain signal’. The closest thing to this is the work done by de Charms et al. (2005) using fMRI for continuous monitoring and display of activity in a brain region known for correlating with experienced pain (anterior cingulate cortex (ACC)). Investigating both chronic pain patients and normal experimental subjects, these researchers found that displaying the moment-to-moment fluctuations in amplitude from the ACC provided an opportunity to influence the signal, which would mean influencing the brain area generating the signal and therefore voluntarily adjusting pain intensity. Whether this pain relief came about via emotional modulation, attentional shifts, or neurological–biochemical changes awaits further research, but the question challenges the mind–body distinction which has oversimplified so much research in this area. Subjects felt their pain intensity reduce, and they felt they were controlling it by doing something to manipulate the graphic display on a video screen. However, the subjects were sitting inside an fMRI device in a research lab, and this would be impractical for large-scale application.
Other than the MRI route, a biofeedback approach generally concentrates on altering a system considered responsible for the pain, or at least correlated with it. Thus we can provide biofeedback from voluntary muscles, feedback from the autonomic nervous system (ANS) variables such as skin temperature, skin conductance, heart rate, heart rate variability (RSA, see below), breathing (rate, rhythm, tidal volume, CO2 level) and EEG, including cerebral blood flow and slow cortical potential. For biofeedback overviews see Schwartz & Andrasik (2003) and Basmajian (1989).
There is support (Arena 2002) for the non-specific use of biofeedback, however, and patients rarely question the lack of specificity. They may readily acknowledge that their pain seems responsive to variation in not only physical activity but emotional stress and depression. Thus the variables of effective or less effective coping, mood modulation in response to the pain, and the amount of co-occurring non-pain distress require consideration in pain management. It may seem that such complex emotional variables could not be detected and fed back via biofeedback devices. But emotions have biological correlates and teaching control over them amounts to gaining leverage over the feeling states themselves. And these feeling states (explored by the placebo researchers cited above) have neurochemical effects on pain intensity.
Pelvic floor biofeedback began with Arnold Kegel (1948), who designed a pressure perineometer to measure contractile force from inside the vagina, with pressure changes displayed on an external gauge. The intent was to improve strength of the pubococcygeus muscle, and it was usually successful. More modern use of biofeedback for pelvic pain usually relies on either manometric feedback (inflatable balloons with adjustable size, placed in the rectum) or surface electromyographic (SEMG) information gained via vaginal or rectal sensors. Information may also be gained from monitoring the external muscles of the lower abdomen, perineum, thighs, and buttocks. Pelvic pain can of course come from many sources, but if dysfunctional muscle activity is suspected, it is simple enough to feed back the continuous muscle amplitude to the patient, opening a channel for voluntary control.
Research applications to pelvic pain problems
Hand temperature and pelvic pain
In a small multiple baseline study (Hart et al. 1981) five women with pain from endometriosis were trained in hand-warming with individual sessions twice weekly over 2 months, with home practice in between sessions. The rationale was to reduce physiological arousal, and the intent was to have the skill generalize as a learned and perhaps automatic response to pain. All but one person learned to voluntarily increase hand temperature, and reports of pain relief were accompanied by decreases in life interference from pain, decrease in affective distress, and increase in ‘life control’. One person could not learn the hand-warming skill; her pain remained the same and some indicators got worse.
Muscle biofeedback and pelvic pain
There are several ways that excess muscle tension can cause pain: prolonged ischaemia, accumulation of metabolites such as lactic acid and potassium; reduced intramuscular circulation, release of bradykinin and serotonin, and various aggravators of inflammation (Mense 2000). In addition, pain intensity is mediated by co-occurring emotional factors. Brain sites such as the anterior cingulate cortex are responsive to allodynia and are also involved in conscious mediation of ‘suffering’ (Yoshino et al. 2009). Therefore, negative affect from any source is likely to make pain worse because at some level different kinds of distress are not differentiated. The most reliable predictors of hyperalgesia seem to be varieties of anxiety, fear of movement, fear of injury, and the tendency toward catastrophic thinking (Boersma & Linton 2006a, 2006b).
Muscle tension is often elevated in chronic pain patients as part of an attempt to brace and protect the body from damage. Muscular rigidity is a primary defensive response to threat, pain and trauma, and this response can be triggered by both relevant and irrelevant sensory and emotional stimuli. SEMG monitoring can detect and quantify the degree of inappropriate pelvic muscle hypertonicity and instability (White et al. 1997, Glazer et al. 1998). Many chronic pain syndromes besides pelvic pain are associated with increased muscle tension (Flor et al. 1992) and can be alleviated in part by better muscle control.
Granot et al. (2002) studied pain sensitivity in a group of women with vulvar vestibulitis using a heated bar on the skin. A matched control group without pain was used for comparison. The researchers collected anxiety measures and estimates of pain intensity and unpleasantness, and blood pressure was also recorded. The pain patients had significantly more state and trait anxiety before the procedure began; they gave higher estimates for pain magnitude, unpleasantness, and had higher systolic blood pressure. The authors’ conclusions were that these subjects were more anxious and had higher systemic pain sensitivity.
The study of Bendaña et al. (2009) used a treatment sample of 52 women having problems with urinary frequency and urgency, interstitial cystitis, CPP, dysuria, and evidence of pelvic floor muscle (PFM) spasm. Initial determination of the levator ani muscle complex condition was done by manual vaginal examination. Subject criteria for selection were bladder dysfunction, including pelvic pain, and evidence of PFM tension. The therapeutic goal was to detect and reduce muscle tension and spasms in the PFMs using transvaginal SEMG and electrical stimulation. During six individual sessions the subjects observed computerized visual feedback which reflected their internal muscle tension from moment to moment. First isolating sensations of the relevant muscles from surrounding pelvic, abdominal and back muscles, they learned to increase control of the pertinent muscles in both tensing and relaxing directions.
The study of Heah et al. (1997) of men with levator ani syndrome (LAS) used manometric rectal balloon biofeedback. Average pain report after completion of biofeedback dropped to around 25% of that before biofeedback, with use of analgesics also significantly reduced.
Grimaud et al. (1991) also used a manometric technique to investigate patients with chronic idiopathic anal pain. In the 12 cases studied, the pressure in the anal canal was significantly higher than in a normal comparison group. After an average of eight biofeedback training sessions, in which patients learned voluntary control of the external sphincter, the pain disappeared, and the anal canal pressure dropped to normal or near-normal.
Cornel et al. (2005) reported treatment of 31 men with chronic prostatitis and CPP. They learned to control and relax PFM tension via biofeedback provided by a rectal SEMG sensor. Average muscle tension before treatment was 4.9 µv, and dropped to 1.7 µv afterward. Corresponding drops in symptom scores (NIH Chronic Prostatitis Symptom Index) went from 23.6 to 11.4.
Chiarioni et al. (2009) reports administering nine sessions of counselling plus electrogalvanic stimulation (EGS), massage or biofeedback randomized to 157 patients suffering from LAS. Outcomes were reassessed at 1, 3, 6 and 12 months. Among patients with LAS, adequate relief was reported by 87% for biofeedback, 45% for EGS, and 22% for massage. Pain days per month decreased from 14.7 at baseline to 3.3 after biofeedback, 8.9 after EGS, and 13.3 after massage. Pain intensity decreased from 6.8 (0–10 scale) at baseline to 1.8 after biofeedback, 4.7 after EGS, and 6.0 after massage. Improvements were maintained for 12 months. The authors conclude that biofeedback is the most effective of these treatments, and EGS is somewhat effective.
Jantos (2008) assessed vulvar pelvic muscle tension in 529 cases of vulvodynia, combined with psychological testing, to examine psychophysiological factors. The study also provided biofeedback-based intervention in the form of daily pelvic muscle exercises based on findings of SEMG using the ‘Glazer Protocol’ (Glazer et al. 1995) This involves use of progressively larger dilators to stretch and relax the vulvar and vaginal muscles, intravaginal EMG biofeedback, and brief psychotherapy aimed at improved psychological (anxiety, depression, fear of sexual activity) and sexual functioning. State and trait anxiety differentiated normals from vulvodynia patients, who also had lower sensory thresholds, more autonomic disturbances, and greater emotional responses. General outcomes after treatment included normalizing of muscle characteristics, capacity to accept larger dilators, and greater likelihood of resuming normal sexual activity. PFM improved in several ways, correlating with degree of improvement in the group as a whole, though not individually. Resting baseline and instability declined by more than 50%; maximum phasic and tonic contractions increased.
A unique finding in the Jantos study was the relationship between duration of symptoms and resting PFM EMG (subject characteristics at the onset of the study): severity of symptoms did not decline with time, but muscle tension did. The authors speculated that this could indicate development of contractures, which resemble muscle spasms upon palpation but are produced locally rather than by corticospinal input. As a result they are electrically silent and would not contribute to pelvic EMG. Such contractures could create pain by producing myofascial trigger points (Bornstein & Simon 2002). Trigger points produce a high local EMG signal that does not generalize to the whole muscle. These structures are sensitive to sympathetic nervous system activity (McNulty et al. 1994, Chen et al. 1998), and therefore can be aggravated by anxiety, apprehension and negative psychological states.
The psychophysiological perspective here gives a more complete understanding of how trigger points respond to physiological and emotional arousal by producing more pain (see also Chapter 4). For this reason, any arousal-reduction technique, including general relaxation methods, should be helpful for pain intensity that arises from trigger points.
Intrapelvic SEMG in the treatment of functional chronic urogenital, gastrointestinal and sexual pain and dysfunction
 Biofeedback meets evidence-based medicine: The Glazer Protocol
Biofeedback meets evidence-based medicine: The Glazer Protocol
This section of the chapter focuses on a specific methodology and protocol, the Glazer Protocol, for the diagnosis and treatment of essential CPP and dysfunction. The methodology involves the use of non-invasive intrapelvic (intravaginal or intra-anal) SEMG. The first factor differentiating this approach from the self-regulation biofeedback approach, discussed earlier, is the emphasis on the electrophysiology of the SEMG signal, rather than the psychophysiogy of self-regulation. This protocol relies more on the bioelectric information derived from the SEMG signal analysis, rather than the traditional biofeedback use of the SEMG signal to teach the patient voluntary self-regulation through enhanced interoceptive awareness. Unlike traditional biofeedback, this protocol is highly operationally defined, including diagnostic criteria (ICD), medical, psychological and sexual history, patient positioning and muscle use training, muscle activation and deactivation sequence, SEMG signal processing, recording and formatting, to create a standardized SEMG report and database. This approach facilitates the development of multicentre, multidiagnosis, multitreatment databases for statistical analysis. This operationally defined procedural and biometric/psychometric approach creates the foundation for evidence-based research and represents a substantial departure from past work in the field of clinical biofeedback (Glazer & Laine 2007).
Evidence-based medicine applies evidence from scientific methodology to health care practice. It assesses the quality of evidence relating to risks and benefits of treatments. The power of clinical evidence lies in its freedom from bias. The most powerful evidence for therapeutic efficacy comes from randomized, double-blind, placebo-controlled trials with operationally defined patient populations, diagnoses and treatment protocols. Patient testimonials, case studies, clinical experience and expert opinion have little value as scientific evidence. This in no way takes away from the importance of traditional clinical practice experience and thinking. Often clinical practice serves as an ‘incubator’ leading to ideas which are then transformed into more evidence-based hypotheses and subject to evidence-based medicine research. The completion of the cycle is the evidence-based research findings returning to clinical practice where their implementation can benefit clinical thinking and patient care (see Chapter 9).
A recent review article (Glazer & Laine 2007), summarizing the peer-reviewed literature in the use of PFM biofeedback for the treatment of functional urinary incontinence, exemplifies this. This review reports a total of 326 studies found in Medline between 1975 and 2005. Only 8.6% of these studies operationally defined independent and dependent variables, utilized prospective randomized trials with parametric statistical analyses, and used patient selection criteria to rule out organic causes of urinary incontinence. Among these 27 studies are six different operational definitions for the diagnosis, eight operational definitions for treatments, 12 operational definitions for biofeedback protocols, and six operational definitions for treatment outcome. In 30 years of peer-reviewed literature only seven studies reported a comparison of biofeedback to a matched, no treatment, control group. For these seven studies, differences in signal processing, biofeedback instrumentation, assessment and treatment protocols, biofeedback modalities, and multiple uncontrolled variables make each of these groups so different that there is no standardized definition of biofeedback. The same is true within each study, since the biofeedback groups are not comparable to their respective control groups due to non-randomized, uncontrolled variables between groups. This pervasive lack of standardization has hampered the scientific assessment of biofeedback by effectively precluding the application of evidence-based medicine standards to the field.
Applications
The Glazer Protocol is used in the diagnosis and treatment of a wide range of PFM-related disorder specialties as shown in Table 10.1.
Table 10.1 Disorders, categorized by medical specialty and ICD codes, treated with pelvic floor muscle SEMG biofeedback
| Medical specialty | Diagnostic ICD-9/-10 code |
|---|---|
| Gynaecology/Dermatology/Psychiatry | |
| 1. Vulvar vestibulitis syndrome | 625.71, 625.0 |
| 2. Dysaesthetic vulvodynia | 625.70, 625.9 |
| 3. Vaginismus | F94.2, 625.1 |
| 4. Dyspareunia (introital N94.1, deep 302.76) | N94.1, 302.76 |
| Female Urology/Neurourology | |
|---|---|
| 5. Dysuria | 306.53, 788.1 |
| 6. Urinary stress incontinence | 625.6 |
| 7. Urinary urge incontinence | N39.41 |
| 8. Urinary incontinence mixed | 788.34 |
| 9a. Detrusor hyperactivity | N32.8 |
| 9b. Neurogenic bladder | N31.9 |
| 10. Urinary retention | N39.8 |
| 11. Interstitial cystitis | N30.1 |
| Gastroenterology/Colorectal Surgery | |
|---|---|
| 12. Functional fecal incontinence | 787.6 R15 |
| 13. Functional constipation | K59.00 |
| 14. Anismus (anorectal pain syndrome, levator ani proctalgia fugax) | K59.4 |
| 15. Irritable bowel syndrome | 564.1 |
| Male Urology/Neurourology | |
|---|---|
| 16. Mixed urinary incontinence | 788.34 |
| 17. Prostatodynia | N42.81 |
| 18. Prostate cancer/prostatectomy | C61 |
| 19. Benign prostatic hypertrophy | 600.0 |
| 20. Chronic pelvic or perineal pain | 625.9 R10.2 |
| Asymptomatic | |
|---|---|
| 21. Healthy male (controls) | |
| Multiple codes limited to absence of specific organic pathology | |
| 22. Healthy female (controls) |
Assessment with the Glazer Protocol
The Glazer Protocol operationally defines both the intrapelvic SEMG assessment and rehabilitation of PFM. The assessment protocol starts by educating the patient on the structure and function of the PFM as they relate to their individual symptom presentation. This is accomplished with a scripted presentation, with responses to any questions the patient presents. The presentation includes instructions on private self-insertion of the intrapelvic sensor, body positioning as a critical factor in SEMG measurement, and teaching the patient the correct method for contracting and relaxing the PFM. This instruction focuses on creating the intravaginal lifting sensation associated with the correct use of PFM while permitting limited co-contractions, or overflow (Glazer & McConkay 1996) to offset fatigue during the initial stages of exercise.
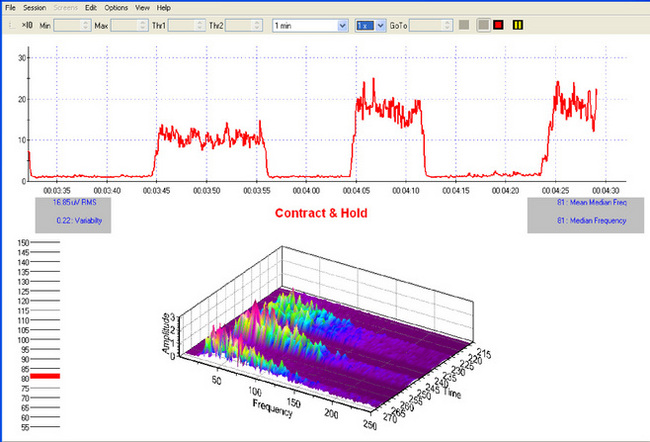
The intrapelvic SEMG assessment consists of a fixed series of PFM contractions and relaxations, directed via an on-screen written script and simultaneous voice presentation. This assessment is a computer-controlled continuous process in which software (Biograph Infiniti with Glazer Protocol) directs both the instructions to the patient and the SEMG biofeedback signal processing device (Myotrac Infiniti), which continuously records raw SEMG data (2048 samples/s) and displays the integrated SEMG signal (20 samples/s) throughout the assessment. The SEMG screen presents a graphic and numeric presentation of the integrated SEMG signal as well as signal variability measures and a three-dimensional fast Fourier transformation power density spectral frequency display of the signal. This allows the clinician to view real-time changes in the spectral frequency distribution of the signal power throughout the conduct of the evaluation (Figure 10.1).
The fixed sequence of muscle activity utilized during the protocol includes pre-baseline rest, phasic contractions, tonic contractions, endurance contraction, and post-baseline rest. This traditional series of PFM assessment contractions were originally intended to reflect sexual, sphincteric and support functions of the pubococcygeus muscle (Kegel 1948, 1952). In the Glazer Protocol, SEMG measures taken continuously throughout the protocol include average SEMG amplitude, muscle recruitment and recovery latencies, median power density spectral frequency, and two measures of SEMG variability: raw (standard deviation) and amplitude corrected (coefficient of variability). Upon completion of the protocol the data are stored in raw SEMG form (2048 samples/s).
Levels of interpretation and applications of SEMG evaluation data include both empirical and pathophysiological perspectives
Physiological
Electrophysiology data derived from SEMG is one manifestation of local and systemic integrated physiology. The functional integration of multiple physiological systems is necessary to understand disorders such as complex regional pain syndromes. It is this physiological integration which allows the use of striated muscle SEMG to better understand the multiple physiological processes contributing to dysfunction. There are several studies which have looked at the relationship between striate muscle fatigue, SEMG, blood flow, PO2, subjective sense of fatigue, and pain (Alfonsi et al. 1999, Yoshitake et al. 2001, Hug et al. 2004, Tachi et al. 2004, Dimitrov et al. 2006). These variables show a complex, non-linear relationship to one another, but these studies basically report that subjective sense of muscle fatigue and localized nociceptive pain are correlated with the following: SEMG increased contractile amplitude, lower median power density spectral frequency, reduced microcirculation in local muscle and surrounding tissue, and hypoxia. On a neurochemical level these changes are associated with release of neurokines, cytokines, lactic acid, interleukin, and tumour necrosis factor-α, representing localized ‘defensive’ responses of ischaemia, inflammation and sensitization (Mense 2004). These markers are all part of an integrated response from the most molecular cellular level to the most molar level of cognition, affect and goal-oriented intentional behaviour.
If we see all responses at all levels as part of an integrated process, it now makes sense to look at the relationships which may exist among any of these components. An example of this is a recently recognized intrapelvic SEMG profile which represents atrophic vaginitis. This oestrogen loss condition may manifest as chronic, fluctuating vulvar dryness, irritation, tissue integrity compromise, dyspareunia, emotional changes, bone density loss, and pelvic organ prolapse (Mehta & Bachman 2008). There is also a highly reliable intrapelvic SEMG profile which correlates with this condition. This profile includes low-amplitude (hypotonicity), low-signal-variability resting tone, slow recruitment and recovery latencies to low-amplitude phasic, tonic, and endurance contractions. These contractions show low signal standard deviations and coefficients of variability, and a high median frequency power density spectrum on sustained isometric contractions. This SEMG pattern as a potential confirmation for oestrogen loss is still undergoing data collection to produce a sample size sufficient for parametric data analysis and publication.
Research summary
SEMG diagnostic studies refer to the use of intrapelvic SEMG data using between-group comparison studies to determine which SEMG variable or combination of variables yield statistical significance in differentiating PFM-related disorders. The first study in this series, entitled ‘Establishing the diagnosis of vulvar vestibulitis’, was published in 1997 (White et al. 1997). This study compared intravaginal SEMG assessment findings from essential and organic vulvar pain patients. Six individual SEMG criteria differentiated vulvar vestibulitis patients from organic vulvar pain patients, with 88% of vulvar vestibulitis patients manifesting three or more of these criteria to a significantly greater degree than the organic vulvar pain patients. The single most statistically significant variable differentiating them was the resting baseline stability as measured by the coefficient of variability of the integrated intrapelvic SEMG. Vulvar vestibulitis patients showed significantly higher resting instability than organic vulvar pain control patients.
Another study ‘Electromyographic comparisons of the pelvic floor in women with dysesthetic vulvodynia and asymptomatic women’ compared SEMG PFM evaluation variables in dysaesthetic vulvodynia patients to matched asymptomatic controls (Glazer et al. 1998). Findings indicated that dysaesthetic vulvodynia patients manifest significantly greater intravaginal SEMG-sustained contractile weakness, resting hypertonicity and instability.
Two related papers (Glazer et al. 1999, Romanzi et al. 1999) studied reliability and clinical predictive validity of intravaginal SEMG. These papers reported the findings of a wide range of symptomatic patients undergoing both manual (digital) and SEMG intrapelvic repeated evaluations. At each administration, the order of procedure and clinician was randomized between a urogynaecologist and a gynaecologist conducting the digital exams and Glazer conducting intrapelvic SEMG evaluations. Reliability within and between evaluators and procedures was statistically significant but digital exam results could not significantly predict any clinical status. Intravaginal SEMG significantly predicts stress and urge incontinence, menstrual status and parity.
Hetrick et al. (2006) studied differences in intra-anal PFM SEMG readings between men suffering from chronic pelvic pain syndrome (CPPS) compared with a matched control group of pain-free men. CPPS patients were found to manifest overall greater PFM electrophysiological instability. This measure as well as chronic prebaseline resting hypertonicity and endurance contraction weakness were statistically significant in differentiating CPPS patients from their asymptomatic matched controls. It is interesting to note the similarity in these pelvic floor SEMG findings to those previously found in women suffering from essential vulvovaginal pain disorders (Glazer et al. 1995, 1998, White et al. 1997).
Intrapelvic SEMG treatment studies use intrapelvic SEMG data to develop PFM biofeedback treatment protocols. The earliest of these studies ‘Treatment of vulvar vestibulitis syndrome with electromyographic biofeedback of pelvic floor musculature’ (Glazer et al. 1995) was the first peer-review published study in the field. It reported a 50% rate of asymptomatic outcome on 6-month follow-up with overall self-reported improvement averaging 83%. Only the standard deviation of tonic resting periods showed significant predictive validity for pain reduction and improvement in sexual desire, arousal and orgasm. This study concluded that PFM electrophysiological stabilization through intrapelvic SEMG biofeedback-assisted exercise produces pain relief and improved sexual functioning for vulvovaginal pain patients.
Another study ‘Dysesthetic vulvodynia, long term follow-up after treatment with surface electromyography-assisted PFM rehabilitation’ (Glazer, 2000) reported on the 3–5-year follow-up status of 43 patients who were asymptomatic at the completion of their PFM rehabilitation treatment for vulvodynia. All 43 patients remained pain-free; recovery of sexual desire, pleasure and frequency, however, progressively improved but remained well below levels experienced prior to the onset of vulvar pain.
A doctoral dissertation from McGill University (Bergeron et al. 2001) described a prospective, randomized treatment design comparing vestibulectomy (surgical removal of a portion of the superficial tissue making up the vestibule of the vagina), Glazer Protocol biofeedback, and group cognitive behaviour therapy in the treatment of vulvar vestibulitis. Surgical outcomes were found superior to both the intrapelvic Glazer SEMG treatment protocol and the couples group cognitive behaviour therapy. When examining patient self-report measures, all three groups did equally well with a small, statistically non-significant, preference for surgery. Before biofeedback and cognitive behavioural therapy were included in the treatment of vulvar pain disorders, surgery was considered the primary treatment and ‘gold standard’ for many years, in spite of the significant adverse consequences as well as absence of patient satisfaction or long-term follow up.
In a study entitled ‘Treating vulvar vestibulitis with electromyographic biofeedback of pelvic floor musculature’ patients with moderate to severe vulvar vestibulitis syndrome underwent the Glazer intrapelvic biofeedback protocol (McKay et al. 2001). Patients received monthly in-office evaluation and daily home-trainer-assisted PFM rehabilitation. Eighty-three percent of patients demonstrated significant reduction in introital tenderness, with 69% resuming sexual intercourse and 48% reporting no discomfort during sexual intercourse.
Glazer & MacConkey (1996) published the first methodological paper ‘Functional rehabilitation of pelvic floor muscles: a challenge to tradition’. Traditional PFM biofeedback-assisted rehabilitation strongly emphasized the exclusive use of the pubococcygeus without the use of supportive or accessory muscles such as gluteal, quadriceps, adductor longus and particularly abdominal muscles. This study demonstrates that the traditional practice of excluding accessory muscles in PFM re-education is not always warranted. Subjects were trained and then tested either with or without abdominal augmented PFM contractions in a 2 × 2 experimental design. Results clearly demonstrated significantly greater contractile amplitude and reduced variability (strength and coordination) in subjects tested with exclusive pubococcygeus contraction after training with abdominals, compared to subjects both trained and tested without training abdominal muscles. Clearly, where up-training and coordination are the training goals, the co-contraction of abdominals during training of the pelvic floor should not be excluded.
Glazer et al. (2002) published the first methodological paper introducing the technology of telemedicine via videoconference over the internet. This paper reported a case history demonstrating the use of a newly developed telemedicine system permitting the remote, real-time use of SEMG of pelvic floor musculature. This browser-based version of the Glazer Protocol offers a reliable and convenient diagnostic and treatment tool that overcomes the barriers of distance and time. As this technology has become more readily available, it has greatly facilitated international education and research collaboration with the standardized procedures critical to research reaching the requirements of evidence-based medicine. It also permits direct patient assessment and treatment by those with most experience in fields just beginning to utilize SEMG intrapelvic biofeedback.
Brown et al. (2003, 2004, 2005, 2006) published a series of papers reporting on the use of botulinum toxin A injected into the pelvic floor musculature as a possible treatment for vulvar vestibulitis syndrome. These studies employed intravaginal SEMG to determine if any clinical findings correlate with this measure. Data suggested that only those patients with elevated resting tone, variability and spectral frequency benefited from the injection. This is consistent with the fact that botulinum toxin A is known to selectively block type II glycolytic fibre. This finding suggests that intravaginal SEMG would serve as a valuable tool in selecting patients who would benefit from this procedure.
Summary of Medline ‘biofeedback’ ‘pelvic pain’ literature search
1. Review articles or meta-snalyses without original primary research reported;
2. Studies in which biofeedback was only one component of a protocol employing multiple treatment components (e.g. biofeedback+estim, biofeedback+neuromodulation, biofeedback+trigger point release, etc.). No subjects received a biofeedback alone treatment;
3. Studies in which there are no biofeedback treatments included (e.g. pelvic floor exercises without biofeedback);
4. Studies in which there are no pelvic pain disorders treated (e.g. disorders treated are urinary or bowel symptoms without a pain component or no pain components were measured or recorded).
6 Single-group biofeedback only
1 Within-group multiple treatments randomized
3 Randomized between group no control (two or more treatment groups)
3 Randomized between group with control (two or more and control groups)
Sample size: range 5–100, mean 32
Age range: range 7–74, mean 41
Gender: 4 males only, 6 females only, 3 males and females
3 Male chronic pelvic pain syndrome
6 Biofeedback effective (single group)
1 Biofeedback superior (within group, multiple treatment)
2 Biofeedback superior (between treatment groups)
Case study 10.1
Systems review
5. Skin and hair: stretch marks, easy bruising, warts, split nails
7. Gastrointestinal: loss of appetite, nausea/vomiting, bloating, constipation
8. Cardiovascular: palpitations, low blood pressure
9. Bowel: greasy stool, rectal pain
10. Bladder: urine retention, incomplete urination, urinary tract infection (UTI), kidney stones, stress urinary incontinence (SUI)
11. Gynaecology: dysmennorrhoea, irregular menses, vaginal discharge, dryness vaginal pain, pelvic pain, dyspareunia, clitoral pain, Bartholin cysts ×3 (self-resolved)
13. Musculoskeletal: back pain
14. Psychological: anxiety, substance abuse
15. Sexual: lack of arousal, pain in genitals, Bartholin cysts ×3
Psychometrics: MMPI-2
2. Clinical scale profile: HY, SI profile frequency 10.4%, profile stability high, supplementary scale profile: MAC-R
3. Content scale profile ANX HEA
4. Additional scales: physical, somatic, negative emotionality, generalized fearfulness, gastrointestinal symptoms, general health concerns
Sexual evaluation
1. Menarche age 12, 28-day cycle, heavy flow, little dysmenorrhoea.
2. No history vaginal yeast, bacteria, viral or STD.
3. Intercourse initiates at age 18.
4. Reports history of variable, moderate to high, levels of desire and arousal daily with masturbation to orgasm thoughout teens.
5. G2P2 vaginal w/episiotomies. Both with large fetus and prolonged second stage labour. Patient reports postnatal recovery of desire/arousal/orgasm after breast feeding.
6. Orgasmic on clitoral stimulation manual/oral, self or partner, rarely with thrusting intercourse during which she conducts clitoral self-stimulation.
7. Since pain onset, patient reports dyspareunia, significantly reduced desire and arousal which is not discussed or ‘managed’ with her husband. She denies experiencing desire, arousal or orgasm since pain onset, but does engage in oral and manual stimulation of husband who also acknowledges frequent masturbation. Patient feels marriage is at risk due to her sexual abstinence.
Intrapelvic SEMG evaluation
1. Glazer Protocol administered with an intravaginal sensor after patient views information/educational and instructional video on correct use of equipment and proper position and action for PFM contractions while limiting co-contractions of leg adductors, lower abdominals and gluteal muscle groups.
2. Protocol consists of signal verification, pre-baseline rest (60 seconds), phasic (flick) contractions, tonic (10 second) contractions, endurance (60 second) contraction, and post-baseline rest (60 seconds). During the protocol pubococcygeal SEMG is taken continuously including amplitude, standard deviations, coefficients of variability, fast Fourier transformation power density spectral frequency, and recruitment/recovery latencies for all contractions and releases. At the completion of the protocol the data from the evaluation are printed out for the patient and stored in the database fore later analysis and comparison.
History and data review and integration with treatment(s) prescribed
Summary of findings
1. Easy bruising, splitting nails and warts.
2. Loss of appetite, nausea/vomiting/bloating and constipation with pain on bowel movements and greasy stool.
3. Urine retention, incomplete voids, kidney stones, vaginal/rectal sensation ‘like a ball pushing out from inside’ and SUI.
4. Dysmenorrhoea, irregular menses, vaginal discharge, dryness, dyspareunia, vulvar, vaginal and clitoral pain spontaneous unprovoked chronic and localized provoked.
5. Reduced sexual desire arousal and orgasms with 17 months abstinence and concerned over marital relations.
6. Psychological evaluation reveals generalized anxiety and a focus on somatic concerns and a predisposition to substance abuse.
7. Gynaecological exam and labs, including complete blood count, are normal. Well-established organic causes of vulvar pain (infections, dermatoses, neuropathies, anatomic changes, pelvic inflammatory disorder, pelvic congestion, etc.) have been ruled out on multiple gynaecological and imaging procedure evaluations.
8. Intrapelvic SEMG evaluation shows mildly elevated and unstable initial rest, slow recruit/recover latencies and low peak amplitudes on phasic contractions, low amplitude, stable, high median frequency for both tonic and endurance contractions, and low-amplitude, stable post baseline.
Interpretation of findings
• Well-established organic causes of vulvar pain have been ruled out.
• Symptoms meet criteria for diagnosis of both DV and VVS, commonly overlapping conditions.
• The role of multiple prescription pain medications and non-prescription supplements to both symptom relief and/or symptom contribution must be reviewed.
• Concurrent systems disturbances include lower gastrointestinal and urogenital symptoms, commonly co-occurring with essential vulvar pain disorders which may suggest a common pathophysiology.
• Psychological factors are consistent with secondary role rather than primary aetiology. Psychological, interpersonal and sexual consequences are significant factors leading to symptomatic maintenance and resistance to functional change.
• Intrapelvic SEMG shows a pattern of pelvic floor dysfunction consistent with menstrual changes (recent onset of irregular menses), voiding disorders (retention, SUI) and essential vulvar pain disorders (DV, VVS).
Treatment
Initiation of manualized 10-session programme of couples group sexual therapy for women suffering from vulvar pain, and their partners. This is an informational, educational, support group conducted by a psychologist using cognitive behavioural techniques and specific home assignments (Bergeron et al. 2001). For those sufferers with more profound sexual disturbances of desire, arousal and orgasm (FSFI) which offer direct interference with compliance to sexual prescriptices, brief individual therapy may also be employed, which is the case with this patient who underwent a course of brief, 10-session, weekly eclectic prescriptive therapy with a focus on addressing her general anxiety (breathing retraining), PTSD (EMDR) from childhood abuse, somatic overconcern (systematic desensitization) and sexual avoidance (dilators, sensate focus, orgasmic restoration, etc.). Both the manualized group couples therapy and the individual therapy were conducted by the first author, who also conducted the intravaginal SEMG biofeedback.
The intrapelvic SEMG pattern most closely matches that pattern shown by perimenopausal women (Glazer et al. 1999, Romanzi et al. 1999). The age of the patient, recent onset of irregular menses, bowel and bladder changes, vulvar dryness, sensations associated with pelvic floor relaxation, and the findings of the endocrinologist all suggest atrophic changes may be contributory to her vulvar pain. The SEMG pattern is also consistent with the symptoms of urinary retention (elevated unstable baseline) and recent onset of SUI (slow and weak urethral closure) as well as functional faecal constipation. Intrapelvic SEMG biofeedback combined with topical and intravaginal HRT has, in my clinical practice, shown positive results with this symptom pattern.
Outcomes
Treatment compliance
She has maintained a high level of compliance with prescribed 20 min ×2/day pelvic floor SEMG biofeedback and has normalized her intravaginal SEMG. The patient still reports that episodic stress from environmental demands can still lead to setbacks with recurrence of vulvar vestibular burning and localized hypersensitivity to contact. The patient will continue her full compliment of treatments for the following 6 months and there are no contraindications to expectations of full recovery (Glazer 2000). However, as with all essential chronic pain disorders in which the pathophysiology is not understood, the patient must be taught a subconscious, habitual, but constant awareness of the presence of risk factors, in order to achieve a balance between prophylaxis while maintaining the highest levels of engagement possible with the least restrictions on the conduct of daily life activities. Keeping this balance between awareness of chronic predisposition (ledger, genetics) and maximization of functional engagement is the key to a healthy and satisfying life for those who suffer from any essential chronic pain disorders.
Alfonsi E., Pavesi R., Merio I.M., et al. Hemoglobin near-infrared spectroscopy and surface EMG study in muscle ischemia and fatiguing isometric contraction. J. Sports Med. Phys. Fitness. 1999;39(2):83-92.
Arena J.G. Chronic pain: psychological approaches for the front-line clinician. J. Clin. Psychol.. 2002;58(11):1385-1396.
Basmajian J.V., editor. Biofeedback: Principles and practice for clinicians, third ed., Baltimore, MD: Williams & Wilkins, 1989.
Bendaña E.E., Belarmino J.M., Dinh J.H., Cook C.L., Murray B.P., Feustel P.J., et al. Efficacy of transvaginal biofeedback and electrical stimulation in women with urinary urgency and frequency and associated pelvic floor muscle spasm. Urol. Nurs.. 2009;29(3):171-176. PMID: 19579410
Bergeron S., Binik Y.M., Khalife S., et al. A randomized controlled comparison of group cognitive-behavioral therapy, surface electromyographic biofeedback and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001;91:297-306.
Boersma K., Linton S.J. Psychological processes underlying the development of a chronic pain problem: a prospective study of the relationship between profiles of psychological variables in the fear-avoidance model and disability. Clin. J. Pain. 2006;22(2):160-166.
Boersma K., Linton S.J. Expectancy, fear and pain in the prediction of chronic pain and disability: a prospective analysis. Eur. J. Pain. 2006;10(6):551-557.
Bornstein J., Simons D.G. Focused review: myofascial pain. Arch. Phys. Med. Rehabil.. 2002;83(3 Suppl. 1):S40-S47. S48–S49
Brown C., Vogt V., Menkes D., Ling F., Glazer H., Curnow J. An open label trial of botulinum toxin type A in treating women with vulvar vestibulitis syndrome. Poster presentation. Amsterdam, Netherlands: The International Society for the Study of Women’s Sexual Health (ISSWSH), 2003.
Brown C., Glazer H., Vogt V., Menkes D. Effect of botulinum toxin type A on sexual function in vestibulodynia. New York, NY: Sexual Medicine Society of North America, 2005. Abstract 179
Brown C.S., Vogt V., Menkes D., Bachmann G., Glazer H. Subjective and objective outcomes of Botulinum Toxin Type A in Vulvar Vestibulitis Syndrome. Vulvodynia and Sexual Pain Disorders in Women Conference,. 2004. Atlanta, GA
Brown C.S., Glazer H.I., Vogt V., Menkes D., Bachmann G. Subjective and objective outcomes of botulinum toxin type A treatment in vestibulodynia: pilot data. J. Reprod. Med.. 2006;51(8):635-641.
Chen J.T., Chen S.M., Kuan T.S., Chung K.C., Hong C.Z. Phentolamine effect on the spontaneous electrical activity of active loci in a myofascial trigger spot of rabbit skeletal muscle. Arch. Phys. Med. Rehabil.. 1998;79(7):790-794.
Chiarioni G., Nardo A., Vantini I., Romito A., Whitehead W.E. Biofeedback is superior to electrogalvanic stimulation and massage for treatment of levator ani syndrome. Gastroenterology, 2009.
Cornel E.B., van Haarst E.P., Schaarsberg R.W., Geels J. The effect of biofeedback physical therapy in men with chronic pelvic pain syndrome type III. Eur. Urol.. 2005;47(5):607-611.
deCharms R.C., Maeda F., Glover G.H., et al. Control over brain activation and pain learned by using real-time functional MR. Proc. Natl. Acad. Sci. U. S. A.. 2005;102(51):18626-18631.
Dimitrov G.V., Arabadzhiev T.I., Mileva K.N., Bowtell J.L., Crichton N., Dimitrova N.A. Muscle fatigue during dynamic contractions assessed by new spectral indices. Med. Sci. Sports Exerc.. 2006;38(11):1971-1979.
Flor H. The modification of cortical reorganization and chronic pain by sensory feedback. Appl. Psychophysiol. Biofeedback. 2002;27(3):215-227.
Flor H., Fydrich T., Turk D.C. Efficacy of multidisciplinary pain treatment centers: a meta-analytic review. Pain. 1992;49(2):221-230.
Gatchel R.J., Pent Y.B., Peters M.L., Fuchs P.N., Turk D.C. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psych. Bull.. 2007;133(4):581-624.
Glazer H.I. Dysesthetic vulvodynia. Long term follow-up after treatment with surface electromyography-assisted pelvic floor muscle rehabilitation. J. Reprod. Med.. 2000;45:798-802.
Glazer H.I., Laine C.D. Pelvic floor muscle biofeedback in the treatment of urinary incontinence: a literature review. Appl. Psychophysiol. Biofeedback. 2007;31(3):187-201.
Glazer H.I., MacConkey D. Functional rehabilitation of pelvic floor muscles: a challenge to tradition. Urol. Nurs.. 1996;16(2):68-69.
Glazer H.I., Rodke G., Swencionis C., Hertz R., Young A.W. Treatment of vulvar vestibulitis syndrome with electromyographic biofeedback of pelvic floor musculature. J. Reprod. Med.. 1995;40(4):283-290.
Glazer H.I., Jantos M., Hartmann E., Swencionis C. Electromyographic comparisons of the pelvic floor in asymptomatic and vulvodynia females. J. Reprod. Med.. 1998;43:959-962.
Glazer H.I., Romanzi L., Polaneczky M. Pelvic floor muscle surface electromyography; reliability and clinical predictive validity. J. Reprod. Med.. 1999;44:779-782.
Glazer H.I., Marinoff S.M., Sleight I.J. The web-enabled Glazer surface electromyographic protocol for the remote, real-time assessment and rehabilitation of pelvic floor dysfunction in vulvovaginal pain disorders. J. Reprod. Med.. 2002;47(9):728-730.
Granot M., Friedman M., Yamitsky D., Zimmer E.Z. Enhancement of the perception of systemic pain in women with vulvar vestibulitis. BJOG. 2002;109(8):863-866.
Grimaud J.C., Bouvier M., Naudy B., Guien C., Salducci J. Manometric and radiologic investigations and biofeedback treatment of chronic idiopathic anal pain. Dis. Colon Rectum. 1991;34(8):690-695.
Hart A.D., Mathisen K.S., Prater J.S. A comparison of skin temperature and EMG training for primary dysmenorrhea. Biofeedback Self Regul.. 1981;6(3):367-373.
Heah S.M., Ho Y.H., Tan M., Leong A.F. Biofeedback is effective treatment for levator ani syndrome. Dis. Colon Rectum. 1997;40(2):187-189.
Hetrick D.C., Glazer H., Liu Y.W., Turner J.A., Frest M., Berger R.E. Pelvic floor electromyography in men with chronic pelvic pain syndrome: a case-controlled study. Neurourol. Urodyn.. 2006;25(1):46-49.
Hug F., Faucher M., Marqueste T., et al. Electromyographic signs of neuromuscular fatigue are comcomitant with further increase in ventilation during static handgrip. Clin. Physiol. Funct. Imaging. 2004;24(1):25-32.
Jantos M. Vulvodynia:a psychophysiological profile based on electromyographic assessment. Appl. Psychophysiol. Biofeedback. 2008;33(1):29-38.
Kegel A.H. Progressive resistance exercise in the functional restoration of the perineal muscles. Am. J. Obstet. Gynecol.. 1948;56(2):238-248.
Kegel A.H. Stress incontinence and genital relaxation: a nonsurgical method of increasing the tone of sphincters and their supporting structures. Ciba Clin. Symp.. 1952;4(2):35-51.
McKay E., Kaufman R., Doctor U., et al. Treatment of vulvar vestibulitis with electromyographic biofeedback of pelvic floor musculature. J. Reprod. Med.. 2001;46(4):337-347.
McNulty W.H., Gevirtz R.N., Hubbard D.R., Berkoff G.M. Needle electromyographic evaluation of trigger point response to a psychological stressor. Psychophysiology. 1994;31(3):313-316.
Mehta A., Bachman G. Vulvovaginal complaints. Clin. Obstet. Gynecol.. 2008;51(3):549-555.
Mense S. Neurobiological concepts of fibromyalgia: the possible role of descending spinal tracts. Scand. J. Rheumatol. Suppl.. 2000;113:24-29.
Mense S. Functional neuroanatomy for pain stimuli, reception, transmission and processing. Schmerz. 2004;18(3):225-237. (German)
Romanzi L., Polaneczky M., Glazer H.I. A simple test of pelvic muscle during pelvic examination: correlation to surface electromyography. J. Neurourol. Urodyn.. 1999;18:603-612.
Schwartz M., Andrasik F. Biofeedback: A practitioner?s guide. New York: Guilford Press, 2003. third ed.
Tachi M., Kouzaki M., Kanejosa J., Fukunaga T. The influence of circulatory difference on muscle oxygenation and fatigue during intermittent static dorsiflexion. Eur. J. Appl. Physiol.. 2004;91(5–6):682-688.
Turk D.C., Dworkin R.H., McDermott M.P., et al. Analyzing multiple endpoints in clinical trials of pain treatments: IMMPACT recommendations Initiative on Methods, Measurement and Pain Assessment in Clinical Trials. Pain. 2008;139(3):485-493.
Wager T.D., Lindquist M., Kaplan L. Meta-analysis of functional neuroimaging data: current and future directions. Soc. Cogn. Affect Neurosci.. 2007;2(2):150-158.
White G., Jantos M., Glazer H.I. Establishing the diagnosis of vulvar vestibulitis. J. Reprod. Med.. 1997;42:157-161.
Yoshino A., Okamoto Y., Onada K., et al. Sadness enhances the experience of pain via neural activation in the anterior ingulate cortex and amygdale: An fMRI study. Neuroimage. 2009.
Yoshitake Y., Ue H., Miyazaki M., Moritani T. Assessment of lower-back muscle fatigue using electromyography, mechanomyography and near-infrared spectroscopy. Eur. J. Appl. Physiol.. 2001;84(3):174-179.
Zubieta J.K., Stohler C.S. Neurobiological mechanisms of placebo responses. Ann. N. Y. Acad. Sci.. 2009;1156:198-210.