Chapter 62 Behavioral, Cognitive, and Social Aspects of Childhood Epilepsy
Children and adolescents with epilepsy and adults with childhood-onset epilepsy often are reported to have social maladjustment, including poor educational attainment, lower than expected occupational status, poorer perceived health and fitness, more frequently reported behavior problems, lower rates of marriage as adults, and higher rates of social isolation at all ages [Camfield et al., 1993; Clement and Wallace, 1990; Hoare, 1984; Jalava and Sillanpaa, 1997; Rutter et al., 1970; Sillanpaa, 1990]. These poor outcomes have multiple causes. In any particular patient, one or more causes of poor functioning may be identified and, at times, remedied. In general, neither epilepsy nor the seizures themselves are the most important cause of cognitive or behavioral disability. The underlying causes of cognitive and behavioral dysfunction may be subtle or obvious, but generally are complex and multifactorial. In some instances, underlying neurologic structural lesions cause both epilepsy and other disabilities, including cognitive dysfunction. In others, such as benign focal epilepsy of childhood, learning and behavioral disorders are more difficult to explain, and the relationship with epilepsy is almost certainly not causal.
Cognitive and Behavioral Disorders
Cognitive Disabilities in Children with Epilepsy
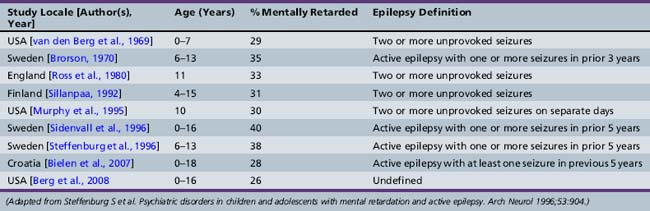
Although not all children with epilepsy have cognitive impairment, epilepsy is more frequent in cognitively handicapped children than in the general population [Britten et al., 1986; Forsgren et al., 1990; Sillanpaa, 1992]. Various population-based prospective and cohort studies of mentally retarded children have documented the prevalence of epilepsy to be 15–35 percent. Children with severe mental retardation and cerebral palsy have the highest rates of epilepsy. Table 62-1 contains information from selected population-based or cohort studies that estimate the prevalence of epilepsy in mentally retarded populations. Caution must be used in interpreting this information, since definition of both epilepsy and mental retardation varies. Several studies divide mentally retarded groups into mild (intelligence quotient [IQ] of 50–70) and moderate to severe mental retardation (IQ under 50). Epilepsy is substantially more prevalent in severely retarded cohorts. Prevalence of epilepsy is highest in cohorts of mentally retarded children with associated cerebral palsy [Curatolo et al., 1995; Sussova et al., 1990]. Even excluding children with major structural brain disease causing epilepsy and associated disabilities, mental retardation is more common in children with epilepsy than in children without epilepsy, with or without other chronic illnesses. Table 62-2 lists selected studies of the prevalence of mental retardation in cohorts of children with epilepsy or in populations surveyed for both mental retardation and epilepsy. Again, definition of both epilepsy and mental retardation is not uniform.
Table 62-1 Rates of Epilepsy in Cognitively Impaired Children and Adolescents: Population and Cohort Studies
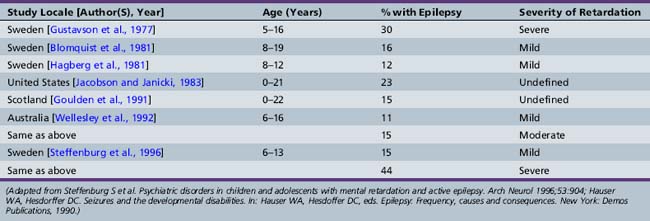
Table 62-2 Frequency of Cognitively Impaired in Children with Epilepsy: Population and Cohort Studies
Cognitive Function in Benign Childhood Epilepsy Syndromes
Many studies have noted subtle cognitive dysfunction in children with epilepsy syndromes known to have a good prognosis, easily attainable seizure control, and no structural brain disease, such as childhood absence epilepsy and benign focal epilepsy of childhood [D’Allessandro et al., 1990; Dieterich et al., 1985; Olsson and Campenhausen, 1993; Piccirilli et al., 1994; Singhi et al., 1992]. The cognitive dysfunction detected in children with childhood absence epilepsies includes deficits in visual sustained attention and execution of visual-motor tasks [Levav et al., 2002], verbal memory and word fluency [Henkin et al., 2005], and nonverbal memory and delayed recall [Pavone et al., 2001]. Children with benign childhood epilepsy with centrotemporal spikes have been documented as having difficulties in memory and phonologic processing skills [Northcott et al., 2005]. Furthermore, this condition has been shown to be strongly comorbid with reading disability and speech sound disorder (development of motor control of speech). These deficits are thought to be independently inherited traits rather than a consequence of the epilepsy itself, with increased odds amongst relatives of the proband [Clarke et al., 2007]. Benign childhood epilepsy with occipital paroxysms has been found to be associated with selective dysfunction in perceptive-visual attentional ability, verbal and visual-spatial memory abilities, visual perception, visual-motor integration, some language tasks, reading, writing abilities, and arithmetic abilities [Germano et al., 2005].
Cognitive Dysfunction Due to Interictal Epileptiform Discharges
Conflicting evidence exists regarding the role of interictal epileptiform discharges in cognitive function, as distinct from ictal effects and from the long-term stable interictal effects caused by the clinical syndrome or the underlying etiology [Aldenkamp et al., 2004]. Studies using sophisticated computerized cognitive test batteries time-locked to electroencephalography (EEG) discharges have noted transient cognitive impairment with slowing of reaction times and decreased perceptual accuracy during epileptiform discharges [Aldenkamp et al., 1996; Binnie, 2003, 1993; Shewmon and Erwin, 1989]. At least one study has shown low incidence of such impairment and questioned its clinical significance [Fonseca et al., 2007]. Refinement of methodology has suggested that a larger proportion of presumed transient cognitive impairment can be attributed to subtle seizures, while interictal epileptic activity has a smaller effect upon cognitive functioning [Aldenkamp et al., 2004]. Finally, the effects of such transient cognitive impairment on more stable tasks, such as reading or intelligence, have not been studied and are unknown.
Learning Disabilities and Academic Underachievement
Learning disability is diagnosed when one or more areas of learning are significantly below expectations, and not explained by overall cognitive level, sensory abnormalities, or lack of opportunity or teaching (see Chapters 43 and 45). Learning disabilities are reported to be more frequent in children with epilepsy. Studies have shown that children with epilepsy are at higher risk for repeating a year in school and over half require special education services. Although the frequency of special education placement was significantly higher in the children with remote symptomatic epilepsy and epileptic encephalopathies, in one study 48.9 percent of those considered neurologically normal received some form of special educational services [Berg et al., 2005].
Educational underachievement in reading, writing, and math has been reported in a variety of settings, comparing children with epilepsy both with their normal peers and with children with other chronic illnesses, such as asthma [Bagley, 1970; Fastenau et al., 2004; Holdsworth and Whitmore, 1974; Mitchell et al., 1991; Seidenberg et al., 1986; Selassie et al., 2008; Stores, 1981; Stores and Hart, 1976; Sturniolo and Galletti, 1994; McNelis et al., 2007]. It is unclear whether the relationship is a direct one, in which the epilepsy, seizures, or medications themselves cause learning disability, or an indirect one, in which an underlying neurologic condition causes both seizures and abnormalities in perception, memory, and visual-motor skills. Educational underachievement may be excessive in subjects drawn from inner-city teaching hospitals because of social factors entirely unrelated to medical conditions [Mitchell et al., 1991]. Parental expectations for a child with epilepsy are often lowered, at times inappropriately [Chavez, 1985; Hartlage and Green, 1972; Hoare and Kerley, 1991; Long and Moore, 1979]. Even after the effects of sociocultural variability have been accounted for, at least some children with epilepsy manifest learning disabilities. In one study comparing children with newly diagnosed epilepsy to children with recently diagnosed moderate asthma, academic underachievement was significantly more common in children with epilepsy, particularly boys with severe epilepsy [Austin et al., 1998]. Earlier seizure onset, generalized nonabsence seizures, and comorbid attention-deficit hyperactivity disorder (ADHD) appear to be risk factors for learning disability amongst children with epilepsy. However, a diagnosis of epilepsy, even when seizures are less severe and controlled, should provide sufficient cause to screen children for learning disability and to monitor academic performance continuously [Fastenau et al., 2008].
Detailed neurocognitive batteries in children with epilepsy demonstrate higher than expected rates of dyslexia, visuospatial difficulties, nonverbal learning difficulties, attention/executive/construction difficulties, verbal memory and learning problems, language difficulties, and slowed processing speeds [Mitchell et al., 1992; Fastenau et al., 2009]. With psychometric testing, almost half of the children with epilepsy met criteria for a learning disability [Seidenberg et al., 1986; Fastenau et al., 2008]. Some of these findings may be related to subtle or overt underlying structural lesions causing both the epilepsy and the learning disability, but not all can be readily explained. Numerous studies attempting to identify demographic, neurological, and seizure-related risk factors for academic underachievment have shown inconsistent results. Inadequate seizure control, early age of onset, longer duration of disorder, and polytherapy have not universally been shown to be associated with academic underachievement. Additionally, in children with normal IQ, no significant relationship between epilepsy type/syndrome and educational problems has been found [Mitchell et al., 1991; Oostrom et al., 2005; McNelis et al., 2007].
Attention Deficit, Impulsivity, and Overactivity
Attention, impulsivity, and activity level can be measured in various ways, ranging from parent and teacher questionnaires to psychometric testing to computerized continuous performance tasks. Regardless of methods used, most studies of children and adults with epilepsy demonstrate an excess incidence of inattention, impulsivity, and slowed reaction time [Kinney et al., 1990; Mitchell et al., 1992; Stores, 1978]. These findings should not imply that clinical ADHD is extremely common in children with epilepsy, although the prevalence is probably somewhat higher than in the general population. As with overall cognitive function in children with epilepsy, simple cause and effect relationships are uncommon. Underlying neurologic conditions may cause both ADHD and epilepsy [Kinney et al., 1990]. Antiepileptic medications may affect attention and impulsivity, both positively and negatively, at least in some persons [Mitchell et al., 1993; Riva and Devoti, 1996]. Measured effects on attention are small, and may not be clinically significant. In rare instances, frequent seizures may affect attention, and seizure control may eliminate an apparent attention deficit. There is some evidence that frequent epileptiform discharges may disrupt attention, which may improve with antiepileptic treatment [Gordon et al., 1996].
Autism and Autistic Spectrum Disorders
Autistic spectrum disorders are associated with an increased incidence of epilepsy, but evidence that one causes the other is lacking [Carlton Ford et al., 1995; Cavazzuti and Nalin, 1990; Olsson et al., 1988; Steffenburg et al., 1996; Wong, 1993]. A number of syndromes are associated with a high incidence of both autistic behavior and seizures (e.g., Angelman’s syndrome, tuberous sclerosis), but the coincidence of seizures and behavioral disorder is due to the underlying condition. A possible rare exception is the child in whom autistic behavior develops along with language regression, accompanied by an epileptiform EEG (continuous spike-and-wave pattern during sleep) [Hirsch et al., 1990; Kyllerman et al., 1996; Perez et al., 1993; Roulet et al., 1991]. This condition has been considered to be a variant of Landau–Kleffner syndrome. Behavior and language may improve with treatment with antiepileptics (rarely), corticosteroids, or corticotropin, or after subpial transection of epileptogenic cortex [Hirsch et al., 1990].
Psychiatric Disorders in Childhood Epilepsy
Little evidence exists to support the notion that severe psychiatric disorders are more common in children with epilepsy. Although major psychiatric illnesses, such as schizophrenia, obsessive-compulsive disorder, or affective disorders, may coexist with childhood epilepsy, the prevalence is not higher than in the general population. Treatment of coexisting severe psychiatric disorder and epilepsy may be complex. Some antiepileptics (carbamazepine, valproic acid) are reported to be beneficial in treatment of certain psychiatric disorders, most notably bipolar affective disorder [Fenn et al., 1996]. In general, however, treatment of epilepsy does not relieve symptoms of major psychiatric illness. Occasionally, “paradoxical normalization,” or “forced normalization,” is reported in children who experience a decrease in psychiatric symptoms when seizures are uncontrolled, with worsening of symptoms when seizures are in good control [Amir and Gross-Tsur, 1994].
Depressive disorders and mood disturbances have been reported more frequently in adolescents and adults with epilepsy than in healthy peers. Prevalence of depression in children and adolescents with epilepsy is significantly higher than in the general pediatric population [Dunn et al., 1999; Ettinger et al., 1998]. Moreover, depressive disorders are often underdiagnosed and undertreated in patients with pediatric epilepsy [Plioplys, 2003]. A number of studies have clearly highlighted that suicidal ideation and attempts are more likely to be seen in children and adolescents with epilepsy than in the general pediatric population [Baker, 2006; Oguz et al., 2002; Thome-Souza et al., 2007]. In addition to depression, anxiety disorders are frequent comorbidities in childhood epilepsies [Ekinci et al., 2009]. The etiology of depressive symptoms may be complex; social stigma, lack of employment opportunities, and lack of social contacts may contribute to depression. Self-reported quality of life is lower in adolescents with epilepsy than in adolescents with asthma. Although this difference was more striking for young persons with active epilepsy, quality of life measures were low, even when seizures were fully controlled or inactive [Austin et al., 1996].
Behavioral Problems, Conduct Disorders, and Delinquency
Behavioral disturbances in children and adolescents with epilepsy may be due to family factors and parental anxiety about epilepsy, rather than a primary result of epilepsy or of the underlying neurologic disorder [Austin et al., 1992; Carlton Ford et al., 1995; Gortmaker et al., 1990; Hoare and Kerley, 1991; Lothman and Pianta, 1993; Mitchell et al., 1994; Pianta and Lothman, 1994]. Self-esteem is reported to be lower and behavioral problems are more frequent in children and adolescents with epilepsy than in peers with or without chronic illnesses such as asthma or diabetes [Apter et al., 1991; Austin, 1989; Hoare and Mann, 1994; Matthews et al., 1982; Westbrook et al., 1991]. When children with epilepsy are assessed at the time of first seizure diagnosis, behavior problems are frequently reported by parents and teachers, particularly in children who had previously unrecognized seizures [Austin et al., 2001, 2002]. Children with epilepsy have higher rates of oppositional-defiant disorder [Dunn et al., 2009; Jones et al., 2007] and conduct disorder [Davies et al., 2003; Dunn et al., 2009] compared to the general population.
Adolescents and young adults with childhood-onset epilepsy have slightly higher than expected rates of delinquency in some studies [Camfield et al., 1993]. It is uncertain whether this propensity is due to underlying brain disease with poor impulse control, stigma, lack of opportunity, or other sociocultural factors. A population-based study in Finland, however, failed to find a relationship between delinquency and epilepsy in males up to age 22 years, although delinquency was associated with a history of central nervous system trauma [Rantakallio et al., 1992].
Cognitive and Behavioral Outcome of Specific Epilepsy Syndromes
Infantile Spasms
Certain pediatric epilepsy syndromes have been associated with significant, sometimes devastating, cognitive or behavioral declines. Perhaps the best studied of the catastrophic childhood epilepsies is infantile spasms. Mental retardation has been reported in up to 80 percent of children with infantile spasms and is described as severe in more than half of the cases [Jambaqué, 1994]. Although many patients exhibit global arrest of development, specific cognitive deficits, such as speech difficulties and impaired visuospatial abilities, have been noted in others [Besag, 2004]. Thirteen percent of those with cryptogenic infantile spasms were reported to exhibit persistent autistic features. In children with infantile spasms due to tuberous sclerosis, rate of autism is higher (58 percent) [Bolton et al., 2002; Hunt and Dennis, 1987; Riikonen and Amnell, 1981]. Effective early treatment of both cryptogenic and symptomatic spasms may improve cognition and behavior [Caplan et al., 2002; Jambaqué et al., 2000; Kivity et al., 2004]. Other prognostic factors for a better cognitive outcome include sustained seizure control with the first medication [Partikian and Mitchell, 2009], age at onset equal to or greater than 4 months, and absence of atypical spasms and partial seizures [Riikonen, 2009].
Epileptic Encephalopathies of Infancy
Many of the epileptic encephalopathies with neonatal or infantile onset are associated with frequent seizures that are notoriously refractory to treatment with both conventional and newer antiepileptic medication. Included in this group are severe myoclonic epilepsy of infancy (Dravet’s syndrome), early infantile epileptic encephalopathy (Ohtahara’s syndrome), neonatal myoclonic encephalopathy (or early infantile myoclonic encephalopathy), and migrating partial seizures of infancy. Delayed development usually is seen by the second year, and interpersonal relationships rarely progress past a level expected for that of a 2-year-old [Besag, 2004]. Behavior in the affected child typically is hyperactive with autistic features. It is difficult to attribute the abnormalities in cognition and behavior fully to the frequent seizures and paroxysmal findings on EEG, when an undiagnosed metabolic or genetic etiology may play a causative or contributory role. In children with tuberous sclerosis or Sturge–Weber syndrome, early control of seizures is associated with a more favorable neurodevelopmental and behavioral outcome [Jambaqué et al., 2000; Kramer et al., 2000].
Lennox–Gastaut Syndrome
Lennox–Gastaut syndrome frequently has been associated with autistic features and cognitive deficits, although published literature specific to this topic is sparse [Besag, 2004]. Long-term follow-up evaluation of these patients commonly reveals slowness of intellectual ability and motor speed, apathy (possibly better described as an inability to engage with the environment secondary to frequent epileptiform discharges), and perseverative behavior [Kieffer-Renaux et al., 2001].
Electrical Status Epilepticus in Sleep and Landau–Kleffner Syndrome
Electrical status epilepticus in sleep is an EEG pattern detected in some cases of pediatric epilepsy that often is associated with specific cognitive and language dysfunction. It frequently is encountered in those syndromes described as continuous spikes and waves (during slow-wave sleep and Landau–Kleffner syndrome). With continuous spike-and-wave activity in sleep, a typical decrease in the IQ or developmental quotient is noted by most investigators [Boel and Casaer, 1989; Roulet-Perez et al., 1993]. Of interest, some 40–60 percent of children with continuous spike-wave sleep exhibit an expressive aphasia, which is in contrast with children with Landau–Kleffner syndrome, who tend to present with a verbal or auditory agnosia [Galanopoulou et al., 2000]. In patients with Landau–Kleffner syndrome, language may recover spontaneously, partially improve with therapy, or unfortunately remain permanently affected despite improvement of the EEG abnormality [Besag, 2004].
Benign Focal Epilepsies of Childhood
Of specific note is the syndrome termed benign childhood epilepsy with centrotemporal spikes (benign rolandic epilepsy). Although this disorder was once thought of as a universally benign syndrome, increasing evidence suggests that a subpopulation of children may present with recent impairment of overall cognitive functioning, or difficulties with visual perception, concentration, and short-term memory [Weglage et al., 1997].
Family, Community, and Cultural Perceptions of Epilepsy
Social acceptance and inclusion of children and adolescents with epilepsy are far from complete, even when seizures are infrequent or fully controlled. In some cultural settings, it is not generally disclosed to friends or extended family that a child has epilepsy [Ju et al., 1990]. Some children are not sent to school if seizures are uncontrolled. Despite laws guaranteeing disabled and medically impaired children full access to education, some schools discourage attendance by children with active seizure disorders. All of these prejudices may further impair social and academic function in children with epilepsy. Fear of stigmatization may contribute to the high frequency of nondisclosure of epilepsy among adolescents [MacLeod and Austin, 2003]. It has been suggested that society’s understanding of epilepsy, as reflected through literature, has changed over time, and the ancient belief that seizures were a supernatural force has given way to the present understanding that epilepsy represents a medical condition [Jones, 2000]. However, a survey of a newer medium, movies released between 1937 and 2003 from four continents, found portrayal of all of the ancient beliefs about epilepsy, including demonic or divine possession, genius, lunacy, delinquency, and general “otherness” [Baxendale, 2003].
Social Adjustment of Adults with Childhood-Onset Epilepsy
Population-based studies from several countries document that social functioning is impaired in adults who had childhood-onset epilepsy, compared with their healthy peers [Farmer et al., 1992; Sillanpaa, 1990]. Marriage is less frequent, employment is less frequent and at less skilled occupations, and social isolation is more frequent. Differences are more striking when adults have on-going seizures, but are present even when complete remission or control has been obtained. Even when studies were restricted to adults with childhood-onset absence epilepsy, a disorder thought to be benign and likely to remit, social functioning continued to be impaired in comparison with that in nonepileptic peers [Dieterich et al., 1985]. Other studies of outcome in adults with childhood-onset epilepsy find substantial maladaptation as well, particularly in social and vocational function. Social functioning is generally much more impaired in the subgroup of adults with on-going seizures than in those who attain complete remission. A population-based study of adults in Nova Scotia, evaluated 25 years after a diagnosis of juvenile myoclonic epilepsy, documented a high frequency of social isolation, unemployment and social impulsiveness, with 74 percent having at least one major unfavorable social outcome [Camfield and Camfield, 2009].
In long-term follow-up (30 years) of a population-based cohort of children with epilepsy in Finland, about 60 percent of subjects were independent in activities of daily living, and 57 percent were employed, most in manual labor or semiskilled positions [Sillanpaa and Helenius, 1993]. Several studies are notable for including only adults with childhood-onset absence [Olsson and Campenhausen, 1993] or mixed generalized seizures (absence plus generalized tonic-clonic) [Dieterich et al., 1985]. Young adults with persisting absence seizures since childhood or adolescence, originally identified during a population-based study of absence epilepsy, were compared with a Swedish reference sample of young adults, assessing the impact of epilepsy on schooling, occupation, leisure-time activities, friends, daily routines, and housing. Although the overall employment rate did not differ from that in the reference subjects, persons with epilepsy were more likely to be employed in an unskilled job or in an occupation below that expected for educational level. Social isolation was reported in 34.5 percent, compared with 7.9 percent of the reference group. A high percentage of subjects (74 percent) reported that epilepsy had affected at least one area of their social functioning [Olsson and Campenhausen, 1993]. In a Finnish study of adults who had uncomplicated childhood-onset epilepsy, quality of life of adults with epilepsy in remission on medication was lower, and rates of unemployment were higher, than in comparison subjects or in adults whose epilepsy was in remission after withdrawal of medication [Sillanpaa et al., 2004].
Effects Of Antiepileptic Drugs on Behavior, Attention, and Mood
General Effects of Antiepileptic Drugs
Cognitive, psychiatric, and behavioral abnormalities in children with epilepsy often are attributed to antiepileptic medications. Most of these effects are unsupported by data from well-controlled, randomized, prospective clinical research. It is clear, however, that idiosyncratic adverse behavioral and cognitive responses can occur with any antiepileptic drug (see Chapter 59). In addressing issues of abnormal cognition and behavior in the management of pediatric epilepsy, several factors need to be recognized. Epilepsy occurring in the developing brain is likely to be substantially different from that in an adult in both its qualities and response to treatment. In addition, the most refractory epilepsies are likely to begin in childhood. The management of severe childhood epilepsy may prove challenging in that the choice of antiepileptic medication depends on the type of syndrome in which the seizures occur, as well as the cognitive and behavioral abnormalities that may occur with these syndromes. Finally, treating a combination of several seizure types in one patient may be difficult, because some anticonvulsants that are effective in treating one type of seizure may be ineffective or even exacerbate another seizure type.
In well-designed, controlled studies, evidence of long-term adverse cognitive effects of antiepileptic medications is generally slight or difficult to document objectively. A few studies have randomized subjects at the onset of seizures to receive one of several antiepileptic drugs [Aikia et al., 2006; Fritz et al., 2005; Forsythe et al., 1991; Mitchell and Chavez, 1987]. Most studies, however, examine patients assigned nonrandomly to receive various antiepileptic drugs when medication is started, changed, or withdrawn [Aldenkamp et al., 1993; Aman et al., 1994; Chen et al., 1996; Mitchell et al., 1993; Sabers et al., 1995; Stores et al., 1992]. The only double-blind, placebo-controlled, long-term studies of the behavioral effects of monotherapy with antiepileptic drugs are limited to studies of phenobarbital for febrile seizures in infants and toddlers [Farwell et al., 1990; Camfield et al., 1979]. There are many observational studies addressing cognitive and behavioral side effects, often comparing newer antiepileptic agents with older ones. These are typically limited to small patient cohorts and short-term follow-up. Comparative, blinded trials of anticonvulsant monotherapy in pediatrics are extremely limited. One exception is a recently completed comparative trial of monotherapy for childhood absence epilepsy compared ethosuximide, lamotrigine and valproic acid [Glauser et al., 2010]. While efficacy was similar for ethosuximide and valproic acid, negative behavioral effects were more frequent with valproate, particularly affecting attention.
Antiepileptic Drugs and Motor Speed
In older children and adults, the major effect of most antiepileptic medications on cognitive function appears to be a slowing of motor and cognitive processing speed. Early reports that phenytoin caused generalized decline in cognitive function were later disputed when further data analysis and research found that the major effect of phenytoin was on motor speed [Aldenkamp et al., 1994; Dodrill and Tempkin, 1989; Duncan et al., 1990]. Other cognitive functions are relatively spared, if analyzed independent of response speed. Many standardized cognitive tests are at least partly dependent on timed performance. Thus, IQ may appear to be lowered by medications, whereas the primary effect is on motor speed. Nevertheless, some patients perceive that their responses are slower and are bothered by this, despite otherwise normal functioning by most other measures.
Conversely, improvements in cognitive function, impulsivity, and behavior have been reported with several antiepileptic drugs. This improvement may occasionally be dramatic, resulting from control of frequent seizures, such as when a child with frequent absence seizures is started on anticonvulsants. Improved behavior and cognitive function as a direct result of antiepileptic medication also has been documented for some antiepileptic drugs, most consistently carbamazepine, lamotrigine, and levetiracetam [Aldenkamp et al., 2002; Aman et al., 1994; Gillham et al., 2000; Mitchell et al., 1993]. Effects are slight, however, and probably not clinically significant in most instances; they may even be disputed [Seidel and Mitchell, 1999].
Excitement or Agitation Due to Sedative Antiepileptic Drugs
Most sedative drugs have the potential for causing excitement and agitation when they are first initiated, but this effect dissipates in most children over a few weeks. Phenobarbital causes sustained behavioral difficulties, primarily overactivity, in some children, and can cause irritability and disturbed sleep, particularly in infants and toddlers [Camfield et al., 1979; Wolf et al., 1977]. Estimates of the number of children who do not tolerate phenobarbital because of resulting overactivity range from 5 to 25 percent. This effect is more frequent in toddlers and preschool-aged children but may occur at any age. Published case reports document a variety of idiosyncratic behavioral adverse reactions to virtually all antiepileptic drugs. Valproic acid occasionally causes a confused state or psychosis [Papazian et al., 1995]. Felbamate also has been reported to cause agitation and significant behavioral effects early in treatment, although overall this drug tends to improve behavior with prolonged treatment [Gay et al., 1995].
Behavioral and Cognitive Effects of the Newer Antiepileptic Agents
Newer medications formulated in the last 20 years for use as antiepileptic drugs generally have undergone at least add-on trials in children, with some studies including behavioral and cognitive assessment. These newer antiepileptic drugs have been reported to cause behavior change, both positive and negative. Very few have included behavioral or cognitive measures in properly designed, blinded monotherapy studies, either in comparison with placebo or in comparison with another antiepileptic. Comparison across studies of antiepileptic drugs is further hampered by varying criteria for selection of participants for studies, and use of differing neuropsychologic tests and study designs [Brunbech and Sabers, 2002]. A recent review of the literature regarding anticonvulsant effects in children summarizes published studies, nearly all of which examined phenobarbital, carbamazepine, or valproic acid [Loring and Meador, 2004]. Several other reviews addressing the effects of the newer antiepileptic agents are limited to the adult patient population [Aldenkamp et al., 2006; Kennedy and Lhatoo, 2008].
Specific Medications
Topiramate
Topiramate has been reported in adult epileptic and nonepileptic persons to cause significant cognitive difficulties, primarily involving word-finding and verbal memory, particularly during initial treatment and titration [Fisher and Blum, 1995; Gomer et al., 2007; Huppertz et al., 2001; Lee et al., 2003; Martin et al., 1999; Thompson et al., 2000]. These adverse effects appeared to dissipate over time, particularly if the dosage is increased slowly, although they may persist [Aldenkamp et al., 2003; Tatum et al., 2001]. However, subsequent studies have shown that a gradual introduction of topiramate does not necessarily prevent effects on cognition, that there is no clear relation between daily dosage and cognitive side effects, and that adverse effects on verbal fluency, verbal memory and cognitive speed may persist until withdrawal of the medication [Gerber et al., 2000; Gomer et al., 2007; Huppertz et al., 2001; Kockelmann et al., 2003]. No clear at-risk group or predicting factor has been identified. In one double-blind, randomized, placebo-controlled trial of topiramate for add-on therapy in 86 children with partial epilepsy, children receiving topiramate had an increased frequency of emotional lability, difficulties with concentration, and fatigue, but the changes were not severe enough to cause any subject to discontinue treatment [Elterman et al., 1999].
Lamotrigine
Since its introduction in the United States in 1994, lamotrigine has received much attention with respect to its potential positive psychotropic effects [Brunbech and Sabers, 2002]. Significant improvements in behavior, cognition, and motor skills (unrelated to seizure control) are noted in pediatric patients, although these effects have not yet been formally studied [Culy and Goa, 2000; Meador and Baker, 1997]. Lamotrigine has been reported to have fewer cognitive effects in adults with newly diagnosed epilepsy than those described for carbamazepine, phenytoin, and topiramate [Blum et al., 2006; Brodie et al., 1995; Meador et al., 2001; Steiner et al., 1999]. A short-term treatment study performed on healthy volunteers using low-dose lamotrigine revealed improvement in cognitive activation and mood, and reported subjective positive effects on quality of life relative to that achieved with use of valproate [Aldenkamp et al., 2002]. In a trial of lamotrigine for add-on therapy in children with Lennox–Gastaut syndrome, responders with improved seizure control were noted to be more alert and attentive, whereas nonresponders were more likely to experience agitation. Lamotrigine exhibited no clinically significant adverse cognitive effects as adjunctive treatment for children with well-controlled or mild epilepsy, when compared to placebo [Pressler et al., 2006].
Clobazam
Clobazam is a 1,5 benzodiazepine, initially formulated as an anxiolytic, but later recognized for its antiseizure potential via open-label, adjunctive trials and double-blind, placebo-controlled, add-on trials [Hentschel and Froscher, 1992]. Clobazam is effective as monotherapy in treating partial seizures with secondary generalization and in some primary generalized tonic-clonic seizures. A randomized, double-blind, prospective multicenter Canadian study addressed the cognitive tolerability of clobazam compared to carbamazepine and phenytoin in children with newly diagnosed epilepsy. There appeared to be no deterioration of intelligence, memory, attention, psychomotor speed, or impulsivity in children on clobazam relative to the other standard monotherapies. Behavioral side effects were similar, as well [Bawden et al., 1999]. There are currently no studies published comparing cognitive or behavioral effects of clobazam to the newer antiepileptic drugs or to placebo in adults or children.
Gabapentin
Gabapentin is similar in structure to gamma-aminobutyric acid (GABA) but does not seem to have effects at the GABA receptors. In both adult volunteer studies and studies with double-blind, randomized, crossover designs, gabapentin produced no significant alteration of psychomotor or memory abilities when used for either monotherapy or add-on treatment [Dodrill et al., 1999; Leach et al., 1997; Martin et al., 1999; Meador et al., 1999]. Gabapentin occasionally has been reported to cause aggressive or agitated behavior [Lee et al., 1996; Tallian et al., 1996]. Children in whom significant adverse behavioral changes occur with use of gabapentin tend to have some degree of documented mental retardation [Khurana et al., 1996]. No published controlled studies have evaluated the cognitive and behavioral effects of gabapentin in children who have epilepsy.
Levetiracetam
Levetiracetam, a structurally and mechanistically novel antiepileptic drug, has a favorable pharmacokinetic profile and is effective in treating partial seizures in both adult and pediatric patients [Callenbach et al., 2008; Dooley and Plosker, 2000; Lagae et al., 2003; Li et al., 2009; Verrotti et al., 2009]. Several observational studies have noted significant improvements in verbal fluency, prospective and working memory, and attention in patients treated with levetiracetam [Gomer et al., 2007; Lopez-Gongora et al., 2008; Piazzini et al., 2006]. However, among adult patients with epilepsy who received levetiracetam, behavioral abnormalities were reported in a significant proportion relative to those patients with anxiety or cognitive disorders but without epilepsy. The behavioral events cited were less common than those reported with other antiepileptic agents [Cramer et al., 2003]. Another recent study reported a dose-independent behavioral change in 59 percent of adult patients, 37 percent of which were negative (loss of self-control, restlessness, sleep disturbance, and aggression) and 22 percent positive (increased energy and activation). The positive effects did not appear to be related to type of epilepsy, co-treatment, dose, or psychiatric history, whereas the negative effects were associated with poorer seizure control, intellectual disability, and underlying impulsiveness [Helmstaedter et al., 2008]. A prospective open-label, add-on trial using levetiracetam to treat refractory partial and generalized seizures in children younger than 16 years of age revealed adverse events in 51 percent of subjects. Most of these events were behavioral in nature, although the majority of children did not require discontinuation of the medication. As with the gabapentin study, many of the children were developmentally delayed or cognitively impaired. In this same study, however, improvement in behavior and/or cognition after the addition of levetiracetam was reported in 25.6 percent [Wheless and Ng, 2002]. A few anecdotal reports have described improved behavior in children with autism treated with levetiracetam, but in the same population, some children experienced an increase in aggressive behavior [Rugino and Samsock, 2002].
Oxcarbazepine
Oxcarbazepine is a keto homolog of carbamazepine, although it has a very distinct metabolic profile. It is labeled for use as monotherapy or adjunctive therapy for partial seizures with or without secondarily generalized seizures in children 6 years of age and older. Although no deterioration in cognitive function test results was reported in one crossover and several comparative monotherapy studies in adult epilepsy patients [Aikia et al., 1992; McKee et al., 1994; Sabers et al., 1995], cognitive function has not been systematically studied in children and adolescents. One open-label comparison study investigating the effect of oxcarbazepine on cognition in children and adolescents aged 6–17 years revealed no differences on cognitive testing relative to carbamazepine and valproate. Results, however, were limited to a 6-month follow-up period [Donati et al., 2006]. A nonrandomized, open-label, observational study using oxcarbazepine as monotherapy in children with benign childhood epilepsy with centrotemporal spikes (BECTS) showed apparent conservation of cognitive functions and behavioral abilities [Tzitiridou et al., 2005].
Tiagabine
Tiagabine acts as a GABA uptake inhibitor in neurons and glial cells, and is effective for add-on therapy in the treatment of refractory partial epilepsy. Data from several randomized, double-blind, placebo-controlled, parallel-group or crossover studies of tiagabine as monotherapy or add-on therapy have been published [Aikia et al., 2006; Dodrill et al., 1997; Kalviainen et al., 1996; Sveinbjornsdottir et al., 1994]. Generally, no significant decline in IQ, reaction speed, attention, or memory has been noted at either low or high doses. One randomized, open, comparative study investigating the cognitive side effects of tiagabine versus topiramate revealed deterioration in verbal memory (delayed free recall) in patients being treated with tiagabine [Fritz et al., 2005]. However, this finding of a decline in only one of three measures of verbal learning and memory was not conclusive evidence that the drug only affects the episodic memory ability, thus suggesting that further study is necessary. No information is available regarding the cognitive effects of tiagabine in the pediatric population.
Zonisamide
No published trials have used formal neuropsychological tests to evaluate cognitive or behavioral function in pediatric patients with either partial or generalized seizures treated with zonisamide. In several observational studies, 26–61 percent of patients reported mild to moderate adverse events, with only a mild development of tolerance over time [Berent et al., 1987; Kothare et al., 2006; Tosches and Tisdell, 2006; Wilfong, 2005]. Cognitive dysfunction, specifically attention, memory, and language changes, was reported in 2–11 percent, often occurring at therapeutic serum levels. One prospective, randomized, open-label investigation of zonisamide as monotherapy in adult patients used several standardized neuropsychological tests for evaluation of cognitive changes. Significantly decreased performance with delayed word recall and verbal fluency were noted, despite the absence of confounding factors, such as intractable epilepsy and polypharmacy [Park et al., 2008]. Reports from Japan, where the medication has been in use for 20 years, suggest that psychotic episodes and behavior changes may occur in children given zonisamide, despite improved seizure control [Hirai et al., 2002; Kimura, 1994].
Vigabatrin
Vigabatrin was recently approved in the United States, after long delays due to reports of vigabatrin-induced retinal toxicity. Approval in the United States is for the treatment of infantile spasms and refractory complex partial seizures in adults. Mild and transient drowsiness, dizziness, and irritability are frequently reported with vigabatrin initiation, but do not appear to affect cognition in a strong, adverse way [Dodrill et al., 1993; McGuire et al., 1992; Monaco et al., 1997]. Both a single review of the cognitive effects of vigabatrin, and a monotherapy study comparing the drug to carbamazepine in adult patients with partial epilepsy, not only revealed an absence of cognitive deterioration, but also reported an improvement in tests of memory, psychomotor speed, and flexibility of mental processing [Monaco, 1996; Kälviäinen et al., 1995].
Rufinamide
Rufinamide is a structurally novel compound that appears to exert its action by limiting the frequency of sodium-dependent neuronal action potentials. Reports during the experimental phase of the drug revealed potential improvement of reaction-timed tests, but a possible impairment of short-term memory at higher doses. One multicenter, double-blind, randomized, placebo-controlled, parallel study of rufinamide in patients aged 15–64 years with partial epilepsy has been performed and revealed no significant deterioration in psychomotor speed, alertness, attention, or working memory [Aldenkamp and Alpherts, 2006]. There have been no published investigations of the cognitive and behavioral effects of this medication in the pediatric population.
Treatment of Cognitive, Social, Academic and Behavioral Problems Associated with Epilepsy
As with all medical treatment, care of the child with epilepsy must be individualized. Nevertheless, several general principles apply. The developmental, cognitive, academic, and behavioral status of the child and the social functioning of the family must be integral and on-going concerns in the management of a child with epilepsy. Brief developmental, cognitive, or academic assessment is appropriate at the time of initial evaluation and at follow-up visits. Although treatment generally will not alter the underlying cognitive capacity of the child, early intervention and referral to appropriate community and school resources will maximize function. Appropriate counseling of the family may minimize later behavioral and adjustment difficulties. Group programs to help families with their children with epilepsy may be effective in altering parental attitudes, reducing fears, and improving the children’s participation in family and community activities [Lewis et al., 1990]. They may also improve self-management and communication skills and health-related quality of life in the social exclusion dimension [Jantzen et al., 2009].
Treatment of Attention-Deficit Disorders (ADD and ADHD) in Children with Epilepsy
Alteration in antiepileptic treatment may improve behavior and attention if the original treatment has substantially affected behavior. However, ADHD may coexist with epilepsy, independent of antiepileptic treatment. Treatment with stimulants (e.g., methylphenidate, pemoline, dextroamphetamine) or with tricyclic antidepressants (e.g., imipramine, desipramine) generally does not compromise seizure control [Gross-Tsur et al., 1997]. Atomoxetine (a nonamphetamine selective norepinephrine reuptake inhibitor with stimulant properties) does not significantly affect seizure control; nor does it have significant pharmacokinetic interactions with antiepileptic medications. Bupropion, an antidepressant drug occasionally useful for ADHD, particularly when associated with mood disorder, may increase tendency toward seizures, particularly at high doses. Most pediatric neurologists avoid its use at doses greater than 100 mg per day in children with epilepsy.
Peer Relationships, Teasing, and Social Isolation
Social isolation and poor peer relationships are a particular problem in school-aged and adolescent children with epilepsy and are difficult to address therapeutically. Nonverbal learning disabilities may make the child socially maladroit and target them for teasing by peers. Children may be particularly at risk for teasing and exclusion by peers if they have had seizures at school or are singled out by the need to leave the classroom to take medication during the school day. Therapeutic or educational programs that emphasize social skills and assertiveness training may be helpful in some children and adolescents [Henriksen, 1990; Lewis et al., 1990; Strang, 1990].
References
![]() The complete list of references for this chapter is available online at www.expertconsult.com.
The complete list of references for this chapter is available online at www.expertconsult.com.
Äikiä M., Jutila L., Salmenperä T., et al. Comparison of the cognitive effects of tiagabine and carbamazepine as monotherapy in newly diagnosed adult patients with partial epilepsy: pooled analysis of two long-term, randomized, follow-up studies. Epilepsia. 2006;47(7):1121-1127.
Äikiä M., Kälviäinen R., Sivenius J., et al. Cognitive effects of oxcarbazepine and phenytoin monotherapy in newly diagnosed epilepsy: one year follow-up. Epilepsy Res. 1992;11:199-203.
Aldenkamp A.P., Alpherts W.C., Blennow G., et al. Withdrawal of antiepileptic medication in children – effects on cognitive function: The Multicenter Holmfrid Study. Neurology. 1993;43:41.
Aldenkamp A.P., Alpherts W.C., Diepman L., et al. Cognitive side effects of phenytoin compared with carbamazepine in patients with localization related epilepsy. Epilepsy Res. 1994;19:37.
Aldenkamp A.P., Alpherts W.C.J. The effect of the new antiepileptic drug rufinamide on cognitive functions. Epilepsia. 2006;47(7):1153-1159.
Aldenkamp A.P., Arends J. Effects of epileptiform EEG discharges on cognitive function: Is the concept of “transient cognitive impairment” still valid? Epilepsy Behav. 2004;5:S25-S34.
Aldenkamp A.P., Arends J., Bootsma H.P.R., et al. Randomized double-blind parallel-group study comparing cognitive effects of a low-dose lamotrigine with valproate and placebo in healthy volunteers. Epilepsia. 2002;43:19.
Aldenkamp A.P., Overweg J., Gutter T., et al. Effect of epilepsy, seizures and epileptiform EEG discharges on cognitive function. Acta Neurol Scand. 1996;93:253.
Aman M.G., Werry J.S., Paxton J.W., et al. Effects of phenytoin on cognitive motor performance in children as a function of drug concentration, seizure type, and time of medication. Epilepsia. 1994;35:172.
Amir N., Gross-Tsur V. Paradoxical normalization in childhood epilepsy. Epilepsia. 1994;35:1060.
Apter A., Aviv A., Kaminer Y., et al. Behavioral profile and social competence in temporal lobe epilepsy of adolescence. J Am Acad Child Adolesc Psychiatry. 1991;30:887.
Austin J.K. Comparison of child adaptation to epilepsy and asthma. J Child Adolesc Psychiatr Ment Health Nurs. 1989;2:139.
Austin J.K., Dunn D.W., Caffrey H.M., et al. Recurrent seizures and behavior problems in children with first recognized seizures: A prospective study. Epilepsia. 2002;43:564.
Austin J.K., Harezlak J., Dunn D.W., et al. Behavior problems in children before first recognized seizures. Pediatrics. 2001;107:115.
Austin J.K., Huberty T.J., Huster G.A., et al. Academic achievement in children with epilepsy or asthma. Dev Med Child Neurol. 1998;40:248.
Austin J.K., Huster G.A., Dunn G.W., et al. Adolescents with active or inactive epilepsy or asthma: A comparison of quality of life. Epilepsia. 1996;37:1228.
Austin J.K., Risinger M.W., Beckett L.A. Correlates of behavior problems in children with epilepsy. Epilepsia. 1992;33:1115.
Bagley C.R. Educational performance of children with epilepsy. Br J Educ Psychol. 1970;40:82.
Baker G.A. Depression and suicide in adolescents with epilepsy. Neurology. 2006;66(6 Suppl 3):S5-S12.
Bawden H.N., Camfield C.S., Camfield P.R., et al. The cognitive and behavioral effects of clobazam and standard monotherapy are comparable. Canadian Study Group for Childhood Epilepsy. Epilepsy Res. 1999;33(2–3):133-143.
Baxendale S. Epilepsy at the movies: possession to presidential assassination. Lancet Neurol. 2003;2(12):764-770.
Berent S., Sackellares J.C., Giordani B., et al. Zonisamide (CI-912) and cognition: results from preliminary study. Epilepsia. 1987;28:61-67.
Berg A.T., Langfitt J.T., Testa F.M., et al. Global cognitive function in children with epilepsy: a community-based study. Epilepsia. April 2008;49(4):608-614.
Berg A.T., Smith S.N., Frobish D., et al. Special education needs of children with newly diagnosed epilepsy. Dev Med Child Neurol. 2005;47:749-753.
Besag F.M. Behavioral aspects of pediatric epilepsy syndromes. Epilepsy Behav. 2004;5:S3.
Bielen I., Cvitanovic-Sojat L., Bergman-Markovic B., et al. Prevalence of epilepsy in Croatia: a population-based survey. Acta, Neurol Scand. 2007 Dec;116(6):361-367.
Binnie C.D. Significance and management of transitory cognitive impairment due to subclinical EEG discharges in children. Brain Dev. 1993;15:23.
Binnie C.D. Cognitive impairment during epileptiform discharges: is it ever justifiable to treat the EEG? Lancet. 2003;2:725-730.
Blomquist H.K., Gustavson K.-H., Holmgren G. Mild mental retardation in children in a northern Swedish county. J Ment Defic Res. 1981;25:169.
Blum D., Meador K., Biton V., et al. Cognitive effects of lamotrigine compared with topiramate in patients with epilepsy. Neurology. 2006;67(3):400-406.
Boel M., Casaer P. Continuous spikes and waves during slow sleep: A 30 months follow-up study of neuropsychological recovery and EEG findings. Neuropediatrics. 1989;20:176.
Bolton P.F., Park R.J., Higgins J.N., et al. Neuroepileptic determinants of autism spectrum Brent disorders in tuberous sclerosis complex. Brain. 2002;125:1247.
Britten N., Morgan K., Fenwick P.B.C., et al. Epilepsy and handicap from birth to age 36. Dev Med Child Neurol. 1986;28:719.
Brodie M.J., Richens A., Yuen A.W.C. Double-blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. Lancet. 1995;345:476.
Brorson L.O.. Epilepsi hos barn och ungdom. Social Department, editor. Epileptikervarden. Stockholm: Allmanna Forlaget; 1970.
Brunbech L., Sabers A. Effect of antiepileptic drugs on cognitive function in individuals with epilepsy. A comparative review of newer versus older agents. Drugs. 2002;62:595.
Callenbach P.M., Arts W.F., ten Houten R., et al. Add-on levetiracetam in children and adolescents with refractory epilepsy: results of an open-label muti-centre study. Eur J Paediatr Neurol. 2008;12(4):321-327.
Camfield C.S., Camfield P.R. Juvenile myoclonic epilepsy 25 years after seizure onset: A population-based study. Neurology. 2009;73:1041-1045.
Camfield C.S., Camfield P.R., Smith B., et al. Biologic factors as predictors of social outcome of epilepsy in intellectually normal children: A population-based study. J Pediatr. 1993;122:869.
Camfield C.S., Chaplin S., Doyle A.V., et al. Side effects of phenobarbital in toddlers; behavior and cognitive aspects. J Pediatr. 1979;95:361.
Caplan R., Siddarth P., Mathern G., et al. Developmental outcome with and without successful intervention. Int Rev Neurobiol. 2002;49:269.
Carlton Ford S., Miller R., Brown M., et al. Epilepsy and children’s social and psychological adjustment. J Health Soc Behav. 1995;36:285.
Cavazzuti G.B., Nalin A. Psychobehavioral disturbance in epileptic children. Childs Nerv Syst. 1990;6:430.
Chavez J.M. The impact of epilepsy on mother-child interaction in Mexican-American families at different levels of acculturation. Diss Abst Int. 1985;46:1741.
Chen Y.J., Kang W.M., So W.C. Comparison of antiepileptic drugs on cognitive function in newly diagnosed epileptic children: A psychometric and neurophysiological study. Epilepsia. 1996;37:81.
Clarke T., Strug L.J., Murphy P.L., et al. High risk of reading disability and speech sound disorder in rolandic epilepsy families: case-control study. Epilepsia. 2007;48:2558-12265.
Clement M.J., Wallace S.J. A survey of adolescents with epilepsy. Dev Med Child Neurol. 1990;32:849.
Cramer J.A., De Rue K., Devinsky O., et al. A systematic review of the behavioral effects of levetiracetam in adults with epilepsy, cognitive disorders, or an anxiety disorder during clinical trials. Epilepsy Behav. 2003;4:124.
Culy C.R., Goa K.L. Lamotrigine: A review of its use in childhood epilepsy. Paediatr Drugs. 2000;2:299.
Curatolo P., Arpino C., Stazi M.A., et al. Risk factors for the co-occurrence of partial epilepsy, cerebral palsy and mental retardation. Dev Med Child Neurol. 1995;37:776.
D’Alessandro P., Piccirilli M., Tiacci C., et al. Neuropsychological features of benign partial epilepsy in children. Ital J Neurol Sci. 1990;11:265.
Davies S., Heyman I., Goodman R. A population survey of mental health problems in children with epilepsy. Dev Med Child Neurol. 2003;45:292-295.
Dieterich E., Doose H., Baier W.K., et al. Long-term follow-up of childhood epilepsy with absences. II. Absence-epilepsy with initial grand mal. Neuropediatrics. 1985;16:155.
Dodrill C.B., Arnett J.L., Hayes A.G., et al. Cognitive abilities and adjustment with gabapentin: results of a multisite study. Epilepsy Res. 1999;35(2):109-121.
Dodrill C.B., Arnett J.L., Sommerville K.W. Evaluation of the effects of vigabatrin on cognitive abilities and quality of life in epilepsy. Neurology. 1993;43(12):2501-2507.
Dodrill C.B., Arnett J.L., Sommerville K.W., et al. Cognitive and quality of life effects of differing dosages of tiagabine in epilepsy. Neurology. 1997;48:1025-1031.
Dodrill C.B., Tempkin N.R. Motor speed is a contaminating factor in evaluating the “cognitive” effects of phenytoin. Epilepsia. 1989;30:453.
Donati F., Gobbi G., Campistol J., et al. Effects of oxcarbazepine on cognitive function in children and adolescents with partial seizures. Neurology. 2006;67:679-682.
Dooley M., Plosker G.L. Levetiracetam: A review of its adjunctive use in the management of partial onset seizures. Drugs. 2000;60:871.
Duncan J.S., Shorvon S.D., Trimble M.R. Effects of removal of phenytoin, carbamazepine, and valproate on cognitive function. Epilepsia. 1990;31:584.
Dunn D.W., Austin J.K., Huster G.A. Symptoms of depression in adolescents with epilepsy. J Am Acad Child Adolesc Psychiatry. 1999;38:1132-1138.
Dunn D.W., Austin J.K., Perkins S.M. Prevalence of psychopathology in childhood epilepsy: categorical and dimensional measures. Dev Med Child Neurol. 2009;51:364-372.
Ekinci O., Titus J.B., Rodopman A.A., et al. Depression and anxiety in children and adolescents with epilepsy: Prevalence, risk factors, and treatment. Epilepsy Behav. 2009;14(1):8-18.
Elterman R.D., Glauser T.A., Wyllie E., et al. A double-blind, randomized trial of topiramate as adjunctive therapy for partial-onset seizures in children. Topiramate YP Study Group. Neurology. 1999;52:1338.
Ettinger A.B., Weisbrot D.M., Nolan E.E., et al. Symptoms of depression and anxiety in pediatric epilepsy patients. Epilepsia. 1998;39:595-599.
Farmer P.J., Placencia M., Junbo L., et al. Effects of epilepsy on daily functioning in Northern Ecuador: Summary of findings of a population-based research project. Neuroepidemiology. 1992;11:180.
Farwell J.R., Lee Y.J., Hirtz D.G., et al. Phenobarbital for febrile seizures: Effects on intelligence and on seizure recurrence. N Engl J Med. 1990;322:364.
Fastenau P.S., Johnson C.S., Perkins S.M., et al. Neuropsychological status at seizure onset in children: risk factors for early cognitive deficits. Neurology. 2009;73:526-534.
Fastenau P.S., Shen J., Dunn D.W., et al. Neuropsychological predictors of academic underachievement in pediatric epilepsy: moderating roles of demographic, seizure, and psychosocial variables. Epilepsia. 2004;45:1261-1272.
Fastenau P.S., Shen J., Dunn D.W., et al. Academic underachievement among children with epilepsy: proportion exceeding psychometric criteria for learning disability and associated risk factors. J Learn Disabil. 2008;41:195-207.
Fenn H.H., Robinson D., Luby V., et al. Trends in pharmacotherapy of schizoaffective and bipolar affective disorders: A 5-year naturalistic study. Am J Psychiatry. 1996;153:711.
Fisher R., Blum D. Clobazam, oxcarbazepine, tiagabine, topiramate, and other new antiepileptic drugs. Epilepsia. 1995;36(Suppl 2):S105.
Fonseca Lineu C., Tedrus Gloria M.A.S., Pacheco Elisabeth M.C. Epileptiform EEG discharges in benign childhood epilepsy with centrotemporal spikes: reactivity and transitory cognitive impairment. Epilepsy Behav. 2007;11(1):65-70.
Forsgren L., Edvinsson S.O., Blomquist H.K., et al. Epilepsy in a population of mentally retarded children and adults. Epilepsy Res. 1990;6:234.
Forsythe I., Butler R., Berg I., et al. Cognitive impairment in new cases of epilepsy randomly assigned to carbamazepine, phenytoin and sodium valproate. Dev Med Child Neurol. 1991;33:524.
Fritz N., Glogau S., Joffmann J., et al. Efficacy and cognitive side effects of tiagabine and topiramate in patients with epilepsy. Epilepsy Behav. 2005;6:373-381.
Galanopoulou A.S., Bojko A., Lado F., et al. The spectrum of neuropsychiatric abnormalities associated with electrical status epilepticus in sleep. Brain Dev. 2000;22:279.
Gay P.E., Mecham G.F., Coskey J.S., et al. Behavioral effects of felbamate in childhood epileptic encephalopathy (Lennox-Gastaut syndrome). Psychol Rep. 1995;77:1208.
Gerber P.E., Hamiwka L., Connolly M.B., et al. Factors associated with behavioral and cognitive abnormalities in children receiving topiramate. Pediatr Neurol. 2000;22:200.
Germano E., Gagliano A., Magazu A., et al. Benign childhood epilepsy with occipital paroxysms: Neuropsychological findings. Epilepsy Res. 2005;64:137-150.
Gillham R., Kane K., Bryant-Comstock L., et al. A double-blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy with health-related quality of life as an outcome measure. Seizure. 2000;9:375-379.
Glauser T.A., Cnaan A., Shinnar S., et al. Childhood Absence Epilepsy Study Group. Ethosuximide, valproic acid and lamotrigine in childhood absence epilepsy. N Engl J Med. 2010;362(9):790-799.
Gomer B., Wagner K., Frings L., et al. The influence of antiepileptic drugs on cognition: a comparison of levetiracetam with topiramate. Epilepsy Behav. 2007;10:486-494.
Gordon K., Bawden H., Camfield P., et al. Valproic acid treatment of learning disorder and severely epileptiform EEG without clinical seizures. J Child Neurol. 1996;11:41.
Gortmaker S.L., Walker D.K., Weitzman M., et al. Chronic conditions, socioeconomic risks, and behavioral problems in children and adolescents. Pediatrics. 1990;85:267.
Goulden K.J., Shinnar S., Koller H., et al. Epilepsy in children with mental retardation. A cohort study. Epilepsia. 1991;32:690.
Gross-Tsur V., Manor O., van der Meere J., et al. Epilepsy and attention deficit hyperactivity disorder: Is methylphenidate safe and effective? J Pediatr. 1997;130:40.
Gustavson K.-H., Holmgren G., Jonsell R., et al. Severe mental retardation in children in a northern Swedish county. J Ment Defic Res. 1977;21:161.
Hagberg B., Hagberg G., Lewerth A., et al. Mild mental retardation in Swedish school children. I. Prevalence. Acta Paediatr Scand. 1981;70:441.
Hartlage L.A., Green J.B. The relation of parental attitudes to academic and social achievement in epileptic children. Epilepsia. 1972;13:21.
Hauser W.A., Hesdorffer D.C. Seizures and the developmental disabilities. In: Epilepsy: Frequency, causes and consequences. New York: Demos Publications; 1990.
Helmstaedter C., Fritz N.E., Kockelmann E., et al. Positive and negative psychotropic effects of levetiracetam. Epilepsy Behav. 2008;13:535-541.
Henkin Y., Sadeh M., Kivity S., et al. Cognitive function in idiopathic generalized epilepsy of childhood. Dev Med Child Neurol. 2005;47:126-132.
Henriksen O. Education and epilepsy: Assessment and remediation. Epilepsia. 1990;31(Suppl 4):S21.
Hentschel B., Froscher W. Clobazam in the treatment of epilepsy. Drugs Today. 1992;28:567-572.
Hirai K., Kimiya S., Tabata K., et al. Selective mutism and obsessive compulsive disorders associated with zonisamide. Seizure. 2002;11:468.
Hirsch E., Marescaux C., Maquet P., et al. Landau-Kleffner syndrome: A clinical and EEG study of five cases. Epilepsia. 1990;31:756.
Hoare P. The development of psychiatric disorder among schoolchildren with epilepsy. Dev Med Child Neurol. 1984;26:3.
Hoare P., Kerley S. Psychosocial adjustment of children with chronic epilepsy and their families. Dev Med Child Neurol. 1991;33:201.
Hoare P., Mann H. Self esteem and behavioural adjustment in children with epilepsy and children with diabetes. J Psychosom Res. 1994;38:859.
Holdsworth L., Whitmore K. A study of children with epilepsy attending ordinary schools; I. Seizure patterns, progress and behavior in school. Dev Med Child Neurol. 1974;16:746.
Hunt A., Dennis J. Psychiatric disorder among children with tuberous sclerosis. Dev Med Child Neurol. 1987;29:190.
Huppertz H.J., Quiske A., Schulze-Bonhage A. Cognitive impairments due to add-on therapy with topiramate. Nervenarzt. 2001;72:275-280.
Jacobson J.W., Janicki M.P. Observed prevalence of multiple developmental disabilities. Ment Retard. 1983;21:87.
Jalava M., Sillanpaa M. Physical activity, health related fitness, and health experience in adults with childhood onset epilepsy: A controlled study. Epilepsia. 1997;38:424.
Jambaqué I. Neuropsychological aspects. In: Dulac O., Chugani H.T., Dalla Bernadina B., editors. Infantile spasms and West syndrome. London: Saunders; 1994:82.
Jambaqué I., Chiron C., Dumas C., et al. Mental and behavioral outcome of infantile epilepsy treated by vigabatrin in tuberous sclerosis patients. Epilepsy Res. 2000;38:151.
Jantzen S., Müller-Godeffroy E., Hallfahrt-Krisl T., et al. FLIP&FLAP – A training programme for children and adolescents with epilepsy, and their parents. Seizure. 2009;18(7):478-486.
Jones J.E., Watson R., Sheth R., et al. Psychiatric comorbidity in children with new onset epilepsy. Dev Med Child Neurol. 2007;49:493-497.
Jones J.M. The falling sickness in literature. South Med J. 2000;93:1169-1172.
Ju S.H., Chang P.F., Chen Y.J., et al. Parental attitude and adjustment to childhood epilepsy. Acta Paediatr Sin. 1990;31:103.
Kälviäinen R., Äikiä M., Saukkonen A.M., et al. Vigabatrin vs carbamazepine monotherapy in patients with newly diagnosed epilepsy: a randomized, controlled study. Arch Neurol. 1995;52(10):989-996.
Kälviäinen R., Äikiä M., Mervaala E., et al. Long-term cognitive and EEG effects of tiagabine in drug-resistant partial epilepsy. Epilepsy Res. 1996;25:291-297.
Kennedy G.M., Lhatoo S.D. CNS adverse events associated with antiepileptic drugs. CNS Drugs. 2008;22(9):739-760.
Khurana D.S., Riviello J., Helmers S., et al. Efficacy of gabapentin therapy in children with refractory partial seizures. J Pediatr. 1996;128:829.
Kieffer-Renaux V., Kaminska A., Dulac O. Cognitive deterioration in Lennox-Gastaut and Doose epilepsy. In: Jambaqué I., Lasonde M., Dulac O., editors. Neuropsychology of childhood epilepsy. New York: Kluwer Academic/Plenum; 2001:185.
Kimura S. Zonisamide-induced behavior disorder in two children. Epilepsia. 1994;35:403.
Kinney R.O., Shaywitz B.A., Shaywitz S.E., et al. Epilepsy in children with attention deficit disorder: Cognitive, behavioral, and neuroanatomic indices. Pediatr Neurol. 1990;6:31.
Kivity S., Lerman P., Ariel R., et al. Long-term cognitive outcomes of a cohort of children with cryptogenic infantile spasms treated with high-dose adrenocorticotropic hormone. Epilepsia. 2004;45:255.
Kockelmann E., Elger C.E., Helmstaedter C. Significant improvement in frontal lobe associated neuropsychological functions after withdrawal of topiramate in epilepsy patients. Epilepsy Res. 2003;54:171-178.
Kothare S.V., Kaleyias J., Mostofi N., et al. Efficacy and safety of zonisamide monotherapy in a cohort of children with epilepsy. Pediatr Neurol. 2006;34:351-354.
Kramer U., Kahana E., Shorer Z., et al. Outcome of infants with unilateral Sturge-Weber syndrome and early onset seizures. Dev Med Child Neurol. 2000;42:756.
Kyllerman M., Nyden A., Praquin N., et al. Transient psychosis in a girl with epilepsy and continuous spikes and waves during slow sleep (CSWS). Eur Child Adolesc Psychiatry. 1996;5:216.
Lagae L., Buyse G., Deconinck A., et al. Effect of levetiracetam in refractory childhood epilepsy syndromes. Eur J Paediatr Neurol. 2003;7(3):123-128.
Leach J.P., Girvan J., Paul A., et al. Gabapentin and cognition: A double blind, dose ranging, placebo controlled study in refractory epilepsy. J Neurol Neurosurg Psychiatry. 1997;62:372.
Lee D.O., Steingard R.J., Cesena M., et al. Behavioral side effects of gabapentin in children. Epilepsia. 1996;37:87.
Lee S., Sziklas V., Andermann F., et al. The effects of topiramate on cognitive function in patients with epilepsy. Epilepsia. 2003;44:339.
Levav M., Mirsky A.F., Herault J., et al. Familial association of neuropsychological traits in patients with generalized and partial seizure disorders. J Clin Exp Neuropsychol. 2002;24:311-326.
Lewis M.A., Salas I., de la Sota A., et al. Randomized trial of a program to enhance the competencies of children with epilepsy. Epilepsia. 1990;31:101.
Li S., Cao J., Xiao N., et al. Efficacy and safety of levetiracetam as an add-on therapy in children aged less than 4 years with refractory epilepsy. J Child Neurol. 2009. Epub ahead of print
Long C.G., Moore J.R. Parental expectations for their epileptic children. J Child Psychol Psychiatry. 1979;20:299.
Lopez-Gongora M., Martinez-Domeno A., Barcia C., et al. Effect of levetiracetam on cognitive functions and quality of life: a one-year follow-up study. Epileptic Disord. 2008;10(4):297-305.
Loring D.W., Meador K.J. Cognitive side effects of antiepileptic drugs in children. Neurology. 2004;62:872.
Lothman D.J., Pianta R.C. Role of child-mother interaction in predicting competence of children with epilepsy. Epilepsia. 1993;34:658.
MacLeod J.S., Austin J.K. Stigma in the lives of adolescents with epilepsy: A review of the literature. Epilepsy Behav. 2003;4:112.
Martin R., Kuzniecky R., Ho S., et al. Cognitive effects of topiramate, gabapentin, and lamotrigine in healthy young adults. Neurology. 1999;52:321.
Matthews W.S., Barabas G., Ferrari M. Emotional concomitants of childhood epilepsy. Epilepsia. 1982;23:671.
McGuire A.M., Duncan J.S., Trimble M.R. Effects of vigabatrin on cognitive function and mood when used as add-on therapy in patients with intractable epilepsy. Epilepsia. 1992;33(1):128-134.
McKee P.J., Blacklaw J., Forrest G., et al. A double-blind, placebo-controlled interaction study between oxcarbazepine and carbamazepine, sodium valproate and phenytoin in epileptic patients. Br J Clin Pharmacol. 1994;37:27-32.
McNelis A.M., Dunn D.W., Johnson C.S., et al. Academic performance in children with new-onset seizures and asthma: A prospective study. Epilepsy Behav. 2007;10:311-318.
Meador K.J., Baker G.A. Behavioral and cognitive effects of lamotrigine. J Child Neurol. 1997;12(Suppl 1):S44.
Meador K.J., Loring D.W., Ray P.G., et al. Differential cognitive and behavioral effects of carbamazepine and gabapentin. Epilepsia. 1999;40:1279.
Meador K.J., Loring D.W., Ray P.G., et al. Differential cognitive and behavioral effects of carbamazepine and lamotrigine. Neurology. 2001;56:1177.
Mitchell W.G., Chavez J.M. Carbamazepine versus phenobarbital for partial onset seizures in children. Epilepsia. 1987;28:56.
Mitchell W.G., Chavez J.M., Lee H., et al. Academic underachievement in children with epilepsy. J Child Neurol. 1991;6:65.
Mitchell W.G., Scheier L.M., Baker S.A. Psychosocial, behavioral, and medical outcomes in children with epilepsy: A developmental risk factor model using longitudinal data. Pediatrics. 1994;94:471.
Mitchell W.G., Zhou Y., Chavez J.M., et al. Reaction time, attention, and impulsivity in epilepsy. Pediatr Neurol. 1992;8:19.
Mitchell W.G., Zhou Y., Chavez J.M., et al. Effects of antiepileptic drugs on reaction time, attention, and impulsivity in children. Pediatrics. 1993;91:101.
Monaco F. Cognitive effects of vigabatrin: a review. Neurology. 1996;47(S1):S6-S11.
Monaco F., Torta R., Cicolin A., et al. Lack of association between vigabatrin and impaired cognition. J Int Med Res. 1997;25(5):296-301.
Murphy C.C., Trevathan E., Yeargin-Allsopp M. Prevalence of epilepsy and epileptic seizures in 10-year-old children: Results from the Metropolitan Atlanta Developmental Disabilities Study. Epilepsia. 1995;36:866.
Northcott E., Connolly A.M., Berroya A., et al. The neuropsychological and language profile with benign Rolandic epilepsy. Epilepsia. 2005;46(6):924-930.
Oguz A., Kurul S., Dirik E. Relationship of epilepsy-related factors to anxiety and depression scores in epileptic children. J Child Neurol. 2002;17:37-40.
Olsson I., Campenhausen G. Social adjustment in young adults with absence epilepsies. Epilepsia. 1993;34:846.
Olsson I., Steffenburg S., Gillberg C. Epilepsy in autism and autistic-like conditions. Arch Neurol. 1988;45:666.
Oostrom K.J., Van Teeseling H., Smeets-Schouten A., et al. Three to four years after diagnosis: cognition and behaviour in children with ‘epilepsy only’: a prospective, controlled study. Brain. 2005;128:1546-1555.
Papazian O., Canizales E., Alfonso I., et al. Reversible dementia and apparent brain atrophy during valproate therapy. Ann Neurol. 1995;38:687.
Park S., Hwang Y., Lee H., et al. Long-term cognitive and mood effects of zonisamide monotherapy in epilepsy patients. Epilepsy Behav. 2008;12:102-108.
Partikian A., Mitchell W.G. Neurodevelopmental and Epilepsy Outcomes in a North American Cohort of Patients With Infantile Spasms. J Child Neurol. 2009. epub: 0883073809341664v1
Pavone P., Bianchini R., Trifiletti R.R., et al. Neuropsychological assessment in children with absence epilepsy. Neurology. 2001;56(8):1047-1051.
Perez E.R., Davidoff V., Despland P.A., et al. Mental and behavioral deterioration of children with epilepsy and CSWS: Acquired epileptic frontal syndrome. Dev Med Child Neurol. 1993;35:661.
Pianta R.C., Lothman D.J. Predicting behavior problems in children with epilepsy: Child factors, disease factors, family stress, and child-mother interaction. Child Dev. 1994;65:1415.
Piazzini A., Chifari R., Canevini M.P., et al. Levetiracetam: an improvement of attention and of oral fluency in patients with partial epilepsy. Epilepsy Res. 2006;68:181-188.
Piccirilli M., D’Alessandro P., Sciarma T., et al. Attention problems in epilepsy: Possible significance of the epileptogenic focus. Epilepsia. 1994;35:1091.
Plioplys S. Depression in children and adolescents with epilepsy. Epilepsy Behav. 2003;4(Suppl):39-45.
Pressler R.M., Binnie C.D., Coleshill S.G., et al. Effect of lamotrigine on cognition in children with epilepsy. Neurology. 2006;66:1495-1499.
Rantakallio P., Koiranen M., Motteonen J. Association of perinatal events, epilepsy, and central nervous system trauma with juvenile delinquency. Arch Dis Child. 1992;67:1459.
Riikonen R., Amnell G. Psychiatric disorders in children with earlier infantile spasms. Dev Med Child Neurol. 1981;23:747.
Riikonen R.S. Favourable prognostic factors with infantile spasms. Eur J Paediatr Neurol. 2009. Apr 10. [Epub ahead of print]
Riva D., Devoti M. Discontinuation of phenobarbital in children: Effects on neurocognitive behavior. Pediatr Neurol. 1996;14:36.
Ross E.M., Peckham C.S., West P.B., et al. Epilepsy in childhood: Findings from the National Child Development Study. BMJ. 1980;280:207.
Roulet E., Deonna T., Gaillard F., et al. Acquired aphasia, dementia, and behavior disorder with epilepsy and continuous spike and waves during sleep in a child. Epilepsia. 1991;32:495.
Roulet-Perez E., Davidoff V., Despland P.A., et al. Mental and behavioural deterioration of children with epilepsy and CSWS: Acquired epileptic frontal syndrome. Dev Med Child Neurol. 1993;35:661.
Rugino T.A., Samsock T.C. Levetiracetam in autistic children: An open-label study. J Dev Behav Pediatr. 2002;23:225.
Rutter M., Graham P., Yule W. A neuropsychiatric study in childhood. Philadelphia: JB Lippincott; 1970.
Sabers A., Moller A., Dam M., et al. Cognitive function and anticonvulsant therapy: Effect of monotherapy in epilepsy. Acta Neurol Scand. 1995;92:19.
Seidel W.T., Mitchell W.G. Cognitive and behavioral effects of carbamazepine in children: Data from benign rolandic epilepsy. J Child Neurol. 1999;14:716.
Seidenberg M., Beck N., Geisser M., et al. Academic achievement in children with epilepsy. Epilepsia. 1986;27:753.
Selassie G.R., Viggedal G., Olsson I., et al. Speech, language, and cognition in preschool children with epilepsy. Dev Med Child Neurol. 2008;50:432-438.
Shewmon D.A., Erwin R.J. Transient impairment of visual perception induced by single interictal occipital spikes. J Clin Exp Neuropsychol. 1989;11:675.
Sidenvall R., Forsgren L., Heijbel J. Prevalence and characteristics of epilepsy in children in Northern Sweden. Seizure. 1996;5:139.
Sillanpaa M. Children with epilepsy as adults: Outcome after 30 years of follow up. Acta Paediatr Scand. 1990;368(Suppl):1.
Sillanpaa M. Epilepsy in children: Prevalence, disability, and handicap. Epilepsia. 1992;33:444.
Sillanpaa M., Haataja L., Shinnar S. Perceived impact of childhood-onset epilepsy on quality of life as an adult. Epilepsia. 2004;45:971.
Sillanpaa M., Helenius H. Social competence of people with epilepsy: A new methodologic approach. Acta Neurol Scand. 1993;87:335.
Singhi P.D., Bansal U., Singhi S., et al. Determinants of IQ profile in children with idiopathic generalized epilepsy. Epilepsia. 1992;33:1106.
Steffenburg S., Gillberg C., Steffenburg U. Psychiatric disorders in children and adolescents with mental retardation and active epilepsy. Arch Neurol. 1996;53:904.
Steiner T.J., Dellaportas C.I., Findley L.J., et al. Lamotrigine monotherapy in newly diagnosed untreated epilepsy: A double-blind comparison with phenytoin. Epilepsia. 1999;40:601.
Stores G. School-children with epilepsy at risk for learning and behavior problems. Dev Med Child Neurol. 1978;20:502.
Stores G. Problems of learning and behavior in children with epilepsy. In: Reynolds E.H., Trimble M.J., editors. Epilepsy and psychiatry. Edinburgh: Churchill Livingstone, 1981.
Stores G., Hart J. Reading skills of children with generalized or focal epilepsy attending ordinary schools. Dev Med Child Neurol. 1976;18:705.
Stores G., Williams P.L., Styles E., et al. Psychological effects of sodium valproate and carbamazepine in epilepsy. Arch Dis Child. 1992;67:1330.
Strang J.D. Cognitive deficits in children: Adaptive behavior and treatment techniques. Epilepsia. 1990;31(Suppl 4):S54.
Sturniolo M.G., Galletti F. Idiopathic epilepsy and school achievement. Arch Dis Child. 1994;70:424.
Sussova J., Seidl Z., Farber J. Hemiparetic forms of cerebral palsy in relation to epilepsy and mental retardation. Dev Med Child Neurol. 1990;32:972.
Sveinbjornsdottir S., Sander J.W., Patsalos P.N., et al. Neuropsychological effects of tiagabine, a potential new antiepileptic drug. Seizure. 1994;3:29-35.
Tallian K.B., Nahata M.C., Lo W., et al. Gabapentin associated with aggressive behavior in pediatric patients with seizures. Epilepsia. 1996;37:501.
Tatum W.O., French J.A., Faught E. Postmarketing experience with topiramate and cognition. Epilepsia. 2001;42:1134.
Thome-Souza M.S., Kuczynski E., Valente K.D. Sertraline and fluoxetine: safe treatments for children and adolescents with epilepsy and depression. Epilepsy Behav. 2007;10:417-425.
Thompson P.J., Baxendale S.A., Duncan J.S., et al. Effects of topiramate on cognitive function. J Neurol Neurosurg Psychiatry. 2000;69:636-641.
Tosches W.A., Tisdell J. Long-term efficacy and safety of monotherapy and adjunctive therapy with zonisamide. Epilepsy Behav. 2006;8:522-526.
Tzitiridou M., Panou T., Ramantani G., et al. Oxcarbazepine monotherapy in benign childhood epilepsy with centrotemporal spikes: a clinical and cognitive evaluation. Epilepsy Behav. 2005;7:458-467.
van den Berg B.J., Yerushalmy J. Studies on convulsive disorders in young children. I. Incidence of febrile and nonfebrile convulsions by age and other factors. Pediatr Res. 1969;3:298.
Verrotti A., Parisi P., Lolacono G., et al. Levetiracetam monotherapy for childhood occipital epilepsy of gastaut. Acta Neurol Scand. 2009;120(5):342-346.
Weglage J., Demsky A., Pietsch M., et al. Neuropsychological, intellectual, and behavioral findings in patients with centrotemporal spikes with and without seizures. Dev Med Child Neurol. 1997;39:646.
Wellesley D.G., Hockey K.A., Montgomery P.D., et al. Prevalence of intellectual handicap in Western Australia. A community study. Med J Aust. 1992;156:94.
Westbrook L.E., Silver E.J., Coupey S.M., et al. Social characteristics of adolescents with idiopathic epilepsy: A comparison to chronically ill and nonchronically ill peers. J Epilepsy. 1991;4:87.
Wheless J.W., Ng Y.T. Levetiracetam in refractory pediatric epilepsy. J Child Neurol. 2002;17:413.
Wilfong A.A. Zonisamide monotherapy for epilepsy in children and young adults. Pediatr Neurol. 2005;32:77-80.
Wolf S.M., Carr A., Davis D.C., et al. The value of phenobarbital in the child who has had a single febrile seizure: A controlled prospective study. Pediatrics. 1977;59:378.
Wong V. Epilepsy in children with autistic spectrum disorder. J Child Neurol. 1993;8:316.


