Bedside Assessment of the Patient
After reading this chapter you will be able to:
 Describe why patient interviews are necessary and the appropriate techniques for conducting an interview.
Describe why patient interviews are necessary and the appropriate techniques for conducting an interview.
 Identify abnormalities in lung function associated with common pulmonary symptoms.
Identify abnormalities in lung function associated with common pulmonary symptoms.
 Identify breathing patterns associated with underlying pulmonary disease.
Identify breathing patterns associated with underlying pulmonary disease.
 Differentiate between dyspnea and breathlessness.
Differentiate between dyspnea and breathlessness.
 Identify terms used to describe normal and abnormal lung sounds.
Identify terms used to describe normal and abnormal lung sounds.
 Describe the mechanisms responsible for normal and abnormal lung sounds.
Describe the mechanisms responsible for normal and abnormal lung sounds.
 Explain why it is necessary to examine the precordium, abdomen, and extremities in patients with cardiopulmonary disease.
Explain why it is necessary to examine the precordium, abdomen, and extremities in patients with cardiopulmonary disease.
 Describe some common abnormalities found during the examination of the precordium, abdomen, and extremities in patients with cardiopulmonary disease.
Describe some common abnormalities found during the examination of the precordium, abdomen, and extremities in patients with cardiopulmonary disease.
Interviewing the Patient and Taking a Medical History
1. To establish a rapport between the clinician and patient
2. To obtain essential diagnostic information
3. To help monitor changes in the patient’s symptoms and response to therapy
For these reasons, interviewing is a crucial aspect of general patient assessment.
Principles of Interviewing
• Sensory and emotional factors
• Verbal and nonverbal components of the communication process
• Cultural and other internal values, beliefs, feelings, habits, and preoccupations of both the health care professional and the patient
Because of the above-listed factors, no two interviews are the same.
Structure and Technique for Interviewing
The ideal interview is one in which the patient feels secure and free to talk about important personal matters. Each interview should begin with the RT introducing himself or herself to the patient and stating the purpose of the visit. The introduction is done in the social space, approximately 4 to 12 feet from the patient. It begins the process of establishing a rapport with the patient and helps the patient feel more comfortable about answering personal questions. Pulling the curtain between the beds of a semiprivate room also may be helpful in making the patient feel more at ease with the interview (Box 15-1).
Using neutral questions and avoiding leading questions during the interview is important. Asking the patient, “Is your breathing better now?” leads the patient toward a desired response and may elicit false information. Asking the patient, “How is your breathing now?” is a better way to get accurate information about the patient’s breathing (Box 15-2).
Common Cardiopulmonary Symptoms
Dyspnea
1. The neural drive to breathe
2. The tension developed in the respiratory muscles
3. The corresponding displacement of the lungs and chest wall
Assessing Dyspnea in the Interview
1. The RT should ask what activities of daily living tend to trigger episodes of dyspnea. For example, is dyspnea triggered by walking on flat surfaces, by climbing stairs, by bathing, by dressing?
2. The RT should ask how much exertion is required for the patient to stop to catch his or her breath with different activities. Does the patient need to stop after walking up one flight of stairs or one step? Dyspnea provoked by less strenuous activities indicates more advanced disease.
3. The RT should ask whether the quality or the sensation of breathing discomfort varies with different activities.
4. To gain a better understanding of the patient’s history, the RT should ask the patient to recall when dyspnea first began and how it has evolved over time. Has dyspnea progressed slowly or rapidly? How long has this progression taken place: over a period of months or years? Has there been a dramatic change in the intensity of dyspnea over recent months, weeks, days, or even within the past few hours?
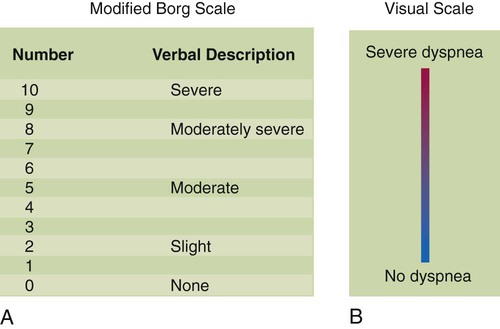
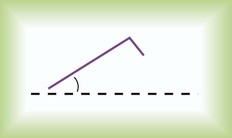
The intensity of dyspnea can be documented using a numeric intensity or visual analog scale (Figure 15-1). Such scales provide a way to evaluate the patient’s response to treatment over time. These scales are important because objective lung function measurements (e.g., pulmonary function tests, PaO2) seldom correlate with the degree of dyspnea in many patients.
Cough
Important characteristics of the patient’s cough to identify include whether it is dry or loose, productive or nonproductive, and acute or chronic and whether it occurs more frequently at particular times (i.e., day or night). Knowledge of such details may help in determining the cause of the cough. A dry, nonproductive cough is typical for restrictive lung diseases such as CHF or pulmonary fibrosis. A loose, productive cough is more often associated with inflammatory obstructive diseases such as bronchitis and asthma. The most common cause of an acute, self-limited cough is a viral infection of the upper airway. Common causes of chronic coughing include asthma, postnasal drip, chronic bronchitis, and gastroesophageal reflux,1 although combinations of these often exist.2 Cough is also associated with the use of certain medications for hypertension (e.g., angiotensin-converting enzyme inhibitors).3
Fever
It was believed for many years that there was a link between fever and atelectasis in postoperative surgical patients. However, more recent evidence has shown no link between the formation of atelectasis and the development of fever (>101.3° F [>38.5° C]) during the first 72 hours after surgery.4
Format for the Medical History
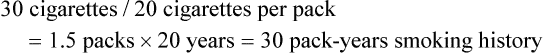
If the patient states that he or she has smoked 15 cigarettes per day for 20 years:
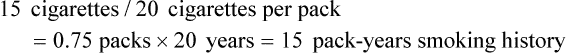
The review of systems is designed to uncover problem areas the patient forgot to mention or omitted. This information is usually obtained in a head-to-toe review of all body systems. For each body system, the interviewer obtains information about current, pertinent symptoms. During a review of the respiratory system, questioning would determine the presence or history of cough, hemoptysis, sputum production, chest pain, shortness of breath, and fever (Box 15-3).
Physical Examination
General Appearance
The first few seconds of an encounter with the patient usually helps reveal the severity of the current problem. For an experienced clinician, these initial impressions determine the course of subsequent assessment. If the patient’s general appearance indicates an acute problem, the rest of the examination may be abbreviated and focused until the patient’s condition is stabilized. If the initial impressions indicate that the patient is stable and not in immediate danger, a more complete assessment can be conducted (Box 15-4). Several indicators are important in assessing the patient’s overall appearance, including the patient’s level of consciousness (see later), facial expression, level of anxiety or distress, positioning, and personal hygiene.
Level of Consciousness
While observing the patient’s overall appearance, the RT should assess the patient’s level of consciousness (alertness). Evaluating the patient’s alertness is a simple but important task. If the patient appears conscious, the RT should assess the patient’s orientation to time, place, person, and situation. This assessment often is called evaluating the sensorium. An alert patient who can correctly tell the interviewer the current date, location, his or her name, and his or her situation (e.g., “I’m in the hospital because I fell and broke my hip”) is said to be “oriented × 4,” and the patient’s sensorium is considered normal. If the patient is not alert, the level of consciousness is assessed. The simple rating scale shown in Box 15-5 allows clinicians to describe the patient’s level of consciousness objectively, using common clinical terms.
Depressed consciousness may occur with poor cerebral blood flow (e.g., hypotension) or when poorly oxygenated blood perfuses the brain. As cerebral oxygenation acutely decreases, the patient initially becomes restless, confused, or disoriented. If hypoxia worsens, the patient may become comatose. However, patients with chronic hypoxia may adapt well and may have normal mental status despite significant hypoxemia. Abnormal consciousness also may occur in chronic degenerative brain disorders, as a side effect of certain medications, and in cases of drug overdose. Additional information on the evaluation of neurologic function is presented in Chapter 46 (see Glasgow Coma Scale score).
Vital Signs
Pulse Rate
The peripheral pulse is evaluated for rate, rhythm, and strength (Box 15-6). The normal adult pulse rate is 60 to 100 beats/min, with a regular rhythm. A condition in which the pulse rate exceeds 100 beats/min is called tachycardia. Common causes of tachycardia are exercise, fear, anxiety, low blood pressure, anemia, fever, reduced arterial blood O2 levels, and certain medications. A condition in which the pulse rate is less than 60 beats/min is called bradycardia. Bradycardia is less common than tachycardia but can occur with hypothermia, as a side effect of medications, with certain cardiac arrhythmias, and with traumatic brain injury.
The radial artery is the most common site used to palpate the pulse. The second and third fingertip pads (but not the thumb) are used to palpate the radial pulse. Ideally, the pulse rate is counted for 1 minute, especially if the pulse is irregular. Essential pulse characteristics that should be noted and documented are described in Box 15-6.
Spontaneous ventilation can influence pulse strength, or amplitude. Normally, a slight decrease in pulse pressure is present with each inspiratory effort. This decrease is caused by negative intrathoracic pressure from respiratory muscle contraction during inspiration. The decrease in blood pressure is the result of decreased left ventricular filling from two mechanisms. First, negative intrathoracic pressure pools blood in the pulmonary circulation, which impedes left heart filling. Second, it simultaneously increases venous return (which increases right ventricular volume and pressure) and limits expansion of the left heart during diastole. This mechanism briefly reduces left ventricular stroke volume and decreases systolic blood pressure during inspiration. The slight decrease in pulse pressure (normally <10 mm Hg) with inspiration may not be noticeable with palpation. A significant decrease in pulse strength (>10 mm Hg) during spontaneous inhalation is called pulsus paradoxus, or paradoxical pulse. Pulsus paradoxus can be quantified with a blood pressure cuff (see later section) and is common in patients with acute obstructive pulmonary disease, especially patients experiencing an asthma attack. During respiratory distress, vigorous inspiratory efforts decrease stroke volume by impeding the strength of left ventricular contraction.5 Pulsus paradoxus also may signal a mechanical restriction of the pumping action of the heart, as can occur with constrictive pericarditis or cardiac tamponade.
Blood Pressure
Blood pressure is determined by the interaction of the force of left ventricular contraction, the systemic vascular resistance, and the blood volume (see Chapter 9). The blood pressure is recorded by listing systolic pressure over diastolic pressure (e.g., 120/80 mm Hg).
Hypertension is defined as arterial blood pressure persistently greater than 140/90 mm Hg. Hypertension is a common medical problem in adults, and in approximately 90% of cases the cause is unknown (primary hypertension). There are two subcategories of hypertension.6 Stage I hypertension occurs when the systolic blood pressure is 140 to 159 mm Hg or the diastolic blood pressure is 90 to 99 mm Hg. Stage II hypertension occurs when the systolic blood pressure is 160 mm Hg or greater or the diastolic blood pressure is 100 mm Hg or greater. In addition, there is a third category known as prehypertension, which is a systolic blood pressure between 120 mm Hg and 139 mm Hg or a diastolic blood pressure between 80 mm Hg and 89 mm Hg. This last category is not a disease state and does not require treatment but rather is used to assess the risk of eventually developing hypertension.
Hypotension is defined as a systolic arterial blood pressure less than 90 mm Hg or a mean arterial pressure less than 65 mm Hg.7 Hypotension also can be defined as a decrease of more than 40 mm Hg from baseline. This expanded definition acknowledges that patients with baseline hypertension may have inadequate tissue perfusion at a blood pressure that may be considered normal for most patients.
Shock is defined precisely as the inadequate delivery of O2 and nutrients to the vital organs relative to their metabolic demand.7 Hypotension is not synonymous with shock. In shock, vital body organs are in imminent danger of receiving inadequate blood flow (underperfusion) and impaired O2 delivery to the tissues (i.e., tissue hypoxia). For this reason, shock is usually treated aggressively with fluids, blood products, or vasoactive drugs, or a combination of these, depending on the cause and severity of shock.
There are two broad categories of hypotension and shock based on whether they are caused by a hypodynamic or hyperdynamic cardiovascular state.8 Hypodynamic states includes left ventricular failure (cardiogenic) and reduced blood volume (hypovolemia or hypovolemic) caused by either hemorrhage or severe fluid loss. Hyperdynamic states occur with profound systemic vasodilation (peripheral vascular failure) associated with overwhelming infection (septic shock), systemic allergic reaction (anaphylaxis), or severe liver failure.
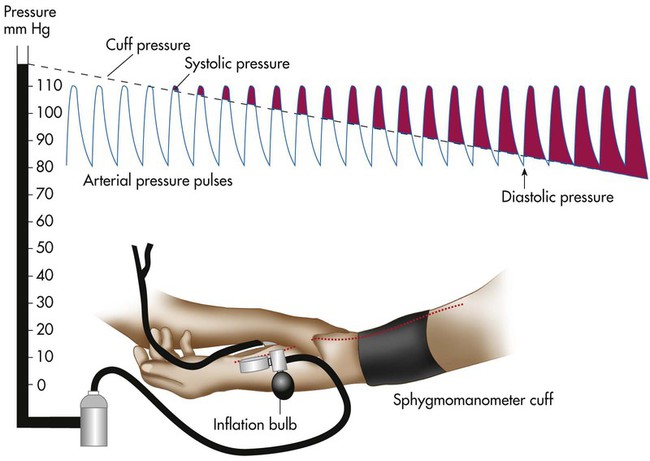
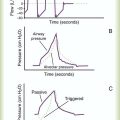
A common technique for measuring arterial blood pressure requires a blood pressure cuff (sphygmomanometer) and a stethoscope (Figure 15-2). When the cuff is applied to the upper arm and pressurized to exceed systolic blood pressure, the brachial artery blood flow stops. As the cuff pressure is slowly released to a point just below the systolic pressure, blood flows intermittently past the obstruction. Partial obstruction of the blood flow creates turbulence and vibrations called Korotkoff sounds. Korotkoff sounds are heard with a stethoscope over the brachial artery distal to the cuff.
The systolic blood pressure usually decreases slightly with normal inhalation. However, a decrease in systolic pressure of more than 6 to 8 mm Hg during a resting inhalation is abnormal and is called paradoxical pulse, or pulsus paradoxus (see Chapter 9). Although simple palpation may be adequate to signal the presence of paradoxical pulse, it can be quantified only by auscultatory measurement. To obtain this measurement, the clinician inflates the cuff until the radial or brachial pulse can no longer be palpated. The clinician slowly deflates the cuff until sounds are heard on exhalation only (point 1). Next, the clinician reduces the cuff pressure until sounds are heard throughout respiration (point 2). The difference between points 1 and 2 indicates the degree of paradoxical pulse.
Examination of the Thorax and Lungs
Inspection
Thoracic Configuration
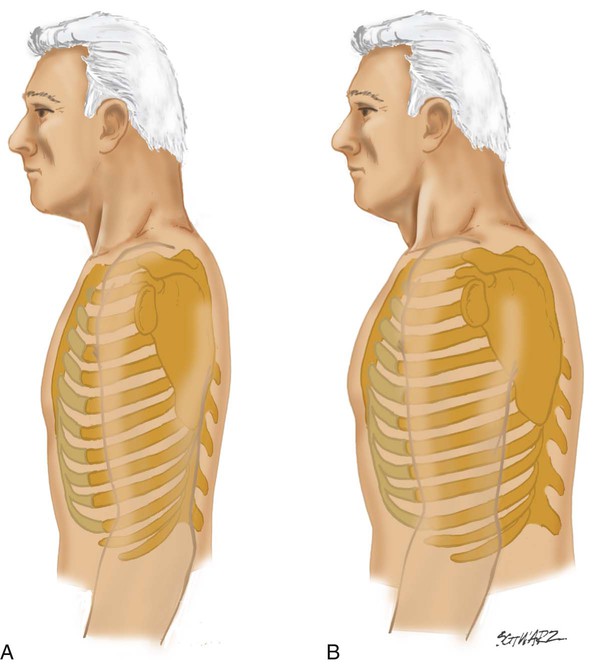
The anteroposterior (AP) diameter of the average adult thorax is less than the transverse diameter. Normally, the AP diameter increases gradually with age but may prematurely increase in patients with COPD. This abnormal increase in AP diameter is called barrel chest and is associated with emphysema. When the AP diameter increases, the normal 45-degree angle of articulation between the ribs and spine is increased, becoming more horizontal (Figure 15-3). Other abnormalities of the thoracic configuration are listed in Table 15-1.
TABLE 15-1
Abnormalities of Thoracic Configuration
| Name | Condition |
| Pectus carinatum | Abnormal protrusion of sternum |
| Pectus excavatum | Depression of part or entire sternum, which can produce a restrictive lung defect |
| Kyphosis | Spinal deformity in which the spine has an abnormal AP curvature |
| Scoliosis | Spinal deformity in which the spine has a lateral curvature |
| Kyphoscoliosis | Combination of kyphosis and scoliosis, which may produce a severe restrictive lung defect as a result of poor lung expansion |
Breathing Pattern and Effort
At rest, a healthy adult has a consistent rate and rhythm of breathing. Breathing effort is minimal on inhalation and passive on exhalation. Abnormal breathing patterns can be broken down into two broad categories. First are breathing patterns directly associated with thoracic or pulmonary diseases that increase work of breathing. Second are patterns primarily associated with neurologic disease (see Chapter 14). Table 15-2 describes common abnormal patterns of breathing.
TABLE 15-2
| Breathing Pattern | Characteristics | Causes |
| Apnea | No breathing | Cardiac arrest, narcotic overdose, severe brain trauma |
| Apneustic breathing | Deep, gasping inspiration with brief, partial expiration | Damage to upper medulla or pons caused by stroke or trauma; sometimes observed with hypoglycemic coma or profound hypoxemia |
| Ataxic breathing | Completely irregular breathing pattern with variable periods of apnea | Damage to medulla |
| Asthmatic breathing | Prolonged exhalation with recruitment of abdominal muscles | Obstruction to airflow out of the lungs |
| Biot’s respiration | Clustering of rapid, shallow breaths coupled with regular or irregular periods of apnea | Damage to medulla or pons caused by stroke or trauma; severe intracranial hypertension |
| Cheyne-Stokes respiration | Irregular type of breathing; breaths increase and decrease in depth and rate with periods of apnea; variant of “periodic breathing” | Most often caused by severe damage to bilateral cerebral hemispheres and basal ganglia (usually infarction); also seen in patients with CHF owing to increased circulation time and in various forms of encephalopathy |
| Kussmaul breathing | Deep and fast respirations | Metabolic acidosis |
| Paradoxical breathing | Abdominal paradox: Abdominal wall moves inward on inspiration and outward on expiration | Abdominal paradox: Diaphragmatic fatigue or paralysis |
| Chest paradox: Part or all of the chest wall moves in with inhalation and out with exhalation | Chest paradox: Typically observed in chest trauma with multiple rib or sternal fractures | |
| Also found in patients with high spinal cord injury with paralysis of intercostal muscles | ||
| Periodic breathing | Breathing oscillates between periods of rapid, deep breathing and slow, shallow breathing without periods of apnea | Same causes as Cheyne-Stokes respiration |
Diaphragmatic fatigue is found in many types of chronic and acute pulmonary diseases. When it occurs acutely, diaphragmatic fatigue often manifests with distinctive breathing patterns.9 The first sign of acute diaphragmatic fatigue is tachypnea. Sometimes tachypnea is followed by a breathing pattern in which the diaphragm and rib cage muscles alternately power breathing in an attempt to give each muscle group some rest (respiratory alternans). This pattern is noted by the upward motion of the diaphragm during inspiration on a series of breaths, followed by diaphragmatic contractions and inward movement of the abdominal wall on the following series of breaths. When the diaphragm is relaxed, contraction of the rib cage muscles sucks the diaphragm upward and the abdomen inward during inspiration. The opposite phenomenon occurs on breaths when the diaphragm is active. When the rib cage muscles are relaxed, the chest wall may appear to sink in as the abdomen protrudes during diaphragmatic contraction; this often gives the impression that the chest has a rocking motion. Finally, abdominal paradox occurs with complete diaphragmatic fatigue, as the diaphragm is drawn upward into the thoracic cavity with each inspiratory effort of the rib cage muscles. An abdominal paradox also occurs when the diaphragm is paralyzed.
These patterns are not always associated with impending muscle fatigue. Rather, they may be adaptations to high workloads when the respiratory muscle strength is normal.10 Also, patients with respiratory distress often have tachypnea, along with recruitment of the expiratory muscles. This situation can make it difficult to discern accurately the presence and type of abnormal breathing pattern. The RT must be careful about offering definitive therapeutic suggestions (e.g., absolute need for mechanical ventilation) when he or she perceives the presence of these abnormal breathing patterns.
Palpation
Thoracic Expansion
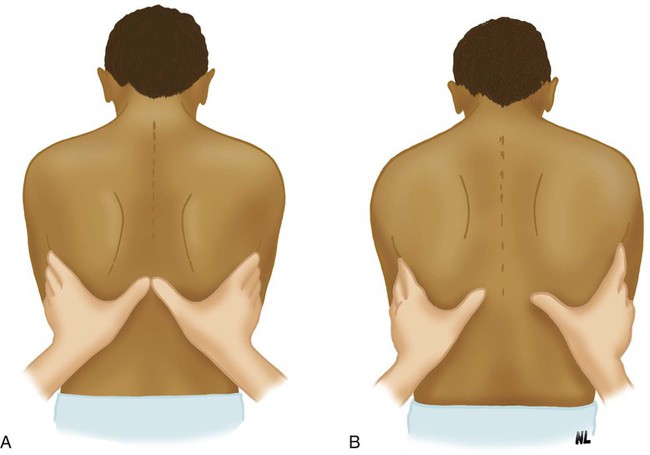
The normal chest wall expands symmetrically during deep inhalation. This expansion can be evaluated on the anterior and posterior chest. To evaluate expansion anteriorly, the RT places his or her hands over the anterolateral chest, with the thumbs extended along the costal margin toward the xiphoid process. To evaluate posteriorly, the RT positions the hands over the posterolateral chest with the thumbs meeting at the T8 vertebra (Figure 15-4). The patient is instructed to exhale slowly and completely. When the patient has exhaled maximally, the RT gently secures his or her fingertips against the sides of the patient’s chest and extends the thumbs toward the midline until the tip of each thumb meets at the midline. The RT next instructs the patient to take a full, deep breath and notes the distance the tip of each of the thumbs moves from midline. Normally, each thumb moves an equal distance of approximately 3 to 5 cm.
Auscultation of the Lungs
Stethoscope
A stethoscope has the following four basic parts: (1) a bell, (2) a diaphragm, (3) tubing, and (4) earpieces (Figure 15-5). The bell detects a broad spectrum of sounds and is very useful for listening to low-pitched sounds (e.g., heart sounds). Proper technique for listening to heart sounds is to place the bell lightly against the chest; this avoids stretching the skin, which makes auscultating heart sounds more difficult by inadvertently filtering out low-frequency sounds. Using the bell to auscultate the lungs is also helpful when emaciation causes rib protrusion that restricts placement of the diaphragm flat against the chest.
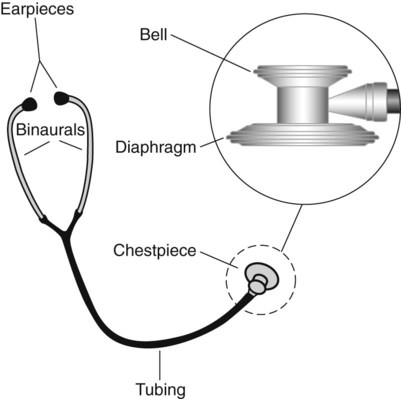
The clinician should examine his or her stethoscope regularly for cracks in the diaphragm, wax or dirt in the earpieces, and other defects that may interfere with the transmission of sound. The stethoscope should always be cleaned with hospital-approved disinfectant after every patient contact to minimize contamination with microorganisms.11,12 Patients who are placed in contact isolation and patients who are in protective isolation because of immunosuppression should have a dedicated stethoscope in the room to prevent cross infection.
Technique
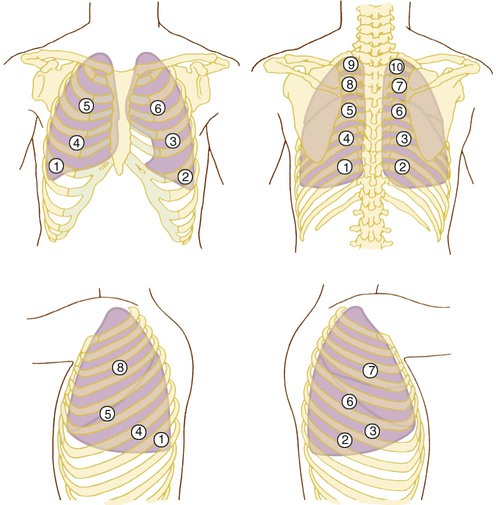
Auscultation of the lungs should be systematic and include all lobes on the anterior, lateral, and posterior chest. Auscultation should begin at the lung bases with comparison of breath sounds side to side, working upward toward the lung apexes (Figure 15-6). It is important to begin at the bases because certain abnormal sounds that occur only in the lower lobes may be altered by several deep breaths. At least one full ventilatory cycle should be evaluated at each stethoscope position. If abnormal sounds are present, the clinician should listen to several breaths to clarify the characteristics.
The clinician should listen for and distinguish among the key features of breath sounds. The clinician should identify the pitch (vibration frequency), the intensity (loudness), and the duration of the inspiratory and expiration components of the sound. The acoustic characteristics of breath sounds can be illustrated in breath sound diagrams (Figure 15-7). The features of normal breath sounds are described in Table 15-3. One must be familiar with normal breath sounds before one can expect to identify the subtle changes that may signify respiratory disease.
TABLE 15-3
Characteristics of Normal Breath Sounds
| Breath Sound | Pitch | Intensity | Location | Diagram |
| Vesicular | Low | Soft | Peripheral lung areas |  |
| Bronchovesicular | Moderate | Moderate | Around upper part of sternum, between the scapulae |  |
| Tracheal | High | Loud | Over the trachea |  |

Terminology
When auscultating over the lung parenchyma of a healthy individual, soft, muffled sounds are heard. These normal breath sounds, referred to as vesicular breath sounds, are lower in pitch and intensity than bronchovesicular breath sounds. Vesicular sounds are heard primarily during inhalation, with an exhalation component approximately one-third the duration of inhalation (see Table 15-3).
Mechanisms and Significance of Lung Sounds
Crackles
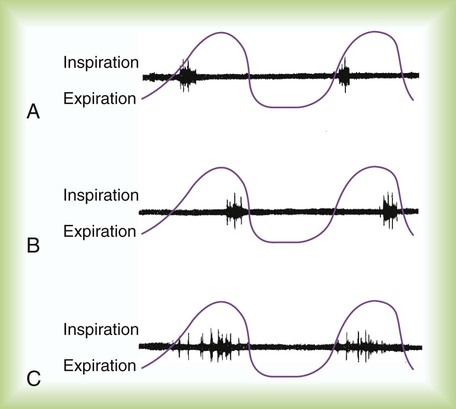
Larger, more proximal bronchi may close during expiration when there is an abnormal increase in bronchial compliance or when the retractile pressures around the bronchi are low. In this situation, crackles usually occur early in the inspiratory phase and are referred to as early inspiratory crackles (Figure 15-8). Early inspiratory crackles are usually scant but may be loud or faint. They often are transmitted to the mouth and are not silenced by a cough or a change in position. They frequently occur in patients with COPD (chronic bronchitis, emphysema, or asthma) and usually indicate severe airway obstruction.
Peripheral airways may close during exhalation when the surrounding intrathoracic pressure increases and when surfactant levels are diminished. Fine, late inspiratory crackles are produced by the sudden opening of peripheral airways, usually late in the inspiratory phase. They are more common in the dependent lung regions, where the peripheral airways are most prone to collapse during exhalation. They may clear with changes in posture or if the patient performs several deep inspirations. Late inspiratory crackles are most common in patients with respiratory disorders that reduce gas volume of the lung, such as atelectasis, pneumonia, pulmonary edema, and pulmonary fibrosis (Table 15-4).
TABLE 15-4
Application of Adventitious Lung Sounds
| Lung Sound | Possible Mechanism | Characteristics | Causes |
| Wheezes | Rapid airflow through obstructed airways | High-pitched, usually expiratory | Asthma, CHF |
| Stridor | Rapid airflow through obstructed upper airway | High-pitched, monophonic | Croup, epiglottitis, postextubation laryngeal edema |
| Coarse crackles | Excess airway secretions moving through airways | Coarse, inspiratory and expiratory | Severe pneumonia, bronchitis |
| Fine crackles | Sudden opening of peripheral airways | Fine, late inspiratory | Atelectasis, fibrosis, pulmonary edema |
Cardiac Examination
Inspection and Palpation
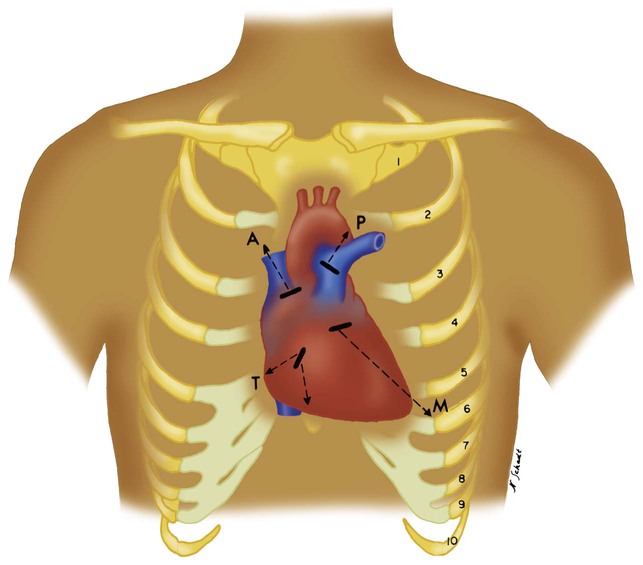
The second left intercostal space near the sternal border is referred to as the pulmonic area and is palpated to identify accentuated pulmonary valve closure. Strong vibrations may be felt in this area with the presence of pulmonary hypertension or valvular abnormalities (Figure 15-9). Rapid blood flow through a narrowed valve or backflow through an incompetent valve may produce palpable vibrations known as thrills. Thrills are usually accompanied by a murmur (see discussion later).
Auscultation of Heart Sounds
Normal heart sounds are created by closure of the heart valves (see Chapter 9). The first heart sound (S1) is produced by closure of the mitral and tricuspid (atrioventricular [AV]) valves during contraction of the ventricles. When systole ends, the ventricles relax, and the pulmonic and aortic (semilunar) valves close, creating the second heart sound (S2). Because pressures in the left side of the heart are higher, mitral valve closure is louder and contributes more to S1. For the same reason, closure of the aortic valve usually is more significant in producing S2. If either the AV valves or the semilunar valves do not close together, a split heart sound is heard. A slight splitting of S2 is normal; it occurs because of increased venous return during spontaneous breathing.
Abnormal Heart Sounds
Pulmonary hypertension may cause two abnormalities in heart sounds. First, it increases the intensity of S2 from a more forceful closure of the pulmonic valve (this is also referred to as a loud P2). Second, S2 splitting may be absent. An increased P2 is identified best over the pulmonic area of the chest (see Figure 15-9).
Abdominal Examination
The abdomen should be inspected and palpated for evidence of distention and tenderness. Abdominal distention and pain impair diaphragmatic movement and may contribute to or cause respiratory insufficiency. Abdominal dysfunction may inhibit deep breathing and coughing and promote atelectasis. Of particular concern is the presence of intraabdominal hypertension, which is defined as intraabdominal pressure greater than 12 mm Hg and is found in 18% of critically ill patients.13 Abdominal compartment syndrome occurs when intraabdominal pressures are greater than 20 mm Hg and often requires emergency decompressive surgery. This syndrome causes profound atelectasis and hypoxemia, hypotension, and renal failure.
Examination of the Extremities
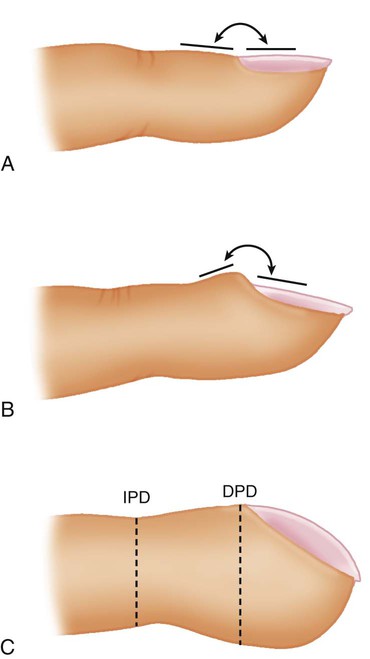
Clubbing
Clubbing of the digits is a significant manifestation of cardiopulmonary disease. Clubbing is a painless enlargement of the terminal phalanges of the fingers and toes that develops over time. As the process advances, the angle of the fingernail to the nail base increases, and the base of the nail feels “spongy.” The profile view of the digits allows easier recognition of clubbing (Figure 15-10), but sponginess of the nail bed is the most important sign. Causes of clubbing include infiltrative or interstitial lung disease, bronchiectasis, various cancers (particularly lung cancer),14 congenital heart problems that cause cyanosis, chronic liver disease, and inflammatory bowel disease. COPD alone, even when hypoxemia is present, does not lead to clubbing. Clubbing of the digits in a patient with COPD indicates that something other than obstructive lung disease is occurring.