Basic Airway Management and Decision Making
Basic airway procedures are often overlooked in favor of more exciting intubation devices and techniques, but basic procedures are critically important and often lifesaving. Establishment of a patent airway, oxygenation, and bag-mask ventilation (BMV) remain the cornerstones of good emergency airway management.1,2 These techniques can be used quickly and in any setting. They allow practitioners to keep apneic patients alive until a definitive airway can be established.3
Extraglottic devices, such as laryngeal mask airways (LMAs) and the King Laryngeal Tube (LT), have also become important for the initial resuscitation of apneic patients and for rescue ventilation when intubation fails.4–6 Another commonly used device is the esophageal-tracheal Combitube, which will be discussed and compared with the King LT. Noninvasive positive pressure ventilation (NPPV) is widely available in both prehospital and emergency department (ED) settings and can be used to optimize oxygenation before intubation or to avoid intubation in carefully selected patients.7,8
Pulse oximetry (Spo2) has greatly improved our ability to monitor the oxygenation of patients at risk for airway or ventilatory compromise.9 These monitors are accurate under most conditions10 and allow clinically subtle deterioration to be recognized quickly (see Chapter 2). Spo2 monitors are standard equipment in all emergency airway settings. The use of waveform capnography in the emergency setting is rapidly increasing but is not yet universally available or applied. This trend should be encouraged because capnography can improve patient safety by rapidly detecting hypoventilation, impending airway obstruction, and risk for apnea before these conditions occur.11
The Challenge of Emergency Airway Management
Although other specialists are sometimes available, most emergency airways are managed by emergency medicine providers.12 Airway management in the ED is quite unique and much different from airway management in the controlled setting of an operating room. Likewise, conventional airway management tools may be ineffective in the uncontrolled emergency environment. Major challenges include an incomplete historical database, hypoxia, shock, full stomach, and the presence of vomit, blood, or excessive secretions in the airway. Many patients are uncooperative and combative, thus making it impossible to properly examine their airway before choosing an intubation technique. Medical history, allergies, and even the current diagnosis are often unknown before emergency airway management begins. Time constraints, lack of patient cooperation, and risk for vomiting limit the use of some techniques, such as awake intubation. In trauma patients, the risk for cervical spine injury limits optimal head and neck positioning for BMV and laryngoscopy. All these factors increase the risk for complications from emergency airway management,12,13 and about 1% of all emergency airways require a surgical approach.14 The popularity of video laryngoscopy and other video airway devices may further reduce the incidence of emergency surgical airways.
Basic Airway Management Techniques
The first concern in the management of a critically ill patient is patency of the airway. Upper airway obstruction most commonly occurs when patients are unconscious or sedated. It can also be due to injury to the mandible or muscles that support the hypopharynx. In these situations, the tongue moves posteriorly into the upper airway when the patient is in a supine position (Fig. 3-1A). Upper airway obstruction caused by the tongue can be relieved by positioning maneuvers of the head, neck, and jaw; the use of nasopharyngeal or oropharyngeal airways; or the application of continuous positive airway pressure (CPAP).
Manual Airway Maneuvers
Airway obstruction in unconscious patients may be due to posterior displacement of the tongue, but research in patients with obstructive sleep apnea using CPAP supports the concept that the airway collapses like a flexible tube.3,15 Upper airway obstruction may cause obvious snoring or stridor, but it may be difficult to appreciate in some patients. All unconscious patients are at high risk for upper airway obstruction.
More than 35 years ago, Guildner16 compared different techniques for opening obstructed upper airways and found that the head-tilt/chin-lift and jaw-thrust techniques were both effective (Fig. 3-1B and C). Modern airway textbooks still describe the head-tilt/chin-lift and jaw-thrust maneuvers but also use the term “triple airway maneuver,” which is a combination of head tilt, jaw thrust, and mouth opening.3,17 Many airway experts believe that the jaw-thrust maneuver (anterior mandibular translation to bring the lower incisors anterior to the upper incisors) is the most important technique for opening the upper airway (Fig. 3-1C).3,18,19
It is widely accepted that the jaw-thrust-only (without head tilt) maneuver should be performed in patients with suspected cervical spine injury,17 but there is no evidence that it is safer than the head-tilt/chin-lift maneuver.20 In 2005, the American Heart Association (AHA)21 concluded that airway maneuvers are safe during manual in-line stabilization of the cervical spine but highlighted evidence that all airway maneuvers cause some spinal movement. Both the chin-lift and the jaw-thrust maneuvers have been shown to cause similar substantial movement of the cervical vertebrae.22–26 The AHA recommended that “in a victim with a suspected spinal injury and an obstructed airway, the head-tilt/chin-lift or jaw-thrust (with head-tilt) techniques are feasible and may be effective for clearing the airway” and emphasized that “maintaining an airway and adequate ventilation is the over-riding priority in managing a patient with a suspected spinal injury.”21
Importantly, the addition of CPAP may relieve airway obstruction when simple manual positioning maneuvers fail. Meier and colleagues15 showed that adding CPAP to the chin-lift and jaw-thrust maneuvers decreased stridor and improved the nasal fiberoptic view of the glottic opening in anesthetized children.
The Head-Tilt/Chin-Lift Maneuver
To perform the head-tilt/chin-lift maneuver, place the tips of the index and middle fingers beneath the patient’s chin (Fig. 3-1B). Lift the chin cephalad and toward the ceiling. The upper part of the neck will naturally extend when the head tilts backward during this maneuver. Apply digital pressure on only the bony prominence of the chin and not on the soft tissues of the submandibular region. The final step in this maneuver is to use the thumb to open the patient’s mouth while the head is tilted and the neck is extended.
The Jaw-Thrust Maneuver
To perform the jaw-thrust maneuver, place the tips of the middle or index fingers behind the angle of the mandible (Fig. 3-1C). Lift the mandible toward the ceiling until the lower incisors are anterior to the upper incisors. This maneuver can be performed in combination with the head-tilt/chin-lift maneuver or with the neck in the neutral position during in-line stabilization.
The Triple Airway Maneuver
The “triple airway maneuver” is described by many authors as the best manual method for maintaining a patent upper airway.3,17 The most common description of this maneuver is head tilt, jaw thrust, and mouth opening.3,17 Other authors describe the triple maneuver differently—as a combination of upper cervical extension (head tilt), lower cervical flexion, and jaw protrusion (jaw lift).19 The triple airway maneuver has been described as a technique for providers with advanced airway skills.17 No studies exist to support the assertion that this technique is more effective than the head-tilt/chin-lift or jaw-thrust maneuvers, but the triple maneuver is commonly mentioned in the anesthesia literature as a valuable technique.
Patient Positioning
The best way to position a patient’s head and neck for opening the upper airway is to mimic how patients position themselves when they are short of breath, with the neck flexed relative to the torso and with atlanto-occipital extension.2 This is known as the “sniffing position” and was described by Magill almost 100 years ago.27 In normal-sized supine adults, this is accomplished by elevating the head about 10 cm while tilting the head back so that the plane of the patient’s face tilts slightly toward the provider at the head of the bed (see Chapter 4, Fig. 4-8).2,3,28–30 Morbidly obese patients require much more head elevation to achieve the proper sniffing position. This can be accomplished by building a ramp of towels and pillows under the upper torso, head, and neck or by using a Troop Elevation Pillow (Mercury Medical, Clearwater, FL) or similar device (Fig. 3-2).31–34 Horizontal alignment of the external auditory meatus with the sternum is the best position for opening the upper airway in morbidly obese patients.33–36
The sniffing position is contraindicated in patients with cervical spine injuries. The best technique for opening the airway in this situation is a simple jaw-thrust maneuver with anterior mandibular translation to bring the lower incisors anterior to the upper incisors (Fig. 3-1C).3,18,19
Airway management is usually easiest when patients are in the supine position, but the lateral position may be best for patients who are actively vomiting and those with excessive upper airway bleeding or secretions. Some evidence suggests that rotating patients to the lateral position may not prevent aspiration.37 Patients with suspected cervical spine injury should have their head immobilized with in-line stabilization if they need to be rolled to the lateral position. Airway management maneuvers may be limited or difficult when patients are in the lateral position.
Foreign Body Airway Obstruction
Abdominal Thrusts (Heimlich Maneuver), Chest Thrusts, and Back Blows (Slaps)
The 2010 International Consensus Conference on Cardiopulmonary Resuscitation and Emergency Cardiopulmonary Care4 evaluated the evidence for different techniques to clear foreign body airway obstruction. They found good evidence for the use of chest thrusts, abdominal thrusts, and back blows or slaps. Insufficient evidence exists to determine which technique is the best and which should be used first. Some evidence indicates that chest thrusts may generate higher peak airway pressure than the Heimlich maneuver does.
The technique of subdiaphragmatic abdominal thrusts to relieve a completely obstructed airway was popularized by Dr. Henry Heimlich and is commonly referred to as the “Heimlich maneuver.”38 The technique is most effective when a solid food bolus is obstructing the larynx. In a conscious patient, stand behind the upright patient. Circle the arms around the patient’s midsection with the radial side of a clenched fist placed on the abdomen, midway between the umbilicus and xiphoid. Then grasp the fist with the opposite hand and deliver an inward and upward thrust to the abdomen (Fig. 3-3A). A successful maneuver will cause the obstructing agent to be expelled from the patient’s airway by the force of air exiting the lungs. Abdominal thrusts are relatively contraindicated in pregnant patients and those with protuberant abdomens. Potential risks associated with abdominal thrusts include stomach rupture, esophageal perforation, and mesenteric laceration, thus compelling the rescuer to weigh the risks and benefits of this maneuver.39–44 Use a chest position for pregnant patients (Fig. 3-3B).

Figure 3-3 A-E, Heimlich maneuvers (see text).
If a choking patient loses consciousness, use chest compressions in an attempt to expel the obstructing agent (Fig. 3-3C).4 The theory is the same as the Heimlich maneuver, with high intrathoracic pressure created to push the obstruction out of the airway. Some data suggest that chest compressions may generate higher peak airway pressure than the Heimlich maneuver.45 After 30 seconds of chest compressions, remove the obstructing object if you see it, attempt 2 breaths, and then continue cardiopulmonary resuscitation (CPR; 30 compressions to 2 breaths). Every time you open the airway to give breaths, look for the object and remove it if possible, and then continue CPR if necessary.
Back blows are recommended for infants and small children with a foreign body obstructing the airway. Some authors have argued that back blows may be dangerous and may drive foreign bodies deeper into the airway, but there is no convincing evidence of this phenomenon.46,47 As with the other techniques, anecdotal evidence suggests that back blows are effective.48–50 No convincing data, however, indicate that back blows are more or less effective than abdominal or chest thrusts. Back blows may produce a more pronounced increase in airway pressure, but over a shorter period than with the other techniques. The AHA guidelines suggest back blows in the head-down position (Fig. 3-3D) and head-down chest thrusts in infants and small children with foreign body airway obstruction (Fig. 3-3E).4 The AHA does not recommend abdominal thrusts in infants because they may be at higher risk for iatrogenic injury. From a practical standpoint, back blows should be delivered with the patient in a head-down position, which is more easily accomplished in infants than in larger children.
Any patient with a complete airway obstruction may benefit from chest compressions, abdominal thrusts, or back blows. It is important to realize that more than one technique is often required to clear obstruction of the airway by a foreign body, so multiple techniques should be applied in a rapid sequence until the obstruction is relieved.21 Perform a finger sweep of the patient’s mouth only if a solid object is seen in the airway. It is recommended that suction be performed on newborns rather than giving them back blows or abdominal thrusts.51
Suctioning
A large-bore dental-type tip device, such as the HI-D Big Stick suction tip, should be readily available at the bedside during all emergency airway management (Fig. 3-4). The large-bore tip allows rapid clearing of vomitus, blood, and secretions.
A limiting feature of many suction catheters is the diameter of the tubing. Vomitus may obstruct the standard 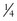 -inch-diameter catheter.52 A
-inch-diameter catheter.52 A 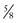 – or
– or 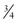 -inch-diameter suction tube (Kuriyama Tubing,
-inch-diameter suction tube (Kuriyama Tubing, 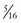 -inch inner diameter, 0.44-inch outer diameter, clear; www.grainger.com) has been shown to significantly decrease suction time for viscous and particulate material (see Fig. 3-4).53
-inch inner diameter, 0.44-inch outer diameter, clear; www.grainger.com) has been shown to significantly decrease suction time for viscous and particulate material (see Fig. 3-4).53
Keep suctioning equipment connected and ready to operate. Everyone participating in emergency airway management should know how to use it. Interposition of a suction trap close to the suction device prevents clogging of the tubing with particulate debris. A trap that fits directly onto a tracheal tube has been described, and use of this device allows effective suctioning during intubation.54
Avoid prolonged suctioning because it may lead to significant hypoxia, especially in children. Do not exceed 15 seconds for suctioning intervals and administer supplemental O2 before and after suctioning. Naigow and Powasner55 found that suctioning consistently induced hypoxia in dogs and that it was best avoided by hyperventilation with high-concentration O2 before and after suctioning.
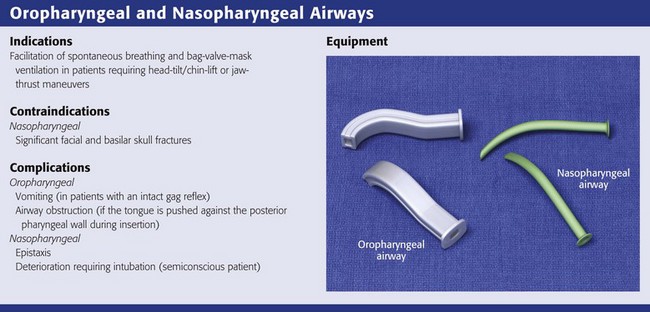
Oropharyngeal and Nasopharyngeal Artificial Airways
Artificial Airway Placement
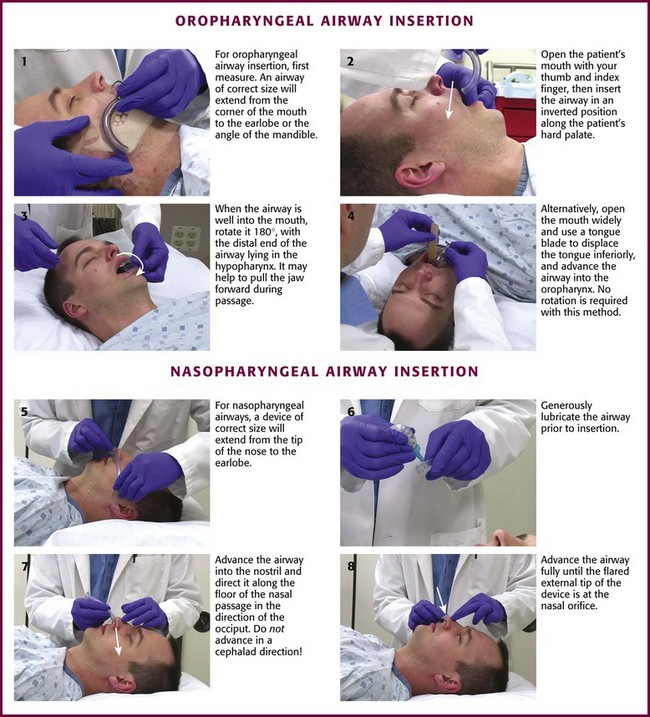
The simplest and most widely available artificial airways are the oropharyngeal and nasopharyngeal airways (Fig. 3-5). Both are intended to prevent the tongue from obstructing the airway by falling back against the posterior pharyngeal wall. The oral airway may also prevent teeth clenching. In cases of severe upper airway edema, such as angioedema caused by an angiotensin-converting enzyme inhibitor, these devices may not function properly or be able to adequately bypass the obstruction. The oropharyngeal airway may be inserted by either of two procedures. One approach is to insert the airway in an inverted position along the patient’s hard palate (Fig. 3-5, step 2). When it is well into the patient’s mouth, rotate the airway 180 degrees and advance it to its final position along the patient’s tongue, with the distal end of the artificial airway lying in the hypopharynx (Fig. 3-5, step 3). A second approach is to open the mouth widely, use a tongue blade to displace the tongue, and then simply advance the artificial airway into the oropharynx (Fig. 3-5, step 4). No rotation is necessary when the airway is placed in this manner. This technique may be less traumatic, but it takes longer.
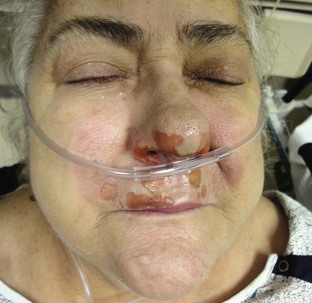
The nasopharyngeal airway is very easy to place. It may be easiest to place it on the patient’s right so that the bevel is facing the septum on insertion. Be sure to lubricate the device before insertion (Fig. 3-5, step 6). Some clinicians use a nasopharyngeal airway to dilate the nasal passages for 20 to 30 minutes before nasotracheal intubation. Simply advance it into the nostril and direct it along the floor of the nasal passage in the direction of the occiput, not cephalad (Fig. 3-5, step 7). Advance it fully until the flared external tip of the airway is located at the nasal orifice (Fig 3-5, step 8).
Both oropharyngeal and nasopharyngeal airways are available in multiple sizes. To find the correct size of either device, estimate its size by measuring along the side of the patient’s face before insertion. An oropharyngeal airway of the correct size will extend from the corner of the mouth to the tip of the earlobe (Fig. 3-5, step 1); a nasopharyngeal airway of the correct size will extend from the tip of the nose to the tip of the earlobe (Fig. 3-5, step 5).
Oxygen Therapy
Indications and Contraindications
Resuscitate all patients in cardiac or respiratory arrest with 100% O2. The most certain indication for supplemental O2 is the presence of arterial hypoxemia, defined as a Pao2 lower than 60 mm Hg or arterial oxygen saturation (Sao2) less than 90%.56 Normal subjects will begin to experience memory loss at an arterial oxygen partial pressure (Pao2) of 45 mm Hg, and loss of consciousness occurs at a Pao2 of 30 mm Hg.57–59 Chronically hypoxemic patients can adapt and function with a Pao2 of 50 mm Hg or lower.60
When tissue hypoxia is present or suspected, give O2 therapy.56,61 Shock states resulting from hemorrhage, vasodilatory states, low cardiac output, and obstructive lesions can all lead to tissue hypoxia and benefit from supplemental O2. Whatever the cause of the shock state, administration of O2 is indicated until the situation can be thoroughly evaluated and cause-specific therapy instituted.
Respiratory distress without documented arterial hypoxemia is a common indication for O2 administration, although no evidence exists to support this practice.62 Oxygen therapy is often recommended for acute myocardial infarction, but there is no difference in outcomes between patients receiving O2 and those receiving room air after myocardial infarction. The AHA has given a class I recommendation for O2 only in patients with hypoxemia, cyanosis, or respiratory distress.56,61,63–65
Although O2 is routinely administered to acute stroke patients, there is no convincing evidence that this practice is beneficial without documented hypoxia, and it is not recommended by current guidelines.66–68 It is reasonable to administer O2 to hypotensive patients and those with severe trauma until tissue hypoxia can definitively be excluded.62
Administer 100% O2 to patients with carbon monoxide poisoning. The half-life of carboxyhemoglobin is 4 to 5 hours in a subject breathing room air but can be decreased to approximately 1 hour by the administration of 100% O2 by non-rebreather face mask at atmospheric pressure.69
There are no contraindications to O2 therapy when a definite indication exists. The risks associated with hypoxemia are grave and undeniable. Never withhold oxygen therapy from a hypoxemic patient for fear of complications or clinical deterioration. Carbon dioxide retention is not a contraindication to O2 therapy. Rather, it demands that the clinician administer O2 carefully and recognize the potential for respiratory acidosis and clinical deterioration. Although the mechanism for the development of respiratory acidosis in patients with chronic obstructive pulmonary disease (COPD) who are administered O2 is debated, its occurrence is not.70,71 Use caution when administering supplemental O2 to hypoxic patients with arterial carbon dioxide pressure (Paco2) higher than 40 mm Hg, but do not withhold it.
Oxygen Administration during Cardiac Arrest and Neonatal Resuscitation
The 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care4 address the potential harm of oxygen therapy and hyperoxemia following cardiac arrest and during neonatal resuscitation. Excerpts from this document are shown in Box 3-1. Although it is still prudent to administer oxygen in the prehospital and ED setting as discussed earlier, additional research may alter current recommendations. See also “Complications of Oxygen Therapy” in this chapter. As a general guideline, fear of oxygen toxicity should not prevent the use of O2 when there is an indication but should encourage the clinician to use the minimum concentration of O2 necessary to achieve the therapeutic goals.
Oxygen Delivery Devices
High-flow delivery systems provide an Fio2 that is relatively constant despite changes in the patient’s respiratory pattern. The Venturi mask is the high-flow delivery device that is most widely available (Fig. 3-6). Room air is entrained into the system through entrainment ports and mixes with the O2 provided from the O2 source. The proportion of entrained air—and therefore Fio2—is constant and determined by the velocity of the O2 jet and the size of the entrainment ports. Because the total gas flow (O2 plus air through the entrainment ports) meets or exceeds the patient’s inspiratory flow rate, no additional entrainment of air occurs around the mask, thereby minimizing changes in Fio2 as the patient’s respiratory pattern changes.72,73 The mask is continuously flushed by the high flow of gas, which prevents the accumulation of exhaled gas in the mask. Venturi masks are packaged with multiple inserts, each with a different size orifice for O2 inflow. Fio2 is determined by selecting the appropriate colored insert and O2 flow rate according to the manufacturer’s instructions. The inspiratory flow rate for a resting adult is about 30 L/min, a rate matched by the total gas flow provided by the Venturi mask at all settings. A patient in respiratory distress may have an inspiratory flow rate of 50 to 100 L/min.73 If the inspiratory flow rate exceeds the total gas flow delivered by the mask, additional air will be entrained around the mask, and Fio2 will decrease. Masks with higher Fio2 ratings entrain less outside air and therefore provide less total flow. Caution should be used with masks rated above 35% in patients with respiratory distress because Fio2 may be significantly reduced with high inspiratory flow rates.
Low-flow delivery devices provide gas flow that is less than the patient’s inspiratory flow rate. The difference between the patient’s inspiratory flow and the flow delivered by the device is met by a variable amount of room air being drawn into the system. Patients with normal respiratory rates and tidal volumes will require less outside air than those in respiratory distress, who typically receive a higher Fio2. As a patient’s inspiratory flow changes, so will the Fio2 that they receive from a low-flow device.72,73
The prongs of a nasal cannula deliver a constant flow of O2 that accumulates in the nasopharynx and provides a reservoir of oxygen-enriched air for inspiration. The Fio2 delivered by nasal cannulas is determined by many factors, including the respiratory rate, tidal volume, pharyngeal geometry, and O2 flow. Most importantly, at a constant O2 flow rate, Fio2 varies inversely with the respiratory rate. Despite this limitation, nasal cannulas are very comfortable for patients and are the most common low-flow O2 delivery device. Although it may seem intuitive, patients using a nasal cannula should be reminded that they should not smoke while oxygen is being delivered (Fig. 3-7).
Procedure
An initial Fio2 of 24% to 28% delivered by Venturi mask is indicated for patients with hypoxemia and chronic respiratory acidosis.62,70
Frequent clinical assessment and Spo2 monitoring are needed in all patients receiving O2 therapy. Periodic blood gas analysis or capnography is imperative for those at risk for respiratory acidosis.74–76 Equilibration of Sao2 after changes in supplemental O2 occurs within 5 minutes.77
Fio2 should be titrated to achieve therapeutic goals while minimizing the risk for complications. An Sao2 of 90% to 95% (Pao2 ≈ 60 to 80 mm Hg) is an appropriate target for most patients receiving supplemental O2.62 Increases above these levels do not add appreciably to the O2 content of blood and are unlikely to confer an additional benefit. One may exceed these parameters in patients with shock and end-organ dysfunction, but the added risk and small potential benefit should be considered on an individual basis. In patients with COPD-associated hypercapnia, an Sao2 of 90% (Pao2 ≈ 60 mm Hg) should be the goal of O2 therapy.74–76 Mechanical ventilation should be considered when oxygenation goals cannot be achieved without progressive respiratory acidosis.
Preoxygenation for Rapid-Sequence Intubation
Preoxygenation is one of the most important aspects of emergency airway management. Preoxygenation before rapid-sequence intubation (RSI) provides much more time for intubation before desaturation occurs and thus significantly increases the chance of successful intubation on the first attempt. Failure to preoxygenate before RSI is often a critical factor when a straightforward emergency airway becomes an airway disaster. Preoxygenation is usually accomplished by providing the maximal Fio2 with a non-rebreather mask for 3 to 5 minutes before intubation. Alternatively, eight vital capacity breaths from a maximal Fio2 system, such as a non-rebreather mask or a bag-valve-mask device, is acceptable when there is no time for standard preoxygenation.78 When using a bag-valve-mask device for preoxygenation, it is important that the exhalation port have a one-way valve so that room air is not drawn into the mask when the patient inhales.
The purpose of preoxygenation is not just to maximize oxygen saturation but to wash out nitrogen from the patient’s lungs and replace it with oxygen. This provides the maximum safe apneic time during RSI. Those at greatest risk for rapid desaturation include obese, pregnant, critically ill, and pediatric patients, and they will benefit the most from good preoxygenation. Most studies addressing preoxygenation have been conducted under ideal conditions in relatively healthy individuals. It is far more challenging to effectively preoxygenate critically ill patients.79
Morbidly obese patients are best preoxygenated in a 25-degree head-up position because significantly higher oxygen tension can be achieved in this position.80 Patients who are hypoxic despite maximal oxygen delivery have been shown to benefit from 3 minutes of NPPV (with 100% O2) just before intubation.81,82
Sometimes patients who need preoxygenation the most are uncooperative with a face mask because of delirium from hypoxia, hypercapnia, or other factors. These patients may benefit from delayed-sequence intubation—careful sedation without blunting of respirations to allow oxygenation with a face mask or NPPV for 2 to 3 minutes before administering a paralytic agent.82 Ketamine (1 to 1.5 mg/kg by slow intravenous push) has been suggested for this technique. Delayed-sequence intubation is a practical way to deal with a difficult problem, but it has not been well studied, and providers using this method should be prepared for clinical deterioration, hypoventilation, or apnea.
Oxygen Therapy during Apnea
Another method to delay desaturation during RSI is nasopharyngeal oxygen insufflation during apnea. Many studies have shown that providing oxygen therapy during apnea is much more beneficial than one might imagine.83–86 During apnea, oxygen continues to be absorbed through the alveoli, and air with a low oxygen concentration is left in the lower airways. Supplying oxygen to the nasopharynx during apnea allows air with a higher oxygen concentration to passively replenish oxygen in the alveoli. Multiple studies, in normal and morbidly obese patients, have shown that nasopharyngeal oxygen insufflation results in a significant delay in desaturation after the onset of apnea.83,86,87 In these studies, nasopharyngeal oxygen insufflation was accomplished by inserting a 10-Fr catheter into the nasopharynx to a distance equal to the distance from the mouth to the tragus of the ear and delivering oxygen at a flow rate of 5 L/min when apnea occurred.83 Using a standard nasal cannula with a nasopharyngeal airway is simpler and would probably provide the same benefit. Also, it is important to keep the upper airway open by using a jaw thrust or artificial airway for this technique to be most beneficial.
Nasal High-Flow Oxygen
High-flow nasal oxygen is a relatively new concept that may have some utility for optimizing oxygenation in critically ill children and adults.88 At lower flow rates, Fio2 is somewhat dependent on the patient’s respiratory rate, but at very high flow rates, Fio2 is consistently very high. In one study, the Fio2 delivered by nasal cannula ranged from about 25% at 1 L/min to 70% to 80% at 15 L/min.89 Commercially available humidified high-flow nasal cannula (HHFNC) systems use flow rates of 5 to 40 L/min and deliver an Fio2 of close to 100%. High-flow oxygen by nasal cannula is not well tolerated unless it is humidified, so commercially available systems (Vapotherm 2000i, Fisher and Paykel Nasal High Flow, AquinOx) deliver oxygen with nearly 100% humidity. HHFNC devices are popular in neonatal and pediatric intensive care units and are commonly used for respiratory support after extubation and for management of respiratory disease.90,91 Some evidence suggests that high-flow nasal oxygen may provide low-grade CPAP.92,93 Several adult studies have shown that high-flow nasal oxygen systems are well tolerated and deliver a higher Fio2 than a high-flow mask in patients with respiratory failure or simulated respiratory failure.94–96
Complications of Oxygen Therapy
Worsening of CO2 retention leading to progressive respiratory acidosis and obtundation in patients with COPD is the complication most likely to be seen in the ED. This phenomenon is well documented and was first described by Barach in 1937.97 It has been attributed to several mechanisms, including loss of hypoxic respiratory drive, ventilation-perfusion (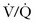 ) mismatch, and decreased hemoglobin affinity for CO2 (Haldane effect). This avoidable complication is best prevented by administering O2 to chronic CO2 retainers only when there is an indication, administering it at the smallest effective dose, and carefully monitoring clinical, capnographic, and arterial blood gas parameters.
) mismatch, and decreased hemoglobin affinity for CO2 (Haldane effect). This avoidable complication is best prevented by administering O2 to chronic CO2 retainers only when there is an indication, administering it at the smallest effective dose, and carefully monitoring clinical, capnographic, and arterial blood gas parameters.
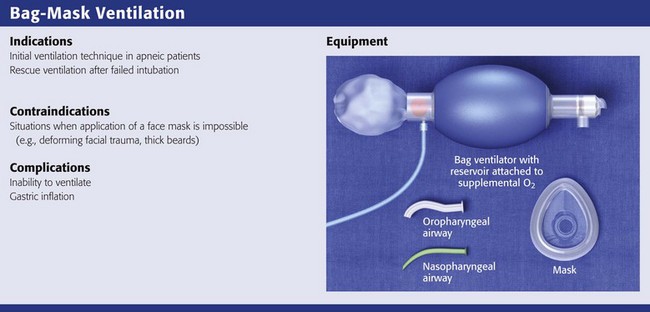
Bag-Mask Ventilation
BMV is the single most important technique for emergency airway management.1,19,98 Bag-mask devices are widely available and are standard equipment in all patient care settings. Although the bag-mask method of ventilation appears to be simple, it can be difficult to perform correctly. Having good BMV skills is a prerequisite to more advanced methods of emergency airway management.1 Manually opening the airway, properly positioning the head and neck, placing an oropharyngeal airway device, and achieving a tight face mask seal are the keys to good BMV.
Indications and Contraindications
BMV is the most common initial technique for ventilation of apneic patients and for rescue ventilation after failed intubation.1,2 Many authors note that BMV is relatively contraindicated in patients with a full stomach, those in cardiac arrest, and those undergoing RSI.2,3 These patients have a high risk for stomach inflation and subsequent aspiration. Unfortunately, these are the patients for whom ED providers most commonly use BMV. In ED situations, the need for ventilation and oxygenation always takes priority over potential aspiration.2
Bag-Mask Ventilation Technique
Achieving adequate ventilation with a bag-mask device requires an open upper airway and a good mask seal. Overly aggressive BMV causes stomach inflation and increases the risk for aspiration. The goal is to achieve adequate gas exchange while keeping peak airway pressure low. Squeezing the bag forcefully creates high peak airway pressure and is more likely to inflate the stomach. Several studies have shown that increased tidal volume is associated with higher peak airway pressure and increased gastric inflation.66,98–101 Data also show that decreased inspiratory time increases peak airway pressure and gastric inflation.102,103 Therefore, the best method of BMV is to provide a tidal volume of about 500 mL delivered over a period of 1 to 1.5 seconds.103 Using a ventilator (instead of a resuscitation bag) to provide the proper tidal volume and inspiratory time is a novel alternative to using a bag-valve device.82 Effective ventilation and oxygenation should be judged by chest rise, breath sounds, Spo2, and capnography.
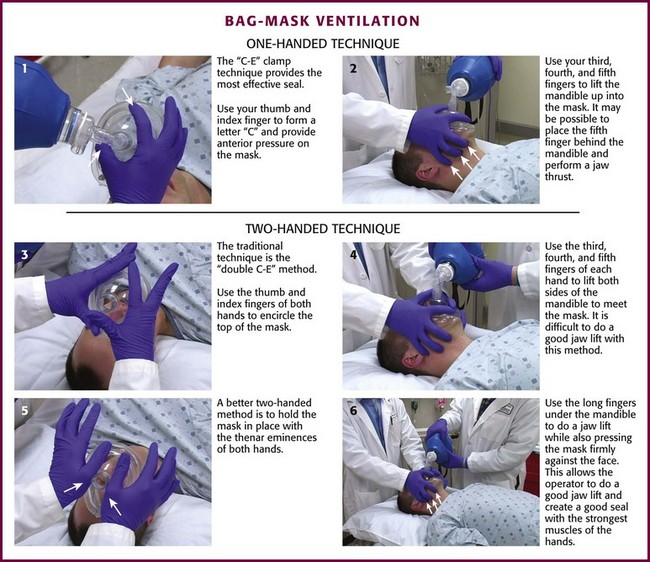
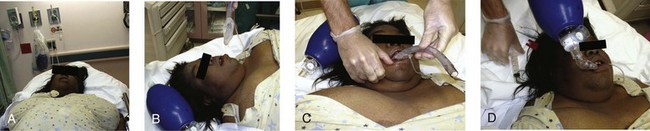
For a single rescuer, only one hand can be used to achieve the seal because the other must squeeze the bag. The rescuer must apply pressure anteriorly while simultaneously lifting the jaw forward. The thumb and index finger provide anterior pressure while the fifth and fourth fingers lift the jaw. The C-E clamp technique is often the most effective: the thumb and index finger form a “C” to provide anterior pressure over the mask, whereas the third, fourth, and fifth fingers form an “E” to lift the jaw (Fig. 3-8, steps 1 and 2). Generally, well-fitting intact dentures should be left in place to help ensure a better seal with the mask.
There are two different methods for two-handed face mask control. The traditional technique is the double C-E method, where the thumb and index finger of both hands encircle the top of the mask (Fig. 3-8, step 3) and the third, fourth, and fifth fingers of both hands form an “E” to lift both sides of the mandible to meet the mask (Fig. 3-8, step 4). The problem with the double C-E technique is that it is difficult to perform a good jaw lift with the hands in this position. Having an assistant lend a third or fourth hand to help lift the jaw is an option, but this can be awkward unless the assistant is a very experienced provider. A better two-handed method is to hold the mask in place with the thenar eminence of both hands (Fig. 3-8, step 5) and use the long fingers under the angle of the mandible to perform a jaw lift while also pressing the mask firmly against the face (Fig. 3-8, step 6). This technique allows the operator to perform a good jaw lift (create mandibular protrusion or an “underbite”) and create a good mask seal with the strongest muscles of the hands.104,105 This method is best for patients with difficult mask ventilation, and it also allows inexperienced providers and those with small hands to do a better job with face mask ventilation.105 In addition, it is important to remember to use oropharyngeal or nasopharyngeal airways (or both) whenever face mask ventilation is difficult.
All bag-mask devices should be attached to a supplemental O2 source (with a flow rate of 15 L/min) to avoid hypoxia. A significant problem with the bag-mask method is the low percentage of O2 achieved with some reservoirs. The amount of O2 delivered is dependent on the ventilatory rate, the volumes delivered during each breath, the O2 flow rate into the ventilating bag, the filling time for reservoir bags, and the type of reservoir used. A 2500-mL bag reservoir and a demand valve are preferred for O2 supplementation during BMV.106
Pediatric bag-mask devices should have a minimum volume of 450 mL. Pediatric and larger bags may be used to ventilate infants with the proper mask size, but care must be maintained to administer only the volume necessary to effectively ventilate the infant. Avoid pop-off valves because airway pressure under emergency conditions may often exceed the pressure of the valve.107
BMV may be the best method of prehospital airway support in trauma patients and children. Murray and coworkers108 performed a large retrospective study suggesting that patients with severe head injury had a higher risk for mortality if they were intubated in the prehospital setting. In the same year, Gausche and associates109 reported that neurologic outcomes and ultimate survival rates after prehospital pediatric resuscitation with BMV by emergency medical service (EMS) providers were as good as those with tracheal intubation.
Complications
The main complications of the bag-mask technique are an inability to ventilate and gastric inflation. Langeron and colleagues110 performed a large prospective study of adults undergoing general anesthesia and reported a 5% incidence of difficult mask ventilation. The incidence is obviously much higher in the emergency setting. Risk factors for difficult BMV include presence of a beard, obesity, lack of teeth, age older than 55 years, history of snoring, short thyromental distance, and limited mandibular protrusion (Box 3-2).110–113 Patients who are not spontaneously breathing but awake enough to interfere with the procedure can also be difficult to bag-mask ventilate. A recent study showed that most patients with difficult BMV became easier to ventilate after paralytic agents were administered and none were more difficult to ventilate after paralytics.114
Cricoid Pressure: Sellick’s Maneuver
In 1961, B.A. Sellick described the use of cricoid pressure to prevent regurgitation during anesthesia, and this technique has since become known as Sellick’s maneuver.115 The purpose of this technique is to apply external force to the anterior cricoid ring to push the trachea posteriorly and compress the esophagus against the cervical vertebrae. In theory, cricoid pressure compresses the distensible upper esophagus but not the airway because the cricoid ring is fairly rigid.
There are no good data that Sellick’s maneuver prevents regurgitation,116,117 but there are data that cricoid pressure prevents gastric inflation during BMV,115,116,118–121 thereby reducing the risk for subsequent regurgitation and vomiting. Several studies have confirmed that Sellick’s maneuver reduces tidal volumes, increases peak inspiratory pressure, and prevents good air exchange when applied during BMV.118,120–129 Sellick’s maneuver also decreases successful insertion of and intubation through LMAs.130–137
BMV can produce gastric inflation, especially if high volume and high pressure are used. To avoid gastric inflation it is best to ventilate with a small volume (500 mL or 6 to 8 mL/kg) and avoid high peak pressure by using a long inspiratory time (1 second). Applying Sellick’s maneuver during BMV may further decrease the risk for gastric inflation and is still recommended by most airway experts.138,139 It should be noted that the routine use of cricoid pressure during BMV of patients in cardiac arrest is not recommended in the 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care.6 It is recommended that cricoid pressure be released immediately if there is any difficulty ventilating with a face mask in an emergency setting.117 In addition, it is reasonable to release or relax cricoid pressure during insertion of an LMA and if ventilation with the LMA is difficult.116,117,136 It may be reasonable to release cricoid pressure during laryngoscopy and tracheal intubation, and this is discussed in Chapter 4.
Some authors believe that improper technique is to blame for the many reported failures of Sellick’s maneuver.116 The proper technique for applying Sellick’s maneuver is to place the thumb and middle finger on either side of the cricoid cartilage with the index finger in the center anteriorly.115 Apply 30 N (6.7 lb) of force to the cricoid cartilage in the posterior direction.116,139 As a reference, about 40 N of digital force on the bridge of the nose will usually cause pain.116
Extraglottic Airway Devices
LMAs
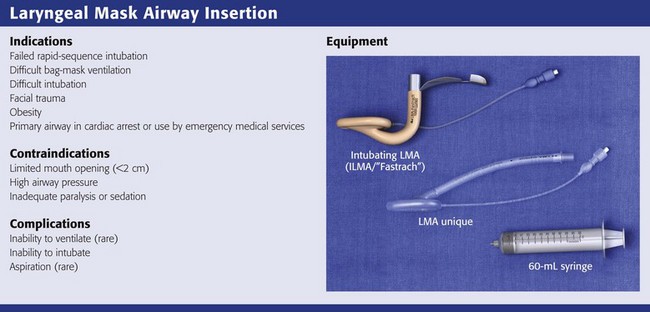
Review Box 3-3 Laryngeal mask airways: indications, contraindications, complications, and equipment.
The first LMA, the LMA Classic (Laryngeal Mask Company, Singapore; www.lmaco.com) became available in 1988. Since then it has been used more than 200 million times and has been described in more than 2500 academic papers.136,140 LMAs are widely considered to be essential adjuncts for rescue ventilation and difficult intubation.141,142 LMAs are primary rescue adjuncts in the difficult airway guidelines put forth by the American Society of Anesthesiologists141 and the Difficult Airway Society in Europe.142 Advanced Cardiac Life Support guidelines suggest that the LMA provides a more secure and reliable means of ventilation than a face mask does.4,6 Pediatric Advanced Life Support guidelines acknowledge the LMA as a potential backup device for difficult pediatric airways.143,144 Several manufacturers now make LMAs since the patent on the LMA Classic expired in 2003. It is important to recognize that all LMAs are not the same and that some of the newer devices have not been well studied.
Either an intubating LMA (ILMA) or a nonintubating LMA can be used in the “cannot-intubate/cannot-ventilate” scenario when face mask ventilation is difficult because of a beard, massive facial trauma, or obesity. Both devices can be inserted in less than 30 seconds and provide effective ventilation in more than 98% of patients.136 In the emergency setting, where obtaining a definitive airway (i.e., tracheal intubation) is the eventual goal, it is more practical to use an ILMA. In addition, the LMA Fastrach (Laryngeal Mask Company, Singapore; www.lmaco.com), the most widely used and well-studied ILMA, is easier to insert than the LMA Classic.145–149 Finally, when the head is in the neutral position, the LMA Fastrach is more likely to allow successful ventilation than the LMA Classic during in-line stabilization of the cervical spine.150–152
The LMA Supreme (Laryngeal Mask Company, Singapore; www.lmaco.com) is the latest offering from the Laryngeal Mask Company; it has a new mask shape that may allow a better mask seal and a gastric evacuation tube. It features a firm curved shape and a bite block handle, so ease of insertion may be similar to that of the Fastrach, and it is available in all sizes from neonate to large adult.153–162 Drawbacks are that it has not been extensively studied and does not facilitate tracheal intubation.
The Cookgas air-Q (Mercury Medical, Clearwater, FL), i-gel (Intersurgical, Berkshire, UK), and Ambu Aura-I (Ambu, Glen Burnie, MD) are promising new LMAs that can facilitate tracheal intubation and are available in pediatric sizes. The air-Q features a large-bore flexible airway tube and an inflatable mask that is stiffer than other LMAs. A few studies in adults and children show that it provides adequate ventilation in nearly all patients, but it has not been extensively tested.163–170 i-gel features a thermoplastic elastomer cuff that does not need inflation, so it may be easier to insert than other LMAs, but it has not been extensively tested.160,171–177 The Ambu Aura-I has some attractive features, but it is very new and almost completely untested.
This chapter describes how to insert the LMA Fastrach and use it for rescue ventilation. Details about intubation with the Fastrach are described in Chapter 4. Use of the LMA Unique (the disposable version of the LMA Classic) and LMA Supreme will also be described because they are cheap, disposable, well tested, and widely available.
Anatomy and Physiology
All the LMA devices consist of an airway tube attached to an oval mask, rimmed by an inflatable cuff. The cuffed mask is designed to form a seal around the glottis when the device is placed properly. The ILMA has a handle that allows the operator to increase seal pressure by lifting the entire device toward the ceiling, like lifting a frying pan (see Review Box 3-3). This is called “Chandy’s” maneuver (see Fig. 3-9, steps 7 and 8). The tip of the LMA sits in the proximal esophagus when it is placed properly. It is common for the epiglottis to become down-folded by the tip of the LMA device on insertion. When using the LMA Fastrach, epiglottic down-folding can be corrected with the “up-down maneuver” (see Fig. 3-9, steps 9 and 10).
Pathophysiology
The cannot-intubate/cannot-ventilate scenario is one reason for using an LMA in the ED. In this situation, failure to adequately ventilate and oxygenate with the LMA occurs in about 6% of cases. Another 6% of patients with difficult airways suffer episodes of hypoxia during attempts to intubate through the LMA.136 There is evidence that the ILMA performs better in the cannot-intubate/cannot-ventilate situation.136 Failure to ventilate with the ILMA occurs in only about 2% of cases, and hypoxia after ILMA placement is very rare. In addition, for difficult airway management, there are more technical difficulties with the LMA than with the ILMA. This is probably due to the fact that the LMA requires more skill for proper insertion and was not specifically designed to facilitate tracheal intubation.
Indications
The LMA Fastrach is indicated as an alternative to BMV or as a conduit for intubation of difficult airways.178 In the cannot-intubate/cannot-ventilate scenario it is a reliable rescue device. In this situation, adequate ventilation with the ILMA is possible in nearly all cases.5,114,136 Ventilation with the ILMA is probably superior to face mask ventilation with inexperienced providers.179 The ILMA can also be used as a primary ventilation and intubation device for patients with difficult airways.178 Tracheal intubation through the ILMA can be accomplished by using a blind technique, with a lightwand, or under fiberoptic guidance (see Chapter 4 for tracheal intubation through the ILMA). Studies of difficult airway management with the ILMA show that almost all patients can be adequately ventilated with the ILMA and 94% to 99% can be intubated through the device.5,136,178,180–187
The LMA Classic (or single-use LMA Unique) is the most extensively tested LMA for children. It may provide a more secure and reliable means of ventilation than a face mask.4 The LMA allows adequate ventilation in 98% of adults with known difficult airways and in 90% to 95% of those with unexpectedly difficult airways.136,188–191 It is also useful as a rescue device in difficult pediatric airways.136 Two descriptive studies and 86 case reports describe use of the LMA for difficult pediatric airways.136,192–198 In these reports, ventilation was adequate with the LMA in nearly all pediatric patients.136,196,198,199 Intubation of pediatric patients through the LMA is usually possible with a small fiberoptic scope.196,198 Case series and case reports also suggest that the LMA can provide an effective rescue airway during neonatal resuscitation if BMV and ET intubation fail.200
Contraindications
Although several studies have shown that the ILMA is safe and effective for ventilation and intubation during in-line cervical spine stabilization, some evidence shows that the ILMA causes posterior pressure on the midportion of the cervical spine.183,201–204 The clinical importance of cervical spine pressure caused by the ILMA is unknown, and the device is generally considered safe in patients with an unstable cervical spine injury. Nevertheless, providers should be aware of this concern and make every effort to stabilize the ILMA in these situations.
Procedure
LMA Fastrach: The first step is to select the appropriate size of ILMA. The ILMA is available in three sizes: size 3 for children weighing 30 to 50 kg, size 4 for small adults weighing 50 to 70 kg, and size 5 for adults weighing 70 to 100 kg (Table 3-1). When there is doubt about which size is appropriate, it is probably better to use the larger size.
TABLE 3-1
Laryngeal Mask Airway, Disposable Laryngeal Mask Airway, and Intubating Laryngeal Mask Airway Size Recommendations Based on Weight*
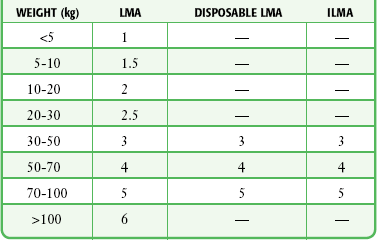
ILMA, intubating laryngeal mask airway; LMA, laryngeal mask airway.
*Note that only a standard LMA is available for patients less than 30 kg.
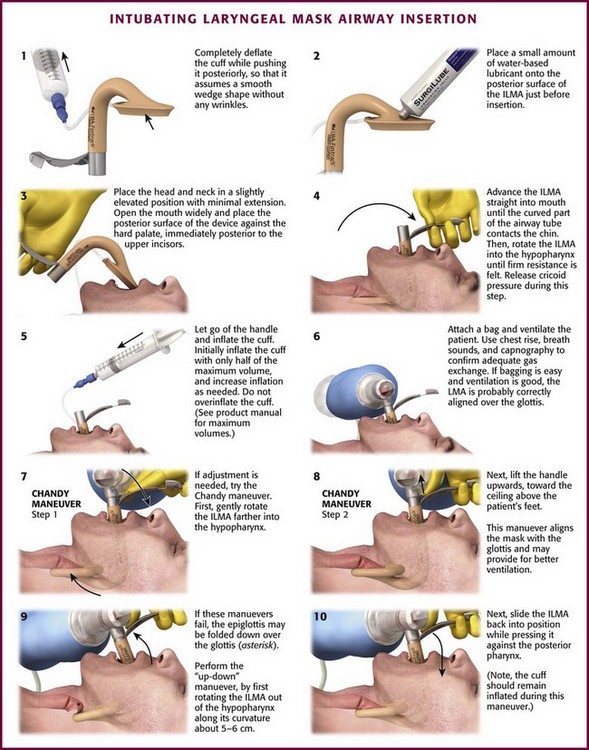
After choosing the correct ILMA, completely deflate the cuff while pushing it posteriorly so that it assumes a smooth wedge shape without any wrinkles (Fig. 3-9, step 1). Place a small amount of water-based lubricant onto the posterior surface of the ILMA just before insertion (Fig 3-9, step 2). Open the patient’s mouth and position the posterior mask tip so that it is flat against the hard palate, immediately posterior to the upper incisors (Fig. 3-9, step 3). Advance the airway straight into the mouth along the hard palate without rotation until the curved part of the airway tube is in contact with the patient’s chin. Then rotate the ILMA completely into the hypopharynx by advancing it along its curved axis. Keep the posterior of the mask firmly applied to the soft palate and posterior pharynx until firm resistance is felt (Fig. 3-9, step 4). Cricoid pressure impedes proper placement of the ILMA, so consider briefly releasing cricoid pressure while the device is rotated into its final position, wedged into the proximal esophagus.133,134,205 After insertion, the airway tube should emerge from the mouth directed somewhat caudally. Without holding the tube or handle, inflate the mask cuff (Fig. 3-9, step 5). The entire device will normally slide backward a bit when the cuff is inflated. Frequently, only half the maximum cuff volume is sufficient to obtain a good mask seal. Do not overinflate the cuff because this may make the seal worse. See the instruction manual for maximum cuff volumes. Attach a bag and ventilate the patient while using chest rise, breath sounds, and capnography to confirm adequate gas exchange. If bagging is easy and ventilation is good, the aperture of the ILMA is probably aligned correctly over the vocal cords.
If optimal ILMA placement is not accomplished initially, adjusting maneuvers can be attempted. The purpose of adjusting maneuvers is to align the aperture of the ILMA with the glottic opening. Proper positioning of the ILMA aperture with the glottic opening allows optimal ventilation and facilitates tracheal intubation. Before adjusting the ILMA, consider the patient’s position and degree of relaxation because both may affect ILMA function. The ILMA works best in the neutral or sniffing position; cervical extension may interfere with proper placement. The patient should not react to ILMA placement with coughing or gagging because this may interfere with proper placement. Have a single operator perform the adjustment maneuvers by gripping the ILMA handle with one hand, in a “frying pan” grip, and providing bag ventilation with the other hand. After each adjustment maneuver, assess the quality of bag ventilation and mask seal. Easy bag ventilation, good chest rise, and absence of an audible mask leak are indications of good ILMA alignment with the glottis (Fig. 3-9, step 6).
To adjust the position of the ILMA, first gently pull the handle toward you without rotation along the ILMA’s curvature. Next, gently push the handle toward the patient’s feet without rotating it. Finally, try the “Chandy maneuver,” which consists of gently rotating the ILMA farther into the hypopharynx and then lifting the handle toward the ceiling above the patient’s feet (Fig. 3-9, steps 7 and 8). If these simple maneuvers do not result in adequate ventilation, consider the “up-down maneuver” (Fig. 3-9, steps 9 and 10). This technique is used to correct down-folding of the epiglottis, which is common with insertion of the ILMA and may interfere with ventilation or intubation. The up-down maneuver is accomplished by rotating the ILMA out of the hypopharynx along its curvature about 5 to 6 cm while the cuff remains inflated and then sliding it back into position while pressing it against the posterior of the pharynx. Do not use excessive force when placing or adjusting the ILMA.
If adjusting maneuvers do not result in adequate ventilation, it is likely that the wrong size ILMA has been used. Incorrect ILMA size is more likely to be a problem if the device is too small; attempting insertion of a larger ILMA is a reasonable first approach. If another ILMA size is not available, external anterior neck manipulation or downward pressure may bring the glottis and ILMA cuff into proper alignment. If the size of the ILMA is not in question, consider completely removing and carefully reinserting the device (see Chapter 4 for intubation through the ILMA and ILMA removal).
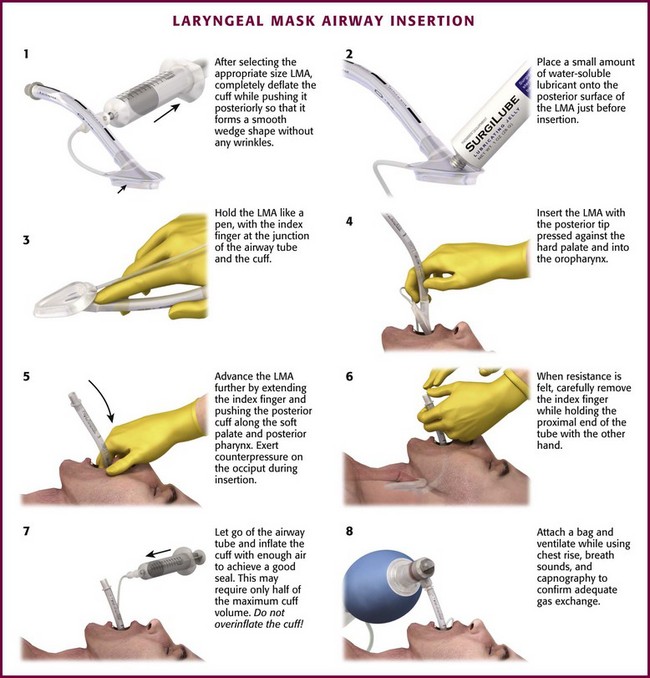
LMA Classic (or Single-Use LMA Unique): The first step is to select the appropriate size LMA. The LMA is available in a wide range of sizes, from size 1 for neonates weighing less than 5 kg to size 6 for adults weighing more than 100 kg. The disposable version is available in sizes 1 through 5, but not size 6. After selecting the proper size, completely deflate the LMA cuff while pushing it posteriorly so that it forms a smooth wedge shape without any wrinkles (Fig. 3-10, step 1). Place a small amount of water-based lubricant onto the posterior surface of the LMA just before insertion (Fig 3-10, step 2). The best patient position for insertion of the LMA is the sniffing position, with the neck flexed and the head extended. The LMA may be inserted via two different techniques, depending on access to the patient. The most common method is the index finger insertion technique. This is accomplished by holding the LMA like a pen, with the index finger at the junction of the airway tube and the cuff (Fig. 3-10, step 3). Have an assistant open the patient’s mouth and insert the LMA with the posterior tip pressed against the hard palate just behind the upper incisors (Fig. 3-10, step 4). Under direct vision, use the index finger to slide the LMA along the hard palate and into the oropharynx (Fig. 3-10, step 5). As the LMA is inserted farther, extend the index finger and push the posterior cuff along the soft palate and posterior pharynx. Exert counterpressure on the back of the patient’s head during insertion. Continue to push the LMA into the hypopharynx until resistance is felt. Use the other hand to hold the proximal end of the LMA tube while removing your index finger from the patient’s mouth (Fig. 3-10, step 6).
An alternative method is the thumb insertion technique. Use this technique when you have limited access to the patient from behind (see www.lmana.com for details). Hold the LMA with your thumb at the junction of the cuff and the airway tube. Place the mask against the hard palate under direct vision as with the index finger technique. Use the thumb to push the LMA into the mouth along the palate and posterior pharynx. Hold the end of the airway tube with the other hand while removing your thumb from the patient’s mouth.
After the LMA is fully inserted, let go of the proximal end of the airway tube and inflate the cuff enough to achieve a good seal over the glottis (Fig. 3-10, step 7). This may require only half the maximum cuff volume. Be careful to not overinflate the LMA cuff (see the product packaging for maximal cuff volumes). Attach a bag and ventilate the patient, with chest rise, breath sounds, and capnography used to confirm adequate gas exchange (Fig. 3-10, step 8). If bagging is easy and ventilation is good, the aperture of the LMA is probably aligned correctly over the glottic opening. Proper positioning of the LMA aperture with the glottic opening allows optimal ventilation.
Aftercare
If the LMA or ILMA will remain in place without tracheal intubation, it can be secured like an ET tube. Removal of the ILMA after tracheal intubation is easy, but more difficult than insertion of the device (see Chapter 4).
Complications
The most important complications associated with using the LMA are aspiration of gastric contents and hypoxia. The LMA does not protect against aspiration and may actually cause vomiting if the patient gags during placement of the device. In fasted anesthetized patients, the incidence of aspiration is very low, about 2 per 10,000 cases.136 There are many descriptive studies and case reports of the use of an LMA for difficult airways with no mention of significant aspiration.136 Although the risk for aspiration is surely higher than 2 per 10,000 when using the LMA in the ED, there is evidence that it provides some protection from passive regurgitation and produces less gastric inflation than BMV does.206
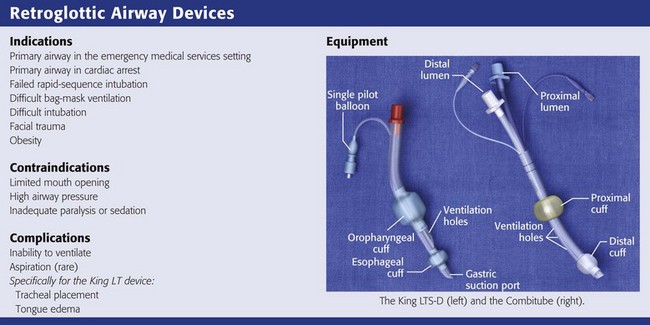
Retroglottic Airway Devices
King LT
The King LT (King Airway-LTS-D EMS, King Systems, Noblesville, IN; www.kingsystems.com) is a retroglottic device that functions similar to the esophageal-tracheal Combitube (see later). Like the Combitube, the LT is designed to isolate the glottic opening between an oropharyngeal cuff and an esophageal cuff (see Review Box 3-4). Unlike the Combitube, the King LT has only one airway lumen and a simplified cuff system, so both cuffs can be inflated from a single port. The literature regarding the King LT is a bit confusing in that many versions of the device have been clinically tested during the last decade. The latest disposable versions are the LT-D and the LTS-D. The LTS-D has an 18-Fr gastric suction port at the tip. The modern LTS-D has been available since 2004 and is also called the LTS II in some literature.207,208
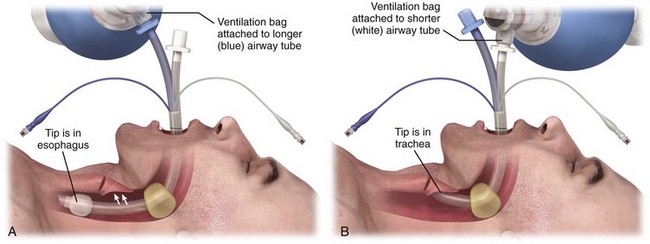
Like the Combitube, the King LT is designed for blind placement and has a large proximal cuff and small distal cuff. Unlike the Combitube, the tip of the King LT is designed to be placed in the esophagus only. The shape of the King LT and the size of the tip in previous versions made it unlikely to be placed into the trachea.209 However, the latest design of the LTS-D has a narrower tip and in one study had a 10% incidence of tracheal placement.210 Interestingly, most patients with tracheal placement of the LTS-D were still able to be adequately ventilated.210
Popularity of the LTS-D has grown rapidly in EMS systems, and it is now widely used by EMS agencies in the United States. Several studies have shown that the LTS-D has a high rate of successful ventilation in the operating room setting.211–213 In addition, there are several case reports of the LT being used as a rescue device for the cannot-intubate/cannot-ventilate scenario and when placement of an LMA failed.214,215 Some data suggest that the LTS-D may be useful in neonates and small infants when direct laryngoscopy fails.216 In the EMS setting, the LT-D has a high success rate (95%) when used for ventilation of out-of-hospital cardiac arrest.217 Finally, an out-of-hospital study by Frascone and colleagues showed that the rate of successful insertion and ventilation with the LTS-D is essentially equivalent to that of standard ET intubation in the hands of paramedics.218
Intubation through the King LT devices is possible with fiberoptics or a bougie, but many who have tried this maneuver in clinical practice have been disappointed. The reason is that the aperture does not necessarily directly align with the glottis, even when the device is ventilating properly.210 In one study the glottis was visualized in only 51% of patients when a fiberscope was passed through the King LT.212
Many EMS agencies that have used the King LT extensively have noticed cases of tongue edema, especially with prolonged use of the device. One case of massive tongue swelling has been reported in the literature.219 Moreover, there is some concern that the large oropharyngeal balloon of the King LT might compress the carotid arteries and be detrimental in patients undergoing CPR, but there is currently no evidence to support this concern.
Indications and Contraindications: In the ED, indications for using the King LT are the same as those for the Combitube. It appears to be a good rescue ventilation device for failed BMV or failed intubation.209,220,221 Because the King LT is a supraglottic airway and is designed to be placed blindly, it is relatively contraindicated in patients with obstruction of the upper airway by a foreign body.
Placement of the King LT: The first step is to choose the proper size King LT. It is available only in adolescent and adult sizes in the United States. Size 3 is yellow and designed for patients 4 to 5 feet in height, size 4 is red and designed for patients 5 to 6 feet in height, and size 5 is purple and designed for patients taller than 6 feet. Several pediatric sizes are available in Europe, but not in the United States.
After determining the appropriate size King LT, check the cuffs and then completely deflate them before placement. Lubricate the device with a water-based lubricant. The best patient position for insertion of the King LT is the sniffing position, but it can be placed with the head in the neutral position if necessary. Hold the LT at the connector with the dominant hand, and hold the mouth open by grasping the chin with the nondominant hand. Introduce the tip of the device into the corner of the mouth while rotating the tube 45 to 90 degrees so that the blue orientation line on the tube is touching the corner of the mouth. Pass the tip of the device into the mouth and under the tongue. As the tip passes under the base of the tongue, rotate the tube back to the midline so that the blue orientation line faces the ceiling. Without exerting force, advance the King LT until the connector is aligned with the teeth. Inflate the cuffs with the minimum volume necessary to create a good seal (see the product brochure for maximum cuff volumes). Ventilate with a bag-valve system and confirm placement with chest rise, breath sounds, and capnography (Fig. 3-11).
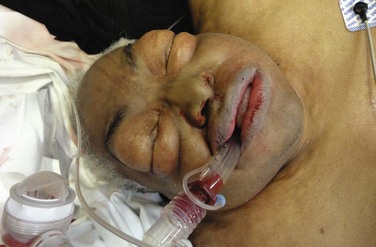
Complications: It is hard to assess the complication rate of the King LT because the device has been modified several times in the last decade and there is no organized surveillance of out-of-hospital airway devices.222 The current LT-D and LTS-D devices have been available since 2004. The LTS-D is referred to as the LTS II in some studies. The most serious complication is tracheal placement, which occurred in 10% of cases in one study and is probably significantly underappreciated and underreported.210 Another complication that is not uncommon and certainly underreported is tongue edema. There is one case report of massive tongue edema occurring 3 hours after placement of the King LT,219 and mild tongue edema is relatively common and not reported in the literature. Finally, there is some concern that the large oropharyngeal balloon of the King LT might compress the carotid arteries and be detrimental in patients undergoing CPR, but no evidence currently supports this concern. Figure 3-12 depicts probable pharyngeal or esophageal perforation, with massive subcutaneous neck and face emphysema following prehospital placement of a King LT. Because an autopsy was denied, the exact injury was never confirmed and may have been related to other interventions during resuscitation.
Combitube and EasyTube
The esophageal-tracheal Combitube (Nellcor, Pleasanton, CA; www.nellcor.com) and the EasyTube (Teleflex Medical–Rusch, Durham, NC) are retroglottic airway devices designed as rescue devices for difficult and emergency airways and can be placed blindly and rapidly.223,224 The EasyTube is very similar to the Combitube and may have some minor advantages,224 but it has not been as well studied. The discussion here will concentrate on the Combitube.
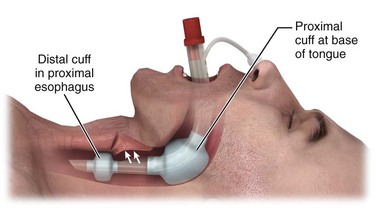
The Combitube has two parallel lumens, a small distal cuff, and a large proximal cuff. When it is placed blindly, the tip will end up in the esophagus in about 95% of cases and in the trachea in about 5% (Fig. 3-13). The longer lumen or tube is used for ventilation when the tip is in the esophagus. It is perforated at the level of the pharynx and occluded at the distal end. The shorter lumen or tube is used for ventilation when the tip is in the trachea. It is open at the distal end, like a standard ET tube. The large proximal cuff or balloon is designed to occlude the pharynx by filling the space between the base of the tongue and the soft palate. The small distal cuff serves as a seal in either the esophagus or the trachea.225–230
The Combitube provides adequate ventilation in about 95% of patients when placed by prehospital providers.225,229,230 When the Combitube is placed by physicians, the success rate approaches 100%.231 It compares favorably with the ET tube with respect to ventilation and oxygenation in cardiac arrest situations.223,228 In unconscious patients, the Combitube may provide protection from aspiration.232
Indications and Contraindications: The Combitube is a good choice as a primary airway in patients who are unresponsive or in cardiac arrest, especially in the uncontrolled prehospital environment. The Combitube can also be used in any emergency airway setting for rescue ventilation after failed BMV or failed intubation. In cases of failed intubation with an unexpectedly difficult airway, the Combitube may be used to provide adequate ventilation and allow time for other methods of intubation or a controlled surgical airway.233,234 The Combitube should not be used in patients with an intact gag reflex and is not recommended in patients shorter than 4 feet. It is contraindicated in patients with suspected caustic poisoning or proximal esophageal disorders.
Placement of the Combitube: The Combitube is available in two sizes. The manufacturer recommends the smaller 37-Fr device for patients 4 feet to 5 feet 6 inches tall and the larger 41-Fr device for patient taller than 5 feet 6 inches. Studies suggest that the smaller 37-Fr Combitube can be used safely in patients up to about 6 feet tall.235,236 The larger 41-Fr device is appropriate for patients taller than 6 feet.
To insert the Combitube, hold the device in the dominant hand and gently advance it caudally into the pharynx while grasping the tongue and jaw between the thumb and index finger of the nondominant hand. Pass the tube blindly along the tongue to a depth that places the printed rings on the proximal end of the tube between the patient’s teeth and the alveolar ridge.237 If resistance is met in the hypopharynx, remove the tube and bend it between the balloons for several seconds to facilitate insertion.237 After insertion, fill the pharyngeal balloon with 100 mL of air and the distal cuff with 10 to 15 mL of air. The large pharyngeal balloon serves to securely seat the Combitube in the oropharynx and create a closed system in the case of esophageal placement. Because about 95% of placements are esophageal, begin ventilation through the longer (blue) airway tube.230
Use chest rise, good breath sounds, and capnography, without gastric inflation, to confirm proper ventilation. Alternatively, use a Wee-type aspirator device on the shorter (clear) lumen to confirm that the tip is in the esophagus before ventilation through the longer (blue) lumen.238 An inability to easily aspirate air confirms esophageal placement. Easy aspiration with the Wee-type device indicates tracheal positioning of the tube and requires changing the ventilation to the shorter (clear) tracheal lumen.
If the Combitube is in the esophageal position, suction the stomach by passing a catheter through the shorter (clear) lumen into the stomach while the patient is being ventilated via the longer (blue) lumen.230
Complications: Inappropriate balloon inflation and incorrect Combitube placement can lead to air leaks during ventilation. The most common placement error is an improper insertion angle. Use a more caudal, longitudinal direction of insertion as opposed to an anteroposterior direction of insertion. The Combitube must also be maintained in the true midline position during insertion to avoid blind pockets in the supraglottic area, which can prevent passage of the tube.230 Attention to aligning the ring markings on the tube at the level of the incisors ensures proper positioning of the tube.
Decision Making in Emergency Airway Management
• Adequacy of current ventilation
• Need for neuromuscular blockade (uncooperative, full stomach, teeth clenching)
Consideration of these factors should guide the clinician in selecting the optimal technique. Choosing the initial approach is often straightforward. Difficulty arises precipitously when the initial approach fails. Time becomes critical as the risk for irreversible hypoxic injury and cardiac arrest rises. Anxiety then increases and the potential for error increases. Forethought and practice are invaluable when managing these situations.
Rapid-Sequence Intubation
RSI in anesthesia has evolved since the introduction of succinylcholine in 1951. RSI was initially used as an abbreviation for rapid-sequence induction but is now synonymous with rapid-sequence intubation. Initially, the main purpose of RSI was to decrease the risk for aspiration in patients with full stomachs who needed emergency intubation. RSI has now become the most common method of emergency airway management because it provides optimal conditions for direct laryngoscopy.14,239–243 Providers using RSI must appreciate the importance of basic skills such as preoxygenation, patient positioning, and BMV.
One of the most important concepts to appreciate when using RSI is that of optimal laryngoscopy to maximize first-pass success.244–246 Preparation, preoxygenation, proper patient positioning, anterior neck maneuvers, and good laryngoscopy skills are all important components of optimal laryngoscopy (see Chapter 4). Just as important as optimal laryngoscopy is the ability to recognize when laryngoscopy (or any technique) has failed and it is time to move to a different approach. It is easier to take a different approach when you follow a simple preconceived algorithm. Patient safety during RSI depends on the provider’s ability to maintain ventilation and oxygenation if the first attempt at intubation fails.246 In this situation, BMV restores oxygenation and keeps the patient stable enough for further intubation attempts. Therefore, the importance of BMV skills cannot be overstated, and mastery of this skill alleviates much of the anxiety associated with difficult emergency airways and improves the chance for successful RSI.3 Also, it is critical to have an EGA device immediately available during every RSI in the event that BMV is difficult or impossible. Finally, all providers who perform RSI should be prepared to perform a surgical airway when all other procedures and devices fail.
Difficult Airways, Failed Intubation, and When to Avoid Rapid-Sequence Intubation
The term difficult airway is popular, but there is no standard definition of this term. It is most commonly used to describe patients who are difficult to intubate with direct laryngoscopy. Subsequently, there has been a significant amount of literature dedicated to predicting difficult direct laryngoscopy. However, even in the best circumstances only about half the instances of difficult laryngoscopy can be predicted.247 Many factors such as Mallampati scoring and measurement of thyromental distance have not been found to accurately predict difficult laryngoscopy, especially in the emergency setting.247–250 Only obvious anatomic and pathologic abnormalities and a history of difficult intubation are accurate predictors of difficult laryngoscopy (see Chapter 4).248
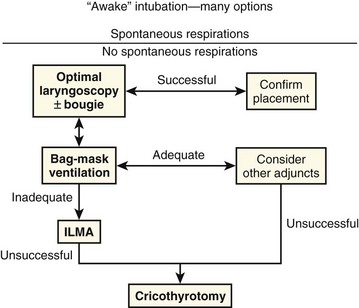
In the emergency setting it is more useful to think of difficult airways as situations in which our usual methods of ventilation and intubation fail. Our goal should be to avoid RSI in patients who cannot be ventilated with a bag-mask device and cannot be intubated by direct or video laryngoscopy. Risk factors for difficult or impossible BMV have been well studied and include the presence of a beard, obesity, lack of teeth, age older than 55 years, a history of snoring, short thyromental distance, and limited mandibular protrusion (see Box 3-2).110–113 Interestingly, one recent study showed that patients who were difficult to ventilate with a face mask usually became easier to ventilate when paralytic agents were administered and that none had worsened ventilation quality when paralyzed.114 Regardless, the realization that we cannot predict all cases of failed BMV and failed laryngoscopy mandates the need for a simple preconceived algorithm that uses proven rescue techniques that are applicable to a broad range of clinical scenarios, such as the bougie and the LMA Fastrach. The value of the bougie is indisputable, and it is clear that use of the LMA Fastrach after failed RSI has decreased the frequency of failed airways and the need for surgical airways (Fig. 3-14).* Finally, if RSI is our usual method of intubation, we must be prepared to perform a surgical airway when laryngoscopy, BMV, and backup devices fail.2,5
Emergency Airway Management Algorithm
The algorithm presented here summarizes the general approach used in the Department of Emergency Medicine at Hennepin County Medical Center (Fig. 3-15, Box 3-3). This algorithm is presented as an example. Individual providers and institutions should determine their own algorithms based on the availability of skills and resources. There are many similarities between this algorithm and those put forth by the American Society of Anesthesiologists and the Difficult Airway Society.141,142 However, this algorithm is simpler and more applicable to emergency airway management. Most published airway algorithms are not ideal for emergency airway management because they do not account for the conditions that are commonly encountered: patients with full stomachs who are critically ill and often uncooperative, and intubations that cannot be canceled if the airway is too difficult. Also, many algorithms resemble wish lists of equipment and skills that are simply not available to many emergency airway providers. Our algorithm is based on the concept that oxygenation, not intubation, is the key.255 It stresses well-proven concepts, procedures, and devices, and it is modeled after a simple algorithm developed by Combes and colleagues that was validated in a large prospective study.5,180,254
References
1. Levitan, R. Mask ventilation, rescue ventilation, and rescue intubation. In: Levitan R, ed. The Airway Cam Guide to Intubation and Practical Emergency Airway Management. Wayne, PA: Airway Cam Technologies, Inc.; 2004:49.
2. Sagarin, MJ, Barton, ED, Chng, YM, et al. Airway management by US and Canadian emergency medicine residents: a multicenter analysis of more than 6,000 endotracheal intubation attempts. Ann Emerg Med. 2005;46:328.
3. Schneider, R, Murphy, M. Bag/mask ventilation and endotracheal intubation. In: Walls R, Murphy M, eds. Manual of Emergency Airway Management. Philadelphia: Lippincott, Williams & Wilkins; 2004:43.
4. Berg, RA, Hemphill, R, Abella, BS, et al. Part 5: adult basic life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl):S685.
5. Combes, X, Jabre, P, Margenet, A, et al. Unanticipated difficult airway management in the prehospital emergency setting: prospective validation of an algorithm. Anesthesiology. 2011;114:105.
6. Neumar, RW, Otto, CW, Link, MS, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(suppl):S729.
7. Ambrosino, N, Vagheggini, G. Noninvasive positive pressure ventilation in the acute care setting: where are we? Eur Respir J. 2008;31:874.
8. Hill, NS, Brennan, J, Garpestad, E, et al. Noninvasive ventilation in acute respiratory failure. Crit Care Med. 2007;35:2402.
9. Maneker, AJ, Petrack, EM, Krug, SE. Contribution of routine pulse oximetry to evaluation and management of patients with respiratory illness in a pediatric emergency department. Ann Emerg Med. 1995;25:36.
10. Lee, WW, Mayberry, K, Crapo, R, et al. The accuracy of pulse oximetry in the emergency department. Am J Emerg Med. 2000;18:427.
11. Waugh, JB, Epps, CA, Khodneva, YA. Capnography enhances surveillance of respiratory events during procedural sedation: a meta-analysis. J Clin Anesth. 2011;23:189.
12. McGee, J, Vender, J. Nonintubation management of the airway: mask ventilation. In: Hagberg C, ed. Benumof’s Airway Management. Philadelphia: Mosby; 2007:345.
13. Bair, AE, Filbin, MR, Kulkarni, RG, et al. The failed intubation attempt in the emergency department: analysis of prevalence, rescue techniques, and personnel. J Emerg Med. 2002;23:131.
14. Sakles, JC, Laurin, EG, Rantapaa, AA, et al. Airway management in the emergency department: a one-year study of 610 tracheal intubations. Ann Emerg Med. 1998;31:325.
15. Meier, S, Geiduschek, J, Paganoni, R, et al. The effect of chin lift, jaw thrust, and continuous positive airway pressure on the size of the glottic opening and on stridor score in anesthetized, spontaneously breathing children. Anesth Analg. 2002;94:494.
16. Guildner, C. Resuscitation—opening the airway: a comparative study of techniques for opening an airway obstructed by the tongue. JACEP. 1976;5:588.
17. Finucane, B, Santora, A. Basic airway management and cardiopulmonary resuscitation. In: Principles of Airway Management. New York: Springer; 2005:21.
18. Barker, TD, Schneider, RE. Supplemental oxygen and bag-mask ventilation. In: Walls RM, Murphy MF, eds. Manual of Emergency Airway Management. Philadelphia: Wolters Kluwer, Lippincott, Williams & Wilkins; 2008:47.
19. Cook, T. Maintenance of the airway during anesthesia: supraglottic devices. In: Calder I, Pearce A, eds. Core Topics in Airway Management. Cambridge: Cambridge University Press; 2005:43.
20. Morikawa, S, Safar, P, Decarlo, J. Influence of the head-jaw position upon upper airway patency. Anesthesiology. 1961;22:265.
21. Adult basic life support. Circulation. 2005;112:III.
22. Aprahamian, C, Thompson, BM, Finger, WA, et al. Experimental cervical spine injury model: evaluation of airway management and splinting techniques. Ann Emerg Med. 1984;13:584.
23. Brimacombe, J, Keller, C, Künzel, KH, et al. Cervical spine motion during airway management: a cinefluoroscopic study of the posteriorly destabilized third cervical vertebrae in human cadavers. Anesth Analg. 2000;91:1274.
24. Donaldson, WF, 3rd., Heil, BV, Donaldson, VP, et al. The effect of airway maneuvers on the unstable C1-C2 segment. A cadaver study. Spine. 1997;22:1215.
25. Donaldson, WF, 3rd., Towers, JD, Doctor, A, et al. A methodology to evaluate motion of the unstable spine during intubation techniques. Spine. 1993;18:2020.
26. Hauswald, M, Sklar, DP, Tandberg, D, et al. Cervical spine movement during airway management: cinefluoroscopic appraisal in human cadavers. Am J Emerg Med. 1991;9:535.
27. Magill, IW. Technique in endotracheal anaesthesia. British Medical Journal. 1930;2:817.
28. Benumof, JL. Comparison of intubating positions: the end point for position should be measured. Anesthesiology. 2002;97:750. [author reply 754].
29. Horton, WA, Fahy, L, Charters, P. Defining a standard intubating position using “angle finder”. Br J Anaesth. 1989;62:6.
30. Park, SH, Park, HP, Jeon, YT, et al. A comparison of direct laryngoscopic views depending on pillow height. J Anesth. 2010;24:526.
31. Brodsky, JB, Lemmens, HJ, Brock-Utne, JG, et al. Anesthetic considerations for bariatric surgery: proper positioning is important for laryngoscopy. Anesth Analg. 2003;96:1841.
32. Collins, JS, Lemmens, HJ, Brodsky, JB, et al. Laryngoscopy and morbid obesity: a comparison of the “sniff” and “ramped” positions. Obes Surg. 2004;14:1171.
33. Levitan, RM, Mechem, CC, Ochroch, EA, et al. Head-elevated laryngoscopy position: improving laryngeal exposure during laryngoscopy by increasing head elevation. Ann Emerg Med. 2003;41:322.
34. Rao, SL, Kunselman, AR, Schuler, HG, et al. Laryngoscopy and tracheal intubation in the head-elevated position in obese patients: a randomized, controlled, equivalence trial. Anesth Analg. 2008;107:1912.
35. El-Orbany, M, Woehlck, H, Salem, MR. Head and neck position for direct laryngoscopy. Anesth Analg. 2011;113:103.
36. Greenland, KB, Edwards, MJ, Hutton, NJ. External auditory meatus-sternal notch relationship in adults in the sniffing position: a magnetic resonance imaging study. Br J Anaesth. 2010;104:268.
37. Adnet, F, Borron, SW, Finot, MA, et al. Relation of body position at the time of discovery with suspected aspiration pneumonia in poisoned comatose patients. Crit Care Med. 1999;27:745.
38. Heimlich, HJ, Hoffmann, KA, Canestri, FR. Food-choking and drowning deaths prevented by external subdiaphragmatic compression. Physiological basis. Ann Thorac Surg. 1975;20:188.
39. Ayerdi, J, Gupta, SK, Sampson, LN, et al. Acute abdominal aortic thrombosis following the Heimlich maneuver. Cardiovasc Surg. 2002;10:154.
40. Dupre, MW, Silva, E, Brotman, S. Traumatic rupture of the stomach secondary to Heimlich maneuver. Am J Emerg Med. 1993;11:611.
41. Meredith, MJ, Liebowitz, R. Rupture of the esophagus caused by the Heimlich maneuver. Ann Emerg Med. 1986;15:106.
42. Tung, PH, Law, S, Chu, KM, et al. Gastric rupture after Heimlich maneuver and cardiopulmonary resuscitation. Hepatogastroenterology. 2001;48:109.
43. Valero, V. Mesenteric laceration complicating a Heimlich maneuver. Ann Emerg Med. 1986;15:105.
44. Visintine, RE, Baick, CH. Ruptured stomach after Heimlich maneuver. JAMA. 1975;234:415.
45. Langhelle, A, Sunde, K, Wik, L, et al. Airway pressure with chest compressions versus Heimlich manoeuvre in recently dead adults with complete airway obstruction. Resuscitation. 2000;44:105.
46. Day, RL, Crelin, ES, DuBois, AB. Choking: the Heimlich abdominal thrust vs back blows: an approach to measurement of inertial and aerodynamic forces. Pediatrics. 1982;70:113.
47. Heimlich, HJ. Heimlich versus a slap on the back. N Engl J Med. 1979;300:990.
48. Ingalls, TH. Heimlich versus a slap on the back. N Engl J Med. 1979;300:990.
49. Redding, JS. The choking controversy: critique of evidence on the Heimlich maneuver. Crit Care Med. 1979;7:475.
50. Vilke, GM, Sith, AM, Ray, LU, et al. Airway obstruction in children aged less than 5 years: the prehospital experience. Prehosp Emerg Care. 2004;8:196.
51. Nadkarni, V, Hazinski, MF, Zideman, D, et al. Pediatric resuscitation: an advisory statement from the Pediatric Working Group of the International Liaison Committee on Resuscitation. Circulation. 1997;95:2185.
52. Vandenberg, JT, Vinson, DR. The inadequacies of contemporary oropharyngeal suction. Am J Emerg Med. 1999;17:611.
53. Vandenberg, JT, Lutz, RH, Vinson, DR. Large-diameter suction system reduces oropharyngeal evacuation time. J Emerg Med. 1999;17:941.
54. Ruben, H, Hansen, E, MacNaughton, FI. High capacity suction technique. A method of reducing the aspiration hazard during induction. Anaesthesia. 1979;34:349.
55. Naigow, D, Powaser, MM. The effect of different endotracheal suction procedures on arterial blood gases in a controlled experimental model. Heart Lung. 1977;6:808.
56. Fulmer, JD, Snider, GL. American College of Chest Physicians/National Heart, Lung, and Blood Institute National Conference on Oxygen Therapy. Heart Lung. 1984;13:550.
57. Boycott, AE, Haldane, JS. The effects of low atmospheric pressures on respiration. J Physiol. 1908;37:355.
58. Harboe, M. Lactic acid content in human venous blood during hypoxia at high altitude. Acta Physiol Scand. 1957;40:248.
59. Hoffmann, C, Clark, RT. Blood oxygen saturations and duration of consciousness in anoxia at high altitudes. Am J Physiol. 1946;145:685.
60. Murphy, R, Driscoll, P, O’Driscoll, R. Emergency oxygen therapy for the COPD patient. Emerg Med J. 2001;18:333.
61. Kallstrom, TJ. AARC Clinical Practice Guideline: oxygen therapy for adults in the acute care facility—2002 revision & update. Respir Care. 2002;47:717.
62. Bateman, NT, Leach, RM. ABC of oxygen. Acute oxygen therapy. BMJ. 1998;317:798.
63. Antman, EM, Anbe, DT, Armstrong, PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation. 2004;110:588.
64. Braunwald, E, Antman, EM, Beasley, JW, et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients with Unstable Angina). Circulation. 2000;102:1193.
65. Rawles, JM, Kenmure, AC. Controlled trial of oxygen in uncomplicated myocardial infarction. Br Med J. 1976;1:1121.
66. Adams, HP, Jr., Adams, RJ, Brott, J, et al. Guidelines for the early management of patients with ischemic stroke: a scientific statement from the Stroke Council of the American Stroke Association. Stroke. 2003;34:1056.
67. Hacke, W, Kaste, M, Bogousslavsky, J, et al. European Stroke Initiative Recommendations for Stroke Management—update 2003. Cerebrovasc Dis. 2003;16:311.
68. Ronning, OM, Guldvog, B. Should stroke victims routinely receive supplemental oxygen? A quasi-randomized controlled trial. Stroke. 1999;30:2033.
69. Tomaszewski, CA, Thom, SR. Use of hyperbaric oxygen in toxicology. Emerg Med Clin North Am. 1994;12:437.
70. Durrington, HJ, Flubacher, M, Ramsay, CF, et al. Initial oxygen management in patients with an exacerbation of chronic obstructive pulmonary disease. QJM. 2005;98:499.
71. Plant, PK, Owen, JL, Elliott, MW. One year period prevalence study of respiratory acidosis in acute exacerbations of COPD: implications for the provision of non-invasive ventilation and oxygen administration. Thorax. 2000;55:550.
72. Bazuaye, EA, Stone, TN, Corris, PA, et al. Variability of inspired oxygen concentration with nasal cannulas. Thorax. 1992;47:609.
73. Slessarev, M, Somogyi, R, Preiss, D, et al. Efficiency of oxygen administration: sequential gas delivery versus “flow into a cone” methods. Crit Care Med. 2006;34:829.
74. National Institute for Clinical Excellence (UK Department of Health). Manangement of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care. Available at www.nice.org.uk/CG101. Accessed October 23, 2012.
75. Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2011. Available at www.goldcopd.org/. Accessed October 23, 2012.
76. Celli, BR, MacNee, W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932.
77. Gruber, P, Kwiatkowski, T, Silverman, R, et al. Time to equilibration of oxygen saturation using pulse oximetry. Acad Emerg Med. 1995;2:810.
78. Baraka, AS, Taha, SK, Aouad, MT, et al. Preoxygenation: comparison of maximal breathing and tidal volume breathing techniques. Anesthesiology. 1999;91:612.
79. Mort, TC. Preoxygenation in critically ill patients requiring emergency tracheal intubation. Crit Care Med. 2005;33:2672.
80. Dixon, BJ, Dixon, JB, Carden, JR, et al. Preoxygenation is more effective in the 25 degrees head-up position than in the supine position in severely obese patients: a randomized controlled study. Anesthesiology. 2005;102:1110.
81. Baillard, C, Fosse, JP, Sebbane, M, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med. 2006;174:171.
82. Weingart, SD. Preoxygenation, reoxygenation, and delayed sequence intubation in the emergency department. J Emerg Med. 2011;40:661.
83. Baraka, AS, Taha, SK, Siddik-Sayyid, SM, et al. Supplementation of pre-oxygenation in morbidly obese patients using nasopharyngeal oxygen insufflation. Anaesthesia. 2007;62:769.
84. Engstrom, J, Hedenstierna, G, Larsson, A. Pharyngeal oxygen administration increases the time to serious desaturation at intubation in acute lung injury: an experimental study. Crit Care. 2010;14:R93.
85. Frumin, MJ, Epstein, RM, Cohen, G. Apneic oxygenation in man. Anesthesiology. 1959;20:789.
86. Taha, SK, Siddik-Sayyid, SM, El-Khatib, MF, et al. Nasopharyngeal oxygen insufflation following pre-oxygenation using the four deep breath technique. Anaesthesia. 2006;61:427.
87. Ramachandran, SK, Cosnowski, A, Shanks, A, et al. Apneic oxygenation during prolonged laryngoscopy in obese patients: a randomized, controlled trial of nasal oxygen administration. J Clin Anesth. 2010;22:164.
88. Kernick, J, Magarey, J. What is the evidence for the use of high flow nasal cannula oxygen in adult patients admitted to critical care units? A systematic review. Aust Crit Care. 2010;23:53.
89. Wettstein, RB, Shelledy, DC, Peters, JI. Delivered oxygen concentrations using low-flow and high-flow nasal cannulas. Respir Care. 2005;50:604.
90. Holleman-Duray, D, Kaupie, D, Weiss, MG. Heated humidified high-flow nasal cannula: use and a neonatal early extubation protocol. J Perinatol. 2007;27:776.
91. Shoemaker, MT, Pierce, MR, Yoder, BA, et al. High flow nasal cannula versus nasal CPAP for neonatal respiratory disease: a retrospective study. J Perinatol. 2007;27:85.
92. Parke, RL, Eccleston, ML, McGuinness, SP. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir Care. 2011;56:1151.
93. Spence, KL, Murphy, D, Kilian, C, et al. High-flow nasal cannula as a device to provide continuous positive airway pressure in infants. J Perinatol. 2007;27:772.
94. Parke, RL, McGuinness, SP, Eccleston, ML. A preliminary randomized controlled trial to assess effectiveness of nasal high-flow oxygen in intensive care patients. Respir Care. 2011;56:265.
95. Roca, O, Riera, J, Torres, F, et al. High-flow oxygen therapy in acute respiratory failure. Respir Care. 2010;55:408.
96. Sim, MA, Dean, P, Kinsella, J, et al. Performance of oxygen delivery devices when the breathing pattern of respiratory failure is simulated. Anaesthesia. 2008;63:938.
97. Westlake, EK, Simpson, T, Kaye, M. Carbon dioxide narcosis in emphysema. Q J Med. 1955;24:155.
98. Dorges, V, Wenzel, V, Knacke, P, et al. Comparison of different airway management strategies to ventilate apneic, nonpreoxygenated patients. Crit Care Med. 2003;31:800.
99. Dorges, V, Ocker, H, Hagelberg, S, et al. Optimisation of tidal volumes given with self-inflatable bags without additional oxygen. Resuscitation. 2000;43:195.
100. Wenzel, V, Idris, AH, Banner, MJ, et al. Influence of tidal volume on the distribution of gas between the lungs and stomach in the nonintubated patient receiving positive-pressure ventilation. Crit Care Med. 1998;26:364.
101. Wenzel, V, Keller, C, Idris, AH, et al. Effects of smaller tidal volumes during basic life support ventilation in patients with respiratory arrest: good ventilation, less risk? Resuscitation. 1999;43:25.
102. von Goedecke, A, Bowden, K, Wenzel, V, et al. Effects of decreasing inspiratory times during simulated bag-valve-mask ventilation. Resuscitation. 2005;64:321.
103. Wenzel, V, Idris, AH, Montgomery, WH, et al. Rescue breathing and bag-mask ventilation. Ann Emerg Med. 2001;37:S36.
104. Joffe, AM, Hetzel, S, Liew, EC. A two-handed jaw-thrust technique is superior to the one-handed “EC-clamp” technique for mask ventilation in the apneic unconscious person. Anesthesiology. 2010;113:873.
105. Reardon, R, Ward, C, Hart, D. Assessment of face-mask ventilation using an airway model. Ann Emerg Med. 2008;52:S114.
106. Campbell, TP, Stewart, RD, Kaplan, RM, et al. Oxygen enrichment of bag-valve-mask units during positive-pressure ventilation: a comparison of various techniques. Ann Emerg Med. 1988;17:232.
107. Guidelines for cardiopulmonary resuscitation and emergency cardiac care. Emergency Cardiac Care Committee and Subcommittees, American Heart Association. Part II. Adult basic life support. JAMA. 1992;268:2184.
108. Murray, JA, Demetriades, D, Berne, TV, et al. Prehospital intubation in patients with severe head injury. J Trauma. 2000;49:1065.
109. Gausche, M, Lewis, RJ, Stratton, SJ, et al. Effect of out-of-hospital pediatric endotracheal intubation on survival and neurological outcome: a controlled clinical trial. JAMA. 2000;283:783.
110. Langeron, O, Mosso, E, Huraux, C, et al. Prediction of difficult mask ventilation. Anesthesiology. 2000;92:1229.
111. El-Orbany, M, Woehlck, HJ. Difficult mask ventilation. Anesth Analg. 2009;109:1870.
112. Kheterpal, S, Han, R, Tremper, KK, et al. Incidence and predictors of difficult and impossible mask ventilation. Anesthesiology. 2006;105:885.
113. Yildiz, TS, Solak, M, Toker, K. The incidence and risk factors of difficult mask ventilation. J Anesth. 2005;19:7.
114. Amathieu, R, Combes, X, Abdi, W, et al. An algorithm for difficult airway management, modified for modern optical devices (Airtraq laryngoscope; LMA CTrach): a 2-year prospective validation in patients for elective abdominal, gynecologic, and thyroid surgery. Anesthesiology. 2011;114:25.
115. Sellick, BA. Cricoid pressure to control regurgitation of stomach contents during induction of anaesthesia. Lancet. 1961;2:404.
116. Brimacombe, JR, Berry, AM. Cricoid pressure. Can J Anaesth. 1997;44:414.
117. Ellis, DY, Harris, T, Zideman, D. Cricoid pressure in emergency department rapid sequence tracheal intubations: a risk-benefit analysis. Ann Emerg Med. 2007;50:653.
118. Lawes, EG, Campbell, I, Mercer, D. Inflation pressure, gastric insufflation and rapid sequence induction. Br J Anaesth. 1987;59:315.
119. Moynihan, RJ, Brock-Utne, JG, Archer, JH, et al. The effect of cricoid pressure on preventing gastric insufflation in infants and children. Anesthesiology. 1993;78:652.
120. Petito, SP, Russell, WJ. The prevention of gastric inflation—a neglected benefit of cricoid pressure. Anaesth Intensive Care. 1988;16:139.
121. Salem, MR, Wong, AY, Mani, M, et al. Efficacy of cricoid pressure in preventing gastric inflation during bag-mask ventilation in pediatric patients. Anesthesiology. 1974;40:96.
122. Allman, KG. The effect of cricoid pressure application on airway patency. J Clin Anesth. 1995;7:197.
123. Georgescu, A, Miller, JN, Lecklitner, ML. The Sellick maneuver causing complete airway obstruction. Anesth Analg. 1992;74:457.
124. Hartsilver, EL, Vanner, RG. Airway obstruction with cricoid pressure. Anaesthesia. 2000;55:208.
125. Ho, AM, Wong, W, Ling, E, et al. Airway difficulties caused by improperly applied cricoid pressure. J Emerg Med. 2001;20:29.
126. Hocking, G, Roberts, FL, Thew, ME. Airway obstruction with cricoid pressure and lateral tilt. Anaesthesia. 2001;56:825.
127. Mac, GPJH, Ball, DR. The effect of cricoid pressure on the cricoid cartilage and vocal cords: an endoscopic study in anaesthetised patients. Anaesthesia. 2000;55:263.
128. Palmer, JH, Yentis, SM. Cricoid pressure application to awake volunteers: discomfort cannot be used to indicate appropriate force. Can J Anaesth. 2005;52:114.
129. Saghaei, M, Masoodifar, M. The pressor response and airway effects of cricoid pressure during induction of general anesthesia. Anesth Analg. 2001;93:787.
130. Ansermino, JM, Blogg, CE. Cricoid pressure may prevent insertion of the laryngeal mask airway. Br J Anaesth. 1992;69:465.
131. Aoyama, K, Takenaka, I, Sata, T, et al. Cricoid pressure impedes positioning and ventilation through the laryngeal mask airway. Can J Anaesth. 1996;43:1035.
132. Asai, T, Barclay, K, McBeth, C, et al. Cricoid pressure applied after placement of the laryngeal mask prevents gastric insufflation but inhibits ventilation. Br J Anaesth. 1996;76:772.
133. Asai, T, Barclay, K, Power, I, et al. Cricoid pressure impedes placement of the laryngeal mask airway and subsequent tracheal intubation through the mask. Br J Anaesth. 1994;72:47.
134. Asai, T, Barclay, K, Power, I, et al. Cricoid pressure impedes placement of the laryngeal mask airway. Br J Anaesth. 1995;74:521.
135. Brimacombe, J. Cricoid pressure and the laryngeal mask airway. Anaesthesia. 1991;46:986.
136. Brimacombe, J. Laryngeal Mask Anesthesia: Priciples and Practice. Philadelphia: Saunders; 2005.
137. Brimacombe, J, White, A, Berry, A. Effect of cricoid pressure on ease of insertion of the laryngeal mask airway. Br J Anaesth. 1993;71:800.
138. Dutton, R, McCunn, M, Anesthsia for trauma. Miller’s Anesthesia. Miller, R, eds. Miller’s Anesthesia, Philadelphia, Elsevier, 2005;Vol 2:2451.
139. Suresh, M, Munnur, U, Wali, A. The patient with a full stomach. In: Hagberg C, ed. Benumof’s Airway Management. Philadelphia: Mosby; 2007:756.
140. Cook, TM. The classic laryngeal mask airway: a tried and tested airway. What now? Br J Anaesth. 2006;96:149.
141. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003;98:1269.
142. Henderson, JJ, Popat, MT, Latto, IP, et al. Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia. 2004;59:675.
143. The International Liaison Committee on Resuscitation (ILCOR) consensus on science with treatment recommendations for pediatric and neonatal patients: pediatric basic and advanced life support. Pediatrics. 2006;117:e955.
144. 2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: pediatric basic life support. Pediatrics. 2006;117:e989.
145. Choyce, A, Avidan, MS, Patel, C, et al. Comparison of laryngeal mask and intubating laryngeal mask insertion by the naïve intubator. Br J Anaesth. 2000;84:103.
146. Choyce, A, Avidan, MS, Shariff, A, et al. A comparison of the intubating and standard laryngeal mask airways for airway management by inexperienced personnel. Anaesthesia. 2001;56:357.
147. Dimitriou, V, Voyagis, GS. Tracheal intubation via the intubating laryngeal mask by inexperienced personnel. Br J Anaesth. 2000;84:538.
148. Levitan, RM, Ochroch, EA, Stuart, S, et al. Use of the intubating laryngeal mask airway by medical and nonmedical personnel. Am J Emerg Med. 2000;18:12.
149. Reeves, MD, Skinner, MW, Ginifer, CJ. Evaluation of the Intubating Laryngeal Mask Airway used by occasional intubators in simulated trauma. Anaesth Intensive Care. 2004;32:73.
150. Asai, T, Neil, J, Stacey, M. Ease of placement of the laryngeal mask during manual in-line neck stabilization. Br J Anaesth. 1998;80:617.
151. Asai, T, Wagle, AU, Stacey, M. Placement of the intubating laryngeal mask is easier than the laryngeal mask during manual in-line neck stabilization. Br J Anaesth. 1999;82:712.
152. Komatsu, R, Nagata, O, Kamata, K, et al. Comparison of the intubating laryngeal mask airway and laryngeal tube placement during manual in-line stabilisation of the neck. Anaesthesia. 2005;60:113.
153. Ali, A, Canturk, S, Turkmen, A, et al. Comparison of the laryngeal mask airway Supreme and laryngeal mask airway Classic in adults. Eur J Anaesthesiol. 2009;26:1010.
154. Cook, TM, Garward, JJ, Handel, J, et al. Evaluation of the LMA Supreme in 100 non-paralysed patients. Anaesthesia. 2009;64:555.
155. Eschertzhuber, S, Brimacombe, J, Hohlrieder, M, et al. The laryngeal mask airway Supreme—a single use laryngeal mask airway with an oesophageal vent. A randomised, cross-over study with the laryngeal mask airway ProSeal in paralysed, anaesthetised patients. Anaesthesia. 2009;64:79.
156. Howes, BW, Wharton, NM, Gibbison, B, et al. LMA Supreme insertion by novices in manikins and patients. Anaesthesia. 2010;65:343.
157. Lee, AK, Tey, JB, Lim, Y, et al. Comparison of the single-use LMA supreme with the reusable ProSeal LMA for anaesthesia in gynaecological laparoscopic surgery. Anaesth Intensive Care. 2009;37:815.
158. Pearson, DM, Young, PJ. Use of the LMA-Supreme for airway rescue. Anesthesiology. 2008;109:356.
159. Seet, E, Rajeev, S, Firoz, T, et al. Safety and efficacy of laryngeal mask airway Supreme versus laryngeal mask airway ProSeal: a randomized controlled trial. Eur J Anaesthesiol. 2010;27:602.
160. Teoh, WH, Lee, KM, Suhitharan, T, et al. Comparison of the LMA Supreme vs the i-gel in paralysed patients undergoing gynaecological laparoscopic surgery with controlled ventilation. Anaesthesia. 2010;65:1173.
161. van Zundert, A, Brimacombe, J. The LMA Supreme—a pilot study. Anaesthesia. 2008;63:209.
162. Verghese, C, Ramaswamy, B. LMA-Supreme—a new single-use LMA with gastric access: a report on its clinical efficacy. Br J Anaesth. 2008;101:405.
163. Erlacher, W, Tiefenbrunner, H, Kastenbauer, T, et al. CobraPLUS and Cookgas air-Q versus Fastrach for blind endotracheal intubation: a randomised controlled trial. Eur J Anaesthesiol. 2011;28:181.
164. Galgon, RE, Schroeder, KM, Han, S, et al. The air-Q((R)) intubating laryngeal airway vs the LMA-ProSeal(TM): a prospective, randomised trial of airway seal pressure. Anaesthesia. 2011;66:1093.
165. Jagannathan, N, Kho, MF, Kozlowski, RJ, et al. Retrospective audit of the air-Q intubating laryngeal airway as a conduit for tracheal intubation in pediatric patients with a difficult airway. Paediatr Anaesth. 2011;21:422.
166. Jagannathan, N, Kozlowski, RJ, Sohn, LE, et al. A clinical evaluation of the intubating laryngeal airway as a conduit for tracheal intubation in children. Anesth Analg. 2011;112:176.
167. Jagannathan, N, Roth, AG, Sohn, LE, et al. The new air-Q intubating laryngeal airway for tracheal intubation in children with anticipated difficult airway: a case series. Paediatr Anaesth. 2009;19:618.
168. Jagannathan, N, Sohn, LE, Mankoo, R, et al. A randomized crossover comparison between the Laryngeal Mask Airway-Unique and the air-Q Intubating Laryngeal Airway in children. Paediatr Anaesth. 2012;22:161.
169. Jagannathan, N, Sohn, LE, Mankoo, R, et al. Prospective evaluation of the self-pressurized air-Q intubating laryngeal airway in children. Paediatr Anaesth. 2011;21:673.
170. Karim, YM, Swanson, DE. Comparison of blind tracheal intubation through the intubating laryngeal mask airway (LMA Fastrach) and the Air-Q. Anaesthesia. 2011;66:185.
171. Bamgbade, OA, Macnab, WR, Khalaf, WM. Evaluation of the i-gel airway in 300 patients. Eur J Anaesthesiol. 2008;25:865.
172. Kleine-Brueggeney, M, Theiler, L, Urwyler, N, et al. Randomized trial comparing the i-gel and Magill tracheal tube with the single-use ILMA and ILMA tracheal tube for fibreoptic-guided intubation in anaesthetized patients with a predicted difficult airway. Br J Anaesth. 2011;107:251.
173. Richez, B, Saltel, L, Banchereau, F, et al. A new single use supraglottic airway device with a noninflatable cuff and an esophageal vent: an observational study of the i-gel. Anesth Analg. 2008;106:1137.
174. Theiler, L, Klein-Brueggeney, M, Urwyler, N, et al. Randomized clinical trial of the i-gel and Magill tracheal tube or single-use ILMA and ILMA tracheal tube for blind intubation in anaesthetized patients with a predicted difficult airway. Br J Anaesth. 2011;107:243.
175. Theiler, LG, Kleine-Bruiggeney, M, Kalser, D, et al. Crossover comparison of the laryngeal mask supreme and the i-gel in simulated difficult airway scenario in anesthetized patients. Anesthesiology. 2009;111:55.
176. Uppal, V, Fletcher, G, Kinsella, J. Comparison of the i-gel with the cuffed tracheal tube during pressure-controlled ventilation. Br J Anaesth. 2009;102:264.
177. Uppal, V, Gangaiah, S, Fletcher, G, et al. Randomized crossover comparison between the i-gel and the LMA-Unique in anaesthetized, paralysed adults. Br J Anaesth. 2009;103:882.
178. Joo, HS, Kapoor, S, Rose, DK, et al. The intubating laryngeal mask airway after induction of general anesthesia versus awake fiberoptic intubation in patients with difficult airways. Anesth Analg. 2001;92:1342.
179. Burgoyne, L, Cyna, A. Laryngeal mask vs intubating laryngeal mask: insertion and ventilation by inexperienced resuscitators. Anaesth Intensive Care. 2001;29:604.
180. Combes, X, Le Roux, B, Suen, P, et al. Unanticipated difficult airway in anesthetized patients: prospective validation of a management algorithm. Anesthesiology. 2004;100:1146.
181. Combes, X, Sauvat, S, Leroux, B. Intubating laryngeal mask airway in morbidly obese and lean patients: a comparative study. Anesthesiology. 2005;102:1106.
182. Ferson, DZ, Rosenblatt, WH, Johansen, MJ, et al. Use of the intubating LMA-Fastrach in 254 patients with difficult-to-manage airways. Anesthesiology. 2001;95:1175.
183. Fukutome, T, Amaha, K, Nakazawa, K, et al. Tracheal intubation through the intubating laryngeal mask airway (LMA-Fastrach) in patients with difficult airways. Anaesth Intensive Care. 1998;26:387.
184. Gerstein, NS, Braude, DA, Hung, O, et al. The Fastrach Intubating Laryngeal Mask Airway: an overview and update. Can J Anaesth. 2010;57:588.
185. Liu, EH, Goy, RW, Lim, Y, et al. Success of tracheal intubation with intubating laryngeal mask airways: a randomized trial of the LMA Fastrach and LMA CTrach. Anesthesiology. 2008;108:621.
186. Martel, M, Reardon, RF, Cochrane, J. Initial experience of emergency physicians using the intubating laryngeal mask airway: a case series. Acad Emerg Med. 2001;8:815.
187. Timmermann, A, Russo, SG, Crozier, TA, et al. Novices ventilate and intubate quicker and safer via intubating laryngeal mask than by conventional bag-mask ventilation and laryngoscopy. Anesthesiology. 2007;107:570.
188. Gataure, PS, Hughes, JA. The laryngeal mask airway in obstetrical anaesthesia. Can J Anaesth. 1995;42:130.
189. Martin, SE, Ochsner, MG, Jarman, RH, et al. Use of the laryngeal mask airway in air transport when intubation fails. J Trauma. 1999;47:352.
190. Parmet, JL, Colonna-Romano, P, Horrow, JC, et al. The laryngeal mask airway reliably provides rescue ventilation in cases of unanticipated difficult tracheal intubation along with difficult mask ventilation. Anesth Analg. 1998;87:661.
191. Silk, JM, Hill, HM, Calder, I. Difficult intubation and the Laryngeal Mask. Eur J Anaesthesiol Suppl. 1991;4:47.
192. Baraka, A. Laryngeal mask airway for resuscitation of a newborn with Pierre-Robin syndrome. Anesthesiology. 1995;83:645.
193. Brimacombe, J, Gandini, D. Airway rescue and drug delivery in an 800 g neonate with the laryngeal mask airway. Paediatr Anaesth. 1999;9:178.
194. Denny, NM, Desilva, KD, Webber, PA. Laryngeal mask airway for emergency tracheostomy in a neonate. Anaesthesia. 1990;45:895.
195. Lavies, NG. Use of the laryngeal mask airway in neonatal resuscitation. Anaesthesia. 1993;48:352.
196. Osses, H, Poblete, M, Asenjo, F. Laryngeal mask for difficult intubation in children. Paediatr Anaesth. 1999;9:399.
197. Trawoger, R, Mann, C, Mortl, M, et al. Use of laryngeal masks in the resuscitation of a neonate with difficult airway. Arch Dis Child Fetal Neonatal Ed. 1999;81:F160.
198. Walker, RW. The laryngeal mask airway in the difficult paediatric airway: an assessment of positioning and use in fibreoptic intubation. Paediatr Anaesth. 2000;10:53.
199. Lopez-Gil, M, Brimacombe, J, Alvarez, M. Safety and efficacy of the laryngeal mask airway. A prospective survey of 1400 children. Anaesthesia. 1996;51:969.
200. Grein, AJ, Weiner, GM. Laryngeal mask airway versus bag-mask ventilation or endotracheal intubation for neonatal resuscitation. Cochrane Database Syst Rev. 2, 2005. [CD003314].
201. Brimacombe, J, Keller, C. Cervical spine instability and the intubating laryngeal mask—a caution. Anaesth Intensive Care. 1998;26:708.
202. Kihara, S, Watanabe, S, Brimacombe, J, et al. Segmental cervical spine movement with the intubating laryngeal mask during manual in-line stabilization in patients with cervical pathology undergoing cervical spine surgery. Anesth Analg. 2000;91:195.
203. Schuschnig, C, Waltl, B, Erlacher, W, et al. Intubating laryngeal mask and rapid sequence induction in patients with cervical spine injury. Anaesthesia. 1999;54:793.
204. Waltl, B, Melischek, M, Schuschnig, C, et al. Tracheal intubation and cervical spine excursion: direct laryngoscopy vs. intubating laryngeal mask. Anaesthesia. 2001;56:221.
205. Reardon, RF, Martel, M. The intubating laryngeal mask airway: suggestions for use in the emergency department. Acad Emerg Med. 2001;8:833.
206. Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 6: advanced cardiovascular life support: section 3: adjuncts for oxygenation, ventilation and airway control. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation. 2000;102:I95.
207. Asai, T, Shingu, K. The laryngeal tube. Br J Anaesth. 2005;95:729.
208. Cook, TM. The laryngeal tube sonda (LTS) and the LTS II. Acta Anaesthesiol Scand. 2006;50:521.
209. Genzwuerker, HV, Hilker, T, Hohner, E, et al. The laryngeal tube: a new adjunct for airway management. Prehosp Emerg Care. 2000;4:168.
210. Kikuchi, T, Kamiya, Y, Ohtsuka, T, et al. Randomized prospective study comparing the laryngeal tube suction II with the ProSeal laryngeal mask airway in anesthetized and paralyzed patients. Anesthesiology. 2008;109:54.
211. Genzwuerker, HV, Altmayer, S, Hinkelbein, J, et al. Prospective randomized comparison of the new Laryngeal Tube Suction LTS II and the LMA-ProSeal for elective surgical interventions. Acta Anaesthesiol Scand. 2007;51:1373.
212. Mihai, R, Knottenbelt, G, Cook, TM. Evaluation of the revised laryngeal tube suction: the laryngeal tube suction II in 100 patients. Br J Anaesth. 2007;99:734.
213. Zand, F, Amini, A, Sadeghi, SE, et al. A comparison of the laryngeal tube-S and Proseal laryngeal mask during outpatient surgical procedures. Eur J Anaesthesiol. 2007;24:847.
214. Asai, T, Matsumoto, S, Shingu, K, et al. Use of the laryngeal tube after failed insertion of a laryngeal mask airway. Anaesthesia. 2005;60:825.
215. Matioc, AA, Olson, J. Use of the Laryngeal Tube in two unexpected difficult airway situations: lingual tonsillar hyperplasia and morbid obesity. Can J Anaesth. 2004;51:1018.
216. Scheller, B, Schalk, R, Byhahn, C, et al. Laryngeal tube suction II for difficult airway management in neonates and small infants. Resuscitation. 2009;80:805.
217. Wiese, CH, Semmel, T, Müller, JJ, et al. The use of the laryngeal tube disposable (LT-D) by paramedics during out-of-hospital resuscitation—an observational study concerning ERC guidelines 2005. Resuscitation. 2009;80:194.
218. Frascone, RJ, Russi, C, Lick, C, et al. Comparison of prehospital insertion success rates and time to insertion between standard endotracheal intubation and a supraglottic airway. Resuscitation. 2011;82:1529.
219. Gaither, JB, Matheson, J, Eberhardt, A, et al. Tongue engorgement associated with prolonged use of the King-LT laryngeal tube device. Ann Emerg Med. 2010;55:367.
220. Agro, F, Galli, B, Ravussin, P. Preliminary results using the laryngeal tube for supraglottic ventilation. Am J Emerg Med. 2002;20:57.
221. Genzwuerker, HV, Dhonau, S, Ellinger, K. Use of the laryngeal tube for out-of-hospital resuscitation. Resuscitation. 2002;52:221.
222. Wang, HE. Safety of the King-LT. Ann Emerg Med. 2010;56:442.
223. Frass, M, Frenzer, R, Rauscha, F, et al. Ventilation with the esophageal tracheal combitube in cardiopulmonary resuscitation. Promptness and effectiveness. Chest. 1988;93:781.
224. Gaitini, LA, Yanovsky, B, Somri, M, et al. Prospective randomized comparison of the EasyTube and the esophageal-tracheal Combitube airway devices during general anesthesia with mechanical ventilation. J Clin Anesth. 2011;23:475.
225. Atherton, GL, Johnson, JC. Ability of paramedics to use the Combitube in prehospital cardiac arrest. Ann Emerg Med. 1993;22:1263.
226. Frass, M, Frenzer, R, Zdrahal, F, et al. The esophageal tracheal combitube: preliminary results with a new airway for CPR. Ann Emerg Med. 1987;16:768.
227. Frass, M, Rödler, S, Frenzer, R, et al. Esophageal tracheal combitube (ETC) for emergency intubation: anatomical evaluation of ETC placement by radiography. Resuscitation. 1989;18:95.
228. Frass, M, Rödler, S, Frenzer, R, et al. Esophageal tracheal combitube, endotracheal airway, and mask: comparison of ventilatory pressure curves. J Trauma. 1989;29:1476.
229. Lefrancois, DP, Dufour, DG. Use of the esophageal tracheal combitube by basic emergency medical technicians. Resuscitation. 2002;52:77.
230. Ochs, M, Vilke, GM, Chan, TC, et al. Successful prehospital airway management by EMT-Ds using the combitube. Prehosp Emerg Care. 2000;4:333.
231. Brimacombe, J. Other extraglottic airway devices. In: Brimacombe J, ed. Laryngeal Mask Anesthesia. Philadelphia: Saunders; 2005:577.
232. Paventi, S, Liturri, S, Colio, B, et al. Airway management with the Combitube during anaesthesia and in an emergency. Resuscitation. 2001;51:129.
233. Blostein, PA, Koestner, AJ, Hoak, S. Failed rapid sequence intubation in trauma patients: esophageal tracheal combitube is a useful adjunct. J Trauma. 1998;44:534.
234. Staudinger, T, Brugger, S, Watschinger, B, et al. Comparison of the Combitube with the endotracheal tube in cardiopulmonary resuscitation in the prehospital phase. Wien Klin Wochenschr. 1994;106:412.
235. Hartmann, T, Krenn, CG, Zoeggeler, A, et al. The oesophageal-tracheal Combitube Small Adult. Anaesthesia. 2000;55:670.
236. Urtubia, RM, Aguila, CM, Cumsille, MA. Combitube: a study for proper use. Anesth Analg. 2000;90:958.
237. Urtubia, RM. ‘Tricks of the trade’ with the Esophageal-Tracheal Combitube. Acta Anaesthesiol Scand. 2002;46:340.
238. Wee, MY. The oesophageal detector device. Assessment of a new method to distinguish oesophageal from tracheal intubation. Anaesthesia. 1988;43:27.
239. Gerardi, MJ, Sacchetti, AD, Cantor, RM, et al. Rapid-sequence intubation of the pediatric patient. Pediatric Emergency Medicine Committee of the American College of Emergency Physicians. Ann Emerg Med. 1996;28:55.
240. Knopp, RK. Rapid sequence intubation revisited. Ann Emerg Med. 1998;31:398.
241. Ma, OJ, Bentley, B, 2nd., Debehnke, DJ. Airway management practices in emergency medicine residencies. Am J Emerg Med. 1995;13:501.
242. McAllister, JD, Gnauck, KA. Rapid sequence intubation of the pediatric patient. Fundamentals of practice. Pediatr Clin North Am. 1999;46:1249.
243. Walls, R. Rapid sequence intubation. In: Walls R, ed. Manual of Emergency Airway Management. Philadelphia: Lippincott: Williams & Wilkins; 2004:22.
244. Levitan, R. A practical approach to emergency airway management. In: Levitan R, ed. The Airway Cam Guide to Intubation and Practical Emergency Airway Management. Wayne, PA: Airway Cam Technologies, Inc.; 2004:3.
245. Levitan, R, Ochroch, EA. Airway management and direct laryngoscopy. A review and update. Crit Care Clin. 2000;16:373.
246. Levitan, RM. Patient safety in emergency airway management and rapid sequence intubation: metaphorical lessons from skydiving. Ann Emerg Med. 2003;42:81.
247. Wilson, ME. Predicting difficult intubation. Br J Anaesth. 1993;71:333.
248. Levitan, RM, Everett, WW, Ochroch, EA. Limitations of difficult airway prediction in patients intubated in the emergency department. Ann Emerg Med. 2004;44:307.
249. Shiga, T, Wajima, Z, Inoue, T, et al. Predicting difficult intubation in apparently normal patients: a meta-analysis of bedside screening test performance. Anesthesiology. 2005;103:429.
250. Yentis, SM. Predicting difficult intubation—worthwhile exercise or pointless ritual? Anaesthesia. 2002;57:105.
251. Calder, I. ‘Difficult airways’: causation and prediction. In: Calder I, Pearce A, eds. Core Topics in Airway Management. Cambridge: Cambridge University Press; 2005:113.
252. Combes, X, Leroux, B, Jabre, P, et al. Out-of-hospital rescue oxygenation and tracheal intubation with the intubating laryngeal mask airway in a morbidly obese patient. Ann Emerg Med. 2004;43:140.
253. Lim, CL, Hawthorne, L, Ip-Yam, PC. The intubating laryngeal mask airway (ILMA) in failed and difficult intubation. Anaesthesia. 1998;53:929.
254. Tentillier, E, Heydenreich, C, Cros, AM, et al. Use of the intubating laryngeal mask airway in emergency pre-hospital difficult intubation. Resuscitation. 2008;77:30.
255. Isono, S, Ishikawa, T. Oxygenation, not intubation, does matter. Anesthesiology. 2011;114:7.

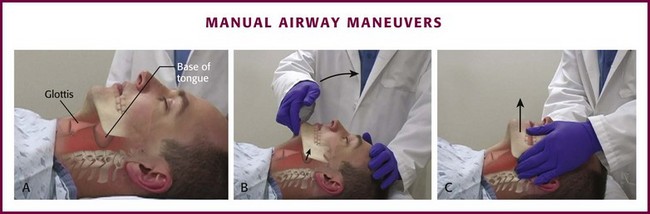
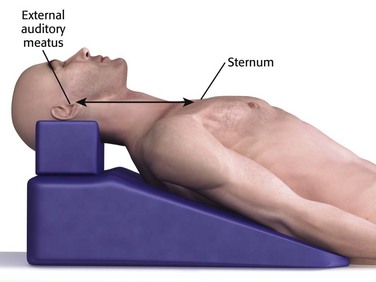
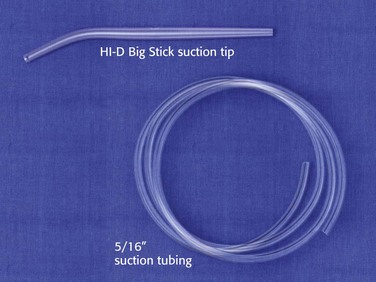
 -inch tubing.
-inch tubing.