B Cell Activation and Signaling
Learning Objectives
• Describe the structure of the B cell receptor (BCR) complex
• Differentiate between the signaling and nonsignaling components of the BCR
• Define the role of CD2 in intracellular signaling
• Explain the usefulness of BCR cross-linking in the intracellular signaling process
• Identify the intracellular signaling pathways activated by cross-linked BCRs
• Recognize the definition of thymus-dependent (TD) antigen
• Identify the definition of a thymus-independent (TI) antigen
• Compare and contrast the molecular structure of TD and TI antigens
• Explain the interactions between TI types I and II antigens and cellular ligands
• Compare and contrast the antibody responses elicited by TI types I and II antigens
• Explain the role(s) of the B lymphocyte stimulator (BLyS) in B cell differentiation to plasma cells
• Identify the role of CD22 in B cell regulation
• Examine the role of atacicept in blocking B cell differentiation
• Explain how belimumab and epratuzumab inhibit B cell differentiation
Key Terms
B cell receptor (BCR)
B lymphocyte stimulator (BLyS)
Brutons tyrosine kinase (BTK)
Plasma cell
Thymus-dependent (TD) antigens
Thymus-independent (TI) antigens
Type I thymus-independent antigens
Type II thymus-independent antigens
X-linked agammaglobulinemia
Introduction
B cells are the major effector cells in an antibody-mediated immune response. The production of antibodies to protein antigens often requires interactions among T cells, B cells, and monocytes. The response is termed thymus-dependent (TD) antibody production. B cells are activated by the engagement of two receptors on the cell surface. However, some antigens can initiate antibody production without antigen processing or the help of T cells. These antigens are termed thymus-independent (TI) antigens. The activation of B cells may require the engagement of one or more receptors. As a consequence of intracellular signaling, B cells differentiate into antibody-producing plasma cells.
B Cell Receptor Complex
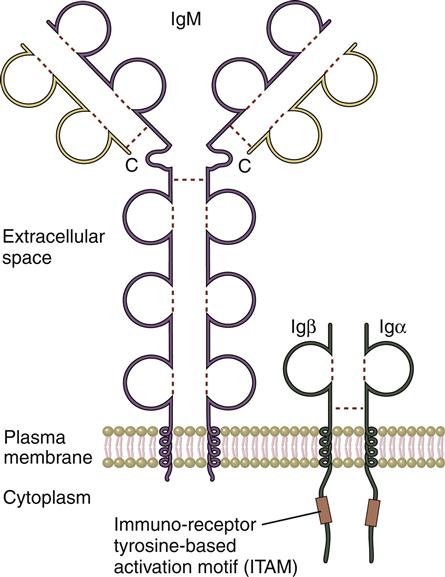
In the initial stage of B cell activation, antigens react with the membrane B cell receptor (BCR), which is a monomeric form of an antibody called immunoglobulin M (IgM; see Chapter 9). Monomeric IgM consists of two mu (μ)–heavy chains and two kappa (κ)– or lambda (λ)–light chains. M–heavy chains have four constant regions (CH1–CH4) and one variable domain (VH). Light chains are connected to the μ-chains and have one constant CL domain and one VL domain. Within the variable regions of both heavy and light chains are hypervariable regions. The combination of hypervariable regions in the heavy and light chains creates a three-dimensional pocket that determines antigen specificity (Figure 8-1). Each B cell expresses a homogeneous population of BCRs that recognize only one antigen. Therefore, 1 × 1013 B cell clones are needed to respond to all known antigens.
The BCR has a short intracytoplasmic tail and cannot transduce signals to the nucleus. To transduce signals, the BCR forms complexes with invariant immunoglobulin-alpha (Ig-α) and immunoglobulin-beta (Ig-β) molecules, which contain multiple immunoreceptor tyrosine-based activation motifs (ITAMs).
B Cell Receptor Signaling
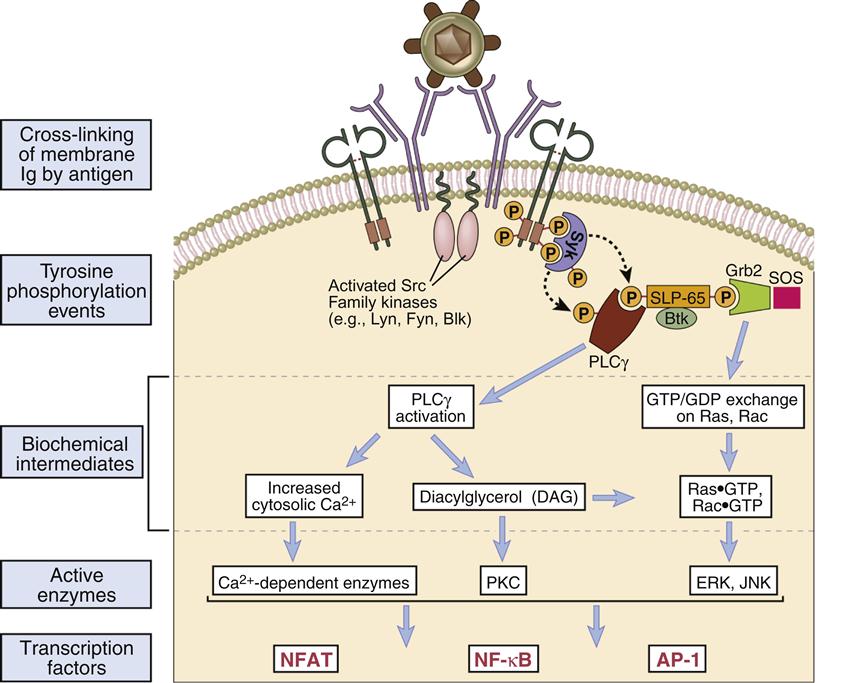
Activating signals are initiated by the cross-linkage of two BCRs by multi-valent antigens. Cross-linkage of two BCRs brings activated a sarcoma (v-src) kinase into the proper position for the activation of cellular signaling. The v-src enzyme phosphorylates the tyrosine residues on the Ig-α and Ig-β ITAMs (Figure 8-2). Phosphorylated ITAMs also act as “docking sites” for syk, an enzyme analogous to ZAP-70 in T cells. Syk activates two downstream signaling pathways whose products promote the synthesis of antibodies.
In one pathway, phosphorylation of phospholipase Cγ2 (the B cell isoform of phospholipase 1) activates the PLC calcium-dependent and DAG pathways. Activation results in the translocation of nuclear factor of activated T cells (NFAT) and nuclear factor–kappaB (NF-κB) to the nucleus. In a second pathway, phosphorlyation of an adaptor protein called SLP-65 (SH-2 binding leukocyte protein) allows interaction with Btk and Grb kinases. The Grb–SLP-65 complex recruits SOS (son of sevenless), which acts as a guanosine triphosphate–guanosine diphosphate (GTP–GDP) exchange protein that activates Ras and Rac. In turn, the MAP (mitogen-activated protein) kinase and the JNK (c-Jun N terminal kinase) pathways are activated with translocation of AP-1 to the nucleus. In B cells, AP-1 accelerates for cell division, which creates B memory cells and B cell differentiation into plasma cells.
Amplification of the B Cell Signal
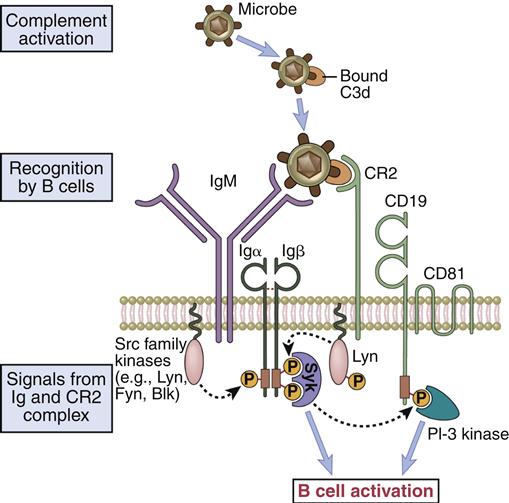
Like T cells, B cells require a second signal for activation. The second signal is provided by a B cell co-receptor complex that consists of CR2, CD19, and CD81 (TAPA-1). The CR2 molecule recognizes a decay product of complement called C3d that is bound to large-molecular-weight antigens or bacteria. Complement is a series of serum proteins that can be activated by polysaccharides (innate immunity) or antigen–antibody complexes (acquired immunity). Complement is discussed in detail in Chapter 11.
Amplification occurs when the multi-valent antigen binds to CD2 and cross-links multiple BCRs. Engagement of the BCR and CR2 activates a lyn kinase that phosphorylates the Ig-α and Ig-β ITAMs. Binding also reorients CD19 and CD81 to bring them into proximity with Ig-α and Ig-β ITAMs (Figure 8-3).
Phosphorylation of CD19 activates a PI-3 kinase, which initiates the Ras–MAP and Jak–STAT signaling pathways that stimulate B cell differentiation, survival, and growth.
Thymus-Dependent Antigens
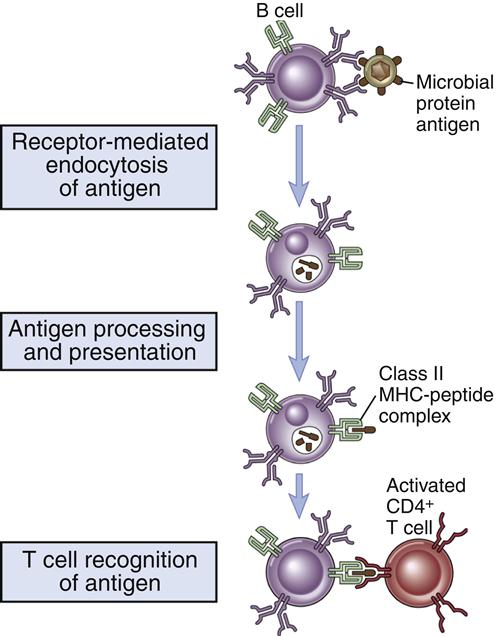
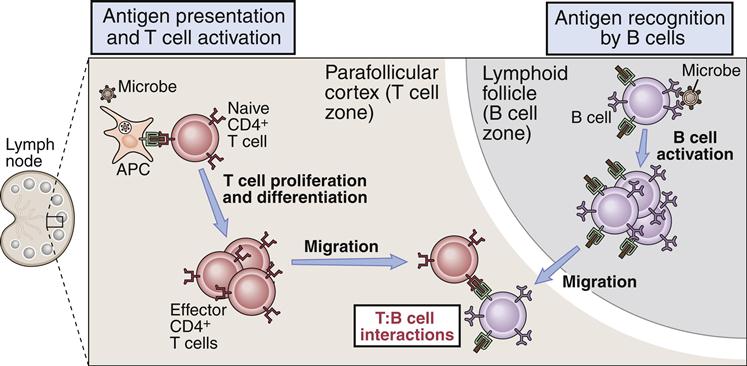
Some antigens require the help of T cells for the stimulation of B cells to produce antibodies. In the parafollicular cortex (T cell zone) of the lymph node, dendritic cells process and present antigens in the context of class II molecules. Antigens are presented to CD4Th2 cells. At this point, the T cell is activated but does not know which B cell needs help to produce antibodies.
B cell activation occurs in the lymph node B cell zone. The unprocessed antigens that activated the T cell bind to antigen-specific BCRs. After interaction between the BCR and antigens, B cells internalize, process, and present epitopes in the context of class II molecules (Figure 8-4).
Under the influence of chemokines, B cells translocate to T cell–rich zones. T cells and B cells come together, and B cells present antigens in the context of class II molecules to the T cell receptors (TCRs) on the activated T cell. As a consequence, activated T cells begin to help B cells to produce antibodies. Secondary signals from CD28–B7-1 and CD40–CD40L interactions are necessary to amplify the help from T cells. Small-molecular-weight cytokines produced by CD4Th2 cells cause the proliferation and differentiation of B cells in defined foci. Within 4 to 7 days, cytokine-stimulated B cells migrate back to lymph node follicles, where they form germinal centers containing proliferating B cells, memory B cells, and plasma cells. A summary of T helper cell–mediated B cell activation is shown in Figure 8-5.
Thymus-Independent Antigens
Antigens that do not require the help of T cells to initiate antibody production are termed thymus-independent (TI) antigens. B cells that respond to TI antigens are found in the spleen, bone marrow, peritoneal cells, and mucosa. In the spleen, specialized subsets of marginal-zone B cells interact with the native, unprocessed TI antigens bound to the surface of marginal-zone macrophages. In the peritoneum and mucosa, another specialized B cell population, called B-1 cells, recognizes TI antigens. B-1 cells are derived from the fetal liver and may represent a separate B cell lineage. Both marginal-zone B cells and B-1 cells produce natural antibodies that are important in the defense against encapsulated bacteria.
In general, TI antigens are linear carbohydrate molecules and have repeating epitopes that engage and cross-link multiple BCRs. On the basis of the necessity for ancillary or secondary receptor interactions, TI antigens can be classified into type I or type II antigens. Bacterial endotoxins, glycolipids, and nucleic acids are type I antigens and require interaction with a BCR and a second signal to activate B cells. Type II antigens directly cross-link multiple BCRs to activate B cells. Natural and synthetic polysaccharides are considered type II antigens. Both type I and type II antigens elicit antibody production, but they are poor inducers of memory cells.
Type I Thymus-Independent Antigens
Lipopolysaccharide (LPS) endotoxins are normal constituents of a gram-negative bacterial cell wall and are released when the bacterium dies or is killed by antibiotics. Endotoxins consist of three domains: (1) the O-polysaccharide region, (2) a core region, and (3) lipid A. The outer O-polysaccharide contains repeating immunogenic epitopes of three to five units that evoke an antibody response. The core polysaccharide region is intermediate between the O-region and lipid A. It contains d-manno-oct-2-ulosonic acid and glycerol-d-manno-heptose. The membrane-anchoring domain, or lipid A, consists of a highly conserved, phosphorylated dimer of N-acetylglucosamine with five to seven attached fatty acids. Both the O-region and lipid A bind to receptors on mammalian cells.
Endotoxin-induced B cell stimulation requires interaction with two receptors. The O-region polysaccharide reacts with antigen-specific BCRs. Lipid A binds to a Toll-like receptor (TLR-4) that has associated ITAMs. In some instances, endotoxins binding is nonspecific and multiple B cell clones are stimulated (polyclonal B cell activation) to produce an antibody called IgM.
Type II Thymus-Independent Antigens
Type II antigens are polysaccharide antigens with repeating epitopes that cross-link multiple BCRs to stimulate B cell activation. For example, capsular polysaccharides from the highly virulent Pneumococcus, Haemophilus, and Meningococcus species are classic type II antigens. Antibodies resulting from type II antigen stimulation are usually low-affinity antibodies, called natural antibodies, which are produced without overt exposure to antigens.
Plasma Cell Differentiation
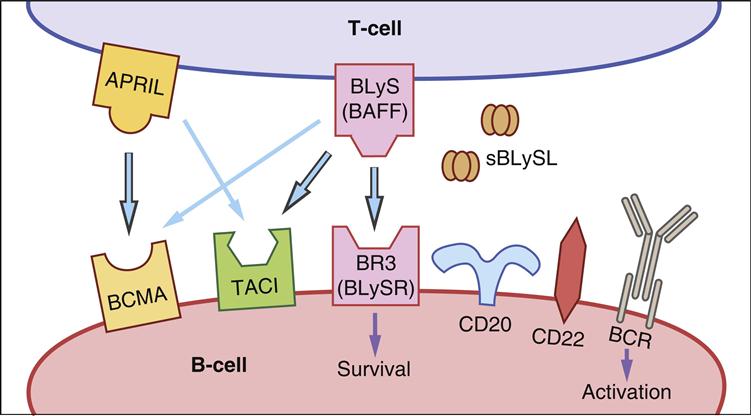
The driving forces for B cell differentiation into plasma cells are two cytokines produced by T cells, monocytes, dendritic cells, and macrophages. (1) One cytokine, called the B lymphocyte stimulator (BLyS), interacts with the TACI (membrane-activated calcium–modulator cyclophilin ligand-interactor) and the BLyS-receptor 3 (BLySR3). Engagement of BLyS with its ligands promotes B cell survival and differentiation into plasma cells. (2) The second cytokine is a proliferation-inducing ligand (APRIL), which binds to the B cell maturation antigen (BCMA). APRIL acts a co-stimulator of B cell proliferation and supports antibody switching from one class to another (Figure 8-6).
Plasma Cell Longevity
Depending on the nature of the antigen, plasma cells are either short lived or long lived. Short-lived plasma cells are generated during TI responses and allow rapid production of protective antibodies to Pneumococcus, Haemophilus, and Meningococcus. These plasma cells are localized in secondary lymphoid tissue such the splenic periarteriolar lymphoid sheath (PALS) and lymph node germinal centers. Long-lived plasma cells are generated after stimulation by TD antigens. Approximately 7 weeks after immunization with a TD antigen, plasma cells in the germinal center migrate to bone marrow and become long-lived plasma cells. These cells continue to produce antibodies for months or years and provide protection against previously encountered antigens. Approximately 80% of antibodies directed at TD antigens found in serum are produced by long-lived plasma cells in bone marrow.
Agents that Block B Cell Differentiation into Plasma Cells
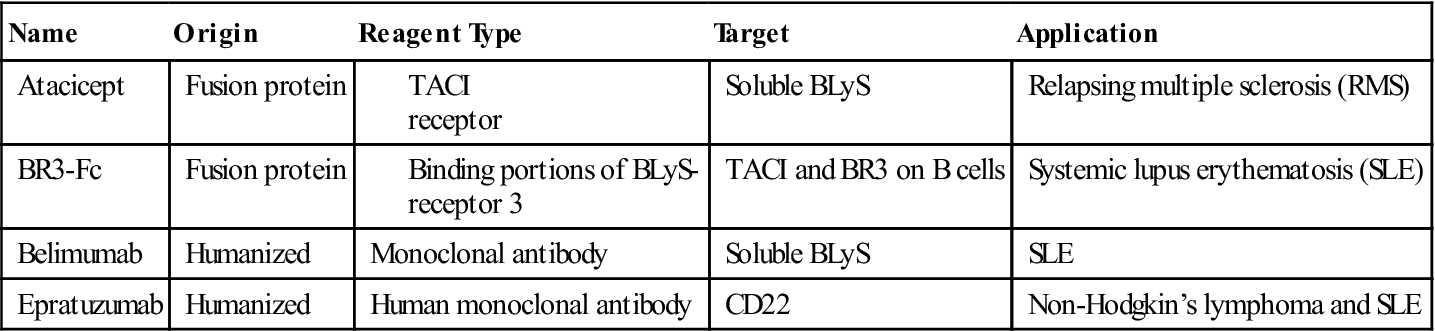
Blockade of B cell differentiation into plasma cells is an important goal of therapy. Inhibition of B cell differentiation can involve the use of fusion proteins or monoclonal antibodies. For example, atacicept is a soluble recombinant fusion protein that contains the binding portion of the B cell TACI receptor that binds both BLyS and APRIL and the distal portion of an antibody molecule. The fusion protein binds and neutralizes soluble BLyS and APRIL cytokines before they can react with stimulatory ligands on the B cell surface. Atacicept has been used successfully in the treatment of systemic lupus erythematosus (SLE) and rheumatoid arthritis.
Two monoclonal antibodies directly prevent B cell differentiation. Belimumab is a humanized monoclonal antibody directed at soluble BLyS. It is currently in clinical trials for the treatment of SLE. Epratuzumab, another humanized monoclonal antibody, is directed at CD22 expressed on the surface on B cells. Antibody engagement of CD22 activates the protein tyrosine phosphatase called SHP1, a negative regulator of BCR signaling. Epratuzumab is useful in the treatment of non-Hodgkin’s lymphoma and SLE (Table 8-1).
Table 8-1
Fusion Proteins and Monoclonal Antibodies That Inhibit B Cell Differentiation
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
TACI, membrane-activated calcium–modulator cyclophilin ligand-interactor.
Immunodeficiencies
Bruton Agammaglobulinema (X-Linked Agammaglobulinemia)
In 1952, Colonel Ogden Bruton, an Army pediatrician, reported the first known immunodeficiency. The patient was a 9-year-old boy, who had no measurable antibody levels. The immune defect was originally named Bruton agammaglobulinemia. Additional studies showed that 90% of the patients were male, and the name was changed to X-linked agammaglobulinemia (XLA), or early-onset agammaglobulinemia. Patients with this immunodeficiency have recurrent episodes of otitis media, pneumonia, and sinusitis caused by Streptococcus pneumoniae or Haemophilus influenzae. Diarrhea caused by Giardia and Campylobacter is also common. The incidence of XLA is unclear; an estimated frequency of 1 per 250,000 live births is considered to be an underestimation.
The immunologic defect has been mapped to the Bruton’s kinase gene (BTK) which is important in B cell maturation and intracellular signaling pathways. In the maturation process, the pro–B cell is the first committed cell in the B cell lineage that is sensitive to interleukin 7 (IL-7). When stimulated with IL-7, the pro–B cell re-arranges BCR heavy chains to increase the antigen-binding repertoire. BTK controls the rearrangement of the μ-chain. A dysfunctional BTK gene restricts the μ-chain re-arrangements, which limits the antigen specificity of the BCR. As a consequence, patients with early-onset agammaglobulinemia fail to respond to most antigens.
Treatment of Early-Onset Agammaglobulinemia
To restore normal antibody levels, patients are treated with pooled immunoglobulin administered by the intravenous or the subcutaneous route. Most preparations contain antibodies to bacterial agents and reduce the incidence of pneumonia, meningitis, and intestinal infections. Some intravenous immunoglobulin preparations (RSV-IVIG) contain high levels of antibodies to respiratory syncytial virus (RSV). RSV infections are common in children under 2 years of age, and usually, RSV does not replicate outside the respiratory tract. However, RSV infection in children with agammaglobulinemia can cause a fatal pneumonia. A more directed therapy for RSV infection is palivizumab, a humanized monoclonal anti-RSV antibody. It is licensed for use in high-risk infants with chronic lung disease and congenital heart diseases.
In patients with chronic sinus infections and respiratory infections, long-term broad-spectrum antibiotic therapy is indicated. Oral antibiotics such as amoxicillin, amoxicillin/clavulanate, and cefuroxime axetil can be used. Intravenous ceftriaxone may be required to treat chronic pulmonary infection or pneumonia.
Summary
• B cells differentiate into plasma cells, which produce antibodies.
• B cell response to protein antigens requires interactions among T cells, B cells, and macrophages.
• B cells can trap, process, and present antigens in the context of class II molecules.
• Endotoxins and polysaccharides can directly activate B cells by cross-linking surface receptors.
• Fusion proteins and monoclonal antibodies can block B cell differentiation into plasma cells.