Chapter 77 Autoimmune Retinopathies
Introduction
Autoimmune retinopathies (AIR) represent a group of inflammatory mediated retinopathies with otherwise unexplained vision loss associated with visual field deficits, photoreceptor dysfunction as evidenced on electroretinography (ERG), and the presence of circulating autoantibodies targeted against retinal antigens. Clinically, the fundus often appears normal, but some patients may show retinal vascular attenuation, diffuse retinal atrophy with or without pigmentary changes or waxy disc pallor. There are usually few or no intraocular inflammatory cells.1,2
AIR presumably results from an immunologic attack on the retina by antibodies directed against retinal antigens. The first case of vision loss and photoreceptor degeneration associated with cancer was described by Sawyer et al. in 1976.3 Paraneoplastic retinopathy, as a term, was first used by Klingele and associates in 1984, and has become the more general term used for autoimmune retinopathies associated with systemic malignancy since then.4 Several forms of autoantibody-mediated retinopathy are described: cancer-associated retinopathy (CAR) syndrome, melanoma-associated retinopathy (MAR) syndrome, or autoimmune retinopathy of other types.3,5–11 AIR can be categorized in two groups: (i) autoimmune retinopathy associated with cancer or other malignancies (paraneoplastic retinopathy or paraneoplastic autoimmune retinopathy); (ii) autoimmune retinopathy without any evidence of malignancy (nonparaneoplastic autoimmune retinopathy). Cancer-associated retinopathy and other paraneoplastic retinopathies will be covered in Chapter 134 (Remote effects of cancer on the retina). Autoimmune retinopathy is the preferred term for an acquired, presumed immunologically mediated retinopathy caused by antiretinal autoantibodies in the absence of a malignancy. This chapter will emphasize the latter.
Epidemiology and mechanisms
The prevalence of AIR is unknown, but the condition is believed to be rare. Clinical reports of nonparaneoplastic AIR consist only of case reports and a few small cohorts.12,13 AIR remains an ill-defined disorder and the lack of standardized diagnostic criteria may be contributing to the underestimation of its prevalence. Though circulating autoantibodies to retinal antigens have been shown to be associated with retinal dysfunction, the mechanisms by which these antibodies cause dysfunction are not entirely understood.14 Multiple retinal proteins have been found to be antigenic, including recoverin, carbonic anhydrase, α-enolase, arrestin, transducin-β, carbonic anhydrase II, TULP1, neurofilament protein, heat shock protein-70, photoreceptor-cell-specific nuclear receptor (PNR), Müller-cell-specific antigen, transient receptor potential cation channel, subfamily M, member 1 (TRPM1) and many other yet-unidentified antigens15,16 (Table 77.1). Some of these antigens are retina-specific, such as recoverin and rhodopsin, while others can be found in nonretinal tissues as well, such as enolase. Among these, recoverin and enolase are the most extensively studied antigens in the context of AIR. Recoverin is a 23 kDa calcium-binding protein found in both rods and cones. Enolase is a 48 kDa glycolytic enzyme whose α- and β-isoforms are found in many tissues, and γ-isoform in neuronal tissues.39 In a large series, more than 30% of patients with antiretinal antibodies tested positive for antienolase antibody.40 Antibodies against α-enolase appear to be fairly ubiquitous; they are found in multiple autoimmune diseases and even in healthy subjects.41–44 This has been, in part, attributed to the multifunctional nature of α-enolase.14,25,45
| Recoverin (23 kD) | CAR, npAIR6,17–24 | |
| Alpha-enolase (46 kD) | CAR, npAIR20,21,25–28 | |
| Carbonic anhydrase II (CA II) (30 kD) | npAIR20,21 | |
| Tubby-like protein 1 (TULP-1) (65 kD) | CAR29 | |
| Heat shock protein 70 (HSC 70) (70 kD) | CAR19 | |
| Transducin (35 kD) | MAR30 | |
| Arrestin (S-Antigen) (48 kD) | MAR, npAIR21,31 | |
| Interphotoreceptor binding protein (IRBP) (141 kD) | npAIR21 | |
| Unknown proteins | 22 kD, 34 kD, 35 kD, 37 kD, 40 kD, 68 kD | npAIR32–34 |
| 34 kD, 40 kD, 46 kD, 60 kD, 70 kD | CAR 22,35–38 | |
Antibodies against the listed proteins were identified in the serum of patients with AIR or AIR-like clinical findings. CAR, cancer-associated retinopathy; MAR, melanoma-associated retinopathy; npAIR, non-paraneoplastic autoimmune retinopathy.
Of all these antigens, recoverin and CAR has the strongest association. Recoverin has been shown to be expressed by the tumor cells of patients with CAR.46 While antirecoverin antibody is most specific to CAR, it has also been found in patients with nonparaneoplastic AIR, as well as patients with small-cell lung carcinoma without any retinopathy.17,18,47 Recoverin and α-enolase have been shown to be highly expressed in cancer cells in patients with paraneoplastic AIR. It is plausible that the disease is triggered by molecular mimicry between retinal proteins and tumor antigens in cases of paraneoplastic AIR and presumed viral or bacterial proteins26 in the case of nonparaneoplastic AIR.26,48,49 Regardless of the presence or absence of malignancy, autoimmune retinopathies appear to share common clinical features.
Experimental studies have attempted to shed light on the pathogenic mechanisms of autoimmune retinopathy. In vitro studies have demonstrated that some of the antiretinal antibodies are, indeed, cytotoxic to retinal cells, and that the cellular internalization of the antibody leads to apoptosis.19,25,27,50–52 This antibody-mediated apoptosis is independent of complement and involves caspase pathways and intracellular calcium influx. Similarly, in vivo studies showed that intravitreal injection of antirecoverin antibody causes apoptosis of retinal cells and a decrease in ERG responses.19,53,54 Recoverin also acts as a uveitogenic antigen and can induce autoimmune retinopathy-like disease in animal models leading to reduced scotopic and photopic ERG responses.55,56 Both in vitro and in vivo studies showed that antirecoverin antibody-triggered apoptosis occurs only in recoverin-positive cells.57 Antirecoverin antibodies target photoreceptor cells and anti-α-enolase antibodies appear to target ganglion cells. Antibodies targeting retinal bipolar cells have been associated with MAR. Additionally, injection of immunoglobulins isolated from serum of patients with MAR into the vitreous of monkeys led to ERG changes similar to those observed in MAR, indicating the pathogenicity of the antiretinal antibodies.58 Evidence from these studies suggests that antiretinal autoantibodies can target virtually any retinal cell type – photoreceptor cells, ganglion cells, bipolar cells – and cause retinal dysfunction.20,50,59 It is still unclear, however, why some patients with antiretinal antibodies develop retinopathy while others do not.
Clinical features
Clinical features of AIR are quite variable. Serum antiretinal autoantibodies have been associated with loss of vision and visual field defects as well as electrophysiologic changes in patients with autoimmune retinopathy, but the exact mechanism has not been fully understood. In particular, the pathogenicity and specificity of these autoantibodies with respect to clinical findings have yet to be determined. Nevertheless, patients with AIR appear to share common clinical features despite the heterogeneity in the detectable circulating antiretinal antibodies.12,20,60
The clinical manifestations of nonparaneoplastic AIR is quite variable, there are no clear guidelines or consensus on the diagnosis of autoimmune retinopathy. Commonly recognized clinical manifestations include:12,13
1. Symptoms: acute, subacute or chronic onset of photopsia, dyschromatopsia, nyctalopia, photoaversion, scotomas, sometimes central vision loss.
2. Fundus findings: normal-appearing fundus, or waxy disc pallor, attenuated retinal vasculature and retinal pigment epithelial (RPE) atrophy or mottling. There are few or no intraocular inflammatory cells.
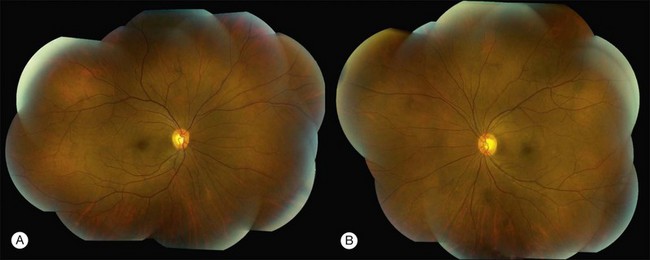
Patients with nonparaneoplastic AIR present with subacute or chronic vision loss. Patients typically complain of color vision changes, photosensitivity or photoaversion, and varying degrees of nyctalopia. Other presenting symptoms include floaters, photopsias, scotomas, most commonly paracentral, and constricted visual fields. Of these, photosensitivity, photoaversion, difficulty seeing in bright light, reduced visual acuity, dyschromatopsia and central scotomas are suggestive of cone dysfunction, whereas nyctalopia (night blindness) and midperipheral scotomas are suggestive of rod dysfunction. Depending on the extent and the distinct retinal cell involvement, visual acuity, particularly in the earlier stages, may be deceivingly good. On presentation to a tertiary care center, most patients may have been diagnosed with nonspecific retinal degeneration or isolated cases of retinitis pigmentosa (RP). Fundus examination can be unremarkable or may show signs of retinal degeneration such as waxy disc pallor, attenuated retinal vasculature with or without pigmentary changes, or diffuse atrophy. There may be mild or no inflammatory cells in the anterior chamber or the vitreous12,13,61 (Fig. 77.1).
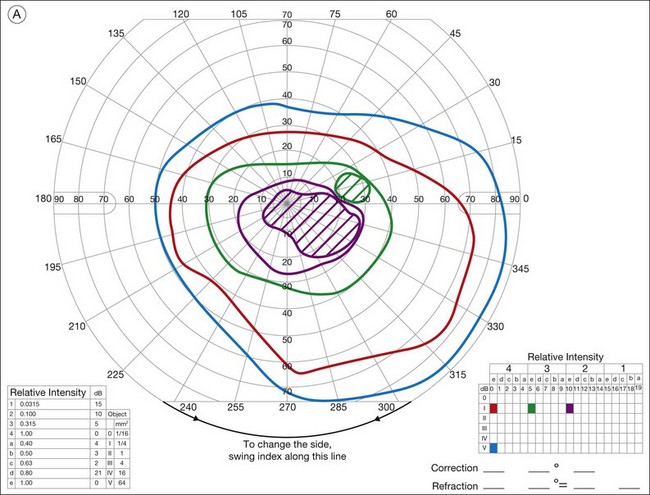
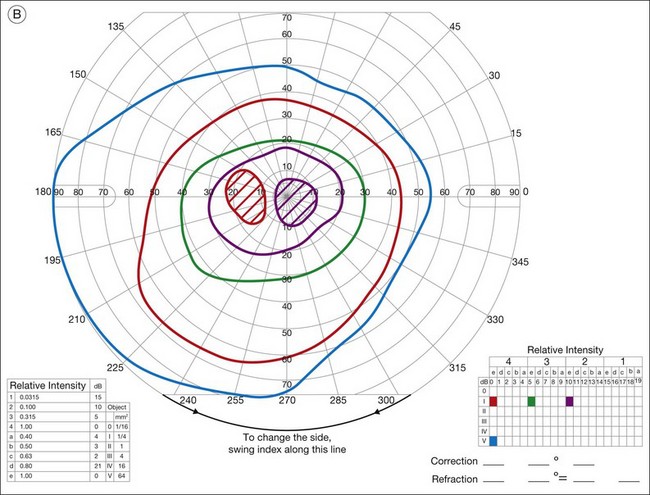
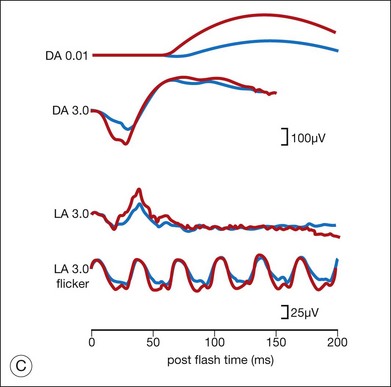
Ancillary testing with fluorescein angiography (FA) or optical coherence tomography (OCT) may show mild retinal vascular staining or leakage, or cystoid macular edema (CME) in some cases.12 Visual field testing may confirm scotomas and constricted visual fields. While static visual field testing may be better for demonstrating and following central and paracentral scotomas, kinetic visual fields are better for measuring peripheral field constriction. However, kinetic visual fields have the disadvantage of examiner dependence, the need for patient cooperation, inter-tester variability, and lack of standardization of parameters.62,63 ERG may demonstrate abnormal rod, cone, Müller cell, and bipolar cell responses and delay in implicit times.12,13,26,32 However, the majority of studies involving ERG findings in AIR come from paraneoplastic retinopathies (Fig. 77.2).
AIR is almost always bilateral, although involvement can be asymmetric. A family or a personal history of systemic autoimmune disease can be common among patients with nonparaneoplastic AIR.12,33 There is a female preponderance (63–66%), and average age at diagnosis appears to range from 51 years to 56 years.12,13,20 The typical patient would be a middle-aged or older adult in their fifth to sixth decades with no history of visual problems prior to the onset of photopsias, scotomas, and other symptoms consistent with AIR, and no family history of RP.
As might be expected for an entity with no consensus in diagnosis, retrospective studies in patients with nonparaneoplastic AIR showed that clinical features vary considerably. In one study, diffuse retinal atrophy was seen in the majority of patients (83%) and pigment deposits in only a small proportion (13%), macular edema was present in approximately half of the cases, while another study showed pigmentary changes in approximately half of the patients and macular edema in 24%. The most common symptoms at onset were subacute vision loss, photopsias, and nyctalopia. Similarly, in a series of 12 patients with antienolase-associated retinopathy, clinical characteristics included visual loss, normal-appearing fundus in most except for vascular attenuation, optic nerve head pallor in some, and mainly abnormal cone responses. In all of these studies, however, there was a female predominance and the age at onset was similar.12,13,20,26,
Diagnosis
The diagnosis of AIR is difficult as there are no definitive or standardized tests. The presumptive diagnosis relies on the presence of the above clinical manifestations, usually more than one, along with demonstration of serum antiretinal antibodies. Most patients will have more than one antiretinal antibody. If the clinical features of autoimmune retinopathy and the circulating antiretinal antibodies are present, and if there is no malignancy at presentation or following a thorough investigation, a diagnosis of nonparaneoplastic AIR is made (Table 77.2).
Table 77.2 Diagnostic criteria for nonparaneoplastic AIR (proposed by the authors)
| Must have all of the criteria | Must have ≥2 of the criteria |
|---|---|
| No evidence of malignancy | Symptoms: Photopsias or scotomas or dychromatopsia or nyctalopia or photoaversion |
| No family history of RP | Fundus changes* |
| (+) serum antiretinal antibodies | Visual field abnormality* |
| ERG abnormality* |
* Fundus changes, visual field or ERG abnormalities as described in clinical manifestations and diagnosis section (see text).
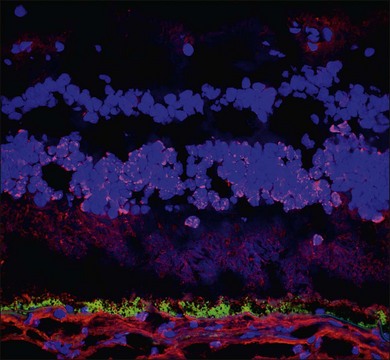
Demonstration of serum antiretinal antibodies can be done using various methods, including Western blot (WB), immunohistochemistry (IHC) or enzyme-linked immunosorbent assay (ELISA). Western blot is a protein immunoblot of patients’ serum incubated with extracts of a normal donor human retina and shows antiretinal IgG bands. It identifies antibody activity based on size of the protein, and the interpretation relies on intensity of protein bands on a photographic film. The results may be affected by multiple technical factors and lack specificity. For example, a 23 kDa band on WB does not necessarily mean antibody against recoverin; there may be other antigens with the same size. The immunohistochemical detection of antiretinal antibodies, on the other hand, involves fixing patient serum against frozen donor human retina (or monkey or mouse retina) using immunohistochemical staining. Sections are then analyzed using light microscopy to determine which layers of the retina show binding of the antiretinal antibody. The advantage with IHC is the ability to localize the specific site of binding within the retina (Fig. 77.3). ELISA is similar, in principle, to WB and IHC and involves adding various dilutions of patient sera into wells coated with specific retinal proteins and binding is detected using secondary antibodies.10,64 All of these techniques, however, lack standardization.15 Moreover, it is important to remember that the mere presence of these autoantibodies does not warrant a diagnosis of AIR, nor does it mean they are pathogenic. Antiretinal antibodies have been detected in patients with other retinal diseases, uveitis, retinal degenerations including age-related macular degeneration, and even in normal controls.21,65–68 Conversely, they can be negative in patients with clinical features of what would be considered AIR.20 Despite the caveats, there are few centers in the United States that perform antiretinal antibody testing. One center provides these services commercially through a CLIA (clinical laboratory improvement amendments) certified laboratory.69 WB and IHC are the more commonly performed techniques.
Autoimmune retinopathy, similar to paraneoplastic retinopathy, can be associated with various antiretinal antibodies. Initially detected autoantibodies in nonparaneoplastic AIR were antirecoverin antibodies.17 Later, antibodies directed against the inner plexiform layer,11 Müller cells (35 kDa) or other unidentified retinal proteins have been described.32,64 One of the most commonly detected antibodies on WB in nonparaneoplastic AIR appears to be one with a molecular weight of about 35 kDa which was detected in about 25% of autoimmune retinopathies.20 Autoimmune retinopathy can be associated with systemic autoimmune diseases, especially those that are autoantibody-mediated. In fact, cases of nonparaneoplastic AIR have been described in the setting of systemic lupus erythematosus.12,70
In a large cohort, only 47% of patients who presented with signs and symptoms compatible with AIR had any detectable antiretinal antibodies. Of these, patients with a history of cancer were more likely to have detectable antiretinal antibodies (63.5%) than those who did not (41.1%).20 In the same study, the authors showed antirecoverin antibodies exclusively in the paraneoplastic AIR group, antibodies against 35 kDa protein were mostly found in nonparaneoplastic AIR patients’ sera whereas anti-α-enolase was somewhat equally distributed in both groups. The fact that half of patients with symptoms and signs compatible with AIR tested negative for any antiretinal antibody with the techniques currently available, highlights the difficulty of diagnosis.
Differential diagnosis
1. Paraneoplastic disorders (e.g., CAR, MAR)
2. White-dot syndrome spectrum disorders (e.g., acute zonal occult outer retinopathy)
3. Retinal degenerative disorders (e.g., retinitis pigmentosa, cone–rod dystrophy)
Paraneoplastic retinopathies, similar to nonparaneoplastic AIR, are characterized by vision loss, photopsias, nyctalopia, and scotomas. Cancer-associated retinopathy is typically associated with antirecoverin antibody, and most commonly associated with small-cell carcinoma of the lung. It is important to remember that a non-neoplastic form of antirecoverin antibody-associated retinopathy has also been described.6,7,17 MAR occurs in patients with cutaneous melanoma and is characterized by a negative waveform on standardized full-field ERG due to reduction in b-wave amplitudes. In addition to similar symptoms as in nonparaneoplastic AIR, patients tend to have diffuse fundus depigmentation. Paraneoplastic retinopathies typically have a more rapid decline; with rapid progression of vision and visual field loss, as well as ERG changes, spontaneous recovery has not been observed. Vision loss can precede the identification of malignancy.60,61 It is important to differentiate paraneoplastic retinopathy from non-paraneoplastic AIR because of significant implications on treatment. The features of paraneoplastic retinopathy are covered in detail in Chapter 134 (Remote effects of cancer on the retina).
White-dot syndromes such as acute zonal occult outer retinopathy (AZOOR) or multiple evanescent white-dot syndrome (MEWDS) have been suggested to be associated with antiretinal antibodies.64,71 AZOOR and MEWDS have overlapping features and cases of MEWDS evolving into AZOOR have been described.72 However, immunohistochemical testing of sera of patients with AZOOR failed to show antiretinal antibodies.73 AZOOR can present with similar symptoms, visual field, and ERG findings to nonparaneoplastic AIR; it is typically bilateral but asymmetric and majority of patients either stabilize or show partial recovery without treatment. MEWDS, despite having similar symptoms, is a unilateral retinopathy which is characterized by afferent pupillary defect, optic nerve swelling and spontaneous recovery, and hence is more readily differentiated from AIR. Both AZOOR and MEWDS may show enlarged blind spot on visual fields. In addition, the majority of eyes affected by AZOOR may show fundus autofluorescence abnormalities which has not been observed in AIR.33,74,75
Antiretinal antibodies have also been reported to occur in patients with RP, a hereditary retinal degeneration. However, up to 60% of patients with RP may lack a family history of retinal degeneration. Indeed, some patients who are eventually diagnosed as nonparaneoplastic AIR are initially diagnosed as isolated cases of RP. Approximately 10% to 37% of patients with RP may have circulating antiretinal antibodies.10,76 Additionally, 90% of patients with RP and macular cysts have circulating antiretinal antibodies on WB, compared with 13% of patients with RP without macular cysts and 6% of controls.21 Most commonly detected antibodies are targeted against carbonic anhydrase II and α-enolase.18 In some cases of RP, rapid progression of visual field loss and CME appears to be associated with presence of antiretinal antibodies.21,65 In retinal degenerations, whether the antibodies precede the onset of retinopathy or whether antiretinal autoantibodies are a consequence of retinal damage is unclear. Additionally, whether the presence of these autoantibodies has a significant impact on clinical course has yet to be determined.
Antiretinal antibodies have also been found in patients with retinal vasculitis, uveitis patients with Vogt–Koyanagi–Harada syndrome (VKH), Behçet disease, and sympathetic ophthalmia. In patients with VKH, antibody reactivity to photoreceptors correlated with disease activity. However, all these syndromes are characterized by significant intraocular inflammation in addition to their unique fundus findings, making the differentiation rather unproblematic. Other rare cases of retinopathies associated with antiretinal antibodies include onchocerciasis and ocular toxoplasmosis. Antibodies to retinal pigment epithelium (RPE), neural retina or photoreceptor layer has been described in these infectious retinopathies.60,68,77 Typical fundus findings in these entities are helpful in differentiating them from nonparaneoplastic AIR. In all of the aforementioned diseases, it is unclear if the antibodies preceded the retinal disease or if the immune reactivity is simply a consequence of the retinal degenerative process.
Treatment and prognosis
Various forms of immunomodulatory approaches have been tried in an attempt to treat autoimmune retinopathy. Theoretically, because of the presumed autoimmune nature of the disease, immunosuppression for nonparaneoplastic AIR is the most sensible treatment strategy. However, because of the ambiguity in diagnosis and lack of standardization in therapeutic outcomes, management of AIR poses an enormous challenge. The immunosuppressive therapy can be considered empiric at this time because of our lack of understanding of this condition. If undertaken, a long-term treatment is needed in most cases and therapy is not helpful once widespread retinal degeneration occurs. Most treatment-related case series are regarding paraneoplastic retinopathy and include chemotherapy, corticosteroids, intravenous immunoglobulin (IVIG), or plasmapheresis.22 Recently, successful treatment of autoimmune retinopathy or associated cancer with more targeted biologic agents have also been reported.35,78,79
Immunosuppressive therapy should be administered by a rheumatologist, immunologist or a uveitis specialist well versed in the management of such therapy. Immunosuppressive agents such as mycophenolate mofetil, cyclosporine or corticosteroids may help improve visual function in some autoimmune retinopathy patients. In a cohort of 24 nonparaneoplastic AIR patients that received therapy with various combinations of prednisone, cyclosporine, azathioprine, mycophenolate mofetil, periocular or intravitreal steroid injections, 15 of the 24 showed varying degrees of improvement in visual acuity or visual field. CME improved in almost half of the patients, unfortunately, ERG was not routinely performed. Decrease in antiretinal antibodies following treatment may be seen in some cases,12,22,60 however clinical significance of this finding is unclear.
In addition to systemic and local corticosteroids, most commonly used immunosuppressive agents in the treatment of AIR include intravenous immunoglobulin (IVIG), antimetabolites such as mycophenolate mofetil, azathioprine, and T-cell inhibitors such as cyclosporine.12 IVIG has multiple mechanisms of action, some of which are not completely understood. When used in the treatment of autoimmune disorders, its effect is believed to be due to its interaction with Fc receptors on effector cells or by acting as anti-idiotypic antibodies directed against idiotypes on circulating autoantibodies. This mechanism may be at play in autoantibody-mediated diseases. Other mechanisms may involve clearance of immune complex deposits, presence of neutralizing antibodies in IVIG, its effect on the number of T-cell subsets, proinflammatory monocytes or regulatory T cells.80 IVIG has been used in several uveitic syndromes refractive to conventional therapy. Its use is limited by cost and long infusions. Typical dose ranges between 1 and 2.5 g/kg each infusion, and the infusions can be administered every 4–8 weeks although different protocols have been used. Major side-effects include hypersensitivity and anaphylactic reactions and thrombotic events.81 Similarly, plasmapheresis has also been used in paraneoplastic autoimmune retinopathy. Plasmapheresis, also known as therapeutic plasma exchange, involves extracorporeal elimination of large molecular weight plasma proteins from the blood. The regimen for plasmapheresis is determined according to the pathologic substance desired to be removed. To replace volume, albumin, albumin–saline combination or fresh-frozen plasma can be used, the latter is preferred to avoid depletion of coagulation factors and immunoglobulins. Its effect is believed to be due to removal of immune complexes and immune reactants. The effect may be rapid but is often short-lived, therefore, more sustainable immunomodulation is often required. Major side-effects include paresthesias, muscle cramps, urticarial and anaphylactic reactions.22
Less frequently, targeted B-cell therapy, such as anti-CD20 monoclonal antibody (Rituximab), has also been used in the treatment of AIR. Rituximab targets CD20 found on B cells which are the precursors of antibody-secreting plasma cells.20,79 Although there is anecdotal evidence, the benefit of immunosuppressive therapy in AIR is also not definite. There are no clear guidelines on how to institute and manage immunosuppressives in patients with AIR, owing to the rarity and ambiguity surrounding this entity. Therefore, the treating physician typically extrapolates from guidelines that are established for other ocular inflammatory disorders.82 These drugs can take several weeks to have an effect and it may take up to several months to observe a clinical effect in the form of improvement or stabilization on visual fields or ERG. More rapid improvement has been reported in CAR patients treated with IVIG.22
The response to treatment is very variable, with more favorable results achieved in paraneoplastic retinopathy, particularly CAR, with a combination of chemotherapy and immunomodulation. It has also been suggested that those with a family history of autoimmune disorders may be less likely to respond to immunosuppression.12,83 Our experience indicates that with treatment only a minority of patients with AIR show improvement in visual function, and some remain stable. This may be due to late presentation to our center with significant loss seen on both ERG and visual fields at presentation. Whether an earlier attempt to treat with immunosuppressives would be more beneficial is not clear. Early treatment attempts would require establishing a clear diagnosis using sensitive and specific assays and more definitive clinical criteria. While most of the immunosuppressives used in the treatment of AIR can be instituted and managed safely, a better understanding of the disease is needed to justify more aggressive and potentially beneficial treatment approaches.
1 Weinstein JM, Kelman SE, Bresnick GH, et al. Paraneoplastic retinopathy associated with antiretinal bipolar cell antibodies in cutaneous malignant melanoma. Ophthalmology. 1994;101:1236–1243.
2 Jacobson DM, Thirkill CE, Tipping SJ. A clinical triad to diagnose paraneoplastic retinopathy. Ann Neurol. 1990;28:162–167.
3 Sawyer RA, Selhorst JB, Zimmerman LE, et al. Blindness caused by photoreceptor degeneration as a remote effect of cancer. Am J Ophthalmol. 1976;81:606–613.
4 Klingele TG, Burde RM, Rappazzo JA, et al. Paraneoplastic retinopathy. J Clin Neuroophthalmol. 1984;4:239–245.
5 Paraneoplastic diseases of neuro-ophthalmologic interest. In: Jacobson DM, Miller NR, Newman NJ. Walsh & Hoyt’s clinical neuro-ophthalmology. 5th ed. Baltimore, MD: Williams & Wilkins; 2008:2497–2551.
6 Thirkill CE, Roth AM, Keltner JL. Cancer-associated retinopathy. Arch Ophthalmol. 1987;105:372–375.
7 Keltner JL, Thirkill CE. Cancer-associated retinopathy vs recoverin-associated retinopathy. Am J Ophthalmol. 1998;126:296–302.
8 Vaphiades MS, Brown H, Whitcup SM. Node way out: Comments by Keltner JL, Thirkill CE. Surv Ophthalmol. 2000;45:77–83.
9 Weinstein JM, Kelman SE, Bresnick GH. Paraneoplastic retinopathy associated with antiretinal bipolar cell antibodies in cutaneous malignant melanoma. Ophthalmology. 1994;101:1236–1243.
10 Potter MJ, Thirkill CE, Dam OM. Clinical and immunocytochemical findings in a case of melanoma-associated retinopathy. Ophthalmology. 1999;106:2121–2125.
11 Mizener JB, Kimura AE, Adamus G. Autoimmune retinopathy in the absence of cancer. Am J Ophthalmol. 1997;123:607–618.
12 Ferreyra HA, Jayasundera T, Khan NW, et al. Management of autoimmune retinopathies with immunosuppression. Arch Ophthalmol. 2009;127:390–397.
13 Larson TA, Gottlieb CC, Zein WM, et al. Autoimmune retinopathy: prognosis and treatment. Invest Ophthalmol Vis Sci. 51, 2010. E-Abstract 6375
14 Adamus G. Autoantibody-induced apoptosis as a possible mechanism of autoimmune retinopathy. Autoimmunity Rev. 2003;2:63–69.
15 Forooghian F, Macdonald IM, Heckenlively JR, et al. The need for standardization of antiretinal antibody detection and measurement. Am J Ophthalmol. 2008;146(4):489–495.
16 Kondo M, Sanuki R, Ueno S, et al. Identification of autoantibodies against TRPM1 in patients with paraneoplastic retinopathy associated with ON bipolar cell dysfunction. PLoS One. 2011;6(5):e19911.
17 Whitcup SM, Vistica BP, Milam AH, et al. Recoverin-associated retinopathy: a clinically and immunologically distinctive disease. Am J Ophthalmol. 1998;126:230–237.
18 Heckenlively JR, Fawzi AA, Oversier J, et al. Autoimmune retinopathy: patients with antirecoverin immunoreactivity and panretinal degeneration. Arch Ophthalmol. 2000;118(11):1525–1533.
19 Ohguro H, Ogawa K, Maeda T, et al. Cancer-associated retinopathy induced by both anti-recoverin and anti-hsc70 antibodies in vivo. Invest Ophthalmol Vis Sci. 1999;40(13):3160–3167.
20 Adamus G, Ren G, Weleber RG. Autoantibodies against retinal proteins in paraneoplastic and autoimmune retinopathy. BMC Ophthalmol. 2004;4:5.
21 Heckenlively JR, Jordan BL, Aptsiauri N. Association of antiretinal antibodies and cystoid macular edema in patients with retinitis pigmentosa. Am J Ophthalmol. 1999;127:565–573.
22 Guy J, Aptsiauri N. Treatment of paraneoplastic visual loss with intravenous immunoglobulin: report of 3 cases. Arch Ophthalmol. 1999;117:471–477.
23 Polans AS, Burton MD, Haley TL, et al. Recoverin, but not visinin, is an autoantigen in the human retina identified with a cancer-associated retinopathy. Invest Ophthalmol Vis Sci. 1993;34:81–90.
24 Keltner JL, Thirkill CE, Tyler NK, et al. Management and monitoring of cancer-associated retinopathy. Arch Ophthalmol. 1992;110:48–53.
25 Adamus G, Amundson D, Seigal GM, et al. Anti-enolase a autoantibodies in cancer-associated retinopathy: epitope mapping and cytoxicity on retinal cells. J Autoimmun. 1998;11:671–677.
26 Weleber RG, Watzke RC, Shults WT, et al. Clinical and electrophysiologic characterization of paraneoplastic and autoimmune retinopathies associated with antienolase antibodies. Am J Ophthalmol. 2005;139:780–794.
27 Ren G, Adamus G. Cellular targets of anti-alpha-enolase autoantibodies of patients with autoimmune retinopathy. J Autoimmun. 2004;23:161–167.
28 Adamus G, Aptsiauri N, Guy J, et al. The occurrence of serum autoantibodies against enolase in cancer-associated retinopathy. Clin Immunol Immunopathol. 1996;78:120–129.
29 Kikuchi T, Arai J, Shibuki H, et al. Tubby-like protein 1 as an autoantigen in cancer-associated retinopathy. J Neuroimmunol. 2000;103:26–33.
30 Potter MJ, Adamus G, Szabo SM, et al. Autoantibodies to transducin in a patient with melanoma-associated retinopathy. Am J Ophthalmol. 2002;134:128–130.
31 Bazhin AV, Dalke C, Willner N, et al. Cancer-retina antigens as potential paraneoplastic antigens in melanoma-associated retinopathy. Int J Cancer. 2009;124:140–149.
32 Peek R, Verbraak F, Coevoet HM, et al. Muller cell-specific autoantibodies in a patient with progressive loss of vision. Invest Ophthalmol Vis Sci. 1998;39:1976–1979.
33 Mantel I, Ramchand KV, Holder GE, et al. Macular and retinal dysfunction of unknown origin in adults with normal fundi: evidence for an autoimmune pathophysiology. Exp Mol Pathol. 2008;84:90–101.
34 Keltner JL, Thirkill CE. The 22-kDa antigen in optic nerve and retinal diseases. J Neuroophthalmol. 1999;19:71–83.
35 Espandar L, O’Brien S, Thirkill C, et al. Successful treatment of cancer-associated retinopathy with alemtuzumab. J Neurooncol. 2007;83:295–302.
36 Ohkawa T, Kawashima H, Makino S, et al. Cancer-associated retinopathy in a patient with endometrial cancer. Am J Ophthalmol. 1996;122:740–742.
37 Murphy MA, Thirkill CE, Hart WM, Jr. Paraneoplastic retinopathy: a novel autoantibody reaction associated with small-cell lung carcinoma. J Neuroophthalmol. 1997;17:77–83.
38 Masaoka N, Emoto Y, Sasaoka A, et al. Fluorescein angiographic findings in a case of cancer-associated retinopathy. Retina. 1999;19:462–464.
39 McAleese SM, Dunbar B, Fothergill JE, et al. Complete amino acid sequence of the neurone-specific gamma isozyme of enolase (NSE) from human brain and comparison with the non-neuronal alpha form (NNE). Eur J Biochem. 1988;178:413–417.
40 Adamus G, Wilson DJ. The need for standardization of antiretinal antibody detection and measurement. Am J Ophthalmol. 2009;147(3):557. author reply 557–8
41 Shin SJ, Kim BC, Kim TI, et al. Anti-alpha-enolase antibody as a serologic marker and its correlation with disease severity in intestinal Behçet’s disease. Dig Dis Sci. 2011;56(3):812–818.
42 Lee JH, Cho SB, Bang D, et al. Human anti-alpha-enolase antibody in sera from patients with Behçet’s disease and rheumatologic disorders. Clin Exp Rheumatol. 2009;27(2 Suppl 53):S63–S66.
43 Forooghian F, Adamus G, Sproule M, et al. Enolase autoantibodies and retinal function in multiple sclerosis patients. Graefes Arch Clin Exp Ophthalmol. 2007;245(8):1077–1084.
44 Vermeulen N, Arijs I, Joossens S, et al. Anti-alpha-enolase antibodies in patients with inflammatory bowel disease. Clin Chem. 2008;54:534–541.
45 Pancholi V. Multifunctional a-enolase: its role in diseases. Cell Mol Life Sci. 2001;58:902–920.
46 Thirkill CE, Tait RC, Tyler NK, et al. Intraperitoneal cultivation of small-cell carcinoma induces expression of the retinal cancer-associated retinopathy antigen. Arch Ophthalmol. 1993;111:974–978.
47 Bazhin AV, Shifrina ON, Savchenko MS, et al. Low titre autoantibodies against recoverin in sera of patients with small cell lung cancer but without a loss of vision. Lung Cancer. 2001;34:99–104.
48 Polans A, Witkowska D, Haley T, et al. Recoverin, a photoreceptor-specific calcium-binding protein, is expressed by the tumor of a patient with cancer-associated retinopathy. Proc Natl Acad Sci U S A. 1995;92:9176–9180.
49 Matsubara S, Yamaji Y, Soto M, et al. Expression of a photoreceptor protein, recoverin, as a cancer-associated retinopathy autoantigen in human lung cancer cell lines. Br J Cancer. 1996;74:1419–1422.
50 Adamus G. Autoantibody-induced apoptosis as a possible mechanism of autoimmune retinopathy. Autoimmun Rev. 2003;2(2):63–68.
51 Chen W, Elias RV, Cao W, et al. Anti-recoverin antibodies cause the apoptotic death of mammalian photoreceptor cells in vitro. J Neurosci Res. 1999;57:706–718.
52 Adamus G, Webb S, Shiraga S, et al. Anti-recoverin antibodies induce an increase in intracellular calcium, leading to apoptosis in retinal cells. J Autoimmun. 2006;26:146–153.
53 Adamus G, Machnicki M, Elerding H, et al. Antibodies to recoverin induce apoptosis of photoreceptor and bipolar cells in vivo. J Autoimmun. 1998;11:523–533.
54 Adamus G, Machnicki M, Seigel GM. Apoptotic retinal cell death induced by antirecoverin autoantibodies of cancer-associated retinopathy. Invest Ophthalmol Vis Sci. 1997;38:283–291.
55 Gery I, Chanaud NP, 3rd., Anglade E. Recoverin is highly uveitogenic in Lewis rats. Invest Ophthalmol Vis Sci. 1994;35:3342–3345.
56 Lu Y, He S, Jia L, et al. Two mouse models for recoverin-associated autoimmune retinopathy. Mol Vis. 2010;16:1936–1948.
57 Williams RC, Peen E. Apoptosis and cell penetration by autoantibody may represent linked processes. Clin. Exp. Rheumatol. 1999;17:643–647.
58 Lei B, Bush RA, Milam AH, et al. Human melanoma-associated retinopathy (MAR) antibodies alter the retinal ON-response of the monkey ERG in vivo. Invest Ophthalmol Vis Sci. 2000;41:262–266.
59 Adamus G, Chan CC. Experimental autoimmune uveitides: multiple antigens, diverse diseases. Int Rev Immunol. 2002;21(2–3):209–229.
60 Hooks JJ, Tso MO, Detrick B. Retinopathies associated with antiretinal antibodies. Clin Diagn Lab Immunol. 2001;8:853–858.
61 Chan JW. Paraneoplastic retinopathies and optic neuropathies. Surv Ophthalmol. 2003;48:12–38.
62 Shapiro LR, Johnson CA. Quantitative evaluation of manual kinetic perimetry using computer simulation. Appl Opt. 1990;29:1445–1450.
63 Keltner JL, Johnson CA, Spurr JO, et al. Comparison of central and peripheral visual field properties in the optic neuritis treatment trial. Am J Ophthalmol. 1999;128:543–553.
64 Heckenlively JR, Ferreyra HA. Autoimmune retinopathy: a review and summary. Semin Immunopathol. 2008;30:127–134.
65 Heckenlively JR, Aptsiauri N, Nusinowitz S, et al. Investigations of antiretinal antibodies in pigmentary retinopathy and other retinal degenerations. Trans Am Ophthalmol Soc. 1996;94:179–200.
66 Cherepanoff S, Mitchell P, Wang JJ, et al. Retinal autoantibody profile in early age-related macular degeneration: preliminary findings from the Blue Mountains Eye Study. Clin Experiment Ophthalmol. 2006;34:590–595.
67 Joachim SC, Bruns K, Lackner KJ, et al. Analysis of IgG antibody patterns against retinal antigens and antibodies to alpha-crystallin, GFAP, and alpha-enolase in sera of patients with “wet” age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2007;245:619–626.
68 Ko AC, Brinton JP, Mahajan VB, et al. Seroreactivity against aqueous-soluble and detergent-soluble retinal proteins in posterior uveitis. Arch Ophthalmol. 2011;129:415–420.
69 Ocular Immunology Laboratory, Oregon Health & Science University [homepage on the Internet]. Portland, OR: OHS; no date [cited 2012 Feb] http//www.ohsu.edu/xd/health/services/casey-eye/clinical-services/diagnostic-services/upload/Ocular-Immunology-Web-2.pdf
70 Cao X, Bishop RJ, Forooghian F, et al. Autoimmune retinopathy in systemic lupus erythematosus: histopathologic features. Open Ophthalmol J. 2009;28(3):20–25.
71 Brown J, Jr., Folk JC. Current controversies in the white dot syndromes. Multifocal choroiditis, punctate inner choroidopathy, and the diffuse subretinal fibrosis syndrome. Ocul Immunol Inflamm. 1998;6(2):125–127.
72 Fine HF, Spaide RF, Ryan EH, Jr., et al. Acute zonal occult outer retinopathy in patients with multiple evanescent white dot syndrome. Arch Ophthalmol. 2009;127(1):66–70.
73 Jacobson SG, Morales DS, Sun XK, et al. Pattern of retinal dysfunction in acute zonal occult outer retinopathy. Ophthalmology. 1995;102:1187–1198.
74 Fujiwara T, Imamura Y, Giovinazzo VJ, et al. Fundus autofluorescence and optical coherence tomographic findings in acute zonal occult outer retinopathy. Retina. 2010;30:1206–1216.
75 Yeh S, Forooghian F, Wong WT, et al. Fundus autofluorescence imaging of the white dot syndromes. Arch Ophthalmol. 2010;128:46–56.
76 Galbraith GM, Emerson D, Fudenberg HH, et al. Antibodies to neurofilament protein in retinitis pigmentosa. J. Clin. Invest. 1986;78:865–869.
77 Chan CC, Nussenblatt RB, Kim MK, et al. Immunopathology of ocular onchocerciasis. 2. Antiretinal autoantibodies in serum and ocular fluids. Ophthalmology. 1987;94:439–443.
78 Sen HN, Chan CC, Caruso RC, et al. Waldenström’s macroglobulinemia-associated retinopathy. Ophthalmology. 2004;111:535–539.
79 Mahdi N, Faia LJ, Goodwin J, et al. A case of autoimmune retinopathy associated with thyroid carcinoma. Ocul Immunol Inflamm. 2010;18:322–323.
80 Kaveri SV, Lacroix-Desmazes S, Bayry J. The antiinflammatory IgG. N Engl J Med. 2008;17(359):307–309.
81 Le Hoang P, Cassoux N, George F, et al. Intravenous immunoglobulin (IVIg) for the treatment of birdshot retinochoroidopathy. Ocul Immunol Inflamm. 2000;8:49–57.
82 Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130:492–513.
83 Jacobson DM, Thirkill CE, Tipping SJ. A clinical triad to diagnose paraneoplastic retinopathy. Ann Neurol. 1990;28(2):162–167. Ophthalmol 1998;126:230–7