Chapter 47 Attention-Deficit Hyperactivity Disorder
Attention-deficit hyperactivity disorder (ADHD) has been described as the most common neurobehavioral disorder in childhood [Cantwell, 1996]. Prevailing opinion characterizes ADHD as a disorder of executive function attributable to abnormal dopamine transmission in the frontal lobes and frontostriatal circuitry. In large part, this concept is based on the clinical efficacy of medications affecting catecholamine transmission in these regions.
The first reference to behavior now associated with ADHD was by George Still in 1902, who referred to a deficit of “moral control.” Within the context of this broad concept, he made the following observation: “A notable feature in many of these cases of moral deficit without general impairment of intellect is a quite abnormal incapacity for sustained attention” [Still, 1902]. Strauss and Lehtinen [1947] used the term “minimal brain damage syndrome” to describe children with cognitive and behavioral deficits. In 1962, Clements and Peters coined the term “minimal brain dysfunction” to describe functional abnormalities in children in whom brain damage could not be demonstrated. Although widely accepted, this concept came under immediate challenge, as it included too heterogeneous a group of children [MacKeith, 1963]. The subsequent emphasis on attention and its neurologic substrate, the frontal lobe and frontostriatal circuitry, represents a refinement of the definition of the condition.
Diagnosis of Attention-Deficit Hyperactivity Disorder
ADHD is a clinical diagnosis based on criteria in the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (Box 47-1). Criteria are divided into two lists of symptoms, one for inattention and another for hyperactive-impulsive behavior. Based on the number of items identified, there are three classifications: ADHD/I (primarily inattentive type), ADHD/HI (primarily hyperactive-impulsive type), and ADHD/C (combined type). The revised diagnostic criteria of DSM-IV, with the inclusion of the three subtypes, increased the number of females, preschoolers, and adults with ADHD [Lahey et al., 1994]. Increased numbers in these groups resulted in an increase in the prevalence of ADHD from 3–5 percent with the DSM-III-R to about 12 percent; ADHD/I alone has been estimated to have a prevalence between 5.4 and 9 percent [Baumgaertel et al., 1995; Wolraich et al., 1996].
Box 47-1 Diagnostic Criteria for Attention-Deficit/Hyperactivity Disorder
Inattention
 often fails to give close attention to details or makes careless mistakes in schoolwork, work, or other activities
often fails to give close attention to details or makes careless mistakes in schoolwork, work, or other activities often does not follow through on instructions and fails to finish schoolwork, chores, or duties in the workplace (not due to oppositional behavior or failure to understand instructions)
often does not follow through on instructions and fails to finish schoolwork, chores, or duties in the workplace (not due to oppositional behavior or failure to understand instructions) often avoids, dislikes, or is reluctant to engage in tasks that require sustained mental effort (such as schoolwork or homework)
often avoids, dislikes, or is reluctant to engage in tasks that require sustained mental effort (such as schoolwork or homework)Hyperactivity
Impulsivity
It has been proposed that the core deficit in ADHD is impairment of behavioral inhibition, which leads to the other symptoms of ADHD. This model of impaired behavioral inhibition is limited to ADHD/HI and ADHD/C (i.e., those with hyperactive or impulsive symptoms) and excludes children with ADHD/I (i.e., those with inattention only) [Barkley, 1997]. The observation that overflow movements were the most discriminating finding between hyperactive boys (without learning disabilities) and normal control subjects seems to support the concept of impaired behavioral inhibition [Denckla and Rudel, 1978]. If this formulation is widely accepted, future classifications may call for separate diagnostic entities, such as attention-deficit disorder and behavioral-inhibition disorder. Some investigators have proposed that all three ADHD subtypes can be explained as disorders of attention or executive function (other than response inhibition), with symptoms of hyperactivity and impulsivity resulting from these impairments [Brown, 2000; Chhabildas et al., 2001; Weiss and Weiss, 2002]. Others also distinguish ADHD/HI and ADHD/C from ADHD/I, but they posit that the symptoms of hyperactivity and impulsivity can result from poor inhibitory control or differences in motivational style characterized by delay aversion [Sonuga-Barke, 2002].
A review of the literature regarding the hypothesis that ADHD represents a primary deficit in executive control defined executive function as comprising “at least four factors: (1) response inhibition and execution, (2) working memory and updating, (3) set-shifting and task-switching and (4) interference control” [Willcutt et al., 2005]. There were significant differences between children with and without ADHD on tasks assessing executive function. Six of eight studies assessing working memory found impaired working memory in children with ADHD. The most consistent effects were observed in measures of response inhibition, vigilance, and planning; children with combined and inattentive types of ADHD differed from controls and did not differ from each other, whereas children with hyperactive-impulsive ADHD had minimal executive function impairment, suggesting that executive function weaknesses are primarily associated with inattention, rather than hyperactivity-impulsivity symptoms. The observation that fewer than half of the children with ADHD had significant impairment of any specific task of executive function, and that the correlation, while significant, tended to be small in magnitude, led the authors to conclude that their findings “do not support the hypothesis that executive functions deficits are the single necessary and sufficient cause of ADHD in all individuals with the disorder. Instead executive function difficulties appear to be one of several important weaknesses that comprise the overall neuropsychological etiology of ADHD” [Willcutt et al., 2005].
Inhibitory deficits and delay aversion in ADHD can be dissociated by specific types of tasks; either deficit alone is only moderately associated with ADHD, whereas these two deficits combined correctly classify nearly 90 percent of children with ADHD. Thus a formulation was proposed in which executive functions (EF) are divided into cognitive aspects, associated with dorsolateral prefrontal cortex (“cool” EF), in contrast to the affective aspects, associated with orbital and medial prefrontal cortex (“hot” EF). Inattention symptoms were attributed to deficits in “cool” EF, whereas hyperactivity-impulsivity symptoms reflected “hot” EF deficits. The authors noted that “the neuroanatomical substrates of cortical-striato-thalamo-cortical circuitry are now revealed to include spirals of one directional information from ‘hot’ ventral-medial/orbital/ventral striatal regions to dorsolateral/superior medial/anterior striatal ‘cool’ regions to even ‘cooler’ premotor and motor circuits” [Castellanos et al., 2006].
The use of the word “often” in the list of symptoms, coupled with qualification “to a degree that is maladaptive and inconsistent with developmental level,” lends an element of subjectivity to this diagnostic schema. Symptom rating scales for parents and teachers have been developed to assist in the ascertainment of diagnostic criteria [Conners, 1997]. The use of broader rating scales, such as the Child Behavior Checklist, provides information regarding the presence of other disorders, such as conduct disorder, oppositional defiant disorder (ODD), and anxiety disorder [Achenbach, 1991; Vaughn et al., 2000], which may warrant diagnoses other than ADHD [Jensen et al., 1997]. The Yale Children’s Inventory was developed to ascertain the presence of attentional deficits and learning disabilities [Shaywitz et al., 1986]. A comprehensive review of evaluation issues in ADHD concluded that no single test can be used to make the diagnosis and that it is up to the clinician “to choose a battery of measures that satisfies what is, to some degree, an individually determined level of diagnostic certainty” [Nass, 2005]. The American Academy of Pediatrics has endorsed the Vanderbilt ADHD rating scales for parents and teachers [Wolraich et al., 2003a, b] and has provided a complete “tool kit,” including a cover letter to teachers and scoring information, on the internet (http://www.nichq.org/about/index.html).
Developmental variability in the presentation of ADHD, and the inconsistency of behavior of children with ADHD in different settings and at different times in the same setting, add to the diagnostic confusion. In preschool children, in particular, the prevalence of ADHD-type symptoms [Blackman, 1999; Palfrey et al., 1985] and the transient nature of such symptoms in many cases [Barkley, 1998] make this a difficult diagnosis. Efforts have been made to provide a more objective basis for the diagnosis of ADHD, such as computerized continuous performance tests [Conners, 1985] or tests of variables of attention [Greenberg, 1993]. However, the correlation of these measures of attention with the behavioral disorder is not sufficient for their use as replacements for the behavioral criteria of DSM-IV [Barkley, 1991].
The motor examination may help distinguish between children with a learning disorder and those with ADHD; it is best to evaluate a child between the age of 5 years and the onset of puberty, a period of rapid change in motor development, when quantitative examination of the motor system, such as the Physical and Neurological Examination for Soft Signs (PANESS) [Denckla, 1985], may demonstrate evidence of motor disinhibition [Denckla, 2003].
Controversies in the Diagnosis of Attention-Deficit–Hyperactivity Disorder
The DSM-IV clinical criteria for diagnosing ADHD (see Box 47-1) indicate a number of qualifications that are too often ignored, resulting in an incorrect diagnosis [American Psychiatric Association, 1994]. The text explicitly states that the findings need to be present “to a degree that is maladaptive and inconsistent with developmental level.” Behavior that may not be typical but is not maladaptive does not warrant a diagnosis of ADHD. Similarly, unreasonable expectations of a child at a young age may result in a false diagnosis. The diagnostic criteria are followed by a number of statements regarding the context of the symptoms. Item C states, “Some impairment from the symptoms is present in two or more settings (e.g., at school [or work] and at home).” This provision allows for the possibility that a child in an inadequate school environment, perhaps with excessive class size, hostile peers, or inexperienced teachers, may present with findings that are unique to that setting rather than represent a disorder of attention. Similarly, a chaotic home environment may explain the child’s presentation. The importance of verifying the presence of symptoms in two or more settings is underscored by a study that found an increase in the incidence of ADHD diagnoses in: states with school accountability laws; students with older or nonwhite teachers; children from a single-parent family, from the lowest income quintile, with a US-born father, or born to a young (<18 years) or older (>38 years) mother [Schneider and Eisenberg, 2006]. Item D reiterates that “there must be clear evidence of clinically significant impairment in social, academic, or occupational function.”
Perhaps most important is item E, which states, “The symptoms do not occur exclusively during the course of a pervasive developmental disorder, schizophrenia, or other psychotic disorder and are not better accounted for by another mental disorder (e.g., mood disorder, anxiety disorder, dissociative disorder, or a personality disorder).” If a child has symptoms that meet the diagnostic criteria for ADHD in the context of these other disorders, treatment should be directed at these other conditions before concluding that the child has a disorder of attention. Not addressed in item E of the DSM-IV criteria are studies that have demonstrated that children with specific neurologic disorders can present with symptoms that meet criteria for ADHD but are attributable to the neurologic disorder rather than a primary disorder of attention. A study by Walters et al. [2000] demonstrated symptoms of impaired attention and hyperactivity in children diagnosed with restless leg syndrome; treatment of the sleep disturbance resolved the so-called ADHD symptoms. Disordered breathing during sleep has also been found to manifest with symptoms consistent with ADHD [Gottlieb et al., 2003]. There are reports of children with focal epileptic discharges having symptoms suggestive of ADHD that resolved when the spike activity was suppressed with antiepileptic drugs [Holtmann et al., 2003; Laporte et al., 2002]. Many symptoms of ADHD are prevalent in individuals with neurogenetic syndromes as part of the behavioral phenotype [Pelc and Dan, 2008]. Future versions of the DSM should add neurological disorders (e.g., sleep disorders, epilepsy, neurogenetic syndromes) to the list of conditions in item E that must be excluded before ADHD is diagnosed. Refining the diagnosis of ADHD will facilitate ascertainment of the physiological and genetic underpinnings of ADHD and its treatment.
The question of conditions coexisting with ADHD is quite complex. Should a diagnosis of ADHD be reserved for individuals with an isolated disorder of attention, hyperactivity, or impulsivity, with an alternative classification used to describe children who meet DSM-IV criteria for ADHD in the context of other neurodevelopmental problems? Denckla [2003] used the term pseudo-ADHD to describe children with comorbidities or confounding factors. In a paper describing a father and son both with orbitofrontal epilepsy and associated attention difficulties and hyperactivity, the term attention-deficit hyperactivity syndrome was used to make a distinction from the specific disorder of ADHD [Powell et al., 1997], analogous to the distinction between Parkinson disease and parkinsonism. It has been proposed that ADHD be divided into subgroups based on the patterns of comorbidity [Biederman et al., 1991].
The presumption that a response to psychostimulant medication indicates that the underlying problem is ADHD can lead to an erroneous diagnosis. Psychostimulant medications can ameliorate depression [Janowsky, 2003], chronic fatigue syndrome [Turkington et al., 2004], and daytime somnolence caused by sleep disorders [Happe, 2003; Ivanenko et al., 2003], and enhance normal individuals’ cognitive functioning and behavior [Rapoport et al., 1978]. A positive response to psychostimulants has no diagnostic significance.
Neurobiology of Attention-Deficit Hyperactivity Disorder
Structural Imaging
Cortical Structures
Reports of reductions in volume of prefrontal regions, more so in the right than left hemisphere, have been described in children with ADHD [Castellanos et al., 1996; Filipek et al., 1997]. A later study further localized involvement to prefrontal and premotor areas [Mostofsky et al., 2002]. In this study of 12 males with ADHD, children with conduct, mood, and anxiety disorders were excluded, but 3 children with coexistent ODD were included. A study involving other brain regions reported reductions in total cerebral volume with a negative correlation between gray-matter volumes and symptom severity [Castellanos et al., 2002]. However, the impact of coexisting conditions on anatomic findings was not considered or described (i.e., it was unclear if there was an association between severity of symptoms and coexisting conditions). Serial examinations found that most volume differences between ADHD and control subjects remained stable; however, the size of the caudate nucleus, which initially was smaller in the ADHD group, became comparable with that in the control group during adolescence. This finding reflected a greater rate of reduction in caudate size in the normal than in the ADHD group. Normalization of the caudate nucleus in adolescents with ADHD may relate to the observation that ratings for hyperactivity and impulsivity are decreased in that age group compared with those in younger children [Hart et al., 1995]. A study using serial magnetic resonance imaging (MRI) scans to measure cortical thickness over time found that typically developing children without ADHD reached peak cortical thickness in the frontal cortex between the ages of 7 and 8 years. In contrast, children with ADHD reached this developmental milestone between the ages of 10 and 11 years. Both groups of children underwent cortical thinning from this peak point of thickness throughout adolescence [Shaw et al., 2007]. A subsequent study compared repeated neuroimaging studies of children with ADHD not taking psychostimulants to an age-matched group of children with ADHD who were taking psychostimulant medication during the inter-scan interval. Comparison was also made to a group of children without ADHD. The decision whether or not to treat with psychostimulants was left up to the treating physician and the family, thus was not randomly assigned. A comparison of the groups taking stimulants and not taking stimulants showed no significant differences in gender, IQ, or clinical characteristics. The neuroanatomic analysis revealed that there was more rapid cortical thinning in the group not taking psychostimulants (0.15 mm/yr) compared to the group taking psychostimulants (0.03 mm/yr). Thus, whereas at baseline there were no significant group differences in cortical thickness, at the end of the study the non-treatment group had a significantly thinner cortex than the treatment group. The authors hypothesize that the “psychostimulant induced increases in age appropriate levels of cognition and action, and perhaps underlying localized fronto-parietal neural activity, might foster cortical development within the normal range” [Shaw et al., 2009].
Subcortical Structures
Findings in the basal ganglia have been inconsistent, with reports of volume reductions in the right caudate nucleus and globus pallidus [Castellanos et al., 1996] or in the left caudate [Filipek et al., 1997]. The study by Castellanos et al. [1996] included children with “mild–moderate” conduct disorder (CD), ODD, anxiety disorder, and reading disorders. However, re-analysis of the data by excluding the children who had CD or ODD found a more robust correlation between volume reductions in the right prefrontal, caudate, and globus pallidus and ADHD. In the study by Filipek et al. [1997], children with coexistent conditions were excluded. In addition to the anatomic differences between children with ADHD and control subjects, this study revealed differences in structural abnormalities between children with ADHD who were considered responders to psychostimulants and those who were not. A study of monozygotic twins discordant for ADHD [Castellanos et al., 2003] revealed reduced caudate volume in the affected twin. In another report on twins discordant for ADHD [Sharp et al., 2003], fathers of twins discordant for ADHD had lower ADHD scores than fathers of ADHD singletons. The rate of breech presentation was greater in affected twins than affected singletons. The data suggested that the discordant twins represented nongenetic instances of ADHD, possibly caused by injury in utero, and that the caudate abnormalities in these individuals might not be pertinent to ADHD that is genetic in nature. No abnormalities have been reported in the putamen, and there have been few studies of the globus pallidus in children with ADHD [Durston et al., 2003]. A study utilizing large deformation diffeomorphic mapping (LDDMM) found that boys with ADHD had significant shape differences and decreases in overall volume of the basal ganglia compared to controls, whereas girls with ADHD did not have volume or shape differences. Children with comorbidities, including other neuropsychiatric disorders, conduct disorders, mood disorder, generalized anxiety disorder, obsessive-compulsive disorder, learning disabilities, or speech and language disorders, were excluded from this analysis [Qiu et al., 2009].
Cerebellum
Reductions in total cerebellar volume [Castellanos et al., 1996, 2002] and in the volume of the cerebellar vermis alone in ADHD compared with control subjects have been described [Castellanos et al., 2001; Mostofsky et al., 1998]. These differences could have been caused by different methods for serially measuring volume, making comparisons between studies difficult. These studies included children who had a high percentage of coexistent conditions, such as ODD, CD, and learning, mood, and anxiety disorders, but the decreased volume of the cerebellar vermis in the ADHD group remained when children with disruptive behavioral disorders were removed from the analysis. However, the subgroup with ADHD and coexisting mood or anxiety disorders had the smallest vermian volumes.
Functional Imaging
The clinical benefit from medications affecting catecholamine levels has led to a focus on frontostriatal circuitry and dopamine pathways in ADHD. Functional magnetic resonance imaging (fMRI) studies have demonstrated abnormal activation of frontostriatal regions in children with ADHD. In normal children, maturation is associated with increased activation of the ventral frontostriatal regions and improved inhibitory control [Durston et al., 2002]. A comparison of ADHD with normal control subjects demonstrated greater frontal activation and lower striatal activation during response inhibition in 10 children with ADHD (8 ADHD/C, 2 ADHD/I; children with high comorbidity scores were excluded). Administration of methylphenidate also resulted in improved performance in a test of response inhibition, associated with increased frontal activation in ADHD children and control subjects, and increased striatal activation in the children with ADHD [Vaidya et al., 1998].
Single-photon emission computed tomography (SPECT) has been used to investigate children with ADHD. One study compared 8 adolescents with “pure” ADHD against 11 with ADHD and coexistent conditions during a test of variables of attention (TOVA) [Lorberboym et al., 2004]. Children with coexistent conditions (e.g., ODD, CD, mood disorders, learning disorder alone or in combination) had decreased temporal lobe perfusion in response to the TOVA compared with the pure ADHD children, who had some but not statistically significant decreases in frontal lobe perfusion. Regional differences in perfusion between the two groups may explain the better rate of response to stimulants in the pure ADHD group and suggests that different treatments for the two groups may be warranted.
Untreated adults with ADHD (with no psychiatric comorbidity) have increased striatal dopamine transporter (DAT) levels compared with normal control subjects (as measured by binding to technetium-99m TRODAT-1, the first 99mTc-labeled ligand identified by SPECT that specifically binds DAT), which decreased after 4 weeks of methylphenidate treatment [Krause et al., 2000]. This finding, along with increased striatal activity on positron emission tomographic (PET) scanning in adolescents with ADHD compared with normal control subjects [Ernst et al., 1999], suggests a role for excess dopaminergic activity in the striatum or nucleus accumbens in persons with ADHD [Solanto, 2002].
Proton MR spectroscopy has also been used to study children with ADHD [Sparkes et al., 2004]. N-acetyl-aspartate (NAA), glutamate/glutamine/γ-aminobutyric acid (Glx), choline, and creatine (Cre) levels in the right prefrontal cortex and left striatum during a test of response inhibition were compared between ADHD children and a control group. A negative correlation between the NAA/Cre ratio and reaction time in the ADHD group was found, compared with a positive correlation in the control group. Children with ADHD with NAA/Cre levels more comparable with those in controls also had much longer reaction times. These findings were thought to reflect preferential use of the prefrontal cortex by children with ADHD during tasks of response inhibition. Of the 8 children with ADHD in this study, 5 had ODD, and 1 had a generalized anxiety disorder; the interpretation of these results, as they apply to ADHD compared with other disorders, is unclear.
Clinical Neurophysiology
Event-related potential (ERP) studies in ADHD children suggest a lack of frontal lobe inhibitory processes, particularly in pathways involving the anterior cingulate cortex. In one study using a Go/NoGo task designed to assess inhibition, no significant performance differences were found between children with ADHD and normal control subjects [Smith et al., 2003]. However, children with ADHD had larger ERPs than the control group to a warning stimulus that provided no information helpful for task performance, suggesting a lack of inhibition to an irrelevant stimulus in the ADHD group. A second study found shorter-latency and higher-amplitude ERPs that were thought to reflect an inhibitory process in the ADHD group [Falkenstein et al., 1999]. These findings suggested that children with ADHD need to trigger inhibition processes earlier and more strongly to achieve the same behavioral performance as control subjects. Individuals in this study likely did not represent a pure ADHD group because they had higher scores in oppositional, delinquent, and aggressive behaviors and social problems. A third study found that the children with ADHD and without coexisting conditions had significantly longer reaction times to target stimuli and made significantly more omission errors than the control group, but did not differ in the number of commission errors [Fallgatter et al., 2004]. The ERP data indicated diminished activation of the anterior cingulate cortex in the Go/NoGo trials in the ADHD group, suggesting deficits in prefrontal response control. This deficit in prefrontal response control was distinguished from deficits in response inhibition. Because the latter study excluded ADHD children with comorbidity, it more strongly suggests that abnormalities in activation of the anterior cingulate cortex may be specific to ADHD.
Genetic Studies
Concise reviews of advances in the genetics of ADHD, including findings that may account for the ADHD subtypes, comorbidities and responses to specific medications, are provided in a commentary and editorial in journal issues devoted to this topic. As summarized by DV Pauls: “there is overwhelming evidence that ADHD is inherited and that genetic factors play a significant role in its manifestation” [Pauls, 2005; Faraone, 2006]. Evidence of dopaminergic involvement has led to molecular genetic studies of dopamine transporter and receptor genes [Kent, 2004]. Pursuit of the DAT gene (SLC6A3, formerly designated DAT1) was in part caused by the fact that psychostimulant medications inhibit activity of DAT. An association between ADHD and the 480-base pair alleles at a variable number tandem repeat (VNTR) in SLC6A3 has been reported [Cook et al., 1995]. A subsequent study confirmed these findings and demonstrated a significant relation between SLC6A3 high-risk alleles and the number of hyperactive-impulsive symptoms, but not inattentive symptoms [Waldman et al., 1998]. The study involved 117 probands, all but one of whom met criteria for ADHD; the remaining child had ODD. Most children with ADHD frequently had symptoms of or were diagnosed with ODD, CD, and depression or dysthymia. Two subsequent studies, one with a similar rate of coexisting conditions [Palmer et al., 1999] and one with a much lower rate [Swanson et al., 2000], failed to replicate the association between SLC6A3 and ADHD.
The dopamine D4 receptor gene (DRD4) has also been associated with ADHD. A 48-base pair VNTR in the third exon of DRD4, also referred to as the DRD4 7-repeat allele, was suggested based on a review of previous studies [Faraone et al., 200l]. Children with ADHD who had the 7-repeat allele had a greater degree of impulsivity (i.e., faster and less accurate responses), were significantly more active (based on Actigraph measures), and had greater total ADHD symptoms scores than those without the allele. However, no differences were seen using measures of attention or response inhibition. The ADHD children with the 7-repeat allele also had higher rates of ODD and CD.
A third dopamine receptor gene, DRD5, has been linked to ADHD. One study that examined a number of candidate genes, including DRD3, DRD4, DRD5, and genes for four enzymes involved in dopamine metabolism, found no significant association between the children with ADHD and genetic polymorphisms [Payton et al., 2001]. However, the 138 ADHD children in this study frequently had coexisting conditions, including ODD (57.5 percent), CD (11.6 percent), and tic (12.3 percent), anxiety (2.7 percent), and depressive (1.4 percent) disorders. Another study also included children with coexistent conditions (Tourette’s syndrome or tics in 34 percent, CD or ODD in 25 percent, anxiety or depression in 8 percent), and linkage to DRD5 only reached significance when restricted to the children who had a response to methylphenidate [Tahir et al., 2000]. Information was not provided about whether the methylphenidate responders had fewer coexisting conditions. Linkage of the DRD4 gene to methylphenidate responders was also observed. However, this study found an inverse relationship between DRD4 and DSM scores and comorbidity ratings.
Studies of DNA from ADHD probands, parents, and healthy controls found a significant association of ADHD with two NET1 single-nucleotide polymorphisms and two DRD1 single-nucleotide polymorphisms. There was no association with polymorphisms in ten other genes previously reported as candidate genes. There were no significant differences in anatomic brain MRI measurements between the children with NET1 or DRD1 gene types; nor was there a relationship between the genetic findings and cognitive or behavioral measures. This study represented the first replication of a previously described association between ADHD and polymorphisms in NET1 and DRD1 genes [Bobb et al., 2005].
In a study of a group of children from families of European descent with an ADHD proband, the ADHD probands were assessed by a child psychiatrist; parental ADHD was assessed through the use of an ADHD self-report scale. The ADHD cohort consisted of 335 parent–child trios of European descent and a set of 2026 ethnically matched, disease-free children as a control group. There was no significant difference in copy number variants (CNV: deletions, duplications, or size) between the patient and control groups. A search for CNVs spanning more than ten consecutive single-nucleotide polymorphisms (SNPs) for deletions, or more than twenty SNPs for duplications present in at least one parent, along with one or more related probands, but not in the controls, yielded 158 deletions and 64 duplications from 154 probands. These CNVs encompassed or overlapped 229 distinct genes, with the largest family of genes affected being the olfactory receptor superfamily. Twenty-two of these genes had previously been implicated in various neurological and neuropsychiatric disorders, including Tourette’s syndrome (2 genes), autism (4 genes), and schizophrenia (15 genes). An additional 8 genes had been recently identified as having structural variants in autism and schizophrenia. Reviewing the gene set for genes associated with nervous system development, function, and behavior, the authors found genes associated with learning, cognition, and hindbrain development. Two genes, the PTPRD and GRM5 genes, were thought to be particularly interesting putative candidate genes for ADHD; one, involving the protein tyrosine phosphatase gene, was detected in four unrelated ADHD probands. Two of the four ADHD probands with the PTPRD deletion reported symptoms consistent with restless leg syndrome. All three children in a family found to have the GRM5 variant met the criteria for ADHD; the GRM5 gene, a glutamatergic receptor gene, has been postulated to play a role in ADHD. Thus the CNVs found in this ADHD were significantly enriched for genes reported as candidate genes in other neuropsychiatric disorders and in neurodevelopmental pathways [Elia et al., 2009].
Other Potential Causes of Attention-Deficit Hyperactivity Disorder
Data reported from the National Longitudinal Survey of Youth [NLSY, 1979] associated hours of television watched per day at ages 1 and 3 years with parental reports of attentional problems at age 7 [Christakis et al., 2004]. The children did not necessarily have clinically diagnosed ADHD; rather, they were scored as having attentional problems by the parents. Although the interaction between environmental influences and genetic endowment is well accepted, such preliminary data suggest the need for further investigation because of issues of cause and effect, limitations in adjusting for confounders, potential for biased reporting, and selective recall.
Coexisting Conditions
Many children who present with symptoms suggestive of ADHD have neurologic or psychiatric conditions that are the cause of those symptoms (e.g., depression, sleep disorders, epilepsy). There are other instances in which multiple conditions coexist. The implications for management are significant. Just as correction of a sleep disorder may resolve the symptoms of inattention, hyperactivity, or impulsivity, addressing a child’s previously undiagnosed learning disability may resolve these symptoms. Alternatively, a child may have both problems, and remediation of the learning disability may still leave him or her with inattention, hyperactivity, or impulsivity that must be independently addressed. It has been proposed that ADHD be divided into subgroups based on the patterns of comorbidity [Biederman et al., 1991]. ADHD and CD have been posited to be distinct disorders [Schachar and Tannock, 1995]. From a practical standpoint, most studies of children with ADHD and coexisting CD treated with psychostimulants demonstrated a reduction in physical and nonphysical aggression and had improvement of ADHD symptoms [Spencer et al., 1996]. Antidepressants also reduced symptoms of aggression and ADHD in these children. Anxiety disorder has been shown to be transmitted independently from ADHD in families [Perrin and Last, 1996], suggesting that these two conditions are distinct disorders. Most studies of children with ADHD and coexisting anxiety or depression found a reduced response in ADHD symptoms when treated with psychostimulants compared with children only with ADHD [Spencer et al., 1996].
Inasmuch as ADHD and mood instability include impulsivity and behavioral problems in their definitions, both involve impairment in executive function, and there are findings of overlapping neuroanatomical abnormalities and treatments, it has been proposed that mood instability be considered a core feature of ADHD, rather than a comorbidity [Skirrow et al., 2009].
A review of the overlapping symptoms associated with ADHD and sleep disorders noted that many children with primary sleep disorders have symptoms highly suggestive of ADHD. Conversely, many children with ADHD are reported to have sleep disturbances, which may be primary, attributable to the side effects of medication, or a result of comorbid conditions, such as ODD, depression, and/or anxiety disorders. A comorbid sleep disorder may significantly increase the daytime impairment in a child with ADHD. It was recommended that all children presenting with ADHD symptoms be clinically assessed for the presence of sleep problems [Owens, 2005].
Reading disability and ADHD are two distinct disorders that may occur together [Shaywitz et al., 1995]. There is evidence of genetic linkage for ADHD and reading disability to the same region on the short arm of chromosome 6. This connection may represent a pleiotropic effect (i.e., the same gene increasing susceptibility to more than one disorder) [Willcutt et al., 2002]. A survey of audiologists and pediatricians found that, although auditory processing disorder and ADHD/I have symptoms in common, there were features that allowed them to be distinguished from each other [Chermak et al., 2002].
A meta-analysis of the literature reporting on tests of overall cognitive ability in ADHD analyzed data from 137 studies in which full-scale IQ scores for children with ADHD were compared to a healthy control group. The ADHD groups had significantly lower full-scale IQ scores relative to the control groups, with an average decrement of 9 points in the full-scale IQ; the verbal and performance IQs were lower in the ADHD group. There was no difference in ADHD subtypes, although the number of children with ADHD/I was small. The authors concluded that these findings “may indicate that the disorder is characterized by mild global cognitive inefficiencies or by multiple specific deficits affecting several cognitive abilities.” They raised the possibility that the decrement could also be attributable to test-taking differences between the groups. In support of this latter possibility, the authors found that the effect size of the full-scale IQ was largest when ability was based on the complete test, as opposed to estimates from subtests; the larger effect on the complete test could possibly be due to longer testing times in studies using a full intellectual assessment battery, with decreasing performance over time caused by deficient sustained attention in the ADHD group. The authors expressed surprise at the finding that for only a few of the measures was the effect size for executive functioning tasks significantly larger than effect sizes for the full-scale IQ. Only the academic achievement tests and CPT (continuous performance tests) measures displayed substantially larger effects than the full-scale IQ; the WCST (Wisconsin card sorting task)-variables, SST (stop signal task)-probability of inhibition, and MFFT (matching familiar figures test)-time tests had smaller effect sizes than full-scale IQ. In a comparison of the mean effect size of tests of executive versus nonexecutive functions, there was greater impairment in the tests of executive function. The authors allowed for the possibility that impairment of executive function accounted for differences in overall ability, inasmuch as measures of overall ability are heavily influenced by executive function. Academic measures of spelling and arithmetic were significantly more sensitive to ADHD than overall cognitive abilities measured by full-scale IQ; the authors commented that achievement measures “may be useful not only for screening co-morbid learning disabilities but also for characterizing behavioral and motivation deficits resulting from executive dysfunction” [Frazier et al., 2004].
Diagnostic Evaluation
ADHD is a clinical diagnosis; there are no diagnostic, laboratory, or cognitive tests [Nichols and Waschbusch, 2004]. A child presenting with symptoms suggestive of ADHD should undergo hearing and vision screening, potentially treatable problems that may be mistaken for ADHD. If the child’s difficulties are predominantly in the school setting, an evaluation for learning disabilities should be pursued, with educational remediation if problems are identified. Social stressors may also be a significant factor [Biederman et al., 2002], which may justify intervention by social services agencies. In general, routine diagnostic testing is not needed in the evaluation of a child for ADHD [American Academy of Pediatrics, 2000]. However, specific testing may be indicated in some circumstances.
Laboratory Studies
Features in the history or on examination may lead to specific tests for disorders manifesting as or coexisting with ADHD, such as hypothyroidism [Rovet, 2002], hyperthyroidism [Suresh et al., 1999], or phenylketonuria [Antshel and Waisbren, 2003]. Reports of an association between lead exposure and ADHD have been inconsistent [Eppright et al., 1997; Tuthill, 1996]. Depending on the results of such laboratory studies, therapy targeting the specific condition may be initiated. An uncontrolled study reported improvement in the parents’, but not the teachers’, Connors Rating Scales scores in children with ADHD treated with iron supplementation, even though they were not iron-deficient [Sever et al., 1997]. Better studies are needed before concluding that routine testing of or supplementation with iron or screening for iron deficiency is advisable.
Electroencephalography
Studies reporting an increased frequency of epileptiform discharges in children with ADHD [Duane et al., 2003; Holtmann et al., 2003; Richer et al., 2002] and reports of ADHD-type symptoms resolving when spike activity was suppressed with antiepileptic drugs [Holtmann et al., 2003; Laporte et al., 2002] have led to proposed guidelines for obtaining an electroencephalogram (EEG). These include a history of clinical events suggesting a seizure (even if only nocturnal or febrile), perinatal stress, head trauma, fluctuating behavioral manifestations, or a family history of epilepsy [Duane, 1996].
Sleep Studies
A sleep history should be obtained. If the results suggest a diagnosis of a sleep disorder or if there is a strong family history of sleep disorders, a sleep study should be considered [Gottlieb et al., 2003; Owens, 2005; Thunstrom, 2002; Walters et al., 2000].
Imaging Studies
There are few clinical indications for performing neuroimaging in children with ADHD. ADHD has been reported in association with head trauma [Gerring et al., 2000; Wassenberg et al., 2004], prematurity [Foulder-Hughes and Cooke, 2003], perinatal injury [Toft, 1999], and neurofibromatosis [Rosser and Packer, 2003]. However, if the child is clinically stable, the presence of ADHD symptoms does not call for imaging studies beyond those indicated for the primary condition.
Treatment
Pharmacologic Therapy
In the 1930s, Charles Bradley administered benzedrine (an amphetamine) to children with a history of neurologic and behavioral problems in whom he had done a lumbar puncture in an attempt to stimulate secretion of cerebrospinal fluid by the choroid plexus and diminish headaches after lumbar puncture. Although benzedrine did not affect the incidence of headaches, the childrens teachers reported major improvement in learning and behavior in a number of children that lasted the entire time they were treated [Gross, 1995]. A subsequent open trial of benzedrine in children with neurologic and behavioral problems who had normal intelligence resulted in improved learning, a greater interest in and a higher quality of their schoolwork, behavioral and social improvements, and increased voluntary control [Bradley, 1937]. However, the use of medication in children was viewed unfavorably in the medical and educational community, and it was not until the 1960s, when methylphenidate was found to be effective in the treatment of attention disorders, that stimulant use was accepted by physicians and parents [Clements and Peters, 1962]. Between 1990 and 1998, there was a 3.7-fold increase in the diagnosis of ADHD, and prescription of stimulants for children 5–18 years old increased from 11.5 to 42 per 1000 [Robison et al., 2002].
Recently, and controversially, the American Heart Association recommended that all children placed on stimulant medications for ADHD should have a screening electrocardiogram (EKG) [Vetter et al., 2008]. The American Academy of Pediatrics concluded that this is neither necessary nor recommended and, instead, recommended cardiovascular screening based on personal, past, and family histories and the cardiovascular examination [Perrin et al., 2008]. Cases of children and adolescents who had a sudden unexplained death were reviewed and a determination was made as to whether toxicology studies revealed evidence of stimulant use at the time of death. Comparison was made to a group of children and adolescents who died as passengers in motor vehicle accidents, in whom toxicology screens were performed. The study excluded children with cardiac conditions, including a history of prolonged QT interval in the deceased or any first-degree relative, history of sudden death among first-degree relatives, conduction disorders in the deceased, and evidence of cardiac disease or any abnormal anatomic findings on autopsy, including cardiomegaly, cardiac hypertrophy, and cardiomyopathy. Ten of 564 sudden unexplained death cases (1.8 percent) had evidence of stimulant use at the time of death, compared to 2 out of 564 motor vehicle accident cases (0.4 percent). The odds ratio of 7.4 was significant at p = 0.02 level of significance. The authors cautioned about potential bias in such a study, but concluded that the finding represents “a significant association or ‘signal’ between sudden unexplained death and the use of stimulant medication, specifically methylphenidate” [Gould et al., 2009]. An accompanying editorial pointed out that sudden unexplained death is a rare event and “that it is not possible to quantify the risk beyond estimating that it is very small,” adding that the findings “underscore the fact that stimulants are not innocuous, their therapeutic use requires careful diagnostic assessment, diligent safety screening and ongoing monitoring,” and that “when making treatment decisions, clinicians need to apply the current, still incomplete, evidence to the care of individual patients by carefully considering the type and severity of symptoms, availability of different treatments, expected benefits and potential risks” [Vitiello and Towbin, 2009]. Given the exclusion criteria in this published review, it would appear that routine EKGs would not have had an impact on the rate of sudden unexplained death.
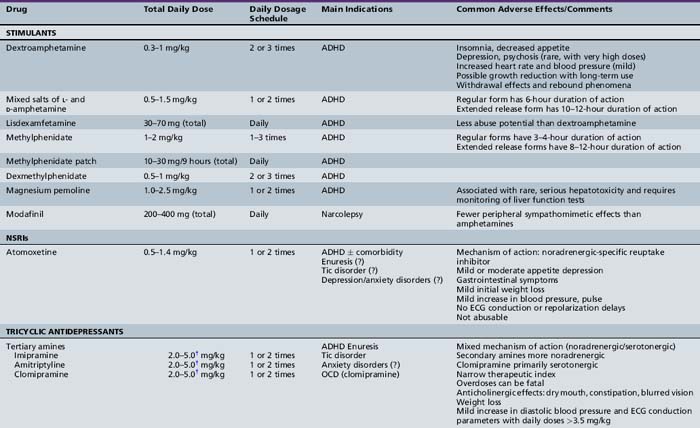
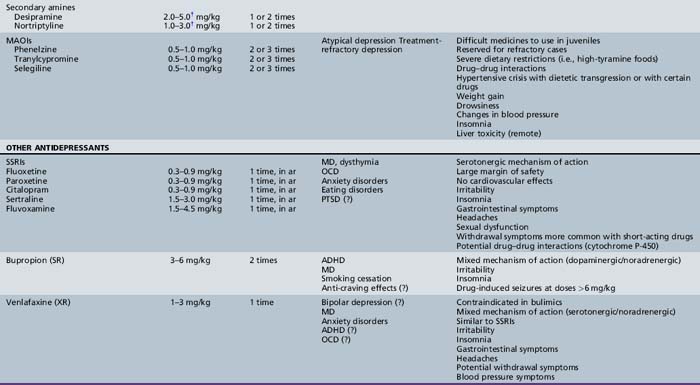
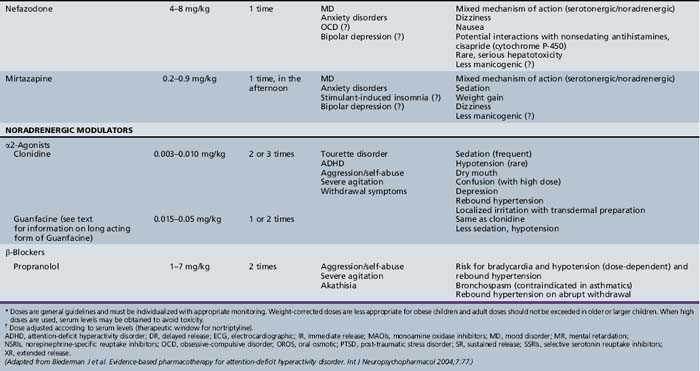
Table 47-1, derived from a review of pharmacotherapy in ADHD [Biederman et al., 2004], summarizes information regarding drug class, dose range, dosing schedule, indications, common adverse effects, and comments by the investigators.
Stimulant Medications
Stimulant drugs, sympathomimetic agents structurally similar to endogenous catecholamines, act centrally and peripherally by enhancing dopaminergic and noradrenergic transmission. Stimulants have been demonstrated to improve cognitive ability, school performance, and behavior [Barkley and Jackson, 1977; Famularo and Fenton, 1987; Rapport et al., 1988]. A study of children with ADHD with a high degree of comorbidity (ODD, 10 percent; CD, 30 percent; anxiety disorder, 17 percent; dyslexia, 32 percent) found differential effects of methylphenidate on various attentional functions at different doses [Konrad et al., 2004]. Specifically, alertness and focused and sustained attention improved in a linear fashion with increasing dose; inhibition and set-shifting were enhanced at a low dose but worsened at a moderate dose; and divided attention did not change at all. The different dose–response relationships for various cognitive and behavioral functions were explained by differential effects of these agents in different brain regions [Solanto, 2002]. The positive effects of methylphenidate on cognitive functions were caused by facilitation of dopaminergic activity in some brain regions, whereas improvement in hyperactivity and impulsivity was mediated by reduction in dopaminergic stimulation in other brain regions. This study did not uncover any differences in the response to methylphenidate between children with ADHD/C versus ADHD/I, nor was there any effect of comorbidity. Such data suggest that a single measure of response to stimulant treatment may be insufficient because different doses may be necessary to improve particular functions.
The response to methylphenidate in a group of 28 preschoolers (3–5 years old), as measured using behavioral ratings by teachers and parents, documented improvement, with 82 percent rated as having normal behavior after treatment, higher than the rate generally achieved in older children [Short et al., 2004]. With the exception of decreased appetite, there were no adverse side effects. The investigators speculate that the higher normalization rate for preschoolers than elementary school children may be a function of fewer demands placed on the preschooler (e.g., shorter school day, no homework).
The most commonly reported side effects of stimulants include appetite suppression and sleep disturbance. Absorption of stimulant medications is not notably affected when taken with or after meals, which may ameliorate appetite suppression [Green, 2001]. Insomnia can be a side effect from the medication but may also be caused by a rebound effect as the medication effect subsides. This distinction is important because, in the latter situation, a late afternoon or evening dose of stimulant medication may ease falling asleep [Chatoor et al., 1983]. Uncommonly, there have been reports of mood disturbances and lethargy after stimulant use [Wilens and Biederman, 1992]. Stimulants may also affect heart rate and blood pressure, but in healthy children, this change is unlikely to have clinical significance [Brown et al., 1984; Short et al., 2004]. There have been reports of psychostimulants inducing or exacerbating tic disorders, but subsequent studies have not found this to be a universal problem [Spencer et al., 1999]. Although this possibility should be discussed with children and their families, the presence of tics in a child with ADHD or a family history of tics is not an absolute contraindication to the use of psychostimulants. Concerns are often expressed regarding an increased risk of substance abuse in children treated with psychostimulants [Biederman et al., 1995b], but there is no supporting evidence. One study found that pharmacologic treatment for ADHD actually decreased the risk of subsequent substance abuse [Biederman et al., 1999]. There also have been reports of a decrease in the height of children taking stimulant medications [Safer et al., 1972], but other studies indicated no effect [Gross, 1976]. The reported decrease in height may reflect a transient maturational delay associated with ADHD, rather than a growth-stunting effect of medication [Spencer et al., 1998]. A longitudinal study [MTA Cooperative Group, 2004] revealed that children treated with medication had a reduced height gain compared with those who were not treated. Growth suppression was still evident during the second year of treatment in the group treated continuously, indicating that this was a persistent effect. The observation that there was less growth suppression in the children who were not treated continuously suggests that interrupting treatment with stimulant medication may limit growth suppression, supporting the concept of drug holidays to address this side effect. However, there have been reports of behavioral deterioration when stimulant medications are abruptly discontinued [Biederman et al., 2004].
The most commonly used drugs in the stimulant class include methylphenidate, dextroamphetamine, and mixed salts of l-and d-amphetamine. Although in the same class, these drugs have slightly different mechanisms of action, and patients may respond differently to each of them [Greenhill et al., 1998]. A review of double-blind, controlled trials of stimulant medications, published from 2005 through 2008, discussed the properties of the various formulations, including immediate, extended-release, and transdermal forms of methylphenidate, dexmethylphenidate, immediate- and extended-release forms of mixed amphetamine salts, and lisdexamfetamine, indicated which preparations can be opened and sprinkled into food and which medications were available as liquid or chewable tablets [Chavez et al., 2009]. The authors emphasized that a lack of response to one stimulant does not predict a response to other stimulants and a trial with a different agent is warranted if the initial treatment fails. ADHD subtype was not felt to dictate treatment choice. Results of studies comparing short- and long-acting preparations have been inconsistent [Fitzpatrick et al., 1992; Pelham et al., 1987, 1990]. Greydanus et al. [2009], in their review of the various classes of medication for treatment of ADHD, conclude: “despite intense pharmacologic advertisements, there are no short-acting or long-acting psychostimulants that are proven to be superior to the others.”
Methylphenidate
Methylphenidate has fewer side effects than amphetamine [Efron et al., 1997]. In the standard formulation, methylphenidate reaches peak concentrations between 1 and 3 hours after oral intake. It is rapidly and extensively metabolized by nonmicrosomal hydrolytic esterases in the liver and other tissues, with an average half-life of 3 hours. In children, the starting dose is 0.3 mg/kg in the morning, rounded to the nearest 5-mg tablet [Drugdex Drug Evaluations, 2009]. It can be useful to have teachers complete a behavior checklist before and after initiation of treatment (preferably without being aware of exactly when the medication is started) to assess efficacy. If, after 1–2 weeks, there is inadequate benefit, the dose can be increased to 0.6 mg/kg. With an average half-life of 3 hours, a morning dose does not persist through the afternoon. Increasing the morning dose may increase the duration of the effect. Alternatively, a second dose 3–4 hours after the initial dose may be necessary. A dose in the middle to late afternoon to facilitate completion of homework may also be warranted. Alternatives to multiple daily doses are the long-acting formulations of methylphenidate. These formulations reach peak concentration 6–8 hours after oral intake, obviating the need for a midday dose. When using the longer-acting formulations, the entire daily dose is given in the morning. If there is no significant improvement in symptoms at a total daily dose of 1–2 mg/kg, alternate medication should be considered.
Methylphenidate can also be administered by the transdermal route, in the form of a methylphenidate patch (Daytrana Transdermal System). The side effect profile is the same as for other forms of methylphenidate; however, the patch allows for a steadier rate of administration over a longer period. The patch is available in 12.5-, 18.75-, 25-, and 37.5-cm2 sizes. The recommendations include applying the patch 2 hours before the desired effect and removing the patch 9 hours after application (or earlier if a shorter duration of effect is desired). For children 6–12 years of age, the recommended dose titration begins with the 12.5 cm2 patch, which delivers 10 mg over 9 hours, at a rate of 1.1 mg/hour and increasing, in steps to the 37.5 cm2 patch, which delivers 30 mg over 9 hours, at a rate of 3.3 mg/hour. A double-blind, placebo-controlled, randomized trial performed for FDA approval of this formulation found no evidence of improved efficacy when the dose was increased from 20 mg to 30 mg over 9 hours [Drugdex Drug Evaluations, 2009].
Dexmethylphenidate
Dexmethylphenidate is the d-threo-enantiomer of methylphenidate. A PET study found specific binding of the d-enantiomer to dopamine transporters in the basal ganglia, whereas the l-enantiomer had widespread, nonspecific binding [Ding et al., 1995]. Studies comparing dexmethylphenidate and methylphenidate have concluded both to be effective in ADHD, but dexmethylphenidate has a longer duration [Keating and Figgitt, 2002]. The time to peak concentrations after oral intake is similar to that of methylphenidate (i.e., between 1 and 3 hours) and, like methylphenidate, it is rapidly and extensively metabolized by nonmicrosomal hydrolytic esterases in liver and other tissues, with an average half-life of about 2 hours. In children, the starting dose of dexmethylphenidate is one-half of the methylphenidate dose (0.15 mg/ kg in the morning, rounded to the nearest 2.5-mg tablet) [Drugdex Drug Evaluations, 2009]. If, after 1–2 weeks, there is inadequate benefit, the dose can be increased in 2.5-mg increments, to a maximum of 20 mg/day. The report of longer clinical efficacy than methylphenidate (despite the similar half-life) may eliminate the need for a midday dose, depending on the clinical response. There is no evidence that giving the d-isomer (dexmethylphenidate) at one-half of the dose of the d,l-enantiomer (methylphenidate) confers any clinical advantage. Dexmethylphenidate is available in 2.5-, 5-, and 10-mg tablets (Focalin).
Lisdexamfetamine
Lisdexamfetamine, a prodrug of dextroamphetamine, is rapidly absorbed in the gastrointestinal tract and converted to dextroamphetamine; thus its efficacy in ADHD is similar to that of dextroamphetamine. The Tmax of lisdexamfetamine is 1 hour, but the Tmax of dextroamphetamine, the active agent, is 3.5 hours after a single dose of lisdexamfetamine. The longer Tmax of dextroamphetamine, when given in the form of lisdexamfetamine, allows for once-a-day dosing and is thought to decrease the abuse potential of this formulation. The recommended starting dose is 30 mg/day, with upward titration by 10–20 mg increments to a maximum dose of 70 mg/day. Lisdexamfetamine comes in 20, 30, 40, 50, 60, and 70 mg capsules [Drugdex Drug Evaluations, 2009].
Dextroamphetamine
Dextroamphetamine has a time to peak concentration of 60–160 minutes and is metabolized in the liver. The average half-life of dextroamphetamine is 10–12 hours, but this varies considerably with urinary pH; at a urine pH of less than 6.6, more than two-thirds of unmetabolized drug is excreted in the urine, whereas at a urine pH greater than 6.7, it is less than one-half. The initial dose of dextroamphetamine is 0.15–0.3 mg/kg (rounded to the nearest 5 mg) [Drugdex Drug Evaluations, 2009]. This dose can be gradually increased to desired effect up to a peak dose of approximately 1 mg/kg/day. Dextroamphetamine’s longer half-life compared with methylphenidate may obviate the need for a midday dose. An extended-release preparation of dextroamphetamine eliminates the need for midday dosing. The regular formulation of dextroamphetamine is available in 5-mg tablets (Dexedrine); the extended-release formulation is available in 5-, 10-, and 15-mg capsules (Dexedrine Spansules).
Noradrenergic Potentiation
Atomoxetine
Atomoxetine (Strattera) is a norepinephrine-specific reuptake inhibitor that is effective in the treatment of children with ADHD. In a study comparing atomoxetine to methylphenidate and placebo, the response rate to atomoxetine and methylphenidate was essentially identical, and both were better than placebo. Appetite suppression was somewhat lower in atomoxetine compared with methylphenidate (22 percent versus 32 percent), and there was significantly less insomnia on atomoxetine (7 percent versus 27 percent) [Spencer et al., 2002]. Atomoxetine is metabolized by the cytochrome P-450 (CYP) 2D6 pathway. Peak plasma concentrations of atomoxetine occur 1–2 hours after oral administration. In extensive metabolizers (most patients), atomoxetine half-life is 4–5 hours. Substantial decreases in clearance and prolongation of the half-life are seen in poor metabolizers. The starting dose is 0.5 mg/kg/day, with gradual increase to a target dose of 1.2 mg/kg/day. In poor metabolizers (about 7 percent of the population), the half-life is substantially longer, and the dose requirement may be much lower. Depending on the response, midday dosing may be required for extensive metabolizers. Food does not affect absorption [Drugdex Drug Evaluations, 2009]. Because atomoxetine is not a controlled substance in the United States, prescriptions with multiple refills can be provided, and renewals can be done over the phone, in contrast to the procedures for stimulant medications. Atomoxetine is available in 10-, 18-, 25-, 40-, and 60-mg capsules. In December 2004 the Food and Drug Administration (FDA) asked the manufacturer to add a bolded warning about severe liver injury to the labeling, indicating that the medication should be discontinued in patients who develop jaundice or laboratory evidence of liver injury. In September 2005 the FDA directed the manufacturer to revise the labeling further to include a boxed warning regarding an increased risk of suicidal thinking in children and adolescents being treated with this drug.
Other agents
Modafinil, a central nervous stimulant, the mechanism of action of which is uncertain, lacks the peripheral sympathomimetic effects seen with amphetamines. Modafinil has been approved for the treatment of excessive daytime sleepiness associated with narcolepsy, obstructive sleep apnea, and shift-work sleep disorder. Studies of its efficacy in ADHD have found it to be more effective than placebo in ameliorating ADHD symptoms and comparable to methylphenidate in efficacy. The doses studied were 340 and 425 mg/day in children weighing less than or more than 30 kg, respectively [Swanson et al., 2006]. Modafinil is available in 100- and 200-mg oral tablets [Drugdex Drug Evaluations, 2009].
Armodafinil, the longer lived, R-enantiomer of modafinil, is available as 50-, 150-, and 250-mg tablets; it has the same mechanism of action and the same clinical indications as Modafinil. No studies have been done on the efficacy of armodafinil in ADHD; presumably it would have efficacy similar to that of modafinil [Drugdex Drug Evaluations, 2009].
Nonstimulant Medications
It is estimated that at least 30 percent of children diagnosed with ADHD do not respond to or tolerate stimulant medications [Spencer et al., 1996]. Most studies have reported a reduced rate of response to psychostimulants in children with ADHD and anxiety or depression [Spencer et al., 1996]. The failure to respond to psychostimulants suggests the possibility of an incorrect diagnosis. However, genetic studies have suggested that children with ADHD respond differently to methylphenidate, depending on whether they were homozygous or heterozygous for the 10-repeat allele at dopamine transporter gene SLC6A3 [Loo et al., 2003; Rhode et al., 2003; Winsberg and Comings, 1999].
Tricyclic Antidepressants
Other agents found to be effective in the treatment of ADHD include tricyclic antidepressants (TCAs). In one study, comorbidity with conduct disorder, depression, or anxiety, or a family history of ADHD did not result in a differential response to desipramine [Biederman et al., 1989]. In studies comparing TCAs with stimulants, TCAs appear to improve behavioral symptoms more consistently than cognitive function [Rapport et al., 1993].
Desipramine
Desipramine, a tricyclic antidepressant, is metabolized in the liver by the CYP-2D6 pathway, with an average half-life of 17.1 hours. For the 7 percent of the population with decreased activity of this enzyme, the half-life may be as long as 77 hours [Drugdex Drug Evaluations, 2009]. The effective dose of desipramine is lower and onset of action sooner for ADHD than for depression [Green, 2007]. The starting dose of desipramine is 1 mg/kg/day, with gradual increments to a maximum of 5 mg/kg/day. This medication may be given once daily or in divided doses, depending on the response. For slow metabolizers, the dose requirement is much lower, and once-daily dosing should be sufficient.
There have been case reports of sudden death in children treated with desipramine [Riddle et al., 1991]. Although a subsequent epidemiologic study did not find greater risk of sudden death with desipramine [Biederman et al., 1995b], it has been suggested that a baseline EKG be obtained before initiating treatment and that serial EKGs be obtained after significant dose increments and periodically during treatment [Biederman et al., 2004].
Alpha-Adrenergic Agonists
The α-adrenergic agents clonidine and guanfacine have been widely used for treatment of ADHD, despite few clinical studies. The success of these agents for Tourette’s syndrome and other tic disorders [Leckman et al., 1991] has made them especially useful in children with ADHD and tic disorders, particularly if a trial of stimulant medication resulted in exacerbation of tics. Reports of three deaths of children taking a combination of methylphenidate and clonidine prompted reviews that found no evidence of an adverse methylphenidate–clonidine interaction [Fenichel, 1995; Popper, 1995]. Nevertheless, if there is a plan to prescribe this combination, a review of this literature and discussion of risks and benefits with the parents are advisable. Guanfacine appears to have an advantage over clonidine because it has a longer half-life and is less sedating [Hunt et al., 1995]. Guanfacine reaches a peak concentration after oral intake in 1–4 hours and is metabolized in the liver, with an average half-life of 17 hours [Drugdex Drug Evaluations, 2009]. The starting dose is 0.015 mg/kg/day (to the nearest 0.5 mg), with a gradual increase to a maximum of 0.05 mg/kg or 4 mg/day, based on the clinical response [Biederman et al., 2004]. The half-life of guanfacine should allow for once-daily dosing, although in clinical studies it was administered in 2–4 divided doses [Chappell et al., 1995; Hunt et al., 1995]. Guanfacine is available in 1 and 2 mg tablets. An extended release form of guanfacine is also available in 1-, 2-, 3-, and 4-mg tablets, which must be swallowed whole. The recommended starting dose is 1 mg/day with therapeutic benefit demonstrated at doses from 0.05 mg/kg/day up to 0.12 mg/kg/day to a maximum of 4 mg/day [Drugdex Drug Evaluations, 2009]. Other agents reported to be effective in ADHD are reviewed in Table 47-1.
Future directions include studies of AMPA (ampakine) receptor modulators, nicotinic acetylcholine receptor antagonists, dopamine agonists (which are effective in the treatment of restless leg syndrome), atypical antipsychotics (e.g., aripiprazole and risperidone), selective noradrenaline reuptake inhibitors and gamma-aminobutyric acid (GABA) B receptor antagonists [Greydanus et al., 2009].
Nonpharmacologic Therapies
Children with ADHD need a school environment with minimal distractions, and with seating that is somewhat isolated and close to the front of the classroom in front of the teacher [Shaywitz and Shaywitz, 1984]. The setting should be fairly structured, with organizational techniques such as checklists and homework assignment pads, and an uncluttered desk at home devoted exclusively to schoolwork.
A multicenter clinical trial of various treatment strategies for ADHD [MTA Cooperative Group, 1999] concluded that stimulants were more effective than behavioral therapies for ADHD symptoms. The combination of stimulants and behavioral therapy resulted in improved social skills but did not significantly improve ADHD symptoms over stimulants alone. A review of treatment modalities of children diagnosed with ADHD in the period from 1995 to 1999 found that among children diagnosed with ADHD, 24 percent also had mental illness. The most frequent treatments were stimulant medication alone (42 percent), stimulant medication combined with psychotherapy or mental health counseling (32 percent), and psychotherapy or mental health counseling alone (10.8 percent). Fifteen percent of children received no treatment other than office visits for initial and follow-up medical care. The percentage of children receiving psychotherapy or mental health counseling alone or in combination with stimulant medication increased with age, and males were more likely than females to receive treatment [Robison et al., 2004].
Biofeedback Programs
Various forms of computer training programs have been studied in treating children with ADHD. Such working memory training programs improved working memory capacity in children with ADHD and adults without ADHD. Improvement generalized to nonpracticed tasks involving prefrontal cortex. In children with ADHD, the improvement in working memory was associated with a decrease in head movements [Klingberg et al., 2002]. Children with ADHD trained to modify their slow cortical potentials also have an increase in contingent negative variation during a continuous performance task compared with those who did not receive training. Associated with this electrophysiologic phenomenon were fewer impulsivity errors on the continuous performance task, suggesting that the contingent negative variation increase represented a neurophysiologic correlate of improved self-regulatory capabilities [Heinrich et al., 2004].
The use of an EEG biofeedback program has been compared with the effectiveness of methylphenidate [Fuchs et al., 2003]. Children were trained to increase the power of the sensory motor rhythm (12 to 15 hertz) and low beta activity (15 to 18 hertz). Assignment to the biofeedback versus methylphenidate group was based on parental preference. Two-thirds of parents chose the biofeedback training program, raising issues of selection bias, and there was no placebo arm in this study. After 3 months, both groups had significant improvements in all four subscales on the TOVA, as well as on a behavior rating scale. Changes in the EEG as a result of biofeedback were not monitored in this study. A previous study using biofeedback reported greater improvement on the TOVA in participants with significant EEG changes than in those without changes (although there were improvements in both groups) [Lubar et al., 1995]. There was no correlation between behavioral changes reported by the parents and changes in the EEG. This study did not include a control group. A study of EEG biofeedback that used a control group (i.e., association between EEG patterns and feedback to the participants was random) found no benefit from EEG biofeedback [Heywood and Beale, 2003]. The investigators noted that, had the data analysis excluded the dropouts and failed to control for behavioral trends unrelated to the EEG biofeedback training, it would have led to the erroneous conclusion that the treatment was effective.
A study assessing the gait of a group of 16 children with ADHD under “usual” and “dual task” conditions found that dual tasking caused a significant decrease in stride time variability in the ADHD group, which was unexpected and the opposite of the anticipated result. After methylphenidate was taken, dual tasking no longer significantly affected stride time variability in the ADHD group. Methylphenidate and dual tasking had similar effects on gait variability, but opposite effects on gait speed, with methylphenidate increasing gait speed and dual tasking slowing it down. It was hypothesized that children with ADHD walk more rhythmically in the dual-tasking condition, opposite to the effect observed in healthy adults, due to a higher attentional level (vigilance) created by presenting the children with a cognitive challenge. Alternatively, the additional cognitive load may have created an “automatic pilot” of gait [Leitner et al., 2007]. This might explain the reported effects of biofeedback and other interventions involving cognitive activity.
Complementary and Alternative Medications
A survey of parents of children referred for evaluation of ADHD reported that 54 percent of the parents used complementary and alternative medicine (e.g., acupuncture, nutritional supplements) for the child’s ADHD symptoms in the prior year [Chan et al., 2003]. Only 11 percent of the parents discussed using such interventions with their child’s physician. A review of the literature on the role of nutritional factors in ADHD, including food additives, sugars, food allergies or sensitivities, and essential fatty acids, identified methodological problems with negative studies without similar discussion of problems with positive studies, possibly revealing bias of the authors [Schnoll et al., 2003]. Nevertheless, the summary statement is reasonably cautious: “There is increasing evidence that there is a subset of children with behavioral problems who are sensitive to one or more food components that may precipitate or contribute to their hyperactive behavior. Research indicates that it is futile to try to identify a specific food or substance that will precipitate negative behavior in all hyperactive children.”
A placebo-controlled, double-blind study of dietary supplementation with omega-3 and omega-6 fatty acids in children with developmental coordination disorder, defined in the DSM-IV as a specific impairment of motor function independent of general ability [American Psychiatric Association, 1994], found a statistically significant improvement in reading, spelling, and ADHD-related symptoms after 3 months in the treatment group, compared to placebo. There was no improvement in the score on a movement assessment battery. Of the 102 children with data at 3 months, there were 32 children who had baseline scores on a parent rating scale exceeding 2 standard deviations above the general population average for DSM-IV criteria for ADHD; of these, 16 were in the treatment group and 16 were in the placebo group. After 3 months, 7 of the 16 children in the treatment group were no longer in the clinical range for ADHD symptoms, while only 1 of 16 in the placebo group was no longer in the clinical range for ADHD [Richardson and Montgomery, 2005]. In contrast, a review of the biology of essential fatty acids (EFAs) in brain function and studies of EFAs in children with ADHD found that uncontrolled, open-label studies reported that EFA supplementation improved ADHD symptoms; however, randomized controlled trials did not show treatment effects; in some cases, there were better results for the placebo group. The authors concluded that the available evidence “does not support the use of EFA supplements as a treatment for children with ADHD” [Raz and Gabis, 2009]. It should be emphasized that the latter review was of studies that recruited children with a diagnosis of ADHD, in contrast to the former study that recruited children with a diagnosis of developmental coordination disorder.
Outcome
ADHD persists into adulthood. The symptoms of ADHD may be less obvious after the individual is older [Hart et al., 1995]. The incidence of ADHD in adults depends on diagnostic criteria and whether historical data are obtained from the patients or their parents [Weiss and Weiss, 2002]. The finding that adolescents and young adults with ADHD had more car accidents with bodily injuries indicates that this is a serious problem, even in older children and adults [Barkley et al., 1993].
References
The complete list of references for this chapter is available online at www.expertconsult.com.
Achenbach T.M. Child behavior checklist for ages 4–18. Burlington, Vt: University of Vermont Department of Psychiatry; 1991.
American Academy of Pediatrics. Committee on quality improvement and subcommittee on attention-deficit/hyperactivity disorder clinical practice guideline: Diagnosis and evaluation of the child with attention deficit/hyperactivity disorder. Pediatrics. 2000;105:1158.
American Psychiatric Association. Diagnostic Statistical Manual of Mental Disorders, ed 4. Washington, DC: American Psychiatric Press; 1994.
Antshel K.M., Waisbren S.E. Timing is everything: Executive functions in children exposed to elevated levels of phenylalanine. Neuropsychology. 2003;17:458.
Barkley R.A. The ecological validity of laboratory and analogue assessment methods of ADHD symptoms. J Abnorm Child Psychol. 1991;19:149.
Barkley R.A. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65.
Barkley R.A. Attention-deficit hyperactivity disorder: A handbook for diagnosis and treatment, ed 2. New York: Guilford Press; 1998.
Barkley R.A., Guevremont D.C., Anastopoulos A.D. Driving related risks and outcome of ADHD in adolescents and young adults: A 3-5 year follow-up survey. Pediatrics. 1993;92:212.
Barkley R.A., Jackson T.l.Jr. Hyperkinesis, autonomic nervous system activity and stimulant drug effects. J Child Psychol Psychiatry. 1977;18:347.
Baumgaertel A., Wolraich M.L., Dietrich M. Comparison of diagnostic criteria for attention deficit disorders in a German elementary school sample. J Am Acad Child Adolesc Psychiatry. 1995;34:629.
Biederman J., Baldessarini R.J., Wright V., et al. A double-blind placebo controlled study of desipramine in the treatment of ADD. I. Efficacy. J Am Acad Child Adolesc Psychiatry. 1989;28:777.
Biederman J., Faraone S.V., Monuteaux M.C. Differential effect of environmental adversity by gender: Rutter’s index of adversity in a group of boys and girls with and without ADHD. Am J Psychiatry. 2002;159:1556.
Biederman J., Newcorn J., Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct depressive anxiety and other disorders. Am J Psychiatry. 1991;148:564.
Biederman J., Spencer T., Wilens T. Evidence-based pharmacotherapy for attention-deficit hyperactivity disorder. Int J Neuropsychopharmacol. 2004;7:77.
Biederman J., Thisted R.A., Greenhill L.L., et al. Estimation of the association between desipramine and the risk for sudden death in 5-to 14-year old children. J Clin Psychiatry. 1995;56:87.
Biederman J., Wilens T., Mick E., et al. Psychoactive substance use disorder in adults with attention deficit hyperactivity disorder (ADHD): Effects of ADHD and psychiatric co-morbidity. Am J Psychiatry. 1995;152:1652.
Biederman J., Wilens T., Mick E., et al. Pharmacotherapy of attention deficit/hyperactivity disorder reduces risk for substance abuse disorder. Pediatrics. 1999;104:e20.
Blackman J.A. Attention-deficit/hyperactivity disorder in preschoolers. Does it exist and should we treat it? Pediatr Clin North Am. 1999;46:1011.
Bobb A.J., Addington A.M., Sidransky E., et al. Support for Association Between ADHD Two Candidate Genes: Net, NET1 and DRD 1. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:67-72.
Bradley C. The behavior of children receiving benzedrine. Am J Psychiatry. 1937;94:577.
Brown R.T., Wynne M.E., Slimmer L.W. Attention deficit disorder and the effect of methylphenidate on attention, behavioral, and cardiovascular functioning. J Clin Psychiatry. 1984;45:473.
Brown T.E. Attention-deficit disorders and co-morbidities in children, adolescents and adults. Washington, DC: American Psychiatric Press; 2000.
Cantwell D.P. Attention deficit disorder: A review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1996;35:978.
Castellanos F.X., Giedd J.N., Berquin P.C., et al. Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2001;58:289.
Castellanos F.X., Giedd J.N., Marsh W.L., et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996;53:607.
Castellanos F.X., Lee P.P., Sharp W., et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740.
Castellanos F.X., Sharp W.S., Gottesman R.F., et al. Anatomic brain abnormalities in monozygotic twins discordant for attention deficit hyperactivity disorder. Am J Psychiatry. 2003;160:1693.
Castellanos F.X., Sonuga-Barke E.J.S., Milham M.P., et al. Characterizing Cognition in ADHD: Beyond Executive Dysfunction. Trends Cogn Sci. 2006;10(3):117-123.
Chan E., Rappaport L.A., Kemper K.J. Complementary and alternative therapies in childhood attention and hyperactivity problems. J Dev Behav Pediatr. 2003;24:4.
Chappell P.B., Riddle M.A., Cahill L., et al. Guanfacine treatment of comorbid attention deficit hyperactivity disorder and Tourette’s syndrome: Preliminary clinical experience. J Am Acad Child Adolesc Psychiatry. 1995;34:1140.
Chatoor I., Wells K.C., Conners C.K., et al. The effects of nocturnally administered stimulant medication on EEG sleep and behavior in hyperactive children. J Am Acad Child Psychiatry. 1983;22:337.
Chavez B., Sopko M.A., Ehret M.J., et al. An Update On Central Nervous System Stimulant Formulations In Children And Adolescents With Attention-Deficit/Hyperactivity Disorder. Ann Pharmacother. 2009;43(6):1084-1095.
Chermak G.D., Tucker E., Seikel J.A. Behavioral characteristics of auditory processing disorder and attention-deficit hyperactivity disorder: Predominantly inattentive type. J Am Acad Audiol. 2002;13:332.
Chhabildas N., Pennington B.F., Willcutt E.G. A comparison of the neuropsychological profiles of the DSM-IV subtypes of ADHD. J Abnorm Child Psychol. 2001;29:529.
Christakis D.A., Zimmerman F.J., DiGiuseppe D.L., et al. Early television exposure and subsequent attentional problems in children. Pediatrics. 2004;113:708.
Clements S.D., Peters J. Task force one: Minimal brain dysfunction in the school aged child. Arch Gen Psychiatry. 1962;6:185.
Conners C.K. ADHD DSM-IV scales for parents and teachers, technical manual. North Tonowanda, NY: Multi Health Systems; 1997.
Conners C.K. The computerized continuous performance test. Psychopharmacol Bull. 1985;21:891.
Cook E.H.Jr, Stein M.A., Krasowski M.D., et al. Association of attention-deficit disorder and the dopamine transporter gene. Am J Hum Genet. 1995;56:811.
Denckla M.B. Revised neurological examination for subtle signs. Psychopharmacol Bull. 1985;21:773.
Denckla M.B. ADHD: Topic update. Brain Dev. 2003;25:383.
Denckla M.B., Rudel R.G. Anomalies of motor development in hyperactive boys. Ann Neurol. 1978;3:231.
Ding Y., Fowler J., Volkow N., et al. Carbon-11-D-threo-methylphenidate binding to dopamine transporter in baboon brain. J Nucl Med. 1995;36:2298.
Drugdex. Klasco R.K., editor. System (electronic version). Thomson Micromedex: Greenwood Village, CO, 2009. Available at http://www.thomsonhc.com
Duane D.D. Valproic acid treatment of learning disorder. J Child Neurol. 1996;11:505.
Duane D.D., Vermilion K., White R. Frequency and character of EEG abnormalities in developmental learning/attention disorders. J Clin Neurophysiol. 2003;20:212.
Durston S., Thomas K.M., Worden M.S., et al. The effect of preceding context on inhibition: An event-related fMRI study. Neuroimage. 2002;16:44.
Durston S., Tottenham N.T., Thomas K.M., et al. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry. 2003;53:871.
Efron D., Jarman F., Barker M. Side effects of methylphenidate and dextroamphetamine in children with attention deficit hyperactivity disorder: A double-blind, crossover trial. Pediatrics. 1997;100:662.
Elia J., Gai X., Xie H.M., et al. Rare Structural Variants Found In Attention-Deficit Hyperactivity Disorder Are Preferentially Associated With Neurodevelopmental Genes. Mol Psychiatry. 2009:1-10. June 23
Eppright T.D., Vogel S.J., Horwitz E., et al. Results of blood lead screening in children referred for behavioral disorders. Mo Med. 1997;94:295.
Ernst M., Zametkin A.J., Matochik J.A., et al. High midbrain DOPA accumulation in children with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1209.
Falkenstein M., Hoormann J., Hohnsbein J. ERP components in Go/No Go tasks and their relation to inhibition. Acta Psychol. 1999;101:267.
Fallgatter A.J., Ehlis A.-C., Seifert J., et al. Altered response control and anterior cingulate function in attention-deficit/hyperactivity disorder boys. Clin Neurophysiol. 2004;115:973.
Famularo R., Fenton T. The effect of methylphenidate on school grades in children with attention deficit disorder without hyperactivity: A preliminary report. J Clin Psychiatry. 1987;48:112.
Faraone S.V. Advances In the Genetics And Neuro-Biology of Attention Deficit Hyperactivity Disorder (Editorial). Biol Psychiatry. 2006;60:1025-1027.
Faraone S.V., Doyle A.E., Mick E., et al. Meta-analysis of the association between the 7-repeat allele of the dopamine D (4) receptor gene and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;58:1052.
Fenichel R.R. Combining methylphenidate and clonidine: The role of post-marketing surveillance. J Child Adolesc Psychopharmacol. 1995;5:155.
Filipek P.A., Semrud-Clikeman M., Steingard R.J., et al. Volumetric MRI analysis comparing attention-deficit hyperactivity disorder and normal controls. Neurology. 1997;48:589.
Fitzpatrick P.A., Klorman R., Brumaghim J.T., et al. Effects of sustained-release and standard preparations of methylphenidate on attention deficit disorder. J Am Acad Child Adolesc Psychiatry. 1992;31:226.
Foulder-Hughes L.A., Cooke R.W. Motor, cognitive, and behavioural disorders in children born very preterm. Dev Med Child Neurol. 2003;45:97.
Frazier T.W., Demaree H.A., Youngstrom E.A. Meta-Analysis of Intellectual and Neuropsychological Test Performance in Attention-Deficit/Hyperactivity disorder. Neuropsychology. 2004;18(3):543-555.
Fuchs T., Birbaumer N., Lutzenberger W., et al. Neurofeedback treatment for attention-deficit/hyperactivity disorder in children: A comparison with methylphenidate. Appl Psychophysiol Biofeedback. 2003;28:1.
Gerring J., Brady K., Chen A., et al. Neuroimaging variables related to development of secondary attention deficit hyperactivity disorder after closed head injury in children and adolescents. Brain Inj. 2000;14:205.
Gottlieb D.J., Vezina R.M., Chase C., et al. Symptoms of sleep-disordered breathing in 5-year-old children are associated with sleepiness and problem behaviors. Pediatrics. 2003;112:870.
Gould M.S., Walsh B.T., Munfakh J.L., et al. Sudden Death and Use of Stimulant Medication in Youths. Am J Psychiatry. 2009;166:992-1001.
Green W.H. Child and adolescent clinical psychopharmacology 4e, Ch7. Philadelphia: Lippincott Williams and Wilkins; 2007.
Green W.H. Child and adolescent clinical psychopharmacology. Philadelphia: Lippincott Williams & Wilkins; 2001.
Greenberg L.M. Test of variables of attention: Continuous performance test. Los Alamitos, Calif: Universal Attention Disorder; 1993.
Greenhill L.L., Pine D., March J., et al. Assessment issues in treatment research of pediatric anxiety disorders: What is working, what is not working, what is missing, and what needs improvement. Psychopharmacol Bull. 1998;34:155.
Greydanus D.E., Nazeer A., Patel D.R. Psychopharmacology of ADHD. In Pediatrics: Current Advances and Issues. Neuropsychiatr Dis Treat. 2009;5:171-181.
Gross M. Growth of hyperkinetic children taking methylphenidate, dextroamphetamine, or imipramine/desipramine. J Pediatr. 1976;58:423.
Gross M.D. Origin of stimulant use for treatment of attention deficit disorder. Am J Psychiatry. 1995;152:298.
Happe S. Excessive daytime sleepiness and sleep disturbances in patients with neurological diseases: Epidemiology and management. Drugs. 2003;63:2725.
Hart E.L., Lahey B.B., Loeber R., et al. Developmental change in attention deficit/hyperactive disorder in boys: A four year longitudinal study. J Abnorm Child Psychol. 1995;23:729.
Heinrich H., Gevensleben H., Freisleder F.J., et al. Training of slow cortical potentials in attention-deficit/hyperactivity disorder: Evidence for positive behavioral and neurophysiological effects. Biol Psychiatry. 2004;55:772.
Heywood C., Beale I. EEG biofeedback vs. placebo treatment for attention-deficit/hyperactivity disorder: A pilot study. J Atten Disord. 2003;7:43.
Holtmann M., Becker K., Kentner-Figura B., et al. Increased frequency of rolandic spikes in ADHD children. Epilepsia. 2003;44:1241.
Hunt R.D., Arnsten A.F., Asbell M.D. An open trial of guanfacine in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995;34:50.
Ivanenko A., Tauman R., Gozal D. Modafinil in the treatment of excessive daytime sleepiness in children. Sleep Med. 2003;4:579.
Janowsky D.S. Depression and dysphoria effects on the interpersonal perception of negative and positive moods and caring relationships: Effects of antidepressants, amphetamine, and methylphenidate. Curr Psychiatry Rep. 2003;5:451.
Jensen P.S., Martin D., Cantwell D.P. Co-morbidity in ADHD: Implications for research, practice, and DSM-V. J Am Acad Child Adolesc Psychiatry. 1997;36:1035.
Keating G.M., Figgitt D.P. Dexmethylphenidate. Drugs. 2002;62:1899.
Kent L. Recent advances in the genetics of attention deficit hyperactivity disorder. Curr Psychiatry Rep. 2004;6:143.
Klingberg T., Forssberg H., Westerberg H. Training of working memory in children with ADHD. J Clin Exp Neuropsychol. 2002;24:781.
Konrad K., Gunther T., Hanisch C., et al. Differential effects of methylphenidate on attentional functions in children with attention deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:191.
Krause K.H., Dresel S.H., Krause J., et al. Increased striatal dopamine transporter in adult patients with attention deficit hyperactivity disorder. Effects of methylphenidate as measured by single proton emission computed tomography. Neurosci Lett. 2000;285:107.
Lahey B.B., Applegate B., McBurnett K., et al. DSM-IV field trials for attention deficit hyperactivity disorder in children and adolescents. Am J Psychiatry. 1994;151:1673.
Laporte N., Sébire G., Gillerot Y., et al. Cognitive epilepsy: ADHD related to focal EEG discharges. Pediatr Neurol. 2002;27:307.
Leckman J.F., Knorr A.M., Rasmusson A.M., et al. Basal ganglia research and Tourette’s syndromes. Trends Neurosci. 1991;14:94.
Leitner Y., Barak R., Giladi N., et al. Gait in Attention Deficit Hyperactivity Disorder: effects of Methylphenidate and dual tasking. J Neurol. 2007;254:1330-1338.
Loo S.K., Specter E., Smolen A., et al. Functional effects of the DAT1 pleomorphism on EEG measures in ADHD. J Am Acad Child Adolesc Psychiatry. 2003;42:986.
Lorberboym M., Watemberg N., Nissenkorn A., et al. Technetium 99m ethylcysteinate dimer single-photon emission computed tomography (SPECT) during intellectual stress test in children and adolescents with pure versus comorbid attention-deficit hyperactivity disorder (ADHD). J Child Neurol. 2004;19:91.
Lubar J.F., Swartwood M.O., Swartwood J.N., et al. Evaluation of the effectiveness of EEG neurofeedback training for ADHD in a clinical setting as measured by changes in T.O.V.A. scores, behavioral ratings, and WISC-R performance. Biofeedback Self Regul. 1995;20:83.
MacKeith R. The types and frequency of antenatal and perinatal cerebral lesions. Bibl Paediatr. 1963;81:1.
Mostofsky S.H., Cooper K.L., Kates W.R., et al. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2002;52:785.
Mostofsky S.H., Reiss A.L., Lockhart P., et al. Evaluation of cerebellar size in attention-deficit hyperactivity disorder. J Child Neurol. 1998;13:434.
MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. A 14 month randomized clinical trial of treatment for attention deficit/ hyperactivity disorder. Arch Gen Psychiatry. 1999;56:1073.
MTA Cooperative Group. National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: Changes in effectiveness and growth after the end of treatment. Pediatrics. 2004;113:762.
Nass R. ADHD: Evaluation and Assessment Issues in the Diagnosis of Attention Deficit Hyperactivity Disorder. Semin Pediatr Neurol. 2005;12:200-216.
National Longitudinal Survey of Youth (NLSY79). US Dept. of Labor; 1979. Available at: www.bls.gov/nls/y79summary.htm
Nichols S.L., Waschbusch D.A. A review of the validity of laboratory cognitive tasks used to assess symptoms of ADHD. Child Psychiatry Hum Dev. 2004;34:297.
Owens J.A. The ADHD and Sleep Conundrum: A Review. J Dev Behav Pediatr. 2005;26(4):312-322.
Palfrey J.S., Levine M.D., Walker D.K., et al. The emergence of attention deficits in early childhood: A prospective study. J Dev Behav Pediatr. 1985;6:339.
Palmer C.G., Bailey J.N., Ramsey C., et al. No evidence of linkage or linkage disequilibrium between DAT1 and attention deficit hyperactivity disorder in a large sample. Psychiatr Genet. 1999;9:157.
Pauls D.V. The Genetics Of Attention-Deficit/Hyperactivity Disorder (Commentary). Biol Psychiatry. 2005;57:1310-1312.
Payton A., Holmes J., Barrett J.H., et al. Examining for association between candidate gene polymorphisms in the dopamine pathway and attention-deficit hyperactivity disorder: A family-based study. Am J Med Genet. 2001;105:464.
Pelc K., Dan B. The ADHD Tetragrammaton Takin in Vain in Neurogenetic Disorders? Acta Paediatr. 2008;97:2-4.
Pelham W.E., Greenslade K.E., Vodde-Hamilton M., et al. Relative efficacy of long-acting stimulants on children with attention deficit-hyperactivity disorder: A comparison of standard methylphenidate, sustained-release methylphenidate, sustained-released dextroamphetamine and pemoline. Pediatrics. 1990;86:226.
Pelham W.E., Sturges J., Hoza J.A., et al. Sustained release and standard methylphenidate effects on cognitive and social behavior in children with attention deficit disorder. Pediatrics. 1987;80:491.
Perrin J.M., Friedman R.A., Knilans T.K., et al. Cardiovascular Monitoring and Stimulant Drugs For Attention Deficit/Hyperactivity Disorder. Pediatrics. 2008;122:451-453.
Perrin S., Last C.G. Relationship between ADHD and anxiety in boys: Results from a family study. J Am Acad Child Adolesc Psychiatry. 1996;35:988.
Popper C. Combining methylphenidate and clonidine: News reports about sudden death. J Child Adolesc Psychopharmacol. 1995;5:157.
Powell A., Yudd A., Zee P., et al. Attention deficit hyperactivity syndrome associated with orbitofrontal epilepsy in a father and son. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10:151.
Qiu A., Crocetti D., Adler M., et al. Basal Ganglia Volume and Shape in Children with Attention Deficit Hyperactivity Disorder. Am J Psychiatry. 2009;166:74-82.
Rapoport J.L., Buchsbaum M.S., Zahn T.P., et al. Dextroamphetamine: Cognitive and behavioral effects in normal prepubertal boys. Science. 1978;199:560.
Rapport M.D., Carlson G.A., Kelly K.L., et al. Methylphenidate and desipramine in hospitalized children. I. Separate and combined effects on cognitive function. J Am Acad Child Adolesc Psychiatry. 1993;32:333.
Rapport M.D., Stoner G., DuPaul G.J., et al. Attention deficit disorder and methylphenidate: A multilevel analysis of dose-response effects on children’s impulsivity across settings. J Am Acad Child Adolesc Psychiatry. 1988;27:60.
Raz R., Gabis L. Essential Fatty Acids and Attention-Deficit-Hyperactivity Disorder: A Systematic Review. Dev Med Child Neurol. 2009:1-13.
Richardson A.J., Montgomery P. The Oxford-Durham Study: A Randomized, Controlled Trial of Dietary Supplementation With Fatty Acids in Children With Developmental Coordination Disorder. Pediatrics. 2005;115:1360-1366.
Richer L.P., Shevell M.I., Rosenblatt B.R. Epileptiform abnormalities in children with attention-deficit-hyperactivity disorder. Pediatr Neurol. 2002;26:125.
Riddle M.A., Nelson J.C., Kleinman C.S., et al. Sudden death in children receiving Norpramin: A review of three reported cases and commentary. J Am Acad Child Adolesc Psychiatry. 1991;30:104.
Robison L.M., Sclar D.A., Skaer T.L., et al. Treatment modalities among US children diagnosed with attention-deficit hyperactivity disorder, 1995–99. Int Clin Psychopharmacol. 2004;19:17.
Robison L.M., Skaer T.L., Sclar D.A., et al. Is attention deficit hyperactivity disorder increasing among girls in the US? Trends in diagnosis and the prescribing of stimulants. CNS Drugs. 2002;16:129.
Rohde L.A., Roman T., Szobot C., et al. Dopamine transporter gene, response to methylphenidate and cerebral blood flow in attention deficit/hyperactivity disorder: A pilot study. Synapse. 2003;48:87.
Rosser T.L., Packer R.J. Neurocognitive dysfunction in children with neurofibromatosis type 1. Curr Neurol Neurosci Rep. 2003;3:129.
Rovet J.F. Congenital hypothyroidism: An analysis of persisting deficits and associated factors. Child Neuropsychol. 2002;8:150.
Safer D., Allen R., Barr E. Depression of growth in hyperactive children on stimulant drugs. N Engl J Med. 1972;287:217.
Schachar R., Tannock R. Test of four hypotheses for the co-morbidity of attention-deficit hyperactivity disorder and conduct disorder. J Am Acad Child Adolesc Psychiatry. 1995;34:639.
Schneider H., Eisenberg D. Who Receives a Diagnosis of Attention-Deficit/Hyperactivity Disorder in the United States Elementary School Population? Pediatrics. 2006;117:e601-e609.
Schnoll R., Burshteyn D., Cea-Aravena J. Nutrition in the treatment of attention-deficit hyperactivity disorder: A neglected but important aspect. Appl Psychophysiol Biofeedback. 2003;28:63.
Sever Y., Ashkenazi A., Tyano S., et al. Iron treatment in children with attention deficit hyperactivity disorder. A preliminary report. Neuropsychobiology. 1997;35:178.
Sharp W.S., Gottesman R.F., Greenstein D.K., et al. Monozygotic twins discordant for attention-deficit/hyperactivity disorder. Ascertainment and clinical characteristics. J Am Acad Child Adolesc Psychiatry. 2003;42:93.
Shaw P., Eckstrand K., Sharp W., et al. Attention-Deficit/Hyperactivity Disorder is Characterized By A Delay In Cortical Maturation. Proc Natl Acad Sci USA. 2007;104:19649-19654.
Shaw P., Sharp W.S., Morrison M., et al. Psycho stimulant Treatment and The Developing Cortex in Attention Deficit Disorder. Am J Psychiatry. 2009;166:58-63.
Shaywitz B.A., Fletcher J.M., Shaywitz S.E. Defining and classifying learning disabilities and attention-deficit/hyperactivity disorder. J Child Neurol. 1995;10:S50.
Shaywitz S.E., Schnell C., Shaywitz B.A., et al. Yale Children’s Inventory (YCI): An instrument to assess children with attention deficits and learning disabilities. I. Scale development and psychometric properties. J Abnorm Child Psychol. 1986;14:347.
Shaywitz S.E., Shaywitz B.A. Diagnosis and management of attention deficit disorder: A pediatric perspective. Pediatr Clin North Am. 1984;31:429.
Short E.J., Manos M.J., Findling R.L., et al. A prospective study of stimulant response in preschool children: Insights from ROC analyses. J Am Acad Child Adolesc Psychiatry. 2004;43:251.
Skirrow C., McLaughlin G., Kuntsi J., et al. Behavioral, Neurocognitive and Treatment Overlap Between Attention-Deficit/Hyperactivity Disorder and Mood Instability. Expert Rev Neurother. 2009;9(4):489-503.
Smith J.L., Johnstone S.J., Barry R.J. Aiding diagnosis of attention deficit/ hyperactivity disorder and its subtypes: Discriminant function analysis of event-related potential data. J Child Psychol Psychiatry. 2003;44:1067.
Solanto M.V. Dopamine dysfunction in AD/HD: Integrating clinical and basic neuroscience research. Behav Brain Res. 2002;130:65. 10
Sonuga-Barke E.J. Interval length and time-use by children with AD/HD: A comparison of four models. J Abnorm Child Psychol. 2002;30:257.
Sparkes S.J., MacMaster F.P., Carrey N.C. Proton magnetic resonance spectroscopy and cognitive function in pediatric attention deficit/hyperactivity disorder. Brain Cogn. 2004;54:173.
Spencer T., Biederman J., Coffey B., et al. The 4-year course of tic disorders in boys with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1999;56:842.
Spencer T., Biederman J., Wilens T. Growth deficits in children with attention deficit hyperactivity disorder. Pediatrics. 1998;102:501.
Spencer T., Biederman J., Wilens T., et al. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35:409.
Spencer T., Heiligenstein J.H., Biederman J., et al. Results from 2 proof-of-concept, placebo-controlled studies of atomoxetine in children with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2002;63:1140.
Still G. Some abnormal psychical conditions in children. Lancet. 1902;1:1166.
Strauss A.A., Lehtinen L.E. Psychopathology and education of the brain-injured child. New York: Grune & Stratton; 1947.
Suresh P.A., Sebastian S., George A., et al. Subclinical hyperthyroidism and hyperkinetic behavior in children. Pediatr Neurol. 1999;20:192.
Swanson J.M., Greenhill L.L., Lopez F.A., et al. Modafinil film coated tablets in children and adolescents with Attention-Deficit/Hyperactivity Disorder: Results of a randomized, double-blind, placebo-controlled, fixed dose study followed by abrupt discontinuation. J Clin Psychiatry. 2006;67:137-147.
Swanson J., Oosterlaan J., Murias M., et al. Attention deficit/hyperactivity disorder children with a 7-repeat allele of the dopamine receptor D4 gene have extreme behavior but normal performance on critical neuropsychological tests of attention. Proc Natl Acad Sci USA. 2000;97:4754.
Tahir E., Yazgan Y., Cirakoglu B., et al. Association and linkage of DRD4 and DRD5 with attention deficit hyperactivity disorder (ADHD) in a sample of Turkish children. Mol Psychiatry. 2000;5:396.
Thunstrom M. Severe sleep problems in infancy associated with subsequent development of attention-deficit/hyperactivity disorder at 5.5 years of age. Acta Paediatr. 2002;91:584.
Toft P.B. Prenatal and perinatal striatal injury: A hypothetical cause of attention-deficit-hyperactivity disorder? Pediatr Neurol. 1999;21:602.
Turkington D., Hedwat D., Rider I., et al. Recovery from chronic fatigue syndrome with modafinil. Hum Psychopharmacol Clin Exp. 2004;19:63.
Tuthill R.W. Hair lead levels related to children’s classroom attention-deficit behavior. Arch Environ Health. 1996;51:214.
Vaidya C.J., Austin G., Kirkorian G., et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: A functional magnetic resonance study. Proc Natl Acad Sci USA. 1998;95:14494.
Vaughn M.L., Riccio C.A., Hynd G.W., et al. Diagnosing ADHD (predominantly inattentive and combined type subtypes): Discriminant validity of the behavior assessment system for children and the Achenbach Parent and Teacher Rating Scales. J Clin Child Psychol. 2000;26:349.
Vetter V.L., Elia J., Erickson C., et al. Cardiovascular Monitoring of Children and Adolescents With Heart Disease Receiving Stimulant Drugs. A Scientific Statement From The American Heart Association Council On Cardiovascular Disease In The Young, Congenital Cardiac Defects Committee And The Council On Cardiovascular Nursing. Circulation. 2008;117:2407-2423.
Vitiello B., Towbin K. Stimulant Treatment of ADHD and Risk of Sudden Death in Children (Editorial). Am J Psychiatry. 2009;166:955-957.
Waldman I.D., Rowe D.C., Abramowitz A., et al. Association and linkage of the dopamine transporter gene and attention-deficit hyperactivity disorder in children: Heterogeneity owing to diagnostic subtype and severity. Am J Hum Genet. 1998;63:1767.
Walters A.S., Mandelbaum D.E., Lewin D.S., et al. Dopaminergic therapy in children with restless legs/periodic limb movements in sleep and ADHD. Pediatr Neurol. 2000;22:182.
Wassenberg R., Max J.E., Lindgren S.D., et al. Sustained attention in children and adolescents after traumatic brain injury: Relation to severity of injury, adaptive functioning, ADHD and social background. Brain Inj. 2004;18:751.
Weiss M., Weiss G. Attention deficit hyperactivity disorder. In: Lewis M., editor. Child and adolescent psychiatry: A comprehensive textbook. Philadelphia: Lippincott Williams & Wilkins, 2002.
Wilens T., Biederman J. The stimulants. Psychiatr Clin North Am. 1992;15:191.
Willcutt E.G., Doyle A.E., Nigg J.T., et al. Validity of The Executive Function Theory of Attention-Deficit/Hyperactivity Disorder: A Meta-Analytic Review. Biol Psychiatry. 2005;57:1336-1346.
Willcutt E.G., Pennington B.F., Smith S.D., et al. Quantitative trait locus for reading disability on chromosome 6p is pleiotropic for attention deficit/hyperactivity disorder. Am J Med Genet. 2002;114:260.
Winsberg B.G., Comings D.E. Association of the dopamine transporter gene (DAT1) with poor methylphenidate response. J Am Acad Child Adolesc Psychiatry. 1999;38:1474.
Wolraich M.L., Hannah J.N., Pinnock T.Y., et al. Comparison of diagnostic criteria for attention-deficit hyperactivity disorder in a county-wide sample. J Am Acad Child Adolesc Psychiatry. 1996;35:319.
Wolraich M.L., Lambert W., Baumgaertel A., et al. Teachers’ screening for attention deficit/hyperactivity disorder: Comparing multinational samples on teacher ratings of ADHD. J Abnorm Child Psychol. 2003;31:445.
Wolraich M.L., Lambert W., Doffing M.A., et al. Psychometric properties of the Vanderbilt ADHD Diagnostic Parent Rating Scale in a referred population. J Pediatr Psychol. 2003;28:559.