Chapter 17 Atrioventricular Nodal Reentrant Tachycardia
Pathophysiology
Anatomy and Physiology of the Atrioventricular Node
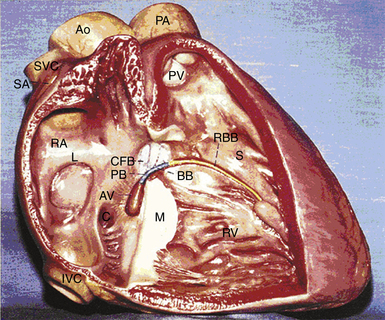
The atrioventricular (AV) junction is a complex structure, located within an area called the triangle of Koch (Fig. 17-1).1 The triangle of Koch is bounded by the coronary sinus ostium (CS os) posteriorly, the tricuspid annulus (the attachment of the septal leaflet of the tricuspid valve) inferiorly, and the tendon of Todaro anteriorly and superiorly. The triangle of Koch is septal in location and constitutes the right atrial (RA) endocardial surface of the muscular AV septum. The compact AV node (AVN) lies just beneath the RA endocardium, at the apex of the triangle of Koch, anterior to the CS os, and directly above the insertion of the septal leaflet of the tricuspid valve, where the tendon of Todaro merges with the central fibrous body. Slightly more anteriorly and superiorly is where the His bundle (HB) lies. The mean distances from the HB electrogram recording site to the upper and lower lips of the CS os are 10 mm (range, 0 to 23 mm), and 20 to 25 mm (range, 9 to 46 mm), respectively. However, the contour of the Koch triangle may be small or even horizontal in some patients.2–4
Functionally, based on activation times during anterograde or retrograde propagation, or both, and on the action potential characteristics from microelectrode recordings in the rabbit AV junction, the cells of the AVN region are frequently described as AN (atrionodal), N (nodal), and NH (nodal-His). The transition from one cell area to the other is gradual, with intermediate cells exhibiting intermediate action potentials with great changes related to the autonomic tone.2–6
The AN region corresponds to the cells in the transitional zone, which are activated shortly after the atrial cells. The transitional cell zone represents the inferior, more open portion of the AVN into which atrial bands gradually merge (i.e., the approaches from the working atrial myocardium to the AVN). Transitional cells are histologically distinct from both the cells of the compact AVN and the working atrial myocytes. Transitional cells are not insulated from the surrounding myocardium but tend to be separated from one another by thin fibrous strands. The connections between atrial and transitional cells are so gradual that no clear anatomical demarcations can be detected.7 Transitional cells do not represent conducting tracts, but rather a bridge funneling of atrial depolarization into the compact AVN through discrete AVN inputs (approaches). In humans and animals, two such inputs are commonly recognized in the right septal region: the anterior (superior) approaches, which travel from the anterior limbus of the fossa ovalis and merge with the AVN closer to the apex of the triangle of Koch, and the posterior (inferior) approaches, located in the inferoseptal RA and which serve as a bridge with the atrial myocardium at the CS os. Although both inputs have traditionally been assumed to be RA structures, growing evidence supports the AV conduction apparatus as a transseptal structure that reaches both atria. A third, middle group of transitional cells has also been identified to account for the nodal connections with the septum and left atrium (LA).2,5,8–12
The N region corresponds to the compact AVN, the region where transitional cells merge with midnodal cells. The N cells represent the most typical of the nodal cells because they are characterized by a less negative resting membrane potential and low action potential amplitude (mediated by the L-type calcium [Ca+2] current), slow rates of depolarization and repolarization, few intercellular connections such as gap junctions, and reduced excitability compared with surrounding cells. The N cells in the compact AVN appear to be responsible for the major part of AV conduction delay. Sodium channel density is lower in the midnodal zone of the AVN than in the AN and NH cell zones, and the inward L-type Ca2+ current is the basis of the upstroke of the N cell action potential. Therefore, conduction is slower through the compact AVN than through the AN and NH cell zones.1,6 Fast pathway conduction through the AVN apparently bypasses many of the N cells by transitional cells, whereas slow pathway conduction traverses the entire compact AVN. Importantly, the recovery of excitability after conduction of an impulse is faster for the slow pathway than for the fast pathway for reasons that are unclear.5,6,13
When traced inferiorly, toward the base of the triangle of Koch, the compact AVN area separates into two extensions, usually with the artery supplying the AVN running between them. The prongs bifurcate toward the CS os and tricuspid annulus (right posterior extension) and toward the mitral annulus (left posterior extension). The right posterior nodal extension has been implicated as the anatomical substrate for the so-called slow pathway in the AVN reentrant tachycardia (AVNRT) circuit. The tachycardia circuit may seldom involve the left posterior nodal extension (see later). The fast pathway is less well defined from an anatomical and structural standpoint. The probable anatomical substrate of this pathway consists of the transitional cell layers located around the compact AVN at the interface between the compact node and transitional cells.3,4,12,14 The HB connects with the distal part of the compact AVN and passes through the fibrous core of the central fibrous body in a leftward direction (away from the RA endocardium and toward the ventricular septum). The HB then continues through the annulus fibrosis, where it is called the nonbranching portion as it penetrates the membranous septum, along the crest of the left side of the interventricular septum, for 1 to 2 cm and then divides into the right bundle branch (RB) and left bundle branch (LB). The HB is insulated from the atrial myocardium by the membranous septum and from the ventricular myocardium by connective tissue of the central fibrous body.1 Viewed from the left ventricle (LV), the HB is marked by the area of fibrous continuity between the aortic and mitral valves adjacent to the membranous septum. Viewed from the aorta, the HB passes beneath the part of the membranous septum that adjoins the interleaflet fibrous triangle between the right and noncoronary sinuses.12
Tachycardia Circuit
The concept of AVN reentry is related, but not identical, to the so-called dual pathway electrophysiology. It is well established that dual AVN physiology is the underlying substrate for AVNRT; however, it is important to recognize that dual AVN physiology characterizes the normal AVN electrophysiology, and the presence of dual (or multiple) AVN pathways is not necessarily indicative of the existence of functional reentry, although it is required to maintain the reentry circuit. Nevertheless, the presence of dual AVN physiology provides the natural substrate for the occurrence of AVN reentry. In the absence of an animal model, however, the exact pathophysiological substrate for AVNRT remains uncertain.4
The AVNRT circuit does not involve the ventricles, but whether the circuit is confined to the compact AVN (subatrial) or involves a component of perinodal atrial myocardium is controversial. There is good evidence that the distal junction of the slow and fast pathways is located in the AVN, with the existence of a region of AVN tissue extending between the distal junction of the two pathways and the HB (called the lower common pathway), at least in a subset of patients (Table 17-1). However, the nature of the proximal link between these pathways is unclear, and the existence of an upper common pathway is still a matter of controversy (Tables 17-2 and 17-3). Based on rare cases of dissociation of atrial activation from the tachycardia (e.g., persistence of AVNRT during different patterns of VA block) and on similarities between fast-slow AV conduction and longitudinal-transverse conduction in nonuniform anisotropy, early studies proposed that AVNRT results from reentry within the compact AVN because of functional longitudinal dissociation within the AVN into fast and slow pathways and suggested the presence of an upper common pathway, at least in a subset of patients.13,15
TABLE 17-1 Evidence against the Necessity of Ventricle in Reentrant Circuit*
| VA Wenckebach CL during ventricular pacing longer than the tachycardia CL |
| HA interval during ventricular pacing at the tachycardia CL longer than that during AVNRT |
| AV block occurring without interruption of AVNRT |
| AES during AVNRT resulting in changes in the relative activation of HB and atrium (i.e., varying HA intervals) |
| VES during AVNRT prematurely depolarizing the HB without affecting the tachycardia |
AES = atrial extrastimulation; AV = atrioventricular; AVNRT = atrioventricular nodal reentrant tachycardia; CL = cycle length; HA = His bundle–atrial; HB = His bundle; VA = ventriculoatrial; VES = ventricular extrastimulation.
* Supporting the presence of a lower common pathway.
From Josephson ME: Supraventricular tachycardias. In Josephson ME, editor: Clinical cardiac electrophysiology, ed 3, Philadelphia, 2002, Lippincott Williams & Wilkins, pp 168-271.
TABLE 17-2 Evidence for the Necessity of Atrium in Reentrant Circuit*
| The finding that AES delivered to the inferior atrial septum close to the CS os during AVNRT just before the expected time of retrograde fast pathway conduction can activate the slow pathway and advance the SVT |
| The finding that cure of AVNRT can be produced by placing ablative lesions in the perinodal atrial myocardium, as much as 10 mm or more from the compact AVN |
| Differences in the site of earliest atrial activation between retrograde conduction over the fast and slow pathways |
| Microelectrode and extracellular recordings and optical mapping of AVN reentrant echo beats in animals |
AES = atrial extrastimulation; AVN = atrioventricular node; AVNRT = atrioventricular nodal reentrant tachycardia; CS os = coronary sinus ostium; SVT = supraventricular tachycardia.
* That is, against the presence of an upper common pathway.
From Josephson ME: Supraventricular tachycardias. In Josephson ME, editor: Clinical cardiac electrophysiology, ed 3, Philadelphia, 2002, Lippincott Williams & Wilkins, pp 168-271.
TABLE 17-3 Evidence against the Necessity of Atrium in Reentrant Circuit*
| Initiation of AVNRT in the absence of an atrial echo |
| AV Wenckebach CL during atrial pacing longer than the tachycardia CL |
| AH interval during atrial pacing at the tachycardia CL longer than that during AVNRT |
| Retrograde VA block occurring without interruption of AVNRT |
| AVNRT occurrence in the presence of AF |
| Depolarization of the atria surrounding the AVN without affecting the tachycardia |
| Resetting of the tachycardia by ventricular stimulation in the absence of atrial activation |
| Heterogeneous atrial activation during AVNRT that is incompatible with atrial participation |
| Changing VA relationship with minimal or no change in the tachycardia CL (suggesting that atrial activation is determined by the functional output to the atrium from the tachycardia and not causally related to it) |
AF = atrial fibrillation; AH = atrial–His bundle; AV = atrioventricular; AVN = atrioventricular node; AVNRT = atrioventricular nodal reentrant tachycardia; CL = cycle length; VA = ventriculoatrial.
* Supporting the presence of an upper common pathway.
From Josephson ME: Supraventricular tachycardias. In Josephson ME, editor: Clinical cardiac electrophysiology, ed 3, Philadelphia, 2002, Lippincott Williams & Wilkins, pp 168-271.
Current evidence, derived from multielectrode recordings and optical mapping studies, supports the role of perinodal tissue and suggests that the fast and slow pathways involved in the reentrant circuit of AVNRT represent conduction over different atrionodal connections, thus making at least a small amount of atrial tissue a necessary part of the reentrant circuit (Video 19  ). As noted, the compact AVN is surrounded by transitional cells whose structure and function are intermediate between those of atrial and compact nodal cells. If one considers the compact AVN and the surrounding transitional cells as a functional AVN unit, which implies that the AVN tissue occupies the bulk of the triangle of Koch, then the AVN reentrant circuit may be considered as confined to the AVN. Therefore, much of the disagreement on the presence or absence of an upper common pathway and the role of the atrium in the genesis of the reentrant circuit may, in part, be related to the definition of the extent of the AVN.16
). As noted, the compact AVN is surrounded by transitional cells whose structure and function are intermediate between those of atrial and compact nodal cells. If one considers the compact AVN and the surrounding transitional cells as a functional AVN unit, which implies that the AVN tissue occupies the bulk of the triangle of Koch, then the AVN reentrant circuit may be considered as confined to the AVN. Therefore, much of the disagreement on the presence or absence of an upper common pathway and the role of the atrium in the genesis of the reentrant circuit may, in part, be related to the definition of the extent of the AVN.16
Nevertheless, the understanding of the AVN as having superior (anterior) and inferior (posterior) inputs that form the fast and slow pathways, respectively, is a simple conceptual framework that seems to enable the clinician to confront most cases. Reentry occurring along these pathways is the basic mechanism for the various subtypes of AVNRT. The proximal atrial insertions of the fast and slow pathways are anatomically distinct during retrograde conduction, and several important functional differences exist between the two pathways (Table 17-4).13,14
TABLE 17-4 Functional Differences between Fast and Slow Atrioventricular Node Pathways
| The fast pathway forms the normal physiological conduction axis, and the AH interval during conduction over the fast pathway is usually no longer than 220 msec. Longer AH intervals can be caused by conduction over the slow pathway. |
| Anterograde ERP of the fast pathway is usually longer than that of the slow pathway. However, many exceptions exist. |
| Adrenergic stimulation tends to shorten the anterograde and retrograde ERP of the fast pathway to a greater extent than that of the slow pathway. Conversely, beta blockers tend to prolong ERP of the fast pathway more than that of the slow pathway. |
| The earliest atrial activation site during retrograde conduction over the fast pathway is in the anterior apex of the triangle of Koch at the same site recording the proximal His potential (although some studies showed the earliest site of atrial activation occurring in the anterior interatrial septum above the tendon of Todaro, outside the triangle of Koch), whereas that over the slow pathway is in the base of the triangle of Koch. |
AH = atrial–His bundle; ERP = effective refractory period.
Types of Atrioventricular Nodal Reentry
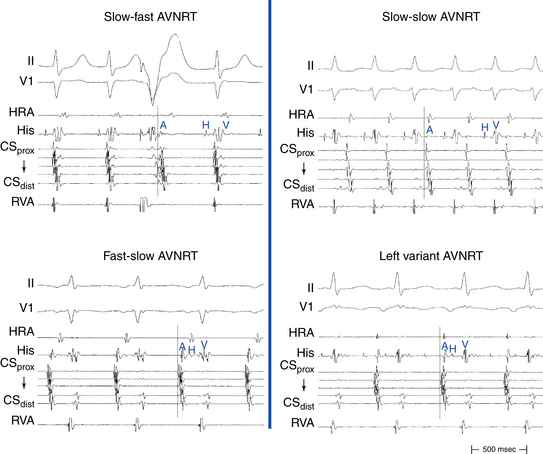
Slow-Fast (Typical, Common) Atrioventricular Nodal Reentrant Tachycardia
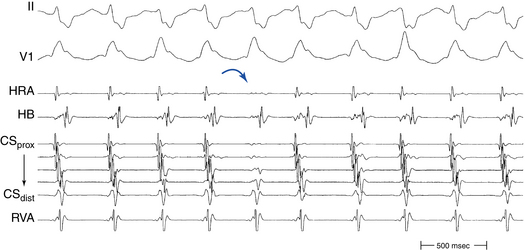
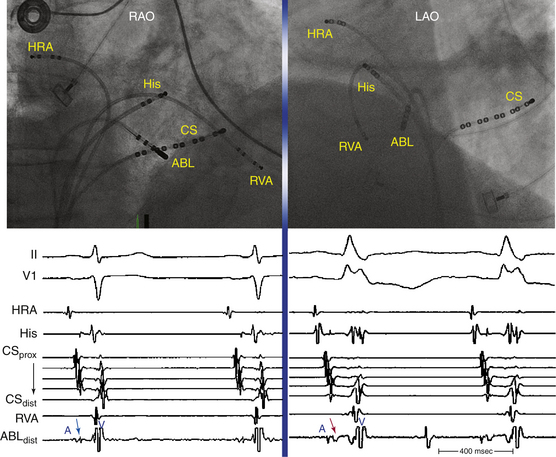
Typical AVNRT accounts for 90% of AVNRTs. The reentrant circuit uses the slow AVN pathway anterogradely and the fast pathway retrogradely. The earliest atrial activation during typical AVNRT is usually in the apex of the triangle of Koch; however, retrograde atrial activation over the fast pathway is heterogeneous and may be found at the CS os or on the left side of the septum in up to 9% of patients (Fig. 17-2).8,13,17,18
The presence of a lower common pathway in typical AVNRT remains controversial. Although some studies suggested the existence of a lower common pathway in most patients with typical AVNRT, others demonstrated that most patients with AVNRT without evidence of a substantial lower common pathway had typical AVNRT (i.e., the lower turnaround is within the proximal HB), and the presence of a lower common pathway was strongly associated with the atypical variants of AVNRT. Nevertheless, it is recognized that the lower common pathway in typical AVNRT, if present, is very short (as assessed by the degree of HB prematurity required for a ventricular extrastimulus [VES] to reset the tachycardia, and by comparing the HB-atrial (HA) interval during AVNRT with that during ventricular pacing at the tachycardia cycle length [CL]).8
Fast-Slow (Atypical, Uncommon) Atrioventricular Nodal Reentrant Tachycardia
The earliest retrograde atrial activation during fast-slow AVNRT is usually in the inferoposterior part of the triangle of Koch. The AH interval is shorter than the HA interval (30 to 185 milliseconds versus 135 to 435 milliseconds), resulting in long RP tachycardia. The lower common pathway is relatively long.8,19
Slow-Slow (Posterior-Type) Atrioventricular Nodal Reentrant Tachycardia
The AH interval is long (more than 200 milliseconds). The HA interval is often short, but it has a much wider range than that in typical AVNRT (–30 to 260 milliseconds, usually more than 70 milliseconds). The ventriculoatrial (VA) interval (as measured from the onset of ventricular activity to the onset of atrial activity by whichever electrode recorded the earliest interval) may be prolonged, ranging from 76 to 168 milliseconds. The AH/HA ratio, however, remains greater than 1. Therefore, this type is sometimes called slow-intermediate AVNRT. The lower common pathway is significantly longer than that in typical AVNRT, a finding that explains the short HA interval seen in many patients with slow-slow AVNRT. These patients often exhibit multiple AH interval jumps during atrial extrastimulus (AES) testing, consistent with multiple slow pathways.8
Left Variant of Atrioventricular Nodal Reentrant Tachycardia
It has been known for some time that the slow pathway may be composed of both rightward and leftward posterior nodal extensions. The rightward extensions travel anatomically in the triangle of Koch between the tricuspid annulus and the CS os. The leftward extensions travel within the myocardial coat of the proximal CS leftward (transseptally) toward the left inferoseptal region and mitral annulus. These leftward extensions can then connect with the rightward extensions in the triangle of Koch anterior to the CS os. The leftward inferior extension can provide an LA input to the AVN, as suggested by functional studies showing preferential access to the AVN from the left inferior septum. Clinically, the leftward inferior nodal extension can behave as the slow pathway and lead to AVNRT. In the typical (slow-fast) form of AVNRT, this would render ablation in the usual inferoseptal RA ineffective and lead to the necessity of ablating inside the CS or on the posterior mitral annulus.8 In atypical forms of AVNRT, if the leftward inferior extension serves as the slow pathway conducting retrogradely, then the earliest atrial activation would be recorded in the LA, thus leading to eccentric CS activation.9
Eccentric retrograde atrial activation sequences that are suggestive of leftward atrionodal extensions of the slow AVN pathway have been described in AVNRT, but the exact incidence is unknown. Various reports have shown a higher incidence of the eccentric CS activation pattern among patients with atypical (14% to 80%) than those with typical (0% to 8%) forms of AVNRT.8,18,20,21 However, even in the presence of eccentric retrograde atrial activation during AVNRT, the significance of such leftward extensions to the AVNRT circuit has been debated. Whether the retrograde left-sided atrionodal connection constitutes the critical component of the reentrant circuit or is only an innocent bystander in atypical AVNRT with the eccentric CS activation pattern is controversial. It may be possible for both the leftward and rightward extensions, either together or separately, to participate in nodal reentry. Right-sided ablation is probably sufficient for most of these patients. However, in some patients, the slow pathway participating in the reentrant circuit cannot be ablated from the posteroseptal RA or the CS os, but it can be eliminated by ablation along the roof of the CS, as much as 5 to 6 cm from the CS os, or mitral annulus. In one report, direct left-sided ablation to the earliest retrograde activation site inside the CS (without ablation at the conventional site of the slow pathway) rendered the tachycardias noninducible in all 18 patients, a finding suggesting that the left-sided slow pathway constituted critical parts of the reentrant circuit.8,22
Superior Variant of Atrioventricular Nodal Reentrant Tachycardia
The superior variant of AVNRT uses a slowly conducting retrograde pathway with a superoseptal atrial exit as a retrograde limb and is associated with evidence of a lower common pathway. The anterograde limb of the circuit is likely the fast pathway. The superior variant of atypical AVNRT has been observed in 3% of all forms of AVNRT, and it is characterized by a shorter AH and longer HA interval during the tachycardia, a longer HA interval during ventricular pacing, and earliest retrograde atrial activation in the superoseptal RA. Whereas classical slow pathway ablation in the inferoseptal RA or proximal CS is highly successful in eliminating most types of AVNRT, it is inefficient in eliminating the superior variant of AVNRT. Instead, the tachycardia was eliminated in most patients in one report by ablation in the midseptal RA (where no His potential is recorded). Those observations suggest that the entire components of the tachycardia circuit in the superior type of atypical AVNRT may be localized to the superior part of the Koch triangle as compared with those in the inferior type. The mechanism by which ablation in the midseptal RA, not in the superoseptal RA where the earliest retrograde atrial activation was recorded, modified the retrograde slow pathway conduction properties and eliminated the tachycardia inducibility of the superior type is unclear.23
Clinical Considerations
Epidemiology
AVNRT is the most common form of paroxysmal supraventricular tachycardia (SVT). The absolute number of patients with AVNRT and its proportion of paroxysmal SVT increase with age. The reason may be related to the normal evolution of AVN physiology over the first two decades of life, as well as to age-related changes in atrial and nodal physiology observed in later decades.24 AVNRT is unusual in children less than 5 years of age, and it typically initially manifests in early life (e.g., in the teens). Conversely, atrioventricular reentrant tachycardia (AVRT) manifests earlier, with an average of more than 10 years separating the time of clinical presentation of AVRT and that of AVNRT. There is also a striking 2:1 predominance of AVNRT in women, in whom symptoms start at a significantly younger age.24,25 In fact, female sex and older age (i.e., teens versus newborns or young children) favor the diagnosis of AVNRT over AVRT.26 Gender differences in the anterograde and retrograde AVN electrophysiological (EP) properties have been observed and may contribute to the pathogenesis of AVNRT.25
Clinical Presentation
About half of patients with typical AVNRT report experiencing a pounding sensation in the neck during tachycardia, which is caused by simultaneous contraction of the atria and ventricles against closed mitral and tricuspid valves. The physical examination correlate of this phenomenon is continuous pulsing cannon A waves in the jugular venous waveform (described as the “frog” sign). This clinical feature has been reported to distinguish paroxysmal SVT resulting from AVNRT from that caused by orthodromic AVRT. Although atrial contraction during AVRT occurs against closed AV valves, the longer VA interval results in separate ventricular and then atrial contraction and a relatively lower RA and venous pressure; therefore, the presence of palpitations in the neck is experienced less commonly (about 17%) in patients with AVRT.26
Initial Evaluation
History, physical examination, and 12-lead ECG constitute an appropriate initial evaluation. In patients with brief, self-terminating episodes, an event recorder is the most effective way to obtain ECG documentation. An echocardiographic examination should be considered in patients with documented sustained SVT to exclude the possibility of structural heart disease.27 Further diagnostic studies (e.g., cardiac stress testing) are indicated only if there are signs or symptoms that suggest structural heart disease.8
Principles of Management
Acute Management
Because maintenance of AVNRT is dependent on AVN conduction, maneuvers or drugs that slow AVN conduction can be used to terminate the tachycardia. Initially, maneuvers that increase vagal tone (e.g., Valsalva maneuvers, gagging, carotid sinus massage) are used.27 When vagal maneuvers are unsuccessful, termination can be achieved with antiarrhythmic drugs whose primary effects increase refractoriness and/or decrease conduction (negative dromotropic effect) over the AVN. Adenosine is the drug of choice and is successful in almost 100% of cases.27 Verapamil, diltiazem, and beta blockers also can terminate AVNRT and prevent induction. Digoxin, which has a slower onset of action than the other AVN blockers, is not favored for the acute termination of AVNRT, except if there are relative contraindications to the other agents. Class IA and IC sodium channel blockers can also be used in treating an acute event of AVNRT, a strategy that is rarely used when other regimens have failed. If AVNRT cannot be terminated with intravenous drugs, electrical cardioversion can always be used. Energies in the range of 10 to 50 J are usually adequate.8
Chronic Management
Because AVNRT is generally a benign arrhythmia that does not influence survival, the primary indication for its treatment relates to its impact on a patient’s quality of life. Factors that contribute to the therapeutic decision include the frequency and duration of tachycardia, tolerance of symptoms, the effectiveness and tolerance of antiarrhythmic drugs, the need for lifelong drug therapy, and the presence of concomitant structural heart disease. Patients who develop a highly symptomatic episode of paroxysmal SVT, particularly if it requires an emergency room visit for termination, can elect to initiate therapy after a single episode. In contrast, a patient who presents with minimally symptomatic episodes of paroxysmal SVT that terminate spontaneously or in response to Valsalva maneuvers may elect to be followed clinically without specific therapy.8,27
Once it is decided to initiate treatment for AVNRT, the question arises whether to initiate pharmacological therapy or to use catheter ablation. Because of its high efficacy (greater than 95%) and low incidence of complications, catheter ablation has become the preferred therapy over long-term pharmacological therapy and can be offered as an initial therapeutic option. It is reasonable to discuss catheter ablation with all patients suspected of having AVNRT. However, patients considering RF ablation must be willing to accept the risk, albeit low, of AV block and pacemaker implantation. For patients in whom ablation is not desirable or available, long-term pharmacological therapy may be effective.27
Most pharmacological agents that depress AVN conduction (including beta blockers and calcium channel blockers) can reduce the frequency of recurrences of AVNRT. If those agents are ineffective, class IA, IC, or III antiarrhythmic agents may be considered. In general, drug efficacy is in the range of 30% to 50%.8
Outpatients can use a single dose of verapamil, propranolol, or flecainide to terminate an episode of AVNRT effectively. This so-called pill in the pocket approach (i.e., administration of a drug only during an episode of tachycardia for the purpose of termination of the arrhythmia when vagal maneuvers alone are not effective) is appropriate to consider for patients with infrequent episodes of AVNRT that are prolonged but well tolerated, and it obviates exposure of patients to long-term and unnecessary therapy between rare arrhythmic events. This approach necessitates the use of a drug that has a short onset of action (i.e., immediate-release preparations).27 Candidate patients should be free of significant LV dysfunction, sinus bradycardia, and preexcitation. Single-dose oral therapy with diltiazem (120 mg) plus propranolol (80 mg) has been shown to be superior to both placebo and flecainide in terminating AVNRT.8,27
Electrocardiographic Features
P Wave Morphology
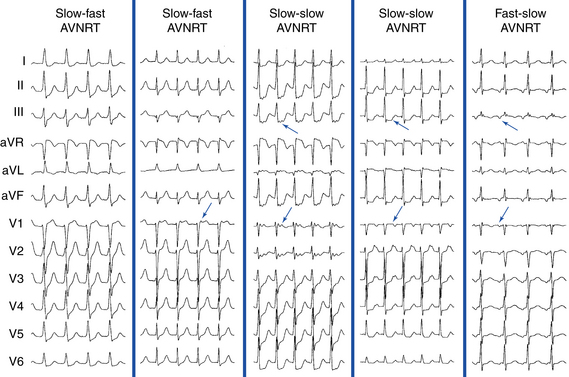
In typical (slow-fast) AVNRT, the P wave is usually not visible because of the simultaneous atrial and ventricular activation. The P wave can distort the initial portion of the QRS (mimicking a q wave in the inferior leads), lie just within the QRS (inapparent), or distort the terminal portion of the QRS (mimicking an s wave in the inferior leads or an r wave in lead V1) (Fig. 17-3).8 When apparent, the P wave is significantly narrower than the sinus P wave and is negative in the inferior leads, findings consistent with concentric retrograde atrial activation over the fast AVN pathway.8 In atypical (fast-slow) AVNRT, the P wave is relatively narrow, negative in the inferior leads, and positive in lead V1 (see Fig. 17-3).8
QRS Morphology
QRS morphology during AVNRT is usually the same as in normal sinus rhythm (NSR). The development of prolonged functional aberration during AVNRT is uncommon, and it usually occurs following induction of AVNRT by ventricular stimulation more frequently than by atrial stimulation, or following resumption of 1:1 conduction to the ventricles after a period of block below the tachycardia circuit.8 At times, alternans of QRS amplitude can occur when the tachycardia rates are rapid. Occasionally, AVNRT can coexist with ventricular preexcitation over an AV bypass tract (BT), whereby the BT is an innocent bystander.8
P-QRS Relationship
In typical (slow-fast) AVNRT, the RP interval is very short (–40 to 75 milliseconds). Variation of the P-QRS relationship with or without block can occur during AVNRT, especially in atypical or multiple-form tachycardias.8 This phenomenon usually occurs when the conduction system and the reentry circuit are unstable during initiation or termination of the tachycardia, likely secondary to decremental conduction in the lower common pathway. The ECG manifestation of P-QRS variations with or without AV block during tachycardia, especially at the initiation of tachycardias or in cases of nonsustained tachycardias, should not be misdiagnosed as atrial tachycardias (ATs); these variations may represent atypical or, rarely, typical forms of AVNRT. Moreover, the variations can be of such magnitude that long RP tachycardia can masquerade for brief periods of time as short RP tachycardia.
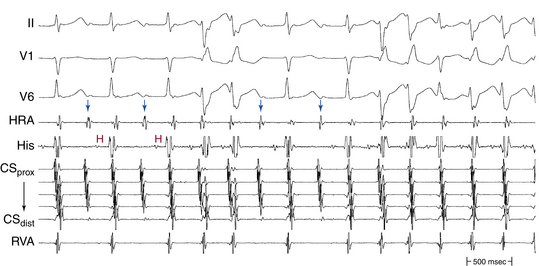
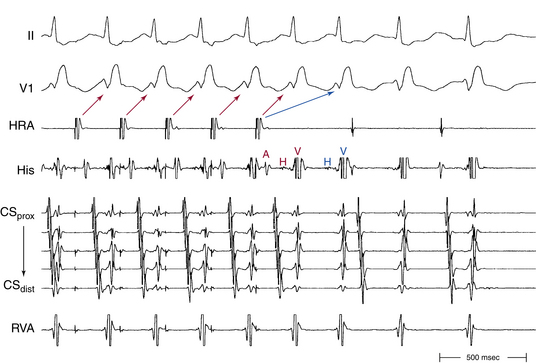
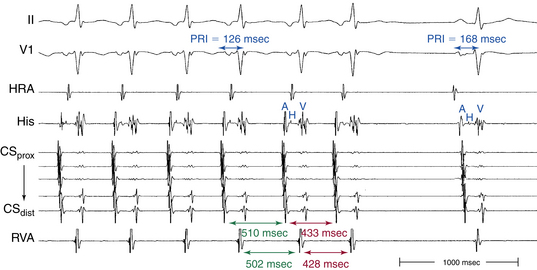
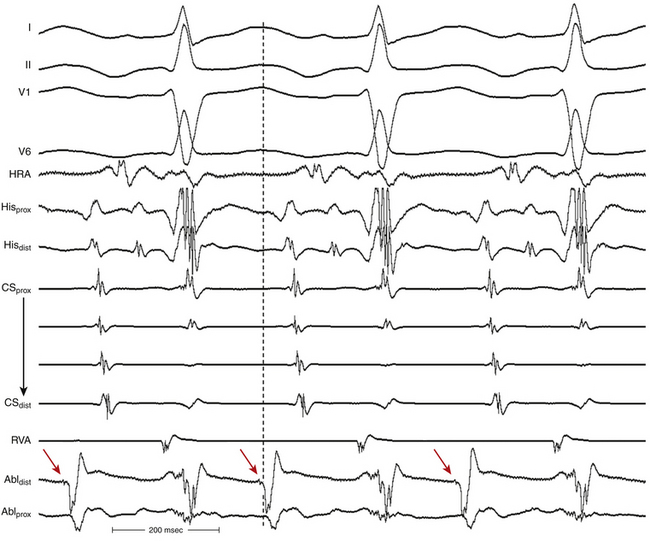
Usually, the A/V ratio during AVNRT is equal to 1; however, 2:1 AV block can be present because of block below the reentry circuit (usually below the HB and, infrequently, in the lower common pathway). In such cases, narrow, inverted P wave morphology in the inferior leads inscribed exactly between QRS complexes strongly suggests AVNRT (Fig. 17-4). The incidence of reproducible sustained 2:1 AV block during induced episodes of AVNRT is approximately 10%. Rarely, VA block can occur because of block in an upper common pathway.8
In atypical (fast-slow) AVNRT, the RP interval is longer than the PR interval. In slow-slow AVNRT, the RP interval is usually shorter than, and sometimes equal to, the PR interval. Occasionally, the P wave is inscribed in the middle of the cardiac cycle, thus mimicking atrial flutter or AT with 2:1 AV conduction (Fig. 17-5).19 Slow-slow AVNRT can be associated with RP intervals and P wave morphology similar to that during orthodromic AV reentrant tachycardia (AVRT) using a posteroseptal AV BT. However, although both SVTs have the earliest atrial activation in the posteroseptal region, conduction time from that site to the HB region is significantly longer in AVNRT than in orthodromic AVRT. The results are a significantly longer RP interval in lead V1 and a significantly larger difference in the RP interval between lead V1 and inferior leads during AVNRT. Therefore, ΔRP interval (V1 – III) of more than 20 milliseconds suggests slow-slow AVNRT (sensitivity, 71%; specificity, 87%).
Electrophysiological Testing
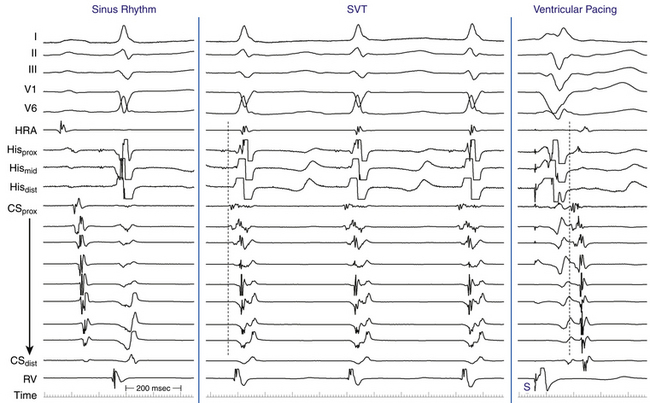
EP testing is used to study the inducibility and mechanism of the SVT and to guide catheter ablation. Typically, three quadripolar catheters are positioned in the high RA, the right ventricular (RV) apex, and the HB region, and a decapolar catheter is positioned in the CS (see Fig. 4-7).
Baseline Observations during Normal Sinus Rhythm
Atrial Extrastimulation and Atrial Pacing During Normal Sinus Rhythm
Anterograde Dual Atrioventricular Nodal Physiology
Demonstration of anterograde dual AVN pathway conduction curves requires a longer effective refractory period (ERP) of the fast pathway than the slow pathway ERP and atrial functional refractory period (FRP), as well as a sufficient difference in conduction times between the two pathways. Dual AVN physiology can be diagnosed by demonstrating the following: (1) a “jump” in the AH interval in response to progressively more premature AES; (2) two ventricular responses to a single atrial impulse; (3) a PR interval exceeding the R-R interval during rapid atrial pacing; and/or (4) different PR or AH intervals during NSR or fixed-rate atrial pacing.28
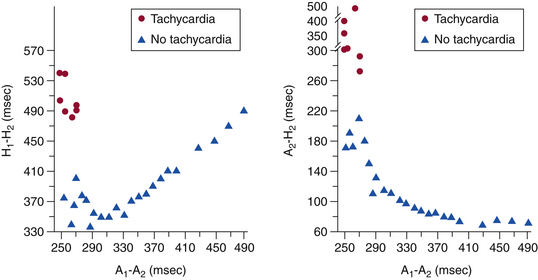
Atrial-His Interval Jump
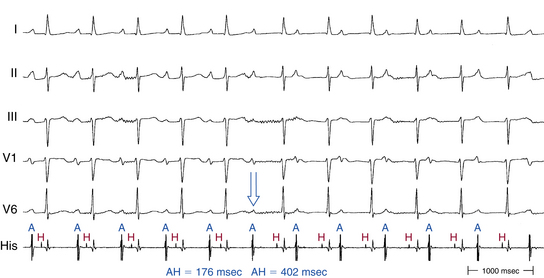
In contrast to the normal pattern of AVN conduction, in which the AH interval gradually lengthens in response to progressively shorter AES coupling intervals, patients with dual AVN physiology usually demonstrate a sudden increase (jump) in the AH interval at a critical AES (A1-A2) coupling interval (Fig. 17-6). Conduction with a short PR or AH interval reflects fast pathway conduction, whereas conduction with a long PR or AH interval reflects slow pathway conduction. The AH interval jump signals block of anterograde conduction of the progressively premature AES over the fast pathway (once the AES coupling interval becomes shorter than the fast pathway ERP) and anterograde conduction over the slow pathway (which has an ERP shorter than the AES coupling interval), with a longer conduction time (i.e., longer A2-H2 interval). A jump in the A2-H2 (or H1-H2) interval of 50 milliseconds or more in response to a 10-millisecond shortening of either the A1-A2 interval (i.e., AES coupling interval) or the A1-A1 interval (i.e., pacing CL) is defined as a discontinuous AVN function curve and is considered evidence of dual anterograde AVN pathways (see Fig. 4-23).8
1:2 Response
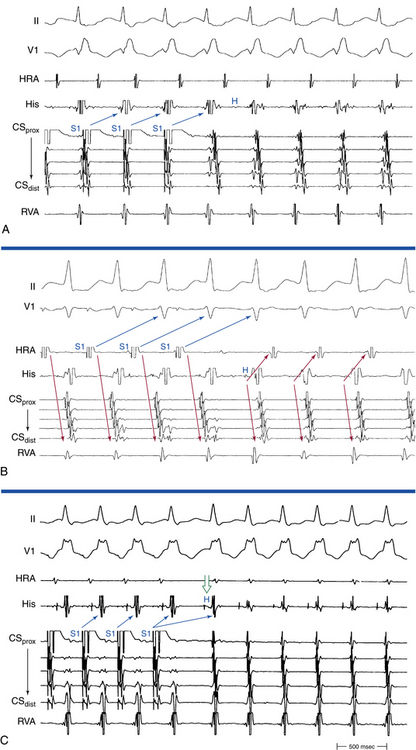
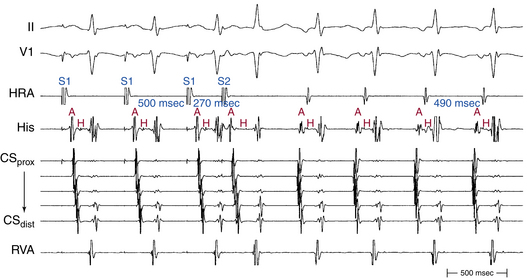
Rapid atrial pacing or AES can result in two ventricular complexes to a single paced atrial impulse. The first ventricular complex is caused by conduction of the atrial impulse over the fast AVN pathway, and the second complex is caused by conduction over the slow AVN pathway (Fig. 17-7). This response requires unidirectional retrograde block in the slow AVN pathway. Typically, in the presence of dual AVN pathways, conduction propagates simultaneously over both fast and slow AVN pathways. However, the wavefront conducting down the fast pathway reaches the distal junction of the two pathways before the impulse conducting down the slow pathway, and, subsequently, it conducts retrogradely up the slow pathway to collide with the impulse conducting anterogradely down that pathway. Thus, the anterograde impulse conducting down the slow pathway does not have the opportunity to reach the HB and ventricle. Rarely, the slow pathway conducts anterogradely only or has a very long retrograde ERP. In this setting, the wavefront traveling anterogradely down the fast pathway blocks (but does not conceal) in the slow pathway retrogradely and fails to retard the impulse traveling anterogradely down that pathway. Consequently, the wavefront traveling down the slow pathway can reach the HB and ventricle to produce a second His potential and QRS in response to a single atrial impulse. Because retrograde block in the slow pathway is a prerequisite to a 1:2 response, when such a phenomenon is present, it indicates that the slow pathway cannot support reentrant tachycardia using the slow pathway as the retrograde limb (i.e., atypical AVNRT cannot be operative). The 1:2 response should be differentiated from pseudo–simultaneous fast and slow pathway conduction, which is a much more common phenomenon during rapid atrial pacing. In the latter case, all paced atrial impulses block anterogradely in the fast pathway and conduct exclusively down the slow pathway with prolonged AH intervals (with PR intervals longer than atrial pacing CL), so that the last paced atrial impulse falls before the His potential caused by conduction of the preceding paced atrial impulse. Thus, the last paced atrial impulse is followed by two His potentials and two ventricular complexes. The last response may then be followed by induction of AVN echo beats or AVNRT, mimicking simultaneous fast and slow pathway conduction (Fig. 17-8).8,29
PR Interval Longer than Pacing Cycle Length During Atrial Pacing
The PR interval gradually prolongs as the atrial pacing rate increases. When a critical pacing rate is reached, the PR interval typically exceeds the R-R interval, with all AVN conduction over the slow AVN pathway (see Fig. 17-8). This manifests as crossing over of the pacing stimulus artifacts and QRSs; that is, the paced atrial complex is conducting not to the QRS immediately following it, but rather to the next QRS, because of a very long PR interval. There should be consistent 1:1 AV conduction that remains stable over the span of several cycles for this observation to be interpreted (i.e., without Wenckebach block). Such slow AVN conduction is seen only when conduction propagates over a slow AVN pathway, and it is not seen in the absence of dual AVN physiology. This phenomenon is diagnostic of the presence of dual AVN physiology, even in the absence of an AH interval jump and therefore is very helpful in patients with smooth AVN function curves. In fact, 96% of patients with AVNRT and smooth AVN function curves have a PR interval/RR interval ratio greater than 1 (i.e., PR interval longer than pacing CL) during atrial pacing at the maximal rate with consistent 1:1 AV conduction (versus 11% in controls).
Different PR or AH Intervals During Normal Sinus Rhythm or At Identical Atrial Pacing Cycle Lengths
This phenomenon can occur when the fast pathway anterograde ERP is long relative to the sinus or paced CL (Fig. 17-9). Such a phenomenon also requires a long retrograde ERP of the fast pathway. Otherwise, AVN echo beats or AVNRT would result, because once the impulse blocks anterogradely in the fast pathway and is conducted down the slow pathway, it would subsequently conduct retrogradely up the fast pathway if the ERP of the fast pathway were shorter than the conduction time (i.e., shorter than the AH interval) over the slow pathway.8
Prevalence of Dual Atrioventricular Nodal Physiology
Another potential reason for the inability to demonstrate dual AVN physiology is block in the fast AVN pathway at the pacing drive CL (i.e., pacing drive CL is shorter than fast pathway ERP). Additionally, atrial FRP can limit the prematurity of the AES. Consequently, AVN activation cannot be adequately advanced to produce block in the fast pathway because a more premature AES would result in more intraatrial conduction delay and less premature stimulation of the AVN. This obstacle can be overcome by the introduction of an AES following a shorter pacing drive CL, introduction of multiple AESs, burst atrial pacing, or stimulation from multiple atrial sites. A typical programmed electrical stimulation protocol used for EP testing in patients with AVNRT is outlined in Table 17-5.28
TABLE 17-5 Programmed Stimulation Protocol for Electrophysiology Testing of Atrioventricular Nodal Reentrant Tachycardia
| Atrial burst pacing from the RA and CS (down to AV Wenckebach CL) |
| Single and double AESs at multiple CLs (600-400 msec) from the high RA and CS (down to atrial ERP) |
| Ventricular burst pacing from the RV apex (down to VA Wenckebach CL) |
| Single and double VESs at multiple CLs (600-400 msec) from the RV apex (down to ventricular ERP) |
| Administration of isoproterenol infusion as needed to facilitate tachycardia induction (0.5-4 µg/min) |
AES = atrial extrastimulus; AV = atrioventricular; CL = cycle length; CS = coronary sinus; ERP = effective refractory period; RA = right atrium; RV = right ventricle; VA = ventriculoatrial; VES = ventricular extrastimulus.
Ventricular Extrastimulation and Ventricular Pacing During Normal Sinus Rhythm
Retrograde Dual Atrioventricular Nodal Physiology
Demonstration of retrograde dual AVN pathway conduction curves requires a longer retrograde ERP of the fast pathway than slow pathway ERP and ventricular and His-Purkinje system (HPS) FRP, as well as a sufficient difference in conduction times between the two pathways. In a pattern analogous to that of anterograde dual AVN physiology, ventricular stimulation can result in discontinuous retrograde AVN function curves, manifesting as a jump in the H2-A2 (or A1-A2) interval of 50 milliseconds or more in response to a 10-millisecond decrement of the VES coupling interval (V1-V2) or ventricular pacing CL (V1-V1). This finding must be distinguished from sudden VA prolongation caused by VH interval (but not HA interval) prolongation related to retrograde functional block in the RB and transseptal activation of HB through the LB (see Fig. 4-27). A 1:2 response (i.e., two atrial responses to a single ventricular stimulus) can also be observed.28
Failure to demonstrate retrograde dual AVN physiology in patients with atypical AVNRT can be caused by similar fast and slow AVN pathway ERPs. Dissociation of refractoriness of the fast and slow AVN pathways may be required and usually can be achieved by any of the following: introduction of VESs at a shorter pacing drive CL; introduction of multiple VESs; burst ventricular pacing; or administration of drugs such as beta blockers, verapamil, or digoxin. In addition, retrograde block in the fast AVN pathway at the pacing drive CL (i.e., the pacing CL is shorter than the fast pathway ERP) and ventricular or HPS FRP interval limiting the prematurity of the VES can also account for such failure.28
Induction of Tachycardia
Initiation by Atrial Extrastimulation or Atrial Pacing
Typical (Slow-Fast) Atrioventricular Nodal Reentrant Tachycardia
Clinical AVNRT almost always can be initiated with an AES that blocks anterogradely in the fast pathway, conducts down the slow pathway, and then conducts retrogradely up the fast pathway. Only when anterograde conduction down the slow pathway is slow enough (critical AH interval) to allow for recovery of the fast pathway to conduct retrogradely does reentry occur (see Fig. 17-6). This critical AH interval is not a fixed interval. It can change with changes in pacing drive CL, changes in autonomic tone, or after drug administration, thus reflecting changes in the fast pathway retrograde ERP.28
Atrial pacing can initiate AVNRT at pacing CLs associated with sufficient AVN conduction delay (see Fig. 17-8), especially during the atypical Wenckebach periodicity, when anterograde block occurs in the fast pathway and conduction shifts to the slow pathway.
Rarely, AES or atrial pacing can produce a 1:2 response with anterograde conduction over both the fast and slow pathways, as explained earlier (see Fig. 17-8).29 Such a response predicts easy induction of slow-fast AVNRT by ventricular stimulation because poor slow pathway retrograde conduction would increase the opportunity for the ventricular stimulus to block in the slow pathway and conduct up the fast pathway to return down the slow pathway and initiate AVNRT.
Atrial echoes and AVNRT usually occur at the same time that dual pathways are revealed (see Fig. 4-23). In 20% of patients, the dual AVN pathway AH interval jump occurs without concurrent occurrence of echo beats or AVNRT because of failure of retrograde conduction up the fast pathway. This failure can be caused by the absence of a distal connection between the two AVN pathways, a long retrograde ERP of the fast AVN pathway, or concealment of the AES anterogradely into the fast AVN pathway (i.e., the AES propagates some distance into the fast pathway before being blocked). The last event results in anterograde postdepolarization refractoriness, which would consequently make the fast pathway refractory to the wavefront invading it in the retrograde direction. The latter phenomenon can be diagnosed by demonstrating that the AH interval following the AES that fails to produce an echo beat is longer than the shortest ventricular pacing CL with 1:1 retrograde conduction. Such a pacing CL is a marker of the fast pathway retrograde ERP. This finding implies that an AES blocking in the fast pathway and conducting over the slow pathway, with an AH interval exceeding fast pathway ERP and still not conducting retrogradely over the fast pathway, is caused by anterograde concealment (and not just block) into the fast pathway.28
Although isolated AVN echoes can occur as long as VA conduction is present, the ability to initiate sustained AVNRT also requires the capability of the slow pathway to sustain repetitive anterograde conduction. In other words, sustenance of AVNRT requires that the tachycardia CL be longer than the ERP of all components of the circuit. Typically, for AVN reentry to occur, the fast pathway should be able to support 1:1 VA conduction at a ventricular pacing CL shorter than 400 milliseconds (i.e., retrograde Wenckebach CL shorter than 400 milliseconds), and the slow pathway should be able to support 1:1 AV conduction at an atrial pacing CL shorter than 350 milliseconds (i.e., anterograde Wenckebach CL shorter than 350 milliseconds). The shorter the AH interval during anterograde conduction over the fast pathway, the better the retrograde conduction over the same pathway (i.e., the shorter the HA interval), and the better the inducibility of AVNRT. Nevertheless, it is important to recognize that during EP testing, these criteria are dependent on the cardiac autonomic tone at that moment, and they can change dramatically by changing the level of patient sedation or the use of isoproterenol or by prolonged periods of rapid pacing (particularly ventricular) that cause hypotension and a reflex increase in adrenergic tone, which then affect inducibility of AVNRT.28
Atypical (Fast-Slow) Atrioventricular Nodal Reentrant Tachycardia
Anterograde dual AVN physiology is usually not demonstrable in patients with atypical AVNRT. Additionally, as noted, the presence of a 1:2 response to AES predicts noninducibility of atypical AVNRT because it indicates failure of the slow pathway to support retrograde conduction, a prerequisite for the atypical AVNRT circuit.19
When atypical AVNRT is initiated with atrial stimulation, it is usually with modest prolongation of the AH interval over the fast pathway and anterograde block in the slow pathway, followed by retrograde slow conduction over the slow pathway (Fig. 17-10). Therefore, a critical AH interval delay is not obvious.19
Initiation by Ventricular Extrastimulation or Ventricular Pacing
Typical (Slow-Fast) Atrioventricular Nodal Reentrant Tachycardia
Ventricular stimulation induces typical AVNRT by different mechanisms. The most common mechanism involves retrograde block of the ventricular stimulus in the slow pathway and retrograde conduction up the fast pathway, followed by anterograde conduction down the slow pathway. This occurs when the retrograde ERP of the slow pathway exceeds that of the fast pathway. This means that induction occurs without the demonstration of retrograde dual AVN physiology, and no critical VA or HA interval is required for induction. Occasionally, an interpolated premature ventricular complex (PVC) can block in the slow pathway retrogradely and penetrate into the fast pathway and cause concealment, so that the fast pathway will be refractory when the next sinus beat occurs. The sinus beat would then block in the fast pathway and conduct down the slow pathway and initiate typical AVNRT. This mechanism is uncommon. Retrograde VA conduction over the fast AVN pathway is usually good, and VA block rarely occurs in patients with typical AVNRT initiated by ventricular stimulation.28
Ventricular stimulation is less effective than atrial stimulation in inducing typical AVNRT (success rate is approximately 10% with VES and 40% with ventricular pacing), whereas atypical AVNRT can be induced almost as frequently by ventricular stimulation as by atrial stimulation. It is difficult for VES to induce typical AVNRT because the prematurity with which the VES arrives at the AVN can be limited by conduction delay in the HPS or in the lower common pathway. The ERP of the HPS may exceed that of the slow AVN pathway. This limitation can usually be overcome by the introduction of multiple VESs, use of a shorter drive CL, or ventricular pacing, which results in adaptation and shortening of the HPS ERP. Additionally, the anterograde ERP of the slow pathway may exceed the ventricular pacing CL so that the slow pathway is incapable of anterograde conduction of the ventricular impulse conducting retrogradely over the fast pathway. Similar retrograde ERPs of the slow and fast pathways also can limit the successful initiation of AVNRT by ventricular stimulation. As noted, manipulation of the autonomic tone with vagal maneuvers or drugs can facilitate dissociation of those ERPs. Another explanation for the lower success rate of AVNRT induction by ventricular stimulation is that the ventricular stimulus can penetrate (and not just block) in the slow pathway retrogradely, thus causing concealment that renders that pathway refractory and incapable of anterograde conduction of the ventricular impulse traveling retrogradely over the fast pathway.28
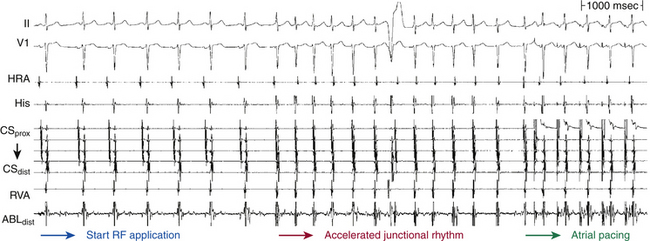
Burst ventricular pacing can overcome many of the problems imposed by HPS refractoriness in the induction of typical AVNRT (Fig. 17-11). During ventricular pacing, the AVN is the primary site of conduction delay. However, block in the lower common pathway and repetitive concealment (not just block) in the slow AVN pathway can still limit the success of ventricular pacing in inducing typical AVNRT.
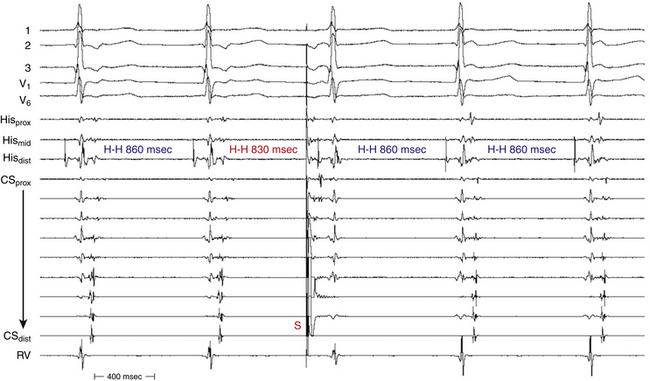
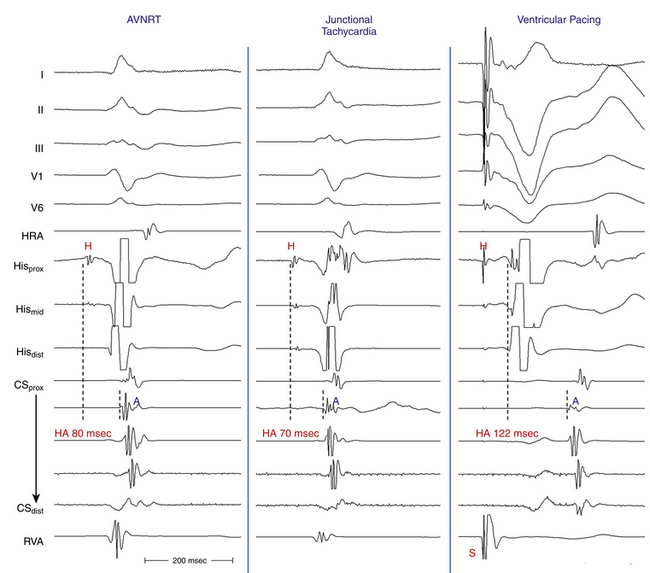
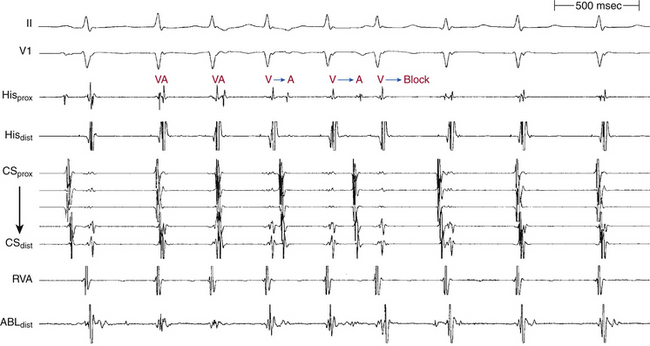
Of note, a VES that initiates an SVT with an HA interval longer than the HA (or VA) interval during the SVT, even though the H-H interval following that particular VES is longer than the H-H interval during the SVT, indicates that the SVT is AVNRT and not orthodromic AVRT. The reason is that both the HA interval during orthodromic AVRT and that following the VES represent sequential conduction duration from the HB up the lower common pathway and fast pathway to the atrium (Fig. 17-12). In contrast, during AVNRT, the HB and the atrium are activated in parallel, resulting in a shortened HA interval.28
Atypical (Fast-Slow) Atrioventricular Nodal Reentrant Tachycardia
Ventricular stimulation can induce atypical AVNRT by different mechanisms. The ventricular impulse can block in the fast pathway and conduct retrogradely over the slow pathway, with a long HA interval, thus allowing for recovery of the fast pathway and subsequent anterograde conduction down this pathway, initiating atypical AVNRT (see Fig. 17-12). This requires the retrograde ERP of the fast pathway to exceed that of the slow pathway. In this case, dual retrograde AVN pathways would be demonstrated with ventricular stimulation. Both VES and ventricular pacing are equally effective in inducing atypical AVNRT by this mechanism. Occasionally, a VES can conduct over both AVN pathways, to produce a 1:2 response, which is subsequently followed by the induction of atypical AVNRT. Ventricular pacing can initiate atypical AVNRT at pacing CLs associated with sufficient retrograde AVN conduction delay, especially during Wenckebach cycles, when retrograde block occurs in the fast pathway and conduction shifts to the slow pathway.19
Inducibility of atypical AVNRT is mostly determined by the retrograde slow pathway conduction. The reason is that the anterograde fast pathway conduction is usually sufficiently fast and its ERP is sufficiently short to allow anterograde conduction of the impulse arriving from the slow pathway.28
Tachycardia Features
Typical (Slow-Fast) Atrioventricular Nodal Reentrant Tachycardia
Atrial Activation Sequence
The initial site of atrial activation is usually recorded in the HB catheter at the apex of the triangle of Koch. In general, the shorter the HA interval is, the more likely the earliest atrial activation is to be recorded in the HB electrograms. As the HA interval prolongs, the earliest atrial activation moves closer to the base of the triangle of Koch or in the CS. However, significant heterogeneity in atrial activation exists, with multiple breakthrough points (unlike AT or orthodromic AVRT). In addition, in approximately 60% of patients, retrograde atrial activation during typical AVNRT can be slightly discordant quantitatively and qualitatively from that during ventricular pacing. Subsequently, the wave of atrial activation propagates radially cephalad and laterally to activate both atria (i.e., concentric atrial activation). This results in a relatively narrow P wave. In fact, the narrowest P wave during any arrhythmia is seen when the atrial activation begins at the apex of the triangle of Koch. The site of earliest atrial activation is not always obvious during AVNRT because of superimposition of atrial and ventricular electrograms. Delivering a VES that advances ventricular activation but does not reset atrial activation usually helps unmask the atrial activation sequence.8
Atrial-Ventricular Relationship
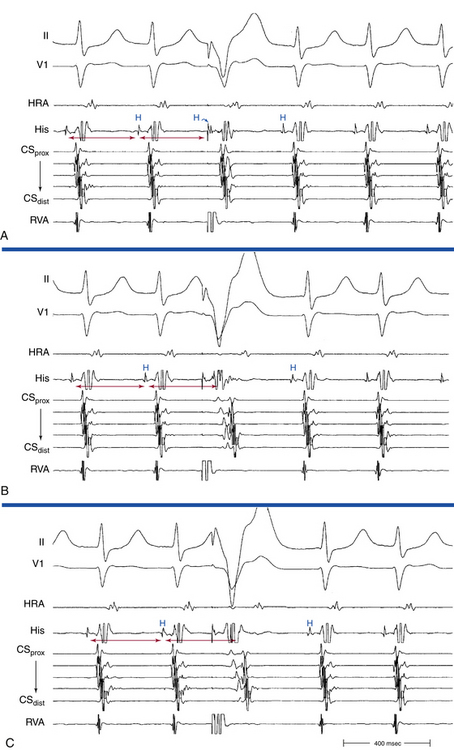
The onset of atrial activation appears before or coincides with the onset of the QRS in approximately 70% of cases. The RP interval is very short (–40 to 75 milliseconds); however, variation of the A/V relationship (with changes in AH, HA, and AH/HA interval ratio) can occasionally occur during initiation or termination of the tachycardia, likely because of decremental conduction in the lower common pathway. Usually, the A/V ratio equals 1. However, AV block can be present because of block below the reentry circuit (usually below the HB and infrequently in the lower common pathway), which can occur especially at the onset of the SVT, during acceleration of the SVT, and following a PVC or VES. Moreover, Wenckebach-type block can occur in the lower common pathway and can result in a changing relationship between the His potential and the atrial electrogram (i.e., retrograde atrial electrogram moves closer to or actually precedes the His potential, until block occurs with no His potential apparent). Reproducible, sustained 2:1 AV block during induced episodes of AVNRT can be observed in approximately 10% of cases (see Fig. 17-4). The His potential is absent in blocked beats in approximately 40% of patients who have 2:1 AV block. In the remaining patients, the His potential can range from being rudimentary to large in amplitude. However, irrespective of whether a His potential is present in blocked beats, the AV block persists after the administration of atropine, a finding suggesting that the site of block is not in the AVN. In addition, a VES introduced during the 2:1 AV block consistently results in 1:1 conduction, indicating that the AV block is functional and that the level of block is infranodal. Therefore, what was previously thought to be 2:1 AVN block in the lower common pathway of the AVNRT circuit (because of the absence of visible His potentials) is more likely to be intra-Hisian block. Rarely, VA block can occur during AVNRT because of block in the upper common pathway (see Fig. 17-11).8,15
Oscillation in the Tachycardia Cycle Length
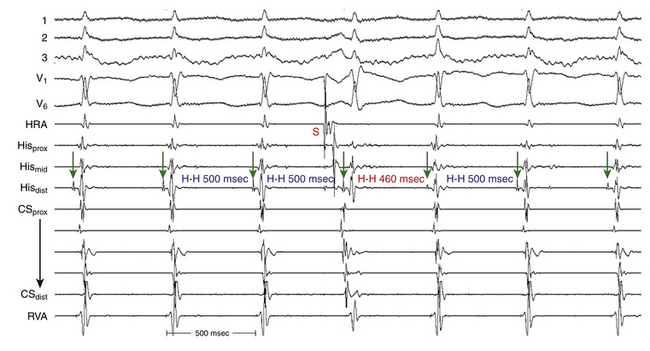
When CL variability is observed during typical AVNRT, it is generally caused by changes in anterograde conduction over the slow AVN pathway. Because retrograde conduction through the fast AVN pathway generally is much less variable, the changes in the ventricular CL that result from variability in anterograde AVN conduction precede the subsequent changes in the atrial CL (Fig. 17-13), and changes in the atrial CL do not predict changes in the subsequent ventricular CL, as is the case in orthodromic AVRT.30
Effect of Bundle Branch Block
The development of prolonged functional aberration during AVNRT is uncommon, and it usually occurs at initiation of the tachycardia or after resumption of 1:1 AV conduction after a period of block in the HB or lower common pathway (see Fig. 17-4). When BBB does occur during AVNRT, it does not influence the tachycardia CL (A-A or H-H intervals) because the ventricles are not required for the tachycardia circuit.
Termination and Response to Physiological and Pharmacological Maneuvers
The tachycardia CL is correlated best to the conduction time down the slow pathway. Spontaneous or pharmacologically mediated changes in the tachycardia CL are also more closely associated with changes in slow pathway conduction. Spontaneous termination of typical AVNRT occurs because of block in the fast or the slow pathway. However, the better the retrograde fast pathway conduction is, the less likely it is to be the site of block. Carotid sinus massage and vagal maneuvers can terminate typical AVNRT with gradual anterograde slowing and then block in the slow pathway, whereas block in the fast pathway is uncommon. AVN blockers (digoxin, calcium channel blockers, and beta blockers) prolong the refractoriness of the fast and slow pathways to similar or different degrees. Such effects mediate termination of AVNRT. However, they can occasionally help dissociate the ERP of the fast and slow pathways and unmask dual AVN physiology, and they can also facilitate inducibility of the AVNRT. Adenosine blocks the slow pathway and terminates AVNRT, but it does not affect the fast pathway. Class IA and IC agents and amiodarone affect both the fast and the slow pathways.28
Atypical (Fast-Slow) Atrioventricular Nodal Reentrant Tachycardia
The earliest site of retrograde atrial activation during atypical AVNRT is usually recorded at the base of the triangle of Koch or CS os, and CS breakthrough is observed in most patients. The CS breakthrough is likely part of or very close to the reentry circuit, as demonstrated by entrainment mapping.21
The RP interval during atypical AVNRT is longer than the PR interval. Additionally, the PR and AH intervals are shorter during AVNRT than during NSR because the atrium and ventricle are activated in parallel with simultaneous conduction up the upper common pathway and down the anterograde fast pathway in atypical AVNRT, but in sequence during NSR (Fig. 17-14).
Usually, the A/V ratio equals 1, as is the case for typical AVNRT. BBB can occur but does not influence the tachycardia CL. In contrast to typical AVNRT, CL variability during atypical AVNRT is usually caused by changes in retrograde conduction over the slow AVN pathway. Anterograde conduction occurs over the more stable fast AVN pathway and is less subject to variability (see Fig. 17-14). Therefore, during atypical AVNRT, changes in the atrial CL predict changes in the subsequent ventricular CL (as is the case in AT).30 Carotid sinus massage, vagal maneuvers, adenosine, and AVN blockers (e.g., digoxin, calcium channel blockers, and beta blockers) generally terminate atypical AVNRT by gradual slowing and then block in the retrograde slow pathway (see Fig. 17-14). Termination of atypical AVNRT with adenosine can also result from block in the fast AVN pathway; however, the value of this observation in distinguishing between atypical AVNRT and orthodromic AVRT using a slow retrograde BT is questionable.
Diagnostic Maneuvers during Tachycardia
Atrial Extrastimulation and Atrial Pacing During Supraventricular Tachycardia
A late-coupled AES usually fails to reach the AVN with adequate prematurity and thus fails to affect the tachycardia, and a full compensatory pause results. However, the AES can anterogradely conceal in the upper common pathway, thereby retarding conduction of the impulse traveling retrogradely up the fast pathway and resulting in a delay in the timing of the next atrial activation. This is usually manifested by an AES that delays the subsequent atrial activation but without affecting the timing of HB and ventricular activation.28
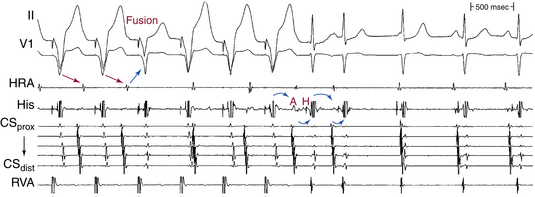
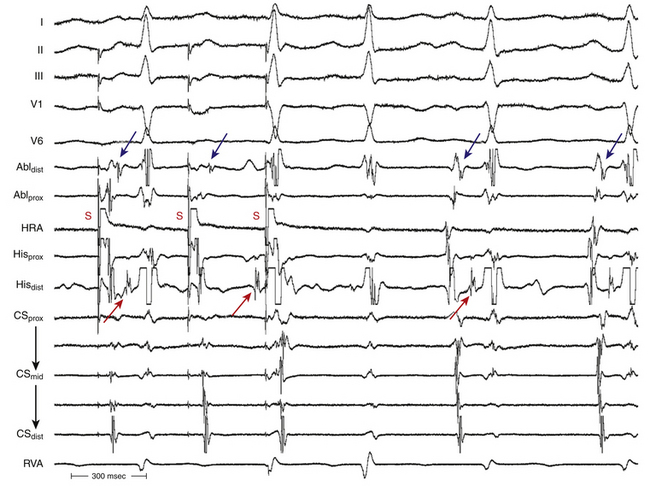
In typical AVNRT, an early-coupled AES frequently penetrates the AVN and resets the reentry circuit, with a resulting compensatory pause that is less than, equal to, or greater than a full compensatory pause, depending on the degree of anterograde conduction delay that the AES encounters down the slow pathway (because of the decremental conduction properties of the AVN). The AES orthodromically propagates through the anterograde slow pathway, with a resulting alteration of the subsequent H-Hˈ interval, whereas it antidromically collides with the preceding tachycardia wavefront traveling retrogradely up the fast pathway (Fig. 17-15). Progressively premature AESs encounter progressive anterograde conduction delay in the slow pathway, and an increasing resetting response pattern occurs. Theoretically, the degree of conduction delay in the slow pathway can exactly compensate for the prematurity of the AES, thus producing a pause that is equal to a full compensatory pause. To verify whether that pause was fully compensatory because the AES failed to penetrate the AVN or because the degree of anterograde conduction delay in the AVN was exactly sufficient to compensate for the prematurity of the AES, the return cycles are evaluated after delivery of additional AESs with different coupling intervals. Similarly, a delay in the slow pathway causes delay in the subsequent His potential timing, and such a delay can be equal to, greater, or smaller than what is needed to compensate for the prematurity of the AES. Therefore, the His potential following an AES can occur early, late, or on time relative to the expected tachycardia His potential. An early-coupled AES can also impinge on the relative refractory period of the lower common pathway and can cause slowed conduction and delay in the timing of the next His potential (i.e., longer A2-H2 than the baseline tachycardia A1-H1). This can occur even without affecting the timing of the next atrial activation, if conduction delay occurs only in the lower common pathway but not in the slow pathway.28 In atypical AVNRT, an early-coupled AES can reset the SVT in a fashion to that seen in typical AVNRT. However, the delay in conduction that the AES will engender is mainly in the retrograde limb of the circuit (i.e., the slow pathway). An AES that accelerates the next His potential and resets tachycardia is indicative of focal junctional tachycardia (Fig. 17-16).
During typical AVNRT, a very early-coupled AES can block anterogradely in the slow pathway, and it usually collides with a retrograde wavefront in the fast pathway to terminate the SVT. However, if the tachycardia CL is sufficiently long, with a wide fully excitable gap, and the AES is appropriately timed, the AES can block anterogradely in the slow pathway and still conduct down the fast pathway to capture the HB and ventricle and terminate the SVT before the SVT wavefront traveling down the slow pathway reaches the lower turnaround point. Therefore, the last HB and ventricular electrograms before termination are advanced and premature, in contrast to termination secondary to an AES blocking anterogradely in the slow and fast pathways, whereby the last HB and ventricular electrograms of the SVT occur on time. This phenomenon occurs more commonly in atypical AVNRT.28 In atypical AVNRT, a very early-coupled AES can conduct down the fast pathway during retrograde slow pathway activation and block retrogradely in the slow pathway. In this case, the AES results in a premature HB and ventricular activation, and the AVNRT is terminated before the expected atrial activation.
Termination
The ability of an AES to terminate AVNRT depends on the following: (1) the tachycardia CL (AVNRT with a CL shorter than 350 milliseconds is rarely terminated by a single AES, unless atrial stimulation is performed close to the AVN); (2) the distance of the site of atrial stimulation from the AVN (which would influence the ability of the AES to arrive to the AVN with adequate prematurity); (3) the refractoriness of the intervening atrial tissue (which can be overcome by delivery of multiple AESs); (4) atrial conduction velocity resulting from the AES; and (5) the size of the excitable gap in the reentrant circuit.28
Entrainment
Atrial pacing at a CL approximately 10 to 30 milliseconds shorter than the tachycardia CL is usually able to entrain AVNRT. In contrast to orthodromic AVRT, entrainment with atrial fusion cannot be demonstrated during AVNRT, a finding suggesting that the reentrant circuit in AVNRT does not have widely separate atrial entry and exit sites. Therefore, during entrainment of AVNRT by atrial pacing, the atrial activation sequence and P wave morphology are always those of pure paced morphology. The inability to demonstrate entrainment with manifest atrial fusion suggests a purely intranodal location of the AVNRT circuit. On the other hand, one study showed orthodromic capture of the atrial electrogram at the HB recording site (i.e., the bipolar HB electrogram morphology is identical to that during the tachycardia and is unaffected by pacing, and the first post-pacing interval [PPI] is identical to the pacing CL) during entrainment from the CS os region, a finding consistent with intracardiac atrial fusion. This suggests the absence of an upper common pathway between a reentrant circuit and atrial tissue surrounding the AVN and supports the concept that the reentrant circuit in AVNRT incorporates the atrial tissue surrounding the AVN. The AH interval during entrainment is usually longer than that during AVNRT because the atrial and the His electrograms are activated in parallel during AVNRT and in sequence during atrial pacing entraining the AVNRT (in response to the presence of intervening atrial tissue and, potentially, an upper common pathway separating the site of atrial stimulation from the reentry circuit).28
Ventriculoatrial Linking
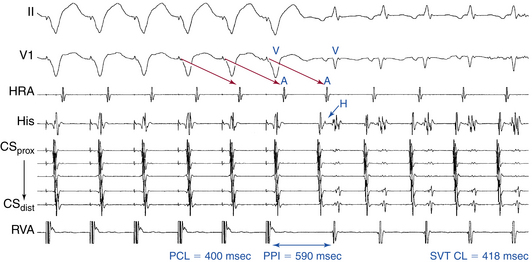
The initial atrial complex following cessation of atrial pacing entraining typical AVNRT is linked to, and cannot be dissociated from, the last captured ventricular complex. As a consequence, the post-pacing VA intervals are fixed and similar to those during tachycardia (with less than 10 milliseconds variation) after different attempts at SVT entrainment, regardless of the site, duration, or CL of the entraining atrial pacing drive. VA linking occurs in the setting of typical AVNRT because the timing of atrial activation is dependent on ventricular activation and is the result of retrograde VA conduction over the AVN fast pathway, which is relatively fixed and constant. VA linking can also be observed in orthodromic AVRT. In contrast, in the setting of AT, the first atrial return cycle following cessation of pacing is dependent on the distance between the AT origin and pacing site, the atrial conduction properties, and the mode of the resetting response of the AT, and it is not related to the preceding ventricular activation. Hence, the post-pacing VA intervals following different attempts at entrainment can vary especially when pacing at different rates or durations or from different atrial sites.31,32
Ventricular Extrastimulation and Ventricular Pacing During Supraventricular Tachycardia
Resetting
For a VES to reset the AVNRT circuit, it needs to advance (prematurely activate) the HB timing by a degree that is dependent on the following: (1) the tachycardia CL, (2) the local ventricular ERP, (3) the time needed for the VES to reach the HB, and (4) the length of the lower common pathway. The longer the lower common pathway is, the more the timing of HB activation must be advanced so that the VES will be able to activate the AVNRT circuit prematurely. Therefore, in slow-fast and slow-slow AVNRT, which typically has a long lower common pathway, the HB activation must be advanced by more than 30 to 60 milliseconds. In contrast, in slow-fast AVNRT, the lower common pathway is shorter, and the tachycardia is typically reset by the VES as soon as the HB activation is advanced.28
A late-coupled VES may block in the HPS or lower common pathway and may not affect the SVT (Fig. 17-17A). A late-coupled VES that resets the SVT without first retrogradely activating the HB (i.e., VES delivered at, after, or within 50 milliseconds of the expected inscription of the anterograde His potential) excludes AVNRT.
An early-coupled VES can reset AVNRT, especially when the tachycardia CL is relatively long (more than 350 milliseconds). The resetting VES antidromically collides with the preceding tachycardia wavefront traveling anterogradely and is conducted through the retrograde pathway to reset the tachycardia (see Fig. 17-17). Resetting of AVNRT with fusion of the QRS cannot be demonstrated because of the shared entry-exit site from the ventricle to the circuit, as discussed earlier.
Termination
Termination of AVNRT with VES is difficult (more so than termination with AES) and is rare when the tachycardia CL is shorter than 350 milliseconds. Such termination favors the diagnosis of orthodromic AVRT. In typical AVNRT, when termination occurs, it is usually caused by block of the VES in the anterograde or retrograde limb of the AVNRT circuit. The slower the SVT is, the more likely the block will be to occur in the anterograde slow pathway. Ventricular pacing can terminate AVNRT more easily than VES because rapid ventricular pacing can modulate and overcome the refractoriness of the intervening HPS. VES always terminates atypical AVNRT by blocking retrogradely in the slow pathway.28
Entrainment
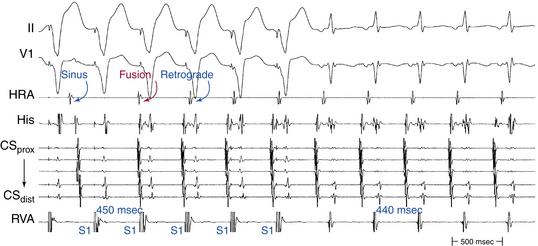
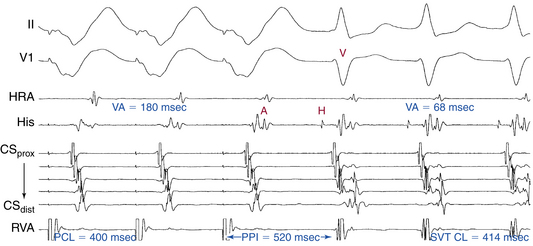
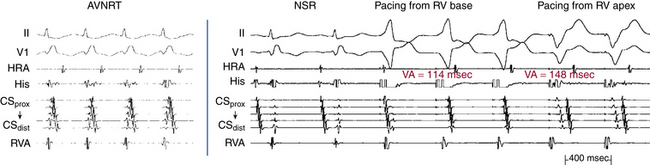
Ventricular pacing at a CL approximately 10 to 30 milliseconds shorter than the tachycardia CL is usually able to entrain AVNRT. Visualization of the His potential before atrial activation during entrainment helps differentiate AVNRT from orthodromic AVRT. If the His potential cannot be visualized during ventricular pacing, two other parameters can be helpful to distinguish AVNRT from orthodromic AVRT: the VA interval during ventricular pacing and the PPI. The VA interval during ventricular pacing is significantly longer than that during AVNRT (ΔVA [VApacing – VASVT] is usually more than 85 milliseconds) because both the ventricle and the atrium are activated in parallel during AVNRT but in sequence during ventricular pacing entraining the AVNRT (Figs. 17-18 and 17-19).
The PPI after entrainment of AVNRT from the RV apex is significantly longer than the tachycardia CL (the [PPI – SVT CL] difference is usually more than 115 milliseconds) because the reentrant circuit in AVNRT (confined above the HB and does not involve the ventricle) is far from the pacing site. In AVNRT, the PPI reflects the conduction time from the pacing site through the RV muscle and HPS, once around the reentry circuit and back to the pacing site. Therefore, the difference between the PPI and tachycardia CL reflects twice the sum of the conduction time through the RV muscle, the HPS, and the lower common pathway. In orthodromic AVRT using a septal BT, the PPI reflects the conduction time through the RV to the septum, once around the reentry circuit and back. In other words, the difference between the PPI and tachycardia CL reflects twice the conduction time from the pacing catheter through the ventricular myocardium to the reentry circuit. Because the ventricle is an essential component of the AVRT circuit, the RV apex is closer to the tachycardia circuit. Therefore, the PPI more closely approximates the SVT CL in orthodromic AVRT using a septal BT ([PPI – SVT CL] difference is usually less than 115 milliseconds) compared with AVNRT. This maneuver was studied specifically for differentiation between atypical AVNRT and orthodromic AVRT using a septal BT, but the principle also applies to typical AVNRT (see Figs. 17-18 and 17-19). For borderline values, ventricular pacing at the RV base can help exaggerate the difference between the PPI and tachycardia CL in the case of AVNRT, but without significant changes in the case of orthodromic AVRT. The reason is that the site of pacing at the RV base is farther from the AVNRT circuit than the RV apex (the paced wavefront has to travel first to near the RV apex before engaging the HPS and conducting retrogradely to the AVN), but it is still close to an AVRT circuit using a septal BT (and in fact is closer to the ventricular insertion of the BT).33
Additionally, overdrive ventricular pacing during entrainment can induce decremental anterograde AVN conduction. Subtracting the increment in AVN conduction time in the first PPI (post-pacing AH interval – prepacing AH interval) from the [PPI – SVT CL] difference (“corrected” [PPI – SVT CL]) has been found to improve the accuracy of this criterion. The difference between the AV intervals (post-pacing AV interval – prepacing AV interval) can be taken for the latter adjustment when a clear His deflection is lacking. A corrected [PPI – SVT CL] of less than 110 milliseconds was found to be highly accurate in identifying orthodromic AVRT from AVNRT.34–36
Differential Right Ventricular Entrainment
As noted, decremental conduction can occur during entrainment of either AVNRT or orthodromic AVRT, and most commonly it occurs during conduction through the AVN, especially the AVN slow pathway. The degree of decrement is dependent on both the pacing rate and the functional refractory properties of the AVN. Therefore, the PPI will prolong if decrement occurs, but the degree of decrement is not expected to be materially different from basal rather than apical pacing as long as the pacing rates are the same or similar. To avoid potential error introduced by decremental conduction within the AVN, correction of the PPI is preferred, and it is obtained by subtracting any increase in the AV interval of the return cycle beat (as compared with the AV interval during SVT). In one study, a differential corrected [PPI – SVT CL] of more than 30 milliseconds after transient entrainment was observed in all cases of AVNRT (i.e., corrected PPI following pacing from the RV base was consistently at least 30 milliseconds longer than that following pacing from the RV apex), and corrected [PPI – SVT CL] of less than 30 milliseconds was observed in all cases of orthodromic AVRT. Additionally, differential VA interval (ventricular stimulus-to-atrial interval during entrainment from RV base versus RV septum) of more than 20 milliseconds was consistent with AVNRT, whereas a differential VA interval of less than 20 milliseconds was consistent with orthodromic AVRT.37
Length of Pacing Drive Required for Entrainment
Careful analysis of the beginning of RV pacing during the SVT can aid in the tachycardia diagnosis. Assessing the timing and type of response of SVT to RV pacing also can help differentiate orthodromic AVRT from AVNRT with high positive and negative predictive values. In the setting of orthodromic AVRT, ventricular tissue is the only intervening tissue between the pacing wavefront and the ventricular insertion site of the BT. Therefore, once ventricular capture is achieved during RV pacing, the paced wavefront propagates to the ventricular insertion site of the BT quickly and resets the tachycardia. Consequently, RV pacing results in a faster response of resetting the tachycardia. In the setting of AVNRT, on the other hand, the pacing wavefront has to penetrate the HPS and AVN tissue prior to resetting the tachycardia. This results in delayed resetting of the tachycardia compared with orthodromic AVRT. After initiation of synchronized RV pacing during SVT at a CL 10 to 40 milliseconds shorter than the tachycardia CL, once constant-appearing paced RV complexes (either pure capture or fixed fusion) are observed (as evidenced by fixed pacing morphology of the surface ECG), the number of RV paced beats required to accelerate the SVT to the pacing CL is determined. The first atrial capture beat accelerated to the pacing CL is identified by demonstrating a fixed ventricular stimulus–to–atrial capture interval. One report demonstrated that using a cutoff of one beat to accelerate the SVT to the pacing CL could identify all orthodromic AVRT cases and essentially exclude all cases of AVNRT with high accuracy. On the contrary, if two beats are required to accelerate SVT to the pacing CL, this can distinguish AVNRT from orthodromic AVRT with very high confidence as well. The major advantage of this method is its independence of tachycardia continuation after cessation of pacing. However, it is likely that these criteria may not be applicable at faster pacing CLs because resetting of the tachycardia may occur earlier in AVNRT in response to a greater degree of penetration into the tachycardia circuit. In addition, these findings may not apply in cases in which AVNRT occurs in the setting of a bystander BT because SVT may be reset using the bystander BT.38
Atrial Resetting During the Transition Zone
On initiation of RV pacing trains during the SVT at a rate slightly faster than the SVT CL, there is a transition zone during which the pacing train fuses with anterograde ventricular activation (i.e., the zone in which the paced QRS complexes show progressive fusion with the SVT complexes) until stable QRS morphology is observed (either completely paced or constantly fused). In patients with AVNRT or AT, acceleration of the timing of atrial activation cannot occur through the AVN during the transition zone. The reason is that the HB is expected to be refractory, as indicated by at least some ventricular activation still occurring by anterograde conduction over the HPS, similar to the concept of entrainment with manifest fusion discussed previously. If perturbation of atrial timing occurs during the transition zone, it indicates the presence of a retrogradely conducting BT, which can be an integral part of the SVT circuit (i.e., orthodromic AVRT) or a bystander. In one report, these criteria showed excellent diagnostic accuracy and could be applied regardless of whether entrainment was achieved or whether the SVT terminated during pacing. Perturbation of atrial timing for 15 milliseconds or longer or a fixed S-A interval measured from last beat of the transition zone was seen in all the patients with orthodromic AVRT and in none of the patients with AVNRT or AT.39
Atrial and Ventricular Electrogram Sequence Following Cessation of Ventricular Pacing
Following ventricular entrainment of typical AVNRT, an “A-V” electrogram sequence is observed after the last paced QRS (see Fig. 17-18). In contrast, following overdrive ventricular pacing (1:1 VA conduction) during AT, retrograde conduction occurs through the AVN. In this setting, the last retrograde P wave resulting from ventricular pacing is unable to conduct back to the ventricle because the AVN is still refractory to anterograde conduction, and the result is an A-A-V response (as discussed in detail in Chap. 11). Importantly, this maneuver is not useful when 1:1 VA conduction during ventricular pacing is absent (see Fig. 11-14). Additionally, a pseudo–A-A-V response can occur during atypical AVNRT because retrograde conduction during ventricular pacing occurs through the slow pathway. This can result in a VA interval that is longer than the pacing CL; hence, the last ventricular paced impulse is followed first by the P wave conducted slowly from the previous paced QRS and then by the P wave resulting from the last paced QRS, thus mimicking an A-A-V response (see Fig. 17-19). Careful identification of the last atrial electrogram that resulted from VA conduction during ventricular pacing avoids this potential pitfall. The last atrial activation resulting from conduction of the last paced ventricular complex follows the preceding P wave with an A-A interval equal to the ventricular pacing CL. A pseudo–A-A-V response can also occur during typical AVNRT with long HV intervals or short HA intervals, or both, in which atrial activation precedes ventricular activation. In the latter setting, using HB activation instead of ventricular activation (i.e., characterizing the response as A-A-H or A-H instead of A-A-V or A-V, respectively) can be more accurate and can help eliminate the pseudo–A-A-V response.40
Diagnostic Maneuvers during Normal Sinus Rhythm after Tachycardia Termination
Atrial Pacing at the Tachycardia Cycle Length
Under comparable autonomic tone status, 1:1 AV conduction over the AVN may or may not be maintained during atrial pacing at the tachycardia CL. AV block can occur during atrial pacing but not during AVNRT because of anterograde block in an upper common pathway. Retrograde conduction properties of the upper common pathway may allow 1:1 conduction from the AVNRT circuit up to the atrium, but the anterograde conduction properties may not allow 1:1 anterograde conduction from the atrium down to the ventricle during atrial pacing at a similar CL. In contrast, 1:1 AV conduction, under comparable autonomic tone status, is typically maintained during atrial pacing at the tachycardia CL in the case of AT and orthodromic AVRT.28 Typically, atrial pacing at the tachycardia CL yields an AH shorter than that during AVNRT as a result of preferential conduction over the fast pathway. To compare slow pathway conduction intervals between AVNRT and atrial pacing more directly, one may have to initiate pacing at a CL less than the tachycardia CL to block in the fast pathway, engage the slow pathway, and then increase the pacing CL to the tachycardia CL.
Ventricular Pacing at the Tachycardia Cycle Length
Ventricular pacing at the tachycardia CL results in HA and VA intervals that are longer during pacing than those during AVNRT when assessed with standard 5-mm spacing quadripolar catheters in the His position. With more closely spaced His recording electrodes, the HA interval during pacing (HApacing) may be less than that during tachycardia (HASVT), a finding implying that part of the proximal HB is part of the AVNRT circuit. Whether or not the HA interval during pacing is longer than that during tachycardia thus depends in large part on how proximal an HB recording is made. The ΔHA interval (HApacing – HASVT) is typically more than –10 milliseconds, because the HA interval during AVNRT is shortened by parallel activation of both the HB and the atrium during the tachycardia, whereas the HB and atrium are activated sequentially during ventricular pacing (Fig. 17-20). The ΔHA interval is even more pronounced in atypical AVNRT, which has a lower common pathway that is longer than that in typical AVNRT. In focal junctional tachycardia, the ΔHA interval is typically close to 0 (see Fig. 17-20).28
Para-Hisian Pacing
Para-Hisian pacing helps exclude the presence of a septal AV BT, which can mediate an orthodromic AVRT with a retrograde atrial activation sequence similar to that during AVNRT. In the absence of a BT, para-Hisian pacing results in a shorter S-A interval when the HB-RB is captured (S-H = 0 and S-A = HA) than the S-A interval when only the ventricle is captured (S-A = S-H+HA) with no change in the atrial activation sequence or HA interval. This response to para-Hisian pacing is termed pattern 1 or AVN/AVN pattern.28
A change in the retrograde atrial activation sequence with loss of HB-RB capture indicates the presence of a retrogradely conducting BT. Similarly, an S-A (VA) interval that is constant regardless of whether the HB RB is being captured indicates the presence of a BT, whereas prolongation of the S-A (VA) interval on loss of HB capture, compared with that during HB capture, excludes the presence of a retrogradely conducting BT, except for slowly conducting and far free-wall BTs. Please refer to Chapter 18 for a more detailed discussion of para-Hisian pacing.28
Differential Right Ventricular Pacing
The response to differential RV pacing can be evaluated by comparing two variables between RV basal and RV apical pacing: the VA interval and atrial activation sequence. In the absence of a septal AV BT, pacing at the RV apex results in rapid access of the paced wavefront to the RB, HB, and AVN, and in a shorter VA interval compared with pacing at the RV base. In the presence of a retrogradely conducting septal AV BT, pacing at the RV base allows the wavefront to access the BT rapidly and activate the atrium with a shorter VA interval than during RV apical pacing, which is farther from the BT ventricular insertion site.28
Occurrence of RB block (RBBB) (but not LB block [LBBB]) can alter the significance of the VA interval criterion, especially when VA conduction propagates over the HPS-AVN. In the presence of retrograde RBBB, VA conduction occurs over the LB-HB. Therefore, the VA interval depends on the distance between the pacing site and the LB rather than the RB, and access of the paced wavefront to the LB can be faster for RV basal or septal pacing compared with pacing from the RV apex (Fig. 17-21).
The atrial activation sequence is similar during RV apical and RV basal pacing in the absence of a retrogradely conducting AV BT because the atrium is activated over the AVN in both cases. In contrast, in the presence of a septal AV BT, the atrial activation sequence may or may not be similar during pacing from the RV apex versus RV base. Atrial activation may propagate only over the AV BT in both situations, or it may propagate over the AV BT during RV basal pacing and over the AVN (or a fusion of conduction over both the AVN and AV BT) during RV apical pacing. Please refer to Chapter 18 for a more detailed discussion on differential RV pacing.
Exclusion of Other Arrhythmia Mechanisms
ATs arising from the anteroseptal region and orthodromic AVRT using superoparaseptal BTs can mimic typical AVNRT. Additionally, ATs arising from the posteroseptal region and orthodromic AVRT using posteroseptal BTs can mimic atypical AVNRT. The various EP testing maneuvers used to exclude these tachycardias are outlined in Tables 17-6 and 17-7.
| Oscillations in SVT CL |
• An ΔAH interval (AHpacing − AHSVT) >40 msec excludes AT.
• If the VA interval following the last entrained QRS is reproducibly constant (with <10 msec variation), despite pacing at different CLs or for different durations (VA linking) and similar to that during SVT CL, AT is unlikely.
• If the VA interval following the last entrained QRS is reproducibly constant (with <14-msec variation), despite pacing at different atrial sites (VA linking), AT is unlikely.
AH = atrial–His bundle; AT = atrial tachycardia; AV = atrioventricular; A-V = atrium-ventricle; AVNRT = atrioventricular nodal reentrant tachycardia; CL = cycle length; NSR = normal sinus rhythm; SVT = supraventricular tachycardia; VA = ventriculoatrial; VES = ventricular extrastimulus.
TABLE 17-7 Exclusion of Orthodromic Atrioventricular Reentrant Tachycardia
AH = atrial–His bundle interval; AV = atrioventricular; AVRT = atrioventricular reentrant tachycardia; BT = bypass tract; CL = cycle length; HA = His bundle–atrial; HB = His bundle; NSR = normal sinus rhythm; PPI = post-pacing interval; RA = right atrium; RB = right bundle branch; RV = right ventricle; S-A = stimulus-to-atrial; S-H = stimulus-to-His bundle; SVT = supraventricular tachycardia; VA = ventriculoatrial; VES = ventricular extrastimulation.
VA block during SVT is a rare phenomenon. As noted, VA block can rarely occur during AVNRT because of block in an upper common pathway (see Fig. 17-13). Other potential mechanisms of SVT with VA block include automatic junctional tachycardia with retrograde VA block and orthodromic AVRT using a nodofascicular or nodoventricular BT for retrograde conduction.41 Intra-Hisian reentry is another potential mechanism, but it is a theoretical entity whose clinical occurrence has not convincingly been demonstrated. All these tachycardias are associated with a concentric atrial activation sequence, mimicking AVNRT. Nonetheless, several criteria can distinguish AVNRT from the other SVT mechanisms. Automatic junctional tachycardia typically cannot be induced or terminated by programmed stimulation; initiation of the tachycardia is usually spontaneous with absence of a critical AH delay and often requires catecholamines. Additionally, automatic junctional tachycardia is characterized by a gradual increase in tachycardia rate (warm-up) following initiation, marked CL variation, and slow decrease in tachycardia rate (cool-down), rather than an abrupt termination. The presence of nodofascicular (nodoventricular) tachycardia should be suspected when intermittent anterograde preexcitation is recorded, when AES fails to capture the tachycardia circuit, and when the SVT can be initiated with a single AES producing two ventricular complexes (mimicking the 1:2 response observed in patients with dual AVN physiology) or by a VES without a retrograde His deflection. Orthodromic AVRT can be diagnosed by demonstrating changes in the tachycardia CL with the development of BBB, and resetting or termination of the SVT by a late-coupled VES delivered when the HB is refractory.15,42
Ablation
Target of Ablation
Historically, the initial approach of RF ablation of AVNRT was modification of AVN conduction by lesions created near the anterosuperior aspect of the triangle of Koch (fast pathway ablation or the “anterior approach”). However, because the fast pathway constitutes the physiological conduction axis and is located in close proximity to the compact AVN and HB, this procedure was associated with an unacceptable risk of AV block or unphysiologically long PR intervals and was consequently abandoned. Since the early 1990s, selective ablation of the slow AVN pathway by lesions created near the posteroinferior base of the triangle of Koch between the CS os and tricuspid annulus (the “posterior approach”) has proved to be a more successful and safer procedure. Additionally, in patients with multiple slow pathways, ablation of the fast pathway can be associated with persistently inducible AVNRT with anterograde conduction over a second slowly conducting pathway. In contrast, successful ablation of the slow pathway can eliminate all variants of AVNRT.43,44
Anatomical Approach
The target of ablation is the isthmus of tissue between the tricuspid annulus and the CS os (Fig. 17-22).28 The sequence of ablation sites chosen for RF delivery is related to the probability of successful slow pathway ablation at each site and the risk of impairing AV conduction. The most common site for effective and safe ablation is along the tricuspid annulus immediately anterior to the CS os; this site has a success rate of 95%. Occasionally, successful ablation can require the catheter positioned along the tricuspid annulus inferior to the CS os. If ablation is not successful at these locations, the catheter is then moved more cephalad along the tricuspid annulus superior to the CS os. Finally, if those sites are unsuccessful, RF delivery at more superior sites (more than halfway between the CS os and HB) can be considered only if the risk of complete AV block can be justified by the severity of clinical symptoms.28
If slow pathway ablation cannot be achieved in the inferoseptal RA, it may be worthwhile to deliver RF energy at sites within the proximal CS, close to the os, with the ablation catheter pointing toward the LV with counterclockwise torque. Rarely, successful slow pathway ablation may require an application of energy on the left side of the posterior septum, along the mitral annulus.28,43
Electroanatomical Approach (Slow Pathway Potentials)
The electroanatomical approach is guided by the identification of slow pathway potentials in conjunction with anatomical landmarks. These potentials have been used by some to define the site of the slow pathway within the triangle of Koch, and they can be used effectively as a guide to target ablation.28
It has been suggested that activation of the slow pathway is associated with inscription of discrete electrical potentials, often referred to as slow pathway potentials. The origin of these potentials is uncertain. Whether they represent nodal tissue activation, anisotropic conduction through muscle bundles in various sites in the triangle of Koch, or a combination of both is unclear. The electrogram morphology of the slow potentials has been variously described as sharp and rapid (representing the atrial connection to the slow pathway; see Fig. 17-22), or slow and broad with low amplitude (representing slow potentials; see Fig. 17-22). The timing of slow potentials during NSR was reported to follow closely (within 10 to 40 milliseconds) local atrial activation near the CS os or to span the AH interval (it can occur as late as the His potential). However, such potentials are specific neither to the triangle of Koch nor to patients with AVNRT and can be recorded near the tricuspid annulus at sites distant from the slow pathway. Despite these observations, the probability of recording putative slow potentials at the site of effective slow ablation is more than 90%.28 For ablation of slow-slow and fast-slow AVNRT, the slow pathway used for retrograde conduction during the tachycardia should be targeted by ablation, and this target can be different from the slow AVN pathway used for anterograde conduction. Therefore, ablation can be guided by the site of earliest retrograde atrial activation during AVNRT or ventricular pacing, which is usually localized to the isthmus of tissue between the tricuspid annulus and the CS os in fast-slow AVNRT and along the anterior aspect of the CS in slow-slow AVNRT (Fig. 17-23).44,45
Ablation Technique
For slow pathway ablation, a quadripolar, 4-mm-tip, deflectable ablation catheter is advanced through a femoral vein. Rarely, a superior vena caval approach (through the internal jugular or subclavian vein) is required because of inferior vena caval obstruction or barriers, and one report demonstrated the feasibility of this approach.46 The target site can be mapped using the anatomical or electroanatomical approach. Large-tip and irrigated-tip catheters have no role in catheter ablation of the slow pathway because the larger lesions they create can increase the risk of AV block.
Anatomical Approach
As noted, the target of slow pathway ablation is the isthmus of tissue between the tricuspid annulus and the CS os.28 Using the right anterior oblique fluoroscopy view, which best displays the triangle of Koch in profile, the ablation catheter is advanced into the RV, moved inferiorly so that it lies anterior to the CS os, and then withdrawn until the distal pair of electrodes records small atrial and large ventricular electrograms (with an A/V electrogram amplitude ratio during NSR of 0.1 to 0.5). Gentle clockwise torque is maintained to keep the catheter in contact with the low atrial septum. This positions the catheter along the tricuspid annulus immediately anterior to the CS os (see Fig. 17-22). Positioning is best performed during NSR, rather than AVNRT, because the atrial and ventricular electrograms at the tricuspid annulus are more easily discerned. The catheter often slips into the CS when one applies even a small amount of torque because the CS is large in many of these patients. If the catheter does not easily reach far enough into the ventricle, yielding an A/V ratio of only 1:1, or if catheter stability is inadequate or falls into the CS repeatedly, a long sheath with a slight septal angulation can be helpful. Moreover, some ablation catheters have asymmetrical bidirectional deflection curves, an option that can prove to be of value for catheter reach and stability in some cases.43
Electroanatomical Approach
The target site is identified by slow pathway potentials (see Fig. 17-22).28 Initially, the triangle of Koch is mapped from the apex (where the HB is recorded) and then moved toward the CS os. This approach also helps evaluate the extension of the zone recording a His potential. Slow pathway potentials are usually recorded at the midanteroseptal position, where they are located in the middle of the isoelectric line connecting the atrial and ventricular electrograms. Moving the mapping catheter inferiorly, the slow pathway potential moves toward the atrial electrogram, and when the optimal site for slow pathway ablation is reached, it merges with the atrial electrogram. Because electrograms with these characteristics can be recorded near the tricuspid annulus at sites distant from the slow pathway, the region explored with the ablation catheter should be limited to the posterior septum, near the CS os.28 In the left anterior oblique fluoroscopy view, using the HB and CS os positions (as defined by the HB and CS catheters) as landmarks, the most common area where the slow potentials are recorded is in the posterior third of the line connecting those two landmarks.45
Validation of slow pathway potentials can be useful and can be achieved by demonstrating that they represent slow and decremental conduction properties. Typically, brief runs of incremental atrial pacing or AES produce a decline in the amplitude and slope, an increase in the duration, and a separation of the slow potential from the preceding atrial electrogram until disappearance of any consistent activity. This is especially helpful in the presence of a sharp slow pathway potential, to distinguish it from a proximal HB recording (Fig. 17-24). Catheter-induced junctional ectopy, when present, indicates that the catheter tip is at a good ablation site. In patients with slow-slow and slow-fast AVNRT, mapping during AVNRT often shows a discrete potential 20 to 40 milliseconds prior to the earliest atrial electrogram; this may indicate the slow pathway and signify a good target site.45
Radiofrequency Energy Delivery
Ablation should be performed during NSR, when it is easier to maintain a stable catheter position. When ablation is carried out during AVNRT, sudden termination of the tachycardia during RF delivery can result in any combination of dislodgment of the ablation catheter, inadvertent AV block, or an incomplete RF lesion.28
Typical RF settings consist of a maximum power of 50 W and a maximum temperature of 55° to 60°C, continued for 30 to 60 seconds, or until the junctional rhythm extinguishes (see later).28 Impedance and ECG should be carefully monitored throughout RF application. However, in the case of slow pathway ablation, the decrement in impedance associated with successful energy applications is usually small (approximately 2.5 Ω); such a small change precludes the clinical usefulness of impedance monitoring.
Junctional Rhythm
An accelerated junctional rhythm typically develops within a few seconds of RF delivery at the effective ablation site (Fig. 17-25). The mechanism of this rhythm is unclear but is likely to be secondary to enhanced automaticity in AVN tissue because of thermal injury. Accelerated junctional rhythm during RF ablation is usually associated with subtle changes in retrograde atrial activation sequence (compared with that during AVNRT). Occurrence of this rhythm is strongly correlated with and sensitive to successful ablation sites; it occurs more frequently (94% versus 64%) and for a longer duration (7.1 versus 5.0 seconds) during successful compared with unsuccessful RF applications. Such a rhythm is, however, not specific for slow pathway ablation and is routinely observed during intentional fast pathway and AVN ablation. More rapid junctional tachycardia, on the other hand, is probably caused by thermal injury of the HB and heralds impending AV block (Fig. 17-26).44,47,48
When an accelerated junctional rhythm occurs, careful monitoring of VA conduction during this rhythm is essential, and overdrive atrial pacing may be performed to ensure maintenance of 1:1 anterograde AV conduction (see Fig. 17-25). Occasionally, atrial pacing at a rate sufficiently fast to override the junctional rhythm results in AV Wenckebach block at baseline, even before the onset of RF energy delivery. In this case, isoproterenol can be used to shorten the AV block CL and maintain 1:1 AV conduction during pacing.48
After an RF application that results in an accelerated junctional rhythm, programmed electrical stimulation (atrial and ventricular stimulation) is performed to determine the presence or absence of slow pathway conduction, AVN echoes, or inducible AVNRT. If the result is unsatisfactory, the catheter is moved to a slightly different site (usually a few millimeters anteriorly), and RF is reapplied.44,48
Several observations should prompt immediate discontinuation of RF application, including sudden impedance rise (more than 10 Ω), prolongation of the PR interval (during NSR or atrial pacing), development of AV block, fast junctional tachycardia (CL shorter than 350 milliseconds), and retrograde conduction block during junctional ectopy (see Fig. 17-26). Although the last observation may herald anterograde AV bock in the case of typical AVNRT and necessitates immediate discontinuation of RF delivery, retrograde block over the fast pathway is a common occurrence in patients with atypical AVNRT, even before ablation. In this setting, the occurrence of junctional rhythm with no VA conduction may not be such an ominous sign; in fact, such an occurrence may indicate successful ablation of the slow pathway and thus should prompt ongoing RF delivery.28 As noted earlier, atrial pacing at a rate sufficient to overdrive junctional rhythm allows monitoring of anterograde fast pathway conduction and helps ensure safe RF application.
Endpoints of Ablation
The optimal endpoint for slow pathway ablation is the complete elimination of slow pathway conduction and dual AVN physiology without impairing the fast pathway. This is evidenced by loss of conduction over the slow pathway (i.e., disappearance of discontinuous AV conduction curves), alteration of Wenckebach CL (shorter or longer), an increase in the ERP of the AVN, and preservation of intact anterograde and retrograde AVN (fast pathway) conduction.49 In many cases, the ERP of the fast pathway can actually shorten, likely secondary to the withdrawal of electrotonic inhibition imposed on the fast pathway by the slow pathway. For atypical AVNRT, elimination of retrograde fast pathway conduction (i.e., complete VA block) is an important additional endpoint.43,44
However, elimination of all evidence of slow pathway conduction is not a necessary requirement for a successful slow pathway ablation procedure.28 It suffices to eliminate inducibility of AVNRT and 1:1 anterograde conduction over the slow pathway, with and without isoproterenol infusion. Complete elimination of all anterograde slow conduction is not essential for clinical success and is actually associated with a slightly higher risk of AV block. In patients with a successful slow pathway ablation procedure, 40% to 50% will still have dual AVN physiology, and 75% of those patients will have single AVN echoes, but AVNRT is not inducible (these patients are said to have undergone slow pathway modification). In most patients who no longer have inducible AVNRT after ablation in the slow pathway region, residual discontinuous AVN function curves and single AVN echoes do not predict an increased risk of AVNRT recurrence.
Therefore, noninducibility of AVNRT (with and without isoproterenol infusion), with residual evidence of dual AVN pathways (AH interval jump) and single AVN echoes with an echo zone shorter than 30 milliseconds, is an acceptable endpoint. However, double AVN echoes or single echoes produced over a wide range of coupling intervals usually predict inducibility of AVNRT with the addition of isoproterenol or atropine, as well as late clinical recurrence of SVT. Therefore, additional RF applications are recommended.43 When AVNRT or dual AVN physiology, or both, is rarely inducible at baseline, it may not be possible to confirm the efficacy of RF ablation. In this case, other parameters can be indicative of a successful ablation, including the disappearance of a PR/RR ratio greater than 1 during rapid atrial pacing with 1:1 AV conduction, prolongation of the Wenckebach CL, and a decrease in fast pathway ERP.8,28,43 If isoproterenol was required for initiation of SVT prior to ablation, isoproterenol should be discontinued during ablation and readministered afterward to assess the efficacy of ablation adequately. If isoproterenol was not necessary for SVT initiation prior to ablation, it need not be used for postablation testing. However, if the presence of single AVN echoes is accepted as an endpoint, isoproterenol infusion is then required during postablation testing to verify noninducibility of double echoes or AVNRT.43,44
Reassessment of tachycardia inducibility is typically repeated 30 minutes after the last successful RF application. However, there are no data supporting the hypothesis that such a waiting period leads to reduced recurrence rates following acutely successful ablation. In fact, one report demonstrated that a procedure-prolonging waiting period may be omitted without compromising the patient’s long-term outcome results.50
Outcome
Acute success rates of AVNRT ablation using the posterior approach are approximately 97% to 99%, and they are similar whether the ablation is guided anatomically or electroanatomically.49 The recurrence rate after apparently successful ablation is approximately 2% to 5%. In 40% of patients with AVNRT recurrences, slow pathway conduction recovers after initial evidence of its abolition. Most AVNRT recurrences take place within the first days to months after ablation.44,49
The most important complication of AVNRT ablation is AV block. AV block occurs in approximately 0.2% to 0.8% of patients who undergo slow pathway ablation using the posterior approach, it generally occurs during RF delivery or within the first 24 hours after ablation, and it is almost always preceded by junctional ectopy with VA block. The level of block is usually in the AVN. Predictors of AV block include proximity of the anatomical ablation site to the compact AVN, the occurrence of fast junctional tachycardia (CL shorter than 350 milliseconds) during RF application, the occurrence of junctional rhythm with VA block, the number of RF applications (related to the amount of tissue damage), and significant worsening of anterograde AV conduction during the ablation procedure.49,51
Subclinical activation of the coagulation system (e.g., elevated plasma levels of the D-dimer) is common during RF ablation. Nevertheless, clinically detectable embolic events are uncommon (0.7%). Aspirin is usually recommended for 6 to 8 weeks following ablation, to minimize the risk of thrombus formation in the RA or vena cavae. Other complications include cardiac tamponade (in 0.2% of patients), hematoma (in 0.2%), and femoral artery pseudoaneurysm (in 0.1%).44
There are several advantages of selective slow pathway ablation over fast pathway ablation, including a significantly lower risk of AV block and avoidance of a long postablation PR interval, which can cause symptoms similar to those of the pacemaker syndrome. Furthermore, in patients with multiple slow pathways, as demonstrated by AVN function curves, ablation of the fast pathway can be associated with persistently inducible AVNRT, with anterograde conduction over a second slowly conducting pathway.44
Selective slow pathway ablation can be technically challenging in several clinical situations. For example, in patients with evidence of impaired fast pathway conduction (usually older patients with tachycardia CL longer than 400 milliseconds), ablation of the slow pathway can result in mandatory conduction through the impaired fast pathway, and Wenckebach block during rest can develop. Whether the fast pathway should be targeted by ablation instead of the slow pathway in these patients is controversial. Similarly, some patients with AVNRT have a markedly prolonged PR interval during NSR, a finding suggesting the possibility of absent anterograde fast pathway conduction and a high risk of complete AV block if the slow pathway is ablated. However, in such patients, the fast pathway can be affected by an electrotonic interaction with the slow pathway, and elimination of this effect by slow pathway ablation often shortens the ERP of the fast pathway. In fact, in many of these patients, the AH interval remains stable or even shortens after slow pathway ablation. Another challenging situation may be experienced in patients with prior unsuccessful attempts at fast pathway ablation, with persistent AVNRT. Slow pathway ablation may then result in high-degree AV block because of impairment of the fast pathway related to the prior ablation attempt. In such patients, it may be wise to confine further ablation efforts to the pathway originally targeted for ablation.43,44
Fast Pathway Modification (Anterior Approach)
Target of Fast Pathway Ablation
The target site of this approach is located closer to the compact AVN and HB in the anterosuperior region of the triangle of Koch. The anterior approach selectively ablates or modifies fast pathway retrograde conduction; however, it can also cause damage to fast or slow pathway anterograde conduction.43,44 Fast pathway ablation is hardly ever the treatment of choice, with the rare exception of patients with clear AVNRT who have a long PR in sinus rhythm (i.e., slow pathway conduction) and no evidence of anterograde fast pathway conduction.
Fast Pathway Ablation Technique
The ablation catheter is initially positioned at the AV junction to obtain the maximal amplitude of the bipolar His potential recorded from the distal pair of electrodes. The catheter is then withdrawn while a firm clockwise torque is maintained until the His potential becomes small or barely visible or disappears while recording a relatively large atrial electrogram (with an A/V electrogram amplitude ratio greater than 1; see Fig. 15-63). At the optimal ablation site, retrograde atrial activation timing occurs earlier or simultaneously with the atrial electrogram in the catheter at the HB position. At this point, the ablation catheter is held steadily, and RF energy is applied (starting at low power outputs of 5 to 10 W) until one of the following events occurs: impedance rise, transient high-grade AV block, prolongation of the PR interval by more than 30% to 50%, or appearance of very rapid junctional rhythm associated with retrograde conduction block. Overdrive atrial pacing may be performed to monitor AV conduction in case a junctional escape rhythm occurs during RF application.43,44
Outcome of Fast Pathway Ablation
Short-term success rates are greater than 95%. However, the anterior approach has a small safety margin and is associated with a higher risk of inadvertent complete AV block (approximately 10%, but ranging from 2% to 20%). Careful monitoring of AV conduction during RF application and prompt discontinuation of energy delivery on evidence of AV block are the keys to minimize the incidence of AV block.
Cryoablation of the Slow Pathway
Cryoablation Technique
Cryomapping
Cryomapping (ice mapping) is designed to verify that ablation at the chosen site will have the desired effect (i.e., block in the slow pathway) and to reassure the absence of complications (i.e., AV block). Cryomapping is performed at a temperature of –30°C. At this temperature, the cryolesion is reversible (for up to 60 seconds), and the catheter is stuck to the atrial endocardium within an ice ball that includes the tip of the catheter (cryoadherence). Formation of an ice ball at the catheter tip and adherence to the underlying myocardium are signaled by the appearance of electrical noise recorded from the ablation catheter’s distal bipole. In the cryomapping mode, the temperature is not allowed to drop to less than –30°, and the duration of energy application is limited to 60 seconds. Once an ice ball is formed, various pacing protocols are performed to test the modification or disappearance of slow pathway conduction.52
Cryomapping is usually performed during NSR in patients with discontinuous anterograde dual AVN conduction curve. For patients without a clear discontinuity in the AVN conduction curve, cryomapping may be performed during AVNRT, which is feasible without the risk of catheter dislodgment on termination of the tachycardia (because of cryoadherence).52
During cryoablation of the slow pathway, no junctional rhythm is observed. Thus, other parameters must be used to validate the potential effectiveness of the ablation site. In fact, the absence of junctional rhythm can be advantageous because it allows the maintenance of NSR during ablation and enables monitoring of the PR interval throughout the procedure. Additionally, the maintenance of NSR during cryoablation allows various pacing maneuvers to be performed during ongoing cryoenergy application, to evaluate the effect of the ablation on slow pathway conduction. Disappearance of the dual AVN physiology, noninducibility of AVNRT, and modification of the fast pathway ERP predict a successful ablation site. When cryoablation is performed during AVNRT, progressive AH interval lengthening followed by termination of AVNRT indicates slow pathway block.52
Slow pathway block usually occurs quickly (within 10 to 20 seconds) at the optimal target site. If cryomapping does not yield the desired result within 20 to 30 seconds or causes unintended AV conduction delay or block, the cryomapping procedure is interrupted. After a few seconds, to allow the catheter to thaw and become dislodged from the tissue, the catheter is moved to a different site, and cryomapping is repeated.52
Cryoablation
When sites of successful cryomapping are identified by demonstrating satisfactory slow pathway block or modification with no modification of the basal anterograde AV conduction, the cryoablation mode is activated, during which a target temperature lower than –75°C is sought (a temperature of approximately –75° to –80°C is generally achieved). The application is then continued for 4 minutes, to create an irreversible lesion. If the catheter tip is in close contact with the endocardium, a prompt drop in catheter tip temperature should be seen as soon as the cryoablation mode is activated. A slow decline in temperature or a very high flow rate of refrigerant during ablation suggests poor catheter tip tissue contact; in such cases, cryoablation should be interrupted and the catheter repositioned.52
Endpoints of Cryoablation
The goal of cryoablation is the complete elimination of slow pathway function. This usually requires delivery of several cryoapplications at closely adjacent sites. If a discontinuity in the anterograde AVN conduction curve and single AVN echoes persist despite multiple cryoapplications, this is still a reasonable endpoint, provided that multiple echoes or SVT are not inducible, even during isoproterenol infusion. If acute procedural success cannot be achieved with a standard 4-mm-tip catheter, a 6-mm-tip catheter can be used, which can help yield larger and deeper cryolesions.52
Outcome of Cryoablation
Previously published reports with cryoablation of AVNRT in pediatric patients demonstrated procedural success rates of 83% to 97% and recurrence rates ranging from 0% to 20%. Although the use of bonus cryoapplications to consolidate the acutely successful cryoablation and the choice of larger-tip cryocatheters (8-mm and 6-mm versus 4-mm tips) to create larger lesions have been associated with fewer recurrences on long-term follow-up without compromising safety, the overall procedural success rate has remained consistently lower than that of RF ablation.51,53–56
Advantages of Cryoablation
One of the distinct advantages of cryothermal technology is the ability to demonstrate loss of function of tissue with cooling reversibly (ice mapping or cryomapping), thereby demonstrating the functionality of prospective ablation sites without inducing permanent injury. Furthermore, once the catheter tip temperature is reduced to less than 0°C, progressive ice formation at the catheter tip causes adherence to the adjacent tissue (cryoadherence), which maintains stable catheter contact at the site of ablation and minimizes the risk of catheter dislodgment during changing cardiac rhythm.52 A disadvantage of cryoablation is that the cryocatheter is not yet as steerable as the conventional RF catheter, and this can potentially limit proper positioning of the catheter tip.52 The procedure is also slightly longer than standard RF ablation.
Cryoablation can be of particular advantage in patients with AVNRT with posterior displacement of the fast pathway or AVN, and those with a small space in the triangle of Koch between the HB and the CS os, when ablation must be performed in the midseptum. However, given the high success rate and low risk of RF slow pathway ablation, it may be difficult to demonstrate a clinical advantage of cryoablation over RF ablation of unselected AVNRT cases. Therefore, the use of cryoablation may be recommended in specific circumstances in which the use of RF can be more likely to cause AVN damage. These circumstances include patients with unusual cardiac anatomy that makes safe RF delivery difficult, those with evidence of impaired AV conduction at baseline, those who need ablation in the close vicinity of the compact AVN following unsuccessful RF ablation at more posterior sites, pediatric patients, and patients in whom even the small risk of AV block associated with RF ablation is considered unacceptable.51–53
1. Tawara S.L. Das Reitzleitungssystem des Saugetierherzens. In: Fischer G., editor. Handbuch der vergleichenden und experimentellen entwicklungslehre der wirbeltiere, Jena. Germany: Semper Bonis Artibus; 1906:136-137.
2. Anderson R., Ho S. The anatomy of the atrioventricular node. Heart Rhythm Society. 2008. http://www.hrsonline.org/Education/SelfStudy/Articles/AndersonHo_Anatomy_AVNode.pdf.
3. Kurian T., Ambrosi C., Hucker W., et al. Anatomy and electrophysiology of the human AV node. Pacing Clin Electrophysiol. 2010;33:754-762.
4. Lee P.C., Chen S.A., Hwang B. Atrioventricular node anatomy and physiology: implications for ablation of atrioventricular nodal reentrant tachycardia. Curr Opin Cardiol. 2009;24:105-112.
5. Mazgalev T. AV nodal physiology. Heart Rhythm Society. 2008. http://www.hrsonline.org/Education/Self Study/Articles/mazgalev.cfm.
6. Shryock J., Belardinelli L. Pharmacology of the AV node. Heart Rhythm Society. 2008. http://www.hrsonline.org/Education/SelfStudy/Articles/shrbel.cfm.
7. Luc P.J. Common form of atrioventricular nodal reentrant tachycardia: do we really know the circuit we try to ablate? Heart Rhythm. 2007;4:711-712.
8. Lockwood D., Nakagawa H., Jackman W. Electrophysiological characteristics of atrioventricular nodal reentrant tachycardia: implications for the reentrant circuit. In: Zipes D.P., Jalife J., editors. Cardiac electrophysiology: from cell to bedside. ed 5. Philadelphia: Saunders; 2009:615-646.
9. Valderrabano M. Atypical atrioventricular nodal reentry with eccentric atrial activation: is the right target on the left? Heart Rhythm. 2007;4:433-434.
10. Racker D.K., Kadish A.H. Proximal atrioventricular bundle, atrioventricular node, and distal atrioventricular bundle are distinct anatomic structures with unique histological characteristics and innervation. Circulation. 2000;101:1049-1059.
11. Mazgalev T.N. The specialized rings and the endless saga of the AV node puzzle. Heart Rhythm. 2009;6:681-683.
12. Basso C., Ho S.Y., Thiene G. Anatomical and histopathological characteristics of the conductive tissues of the heart. In: Gussak I., Antzelevitch C., editors. Electrical diseases of the heart: genetics, mechanisms, treatment, prevention. London: Springer; 2008:37-51.
13. Katritsis D.G., Becker A. The atrioventricular nodal reentrant tachycardia circuit: a proposal. Heart Rhythm. 2007;4:1354-1360.
14. Mazgalev T.N., Ho S.Y., Anderson R.H. Anatomic-electrophysiological correlations concerning the pathways for atrioventricular conduction. Circulation. 2001;103:2660-2667.
15. Morihisa K., Yamabe H., Uemura T., et al. Analysis of atrioventricular nodal reentrant tachycardia with variable ventriculoatrial block: characteristics of the upper common pathway. Pacing Clin Electrophysiol. 2009;32:484-493.
16. Otomo K., Okamura H., Noda T., et al. Unique electrophysiologic characteristics of atrioventricular nodal reentrant tachycardia with different ventriculoatrial block patterns: effects of slow pathway ablation and insights into the location of the reentrant circuit. Heart Rhythm. 2006;3:544-554.
17. Katritsis D.G., Ellenbogen K.A., Becker A.E. Atrial activation during atrioventricular nodal reentrant tachycardia: studies on retrograde fast pathway conduction. Heart Rhythm. 2006;3:993-1000.
18. Otomo K., Okamura H., Noda T., et al. “Left-variant” atypical atrioventricular nodal reentrant tachycardia: electrophysiological characteristics and effect of slow pathway ablation within coronary sinus. J Cardiovasc Electrophysiol. 2006;17:1177-1183.
19. Lee P.C., Hwang B., Tai C.T., et al. The electrophysiological characteristics in patients with ventricular stimulation inducible fast-slow form atrioventricular nodal reentrant tachycardia. Pacing Clin Electrophysiol. 2006;29:1105-1111.
20. Verma A. Guilty culprit or innocent bystander? J Cardiovasc Electrophysiol. 2006;17:1184-1186.
21. Nam G.B., Rhee K.S., Kim J., et al. Left atrionodal connections in typical and atypical atrioventricular nodal reentrant tachycardias: activation sequence in the coronary sinus and results of radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2006;17:171-177.
22. Otomo K., Nagata Y., Uno K., et al. Atypical atrioventricular nodal reentrant tachycardia with eccentric coronary sinus activation: electrophysiological characteristics and essential effects of left-sided ablation inside the coronary sinus. Heart Rhythm. 2007;4:421-432.
23. Otomo K., Nagata Y., Taniguchi H., et al. Superior type of atypical AV nodal reentrant tachycardia: incidence, characteristics, and effect of slow pathway ablation. Pacing Clin Electrophysiol. 2008;31:998-1009.
24. Porter M.J., Morton J.B., Denman R., et al. Influence of age and gender on the mechanism of supraventricular tachycardia. Heart Rhythm. 2004;1:393-396.
25. Suenari K., Hu Y.F., Tsao H.M., et al. Gender differences in the clinical characteristics and atrioventricular nodal conduction properties in patients with atrioventricular nodal reentrant tachycardia. J Cardiovasc Electrophysiol. 2010;21:1114-1119.
26. Gonzalez-Torrecilla E., Almendral J., Arenal A., et al. Combined evaluation of bedside clinical variables and the electrocardiogram for the differential diagnosis of paroxysmal atrioventricular reciprocating tachycardias in patients without pre-excitation. J Am Coll Cardiol. 2009;53:2353-2358.
27. Blomstrom-Lundqvist C., Scheinman M.M., Aliot E.M., et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop guidelines for the management of patients with supraventricular arrhythmias). Circulation. 2003;108:1871-1909.
28. Josephson M.E. Supraventricular tachycardias. In: Josephson M.E., editor. Clinical cardiac electrophysiology. ed 4. Philadelphia: Lippincott Williams & Wilkins; 2008:175-284.
29. Kertesz N.J., Fogel R.I., Prystowsky E.N. Mechanism of induction of atrioventricular node reentry by simultaneous anterograde conduction over the fast and slow pathways. J Cardiovasc Electrophysiol. 2005;16:251-255.
30. Crawford T.C., Mukerji S., Good E., et al. Utility of atrial and ventricular cycle length variability in determining the mechanism of paroxysmal supraventricular tachycardia. J Cardiovasc Electrophysiol. 2007;18:698-703.
31. Maruyama M., Kobayashi Y., Miyauchi Y., et al. The VA relationship after differential atrial overdrive pacing: a novel tool for the diagnosis of atrial tachycardia in the electrophysiologic laboratory. J Cardiovasc Electrophysiol. 2007;18:1127-1133.
32. Knight B.P., Ebinger M., Oral H., et al. Diagnostic value of tachycardia features and pacing maneuvers during paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 2000;36:574-582.
33. Platonov M., Schroeder K., Veenhuyzen G.D. Differential entrainment: beware from where you pace. Heart Rhythm. 2007;4:1097-1099.
34. Gonzalez-Torrecilla E., Arenal A., Atienza F., et al. First postpacing interval after tachycardia entrainment with correction for atrioventricular node delay: a simple maneuver for differential diagnosis of atrioventricular nodal reentrant tachycardias versus orthodromic reciprocating tachycardias. Heart Rhythm. 2006;3:674-679.
35. Veenhuyzen G.D., Stuglin C., Zimola K.G., Mitchell L.B. A tale of two post pacing intervals. J Cardiovasc Electrophysiol. 2006;17:687-689.
36. Kannankeril P.J., Bonney W.J., Dzurik M.V., Fish F.A. Entrainment to distinguish orthodromic reciprocating tachycardia from atrioventricular nodal reentry tachycardia in children. Pacing Clin Electrophysiol. 2010;33:469-474.
37. Segal O.R., Gula L.J., Skanes A.C., et al. Differential ventricular entrainment: a maneuver to differentiate AV node reentrant tachycardia from orthodromic reciprocating tachycardia. Heart Rhythm. 2009;6:493-500.
38. Dandamudi G., Mokabberi R., Assal C., et al. A novel approach to differentiating orthodromic reciprocating tachycardia from atrioventricular nodal reentrant tachycardia. Heart Rhythm. 2010;7:1326-1329.
39. Al Mahameed S.T., Buxton A.E. Michaud GF: New criteria during right ventricular pacing to determine the mechanism of supraventricular tachycardia. Circ Arrhythm Electrophysiol. 2010;3:578-584.
40. Vijayaraman P., Lee B.P., Kalahasty G., et al. Reanalysis of the “pseudo A-A-V” response to ventricular entrainment of supraventricular tachycardia: importance of His-bundle timing. J Cardiovasc Electrophysiol. 2006;17:25-28.
41. Lau E.W. Infraatrial supraventricular tachycardias: mechanisms, diagnosis, and management. Pacing Clin Electrophysiol. 2008;31:490-498.
42. Issa Z.F. Mechanism of paroxysmal supraventricular tachycardia with ventriculoatrial conduction block. Europace. 2009;11:1235-1237.
43. McElderry H., Kay G.N. Ablation of atrioventricular nodal reentrant tachycardia and variants guided by intracardiac recordings. In: Huang S, Wood M.A., editors. Catheter ablation of cardiac arrhythmias. Philadelphia: Saunders; 2006:347-367.
44. Snowdon R.L., Kalman J.M. Catheter ablation of supraventricular arrhythmias. In: Zipes DP Jalife J., editor. Cardiac electrophysiology: from cell to bedside. ed 5. Philadelphia: Saunders; 2009:1083-1092.
45. Gonzalez M.D., Rivera J. Ablation of atrioventricular nodal reentry by the anatomical approach. In: Huang S, Wood M.A., editors. Catheter ablation of cardiac arrhythmias. Philadelphia: Saunders; 2006:325-346.
46. Salem Y.S., Burke M.C., Kim S.S., et al. Slow pathway ablation for atrioventricular nodal reentry using a right internal jugular vein approach: a case series. Pacing Clin Electrophysiol. 2006;29:59-62.
47. Lee S.H., Tai C.T., Lee P.C., et al. Electrophysiological characteristics of junctional rhythm during ablation of the slow pathway in different types of atrioventricular nodal reentrant tachycardia. Pacing Clin Electrophysiol. 2005;28:111-118.
48. McGavigan A.D., Rae A.P., Cobbe S.M., Rankin A.C. Junctional rhythm: a suitable surrogate endpoint in catheter ablation of atrioventricular nodal reentry tachycardia? Pacing Clin Electrophysiol. 2005;28:1052-1054.
49. Estner H.L., Ndrepepa G., Dong J., et al. Acute and long-term results of slow pathway ablation in patients with atrioventricular nodal reentrant tachycardia: an analysis of the predictive factors for arrhythmia recurrence. Pacing Clin Electrophysiol. 2005;28:102-110.
50. Steven D., Rostock T., Hoffmann B.A., et al. Favorable outcome using an abbreviated procedure for catheter ablation of AVNRT: results from a prospective randomized trial. J Cardiovasc Electrophysiol. 2009;20:522-525.
51. Opel A., Murray S., Kamath N., et al. Cryoablation versus radiofrequency ablation for treatment of atrioventricular nodal reentrant tachycardia: cryoablation with 6-mm-tip catheters is still less effective than radiofrequency ablation. Heart Rhythm. 2010;7:340-343.
52. Friedman P.L. How to ablate atrioventricular nodal reentry using cryoenergy. Heart Rhythm. 2005;2:893-896.
53. Chanani N.K., Chiesa N.A., Dubin A.M., et al. Cryoablation for atrioventricular nodal reentrant tachycardia in young patients: predictors of recurrence. Pacing Clin Electrophysiol. 2008;31:1152-1159.
54. Silver E.S., Silva J.N., Ceresnak S.R., et al. Cryoablation with an 8-mm tip catheter for pediatric atrioventricular nodal reentrant tachycardia is safe and efficacious with a low incidence of recurrence. Pacing Clin Electrophysiol. 2010;33:681-686.
55. Rivard L., Dubuc M., Guerra P.G., et al. Cryoablation outcomes for AV nodal reentrant tachycardia comparing 4-mm versus 6-mm electrode-tip catheters. Heart Rhythm. 2008;5:230-234.
56. Drago F., Russo M.S., Silvetti M.S., et al. Cryoablation of typical atrioventricular nodal reentrant tachycardia in children: six years’ experience and follow-up in a single center. Pacing Clin Electrophysiol. 2010;33:475-481.