Chapter 9 Atrioventricular Conduction Abnormalities
General Considerations
Anatomy and Physiology of the Atrioventricular Junction
Internodal and Intraatrial Conduction
Evidence indicates the presence of preferential impulse propagation from the sinus node to the atrioventricular node (AVN)—that is, higher conduction velocity between the nodes in some parts of the atrium than in other parts. However, whether preferential internodal conduction is caused by fiber orientation, size, or geometry or by the presence of specialized preferentially conducting pathways located between the nodes has been controversial.1–3
Atrioventricular Node
The AVN is an interatrial structure, measuring approximately 5 mm long, 5 mm wide, and 0.8 mm thick in adults. The AVN is located beneath the RA endocardium at the apex of the triangle of Koch. The triangle of Koch is septal and constitutes the RA endocardial surface of the muscular AV septum. It is bordered anteriorly by the annulus of the septal leaflet of the tricuspid valve, posteriorly by the tendon of Todaro, and inferiorly by the orifice of the CS ostium (CS os) (see Fig. 17-1). The central fibrous body is composed of a thickened area of fibrous continuity between the leaflets of the mitral and aortic valves, termed the right fibrous trigone, together with the membranous component of the cardiac septum. The tendon of Todaro runs within the eustachian ridge and inserts into the central fibrous body; the annulus of the septal leaflet of the tricuspid valve crosses the membranous septum.4–6
The compact AVN lies anterior to the CS os and directly above the insertion of the septal leaflet of the tricuspid valve, where the tendon of Todaro merges with the central fibrous body. Slightly more anteriorly and superiorly is where the His bundle (HB) penetrates the AV junction through the central fibrous body and the posterior aspect of the membranous AV septum.7 The compact node is adjacent to the central fibrous body on the right side but is uninsulated by fibrous tissue on its other sides, thus allowing contiguity with the atrial myocardium. Because the AV valves are not isoplanar (the attachment of the tricuspid valve into the most anterior part of the central body is a few millimeters apically relative to the mitral valve), the AVN lies just beneath the RA endocardium. When traced inferiorly, toward the base of the triangle of Koch, the compact AVN area separates into two extensions, usually with the artery supplying the AVN running between them. The prongs bifurcate toward the tricuspid and mitral annuli, respectively. The rightward posterior extensions have been implicated in the so-called slow pathway in AVN reentrant tachycardia (AVNRT) circuit.4–6
The normal AV junctional area can be divided into distinct regions: the transitional cell zone (which represents the approaches from the working atrial myocardium to the AVN), the compact AVN, and the penetrating part of the HB.8 The AVN and perinodal area are composed of at least three electrophysiologically distinct cells: the atrionodal (AN), nodal (N), and nodal-His (NH) cells. The AN region corresponds to the cells in the transitional region that are activated shortly after the atrial cells. Transitional cells are histologically distinct from both the cells of the compact AVN and the working atrial myocytes, and they are not insulated from the surrounding myocardium, but tend to be separated from one another by thin fibrous strands. Transitional cells do not represent conducting tracts but a bridge funneling atrial depolarization into the compact AVN via discrete AVN inputs (approaches). Transitional cells approaches connect the working atrial myocardium from the left and right sides of the atrial septum to the left and right margins of the compact node, with wider extensions inferiorly and posteriorly between the compact node and the CS os and into the eustachian ridge. In humans and animals, two such inputs are commonly recognized in the right septal region: the anterior (superior) approaches, which travel from the anterior limbus of the fossa ovalis and merge with the AVN closer to the apex of the triangle of Koch; and the posterior (inferior) approaches, which are located in the inferoseptal RA and serve as a bridge with the atrial myocardium at the CS os. Although both inputs have traditionally been assumed to be RA structures, growing evidence supports the AV conduction apparatus as a transseptal structure that reaches both atria. A third, middle group of transitional cells has also been identified to account for the nodal connections with the septum and LA.4–6
The N region corresponds to the region where the transitional cells merge with midnodal cells. The N cells represent the most typical of the nodal cells, which are smaller than atrial myocytes, are closely grouped, and frequently are arranged in an interweaving fashion. The N cells in the compact AVN appear to be responsible for the major part of AV conduction delay and exhibit decremental properties in response to premature stimulation because of their slow rising and longer action potentials. Fast pathway conduction through the AVN apparently bypasses many of the N cells by transitional cells, whereas slow pathway conduction traverses the entire compact AVN. Importantly, the recovery of excitability after conduction of an impulse is faster for the slow pathway than for the fast pathway, for reasons that are unclear.4
The NH region corresponds to the lower nodal cells, connecting to the insulated penetrating portion of the HB. Sodium (Na+) channel density is lower in the midnodal zone of the AVN than in the AN and NH cell zones, and the inward L-type calcium (Ca2+) current is the basis of the upstroke of the N cell action potential. Therefore, conduction is slower through the compact AVN than the AN and NH cell zones.9,10
The AVN is the only normal electrical connection between the atria and the ventricles; the fibrous skeleton acts as an insulator to prevent electrical impulses from entering the ventricles by any other route. The main function of the AVN is modulation of atrial impulse transmission to the ventricles, thereby coordinating atrial and ventricular contractions. The AVN receives, slows down, and conveys atrial impulses to the ventricles. A primary function of the AVN is to limit the number of impulses conducted from the atria to the ventricles. This function is particularly important during fast atrial rates (e.g., during atrial fibrillation [AF] or atrial flutter), in which only a few impulses are conducted to the ventricles, and the remaining impulses are blocked in the AVN. Additionally, fibers in the lower part of the AVN can exhibit automatic impulse formation, and the AVN may serve as a subsidiary pacemaker.5,6,9
The AVN region is innervated by a rich supply of cholinergic and adrenergic fibers. Sympathetic stimulation shortens AVN conduction time and refractoriness, whereas vagal stimulation prolongs AVN conduction time and refractoriness. The negative dromotropic response of the AVN to vagal stimulation is mediated by activation of the inwardly rectifying potassium (K+) current IKACh, which results in hyperpolarization and action potential shortening of AVN cells, increased threshold of excitation, depression of action potential amplitude, and prolonged conduction time. The positive dromotropic effect of sympathetic stimulation arises as a consequence of activation of the L-type Ca2+ current.7
His Bundle
The HB connects with the distal part of the compact AVN and passes through the fibrous core of the central fibrous body in a leftward direction (away from the RA endocardium and toward the ventricular septum). The HB then continues through the annulus fibrosis (where it is called the nonbranching portion) as it penetrates the membranous septum, along the crest of the left side of the interventricular septum, for 1 to 2 cm and then divides into the right and left bundle branches.4
Proximal cells of the penetrating portion are heterogeneous and resemble those of the compact AVN; distal cells are larger, similar to cells in the proximal bundle branches and ventricular myocytes. The HB is insulated from the atrial myocardium by the membranous septum and from the ventricular myocardium by connective tissue of the central fibrous body, thus preventing atrial impulses from bypassing the AVN. The area of fibrous continuity between the aortic and mitral valves adjacent to the membranous septum marks the HB as viewed from the left ventricle (LV). Viewed from the aorta, the HB passes beneath the part of the membranous septum that adjoins the interleaflet fibrous triangle between the right and the noncoronary sinuses.4
Pathophysiology of Atrioventricular Block
Congenital Atrioventricular Block
Congenital complete AV block is thought to result from embryonic maldevelopment of the AVN (and, much less frequently, the His-Purkinje system [HPS]), mainly secondary to a lack of connection between the atria and the peripheral conduction system, with fatty replacement of the AVN and nodal approaches.4 The incidence of congenital complete AV block varies from 1 in 15,000 to 1 in 22,000 live births. The defect usually occurs proximal to the HB, and QRS duration is shorter than 120 milliseconds.11 Maternal lupus, caused by antibodies targeting intracellular ribonucleoproteins that cross the placenta to affect the fetal heart but not the maternal heart, is responsible for 60% to 90% of cases of congenital complete AV block.12
Approximately 50% of patients with congenital AV block have concurrent congenital heart disease (e.g., congenitally corrected transposition of the great vessels, AV discordance, ventricular septal defects, AV canal defect, tricuspid atresia, and Ebstein anomaly of the tricuspid valve).13 The AV conduction system may be displaced if atrial and ventricular septa are malaligned, AV arrangements are discordant, or the heart is univentricular. Generally, if the AV conduction system is displaced, it will also tend to be more fragile and susceptible to degeneration, thus placing patients at greater risk for AV block.14
Hereditary Progressive Cardiac Conduction Disease
Cardiac ion channelopathies have been described as a rare cause of familial forms of AV block. Mutations in the SCN5A gene (encoding the alpha-subunit of the cardiac Na+ channel) and the KCNJ2 gene (encoding the inward rectifier Kir2.1, a critical component of the cardiac inward K+ rectifier current, IK1) have been associated with AV block. Additionally, mutations in the PRKAG2 gene (encoding the gamma2 regulatory subunit of adenosine monophosphate–activated protein kinase) have been described in patients with Wolff-Parkinson-White syndrome and AV conduction block.15,16
Acquired Atrioventricular Block
Drugs
Various drugs can impair conduction and cause AV block. Digoxin and beta blockers act indirectly on the AVN through their effects on the autonomic nervous system. Calcium channel blockers and other antiarrhythmic drugs, such as amiodarone and dronedarone, act directly to slow conduction in the AVN. Class I and III antiarrhythmic drugs can also affect conduction in the HPS that results in infranodal block. These effects, however, typically occur in patients with preexisting conduction abnormalities. Patients with a normal conduction system function rarely develop complete heart block as a result of using antiarrhythmic agents.17
Acute Myocardial Infarction
AV block occurs in 12% to 25% of all patients with acute myocardial infarction (MI); first-degree AV block occurs in 2% to 12% second-degree AV block occurs in 3% to 10%, and third-degree AV block occurs in 3% to 7%. First-degree AV block and type 1 second-degree (Wenckebach) AV block occur more commonly in inferior MI, usually caused by increased vagal tone and generally associated with other signs of vagotonia, such as sinus bradycardia and responsiveness to atropine and catecholamine stimulation. Wenckebach AV block in the setting of acute inferior MI is usually transient (resolving within 48 to 72 hours of MI) and asymptomatic, and it rarely progresses to high-grade or complete AV block. Wenckebach AV block occurring later in the course of acute inferior MI is less responsive to atropine and probably is associated with ischemia of the AVN or the release of adenosine during acute MI. In this setting, Wenckebach AV block rarely progresses to more advanced block and commonly resolves within 2 to 3 days of onset. The site of conduction block is usually in the AVN.
Complete AV block occurs in 8% to 13% of patients with acute MI. It can occur with anterior or inferior acute MI. In the setting of acute inferior MI, the site of the block is usually at the level of the AVN, and it results in a junctional escape rhythm with a narrow QRS complex and a rate of 40 to 60 beats/min. The block tends to be reversed by vagolytic drugs or catecholamines and usually resolves within several days. Development of complete AV block in the setting of acute anterior MI, however, is associated with a higher risk of ventricular tachycardia (VT) and ventricular fibrillation, hypotension, pulmonary edema, and in-hospital mortality. In this setting, the block is usually associated with ischemia or infarction of the HB or bundle branches and is less likely to be reversible. Complete AV block during acute anterior MI is often preceded by bundle branch block (BBB), fascicular block, or type 2 second-degree AV block. The escape rhythm usually originates from the bundle branch and Purkinje system, with a rate less than 40 beats/min and a wide QRS complex. In general, patients who develop transient or irreversible AV block are older and have a larger area of damage associated with their acute MI.11,18
Degenerative Diseases
Progressive cardiac conduction disease (including Lev disease or Lenègre disease) manifests as progressive slowing of electrical conduction through the atria, AVN, HB, Purkinje fibers, and ventricles, accompanied by an age-related degenerative process, in which fibrosis affects only the cardiac conduction system. Complete AV block can develop and cause syncope or sudden death. Lev disease is a result of proximal bundle branch calcification or fibrosis and is often described as senile degeneration of the conduction system. It is postulated as a hastening of the aging process by hypertension and arteriosclerosis of the blood vessels supplying the conduction system. Lenègre disease is a sclerodegenerative process that occurs in a younger population and involves the more distal portions of the bundle branches. As noted, in heritable progressive cardiac conduction disease (referred to as hereditary Lenègre disease, progressive cardiac conduction disease, and familial AV block), conduction slowing may be attributed to loss-of-function mutations in SCN5A. Whether age-dependent fibrosis of the conduction system is a primary degenerative process in progressive cardiac conduction disease or a physiological process that is accelerated by Na+ current (INa) reduction is still unknown.16
Calcification of the aortic or (less commonly) mitral valve annulus can extend to the nearby conduction system and produce AV block. As noted, the HB penetrates the central fibrous body adjacent to the fibrous continuity between the aortic and mitral valves that is the usual site of dystrophic calcification, and extension of calcification can directly involve the HB or the origin of the left bundle branch, or both.4,11,18
Neuromyopathies
AV conduction disturbance is usually the major cardiac manifestation of neuromuscular diseases, including Becker muscular dystrophy, peroneal muscular dystrophy, Kearns-Sayre syndrome, Erb dystrophy, and myotonic muscular dystrophy. AV block can be an important cause of mortality in such cases.19
Infectious Diseases
Infective endocarditis (especially of the aortic valve) and myocarditis of various viral, bacterial, and parasitic causes (including Lyme disease, rheumatic fever, Chagas disease, tuberculosis, measles, and mumps) result in varying degrees of AV block. Complete AV block occurs in 3% of cases.17 Lyme carditis is of particular importance because in most cases, AV block resolves completely within weeks.
Iatrogenic
Cardiac surgery can be complicated by varying degrees of AV block caused by trauma and ischemic damage to the conduction system. AV block is most frequently associated with aortic valve replacement; less commonly, it occurs following coronary artery bypass grafting.20 Repair of congenital heart defects in the region of the conduction system, such as endocardial cushion malformations, ventricular septal defects, and tricuspid valve abnormalities, can lead to transient or persistent AV block.13,21,22 The block is usually temporary and is thought to be secondary to postoperative local inflammation. However, AV block can appear years later, usually in patients who had transient block just after the operation.23,24 Intracardiac catheter manipulation can inadvertently produce varying degrees of heart block, which is usually temporary. Alcohol septal ablation in patients with obstructive hypertrophic cardiomyopathy also can be complicated by AV block. Complete heart block can occur during right-sided heart catheterization in a patient with preexisting left BBB (LBBB) or during LV catheterization (LV angiography or ablation procedures) in a patient with preexisting right BBB (RBBB).25 AV block can also complicate catheter ablation of AVNRT, bypass tracts and atrial tachycardias in the AVN vicinity, as well as VTs originating in the interventricular septum adjacent to the HB.26
Vagally Mediated Atrioventricular Block
Vagally induced AV block can occur in otherwise normal patients, in those with cough or hiccups, and during swallowing or micturition when vagal discharge is enhanced.27 Vagally mediated AV block occurs in the AVN, is associated with a narrow QRS complex, and is generally benign. The block is characteristically paroxysmal and is often associated with clearly visible sinus slowing on the ECG because the vagal surge can cause simultaneous sinus slowing and AVN block. Additionally, transient AV block can occur secondary to enhanced vagal tone caused by carotid sinus massage, hypersensitive carotid sinus syndrome, or neurocardiogenic syncope. AV block in athletes is typically type 1 second-degree block, probably an expression of hypervagotonia related to physical training, and it resolves after physical deconditioning. This form of AV block may or may not be associated with sinus bradycardia because the relative effects of sympathetic and parasympathetic systems on the AVN and sinus node can differ.
Clinical Presentation
Symptoms in patients with AV conduction abnormalities are generally caused by bradycardia and loss of AV synchrony. Individuals with first-degree AV block are usually asymptomatic; however, patients with marked prolongation of the PR interval (longer than 300 milliseconds) can experience symptoms similar to those with pacemaker syndrome caused by loss of AV synchrony and atrial contraction against closed AV valves. Additionally, in patients with LV dysfunction, severe first-degree AV block can cause worsening of heart failure symptoms.28 Symptoms caused by more advanced AV block can range from exercise intolerance, easy fatigability, dyspnea on exertion, angina, mental status changes, dizziness, and near syncope to frank syncope.11,18 In patients with paroxysmal or intermittent complete heart block, symptoms are episodic, and routine ECGs may not be diagnostic.
Congenital AV block can be apparent in utero or at birth; however, many individuals have few or no symptoms and reach their teens or young adulthood before the diagnosis is made. Because of the presence of reliable subsidiary HB pacemakers with adequate rates (especially in the presence of catecholamines), syncope is rare with congenital complete AVN block. Some patients become symptomatic only when aging produces chronotropic incompetence of the HB rhythm.11,18
Natural History of Atrioventricular Block
The natural history of patients with AV block depends on the underlying cardiac condition; however, the site of the block and the resulting rhythm disturbances themselves contribute to the prognosis. Patients with first-degree AV block have an excellent prognosis, even when the condition is associated with chronic bifascicular block, because the rate of progression to third-degree AV block is low.28 Type 1 second-degree AV block is generally benign; however, when type 1 AV block occurs in association with bifascicular block, the risk of progression to complete heart block is significantly increased because of probable infranodal disease. Type 2 second-degree AV block, usually seen with BBB, carries a high risk of progression to advanced or complete AV block. The prognosis of 2:1 AV block depends on whether the site of block is within or below the AVN.11
The prognosis for patients with symptomatic acquired complete heart block is very poor in the absence of pacing, regardless of the extent of underlying heart disease. Once appropriate pacing therapy has been established, however, the prognosis depends on the underlying disease process.17 Complete heart block secondary to anterior MI carries a poor prognosis because of the coexisting extensive infarction and pump failure. In contrast, complete heart block secondary to idiopathic fibrosis of the conduction system in the absence of additional cardiac disease carries a more benign prognosis.11 AV block after valve surgery can recover; however, if conduction has not recovered by 48 hours after surgery, permanent pacing will likely be necessary.29
Diagnostic Evaluation of Atrioventricular Block
Autonomic Modulation
Whereas the AVN is richly innervated and highly responsive to both sympathetic and vagal stimuli, the HPS is influenced minimally by the autonomic nervous system. Carotid sinus massage increases vagal tone and worsens second-degree AVN block, whereas exercise and atropine improve AVN conduction because of sympathetic stimulation or parasympatholysis, or both. In contrast, carotid sinus massage can improve second-degree infranodal block by slowing the sinus rate and allowing HPS refractoriness to recover. Also, exercise and atropine worsen infranodal block because of the enhanced function of the sinus node and AVN and, as a consequence, the increased rate of impulses conducted to the HPS without changing HPS refractoriness.11,18
Electrocardiographic Features
First-Degree Atrioventricular Block (Delay)
Site of Block
The degree of PR interval prolongation and QRS duration can help predict the site of conduction delay. Very long (more than 300 milliseconds) or highly variable PR intervals suggest involvement of the AVN. Normal QRS duration also suggests involvement of the AVN.17,30
Atrioventricular Node
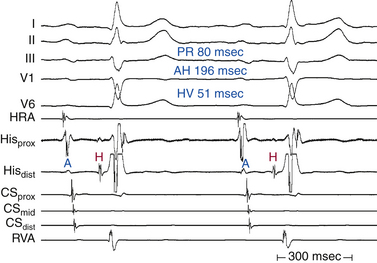
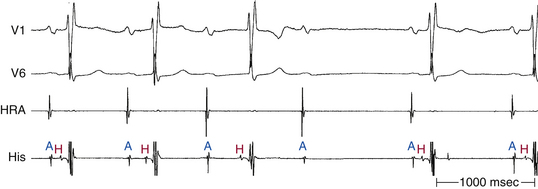
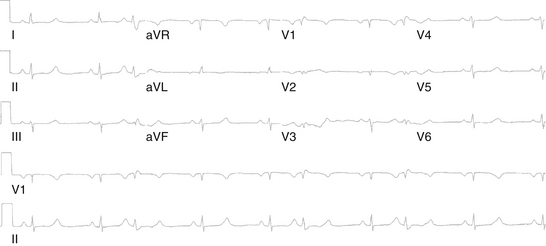
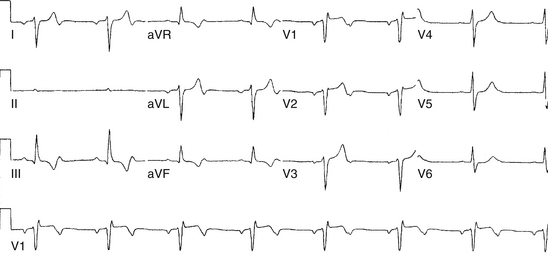
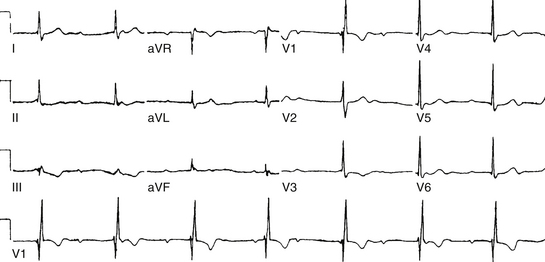
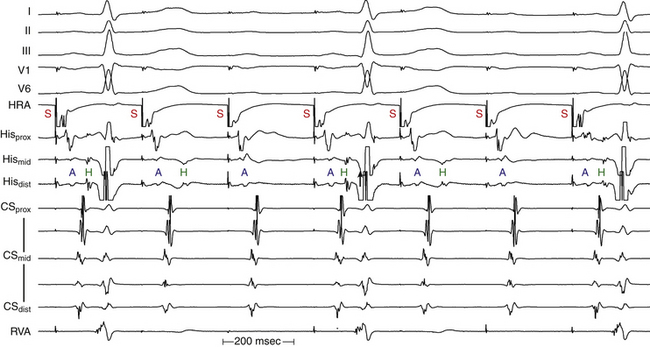
Although conduction delay can be anywhere along the AVN-HPS, the AVN is the most common site of delay (87% when the QRS complex is narrow, and more than 90% when the PR interval is longer than 300 milliseconds; Fig. 9-1).
His-Purkinje System
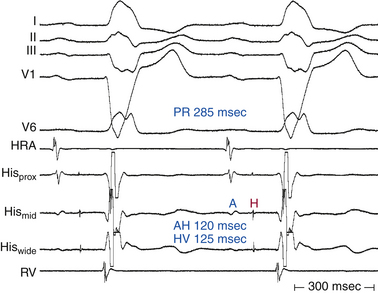
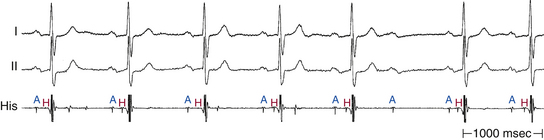
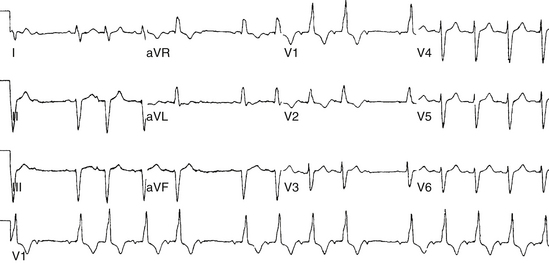
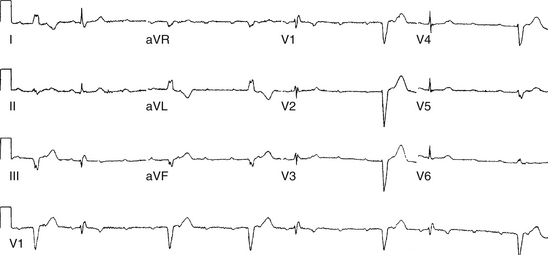
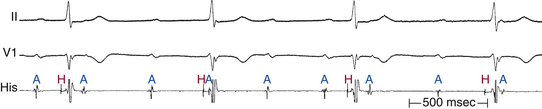
Intra-Hisian conduction delay or HPS disease can cause first-degree AV block. First-degree AV block in the presence of BBB is caused by infranodal conduction delay in 45% of cases. A combination of delay within the AVN and in the HPS must also be considered (Fig. 9-2).
Atrium
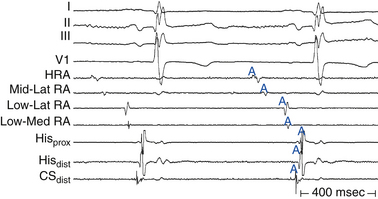
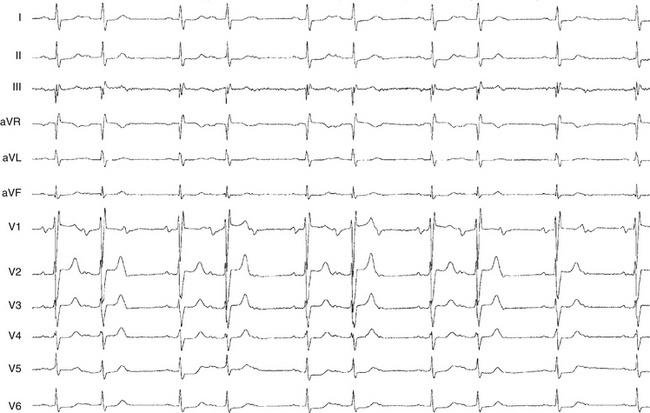
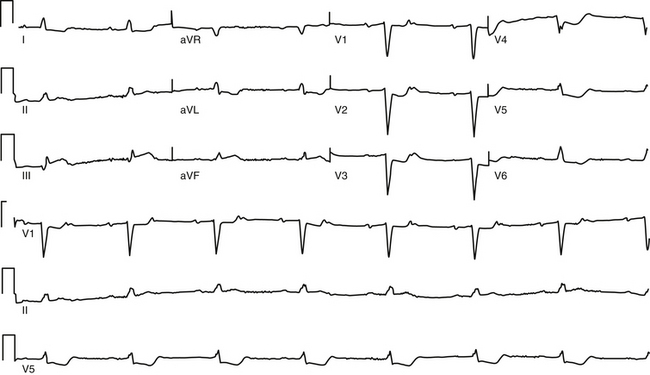
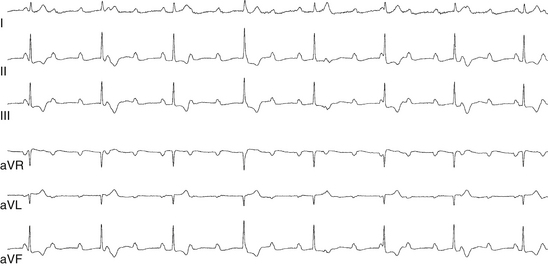
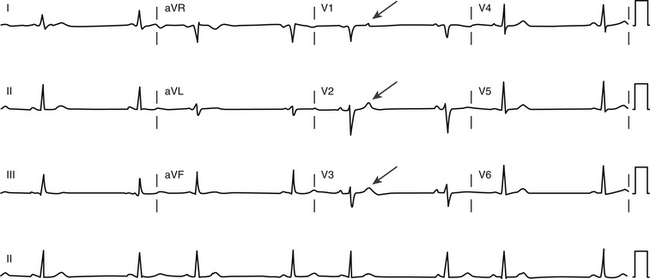
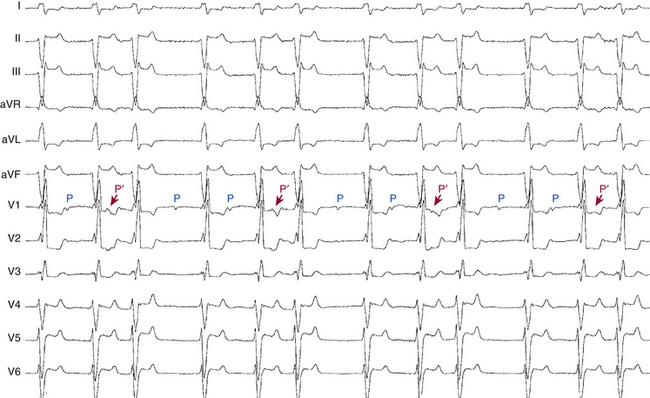
First-degree AV block caused by intraatrial or interatrial conduction delay is not uncommon. An LA enlargement pattern on the ECG (i.e., prolonged P wave duration) reflects the presence of interatrial conduction delay. RA enlargement can prolong the PR interval (Fig. 9-3). In certain cases of congenital structural heart disease, such as Ebstein anomaly of the tricuspid valve or endocardial cushion defects, intraatrial conduction delay can cause first-degree AV block.31
Second-Degree Atrioventricular Block
The term second-degree AV block is applied when intermittent failure of AV conduction is present (i.e., one or more atrial impulses that should be conducted fail to reach the ventricles). This term encompasses several conduction patterns. Types 1 and 2 AV block are ECG patterns that describe the behavior of the PR intervals (in sinus rhythm) in sequences (with at least two consecutively conducted PR intervals) in which a single P wave fails to conduct to the ventricles. The anatomical site of block should not be characterized as either type 1 or type 2 because type 1 and type 2 designations refer only to ECG patterns.11
Type 1 Second-Degree Atrioventricular Block
The behavior of Wenckebach block can be simplified by relating it to an abnormally long relative refractory period of the AVN. In this setting, the rate of AVN conduction depends on the time that the impulse arrives at the AVN. The earlier it arrives at the AVN, the longer it takes to propagate through the AVN, and the longer the PR interval will be; the later it arrives, the shorter the conduction time, and the shorter the PR interval. Thus, Wenckebach periodicity develops because each successive atrial impulse arrives earlier and earlier in the relative refractory period of the AVN, thus resulting in longer and longer conduction delay and PR interval, until one impulse arrives during the absolute refractory period and fails to conduct, with a consequent ventricular pause. In other words, the shorter the RP interval is, the longer the PR interval will be; and the longer the RP interval is, the shorter the PR interval will be. This is referred to as RP-PR reciprocity or RP-dependent PR interval. Using this concept, it is easy to explain the behavior of the PR interval during the Wenckebach periodicity. The first atrial impulse conducting after the pause has an unusually long RP interval, whereas with the following atrial impulse, the RP interval dramatically shortens, thus resulting in prolongation of the PR interval. Although the following atrial impulses have shorter RP intervals, such shortening is not as dramatic, and consequently the progressive prolongation of the PR interval is of lesser degree. In other words, although each successive PR interval prolongs, it does so at a decreasing increment. So, for example, if the PR interval following the first conducted P wave in the cycle increased by 100 milliseconds, the PR interval of the next beat would increase by 50 milliseconds, and so forth.
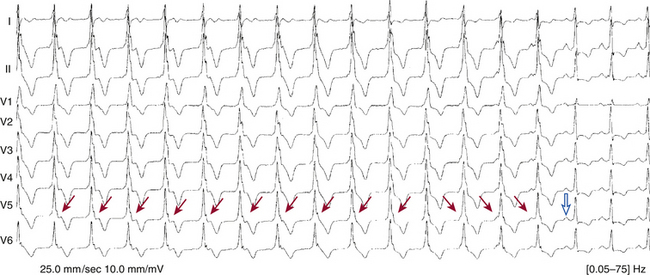
Features of typical Wenckebach periodicity include the following: (1) progressive lengthening of the PR interval throughout the Wenckebach cycle; (2) lengthening of the PR interval occurring at progressively decreasing increments and resulting in progressively shorter R-R intervals; (3) a pause between QRS complexes encompassing the nonconducted P wave that is less than the sum of R-R intervals of any two consecutively conducted beats; (4) shortening of the PR interval after block, compared with the PR interval just preceding the blocked cycle; and (5) group beating, which offers a footprint that identifies Wenckebach periodicity (Fig. 9-4). It is important to recognize that, during Wenckebach periodicity, the atrial impulse conducted to the ventricles is not always represented by the P wave that immediately precedes the QRS complex; the PR interval can be very long and exceed the P-P interval.11,18
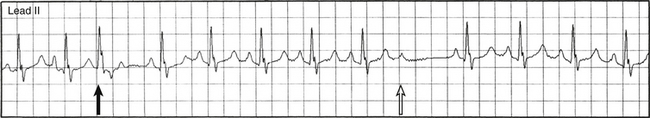
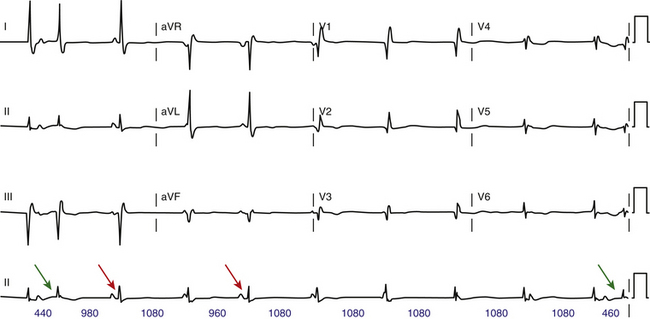
Less than 50% of type 1 AV block cases follow this typical pattern. Typical Wenckebach periodicity is more frequently observed during pacing-induced AV block. Atypical patterns are more likely found with longer Wenckebach periods (more than 6:5). In patients with dual AVN physiology, Wenckebach cycles are almost always atypical; the greatest increment in the AH interval occurs when block occurs in the fast pathway, whichever beat this may be. Differentiating atypical from typical patterns is of little clinical significance. However, an atypical pattern can be misdiagnosed as type 2 second-degree AV block. Some possible atypical features of Wenckebach periodicity include the following: (1) the second (conducted) PR interval (after the pause) often fails to show the greatest increment, and the increment may actually increase for the PR interval of the last conducted beat in the cycle (Fig. 9-5); (2) very little incremental conduction delay and no discernible change in the duration of the PR intervals for a few beats just before termination of a sequence (this is seen most often during long Wenckebach cycles and in association with increased vagal tone, and is usually accompanied by slowing of the sinus rate; Fig. 9-6); (3) the PR interval can actually shorten and then lengthen in the middle of a Wenckebach sequence; and (4) a junctional escape beat can end the pause following a nonconducted P wave, resulting in an apparent shortening of the PR interval.30,32
AVN block usually can be reversed completely or partially by altering the autonomic tone (e.g., with atropine). However, these measures fail occasionally, especially in the presence of structural damage (congenital heart disease or inferior wall MI) to the AVN. In such cases, progression to complete AV block can occur, although such an event is more likely to occur with block in the HPS.
Site of Block
The degree of PR interval prolongation and QRS duration can help predict the site of block. A normal QRS duration usually suggests AVN involvement, whereas the presence of BBB suggests (but does not prove) HPS involvement. Furthermore, short baseline PR interval and small PR interval increments preceding the block suggest HPS involvement.30,32
Type 2 Second-Degree Atrioventricular Block
Type 2 second-degree (Mobitz type II) AV block is characterized on the surface ECG by a constant (normal or prolonged) PR interval of all conducted P waves, followed by sudden failure of a P wave to be conducted to the ventricles (Fig. 9-9). RP-PR reciprocity, the hallmark of type 1 block, is absent in type 2 block. Consequently, the PR interval following a long RP interval (immediately following the pause) is identical to that following a short RP interval (immediately preceding the pause). Type 2 block cannot be diagnosed if the first P wave after a blocked beat is absent or if the PR interval following the pause is shorter than all the other PR intervals of the conducted P waves, regardless of the number of constant PR intervals before the block (see Fig. 9-6). The P-P intervals remain constant, and the pause encompassing the nonconducted P wave equals twice the P-P interval.
A true Mobitz type II AV block in conjunction with a narrow QRS complex is relatively rare and occurs without sinus slowing and without the characteristic Wenckebach sequences. Atypical forms of Wenckebach block with only minimal PR interval variation should be excluded (see Fig. 9-6). Apparent type 2 second-degree AV block can be observed under the influence of increased vagal tone during sleep, in which case Wenckebach block without discernible or measurable increments in the PR intervals is the actual diagnosis; sinus slowing with AV block essentially excludes Mobitz type II block.30 When an apparent Mobitz type II–like pattern with a narrow QRS complex occurs with intermittent Wenckebach AV block sequences (as in Holter recordings), a true Mobitz type II block can be safely excluded because narrow QRS type 1 and type 2 second-degree AV blocks almost never coexist within the HB. Sustained advanced second-degree AV block is far more common in association with true Mobitz type II block than with Wenckebach block or its variant.
Apparent Mobitz type II AV block can also be caused by concealed junctional extrasystoles (confined to the specialized conduction system and not propagated to the myocardium) and junctional parasystole (Fig. 9-10). Exercise-induced second-degree AV block is most commonly infranodal and rarely is secondary to AVN disease or cardiac ischemia.
Site of Block
His-Purkinje System
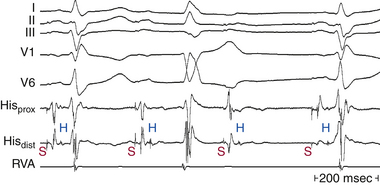
Type 2 second-degree AV block is almost always below the AVN, occurring in the HB in approximately 30% of cases and in the bundle branches in the remainder. Infrequently, type 2 second-degree AV block is found with a narrow QRS complex and is caused by intra-Hisian block (Fig. 9-11; see Fig. 9-9).
2:1 Atrioventricular Block
When only alternate beats are conducted, resulting in a 2:1 ratio, the PR interval is constant for the conducted beats, provided that the atrial rhythm is regular (Fig. 9-12). A 2:1 AV block cannot be classified as type 1 or type 2; using the term type 1 to describe 2:1 AV block when the lesion is in the AVN or when there is evidence of decremental conduction and using the term type 2 to describe 2:1 AV block when it is infranodal or when there is evidence of all-or-none conduction should be discouraged because this practice violates the well-accepted traditional definitions of types 1 and 2 block based on ECG patterns, not on the anatomical site of block. Both types 1 and 2 block can progress to a 2:1 AV block, and a 2:1 AV block can regress to type 1 or 2 block.
Site of Block
1. 2:1 AV block associated with a narrow QRS complex is likely to be intranodal, whereas that associated with a wide QRS complex is likely to be infranodal, but it could still be at the level of the AVN (Fig. 9-13; see Fig. 9-12).
2. Fixed 2:1 AV block with PR intervals shorter than 160 milliseconds suggests intra-Hisian or infra-Hisian block, whereas very long PR intervals (more than 300 milliseconds) suggest AVN block.
3. If the PR interval of all the conducted complexes is constant despite a varying RP interval, infranodal block is likely.
4. The presence of Wenckebach block before or after episodes of 2:1 AV block is highly suggestive of block at the AVN level (see Fig. 9-7).
5. Improvement of block with atropine or exercise suggests AVN block; however, the absence of such response does not exclude intranodal block.30
High-Grade Atrioventricular Block
Failure of conduction of two or more consecutive P waves when AV synchrony is otherwise maintained is sometimes termed high-grade AV block or advanced second-degree AV block (Fig. 9-14).17 This block must happen because of the existing block itself, and not because of retrograde concealment in the AVN or HPS resulting from junctional or ventricular escape complexes that prevent conduction.
Third-Degree (Complete) Atrioventricular Block
AV block is termed complete when all P waves fail to conduct, despite having ample opportunity for conduction. Therefore, if there is less than optimal opportunity for the AVN-HPS to conduct, it cannot be regarded as a failure if it does not conduct. Third-degree AV block is seen on the surface ECG as completely dissociated P waves and QRS complexes, each firing at its own pacemaker rate, with a continuously changing P-R relationship as the P waves march through all phases of the ventricular cycle in the presence of a regular ventricular rhythm (Fig. 9-15). Every possible chance for conduction is afforded, with the P waves occurring at every conceivable RP interval, but the atrial impulse is never conducted to the ventricles. The atrial rate is always faster than the ventricular rate (Fig. 9-16).30,32
Site of Block
Atrioventricular Node
Most cases of congenital third-degree AV block are localized to the AVN (see Fig. 9-15), as is transient AV block associated with acute inferior wall MI, beta blockers, calcium channel blockers, and digitalis toxicity. Complete AVN block is characterized by a junctional escape rhythm with a narrow QRS complex and a rate of 40 to 60 beats/min, which tends to increase with exercise or atropine. However, in 20% to 50% of patients with chronic AV block, a wide QRS escape rhythm may occur. Rhythms originating in the distal HB may have a wide QRS. Those rhythms are usually slower and nonresponsive to atropine.30
Paroxysmal Atrioventricular Block
Clinical Presentation
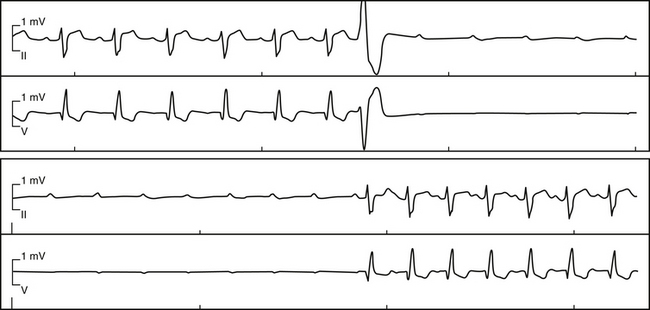
Paroxysmal AV block is characterized by abrupt and sustained AV block in the absence of structural heart disease.32 The block usually starts following a conducted or nonconducted premature atrial complex (PAC) or premature ventricular complex (PVC), and it persists until another PAC or PVC terminates it (Fig. 9-18). Episodes of AV block are commonly associated with prolonged periods of ventricular asystole (of unpredictable duration) precipitating presyncope or syncope or even sudden death. The natural history of paroxysmal AV block is unknown, and it is unclear whether paroxysmal AV block is reversible.33
Mechanism
Paroxysmal AV block is a unique disorder of the HPS and is believed to be caused by local phase 4 block in the HB or in the bundle branches after a critical change in the H-H interval (see Chap. 10). During a long pause (prolonged diastolic period), the fibers of the often-diseased HPS spontaneously depolarize (membrane potential becomes less negative) and become less responsive to subsequent impulses as a result of Na+ channel inactivation. Once such critical diastolic membrane potential is reached, conduction may no longer resume without an appropriately timed escape beat or premature beat (sinus or ectopic) that can reset the transmembrane potential to its maximal resting value. Nonetheless, this explanation is controversial because experimental data indicate that partial membrane depolarization can actually improve conduction, given that the voltage is closer to threshold. Prolongation of the H-H interval can result from spontaneous sinus rate slowing or post-extrasystolic pauses following atrial, ventricular, or His extrasystoles or tachycardia.33
Diagnostic Evaluation
No specific or optimal tests exist to diagnose paroxysmal AV block. Patients with paroxysmal AV block may not have structural heart disease at baseline, and conduction abnormalities may not be evident on resting ECGs. Although no established predictors for paroxysmal AV block exist, evidence of distal conduction disease at baseline is often present, with RBBB the most common finding. A positive response to carotid sinus massage for paroxysmal AV block includes P-P lengthening without changes in preceding PR intervals before heart block (in contrast to a vagally mediated AV block, for which the PR interval lengthens before the block). Additional workup for arrhythmogenic causes including long-term ambulatory monitoring or an implantable loop recorder can also be of value.33,34
The role of EP testing remains uncertain because there is no predictable marker for identifying patients at risk for paroxysmal AV block. EP testing is recommended when suspicion for arrhythmic syncope remains high despite a negative noninvasive workup. Pharmacological provocation (using ajmaline or procainamide) of infranodal block or HV lengthening (via stressing the HPS) was reported to be a potentially useful tool in diagnosing infra-Hisian AV block. However, a positive ajmaline or procainamide response simply suggests infranodal conduction disorder and is not specific for identifying patients at risk of developing paroxysmal AV block over other types of acquired AV block. Paroxysmal AV block may also be reproduced in an EP laboratory via critically timed atrial or ventricular extrastimulus and during rapid ventricular pacing. However, a negative EP study result does not exclude the diagnosis of paroxysmal AV block; at least 10% of patients with baseline BBB and syncope developed paroxysmal AV block during a 3-year follow-up despite a negative EP study result. Furthermore, a normal HV interval cannot rule out a risk for paroxysmal AV block. EP testing is therefore a specific test with low sensitivity, and it should be used in conjunction with supplementary clinical data obtained from personal history and ambulatory and implantable loop recordings.33
Paroxysmal Versus Vagal Atrioventricular Block
Distinction between paroxysmal AV block and the often benign and reversible vagal AV block (whereby an abrupt complete AV block can be precipitated by heightened vagal tone) has important prognostic and therapeutic implications. Unlike vagal AV block, paroxysmal AV block is often initiated by a PAC or PVC or by tachycardia, with sinus acceleration occurring during ventricular asystole without affecting the block (see Fig. 9-18). In contrast, a classic vagal effect on the conduction system includes gradual slowing of the sinus rate and AV conduction (first-degree or Wenckebach block), occasionally followed by sinus arrest or complete AV block (although a more prominent AV response with sudden block can also occur with heightened vagal tone). Subsequently, the sinus rate continues to slow down during ventricular asystole, followed by gradual resumption of AV conduction and sinus acceleration. Additionally, the clinical history, such as during micturition and phlebotomy, among others, can be highly suggestive of heightened vagal tone.33,34
Electrophysiological Testing
Role of Electrophysiological Testing
Nevertheless, EP testing can help diagnose an equivocal ECG pattern or delineate the site of conduction abnormality, if that is required for decision making. EP testing is indicated in a patient with suspected high-grade AV block as the cause of syncope or presyncope when documentation cannot be obtained noninvasively. Similarly, in patients with coronary artery disease, it can be unclear whether symptoms are secondary to AV block or VT; therefore, EP testing can be useful in establishing the diagnosis. Some patients with known second- or third-degree block can benefit from an invasive study to localize the site of AV block to help determine therapy or assess prognosis.30,32
Normal Atrioventricular Conduction
The normal PR interval is 120 to 200 milliseconds. This interval reflects the conduction time from the high RA to the point of ventricular activation (i.e., QRS onset), and it includes activation of the atrium, AVN, HB, bundle branches and fascicles, and terminal Purkinje fibers. To measure the different components of the conduction system contained in the PR interval, intracardiac tracings from the high RA and HB region are required (see Fig. 4-19).
Localization of the Site of Atrioventricular Block
Site of First-Degree Atrioventricular Block
Atrioventricular Node
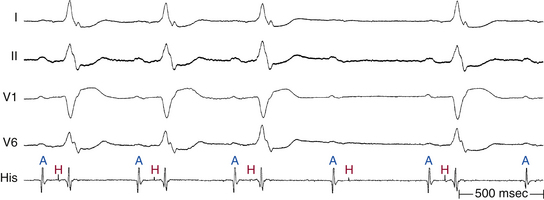
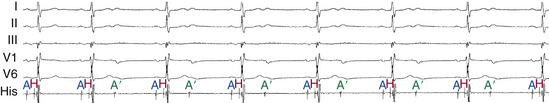
An AH interval longer than 130 milliseconds with a normal HV interval indicates intranodal conduction delay (see Fig. 9-1). Dual AVN physiology can produce transient, abrupt, or alternating first-degree block caused by block in the fast AVN pathway and conduction down the slow pathway. The change in the PR interval seen on the surface ECG corresponds to a jump in the AH interval viewed on the HB electrogram.30
His-Purkinje System
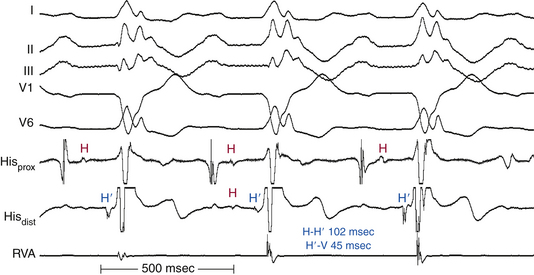
As long as at least one fascicle conducts normally, the HV interval should not exceed 55 milliseconds (or 60 milliseconds in the presence of LBBB). A prolonged HV interval (more than 55 to 60 milliseconds) with or without prolonged His potential duration (more than 30 milliseconds) or a split His potential is diagnostic for HPS disease, even in the presence of a normal PR interval (Fig. 9-19; see Fig. 9-2). A prolonged HV interval is almost always associated with an abnormal QRS because the impairment of intra-Hisian conduction is not homogeneous. Most patients have an HV interval of 60 to 100 milliseconds, and occasionally more than 100 milliseconds. With pure intra-Hisian conduction delay, the atrial-to-proximal His (AH) interval and the distal His-to-ventricular (H′-V) interval are normal, whereas the duration of the His potential is longer than 30 milliseconds, with a notched, fragmented, or split His potential. In this case, verification of the origin of the “split H” from the HB (and not part of the atrial or ventricular electrograms) is critical. This can be achieved by dissociation of the His potential from atrial activation with atrial pacing, adenosine, or vagal stimulation and dissociation of the His potential from ventricular activation by documenting that the HV interval is longer than 30 milliseconds.30
Site of Type 1 Second-Degree Atrioventricular Block
Site of Type 2 Second-Degree Atrioventricular Block (Mobitz Type Ii Block)
His-Purkinje System
The blocked cycle features atrial and HB deflections without ventricular depolarization (Fig. 9-20). The conducted beats usually show evidence of infranodal conduction system disease, with a prolonged HV interval, or even a split His potential, and BBB. Multilevel AV block (intranodal and infranodal) can also occur, especially during rapid atrial tachycardias or atrial pacing. Typically, infranodal AV block occurs in the presence of prolonged HPS refractoriness caused by antiarrhythmic agents or during Wenckebach cycles in the AVN (Fig. 9-21).
Site of Third-Degree (Complete) Atrioventricular Block
Atrioventricular Node
Complete heart block at the AVN level is usually seen on the intracardiac tracings as His potentials consistently preceding each ventricular electrogram. The atrial electrograms are dissociated from the HV complexes (Fig. 9-22). Most often, the escape rhythm originates in the HB (with normal QRS preceded by a His potential and normal HV interval); however, in 20% to 50% of patients with chronic AV block, a wide QRS escape rhythm can occur. Rhythms originating in the distal HB can have a QRS preceded by a retrograde His potential or no His potential at all. Those rhythms are usually slower and nonresponsive to atropine. The stability of the HB rhythm can be assessed by noting the effects of overdrive suppression produced by ventricular pacing (in a manner analogous to testing sinus node function); prolonged pauses (i.e., the lack of HB escapes) herald failure of the escape rhythm.30
Exclusion of Other Phenomena
Nonconducted Premature Atrial Complexes
Early PACs can arrive at the AVN during the absolute refractory period and fail to conduct to the ventricle. This condition can be misdiagnosed as type 1 or type 2 second-degree AV block. Similarly, atrial bigeminy, with failure of conduction of the PACs, can be misinterpreted as 2:1 AV block (Fig. 9-23). In type 2 second-degree AV block, the atrial rhythm is regular, the P-P interval is constant, the nonconducted P wave occurs on time as expected, and P wave morphology is constant. On the other hand, in the setting of nonconducted PACs, the P wave occurs prematurely and usually has a different morphology from that of the baseline atrial rhythm. Nonconducted PACs can often be hidden in the preceding T wave (Fig. 9-24). Additionally, the mere occurrence of PACs in a trigeminal or quadrigeminal pattern can produce a periodicity mimicking Wenckebach periodicity (Fig. 9-25).
Concealed Junctional Ectopy
Ectopic beats arising from the HB that fail to conduct to both the atria and ventricles, with retrograde concealment in the AVN, slowing or blocking conduction of the following sinus P wave, can manifest as type 2 second-degree AV block. Such a phenomenon can be difficult to differentiate from actual block without EP testing. ECG clues to concealed junctional extrasystoles causing such unexpected events include the following: (1) abrupt, unexplained prolongation of the PR interval; (2) the presence of apparent Mobitz type II block in the presence of a normal QRS; (3) the presence of types 1 and 2 AV block in the same tracing; and (4) the presence of manifest junctional extrasystoles elsewhere in the tracing (see Fig. 9-10).
Atrioventricular Dissociation
The distinction between AV dissociation and complete AV block is important. AV dissociation is present when the atria and ventricles depolarize independently of each other. The ventricles are activated by a nonatrial source and are uninfluenced by atrial activity. By definition, there is no retrograde conduction from the ventricles to the atria.17 AV dissociation can occur secondary to complete AV block, atrial bradycardia with a faster independent junctional-ventricular escape rhythm, or increased discharge rate of a subsidiary pacemaker that takes control of the ventricular rhythm.11,35
In complete AV block, the atrial rate is faster than the ventricular rate. For AV block to be diagnosed, the P waves must fail to conduct, given every opportunity for optimal conduction. Thus, failure of conduction of all the P waves, even those occurring at long RP intervals and throughout the phases of the ventricular cycle, has to be documented. Occasionally, the rate of the junctional or ventricular rhythm during AV dissociation is only slightly different from that of the atrial rhythm. In this case, the standard ECG may not provide a recording opportunity long enough to verify failure of conduction, because all the P waves recorded on a single ECG recording may not occur at an appropriate time to allow conduction. Thus, obtaining ECG recording for an adequate length of time is important (Fig. 9-26). Regularity of both atrial and ventricular rhythms with constantly changing P-R relationships, despite the fact that the P wave falls at every conceivable RP interval, and an independent ventricular rate of 40 beats/min or less (faster in congenital complete AV block) are diagnostic of complete AV block. On the other hand, some irregularity of the ventricular rhythm should immediately draw attention to the possibility of intermittent conduction of P waves, which may reflect lesser degrees of AV block or incomplete AV dissociation. Moreover, with complete AV block, the ventricular rate is almost always slower than the atrial rate, whereas in other forms of AV dissociation, the reverse is true.11,35 Therefore, complete AV block with a junctional or ventricular escape rhythm is one form of AV dissociation. However, AV dissociation (complete or incomplete) can occur in the absence of AV block.
In the setting of atrial bradycardia, the atrial rate can become slower than a subsidiary escape focus from the AV junction or ventricle. When the faster junctional or ventricular escape rhythm is associated with VA block, it results in failure of the atrial impulses to conduct anterogradely secondary to retrograde concealment by the escape rhythm impulses (see Fig. 9-16).
An increase in the discharge rate of a subsidiary pacemaker, such as accelerated junctional rhythm, accelerated idioventricular rhythm, or VT, which then exceeds the normal sinus rate, can result in a competing junctional or ventricular rhythm, in which case the atrial rate is always slower than the ventricular rate (see Fig. 9-26).
Atrial Tachyarrhythmias
Failure of the AVN to conduct during fast atrial tachyarrhythmias (atrial tachycardia or flutter) should not be considered pathological AV block. One of the main physiological roles of the AVN is to safeguard the ventricles from rapid atrial rates. Therefore, failure of the AVN to conduct every atrial impulse occurring at a fast rate should be considered a normal physiological finding caused by normal refractoriness. In such situations, terms such as 3:2 and 2:1 AV conduction are more appropriate than 3:2 or 2:1 AV block.
Atrial Fibrillation With Slow Ventricular Rate
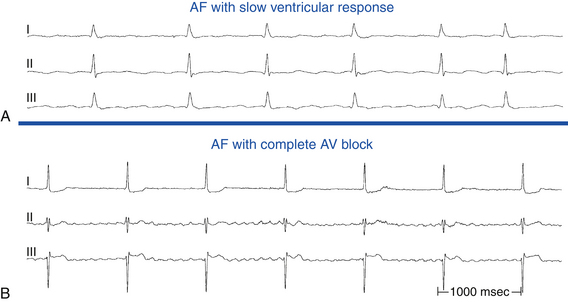
AF with slow ventricular response can be misinterpreted as complete AV block. Verification of the regularity of the slow ventricular rhythm is critical. When AV block is present, the escape rhythm is regular, whereas in AF associated with very slow ventricular response, the ventricular rhythm is a result of conducted atrial beats and is irregular (Fig. 9-27).
Ventriculophasic Sinus Arrhythmia
Ventriculophasic sinus arrhythmia can be observed whenever there is second- or third-degree AV block, and it is manifest as intermittent differences in the P-P intervals based on their relationship with the QRS complex. The two P waves surrounding a QRS complex have a shortened interval or occur at a faster rate when compared with two P waves that occur sequentially without an intervening QRS complex (see Fig. 9-22). The mechanism of this phenomenon is not certain. However, it has been suggested that ventricular contractions enhance sinus node automaticity by increasing the pulsatile blood flow through the sinus nodal artery and by mechanical stretch on the sinus node.
Principles of Management
Pacing is the mainstay of treatment for symptomatic AV block (Table 9-1). Identifying transient or reversible causes for AV conduction disturbances is the first step in management. Withdrawal of any offending drugs, correction of any electrolyte abnormalities, or treatment of any infectious processes or myocardial ischemia should be considered prior to permanent pacing therapy. Pharmacological therapy (atropine, isoproterenol) can be effective in intranodal AV block but only as a short-term emergency measure until pacing can be accomplished. Temporary percutaneous or transvenous pacing is necessary in patients with hemodynamically significant AV block and bradycardia to provide immediate stabilization prior to permanent pacemaker placement or to provide pacemaker support when the block is precipitated by what is presumed to be a transient event, such as ischemia or drug toxicity.36
TABLE 9-1 Indications for Permanent Pacing in Acquired Atrioventricular Block in Adults
Asymptomatic second-degree AV block at intra-Hisian or infra-Hisian levels found at electrophysiological study
First- or second-degree AV block with symptoms similar to those of pacemaker syndrome or hemodynamic compromise
Asymptomatic type II second-degree AV block with a narrow QRS; when type II second-degree AV block occurs with a wide QRS, including isolated right bundle branch block, pacing becomes a class I recommendation
AV block in the setting of drug use and/or drug toxicity when the block is expected to recur even after the drug is withdrawn
Asymptomatic type I second-degree AV block at the AVN level or that which is not known to be intra-Hisian or infra-Hisian
AV block that is expected to resolve and is unlikely to recur (e.g., drug toxicity, Lyme disease, or transient increases in vagal tone, or during hypoxia in sleep apnea syndrome in the absence of symptoms)
AV = atrioventricular; AVN = atrioventricular node.
From Epstein AE, DiMarco JP, Ellenbogen KA, et al: ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices). Developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation 117:e350-408, 2008.
Permanent pacemaker implantation is indicated in most patients with symptomatic advanced heart block, regardless of the site of block. Permanent pacemakers are also indicated in asymptomatic patients with complete heart block or infra-Hisian second-degree AV block, especially those with documented ventricular pauses longer than 3 seconds or a ventricular escape rhythm of less than 40 beats/min.36
In children with congenital heart block, permanent pacing is recommended in those with exercise intolerance, abrupt pauses in the intrinsic rate (longer than two to three times the basic CL), or inappropriately slow average ventricular rate (less than 50 beats/min). Furthermore, prophylactic pacing can considerably reduce the incidence of syncope and sudden death in asymptomatic patients with congenital heart block with severe bradycardia (resting heart rate less than 40 beats/min), ventricular pauses longer than 3 seconds, wide QRS escape rhythm, complex congenital heart disease, ventricular dysfunction, complex ventricular ectopy, or a long QT interval.22,36
Dual-chamber pacing can be beneficial in some patients with marked first-degree AV block (more than 300 milliseconds) and symptoms similar to pacemaker syndrome, as well as in patients with LV dysfunction and heart failure symptoms in whom a shorter AV interval results in hemodynamic improvement, presumably by decreasing LA filling pressure. The latter recommendation, however, is now questionable because a conventional DDD(R) pacemaker with an optimized AV delay would have to pace the ventricle almost 100% of the time. The benefit of pacing with optimized AV synchrony (with a shorter AV delay) should be weighed against the impairment of LV function produced by right ventricular pacing with resultant LV dyssynchrony. However, such a determination can be difficult or impossible.28
Temporary pacing is sometimes required in patients with acute MI (more often in anterior than inferior wall MI). Patients with asymptomatic first-degree or type 1 second-degree AV block do not require pacing. However, patients with type 2 second-degree or complete AV block should be temporarily paced, even if they are asymptomatic. In the setting of MI, the criteria for permanent pacing depends less on the presence of symptoms (Table 9-2). If type 2 second-degree or complete AV block persists once past the peri-infarct period, permanent pacing is indicated. Even if the type 2 or third-degree AV block was transient but associated with BBB that persists following resolution of the AV block, permanent pacing of the post-MI patient improves long-term survival.36
TABLE 9-2 Indications for Permanent Pacing in Atrioventricular Block with Acute Myocardial Infarction
| Class I |
| Persistent second-degree AV block in the His-Purkinje system with bilateral bundle branch block or third-degree AV block within or below the His-Purkinje system after acute MI Transient advanced (second- or third-degree) infranodal AV block and associated bundle branch block; if the site of block is uncertain, an electrophysiology study may be necessary Persistent and symptomatic second- or third-degree AV block |
| Class IIb |
| Persistent second- or third-degree AV block at the AV node level, even in the absence of symptoms |
| Class III |
| Transient AV block in the absence of intraventricular conduction defects Transient AV block in the presence of isolated left anterior fascicular block Acquired left anterior fascicular block in the absence of AV block Persistent first-degree AV block in the presence of bundle branch block that is old or age indeterminate |
AV = atrioventricular; MI = myocardial infarction.
1. Cosio F.G., Anderson R.H., Kuck K.H., et al. Living anatomy of the atrioventricular junctions: a guide to electrophysiologic mapping. A Consensus Statement from the Cardiac Nomenclature Study Group, Working Group of Arrhythmias, European Society of Cardiology, and the Task Force on Cardiac Nomenclature from NASPE. Circulation. 1999;100:e31-e37.
2. Lockwood D., Nakagawa H., Jackman W. Electrophysiological characteristics of atrioventricular nodal reentrant tachycardia: implications for the reentrant circuit. In: Zipes D.P., Jalife J., editors. Cardiac electrophysiology: from cell to bedside. ed 5. Philadelphia: Saunders; 2009:615-646.
3. Valderrabano M. Atypical atrioventricular nodal reentry with eccentric atrial activation: is the right target on the left? Heart Rhythm. 2007;4:433-434.
4. Basso C., Ho S.Y., Thiene G. Anatomical and histopathological characteristics of the conductive tissues of the heart. In: Gussak I., Antzelevitch C., editors. Electrical diseases of the heart: genetics, mechanisms, treatment, prevention. London: Springer; 2008:37-51.
5. Kurian T., Ambrosi C., Hucker W., et al. Anatomy and electrophysiology of the human AV node. Pacing Clin Electrophysiol. 2010;33:754-762.
6. Lee P.C., Chen S.A., Hwang B. Atrioventricular node anatomy and physiology: implications for ablation of atrioventricular nodal reentrant tachycardia. Curr Opin Cardiol. 2009;24:105-112.
7. Anderson R., Ho S. The anatomy of the atrioventricular node. Heart Rhythm Society. 2008. http://www.hrsonline.org/Education/SelfStudy/Articles/Anderson_ho1.cfm.
8. Luc P.J. Common form of atrioventricular nodal reentrant tachycardia: do we really know the circuit we try to ablate? Heart Rhythm. 2007;4:711-712.
9. Mazgalev T. AV nodal physiology. Heart Rhythm Society. 2008. http://www.hrsonline.org/Education/SelfStudy/Articles/mazgalev.cfm.
10. Shryock J., Belardinelli L. Pharmacology of the AV node. Heart Rhythm Society. 2008. http://www.hrsonline.org/Education/SelfStudy/Articles/shrbel.cfm.
11. Schwartzman D. Atrioventricular block and atrioventricular dissociation. In: Zipes D.P., Jalife J., editors. Cardiac electrophysiology: from cell to bedside. ed 4. Philadelphia: Saunders; 2004:485-489.
12. Friedman D.M., Rupel A., Buyon J.P. Epidemiology, etiology, detection, and treatment of autoantibody-associated congenital heart block in neonatal lupus. Curr Rheumatol Rep. 2007;9:101-108.
13. Walsh E.P. Interventional electrophysiology in patients with congenital heart disease. Circulation. 2007;115:3224-3234.
14. Khairy P., Balaji S. Cardiac arrhythmias in congenital heart diseases. Indian Pacing Electrophysiol J. 2009;9:299-317.
15. Smits J.P., Veldkamp M.W., Wilde A.A. Mechanisms of inherited cardiac conduction disease. Europace. 2005;7:122-137.
16. Amin A.S., Asghari-Roodsari A., Tan H.L. Cardiac sodium channelopathies. Pflugers Arch. 2010;460:223-237.
17. Wolbrette D., Naccarelli G. Bradycardias: sinus nodal dysfunction and atrioventricular conduction disturbances. In: Topol E., editor. Textbook of cardiovascular medicine. ed 2. Philadelphia: Lippincott Williams & Wilkins; 2002:1385-1402.
18. Wellens H.J. Atrioventricular nodal and subnodal ventricular disturbances. In: Willerson J., Cohn J., Wellens H.J., Holmes D., editors. Cardiovascular medicine. New York: Springer; 2007:1991-1998.
19. Sovari A.A., Bodine C.K., Farokhi F. Cardiovascular manifestations of myotonic dystrophy-1. Cardiol Rev. 2007;15:191-194.
20. Huynh H., Dalloul G., Ghanbari H., et al. Permanent pacemaker implantation following aortic valve replacement: current prevalence and clinical predictors. Pacing Clin Electrophysiol. 2009;32:1520-1525.
21. Gross G.J., Chiu C.C., Hamilton R.M., et al. Natural history of postoperative heart block in congenital heart disease: implications for pacing intervention. Heart Rhythm. 2006;3:601-604.
22. Villain E. Indications for pacing in patients with congenital heart disease. Pacing Clin Electrophysiol. 2008;31(Suppl 1):S17-S20.
23. Tucker E.M., Pyles L.A., Bass J.L., Moller J.H. Permanent pacemaker for atrioventricular conduction block after operative repair of perimembranous ventricular septal defect. J Am Coll Cardiol. 2007;50:1196-1200.
24. Liberman L., Pass R.H., Hordof A.J., Spotnitz H.M. Late onset of heart block after open heart surgery for congenital heart disease. Pediatr Cardiol. 2008;29:56-59.
25. Padanilam B.J., Morris K.E., Olson J.A., et al. The surface electrocardiogram predicts risk of heart block during right heart catheterization in patients with preexisting left bundle branch block: implications for the definition of complete left bundle branch block. J Cardiovasc Electrophysiol. 2010;21:781-785.
26. Topilski I., Rogowski O., Glick A., et al. Catheter-induced mechanical trauma to fast and slow pathways during radiofrequency ablation of atrioventricular nodal reentry tachycardia: incidence, predictors, and clinical implications. Pacing Clin Electrophysiol. 2007;30:1233-1241.
27. Shirayama T., Hadase M., Sakamoto T., et al. Swallowing syncope: complex mechanisms of the reflex. Intern Med. 2002;41:207-210.
28. Barold S.S., Ilercil A., Leonelli F., Herweg B. First-degree atrioventricular block: clinical manifestations, indications for pacing, pacemaker management and consequences during cardiac resynchronization. J Interv Card Electrophysiol. 2006;17:139-152.
29. Kim M.H., Deeb G.M., Eagle K.A., et al. Complete atrioventricular block after valvular heart surgery and the timing of pacemaker implantation. Am J Cardiol. 2001;87:649-651. A10
30. Josephson M.E. Atrioventricular conduction. In: Josephson M.E., editor. Clinical cardiac electrophysiology. ed 4. Philadelphia: Lippincott Williams & Wilkins; 2008:93-113.
31. Spodick D.H., Ariyarajah V. Interatrial block: the pandemic remains poorly perceived. Pacing Clin Electrophysiol. 2009;32:667-672.
32. Fisch C., Knoebel S. Atrioventricular and ventriculoatrial conduction and blocks, gap, and overdrive suppression. In: Fisch C., Knoebel S., editors. Electrocardiography of clinical arrhythmias. Armonk, NY: Futura; 2000:315-344.
33. Lee S., Wellens H.J., Josephson M.E. Paroxysmal atrioventricular block. Heart Rhythm. 2009;6:1229-1234.
34. Silver E.S., Pass R.H., Hordof A.J., Liberman L. Paroxysmal AV block in children with normal cardiac anatomy as a cause of syncope. Pacing Clin Electrophysiol. 2008;31:322-326.
35. Fisch C., Knoebel S. Atrioventricular conduction abnormalities. In: Fisch C., Knoebel S., editors. Electrocardiography of clinical arrhythmias. Armonk, NY: Futura; 2000:129-149.
36. Epstein A.E., DiMarco J.P., Ellenbogen K.A., et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices). Developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350-e408.