Endovascular Approaches to Arteriovenous Fistula
Etiology
Acquired AVF
These fistulae result from a breach of vascular integrity between an adjacent artery and vein. This defect is usually discrete and identifiable. The inciting event may be penetrating or blunt trauma or erosion of an artery into a vein as the result of an aneurysm or infection. Increasingly, the source of trauma involves invasive medical procedures such as cardiac catheterization, nonvascular surgery, central venous catheterizations, and intra-abdominal organ biopsies [1]. A discrete site of communication is usually present and identifiable, which is critical to planning effective management of these lesions.
Congenital AVF or AVMs
AVMs constitute approximately 15% of all congenital vascular malformations as defined by the Hamburg International Conference on vascular malformations [2]. Most congenital malformations are sporadic, although there are those that are associated with known genetic abnormalities, such as Rendu-Weber-Osler syndrome, an autosomal dominant disorder also known as hereditary hemorrhagic telangiectasia, which results in vascular dysplasia and gastrointestinal hemorrhage and epistaxis [3]. Inherited disorders often affect multiple vascular beds.
Pathophysiology of AVFs
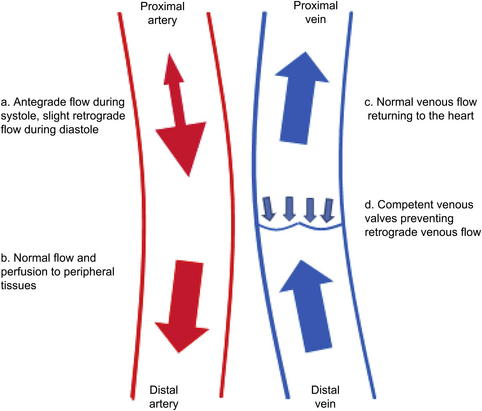
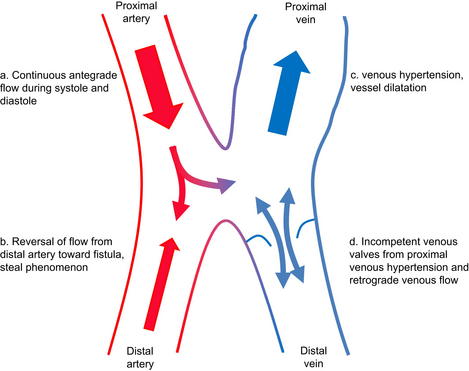
Abnormal patterns of blood flow with shunting of blood from the high-resistance arterial system to the low-resistance venous system, bypassing the capillary beds, is common to all AVF, although it may be less prominent in some extratruncular AVMs. The normal arterial and venous flow, as well as the abnormalities that accompany an AVF, are depicted in Figs. 1 and 2. The symptoms that result from this phenomenon depend on the level at which the fistula exists and the size of the abnormal connection. The more central the fistula and the larger the degree of shunt, the more likely it is to become symptomatic. As a general rule, the multiple small communications associated with congenital AVMs lead to a more indolent clinical course than is associated with large acquired AVF. However, inexorable progression, albeit at varying rates, is the clinical pattern in all of these conditions, which has important implications for therapy. As a rule, arteriovenous shunting is greatest through acquired AVFs and truncular AVMs, and least through extratruncular AVMs.
Increased flow through the arterial system associated with reduced resistance in the venous system leads to loss of the normal reversal of arterial flow during diastole with continuous antegrade flow via the proximal artery into the AVF (see Fig. 2a). There is also an increase of blood flow in the artery proximal to the AVF. These hemodynamic changes lead to increased shear stress on the arterial wall. To accommodate that shear stress, the proximal artery dilates. In extreme cases, this dilation may become significant and the artery may become attenuated and/or aneurysmal. Initially blood flow is maintained in the distal arteries but, as the fistulous connections enlarge, peripheral arterial perfusion decreases and in some cases may be reversed. This process can lead to peripheral arterial ischemia, known as steal (see Fig. 2b) [4].
Blood flow also increases in the central vein. The shear stress of the pulsatile arterial blood flow through the vein leads to thickening, or arterialization, of the vessel wall, as well as dilatation and elongation. Moreover, with increased venous volume and pressure in the proximal vein, distal venous blood flow slows, resulting in valvular incompetence, reversal of venous blood flow, and venous hypertension. (see Fig. 2c, d) [5].
Systemic effects on the circulation become manifest as the fistula enlarges. There is an overall increase in the volume of blood in the venous system caused by the large reserve venous capacity. This increase results in increased return of blood to the heart, a decrease in peripheral blood pressure, and a subsequent increase in cardiac output by increasing both the heart rate and the stroke volume [6]. Because there is increased venous blood volume and a subsequent reduction of arterial blood volume, the renin-angiotensin-aldosterone system is activated, resulting in a further increase in blood volume via retention of sodium and water [4]. If the heart is unable to compensate for the increase of blood volume, high-output heart failure may result.
Signs and symptoms of AVFs
The signs and symptoms from AVFs are the result of increased shunting, venous hypertension, arterial ischemia, and compression of or impingement on adjacent structures. The severity of the signs and symptoms are related to the location and the size of the abnormality. Peripherally located fistulae more often result in locally recognized effects, whereas centrally located fistulae are linked to systemic symptoms. The signs and symptoms from AVF are listed in Box 1.
The most common presenting symptom of an AVF is a palpable thrill or audible bruit. There may be warmth of the skin overlying the fistula as well as a palpable mass [5]. As the vessels dilate and the size of the fistula increases, an increased amount of blood is shunted directly from the arterial system into the venous system. If a significant amount of blood is shunted away from the peripheral tissues, arterial insufficiency distal to the fistula may occur. This insufficiency can result in arterial insufficiency of varying degrees, from intermittent claudication, rest pain, tissue loss, and even gangrene. In addition to the complications of decreased distal arterial blood flow, there are complications of increased venous pressure. Extremities can develop evidence of chronic venous hypertension and insufficiency as seen by peripheral edema, skin hyperpigmentation, venous varicosities, and even venous ulceration [4].
A patient with a high-flow central AVF may initially present with signs and symptoms such as dyspnea on exertion, fatigue, and peripheral edema, which all indicate high-output cardiac failure. Brewster and colleagues [6] found symptoms of congestive heart failure in one-third of patients with aortocaval or iliocaval fistula. Renal insufficiency, hematuria, and peripheral edema are also common in this clinical scenario [1,6]. Patients may also present with lower extremity edema, venous hypertension, and pulsating varicose veins [1].
Diagnosis of AVFs
Physical examination
A thorough cardiac examination is critical to identify the presence of systemic signs or high-output heart failure. Distended neck veins, peripheral edema, presence of gallop, and detection of a lateral point of maximal impulse all suggest underlying cardiac disorders, possible cardiomegaly, and heart failure. Tachycardia is often the first sign of increased cardiac output. Compression of large fistulae with complete obliteration of blood flow results in subsequent reflex bradycardia, which is called the Branham-Nicoladoni sign [4].
Extremities should be carefully examined, including inspection, auscultation, and palpation. Inspection may reveal hyperpigmentation of the distal extremity. Comparison and measurement of the affected extremity with its normal counterpart may identify limb discrepancy and peripheral edema. A bruit may be auscultated, which is described as a continuous, machinerylike murmur. This indicates turbulent flow from the arterial segment into the venous segment. Palpation of the lesion reveals the accompanying thrill. A thrill palpated only during systole suggests a proximal arterial stenosis, allowing the thrill only to be detected at peak arterial flow [7].
Proximal and distal pulses should be evaluated along with the examination of the fistula. A pulse is not usually felt in the fistula. If a pulse is present, this may be a sign of a stenosis at the fistula or central vein. This distal pulse should be evaluated before and after compression of the fistula. Distal pulse augmentation after compression of the fistula indicates a significant portion of arterial inflow diverting into the vein [7].
A search for skin lesions is important to rule out some of the more complicated vascular malformations such as Sturge-Weber Syndrome, Klippel-Trenaunay Syndrome, or Rendu-Weber-Osler Syndrome. Birthmarks, including port-wine stains and cavernous hemangiomas, are noted in one-half of patients with congenital AVMs [8]. Skin temperature is often increased overlying the malformation as a result of the increased blood flow in that area. An associated mass may be apparent with AVMs, but is rarely seen with an AVF. This mass may continue to grow with time, secondary to elongation and expansion of the vessels, resulting in compressive symptoms on surrounding structures [3]. The mass is typically firm, spongy, and can be either completely nontender or exquisitely painful with prominent and pulsatile draining veins.
Imaging
Duplex ultrasonography
After physical examination, duplex ultrasonography is a useful tool in the diagnosis of arteriovenous abnormalities, when the lesion is amenable to duplex interrogation. It is used to identify involved vessels as well as to evaluate and characterize blood flow by velocity spectral analysis. Continuous antegrade flow can be seen in the proximal artery during both systole and diastole instead of the normal pattern of arterial flow, which includes slight retrograde flow during diastole. Arterial pulsations and venous turbulence can also be seen in the venous segment proximal to the fistula. Compression of the fistula should result in increased flow in the distal artery [4].
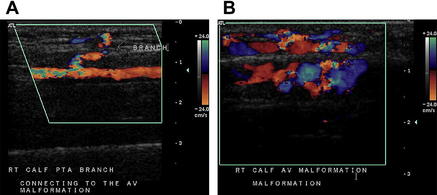
There are major and minor diagnostic criteria for evaluating and diagnosing AVF by color Doppler ultrasonography. Major criteria include a junction of low-resistance and high-resistance flow in the arterial inflow, a high-velocity arterial waveform in the venous outflow, and a turbulent, high-velocity flow at the level of the fistula. Minor diagnostic criteria include visualizing a direct communication between the artery and the vein, an enlarged diameter of the arterial inflow vessel, focal venous dilatation, and a focal perivascular color artifact [9]. Fig. 3 depicts the direct communication between an artery and vein seen on ultrasound. Adjuncts to duplex ultrasonography include segmental Doppler examination and pulse-volume recording (PVR), which are useful in identifying decreased arterial blood perfusion distal to the fistula.
Benefits of Duplex ultrasonography include its noninvasive nature, lack of exposure to contrast or radiation, and easy repeatability, making it ideal for follow-up and surveillance of lesions [10]. The study provides information on the flow patterns and velocities in the vessels involved in the abnormality. However, ultrasonography is highly operator dependent and may be time consuming when the area of interest is large. It is of limited value for lesions in the cranium, chest, or abdomen and must be supplemented by other modalities in those circumstances. Turbulent flow in high-output fistulae may obscure some anatomic detail. The study may also be limited by other factors such as surrounding hematoma, bleeding from the fistula, and patient habitus. Duplex ultrasound is most useful in acquired fistulae of the extremities.
Magnetic resonance imaging and angiography
The imaging modalities of magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) are the studies of choice for the assessment of congenital AVMs. These tests are noninvasive and provide a great amount of anatomic detail needed for planning a surgical intervention. They allow the surgeon to accurately define the anatomy of the lesion, including the extent of the lesion, the vessels involved, and the relationship to the surrounding organs, nerves, tendons, and muscles [11].
There are several advantages of MRI/MRA. MRA images can be reconstructed to give a three-dimensional image for better anatomic detail. There is no exposure to radiation, and it is easily repeatable for use in follow-up. The T2-weighted sequence can accurately distinguish low-flow (enhancing) from high-flow (flow-void) malformations [12]. However, it is a time-consuming test, sometimes lasting 2 hours or more for acquisition of all necessary images. In addition, use of the gadolinium contrast agent has been associated with nephrogenic systemic fibrosis, a severely debilitating disease, more commonly occurring in patients with baseline renal insufficiency. Time-of-flight techniques allow use of the noninvasive MRA imaging modality without the risk of the contrast load. Dynamic MRI can be extremely useful in determining both the quantity and direction of blood flow.
Computed tomography
Advantages of CT include the noninvasive nature of the imaging study as well as the short time it takes to complete the study, even when imaging multiple areas of the body in various phases of contrast injection. However, CT requires exposure to ionizing radiation, and repeated studies result in significant contrast load and radiation exposure. Overall, images are accurate and generally independent of the operator. However, results are highly dependent on imaging protocols used and timing of contrast administration [13]. Both the contrast load and radiation exposure of CTA need to be considered in patients who may require repeated exposure to both agents.
Arteriography
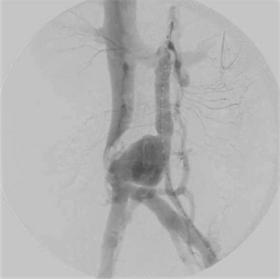
Digital subtraction angiography (DSA) is usually not used in the initial diagnosis of AVF, whereas either duplex ultrasound or MRI is preferred. DSA is best used after noninvasive imaging such as duplex ultrasonography or MRI, when the lesion has been evaluated and endovascular treatment is a viable option. Angiography allows identification of the feeding arteries to the fistulae as well as the draining veins for creating a roadmap of the vascular abnormality for planning and initiating endovascular therapy [1] such as embolization and sclerotherapy. Fig. 4 illustrates the use of arteriography in the diagnosis of an aortocaval fistula that was ultimately repaired in an open fashion.
Management of AVFs: the role of endovascular therapy
The decision of whether and how to treat congenital AVMs is more complex. Because most of these lesions are progressive, treatment of some sort will eventually be required. However, unlike acquired AVFs, vascular connections are often multiple, treatment is usually more complex, and recurrence is common. As a consequence, multimodality treatment is usually required and, in many cases of extratruncular AVM, palliation rather than cure is the most realistic goal. The indications for treatment of congenital AVFs are listed in Box 2. Early diagnosis and treatment greatly improve the outcomes of patients with AVFs. Recommendations for treatment of specific clinical scenarios emphasizing the role of endovascular intervention are discussed later.
Acquired AVF
Endovascular techniques are particularly useful for patients who are high-risk open surgical candidates because of comorbidities or difficult lesion access. Examples include patients at poor risk with lesions of the major proximal vessels, those with high carotid or intracranial vascular lesions, and those with intraparenchymal fistula. Patients with aortocaval or iliocaval fistulae may also be candidates for an endovascular first approach because of the high morbidity, mortality, and blood loss associated with open repair, even when arterial control is obtained [6].
The 2 mainstays of endovascular treatment of AVFs include placement of covered stents and transcatheter embolization. The prerequisite for endovascular repair of an AVF is that the inflow and outflow vessels be accessible for catheter placement of a covered stent or injection of embolization material [5].
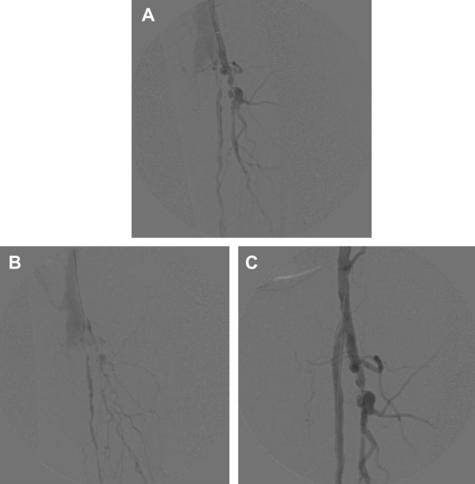
The preferred approach, when feasible, is placement of a covered stent to exclude the connection between the artery and vein. This approach is most successful in traumatic AVF of large vessels, for which the stent graft can be expected to completely obliterate the connection, resolve the problem, and maintain vascular continuity (see Fig. 4A–C). Although this approach may also be used in the treatment of entities such as aneurysmal aortocaval fistula, the artery itself is dilated in these cases and an arterial stent graft may not completely occlude the fistulous cavity. In such cases, transvenous coil embolization of a residual cavity and/or placement of a covered stent in the venous system may be required [14,15]. One must remember the potential for central venous embolization whenever any material is placed to occlude the fistula, and suitable measures must be undertaken to prevent this (eg, venous stent, inferior vena cava filter) before or at the time of embolization. Fig. 5 illustrates an example of successful treatment of an acquired AVF with a covered stent.
When covered stents cannot be deployed because of unsuitable anatomy or small vessels that must be preserved, placement of an uncovered stent to act as a scaffold for coil embolization of the venous outflow is an appropriate alternative. Good examples of such circumstances include the treatment of carotid cavernous fistula or fistula between the main hepatic artery and portal vein or a major branch of the renal artery and vein. In such cases, an uncovered stent is placed first, and coils are then placed through the stent interstices into the fistulous cavity to obliterate it [14]. When embolization is the chosen approach, control of arterial inflow, and often venous outflow, is critical to avoid central embolization of coils or other particulate matter.
When using open or endovascular techniques in the treatment of an AVF, it is critical that the connection between the artery and the vein be obliterated. This principle specifically interdicts proximal placement of ligatures or occluding materials/devices that allow persistence of the fistula from collateral flow. Failure to adhere to this principle not only leads to recurrence of the condition but inhibits future efforts at endoluminal repair because vascular access to the communication has been compromised. It may be necessary to reduce flow in high-output fistulae by temporary occlusion of the arterial inflow and/or the venous outflow so that the precise origin of the fistulae can be identified and the precise endovascular occlusion can be accomplished. The principles of endovascular therapy for acquired AVF are presented in Box 3.
AVMs
The treatment of congenital AVF, or AVMs, is more complex than the treatment of acquired lesions. These lesions have multiple connections between the arterial and venous system that are often difficult to define and to access. Treatment usually requires multiple interventions and recurrence or persistence of the abnormality is common. The mesenchymal cells of extratruncular lesions are stimulated by hypoxia and incomplete treatment may cause them to proliferate. Multimodality therapy is required and surgical removal is never the first line of therapy. Because treatment is complex and recurrence or failure of complete obliteration is common, decision to treat congenital AVF must be individualized. General indications for treatment are included in Box 2. Because the cause and natural history of truncular and extratruncular lesions are different, their therapy is discussed separately.
Truncular lesions
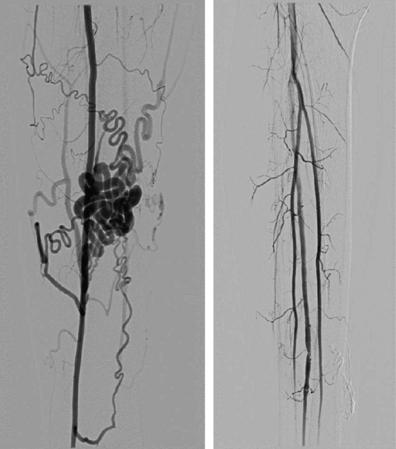
As stated earlier, truncular lesions result from arrested embryologic development after the stage of arterial and venous differentiation. Although there are multiple arteriovenous connections that may be hard to define and obliterate, the lesions themselves are static rather than dynamic. They enlarge by changes in the existing vessels in response to increased blood flow (see earlier) rather than by development of new vessels. These lesions are often extensive and may abut major structures including solid organs and nerves, but they are not infiltrative. Endovascular therapy is the first line of treatment of these lesions and is directed at identifying and obliterating as many arteriovenous connections as possible. This result is accomplished by the use of microcatheters to access the connections as close as possible to their origin and apply a combination of occluding coils, sclerosing agents such as ethanol, polidocanol, ethanolamine oleate, polyvinyl foam powder, and superabsorbent polymer microspheres [16], and glues such as cyanoacrylate or Onyx. A detailed discussion of the indications for using each of these agents can be found in several of the references appended [17]. Fig. 6 shows the arteriogram of a young patient with a congenital AVM before and after treatment with particulate embolization and intravascular occlusion with Onyx.
Extratruncular lesions
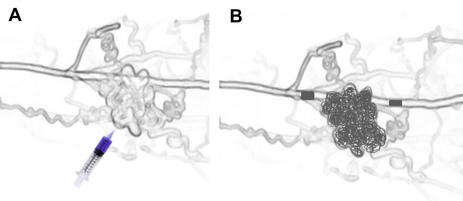
Ligation or embolization of feeding arteries and veins should be avoided as sole or initial therapy because it often stimulates further growth of the lesion by inducing hypoxia. The nidus of the malformation should be the first target of therapy (Fig. 7) and can be accessed through the arterial or venous system, or by direct puncture. This nidus is filled with the sclerosants or glues mentioned earlier, in an attempt to destroy as much of the nidus as possible. It is important to limit the sclerosants and glues to the nidus and immediate draining veins and to avoid spillover into the systemic circulation. In high-flow lesions, this may require a combination of inflow and outflow occlusion using temporary balloon occlusion while the sclerosant or glue is being delivered. Whenever possible, permanent occlusion of feeding vessel should only be undertaken after the nidus has been occluded, because this will limit future access to the lesion.
The principles of endovascular treatment of congenital AVF are presented in Box 4.