Aortic stenosis
Clinical features
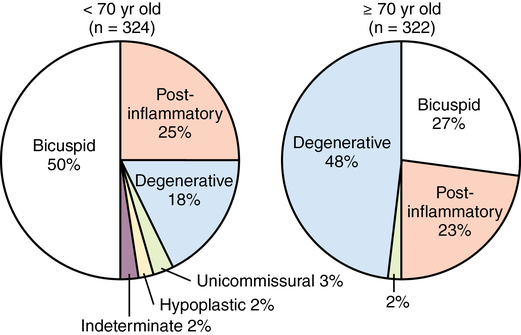
Eighty percent of patients with symptomatic AS are men, approximately 50% will have coronary artery disease, and most of these patients will be at least 70 years old (AS due to a bicuspid valve tends to occur in patients younger than 70 years of age [Figure 149-1]). Overall, however, AS is a disease of the elderly, with a prevalence of more than 4% in North American adults older than 75 years. In a study of 5201 men and women over the age of 65 years, 26% had aortic sclerosis (a thickening of the valve without hemodynamic sequelae), and 2% had AS. The prevalence of aortic sclerosis increased with age in this study: 20% in patients aged 65 to 75 years, 35% in those aged 75 to 85 years, and 48% in patients older than 85 years (AS rates were 1.3%, 2.4%, and 4%, respectively, in these groups).
Natural history
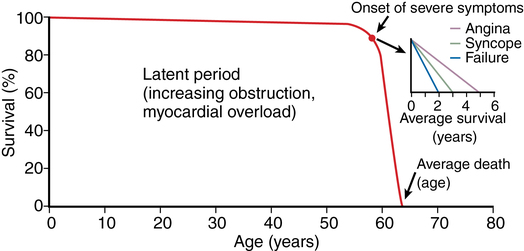
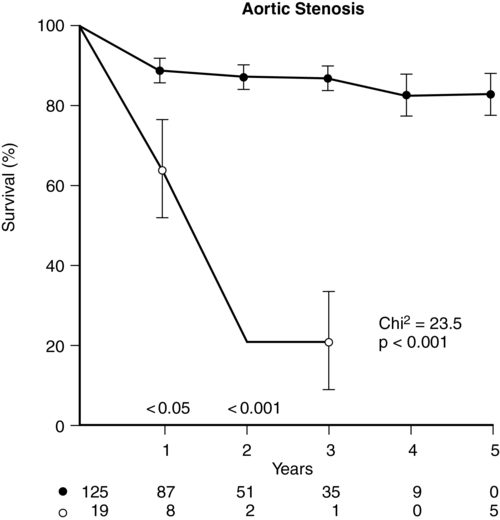
Patients with AS are at increased risk of dying suddenly (likely from cardiac arrhythmia due to ischemia from mismatching of O2 supply and demand), yet the typical natural history of AS is a gradual onset of symptoms manifesting in the fifth to seventh decades of life. Aortic sclerosis is not an uncommon finding in patients older than 65 years, but about 16% of patients with sclerosis develop AS within 7 years. Patients with aortic sclerosis are asymptomatic, but once the pressure gradient across the valve exceeds the upper limits of normal, exertional dyspnea, angina, and syncope–the cardinal symptoms of AS–can appear within 5 years. The mortality rate is approximately 25% per year among symptomatic patients (Figure 149-2), with three quarters of those whose AS is untreated dying within 3 years of the onset of symptoms (Figure 149-3). Asymptomatic patients, on the other hand, even those with severe disease, have a more favorable outlook (risk of death <1% per year).
Anatomic considerations
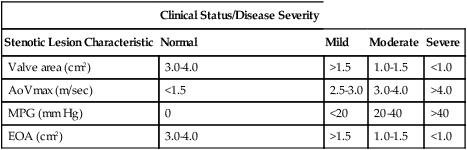
The internal cross-sectional area of a normal aortic valve during systole is 3.0 to 4.0 cm2; significant hemodynamic obstruction does not occur until the valve area is less than 1.5 cm2. Based upon measurements of valve area, peak blood flow velocity across the valve (AoVmax), mean pressure gradient, and effective orifice area, the degree of AS is categorized as mild, moderate, severe, or critical and is most commonly assessed with echocardiography (Table 149-1). The measurement of pressure gradients is accurate less than 50% of the time because the pressure gradients are flow dependent. Measuring the valve area is the most reliable method of assessing severity of AS because it depends less on ventricular contractility than do pressure gradients, but measuring valve area using two-dimensional echocardiography has several factors that may limit its usefulness, including the difficulty in obtaining the correct short-axis view, the presence of calcifications that create shadowing on the image, and the inability with a “pinhole” valve to identify the orifice during systole. Therefore, the effective valve area or the effective orifice area is calculated using the following continuity equation:
Table 149-1
Aortic Stenosis: Measurements and Severity of Disease
| Clinical Status/Disease Severity | ||||
| Stenotic Lesion Characteristic | Normal | Mild | Moderate | Severe |
| Valve area (cm2) | 3.0-4.0 | >1.5 | 1.0-1.5 | <1.0 |
| AoVmax (m/sec) | <1.5 | 2.5-3.0 | 3.0-4.0 | >4.0 |
| MPG (mm Hg) | 0 | <20 | 20-40 | >40 |
| EOA (cm2) | 3.0-4.0 | >1.5 | 1.0-1.5 | <1.0 |
< ?xml:namespace prefix = "mml" />
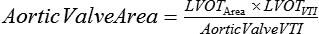
Anesthetic considerations for aortic valve replacement
In addition to routine American Society of Anesthesiologists recommended monitors, arterial and pulmonary artery catheters (the latter often inserted more for postoperative care than intraoperative care) and a transesophageal echocardiographic probe are used. Echocardiography permits assessment of preload, left ventricular function, valve gradients, and prosthetic valve function and provides real-time information to the surgical team. Arrhythmias and hypotension should be treated aggressively. Anesthetic goals are summarized in Box 149-1.
Anesthesia for patients with aortic stenosis undergoing noncardiac operations
Patients with AS who undergo noncardiac operations are also at increased risk of developing perioperative myocardial infarction, congestive heart failure, and arrhythmia. An adequate history for symptoms should be obtained and appropriate diagnostic testing should be performed before patients with AS undergo elective operations. Anesthetic goals for noncardiac operations are similar to those for aortic valve replacement (see Table 149-1). Given the potential for deleterious effects of a reduced systemic vascular resistance and reduced coronary perfusion, the use of spinal or epidural anesthesia is relatively contraindicated.