Antigen-Presenting Cells
Learning Objectives
• Differentiate between professional and amateur antigen-presenting cells
• Compare and contrast monocytes and macrophages
• Recognize dendritic cell subpopulations
• Explain the roles of plasmacytoid dendritic cells in innate immunity and adaptive immunity
• Recognize the differences between a phagosome and an endosome
• Explain the roles of the invariant chain protein in antigen presentation
• Define class II-associated invariant chain peptide (CLIP)
• Recognize the biological roles of HLA-DM and HLA-DO in antigen presentation
• Identify the differences between phagocytosis and receptor-mediated endocytosis
• Discuss the concept of dendritic cell cross-priming and its usefulness in developing cancer vaccines
Key Terms
Class II-associated invariant chain peptide (CLIP)
Cross-priming
Dendritic cell
Endosome
Follicular dendritic cell
Iccosomes
Interstitial dendritic cell
Invariant chain
Langerhans cell
Macrophage
Monocyte
Phagosome
Plasmacytoid dendritic cell
Receptor-mediated endocytosis
Introduction
The previous chapter discussed molecules that present antigens to T cells. Prior to loading class I and II molecules with antigens, large-molecular-weight proteins must be degraded to a length of 8 to 30 amino acids. This chapter discusses the cells involved in antigen recognition and the processing of exogenous antigens. The processing of endogenous antigens is discussed later in Chapter 18.
To generate the small peptides, inhaled, ingested, or injected antigens are internalized by specialized antigen-presenting cells (APCs) and processed in an endocytic pathway.
APCs can be divided into “professional” and “amateur” subsets. Professional APCs such as monocytes, macrophages, dendritic cells, and B cells are fully committed to antigen presentation as an integral part of their function in the generation of the immune response. Other cells such as endothelial cells, fibroblasts, glial cells, pancreatic β- cells, keratinocytes, and thyroid cells present antigens only under select conditions. These cells are considered “amateur” APCs.
Professional Antigen-Presenting Cells
Monocytes and Macrophages
Monocytes and macrophages are responsible for the digestion of foreign material and for the presentation of antigenic epitopes to immunocompetent cells. Monocytes circulate in the blood, whereas macrophages are found in most tissues, for example, the brain (microglia), bone (osteoclasts), and connective tissue (histiocytes). Kupffer cells in the liver are the largest concentration of macrophages in the body.
Macrophages participate in both innate and adaptive immune responses. As part of innate immunity, macrophages are activated by antigens that are bound to PAMP, Toll-like, scavenger, and mannose receptors. In an adaptive response, antigens are recognized using receptors for intermediary molecules, such as antibody and complement fragments.
Dendritic Cells
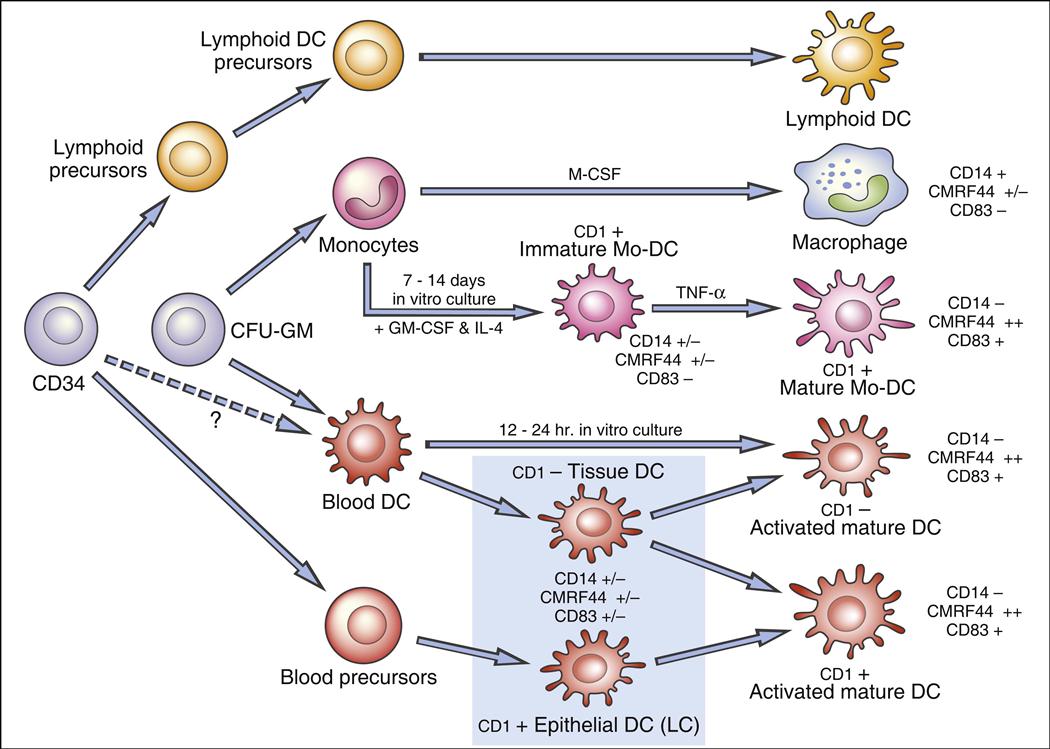
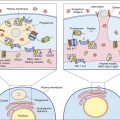
In 1973, Steinman described a rare peripheral blood cell with membrane dendrites similar to a neuron. Other studies demonstrated that the dendritic cell (DC) is a “professional” antigen-producing cell present in lymphoid and nonlymphoid tissues. Dendritic cell precursors arise in bone marrow from committed CD34+ stem cells; differentiate into immature myeloid, monocytic, and lymphoid DCs; and are seeded into blood. Because of heterogeneity in cell surface markers, populations of DCs are difficult to identify. However, a putative hematopoietic differentiation pathway for DCs is shown in Figure 5-1.
Several DC subsets localize in tissues or circulate in peripheral blood. Two populations of dendritic cells are derived from a myeloid stem cell: (1) One population migrates to the lymph node where it becomes follicular dendritic cells. (2) A second population is found in nonlymphoid tissue and is referred to as interstitial dendritic cells. Lymphoid or plasmacytoid dendritic cells localize in the T cell compartment within the lymph node. Monocyte precursors may remain in peripheral blood as monocytic dendritic cells, although this concept is still being debated.
Dendritic cells are uniquely suited for antigen presentation. High numbers are concentrated at likely sites of microbial entry into the body (e.g., intestine and respiratory tract). Most DCs can efficiently capture and process antigen for presentation to T cells. Moreover, they are mobile and can migrate through tissue or stimulate immune responses in the regional lymph nodes.
Follicular Dendritic Cells
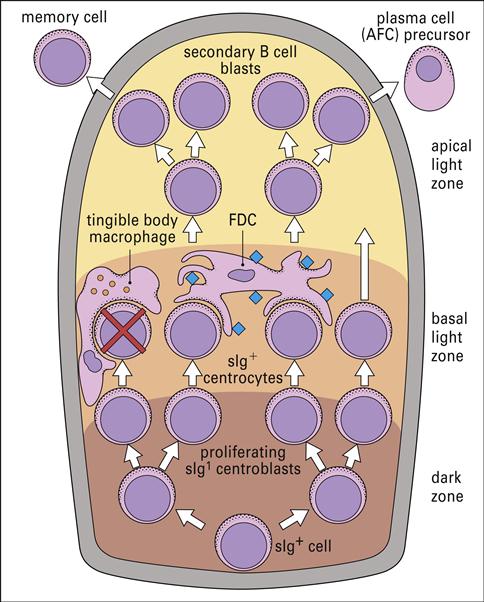
Follicular dendritic cells (FDCs) are found in the lymph node germinal follicles (Figure 5-2) and have several different functions, including activation of B cells and maintenance of immunologic memory.
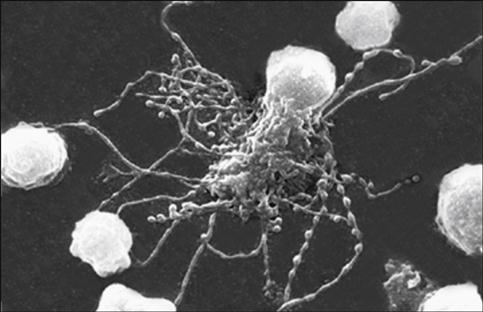
Unlike other DCs, FDCs do not process antigen for presentation to T cells. Rather, they stimulate a CD4Th2 response and maintain memory by a unique mechanism. Using a repertoire of receptors, FDCs bind processed and unprocessed antigens onto beaded, three-dimensional structures called iccosomes (Figure 5-3).
Antigens may be retained in iccosomes for months and perhaps years. To stimulate antibody production, an antigen is slowly released, processed by B cells, and presented to T cells in the context of class II molecules. Cytokines produced from FDCs also contribute to the activation and differentiation of B cells into plasma cells. As a consequence of the continual stimulation of B cells, antibodies and memory cells are continually produced.
Interstitial Dendritic Cells
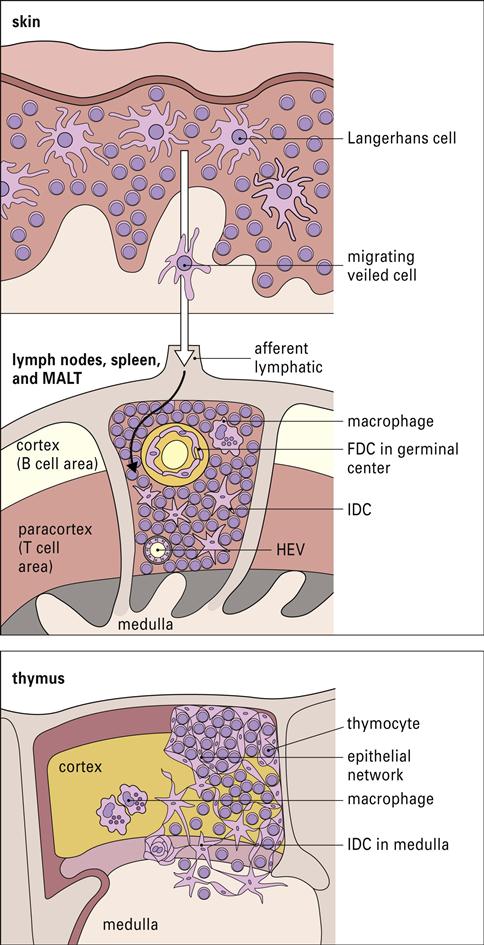
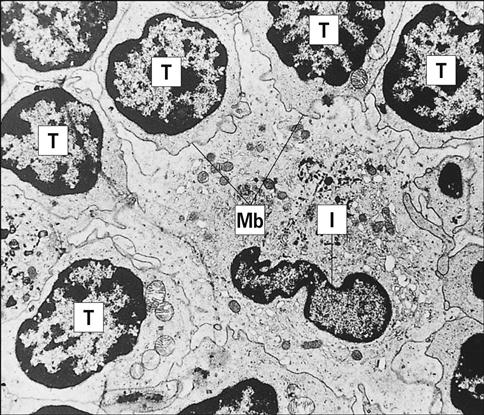
Interstitial dendritic cells are found in most tissue, with the exception of the brain and the eye. These cells serve as a sentinel for detecting foreign or antigenic molecules. The largest reservoir of IDCs resides in the skin, where resident Langerhans cells (LC) occupy 25% of the skin surface area, but only 2% to 3% of skin cells. Resting or immature LCs reside in the suprabasilar epidermis and are identified by the presence of cytoplasmic Birbeck granules and by the expression of CD1, class I, class II molecules, and abundant receptors for pathogen-associated microbial patterns (PAMPs) and mannose. When antigens are captured, the immature LCs disengage from the epithelium and migrate to the paracortex or T cell zones of the regional lymph nodes. During migration, LCs undergo a transformation into “veiled cells.” After entering the lymph node, the “veiled cells” assume the role of resident interdigitating dendritic cells that present antigen to naïve CD4 cells (Figure 5-4).
Resident lymph node IDCs can also process soluble antigens entering the lymph node via the lymph fluid. Lymph-borne soluble antigens percolate through the node in a manner that ensures contact with IDCs. Following internalization and processing, antigen is presented to naïve and effector CD4 T cells, which have close contact with the IDCs (Figure 5-5).
Plasmacytoid Dendritic Cells
Plasmacytoid dendritic cells (pDCs) resemble antibody-secreting plasma cells and are believed to arise from a lymphoid progenitor. pDCs are found in blood and in lymphoid tissues such as lymph nodes, tonsils, spleen, thymus, and Peyer’s patches. Activated pDCs link innate immunity and adaptive immunity to viruses. In the innate response, Toll-like receptors 7 and 9 bind viral deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) from herpes simplex virus (HSV), Sendai virus, human immunodeficiency virus type 1 (HIV-1), and influenza virus. Receptor activation of pDCs initiates synthesis of α-interferon and β-interferon. Interferon prevents the spread of the virus to uninfected cells and also activates natural killer cells. In the adaptive response, pDCs process and present antigens to T cells. Cytokines produced by pDCs also induce the expansion of antigen-specific CD8 memory cells and unique CD4Th1 cells responding to endogenous antigens.
B Cells
Under certain circumstances, B cells are able to present antigens to T cells. Antigens bind to an antigen-specific B cell receptor (BCR) which is a modified antibody. Cross-linking of BCRs internalizes antigens, which are degraded in the cytoplasm and presented to T cells in context with class II markers. B cells present antigens at 100 to 10,000 times lower concentration than that required for macrophage presentation.
Presentation of Exogenous Antigens
Exogenous antigens are degraded in the endocytic pathway and loaded onto class II molecules. The mechanism for class II molecule delivery differs with macrophages and B cells. Macrophages and dendritic cells bind antigens using a number of different receptors for PAMPs, complement fragments, or antibodies. Opsonized bacteria and antigens are ingested by a process called phagocytosis. During phagocytosis, a re-arrangement of cytoskeleton generates long membrane evaginations called pseudopodia, which surround and engulf membrane-bound material to form an internal vacuole.
B cells use receptor-mediated endocytosis to ingest foreign material. B cell receptors are localized in areas containing membrane clathrin. Receptor binding activates the clathrin and facilitates an inward folding of the cell membrane to form a vesicle. After recycling the clathrin molecules and the B cell receptors to the cell surface, the vesicle becomes a membrane-bound vacuole (Figure 5-6).
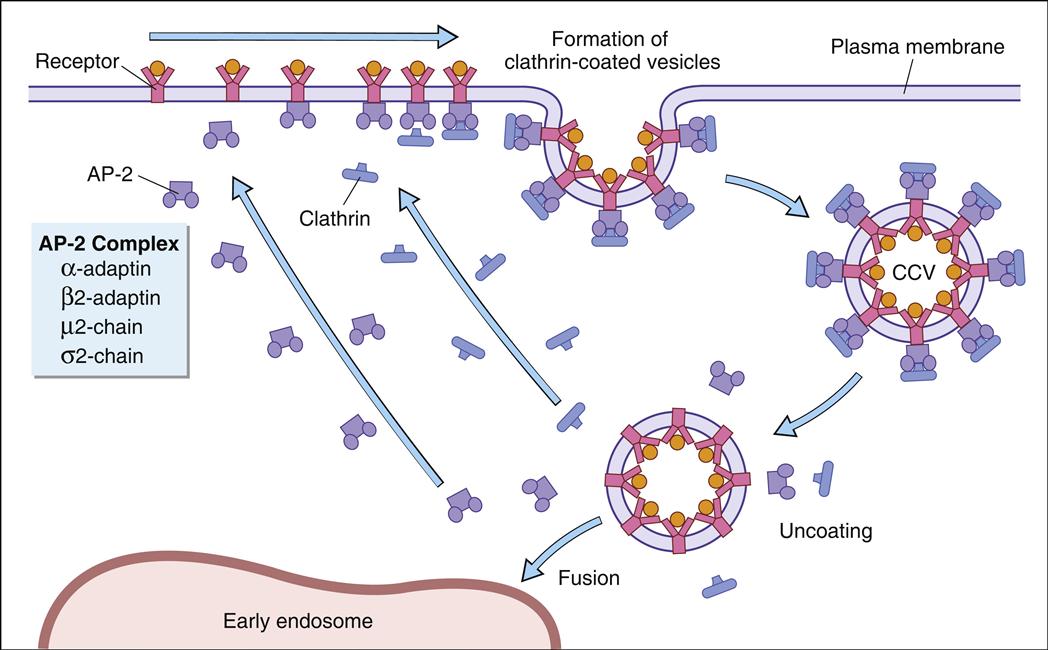
Different terms are used to describe the vacuoles in macrophages and B cells. In monocytes and macrophages, the vacuole is called a phagosome. Cytoplasmic lysosomal vesicles containing hydrolytic enzymes fuse with the phagosome membrane and empty their contents into the phagosome, which is now termed a phagolysosome. In B cells, endocytosis forms a vacuole called early endosome. In the endocytic pathway, early endosomes travel through a series of tubes and vesicles from the periphery to deep inside the cell (late endosome).
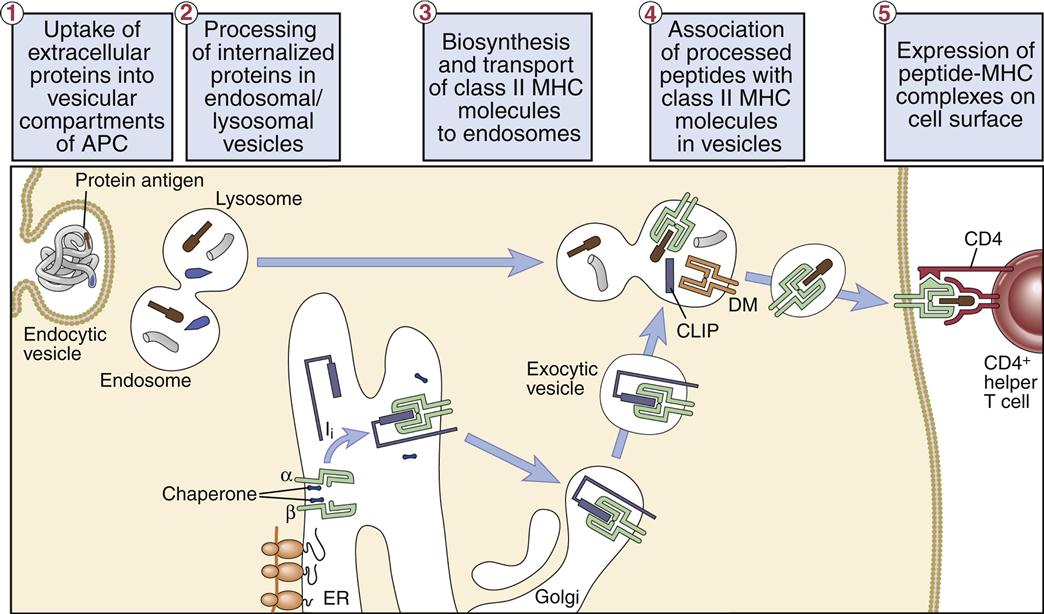
Class II molecules synthesized in the endoplasmic reticulum rapidly associate with a 30-kilodalton (kDal) invariant peptide chain (Ii). Homotrimers of Ii chain associate with three α-heterodimers or β-heterodimers of class II molecules. The Ii has two roles in antigen presentation: (1) It contains a signaling sequence that directs the class II molecule—into the endosome or the phagolysosome. (2) The Ii also prevents the loading of peptides into the binding groove until the class II molecule enters the endosome or the phagolysosome. The Ii may also contribute to the formation of the binding cleft and the overall structure of the class II molecule recognized by T cells. After entry into antigen-containing endosome or phagolysosome, the Ii is removed in an orderly proteolytic reaction (Figure 5-7).
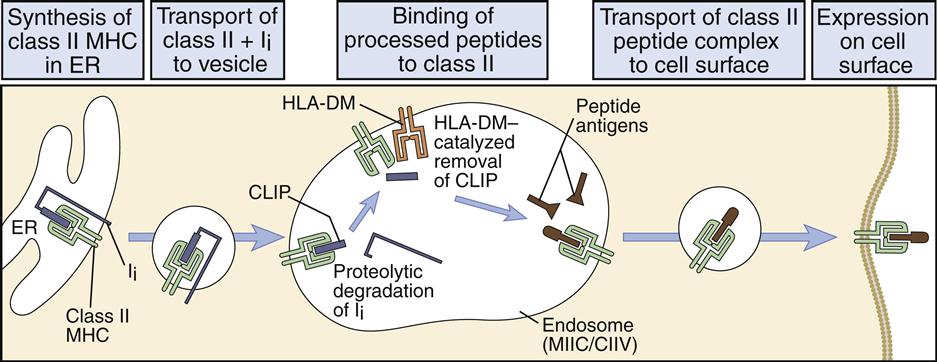
In the endosome or phagolysosome, the invariant chain is truncated to a 3-kDal peptide called the class II-associated invariant chain peptide, or CLIP. In humans, the disassociation of the CLIP is facilitated by the HLA-DM (monocytes) or the HLA-DO (B cells), which also stabilize the class II molecule and assist in peptide selection (Figure 5-8).
Stabilization allows the binding of peptides with low and high structural stability. Low-stability peptides are released from class II molecules. The interaction between high-stability peptides and the binding groove on class II molecules creates a stable peptide–class II molecule complex. Large and small peptides may bind to the open-ended class II binding cleft. Proteolytic enzymes trim the larger peptide to 10 to 30 amino acids.
Dendritic Cells and Cancer Vaccines
Tumor cells are usually not immunogenic because HLA molecules are downregulated and tumor-specific antigens cannot be presented to T cells. Vaccinologists use dendritic cell cross-priming to increase the immunogenicity of tumor cells for use in vaccines. In cross-priming, dendritic and tumor cells are obtained from the patient and purified. In the laboratory, DCs and tumor cells are mixed together and incubated for several days. DCs ingest intact tumor cells and process the appropriate antigens for presentation in the context of class I molecules. Administration of antigen-pulsed dendritic cells to the patient evokes a vigorous CD8 cytotoxic cell response to tumor cells. This concept is being used to develop autologous pulsed dendritic cell vaccines against tumors. From a theoretical perspective, dendritic cell vaccines could be used to treat all cancers. However, clinical experience shows that at the present time, the efficacy of dendritic cell vaccination is limited to melanoma and renal cancer treatments.
Dendritic Cells and Disease
Dendritic cells may play a critical role in psoriasis. Interactions between dendritic cells and T cells play a critical role in the formation of plaques in chronic psoriasis. Plaques contain high numbers of pDCs, myeloid DCs, and inflammatory dendritic skin cells. Interactions between DCs and CD4 cells lead to the clonal expansion of CD8 cells in skin lesions. CD8 cells produce proinflammatory cytokines that are implicated in psoriasis.
Dendritic cells also play a role in the pathogenesis of human immunodeficiency virus (HIV) infections. FDCs can concentrate infectious viruses for extended periods. Moreover, FDCs promote the migration of T cells into the germinal center and the transfer of virus to T cells. At the same time, IDCs are infected and support viral replication for 45 to 60 days.