Antigen Presentation for Cell-Mediated Response
Learning Objectives
• Draw the structure of a class I antigen-presenting molecule
• Explain antigen processing and presentation to CD8 T cells
• Recognize the role of ubiquitin in antigen processing
• Identify the role of heat shock protein 70 (HSP70) in antigen processing
• Compare and contrast the function of class I and CD1 molecules
Key Terms
Calreticulin
Cell-mediated immunity
CD1
Class I molecules
Class II molecules
Cross-priming
Endogenous antigen
Exogenous antigen
Proteasome
Transporter-associated antigen processing (TAP) protein
Tapasin
Ubiquitination
Introduction
Previous chapters in this text focused on antibody responses initiated by class II molecule antigen presentation to CD4Th2 cells. Although antibodies are efficient in dealing with exogenous antigens or extracellular microbes, they are ineffective in eradicating intracellular microbes, viruses, and tumor cells. A cell-mediated response, which is usually mounted in response to these endogenous antigens, consists of lymphocytes, activated macrophages, and natural killer (NK) cells. The nature of the cellular response is a reflection of antigen presentation. If antigens are presented in the context of class II or CD1 molecules, CD4Th1 cells promote an inflammatory response that involves macrophages, lymphocytes, and fibroblasts. When antigens are presented by class I molecules, cytotoxic T cells are activated. These cells are able to lyse target cells following cell-to-cell contact.
Class I Proteins
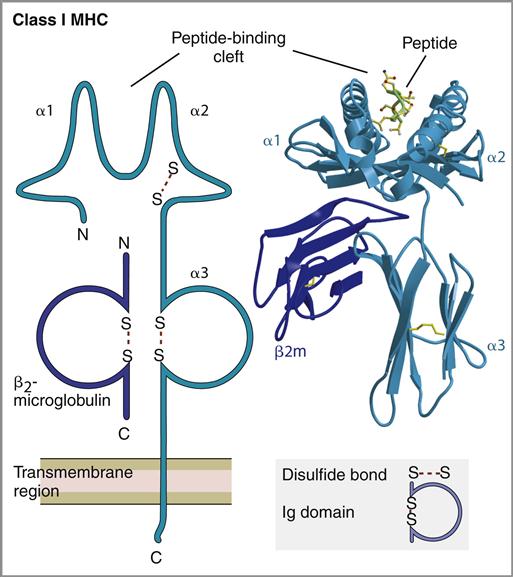
Class I molecules are found on all the nucleated cells in the body and present antigen to CD8 T cells. They consist of a single, α-chain transmembrane protein that is stabilized by a second protein known as β2-microglobulin (see Chapter 4).
Because the processed antigens are bound to class I molecules at the ends, the length of the antigen is restricted to 9 to 11 amino acids (Figure 18-1).
Class I Molecules and Antigen Presentation
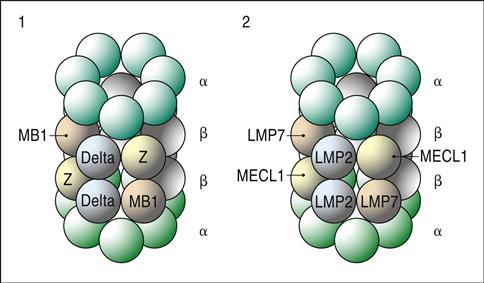
Endogenous antigens are generally large molecules that must be digested and fragmented before presentation to lymphocytes. Antigens are marked for enzymatic degradation by a process known as ubiquitination. In the process, molecules of ubiquitin are attached to the antigen by covalent bonding between amino groups of ubiquitin and lysine amino acids in the antigen. When four or more ubiquitin molecules bind to the antigen, the complex is shuttled to a series of proteasomes for fragmentation. During transport through the cytoplasm, heat shock protein 70 (HSP70) interacts with the ubiquitinated protein and protects it from enzymatic digestion. Initial digestion takes place in a 20S proteasome. The low-activity 20S proteasome consists of two α-subunits and two β-subunits, each having seven globular protein subsets. Only three β-subunits (MB1, delta, and zeta) are enzymatically active. Antigen fragments produced by the 20S proteasome vary widely in length (Figure 18-2).
To increase the rate of antigen fragmentation and to generate more homogeneous antigen fragments, the three β-proteasome subsets are replaced with high-activity enzymes (LMP2, LMP7, and MECL1). Additional subunits, which accelerate antigen processing, are also added to create a 26S proteasome. Digestion in the 26S proteasome produces 9 to 11 amino acid sequences, which are suitable for class I loading.
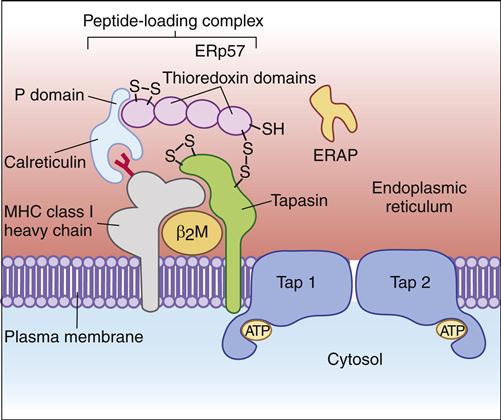
Antigens fragmented in the cytosol must be transported to the endoplasmic reticulum (ER) before they can be loaded onto class I molecules. Transporter-associated antigen processing (TAP) proteins are used to move antigenic fragments from the cytoplasm into the ER. TAP is actually a heterodimer of TAP1 and TAP2. Both isoforms are required for antigen transport and for the stabilization of class I molecules. With the exception of fragments with C-terminal proline and glycine, all peptides will bind to TAPs. Peptide fragments are loaded onto class I molecules in the ER.
Prior to antigen loading, the class I molecule forms complexes with three proteins: (1) a transmembrane-associated protein, called the TAP-binding protein, or tapasin, (2) a chaperone protein called calreticulin, and (3) a thiol oxidoreductase (ERp57). The oxidoreductase attaches to calreticulin and covalently links to tapasin by using disulfide bonds (Figure 18-3). In addition to stabilizing the class I–β2-microglobulin complex, tapasin may encourage the binding of high-affinity antigens to the class I molecule. Antigenic fragments are loaded onto the class I molecule after it is stabilized.
If the antigenic fragments are longer than 9 to 11 amino acids, an ER aminopeptidase (ERp57) trims the antigen’s hydrophobic N-terminus so that the overall length is between 9 and 11 amino acids. The peptide-loaded class I molecules are transferred to the Golgi apparatus, which functions to package proteins for secretion. From the Golgi apparatus, specialized vesicles transport the antigen-loaded class I molecules to the cell surface.
CD1 Proteins
The CD1 family is unrelated to class I and II molecules and represents a third lineage of antigen-presenting molecules that are expressed by B cells, monocytes, and dendritic cells (see Chapter 4). They differ in intracellular trafficking, antigen processing, and antigen loading. Peptides binding to CD1 are much longer than the typical 9 to 11 amino acids found in class I binding clefts because CD1 has three anchor positions, one at each end and one in the middle.
CD1 binds lipid molecules from bacteria, self-antigens, and synthetic antigens. Bacterial antigens are highly conserved mycolyl, diacylglycerols, and lipopeptides from pathogenic Mycobacterium, Plasmodium, and Leishmania species. CD1-restricted T cells play a critical role in resolution of infections by these organisms. Autoimmune disease may occur when CD1s are loaded with self-antigens such as phospholipids, phosphatidylinositol, or phosphatidylethanolamine.
CD1 and Antigen Presentation
Antigen processing is similar to that described for exogenous antigens, but antigen loading differs significantly. CD1s are synthesized in the ER and most move directly to the cell surface without being antigen loaded. Antigens are captured by mannose receptors and internalized into endosomes. The endsomal pathway is necessary for efficient T cell stimulation by all known bacterial lipid antigens. In the endosome, the presence of saposon lipid transfer proteins and a low pH allow transfer of the lipid antigens to CD1 molecules that have been recycled from the cell membrane to the endosome. Following stabilization, the antigen-loaded CD1 enters the secretory pathway and is redistributed to the cell surface.
Antigen presentation by CD1 group I and group II family members elicits different immune responses. For example, antigen presentation by CD1a, CD1b, and CD1c initiate a cellular inflammatory response that culminates in granulomatous lung disease. Conversely, antigen presented by C1d drives the response toward T helper cell (CD4Th2)–driven B cell–mediated responses.
Cross-Priming and Antigen Presentation
The fundamental paradigm of antigen presentation is that exogenously derived peptides are presented to Th2 cells by class II molecules, and CD8 cytotoxic T cells are activated after presentation of endogenous peptides by class I molecules. However, the paradigm fails to explain the generation of CD4Th1 cellular inflammatory responses to endogenously derived antigens or the CD8 cytotoxic response to exogenous antigens.
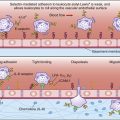
Both mechanisms can be explained by the concept of cross-priming by dendritic cells. As a consequence of normal microbial metabolism, endogenous proteins are excreted from infected cells and become exogenous antigens. Intact tumor cells are also considered exogenous antigens. Following ingestion, cells and excreted proteins are placed in phagosomes (Figure 18-4).
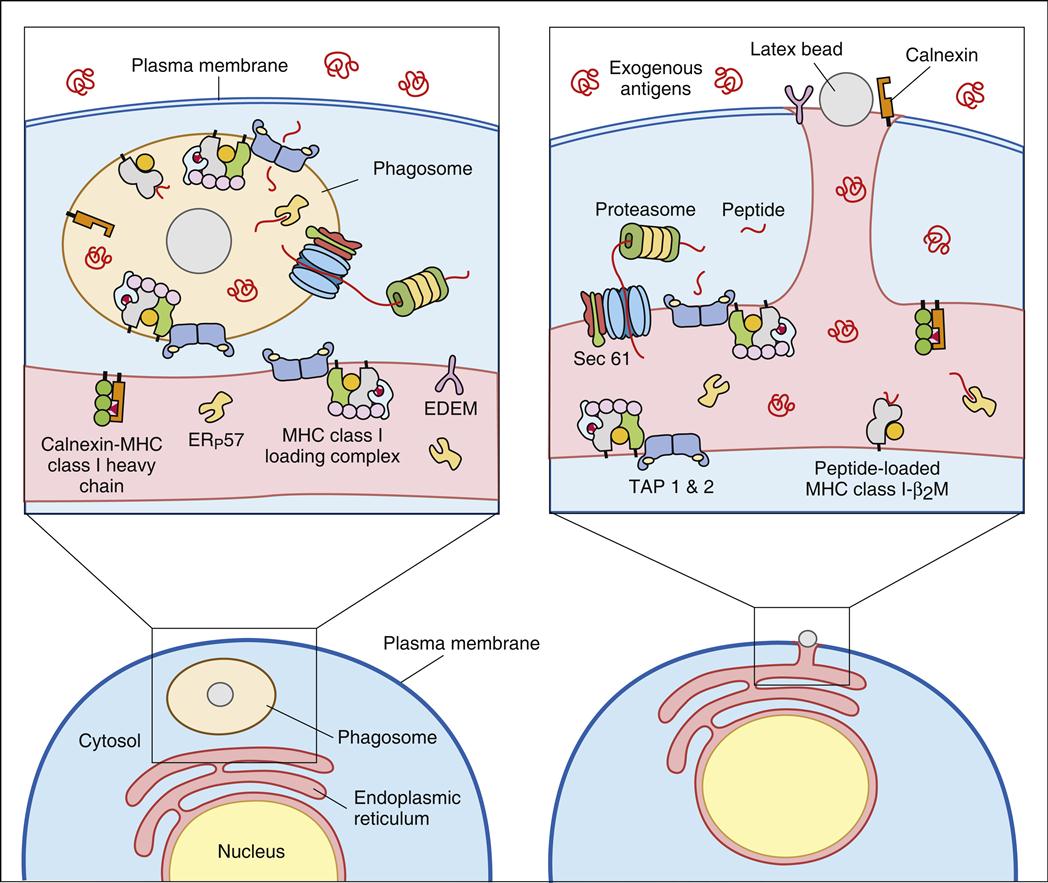
In the generation of cytotoxic T cells, the early phagocytic vesicles or phagosomes fuse with the ER membrane and use the protein translocation channel Sec61 to funnel partially degraded antigen into the cytoplasm, where it is digested by proteasomes. Following association with TAP, peptides are loaded onto class I molecules recycled from the cell surface or from a constitutive pool of class I molecules retained in the cytoplasm of dendritic cells.
The generation of a CD4Th1 inflammatory response to an endogenous antigen is a relatively simple process. Excreted microbial proteins are fragmented in the endocytic pathway and loaded onto class II molecules (see Chapter 5). CD4Th1 cells are selected for antigen-driven activation and proliferation by the expression of B7.2 co-stimulatory molecules on dendritic cells.
Drugs and Antigen Presentation
Little information on drugs that inhibit antigen processing or presentation is available. However, two fungal derivatives are known to block CD1 presentation: (1) Brefeldin A, an antibiotic lactone produced by Eupenicillin brefeldianum, prevents the delivery of antigen-loaded class I and CD1 molecules to the cell surface. (2) Bafilomycin A, produced by Streptomyces griseus, inhibits the transport of antigens from the late endosomes to the ER. As a consequence, the immune system cannot be stimulated.
Summary