Recent Posts
Categories
- Allergy and Immunology
- Anesthesiology
- Basic Science
- Cardiothoracic Surgery
- Cardiovascular
- Complementary Medicine
- Critical Care Medicine
- Dermatology
- Emergency Medicine
- Endocrinology, Diabetes and Metabolism
- Gastroenterology and Hepatology
- Hematology, Oncology and Palliative Medicine
- Internal Medicine
- Medical Education
- Neonatal – Perinatal Medicine
- Nephrology
- Neurology
- Neurosurgery
- Nursing & Midwifery & Medical Assistant
- Obstetrics & Gynecology
- Opthalmology
- Orthopaedics
- Otolaryngology
- Pathology
- Pediatrics
- Physical Medicine and Rehabilitation
- Plastic Reconstructive Surgery
- Psychiatry
- Pulmolory and Respiratory
- Radiology
- Rheumatology
- Sleep Medicine
- Surgery
- Test
Home » Anticoagulation and reversal for cardiopulmonary bypass
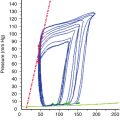
Anticoagulation and reversal for cardiopulmonary bypass
Published on 07/02/2015 by admin
Filed under Anesthesiology
Last modified 22/04/2025
Print this page