Antibodies and In Vitro Research and Diagnostic Assays
Learning Objectives
• Recognize the components of a flow cytometry (FCM)
• Compare and contrast forward and side scatter measurements
• Differentiate between absorbance and emission spectra
• Identify common fluorochromes used in lymphocyte enumeration
• Understand the concept of dual labeling and four-quadrant analyses
• Explain the lateral flow technology used in home pregnancy tests
• Recognize the function of sodium dodecyl sulfate in Western blotting
Key Terms
Enzyme-linked immunosorbent assay (ELISA)
Flow cytometer (FCM)
Fluorescence-activated cell sorter (FACS)
Sodium dodecyl sulfate
Western blotting
Introduction
Polyclonal and monoclonal antibodies are used in a wide range of in vitro assays in many different formats. The formats of the technology range from sophisticated flow cytometry (FCM) to simple enzyme-linked immunosorbent assay (ELISA). Data from antibody-facilitated assays are often used to support a tentative clinical diagnosis, evaluate disease progress, and provide prognostic information. In vitro assays are also used to determine the presence of bacteria, viruses, and antigens in biologic fluids. Increased hormone levels characteristic of disease states or pregnancy are determined by antibody-facilitated assays.
Flow Cytometry
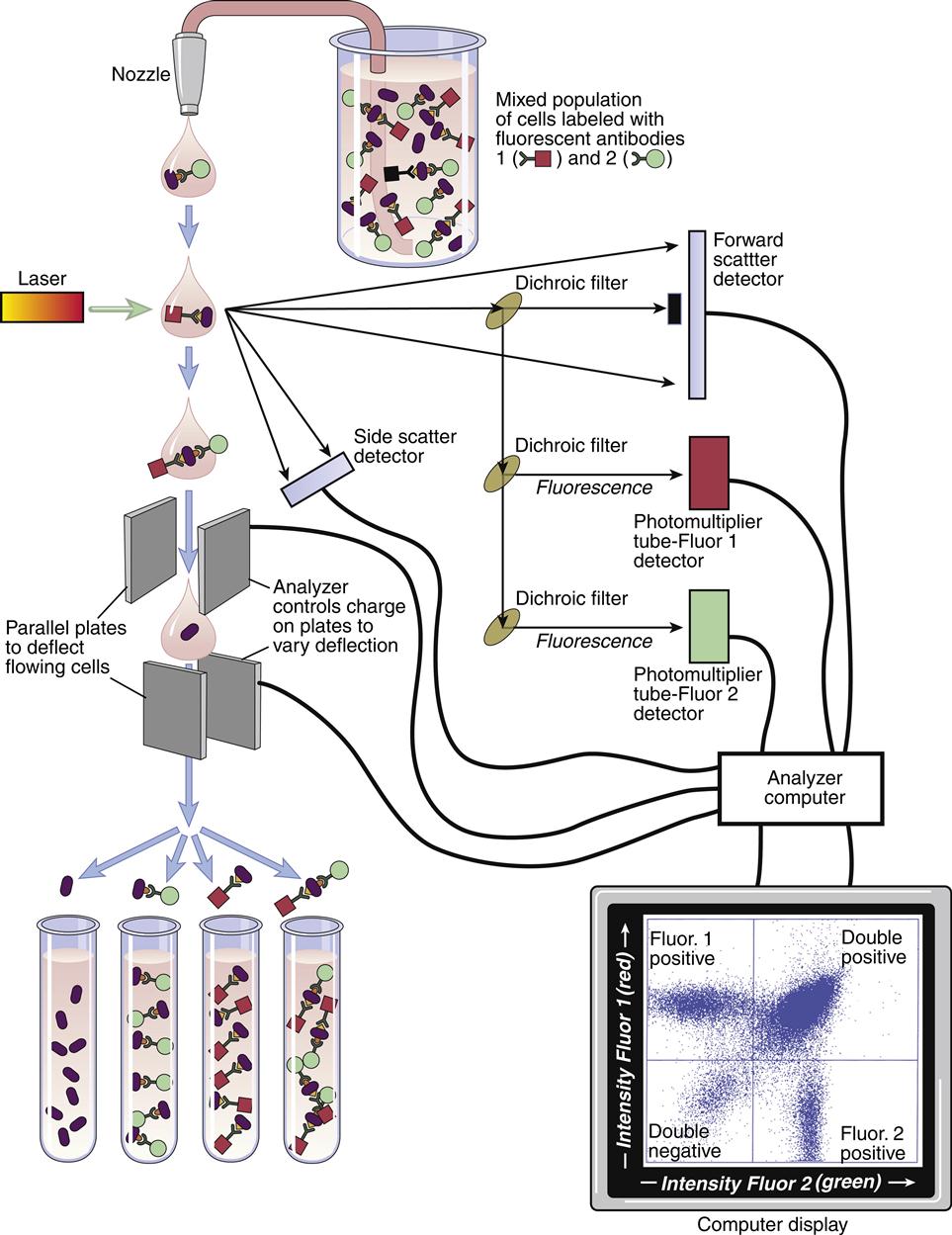
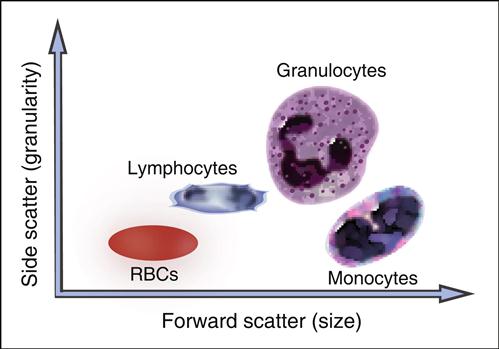
A flow cytometer consists of four components: (1) a fluidics system, (2) single or dual lasers, (3) optical detectors, and (4) an on-board computer system that collects and analyzes data. As part of FCM, cells are placed in a fluidic system that contains sheath fluid or saline. Using hemodynamic focusing, cells are concentrated at a nozzle and passed in a single file through a laser (the interrogation point) at a rate of several thousand cells per second. Lasers are usually of high-power, water-cooled argon or krypton. The computer records each cell as a dot on a linear or logarithmic “x–y” graph. As the cell is passed through the interrogation point, light is scattered in all directions. One optical detector collects forward, or low-level, light scatter. Forward scatter is laser light that passes around the cells and is roughly proportional to cell size. An optical detector converts the light passing around the cell to voltage impulses, which are stored in the computer. In essence, small cells generate small voltages, and large cells generate large voltages. Another detector collects light scattered from the laser at a 90-degree angle, which is called side scatter. Side scatter is a measure of the internal complexity or granularity of a cell. Since peripheral blood cells vary in both size and granularity, a linear plot of side scatter versus forward scatter is used to separate peripheral blood cells into lymphocyte, monocyte, and granulocyte populations (Figure 14-1).
Enumeration of Lymphocyte Subpopulations
Lymphocyte phenotyping (lymphocyte subset enumeration) is a routine test used to screen for immunodeficiencies, evaluate disease progress, support a tentative diagnosis, and provide prognostic information. In patients with acquired immuno deficiency syndrome (AIDS), FCM is used to monitor CD4 cell numbers.
An inverse correlation exists between CD4 numbers and the risk for specific bacterial and fungal infections. Clinicians use CD4 data to determine when and what type of prophylactic antibiotic therapy should be implemented. FCM is also used to differentiate between acute B cell leukemia (ABCL) and myelogenous leukemia, to diagnose chronic lymphocytic leukemia (CLL), and to differentiate between hairy cell leukemia and mantle cell lymphoma.
Proper identification and classification of hematopoietic neoplasms are critical because treatment of leukemia and lymphoma are radically different. For example, CLL is usually treated with nucleoside analogues and alemtuzumab directed at CD52 expressed by leukemic cells. First-line therapy for hairy cell leukemia is chlorodeoxyadenosine (2-CdA). Conversely, mantle cell lymphoma is treated with hyperfractionated cyclophosphamide, doxorubicin, vinblastine, and dexamethasone, with or without rituximab.
Dual staining of malignant cells has some prognostic value. For example, patients with ABCL have a poor prognosis if the malignant cells express CD10, CD34, and CD13. Conversely, the prognosis is excellent and so is response to chemotherapy when ABCL cells express CD45, CD135, and CD34.
In the enumeration of lymphocytes, size and granularity limits are used to draw an electronic “gate” around the lymphocyte population. Any subsequent data are derived solely from the “gated” area. The presence of lymphocytes in the gated area is verified by using fluorochrome-labeled monoclonal antibodies. Fluorochromes have high intensity and minimal overlapping spectra. As labeled antibodies pass the interrogation point, the fluorochrome absorbs light (excitation) at a specific wavelength. When the excited fluorochrome returns to a ground state, light is emitted at a different wavelength. Using mirrors and filters, the emitted light is delivered to photomultiplier detectors. In the detectors, fluorescence is converted to voltage intensity and stored in the computer as a dot on an x–y graph. Fluorescence yields information on the number of cells with fluorochrome, and the fluorochrome intensity is an index of the number of fluorescent markers per cell.
Although numerous fluorochromes can be used in FCM, fluorescein isothiocyanate (FITC) and phycoerythrin (PE) are commonly used in laboratory studies. FITC is a fluorescein derivative that is easily conjugated to antibodies via primary amines. In most conjugations, three to six FITC molecules are covalently linked to a single antibody. FITC is excited at 488 nanometer (nm) by an argon laser, and light is emitted at 530 nm. PE is a large-molecular-weight protein commonly found in cyanobacteria and red-green algae. Only one molecule of PE can be conjugated to an antibody. It is maximally excited at 533 nm and emits a very bright light at 570 nm.
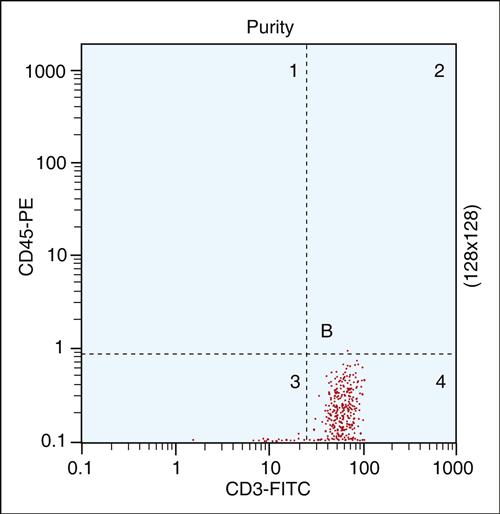
To verify the percent of lymphocytes in the “gated” population, cells are labeled with two different antibodies—usually CD45-PE and CD3-FITC. The former is a pan-leukocyte marker, and the latter reacts only with T cells. Using a histogram of FITC versus PE immunofluorescence, cells are electronically placed into four quadrants: (1) Cells lacking both markers are placed in the lower left quadrant. (2) Cells expressing only the CD45 marker are placed in the upper left quadrant. (3) CD3-positive cells are shown in the lower right quadrant. (4) Cells expressing both markers are placed in the upper right quadrant. Generally, 95% of lymphocytes are found in the lower right quadrant (Figure 14-2).
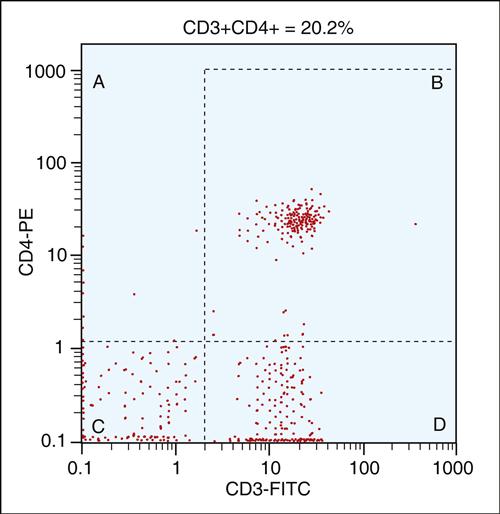
After the lymphocyte population is verified, total T cell, CD4, and B cell populations can be enumerated using dual staining techniques, as shown in Table 14-1. Dual-color immunofluorescence enumerations of the peripheral blood CD4 subset are shown in Figure 14-3. Cells are incubated with two monoclonal antibodies conjugated to two different fluorochromes. FITC-conjugated antibodies are directed at CD3, and PE is linked to an anti-CD4 antibody. Dual-labeled CD4 helper/amplifier cells are shown in the upper right quadrant. The study is repeated using CD3–CD8 dual staining for T cytotoxic cells and CD16–CD56 staining for natural killer (NK) cells. B cells are identified by staining with anti-CD19.
Table 14-1
Minimum Monoclonal Panel Used to Determine Lymphocyte Subsets
| Monoclonal Antibody Combinations (FITC/PE) | Cell Type Enumerated |
| Nonspecific IgG1/IgG2 | Isotype control |
| CD45/CD14 | % lymphocytes in gated region |
| CD3 | Total T cells |
| CD3/CD4 | Helper T cells |
| CD45RA | Naïve helper T cells |
| CD45RO | Memory T cells |
| CD3/CD8 | Cytotoxic T cells |
| CD19 | Total B cells |
| CD16/CD56 | Natural killer cells |
FITC, Fluorescein isothiocyanate; IgG, immunoglobulin G; PE, phycoerythrin.
Fluorescence-Activated Cell Sorter
With several modifications, the flow cytometer can act as a fluorescence-activated cell sorter (FACS) capable of isolating pure populations of cells. In the FACS configuration, a vibrating mechanism breaks the cell stream into individual droplets immediately after they exit a nozzle. Droplets meeting the sorting criteria in terms of fluorescence are given a positive or negative electrical charge. For example, cells coated with FITC-labeled anti-CD3 can be isolated by giving the cells a positive charge as they pass through the laser. The charged droplet passes through deflection plates that have a positive or negative charge. Depending on the droplet’s polarity, cells are deflected to the right or to the left into collection vessels. Uncharged cells are not deflected, and they enter a waste stream (Figure 14-4). Purified CD3 cells can be used in assays that assess the function of T cells.
Identification of Bacteria and Viruses by Flow Cytometry
FCM is often used in clinical microbiology because of its high sensitivity, specificity, and quick turnaround time. Bacteria and bacterial spores have a size range of 0.25 to 3.0 μm and can be detected in biologic fluids by using forward scatter measurements. Gated bacterial populations can be identified by labeled monoclonal antibodies directed at genus-specific antigens. FCM has been used to detect Salmonella, Mycobacteria, Brucella, Mycoplasma, Legionella, and Escherichia coli in biologic fluids. The method is extremely sensitive and can detect 10 to 100 bacteria/mL of sample. Other direct fluorochromes or substrates can be used to discriminate among viable, viable but non-culturable, and nonviable organisms. FCM also offers a rapid alternative for viral identification. Extracellular and intracellular viral antigens can be detected in biologic fluids. FCM has been useful in the rapid diagnosis of blood-borne cytomegalovirus (CMV), which is a common infection in transplant recipients and patients with AIDS. Identification of intracellular viruses is more difficult. Studies on infected cells require permeabilization to allow the entry of labeled antibodies into the cytoplasm. Since FCM requires isolated cells, it is best suited to the study of respiratory infections by using bronchoalveolar lavage fluids.
Immunochromatographic Tests
Pregnancy Testing
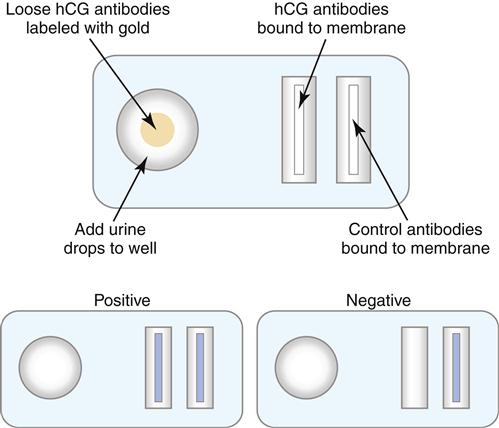
Most immunochromatographic test formats use lateral flow through a semi-permeable membrane. Antibodies are used as both capture and detection agents. The prototypic assay is the home pregnancy test that detects human chorionic gonadotropin (hCG) in urine. hCG is secreted by the developing placenta following conception and can be detected (5–50 mIU/mL) after 1 week of gestation.
In the test, urine is drawn through a membrane by capillary action into three distinct zones (reaction, test, and control zones). If the urine contains hCG, the color-labeled murine monoclonal antibodies react with hCG in the reaction zone, and the complex migrates to the test zone. Unlabeled polyclonal antibodies in the test zone recognize a second epitope on hCG. Labeled antibodies bound to hCG localize in the test zone, and the colored band suggests a positive test. Labeled and unlabeled antibodies continue to migrate into the control zone, which contains antimurine antibody. A colored band in the control zone confirms the proper capillary migration of urine. Positive reactions in both test and control zones confirm a positive test for elevated hCG (Figure 14-5).
Enzyme-Linked Immunosorbent Assay
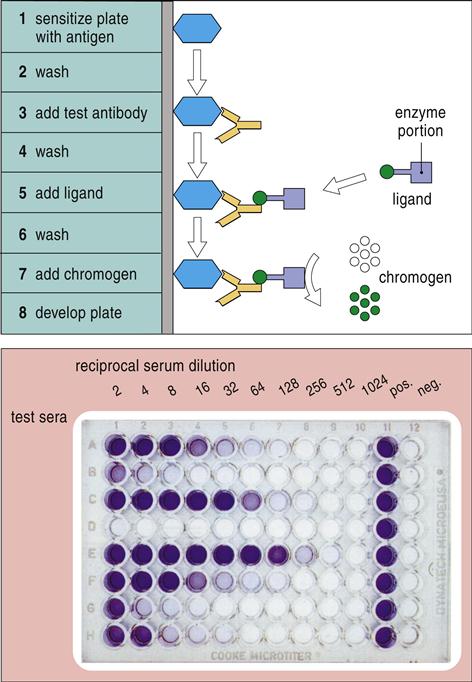
ELISA is a diagnostic tool that can be used to detect either antigens or antigen-specific antibodies in a biologic sample. In the simplest format, antigens are immobilized in 96-well microtiter plates. Test antibodies are then added to the mixture. To begin the detection process, an enzyme ligand is bound to the antibodies. After the removal of unbound antibodies and the unbound ligand, an enzyme substrate is added to produce a visible color change. Since the only variable in the assay is the concentration of antigens, the intensity of the color is directly proportional to the antigen concentration. Newer formats use fluorochrome or enzyme-conjugated antibodies to increase the sensitivity of the assay (Figure 14-6).
Several alternative ELISA formats include the indirect assay and the sandwich assay. In the indirect assay, antigens are immobilized, and unlabeled primary antibodies are incubated with the antigens. After the removal of the unbound antibodies, enzyme-conjugated secondary antibodies directed at the Fc region of the primary antibodies are added to the mixture. Addition of a colorimetric enzyme substrate (see Figure 14-6) yields a colorimetric endpoint.
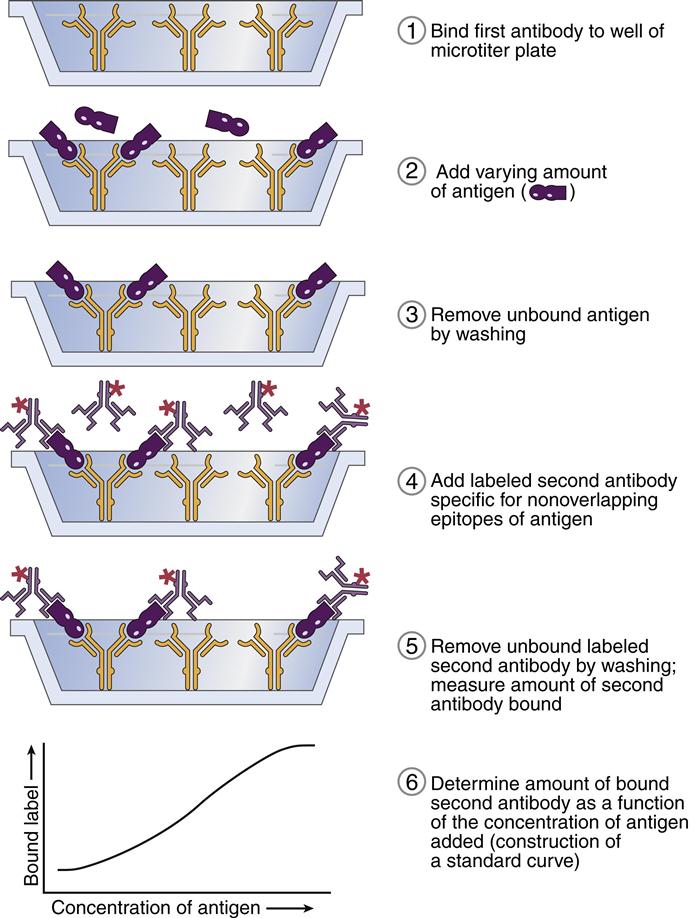
The sandwich assay is another common ELISA format. In this configuration, antigens are captured by antibodies immobilized on a polystyrene substrate. Enzyme-conjugated antibodies directed at a second or different antigen epitope are added to the mixture. The addition of the enzyme substrate yields a visible color (Figure 14-7).
ELISA is commonly used in the laboratory as an adjunct in the diagnosis of bacterial, viral, and parasitic infections. Serial ELISA assays are used to measure antigen-specific antibody levels in serum. Generally, antibody levels increase over time during an active infection and decrease when the infection abates. A representative list of diagnostic ELISA formats used in clinical medicine is provided in Table 14-2.
Table 14-2
Diseases for Which ELISA Is Used to Support a Clinical Diagnosis
| Microbe | Disease |
| Bordetella pertussis | Whooping cough |
| Borrelia burgdorferi | Lyme disease |
| Brucella abortus | Brucellosis |
| Chlamydia trachomatis | Chlamydial infections |
| Corynebacterium diphtheria | Diphtheria |
| Helicobacter pylori | Stomach ulcers |
| Mycoplasma pneumoniae | Atypical pneumonia |
| Clostridium tetani | Tetanus |
| Treponema pallidum | Syphilis |
| Legionella spp. | Legionnaires’ disease |
| Toxoplasma gondii | Toxoplasmosis |
| Entamoeba histolytica | Amebiasis |
Western Blotting
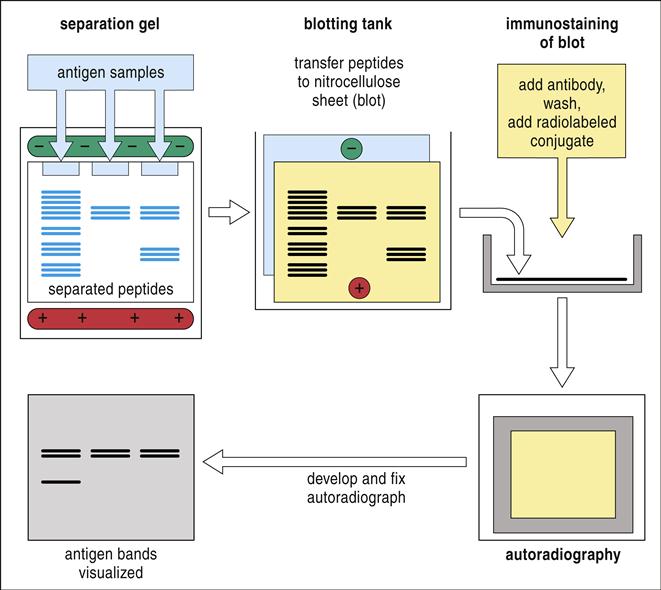
Western blotting is used to identify proteins within a complex mixture. In the protocol, proteins are denatured using sodium dodecyl sulfate (SDS). Generally, the amount of SDS bound to protein is directly proportional to the size and the negative charge of the protein. In an electrical field, denatured protein moves through a polyacrylamide mesh toward a positive electrode at a rate dependent on both protein size and charge. The technique is called SDS-polyacrylamide gel electrophoresis, or SDS-PAGE. To visualize the separated protein bands, the proteins are imprinted onto a nitrocellulose membrane by using electroblotting or transverse electrophoresis. To detect specific protein bands, unlabeled antibodies directed at a specific protein are added to the nitrocellulose. In classic protocols, a radiolabeled antibody directed at the Fc portion of the primary antibody is added to the nitrocellulose (Figure 14-8). After the immunoblot is washed, it is transferred to an x-ray film. When the x-ray film is developed, the protein bands with attached antibodies are immediately visible. Newer technology has replaced radioactivity with colorimetric dyes.
Western blotting has several limitations. Conformational epitopes are irreversibly denatured by SDS and cannot be detected. Linear epitopes can be detected, but this requires the use of polyclonal antibodies. Directing polyclonal antibodies at multiple and different linear epitopes on the same protein increases the sensitivity of the assay. However, polyclonal antibodies are a finite source of reagents, and antibody pools must be restandardized at regular intervals.
A combination of ELISA and Western blotting is commonly used in the diagnosis of HIV infections. ELISA is used as a screening assay for antibodies directed at human immunodeficiency virus (HIV). However, false-positive reactions in the ELISA are often observed in patients with syphilis, Lyme disease, hemophilia, systemic lupus erythematosus (SLE), and alcohol-related hepatitis. Western blotting, which detects HIV surface antigens, is used to confirm the ELISA results. The presence of viral antigens in biologic fluids and the presence of antibodies confirm an active HIV infection.