CHAPTER 300 Anterior Thoracic Instrumentation
Historical Background
The impetus for developing thoracic instrumentation can be found in the literature as far back as 1928, when Royle described anterior decompression of the spine for scoliotic deformities.1 Hodgson and Stock later described similar decompressive procedures for Pott’s disease.2 These efforts did not include spinal reconstruction; as a result, their patients suffered from postoperative instability and progressive deformity. In 1958, Humphries and Hawk published one of the first reports on ventral instrumentation developed for stabilization after transperitoneal débridement in a patient with Pott’s disease.3 Their ventral plate and screw construct provided little biomechanical support and was later abandoned. In the 1970s, advances in construct design were introduced by Dwyer,4,5 Zielke,6 and colleagues. The screw-cable construct of Dwyer and the screw-rod construct of Zielke successfully corrected scoliotic deformities. However, these constructs were not rigid enough to provide structural support in the setting of significant spinal instability. In the late 1970s, Dunn developed a double-rod, double-screw construct that provided adequate stability for anterior thoracolumbar reconstruction.7,8 In 1986, however, the Dunn device was removed from clinical use after reports of great vessel erosion.9 Since the Dunn device, improved material properties and implant designs have led to lower profile constructs, which essentially eliminated the risk for long-term vessel erosion, as well as magnetic resonance imaging–compatible implants. Today’s spine surgeon has numerous options to effectively stabilize the anterior thoracic spine.
Indications for Operative Intervention
Neoplasm
Most neoplastic disorders affecting the spinal column are malignant. Spinal neoplasms can be manifested as pain, progressive deformity, or neurological deficits. Operative intervention is appropriate for tissue diagnosis, neurological decompression, and spinal stabilization. Other indications include resection of radioresistant neoplasms, resection of isolated recurrences, and neurological deterioration after adjuvant treatment. Less optimal outcomes with an increased incidence of postoperative complications have traditionally been associated with posterior decompression for anterior metastatic disease.10 Clinical outcomes were so poor that radiotherapy became the treatment of choice.10–14
Advances in surgical technique led to the development of ventral spinal approaches without excessive complications. An anterior approach provides a direct means of addressing ventral pathology and reconstructing the spine at the primary site of instability.10 Currently, clinical outcomes in patients with metastatic spinal disease after anterior decompression are superior to those after posterior decompression with or without adjuvant radiotherapy.15–22 However, selection of appropriate patients for surgical intervention remains a challenge.
Important considerations for appropriate patient selection include age, preoperative functional status, presence of medical comorbid conditions, life expectancy, and need for tissue diagnosis.23–25 Typically, surgical intervention is reserved for patients with a life expectancy of at least 6 to 12 months; however, surgery should not be withheld if it will significantly improve quality of life. The value of restoring neurological function, such as ambulation or sphincter control, must be judged on an individual basis. Other considerations in selecting patients for operative intervention include the type of tumor, compromise of the spinal canal, extent of neurological deficits, level of pain, and degree of instability. Adjuvant treatment such as radiotherapy, chemotherapy, or hormonal therapy may be indicated in specific cases. Additionally, nonoperative intervention remains an option for patients with stable myelopathy despite spinal cord compression.11,26,27 The surgeon must take all these factors into consideration on a case-by-case basis.
Neurological deterioration is rare after operative intervention for metastatic spinal disease. Perioperative mortality rates range from 6% to 8%, and overall complication rates range from 8% to 11%.15 In these reports, some patients were not treated initially with an anterior approach, and it is possible that an anterior approach alone would have decreased the combined rate of morbidity and mortality.15
Trauma
Most thoracic spine fractures occur at the thoracolumbar junction; about 50% to 80% occur between T10 and L2.28,29 The thoracolumbar region is particularly susceptible to traumatic injury for several reasons: instability associated with transition from the stable thoracic spine to the mobile lumbar region, loss of rigidity provided by the intact rib cage, instability associated with transition from kyphosis to lordosis, and change in orientation of the facet joints.30,31 Based on the three-column model of stability, Denis categorized these fractures into four classes: compression fractures, burst fractures, seat belt–type fractures, and fracture-dislocations.32 The most common types of fractures are compression and burst fractures.32 Traditionally, fractures that produce a 40% loss in height of the vertebral body, a 50% compromise of the spinal canal without neurological deficits, a kyphotic deformity of 30 degrees, or neurological deficits require operative intervention.33,34
The choice of surgical procedure remains controversial. Posterior fusion and fixation may be appropriate in the absence of a ventral deformity or anterior spinal canal compromise. Lesions producing ventral compression or significant biomechanical compromise of the anterior and middle columns are indications for an anterior approach. Previous reports have documented better outcomes with an anterior approach than with isolated posterior stabilization.33,35–37 If the posterior elements have been compromised, however, an isolated anterior construct may be insufficient to resist flexion forces. Loss of the posterior tension band may require supplementation with a posterior stabilization construct. Patients with a complete spinal cord injury may still benefit from spinal stabilization. In such circumstances, surgical stabilization optimizes the rehabilitation process and possibly decreases the length of hospitalization.33–35,38–41
Infection
Hodgson and Stock were among the first to describe successful anterior decompression in patients with Pott’s disease.2 With advances in diagnosis and antibiotics, most cases of vertebral osteomyelitis and diskitis can now be treated effectively without surgical intervention. As many as 90% of patients respond to intravenous antibiotics and immobilization.42 Surgical débridement and stabilization are necessary when the infection is resistant to intravenous antibiotics, the causative organisms cannot be identified, neurological deficits progress in the presence of a ventral collection, and severe osseous destruction leads to a significant deformity or intractable pain.
Historically, there has been concern regarding the insertion of instrumentation into an infected spine; however, clinical series have demonstrated that the presence of an active infection does not appear to be a contraindication to insertion of spinal implants,43–45 and in fact, anterior instrumentation has been shown to be safe for the treatment of patients with pyogenic vertebral osteomyelitis of the thoracic and lumbar spine after anterior débridement and autogenous bone grafting.46 In addition, it has also been shown to provide segmental stability, correction of kyphotic deformity, and promotion of bony fusion in patients with spinal tuberculosis.47
Deformity and Degenerative Conditions
Symptomatic deformity of the thoracic spine can occur as a late complication of trauma, infection, neoplasm, or previous surgery. Surgery is often reserved for kyphosis greater than 30 degrees, as well as radiographic evidence of progression of the deformity. Clinical indications for operative intervention include intractable axial or radicular pain and progressive neurological deficits. At the thoracolumbar junction, instrumentation is necessary to resist the dynamic biomechanical forces encountered at this level.48–51
In the spine, the overwhelming majority of degenerative disease is encountered rostral or caudal to the thoracic region. Disk herniations are the most common type of degeneration at the thoracic level. Most of these rare lesions can be resected successfully through a posterolateral approach without the addition of fusion and fixation.52
Surgical Approaches to the Anterior Thoracic Spine
Approach to the Upper Thoracic Spine
Depending on the patient’s body habitus, exposure of the first or second thoracic vertebrae can occasionally be accomplished through a traditional anterior cervical approach. A more extensive sternal-splitting approach is usually necessary for anterior access to the lower vertebrae, typically up to the fourth or fifth thoracic vertebrae, or for patients with short stout necks. These exposures should be performed with the assistance of an experienced thoracic surgeon and are beyond the scope of this text.53–57 Numerous references are available that describe these approaches in more detail.55,58–61
Lateral Extracavitary Approach
The lateral extracavitary approach introduced by Larson and colleagues62 in 1976 is a derivative of the lateral costotransversectomy. It is ideally suited for lesions with a significant anterior paraspinal extension, with or without a substantial intraspinal component. This approach provides a familiar exposure and orientation for neurosurgeons and extensive access to all three spinal compartments.63 The lateral extracavitary approach is entirely extrapleural and avoids the perioperative complications associated with transthoracic exposure. In addition, circumferential stabilization of the spine is possible through a single incision. The lateral extracavitary approach is versatile and can be modified according to the size of the tumor, level of involvement, relationship to neural structures, and need for spinal stabilization.
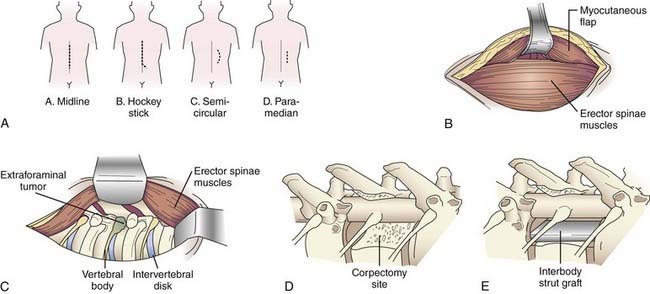
A variety of incisions can provide access via the lateral extracavitary approach, including midline, hockey stick, semicircular, and paramedian incisions (Fig. 300-1A). If a “hockey stick” type of incision is used, adequate caudal exposure is needed before the incision is extended laterally, or the caudal extent of the exposure will be limited. Routine subperiosteal dissection of the paraspinal muscles is performed with a Cobb dissector and monopolar cauterization. The takedown is performed bilaterally if posterior exposure is required for laminectomy or instrumentation; otherwise, unilateral exposure is sufficient. The lateral limb of the “hockey stick” incision is opened by transecting the transversely oriented thoracic muscles (i.e., latissimus dorsi, trapezius, rhomboid) in line with the skin incision. The myocutaneous flap is elevated to expose the longitudinally oriented erector spinae musculature (Fig. 300-1B). An extended midline incision can provide similar access with less musculoligamentous injury and potentially an improved cosmetic result.
Once the transversely oriented muscles are retracted, the lateral margin of the erector spinae is identified and dissected medially to expose the underlying ribs. This dissection is then carried over the facet joint and eventually communicates with the posterior dissection. Mobilization of the erector spinae muscle allows access to the lateral spinal compartment (Fig. 300-1C). The longitudinal muscle mass is wrapped in a moist sponge to avoid desiccation and positioned medially or laterally to allow the surgeon to work on either side.
Once the rib and transverse processes are removed, the neurovascular bundle is identified within the endothoracic fascia, deep to the intercostal musculature. The segmental nerves above and at the level of the pathology are dissected free and divided, with the proximal stump elevated medially toward the intervertebral foramen. Unlike the situation at the lumbar levels, nerves at the thoracic levels can be sacrificed without incurring a functional deficit. Once the nerve is free, the parietal pleura is displaced ventrally to provide access to the anterolateral spinal compartment. The parietal pleura is kept out of the operative field with a table-mounted retractor system. A padded, wide retractor blade helps avoid a pleural tear. The parietal pleura, diaphragm, or both are bluntly dissected free from the ventrolateral aspect of the vertebral bodies with a Cobb elevator. Sharp dissection may be required at the level of the disk space if more adherent connective tissue is present. The sympathetic chain is identified along the ventrolateral aspect of the vertebral bodies. The rami communicantes are transected at the involved spinal segments, and the sympathetic chain is mobilized by subperiosteal dissection. The dorsal and foraminal vessels are identified, cauterized, and transected. The previously elevated nerve stump facilitates dissection to the foramen and pedicle. Resection of the pedicle allows identification of the ventral spinal canal. A distinct advantage of the lateral extracavitary approach is the simultaneous access provided to both the anterolateral and the posterior spinal compartments. The spinal canal can be decompressed by resection of both the posterior and anterior elements (Fig. 300-1D). Once the pathology has been resected, an interbody spacer can be placed (Fig. 300-1E) and circumferential spinal stabilization can be performed. The parietal pleura is inspected for air leaks. Minor leaks can be repaired primarily. A significant leak mandates placement of a chest tube during the immediate postoperative period. Multilayer wound closure is performed while making sure to reattach the appropriate muscle layers.
Retropleural Thoracotomy
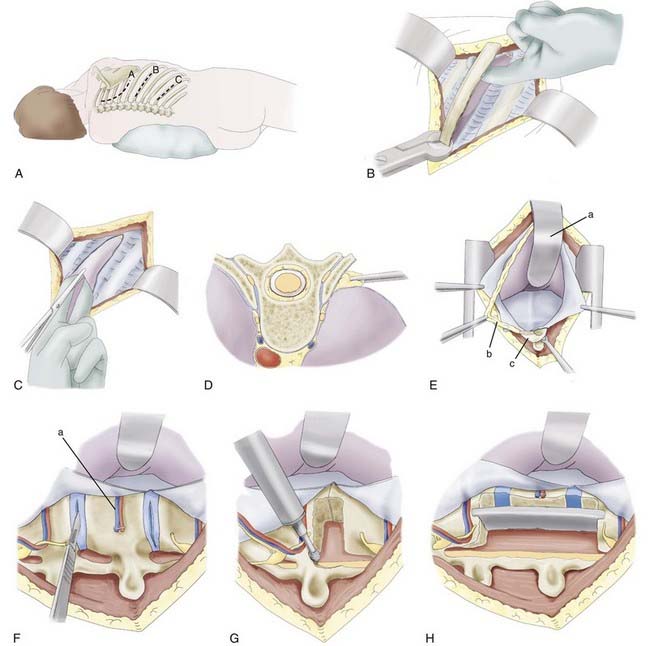
The patient is placed in a lateral position with an axillary roll and appropriate padding to prevent pressure neuropathies. Because of the caudal rib angulation, the incision for an anterolateral approach should be made two levels above the pathology. For example, a lesion at T8 would be approached through an incision over the sixth rib. The incision for a retropleural approach starts approximately 4 cm from the midline over the rib of interest and extends to the midaxillary line (Fig. 300-2A). The subcutaneous tissue and underlying muscles are transected with monopolar cauterization to expose the periosteum of the rib. The surrounding soft tissue is detached from the dorsal aspect of the rib with an Addison dissector while preserving the intercostal neurovascular bundle. A Doyen dissector is used to free the ventral periosteum along the length of the rib. The dissection continues as far medially as possible, usually 1 to 2 cm lateral to the costotransverse joint. The section of rib is resected and saved for possible grafting (Fig. 300-2B).
The endothoracic fascia is identified as the shiny tissue layer deep to the rib periosteum but superficial to the parietal pleura. This fascial layer is sharply incised in line with the rib bed (Fig. 300-2C). The underlying parietal pleura is freed in all directions by blunt dissection with a finger or sponge dissector (Fig. 300-2D). This maneuver exposes the costotransverse joint and anterolateral aspect of the vertebral bodies. For exposure of the lower thoracic region and thoracolumbar junction, the lateral attachments of the diaphragm must be divided so that the retropleural and retroperitoneal spaces are in communication. Instead of incising the diaphragm circumferentially from the anterior chest wall, the 11th and 12th rib attachments to the diaphragm are dissected subperiosteally through a smaller, more caudal diaphragmatic opening. The dissection continues medially to elevate the lateral and medial arcuate ligaments from the underlying muscles. At the transverse process of L1, a cuff of muscle is left intact to help reapproximate the diaphragm. Finally, the left crus is divided to complete the communication of the retropleural and retroperitoneal cavities. The peritoneum is gently swept from the posterior abdominal wall, with special attention paid to the area near the central tendon, where the peritoneum is thinner. The hemiazygos and accessory azygos veins should be identified and may require cauterization or clipping. The proximal rib head is resected sharply to expose the lateral aspect of the vertebral body (Fig. 300-2E). A table-mounted retractor is positioned and used to retract the lung and peritoneal contents. Use of a well-padded retractor blade avoids injury to the underlying structures. Once stabilization is complete, multilayer closure is performed.
Corpectomy and Fusion
After adequate exposure is achieved, the corpectomy is performed. Before the vertebral body is resected, the rostral and caudal intervertebral disks are removed. The lateral surface of the disk is incised, and the disk material is extracted with pituitary rongeurs and curets (Fig. 300-2F). After removal of the disk, the pedicle and facet joint are removed with either a high-speed drill or a rongeur. During these maneuvers, the epidural space is probed with a nerve hook or blunt dissector to ensure that the thecal sac and nerve roots are freed. While drilling, adequate irrigation is necessary to avoid thermal injury to the underlying nerve root and spinal cord.
Ventral decompression is achieved by first drilling the anterior aspect of the vertebral body to create a ventral defect (Fig. 300-2G). With a reverse-angle curet, the remaining dorsal bone is delivered into the trough created, and the corpectomy is completed. Considerable blood loss is possible because of the proximity of the perineural venous plexus. The anesthesiologist should be advised of this possibility so that excessive blood loss can be replaced adequately. A lesion within the ventral spinal canal can be delivered into the corpectomy site with the use of a reverse-angle or down-angled curet. Contralateral visualization of the spinal canal can be obtained with an angled dental mirror.
The height of the corpectomy defect and width of the rostral and caudal end plates are measured to determine the appropriate graft size. Many devices are now equipped with templates and calipers to make these calculations more precise. The graft is cut or constructed to the appropriate size, and a trial insertion is performed. Ideally, a nonexpandable graft should be slightly longer than the relaxed corpectomy site so that retraction of the distracted ligaments will hold the graft in place. The vertebral bodies are distracted, and the graft is inserted with a mallet and impactor. The graft can be mortised into the end plates to help prevent dislodgment of the graft (Fig. 300-2H). Alternatively, an expandable cage can be inserted in the reduced configuration and, according to manufacturer guidelines, expanded to engage the end plates. Excessive distraction of the expandable cage should be avoided because the expansion mechanism typically provides the surgeon with a biomechanical advantage that may not be appreciated during insertion; several reports imply that this biomechanical advantage leads to an increased incidence of graft subsidence.90,91,93 After placement, the graft should lie flush with or slightly recessed from the ventral edges of the vertebral bodies. Corticocancellous bone chips are placed ventral to the graft to enhance the potential for fusion. The posterior border is checked with a nerve hook to ensure that the graft does not impinge on the spinal cord.
Fusion Techniques of the Anterior Thoracic Spine
Anterior Interbody Graft Materials
The most commonly used material for interbody grafting is bone, either autograft or allograft. Autogenous bone is the “gold standard” for successful arthrodesis because of its osteoconductive, osteogenic, and osteoinductive properties.64–66 The size and shape required to support the anterior thoracic spine, however, make harvesting an adequate structural autograft difficult. The resected rib is an excellent source of autograft, but with the evolution of synthetic interbody cages, harvested autograft can be morselized and inserted into the interbody cage. The complication rate associated with harvesting autologous bone from a separate site, primarily the iliac crest, ranges from 9.4% to 49%.67–71 Severe complications are rare but can occur as a result of injury to the lateral femoral cutaneous nerve, damage to the peritoneum, hip fracture, donor site pain, hematoma formation, and infection.72
Structural allograft, such as fibula or humerus, is commonly used, but their fusion potential is less than that of autologous bone.73–77 It may be enhanced with the application of morselized autograft or fusion extenders.66,78–82 Several of these extenders, however, have not been approved by the Food and Drug Administration for this application, and the effect on neoplastic or infectious tissue is currently unknown. The reduced fusion potential of allograft bone increases the risk for pseudarthrosis and the formation of stress fractures. Allograft bone also tends to have a higher modulus of elasticity, a measure of stiffness, which increases the risk for subsidence and telescoping into the adjacent vertebral bodies. Although the risk for viral transmission is low, it may still serve as an unnecessary source of anxiety for certain patients.83,84 Under normal circumstances, the difference in fusion rates achieved with autograft and allograft is likely to be clinically irrelevant.
For patients with malignant disease, using bone as an interbody graft has several disadvantages. If life expectancy is limited, there may not be enough time or need for a solid arthrodesis. Adjuvant treatments, such as radiation therapy and chemotherapy, will adversely affect fusion potential.66 Consequently, patients with malignant disease are candidates to receive synthetic graft material. The traditional material used for patients with metastatic disease has been polymethyl methacrylate (PMMA). Acrylic provides the structural support; however, no osteointegration occurs at the interface between acrylic and bone. Because the acrylic-bone interface can loosen over time, grafting with PMMA is often supplemented with Steinmann pins.85,86
In recent years a wide range of synthetic implants have become available. Various materials have been incorporated into interbody struts, including ceramics, hydroxyapatite, titanium, carbon fiber, and polyetheretherketone (PEEK).66,86 The evolution of interbody cages has led to the development of expandable corpectomy cages.87–89 These devices are considered by some to be easier to insert because the graft does not have to be cut or contoured to the exact size of the interbody defect. These constructs are typically modular with two end plates and a central core. The end plates are available in multiple sizes and angles to accommodate the patient’s anatomy. In addition, the surgeon is able to directly apply a distracting force from within the corpectomy defect, thereby potentially increasing the ability to reduce a kyphotic deformity. The Synex system (Synthes, Paoli, PA) uses an expandable titanium mesh, the XPand system (Globus, Audubon, PA) uses a titanium corpectomy spacer, and the XPand-R system (Globus) is constructed from radiolucent PEEK material. A recent report on expandable cages has shown that design variations of expandable cages are of little importance.90 Additionally, no significant difference could be determined between the biomechanical properties of expandable and nonexpendable cages.90
Expandable cages provide adequate space for packing with bone graft, and supplemental fixation can then be performed. Potential advantages of these systems include a broad end plate surface area and smoother edges, which help prevent subsidence of the cage. Expandable spacers have been shown to be easy to use because they permit optimal fit and correction of deformity as a result of in vivo expansion of the cage.91,92 Isolated reports, however, have demonstrated that expandable cages can cause adjacent-level vertebral body fractures. The failure pattern of expandable cages has been suggested to be more destructive than the failure pattern of nonexpandable cages. Avoiding aggressive vertebral body expansion, especially in osteoporotic patients and in cases in which the cage-to–end plate contact is less than ideal, might prevent adjacent-level vertebral body fractures.93 However, further long-term studies and follow-up are required to show the lasting effects and possible complications of expandable cages.
Instrumentation of the Anterior Thoracic Spine
Biomechanical Considerations
Detailed discussion of biomechanical concepts is beyond the scope of this text; however, some basic concepts apply to the most rudimentary exercise in anterior thoracic stabilization. The anterior and middle columns provide the most resistance to axial loads. Therefore, destruction of the anterior or middle column can lead to spinal instability and a progressive kyphotic deformity. Subsequently, the spinal cord can be injured by dorsally extruded bone fragments or tethering of the spinal cord over the kyphotic deformity.32,94
A ventral approach is optimal for reconstruction of the anterior and middle columns. Insertion of the interbody strut in line with the instantaneous axis of rotation places the anterior and middle columns in the optimal position to resist an axial load. However, an adequate posterior column is necessary to maximize resistance to flexion, extension, or rotational forces. The surgeon must determine the integrity of the posterior column. If significant compromise of the posterior elements exists, a circumferential approach is required to achieve maximal stability.95–97
General Principles of Implantation
Despite specifics for both rod and plate constructs, the basic principles for insertion are the same.72,98,99 The systems available today are implanted primarily along the ventrolateral aspect of the vertebral bodies. The goals are to provide the greatest degree of stability with the lowest profile and to maximize implant-bone contact. Unequal stresses at the various bone-implant surfaces should be avoided by contouring the implant as closely as possible to the vertebral body surface and completely removing the costovertebral articulation and any protruding osteophytes. A recent study has shown that for load sharing and load stiffness, the design of the instrumentation system has more of an impact than whether it is a plate or rod type of system. Both the plate and rod systems were able to stabilize a corpectomy reconstruction model; in fact, the graft contributed to the overall stiffness of the construct. From this study it seems reasonable to conclude that because most of the common anterior instrumentation designs share similar characteristics, ease of use and surgeon familiarity with a particular system may be more important than the material capabilities of each particular implant.100
Because of the lateral insertion of the screws into the vertebral body, a clear understanding of the anatomy is essential to avoid compromising the spinal canal. The concave surface of the dorsal cortical wall may lead to compromise of the canal if the posterior screws are not angled anteriorly.101 Ideally, the posterior screw or bolt is inserted at a point 4 to 5 mm anterior to the posterior margin of the vertebral body and angled slightly ventrally (approximately 10 degrees away from the thecal sac) to avoid the spinal canal. The initial screw trajectory is created with an awl or drill. The cancellous bone of the vertebral body allows the use of a threaded tap to complete the trajectory. The anterior screws are placed either perpendicular to the posterior wall or slightly dorsal. Such placement triangulates the screws, thereby increasing resistance to screw pullout and preventing a parallelogram deformity. Careful evaluation of preoperative images allows an estimation of the screw length needed; however, this measurement is confirmed during surgery by inserting a ball-tipped probe into the screw hole. Depending on the construct used, unicortical screw purchase may be acceptable, but bicortical purchase will maximize stability. Recent biomechanical research has demonstrated that the stability afforded by a triangulated two-screw, dual-rod system is only slightly better than that provided by a single bolt and plate system (not statistically significant). This suggests that the single bolt system may be useful in certain clinical situations, such as thoracoscopic spine surgery.102
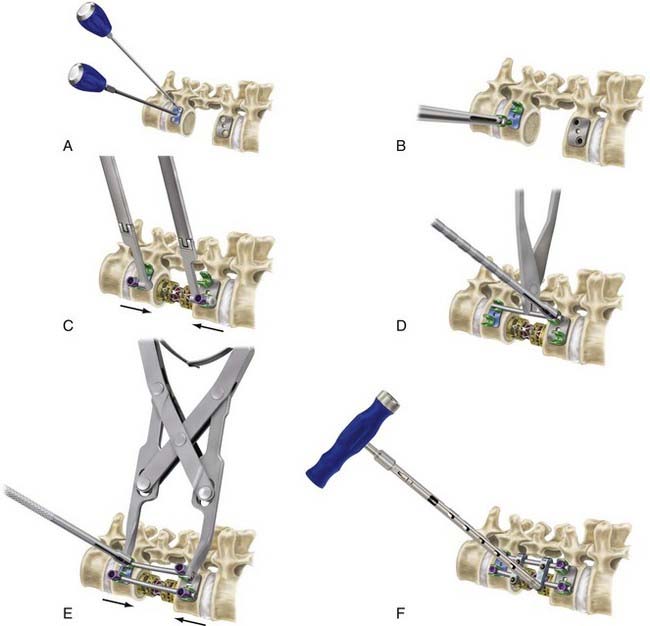
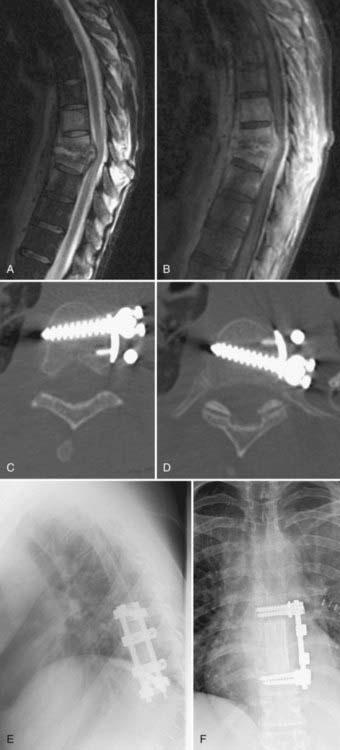
An intrinsic bending moment between the rods can cause a translational deformity with dual-rod constructs.103 This problem can be avoided by cross-fixation of the parallel rods and triangulation of the screws within the vertebral body. Some of the newer generation constructs avoid a parallelogram construct by offsetting the screws in a rostral-caudal orientation within a single vertebral body. Distraction and compression forces can be applied to the screws before and after the graft is inserted. Excessive distraction across the defect is to be avoided because it can cause spinal cord injury from stretch and vascular compromise. Distraction is therefore limited, especially if distraction-resisting ligaments, such as the anterior longitudinal ligament, are incompetent. Figure 300-3 demonstrates the general principles of insertion of vertebral body screws and dual-rod constructs. Postoperative imaging, especially computed tomography, is then used to ensure appropriate positioning of the implants (Fig. 300-4).
Dual-Rod Constructs
The Kostuik-Harrington device was developed to improve the poor results obtained with posterior Harrington distraction rods.104 Unlike plate systems, the Kostuik-Harrington system allows the application of distractive forces in addition to fixation. This system provides adequate stabilization as long as it is constructed in a rectangular or parallelogram fashion. The construct consists of a standard Harrington distraction system along with a compression rod. Two types of collar-ended screws are crimped over the compression rod and ratcheted to fit onto the distraction rod. With no toggle at the screw-rod interface, this system is classified as a rigid distraction construct. Clinical success has been reported with the Kostuik-Harrington device,49,104–106 but newer constructs tend to be less cumbersome and easier to implant.
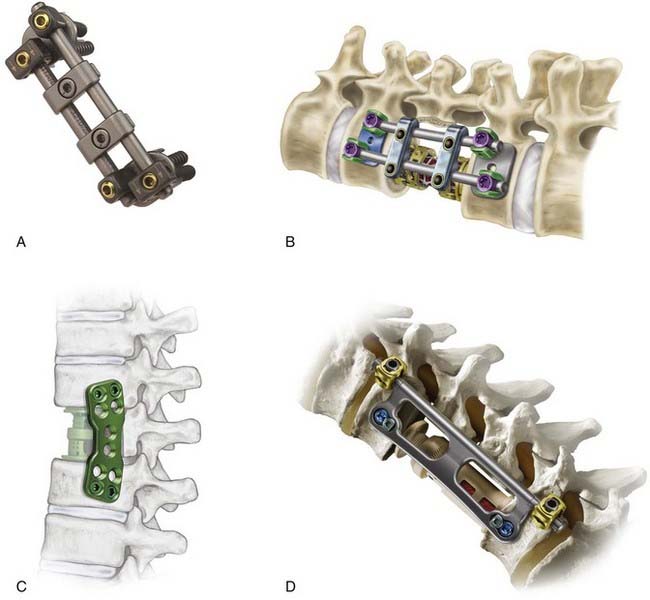
The Kaneda SR Anterior Spinal System (Depuy Acromed, Raynham, MA) was developed in 1984 for the treatment of thoracolumbar fractures (Fig. 300-5A).107 Many of the newer generation screw-rod constructs are a variation on the general theme introduced by the Kaneda device. This device consists of spiked vertebral plates, cancellous screws, a smooth rod, and transverse couplers. The vertebral plate has four spikes on its undersurface and is impacted into the ventrolateral surface of the vertebral body. These plates serve as templates for screw placement, prevent screw migration, and help resist axial loads. After the rod is inserted, both compressive and distractive forces can be applied to the construct before final tightening of the vertebral body screw. The transverse coupler increases the stability of the construct against both rotational and flexion-extension forces. The Kaneda device also allows multisegmental fixation, with intervening vertebral bodies fastened to the construct through separate screws. The indications for this construct are identical to those for the Kostuik-Harrington construct and include correction of scoliotic deformities. The fusion rate is greater than 95%.108
The CD Horizon Legacy Anterior Spinal System (Medtronic Sofamor Danek, Memphis, TN) is similar to the Kaneda system and is an update of the previous-generation CD Horizon Antares dual-rod system (Fig. 300-5B). It consists of a series of vertebral body staples, rods, screws, cross-link plates, and connecting components that permit distraction to accommodate the interbody graft. This recent version includes staples contoured to fit the lateral surface of the vertebral body to provide greater stability.109
Screw-Plate Constructs
The Anterior Thoracolumbar Locking Plate (Synthes, Paoli, PA) is designed to stabilize the spine across the thoracolumbar junction and lumbar spine. Despite the construct’s rigidity, the graft can be compressed during plate insertion with a separate set of screws. Two cancellous bone screws are inserted into the angled dynamic compression plate holes and sequentially tightened to produce temporary compression. This places compressive force on the vertebral body before insertion of the locking screws. Once the temporary fixation is complete, the locking screws are inserted and permanently maintain the compression placed across the graft. The plate hole is threaded so that the locking screw trajectory is perpendicular to the plate to ensure proper insertion. Therefore, this system is rigid.110 Clinical results with this system have been favorable and support its use as a safe and effective anterior fixation construct.111 The Synthes Thoracolumbar Spine Locking Plate system has been released recently and is the update to the Anterior Thoracolumbar Locking Plate (Fig. 300-5C). Adjustments include multiple screw hole locations, as well as conical screw holes that allow up to 5 degrees of angulation during screw insertion.112
The Gateway Thoracolumbar Plate System (Globus, Audubon, PA) is a hybrid lateral thoracolumbar system that combines the features of a plate and dual-rod system (Fig. 300-5D). Windows in the plate allow visualization and access to the interbody strut graft. The device is secured with top-loading screws on the posterior side (rod portion) of the construct. Distraction and compression can be applied across the posterior screws. Before tightening these posterior screws, the plate can be rotated to allow continued access to the corpectomy site and interbody graft. Once the plate is seated on the lateral surface of the vertebral body, anterior screws are inserted to lock the implant into position.113
Complications Related to Operative Intervention
The key to avoiding complications related to ventral instrumentation of the thoracic spine is appropriate preoperative planning, including comprehensive preoperative medical and pulmonary evaluation if needed. Complications related to the implantation of ventral thoracic instrumentation can occur at any point during the procedure.114 Rare injuries to internal organs (lungs, diaphragm, kidneys, ureters) or major vessels (aorta, vena cava) have been reported.115 During a transcavitary exposure, the assistance of a thoracic or general surgeon may significantly reduce the incidence of such injuries. Damage to the neural elements is more likely to occur during decompression and reconstruction. Excessive distraction of the deformity can lead to a stretch injury or vascular compromise and should be avoided. Neurological changes can also occur secondary to epidural hematoma formation or dislodgment of the graft. Oftentimes, ipsilateral hip flexion weakness is seen and is secondary to iliopsoas dissection.72 If the dura is violated, every attempt should be made to obtain watertight closure. Persistent tears in the dura significantly increase the risk for infection and interfere with wound healing, particularly if a chest tube must be inserted. In such a scenario, greater than 20–cm H2O suction should be avoided, and transition to a water seal as soon as possible is recommended to prevent the formation of a cerebrospinal fluid fistula.
Complications related to the instrumentation or fusion usually occur during the postoperative period or after recovery.114 Instrumentation failure, including fracture, loosening, or pullout, can be reduced by appropriate selection of constructs and insertion techniques. Despite these precautions, instrumentation fails in as many as 15% of cases.116
Boden SD, Schimandle JH. Biologic enhancement of spinal fusion. Spine. 1995;20:113S-123S.
Chou D, Lu DC, Weinstein P, et al. Adjacent-level vertebral body fractures after expandable cage reconstruction. J Neurosurg Spine. 2008;8:584-588.
Cooper PR, Errico TJ, Martin R, et al. A systematic approach to spinal reconstruction after anterior decompression for neoplastic disease of the thoracic and lumbar spine. Neurosurgery. 1993;32:1-8.
Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8:817-831.
Ebraheim NA, Xu R, Ahmad M, et al. Anatomic considerations of anterior instrumentation of the thoracic spine. Am J Orthop. 1997;26:419-424.
Ikard RW. Methods and complications of anterior exposure of the thoracic and lumbar spine. Arch Surg. 2006;141:1025-1034.
Johnson J, Pare LS, Torres R. Thoracolumbar vertebral body replacement: materials and techniques. Contemp Neurosurg. 1998;20:1-8.
Kaneda K, Abumi K, Fujiya M. Burst fractures with neurologic deficits of the thoracolumbar-lumbar spine. Results of anterior decompression and stabilization with anterior instrumentation. Spine. 1984;9:788-795.
Kostuik JP, Matsusaki H. Anterior stabilization, instrumentation, and decompression for post-traumatic kyphosis. Spine. 1989;14:379-386.
Lange U, Edeling S, Knop C, et al. Anterior vertebral body replacement with a titanium implant of adjustable height: a prospective clinical study. Eur Spine J. 2007;16:161-172.
McCormick PC. Surgical management of dumbbell and paraspinal tumors of the thoracic and lumbar spine. Neurosurgery. 1996;38:67-74.
McCormick PC. Retropleural approach to the thoracic and thoracolumbar spine. Neurosurgery. 1995;37:908-914.
Pflugmacher R, Schleicher P, Schaefer J, et al. Biomechanical comparison of expandable cages for vertebral body replacement in the thoracolumbar spine. Spine. 2004;29:1413-1419.
Pointillart V, Aurouer N, Gangnet N, et al. Anterior approach to the cervicothoracic junction without sternotomy: a report of 37 cases. Spine. 2007;32:2875-2879.
Prolo DJ, Rodrigo JJ. Contemporary bone graft physiology and surgery. Clin Orthop Relat Res. 1985;200:322-342.
Riaz S, Fox R, Lavoie MV, et al. Vertebral body reconstruction for thoracolumbar spinal metastasis—a review of techniques. J Ayub Med Coll Abbottabad. 2006;18:70-77.
Rodts G, Mummaneni P, Haid RJr, et al. Ventral and lateral thoracic and lumbar fixation techniques, 2nd ed. Benzel E, editor. Spine Surgery: Techniques, Complication Avoidance, and Management, Vol 2. New York: Churchill Livingstone. 2004:1486-1503.
Sundaresan N, Krol G, Steinberger AA, et al. Management of tumors of the thoracolumbar spine. Neurosurg Clin N Am. 1997;8:541-553.
Thongtrangan I, Balabhadra RS, Le H, et al. Vertebral body replacement with an expandable cage for reconstruction after spinal tumor resection. Neurosurg Focus. 2003;15(5):E8.
Yao K, Guiot B, Mendel E. Anterior thoracolumbar instrumentation. In: Wolfa C, Resnick D, editors. Neurosurgical Operative Atlas, Spine and Peripheral Nerves. 2nd ed. New York: Thieme; 2007:216-221.
1 Royle N. The operative removal of an accessory vertebra. Med J Aust. 1928;1:467-468.
2 Hodgson A, Stock F. Anterior spinal fusion: a preliminary communication on radical treatment of Pott’s disease and Pott’s paraplegia. Br J Surg. 1956;44:266-275.
3 Humphries A, Hawk W. Anterior fusion of the lumbar spine using an internal fixative device. Surg Forum. 1958;9:770-773.
4 Dwyer A, Newton N, Sherwood A. Anterior approach to scoliosis: a preliminary report. Clin Orthop Relat Res. 1969;69:192-202.
5 Dwyer A, Schafer M. Anterior approach to scoliosis: results of treatment in fifty-one cases. J Bone Joint Surg Br. 1974;56:218-224.
6 Zielke K, Stunkat R, Beaujean F. [Ventrale derotations-spondylodesis.]. Arch Orthop Unfallchir. 1976;85:257-277.
7 Dunn H. Anterior stabilization of thoracolumbar injuries. Clin Orthop Relat Res. 1984;189:116-124.
8 Dunn H. Anterior spine stabilization and decompression for thoracolumbar injuries. Orthop Clin North Am. 1986;17:113-119.
9 Brown L, Bridwell K, Holt R. Aortic erosions and lacerations associated with the Dunn anterior spinal instrumentation. Paper presented at the Scoliosis Research Society, San Diego, Ca, 1985.
10 Findlay G. Adverse effects of the management of malignant spinal cord compression. J Neurol Neurosurg Psychiatry. 1984;47:761-768.
11 Gilbert R, Kim J, Posner J. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1978;4:40-51.
12 Black P. Spinal metastasis: current status and recommended guidelines for management. Neurosurgery. 1979;5:726-746.
13 Young R, Post E, King G. Treatment of spinal epidural metastases: randomized prospective comparison of laminectomy and radiotherapy. J Neurosurg. 1980;53:741-748.
14 Wong D, Fornasier V, MacNab I. Spinal metastasis: the obvious, the occult, and the impostors. Spine. 1990;15:1-4.
15 Sundaresan N, Digiacinto G, Hughes J. Surgical treatment of spinal metastases. Clin Neurosurg. 1986;33:503-522.
16 Johnson J, Leatherman K, Holt R. Anterior decompression of the spinal cord for neurological deficit. Spine. 1983;8:396-405.
17 Siegal T, Tiqua P, Siegal T. Vertebral body resection for epidural compression by malignant tumors: results of forty-seven consecutive operative procedures. J Bone Joint Surg Am. 1985;67:375-382.
18 Harrington K. Anterior decompression and stabilization of the spine as a treatment for vertebral collapse and spinal cord compression from metastatic malignancy. Clin Orthop Relat Res. 1988;233:177-197.
19 Sundaresan N, Digiacinto V, Hughes J, et al. Treatment of neoplastic spinal cord compression: results of a prospective study. Neurosurgery. 1991;29:645-650.
20 Cooper PR, Errico TJ, Martin R, et al. A systematic approach to spinal reconstruction after anterior decompression for neoplastic disease of the thoracic and lumbar spine. Neurosurgery. 1993;32:1-8.
21 Sonntag V, Marcotte P. A systematic approach to spinal reconstruction after anterior decompression for neoplastic disease of the thoracic and lumbar spine. Comment on Cooper PR, et al. Neurosurgery. 1993;32;:1-8.
22 Sundaresan N, Krol G, Steinberger AA, et al. Management of tumors of the thoracolumbar spine. Neurosurg Clin N Am. 1997;8:541-553.
23 Karnofsky D, Abelmann W, Craver L, et al. The use of nitrogen mustards in the palliative treatment of carcinoma: with particular reference to bronchogenic carcinoma. Cancer. 1948;1:634-656.
24 Mahaley MSJr, Mettlin C, Natarajan N, et al. National survey of patterns of care for brain-tumor patients. J Neurosurg. 1989;71:826-836.
25 Tokuhashi Y, Matsuzaki H, Toriyama S, et al. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine. 1990;15:1110-1113.
26 Greenberg H, Kim J, Posner J. Epidural spinal cord compression from metastatic tumor: results with a new treatment protocol. Ann Neurol. 1980;8:361-366.
27 Boland PJ, Lane JM, Sundaresan N. Metastatic disease of the spine. Clin Orthop Relat Res. 1982;169:95-102.
28 Keene JS. Radiographic evaluation of thoracolumbar fractures. Clin Orthop Relat Res. 1984;189:58-64.
29 Mumford J, Weinstein JN, Spratt KF, et al. Thoracolumbar burst fractures. The clinical efficacy and outcome of nonoperative management. Spine. 1993;18:955-970.
30 Errico T, Bauer R, Waugh T. Spinal Trauma. Philadelphia: JP Lippincott; 1990.
31 Berg EE. The sternal-rib complex. A possible fourth column in thoracic spine fractures. Spine. 1993;18:1916-1919.
32 Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8:817-831.
33 Bohlman HH. Treatment of fractures and dislocations of the thoracic and lumbar spine. J Bone Joint Surg Am. 1985;67:165-169.
34 Willen J, Lindahl S, Nordwall A. Unstable thoracolumbar fractures. A comparative clinical study of conservative treatment and Harrington instrumentation. Spine. 1985;10:111-122.
35 Esses SI, Botsford DJ, Kostuik JP. Evaluation of surgical treatment for burst fractures. Spine. 1990;15:667-673.
36 Bohlman HH, Freehafer A, Dejak J. The results of treatment of acute injuries of the upper thoracic spine with paralysis. J Bone Joint Surg Am. 1985;67:360-369.
37 Kostuik JP. Anterior fixation for burst fractures of the thoracic and lumbar spine with or without neurological involvement. Spine. 1988;13:286-293.
38 Gertzbein SD, Macmichael D, Tile M. Harrington instrumentation as a method of fixation in fractures of the spine. J Bone Joint Surg Br. 1982;64:526-529.
39 Moreland DB, Egnatchik JG, Bennett GJ. Cotrel-Dubousset instrumentation for the treatment of thoracolumbar fractures. Neurosurgery. 1990;27:69-73.
40 Zou D, Yoo JU, Edwards WT, et al. Mechanics of anatomic reduction of thoracolumbar burst fractures. Comparison of distraction versus distraction plus lordosis, in the anatomic reduction of the thoracolumbar burst fracture. Spine. 1993;18:195-203.
41 Dekutoski MB, Conlan ES, Salciccioli GG. Spinal mobility and deformity after Harrington rod stabilization and limited arthrodesis of thoracolumbar fractures. J Bone Joint Surg Am. 1993;75:168-176.
42 Cahill DW, Love LC, Rechtine GR. Pyogenic osteomyelitis of the spine in the elderly. J Neurosurg. 1991;74:878-886.
43 Baker AS, Ojemann RG, Swartz MN, et al. Spinal epidural abscess. N Engl J Med. 1975;293:463-468.
44 Kostuik JP. Anterior spinal cord decompression for lesions of the thoracic and lumbar spine, techniques, new methods of internal fixation results. Spine. 1983;8:512-531.
45 Oga M, Arizono T, Takasita M, et al. Evaluation of the risk of instrumentation as a foreign body in spinal tuberculosis. Clinical and biologic study. Spine. 1993;18:1890-1894.
46 Dai LY, Chen WH, Jiang LS. Anterior instrumentation for the treatment of pyogenic vertebral osteomyelitis of thoracic and lumbar spine. Eur Spine J. 2008;17:1027-1034.
47 Jin D, Qu D, Chen J, et al. One-stage anterior interbody autografting and instrumentation in primary surgical management of thoracolumbar spinal tuberculosis. Eur Spine J. 2004;13:114-121.
48 Malcolm BW, Bradford DS, Winter RB, et al. Post-traumatic kyphosis. A review of forty-eight surgically treated patients. J Bone Joint Surg Am. 1981;63:891-899.
49 Roberson JR, Whitesides TEJr. Surgical reconstruction of late post-traumatic thoracolumbar kyphosis. Spine. 1985;10:307-312.
50 Kostuik JP, Matsusaki H. Anterior stabilization, instrumentation, and decompression for post-traumatic kyphosis. Spine. 1989;14:379-386.
51 Chang KW. Oligosegmental correction of post-traumatic thoracolumbar angular kyphosis. Spine. 1993;18:1909-1915.
52 Le Roux PD, Haglund MM, Harris AB. Thoracic disc disease: experience with the transpedicular approach in twenty consecutive patients. Neurosurgery. 1993;33:58-66.
53 Sar C, Hamzaoglu A, Talu U, et al. An anterior approach to the cervicothoracic junction of the spine (modified osteotomy of manubrium sterni and clavicle). J Spinal Disord. 1999;12:102-106.
54 Darling GE, McBroom R, Perrin R. Modified anterior approach to the cervicothoracic junction. Spine. 1995;20:1519-1521.
55 Kurz LT, Pursel SE, Herkowitz HN. Modified anterior approach to the cervicothoracic junction. Spine. 1991;16:S542-547.
56 Pointillart V, Aurouer N, Gangnet N, et al. Anterior approach to the cervicothoracic junction without sternotomy: a report of 37 cases. Spine. 2007;32:2875-2879.
57 Cohen ZR, Fourney DR, Gokaslan ZL, et al. Anterior stabilization of the upper thoracic spine via an “interaortocaval subinnominate window”: case report and description of operative technique. J Spinal Disord Tech. 2004;17:543-548.
58 Cauchoix J, Binet JP. Anterior surgical approaches to the spine. Ann R Coll Surg Engl. 1957;21:234-243.
59 Standefer M, Hardy RWJr, Marks K, et al. Chondromyxoid fibroma of the cervical spine—a case report with a review of the literature and a description of an operative approach to the lower anterior cervical spine. Neurosurgery. 1982;11:288-292.
60 Micheli LJ, Hood RW. Anterior exposure of the cervicothoracic spine using a combined cervical and thoracic approach. J Bone Joint Surg Am. 1983;65:992-997.
61 Nazzaro JM, Arbit E, Burt M. “Trap door” exposure of the cervicothoracic junction. Technical note. J Neurosurg. 1994;80:338-341.
62 Larson SJ, Holst RA, Hemmy DC, et al. Lateral extracavitary approach to traumatic lesions of the thoracic and lumbar spine. J Neurosurg. 1976;45:628-637.
63 McCormick P, Holtzman RN, Stein BM, Farcy JP. The lateral extracavitary approach to the thoracic and lumbar spine. In: Spinal Instability. New York: Raven Press; 1993.
64 Heiple KG, Chase SW, Herndon CH. A comparative study of the healing process following different types of bone transplantation. J Bone Joint Surg Am. 1963;45:1593-1616.
65 Prolo DJ, Rodrigo JJ. Contemporary bone graft physiology and surgery. Clin Orthop Relat Res. 1985;200:322-342.
66 Boden SD, Schimandle JH. Biologic enhancement of spinal fusion. Spine. 1995;20:113S-123S.
67 Laurie SW, Kaban LB, Mulliken JB, et al. Donor-site morbidity after harvesting rib and iliac bone. Plast Reconstr Surg. 1984;73:933-938.
68 Keller E, Triplett W. Iliac bone graft: review of 160 consecutive cases. J Oral Maxillofac Surg. 1987;45:11-14.
69 Summers BN, Eisenstein SM. Donor site pain from the ilium. A complication of lumbar spine fusion. J Bone Joint Surg Br. 1989;71:677-680.
70 Younger E, Chapman M. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192-195.
71 Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine. 1995;20:1055-1060.
72 Yao K, Guiot B, Mendel E. Anterior thoracolumbar instrumentation. In: Wolfa C, Resnick D, editors. Neurosurgical Operative Atlas, Spine and Peripheral Nerves. 2nd ed. New York: Thieme; 2007:216-221.
73 Bradford DS. Anterior vascular pedicle bone grafting for the treatment of kyphosis. Spine. 1980;5:318-323.
74 McBride GG, Bradford DS. Vertebral body replacement with femoral neck allograft and vascularized rib strut graft. A technique for treating post-traumatic kyphosis with neurologic deficit. Spine. 1983;8:406-415.
75 Shaffer JW, Field GA, Goldberg VM, et al. Fate of vascularized and nonvascularized autografts. Clin Orthop Relat Res. 1985;197:32-43.
76 Blumenthal SL, Baker J, Dossett A, et al. The role of anterior lumbar fusion for internal disc disruption. Spine. 1988;13:566-569.
77 Hanley E, Harvell J, Shapiro D, et al. Use of allograft bone in cervical spine surgery. Semin Spine Surg. 1989;1:262-270.
78 Einhorn TA, Lane JM, Burstein AH, et al. The healing of segmental bone defects induced by demineralized bone matrix. A radiographic and biomechanical study. J Bone Joint Surg Am. 1984;66:274-279.
79 Dahners LE, Jacobs RR. Long bone defects treated with demineralized bone. South Med J. 1985;78:933-934.
80 Gepstein R, Weiss RE, Hallel T. Bridging large defects in bone by demineralized bone matrix in the form of a powder. A radiographic, histological, and radioisotope-uptake study in rats. J Bone Joint Surg Am. 1987;69:984-992.
81 Hulth A, Johnell O, Henricson A. The implantation of demineralized fracture matrix yields more new bone formation than does intact matrix. Clin Orthop Relat Res. 1988;234:235-239.
82 Urist MR, Dowell TA, Hay PH, et al. Inductive substrates for bone formation. Clin Orthop Relat Res. 1968;59:59-96.
83 Leads from the MMWR. Transmission of HIV through bone transplantation: case report and public health recommendations. JAMA. 1988;260:2487-2488.
84 Buck BE, Malinin TI, Brown MD. Bone transplantation and human immunodeficiency virus. An estimate of risk of acquired immunodeficiency syndrome (AIDS). Clin Orthop Relat Res. 1989;240:129-136.
85 Arbit E, Galicich JH. Vertebral body reconstruction with a modified Harrington rod distraction system for stabilization of the spine affected with metastatic disease. J Neurosurg. 1995;83:617-620.
86 Johnson J, Pare LS, Torres R. Thoracolumbar vertebral body replacement: materials and techniques. Contemp Neurosurg. 1998;20:1-8.
87 Ernstberger T, Kogel M, Konig F, et al. Expandable vertebral body replacement in patients with thoracolumbar spine tumors. Arch Orthop Trauma Surg. 2005;125:660-669.
88 Uchida K, Kobayashi S, Nakajima H, et al. Anterior expandable strut cage replacement for osteoporotic thoracolumbar vertebral collapse. J Neurosurg Spine. 2006;4:454-462.
89 Lange U, Edeling S, Knop C, et al. Anterior vertebral body replacement with a titanium implant of adjustable height: a prospective clinical study. Eur Spine J. 2007;16:161-172.
90 Pflugmacher R, Schleicher P, Schaefer J, et al. Biomechanical comparison of expandable cages for vertebral body replacement in the thoracolumbar spine. Spine. 2004;29:1413-1419.
91 Thongtrangan I, Balabhadra RS, Le H, et al. Vertebral body replacement with an expandable cage for reconstruction after spinal tumor resection. Neurosurg Focus. 2003;15(5):E8.
92 Riaz S, Fox R, Lavoie MV, et al. Vertebral body reconstruction for thoracolumbar spinal metastasis—a review of techniques. J Ayub Med Coll Abbottabad. 2006;18:70-77.
93 Chou D, Lu DC, Weinstein P, et al. Adjacent-level vertebral body fractures after expandable cage reconstruction. J Neurosurg Spine. 2008;8:584-588.
94 White AI, Panjab IM. Clinical Biomechanics of the Spine. Philadelphia: JB Lippincott; 1990.
95 Benzel E. Spinal deformities. In: Benzel E, editor. Biomechanics of Spine Stabilization: Principles and Clinical Practice. New York: McGraw-Hill; 1995:76-77.
96 Benzel E. Spinal fusion. In: Benzel E, editor. Biomechanics of Spine Stabilization: Principles and Clinical Practice. New York: McGraw-Hill; 1995:103-105.
97 Kanayama M, Ng JT, Cunningham BW, et al. Biomechanical analysis of anterior versus circumferential spinal reconstruction for various anatomic stages of tumor lesions. Spine. 1999;24:445-450.
98 Isaacs R. Anterolateral graft and plate reconstruction. In: Fessler R, Sekhar L, editors. Atlas of Neurosurgical Techniques: Spine. New York: Thieme; 2006:436-440.
99 Rodts G, Mummaneni P, Haid RJr, et al. Ventral and lateral thoracic and lumbar fixation techniques, 2nd ed. Benzel E, editor. Spine Surgery: Techniques, Complication Avoidance, and Management, Vol 2. New York: Churchill Livingstone. 2004:1486-1503.
100 Brodke DS, Gollogly S, Bachus KN, et al. Anterior thoracolumbar instrumentation: stiffness and load sharing characteristics of plate and rod systems. Spine. 2003;28:1794-1801.
101 Ebraheim NA, Xu R, Ahmad M, et al. Anatomic considerations of anterior instrumentation of the thoracic spine. Am J Orthop. 1997;26:419-424.
102 Chou D, Larios AE, Chamberlain RH, et al. A biomechanical comparison of three anterior thoracolumbar implants after corpectomy: are two screws better than one? J Neurosurg Spine. 2006;4:213-218.
103 Benzel E. Anterior cantilever beam fixation and related techniques. In: Benzel E, editor. Biomechanics of Spine Stabilization: Principles and Clinical Practice. New York: McGraw-Hill; 1995:187-189.
104 Kostuik J. Anterior Kostuik-Harrington distraction systems. In: An H, Cotler J, editors. Spinal Instrumentation. Baltimore: Williams & Wilkins; 1992:359-377.
105 Oglivie J. Zielke instrumentation of the spine. In: An H, Cotler J, editors. Spinal Instrumentation. Baltimore: Williams & Wilkins; 1992:353-358.
106 Kostuik JP. Anterior fixation for fractures of the thoracic and lumbar spine with or without neurologic involvement. Clin Orthop Relat Res. 1984;189:103-115.
107 Kaneda K, Abumi K, Fujiya M. Burst fractures with neurologic deficits of the thoracolumbar-lumbar spine. Results of anterior decompression and stabilization with anterior instrumentation. Spine. 1984;9:788-795.
108 Kaneda K. Kaneda anterior spinal instrumentation for the thoracic and lumbar spine. In: An H, Cotler J, editors. Spinal Instrumentation. Baltimore: Williams & Wilkins; 1992:413-433.
109 Schwartz D, Marciano F, Anderson D, et al. CD Horizon Legacy Anterior Spinal System Tumor/Trauma Surgical Technique. Memphis, TN: Medtronic Sofamor Danek; 2007.
110 Aebi M, Thalgott J, Webb J. Stabilization techniques: Thoracolumbar spine. In: Aebi M, Thalgott J, Webb J, editors. AO ASIF Principles in Spine Surgery. Berlin: Springer; 1998:85-88.
111 Thalgott JS, Kabins MB, Timlin M, et al. Four year experience with the AO anterior thoracolumbar locking plate. Spinal Cord. 1997;35:286-291.
112 The Thoracolumbar Spine Locking Plate (TSLP) System: Technique Guide. Paoli, PA: Synthes Spine, 2004.
113 GATEWAY Thoracolumbar Plate System, Surgical Technique. Audubon, PA: Globus Medical, 2007.
114 Ikard RW. Methods and complications of anterior exposure of the thoracic and lumbar spine. Arch Surg. 2006;141:1025-1034.
115 Matsuzaki H, Tokuhashi Y, Wakabayashi K, et al. Penetration of a screw into the thoracic aorta in anterior spinal instrumentation. A case report. Spine. 1993;18:2327-2331.
116 McLain RF, Burkus JK, Benson DR. Segmental instrumentation for thoracic and thoracolumbar fractures: prospective analysis of construct survival and five-year follow-up. Spine J. 2001;1:310-323.