CHAPTER 302 Anterior Lumbar Instrumentation
Indications
Neoplasm
The spinal column is the most frequent site of bony metastases1; up to two thirds of these arise from breast, prostate, lung, and hematopoietic cancers.1–6 Primary tumors of the spine, on the other hand, are extremely rare. Less than 10% of reported bone tumors and soft tissue sarcomas involve the spine.7,8 Clinical manifestations of spinal tumors result from expansion of the periosteum or cortex, pathologic compression fractures, spinal instability, spinal deformity, and direct compression of neural elements. By far the most common symptom produced by either primary or metastatic lesions is pain,9–12 stereotypically a constant pain that is not relieved by rest and intensifies at night. Progression of the tumor beyond the confines of the vertebral body may produce myeloradiculopathy because of direct compression of neural elements or compromise of vascular supply. Acute partial loss of neurological function may be an indication for urgent surgical intervention because studies have shown improvement in neurological function following early surgical treatment in such circumstances.13,14
Numerous series have reported the successful treatment of spinal neoplastic disease with a ventral or ventrolateral approach.6,14–22 Prognostic factors, including tumor type and location, preoperative neurological status, overall life expectancy and medical condition correlate with surgical outcome23,24 and should be carefully considered in formulating a management strategy for each patient.
Trauma
Compared with fractures of the thoracolumbar junction, fractures of the lumbar spine distal to L2 are relatively uncommon, with less than 4% of all fractures involving L3-5.25,26 Certain mechanisms of high-energy traumatic injury tend to be associated with both thoracolumbar and lumbar fractures. Falls from a significant height that produce a substantial axial load may result in unstable burst fractures. Passengers wearing lower abdominal seat belts during motor vehicle accidents may sustain flexion or flexion-distraction injuries to the lumbar or thoracolumbar spine, with the seat belt acting as a fulcrum.
The decision to treat a lumbar spine trauma patient operatively is based on multiple factors. In addition to the presence of a neurological deficit, the extent of spinal canal encroachment or compromise, loss of vertebral body height, degree of local kyphotic deformity, and fracture pattern may guide treatment decision making.27–38 Various classification schemes have been proposed for spinal fractures, many of which are based on Denis’ three-column model of spinal stability and on the mechanism of spinal injury.39–43 Generally, a fracture that compromises the anterior and middle columns is likely to be unstable, but isolated anterior column involvement causing significant kyphosis may also warrant surgical intervention to prevent the development of a progressive deformity.44 The surgeon must also decide whether a stand-alone anterior construct is appropriate or if a circumferential (anterior-posterior) construct and arthrodesis are necessary.
Surgical decompression is the first-line treatment for a patient with an acute partial neurological deficit due to neural compression following trauma. Emergent decompression is recommended for a progressive neurological deficit and a grossly unstable spine. The indications for operative treatment of patients with fixed neurological deficits are less clear. Advocates who favor delaying surgery believe that there is a reduced risk of neural injury and intraoperative blood loss with surgery after the acute period.45,46 Proponents of early surgery, on the other hand, contend that it provides the best opportunity for neurological recovery and that delaying intervention may make it more difficult to achieve fracture reduction or spinal correction. Patients may also be mobilized early after surgery, thereby avoiding the complications of prolonged bed rest.47
Degenerative Disease
Degenerative disease is a common condition of the lumbar spine, which is often characterized by chronic, progressive instability.48 Reconstruction of the anterior column can effectively improve sagittal balance, restore lumbar lordosis, and enlarge the neural foramina, reversing the pathologic consequences of degenerative processes.49,50 Anterior interbody fusion may be indicated for a wide range of degenerative pathology including degenerative disk disease, lumbar instability, iatrogenic instability, pseudoarthrosis following posterior arthrodesis, or a grade I or II spondylolisthesis.50–58 There are, however, several relative contraindications to anterior interbody fusion procedures including severe osteoporosis, grades III and IV spondylolisthesis, and extensive vertebral body destruction. In cases of severe degenerative destruction of vertebral bodies, or a significant local spinal deformity, multisegmental ventral instrumentation constructs may be necessary, either alone or in conjunction with posterior fixation.
Infection
Bacterial organisms, usually Staphylococcus aureus, are the most common causes of spinal infections, and most spinal infections are treated with intravenous antibiotics and immobilization before the onset of a neurological deficit or spinal deformity.59–61 Operative indications include a progressive neurological deficit, a significant spinal deformity, intractable pain, or, more rarely, progression of the infection despite appropriate antibiotic therapy. Because infections of the vertebral body affect the anterior and middle columns, the surgical approach must provide access to the ventral spine to allow complete débridement of the active infection. This often involves discectomies and a corpectomy of the involved vertebral body followed by a fusion. Evidence suggests that the rate of postoperative infection is not increased in the presence of metallic implants provided débridement has been adequately performed.59,62
Surgical Approaches
The decision of which approach to use is influenced primarily by which lumbar levels are involved and by the goals of surgery. Over the years, several ventral approaches for access to the anterior lumbar spine have been developed and refined.58,63,64 The approach selected should provide maximal visualization of the lesion and regional anatomy. For instance, exposure of the thoracolumbar junction for access to L1 requires a thoracoabdominal approach and release of the diaphragm. Lesions located between L2 and L5 can be addressed through a flank or paramedian incision and a retroperitoneal approach. If midline exposure of L2 to L5 is required, a transperitoneal approach is often most direct. The side of the approach is ultimately dictated by the location and nature of the pathology, but a left-sided approach is usually preferred because the liver is avoided and the aorta is easier to mobilize and less susceptible to injury than the vena cava. Our practice is to use a general or vascular surgeon for anterior approaches to the lumbar spine to ensure a safe and adequate exposure. We scrub in, however, as assistants, to gain experience with the approaches and to help tailor the exposure to the requirements of the individual case.
Thoracoabdominal Approach (T11-L2)
The thoracoabdominal approach provides for the best exposure of T12-L2. This approach requires a rib resection; generally, resection of the rib two levels above the level of primary pathology is performed.65–67 Hence, resection of the ninth rib provides the best window of access to T11-12, and is accomplished through a transthoracic approach, whereas exposure of T12-L1 may be accomplished via a thoracoabdominal 10th rib approach. Generally, thoracoabdominal exposure can be extended distally to gain access to additional lumbar levels without significant difficulty.
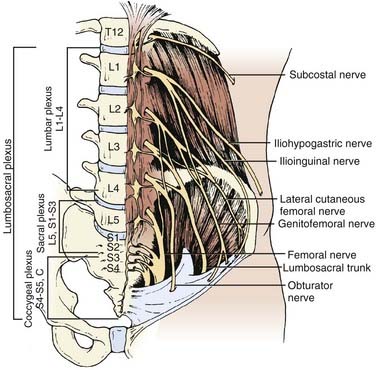
The patient is placed in the lateral decubitus position. A left-sided approach is usually preferred to avoid the inferior vena cava and the liver. An incision is marked using fluoroscopy and is made from the lateral border of the paraspinal musculature along the 10th rib to the junction of the rib and the costal cartilage.68 A circumferential subperiosteal rib dissection is performed using monopolar cautery and Doyen dissectors with care taken to avoid the neurovascular bundle that lies on the inferior aspect of the rib. The rib is divided posteriorly at the angle of the rib and at the junction of the rib and costal cartilage. The rib is removed, the remaining costal cartilage is divided lengthwise with a knife or scissors, and the flaps of cartilage are retracted. The retroperitoneal space is identified with blunt dissection deep to the costal cartilage and the peritoneum is dissected off the inferior surface of the diaphragm. The peritoneum is then retracted medially, and the abdominal musculature is divided in layers, revealing the diaphragm. The endothoracic fascia within the rib bed is divided along with the parietal pleura, which is deep to it. The soft tissue is swept off the thoracic and abdominal surfaces of the diaphragm, which is then incised circumferentially leaving a cuff of muscle of approximately attached to the chest wall. The crus of the diaphragm is cut and elevated off the spinal column. Alternatively, the diaphragm is bluntly stripped from the chest wall with a Cobb elevator or sponge stick. This technique obviates the necessity of reapproximating the diaphragm at closure. A large Deaver retractor is used to retract the peritoneal sac anteromedially, and a large rib retractor opens the 10th rib space to reveal the thoracolumbar junction. In the lumbar spine, the psoas must be elevated dorsolaterally off the vertebral bodies to allow full visualization as far distally as the lesion dictates, taking care not to injure the lumbar plexus that runs within the muscle or the genitofemoral nerve that runs on its surface. A table-mounted retractor with multiple adjustable arms is used to maintain exposure during the procedure.
Retroperitoneal Flank Approach (L2-4)
The patient is placed in a lateral decubitus position with appropriate padding to prevent pressure ulcerations and neuropathies. If an electric operating table is used, the patient is positioned so that flexion of the operating table opens the space between the iliac crest and costal margin; otherwise a small rolled towel or pad may be used to achieve the same position. The incision begins in the midaxillary line between the inferior margin of the ribs and the iliac crest and follows an inferior oblique course to the lateral edge of the rectus sheath. The level of the incision is determined using fluoroscopy based on the desired level of exposure (Fig. 302-1). For lesions of the upper lumbar spine, the incision should be made above the umbilicus along the 11th or 12th rib. Occasionally a portion of the rib may be osteotomized or excised to improve exposure and can then be subsequently used as graft material. For lesions in the midlumbar spine, the incision starts at the level of the umbilicus. The lower lumbar spine is accessed through an incision superior to the midpoint between the umbilicus and the symphysis pubis. If exposure distal to L4 is required, the medial aspect of the incision can be extended caudally, essentially adding a paramedian approach type of incision to the traditional flank incision.
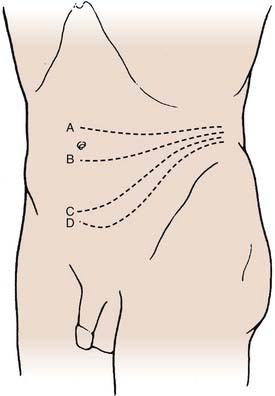
FIGURE 302-1 Transverse incisions for the retroperitoneal approach: L2-3 (A), L3-4 (B), L4-5 (C), and L5-S1 (D).
(From Benzel EC. Spine Surgery: Techniques, Complication Avoidance, and Management. Philadelphia: Churchill Livingstone; 1999.)
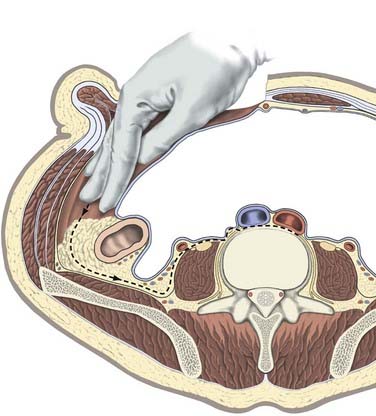
The underlying musculature, including the latissimus dorsi, serratus posterior inferior, external oblique, and internal oblique muscles, are transected in line with the skin incision. The transversalis fascia is identified and divided to enter the retroperitoneal space. The underlying peritoneum is identified as a semitranslucent layer and carefully separated from the abdominal wall using blunt dissection with a finger or a sponge stick. Adhesions, particularly toward the midline at the rectus sheath, may require sharp dissection. A plane between the quadratus lumborum muscle and retroperitoneal organs is developed, and the viscera, including the kidney, perirenal fat, and ureter, are retracted medially (Fig. 302-2). Dissection dorsal to the psoas muscle is avoided because this plane ends in a blind pouch. The dissection is continued caudally, mobilizing the peritoneum off the posterior abdominal wall to the level of the sacrum. The retroperitoneal organs and peritoneal contents are padded with moist sponges and retracted medially and positioned outside the operative field using a table-mounted retractor system. The dissection continues dorsomedially to identify the psoas muscle and genitofemoral nerve. This nerve lies along the ventral surface of the psoas muscle and should not be injured (Fig. 302-3). The psoas muscle may require dorsolateral mobilization with a Cobb elevator and monopolar coagulation to expose the spine. Overly aggressive retraction of the psoas should be avoided to prevent injury to the lumbosacral plexus located within the muscle. The sympathetic trunk, which lies just medial to the psoas along the ventrolateral surface of the vertebral bodies, should be preserved if possible (see Fig. 302-3). Division of the sympathetic trunk may result in unilateral decreased sympathetic tone in the lower extremity. Patients may complain of a cold contralateral leg because the retained sympathetic activity in the normal limb maintains vasoconstriction. At the lower lumbar regions, particularly L4-5, the common iliac vessels lie along the lateral aspect of the vertebral bodies and may require medial or lateral mobilization to increase exposure.
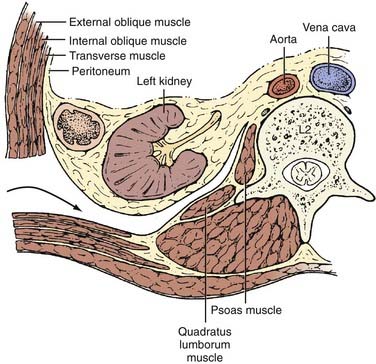
FIGURE 302-2 Axial view of ventrolateral retroperitoneal approach.
(From Benzel EC. Spine Surgery: Techniques, Complication Avoidance, and Management. Philadelphia: Churchill Livingstone; 1999.)
Transpsoas Approach (L1-5)
In recent years the field of minimally invasive surgery has expanded to involve surgical approaches to the spine as surgeons and patients seek novel methods to reduce tissue trauma during surgery, lessen postoperative discomfort, and shorten hospital stays. To this end, a novel approach through the psoas muscle offers a lateral approach that may be accomplished through one or two small (3- to 4-cm) incisions using tubular retractors and avoids the need for either a transperitoneal or retroperitoneal anterior approach.69
Paramedian Retroperitoneal Approach (L3-S1)
The plane between the transversalis fascia and peritoneum is developed with blunt dissection, and the parietal peritoneum is freed from the lateral abdominal wall. The abdominal contents and peritoneum, along with the kidney and ureter, are reflected medially to expose the ventral lumbar spine (Fig. 302-4). Dissecting the peritoneum off the ventral abdominal wall allows full mobilization of the peritoneal contents. The remainder of the dissection is similar to the ventrolateral retroperitoneal approach.
Transperitoneal Approach (L4-S1)
The abdominal contents are packed in a moist sponge and retracted into the upper abdomen to expose the posterior peritoneum. The peritoneum is incised in the midline over the aorta. The incision continues caudally over the right common iliac vessels to enter the retroperitoneal space. At the level of the iliac vessels, the incision proceeds medially to avoid the ureter. The peritoneal flaps are retracted laterally over the iliac vessels. The retroperitoneal portions of the large bowel are mobilized and retracted to the left to expose the aortic bifurcation and sacral promontory. Adjacent to the aorta, the ureters and hypogastric plexus are also visualized and treated with caution. Typically, the aortic bifurcation lies at L4 and the inferior vena cava origin starts at L5, but variations are common. The surgeon must be aware of these anatomic variants and adjust the operative plan as needed. Anterior branches of the aorta, including the inferior mesenteric artery, the middle sacral artery, and the segmental lumbar arteries, are also encountered with the transperitoneal approach. A higher incidence of hypogastric plexus injury and subsequent retrograde ejaculation have been reported with the transperitoneal approach versus the retroperitoneal approaches.70 Judicious use of bipolar cautery and sharp and blunt dissection of the tissues ventral to the annulus may minimize this risk. Monopolar cautery should only be used extensively within the disk space.
Biomechanics of Anterior Lumbar Instrumentation
Most anterior lumbar fixation constructs are variations on the rigid cantilever beam design.71 Screws are placed into the vertebral bodies above and below the pathology for single-level disease, or segmentally for more extensive procedures not requiring corpectomies, such as thoracolumbar scoliosis correction. The screws are attached rigidly to longitudinal members, typically either a rod or a plate. Similar to the interbody grafts, the cantilever beam construct functions in distraction most of the time by resisting compressive forces. Because of its rigid attachment to the vertebrae, however, it also resists extension, axial rotation, and lateral bending.
Expandable vertical cages of titanium and polyetheretherketone (PEEK) are currently available in sizes appropriate for use in the lumbar spine following a single or multilevel corpectomy. These offer the advantage of ease of placement because they can be placed in the intervertebral defect and then be expanded to engage the adjacent end plates. It may be possible, particularly in the setting of ligamentous laxity or dorsal column disruption, to overdistract the cage and create a segmental deformity. Also, although distraction of the device may initially create significant pullout resistance, ligamentous creep will occur and the device may loosen. Although vertebral fractures adjacent to expandable cages have been reported, a causal relationship has not been established.72 The role of expandable cages in anterior lumbar reconstructive surgery and their merits and disadvantages relative to fixed devices remains to be elucidated.
Instrumentation
General Principles
A translational deformity can occur with two-rod constructs because of an intrinsic bending moment between the rods.71 This can be avoided by cross-fixation of parallel rods and by triangulating the screws within the vertebral body. Some of the newer constructs avoid a parallelogram configuration by offsetting the screws in the sagittal and coronal planes within a single vertebral body. Distraction and compression are applied to the screws before and after the graft is inserted. Excessive distraction across the defect can cause neurological injury from stretch and vascular compromise. Particular care with distraction should be taken if the anterior or posterior longitudinal ligament is incompetent.
Instrumentation Constructs
Screw-rod constructs generally consist of vertebral body plates, cancellous screws, paired rods, and a method of fixing the rods to the screws, either directly or through vertebral body plates. The screws provide a means for distraction across the defect during insertion of an interbody strut graft. A transverse coupler or parallel connector can be used to increase the stability of the construct against both rotatory and flexion-extension forces. Most of these devices allow multisegmental fixation.73
The screw-plate constructs generally consist of a titanium locking plate, cancellous vertebral body screws, and compression screws. With earlier plating systems, distraction across the corpectomy site was obtained with a separate instrument because the plate provides no anchor for distraction. Once the graft is in place, compression is applied through a separate set of screws, which can provide a limited degree (typically up to 3 mm) of compression.73 However, newer plating systems incorporate slotted plates, allowing greater compression across the corpectomy site after the plate is positioned for precise seating of the graft.
An interbody device may be used when intervertebral support is desired following a diskectomy or corpectomy, particularly if an appropriate structural bone graft, either autologous or allogeneic, is not available. Compared with more traditional posterior fusion constructs, anterior interbody fusion has some surgical and biomechanical advantages.74–78 The goals of ventral placement of an interbody device or graft are to restore disk height and lumbar lordosis, to distract the intervertebral foramen, and to provide a favorable fusion environment, all without disturbing the posterior tension band.
Complications
Potential complications related to anterior lumbar instrumentation can be divided into major and minor and classified as visceral, vascular, neurological, and construct-related. Complications related to the graft and instrumentation include pseudarthrosis, graft or construct dislodgment, and instrumentation failure. The cumulative incidence of complications for anterior spinal fusion has been reported to be as high as 40%.79,80 Major catastrophic complications occur less often.
Vascular injuries have been reported to follow anterior lumbar fusion in up to 15% of cases.81,82 Such injuries are more common with a rectus incision than with a flank incision.81 The aorta, inferior vena cava, common iliac vessels, and their associated branches are all potentially at risk, depending on the specific approach used and the vertebral levels exposed. The left-sided approach is favored because arterial structures are easier to mobilize than venous structures and also because hemorrhage from an arterial vessel is usually easier to control. Inflammatory, neoplastic, or degenerative processes or adjuvant therapy for neoplastic disease, such as radiation or chemotherapy, may increase scarring around the vessels, making dissection more difficult. Bleeding from either type of vessel should be managed directly and expeditiously. Digital pressure is often the initial step in controlling a vessel laceration. Temporary hemostatic clamps can also be placed to control bleeding and to allow repair of the defect. Excessive lateral retraction of the iliac vessels can lead to spasm or thrombosis. If a thrombus occurs, the assistance of a vascular surgeon may be required to perform a thrombectomy.
Most visceral complications consist of bowel and ureteral injuries. Bowel perforations, most often encountered with a transperitoneal approach, should be repaired by a general surgeon. Inadequate closure can lead to peritonitis, sepsis, or abscess formation. A functional ileus is common after intra-abdominal surgery and typically resolves spontaneously within 2 to 3 days.83 A mechanical ileus may result if the bowels are not returned to their normal anatomic location. Failure to recognize a mechanical obstruction can compromise blood flow and lead to the devastating consequences of bowel ischemia. The ureter is frequently manipulated during a retroperitoneal approach but is usually lateral to the transperitoneal exposure. Excessive mobilization or traction can lead to injury or fibrosis. If mobilization is required, particularly with a rostral lumbar exposure, a generous cuff of soft tissue should be left surrounding the ureter to preserve blood flow.
Various neurological injuries are associated with an anterior lumbar approach. The preaortic sympathetic plexus forms the superior hypogastric plexus distal to the aortic bifurcation ventral to the L5-S1 intervertebral disk space, which provides innervation to the sphincter urethrae muscle. Deinnervation of this muscle causes retrograde ejaculation in men, a significant complication that can result in functional sterility. The reported incidence of this complication ranges from 5% to 22%.81,84,85 Within 1 to 2 years, function returns completely or partially in as many as one third of these patients. If a ventral approach to L5-S1 is planned in a male patient, this potential complication is discussed frankly and the advisability of sperm banking explained to him. Penile erection is mediated through the parasympathetic plexus and should not be injured when standard anterior approaches are used.86 If erectile dysfunction is associated with an anterior lumbar procedure, it is usually nonorganic. Injury to the lumbosacral plexus and to the femoral and genitofemoral nerves is possible during dissection or retraction of the psoas muscle; ipsilateral leg weakness or paresthesias result. Decompression and graft insertion can injure the exiting nerve roots and cauda equina, producing lower extremity or bowel and bladder deficits. Careful technique and planning can help avoid such injuries.
Arbit E, Galicich JH. Vertebral body reconstruction with a modified Harrington rod distraction system for stabilization of the spine affected with metastatic disease. J Neurosurg. 1995;83:617-620.
Baker JK, Reardon PR, Reardon MJ, et al. Vascular injury in anterior lumbar surgery. Spine. 1993;18:2227-2230.
Benzel EH. Anterior cantilever beam fixation and related techniques. In: Benzel EH, editor. Biomechanics of Spine Stabilization: Principles and Clinical Practice. New York: McGraw-Hill; 1995:187-189.
Blumenthal SL, Baker J, Dossett A, et al. The role of anterior lumbar fusion for internal disc disruption. Spine. 1988;13:566-569.
Chou D, Lu DC, Weinstein P, et al. Adjacent-level vertebral body fractures after expandable cage reconstruction. Report of 4 cases. J Neurosurg Spine. 2008;8:584-588.
Cooper PR, Errico TJ, Martin R, et al. A systematic approach to spinal reconstruction after anterior decompression for neoplastic disease of the thoracic and lumbar spine. Neurosurgery. 1993;32:1-8.
Crock HV. Anterior lumbar interbody fusion: indications for its use and notes on surgical technique. Clin Orthop Relat Res. 1982;165:157-163.
Denis F. Spinal instability as defined by the three-column spine concept in acute spinal trauma. Clin Orthop Relat Res. 1984;189:65-76.
Dunn HK. Anterior spine stabilization and decompression for thoracolumbar injuries. Orthop Clin North Am. 1986;17:113-119.
Faciszewski T, Winter RB, Lonstein JE, et al. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults: a review of 1223 procedures. Spine. 1995;20:1592-1599.
Inoue S, Watanabe T, Hirose A, et al. Anterior discectomy and interbody fusion for lumbar disc herniation: a review of 350 cases. Clin Orthop Relat Res. 1984;183:22-31.
James KS, Wenger KH, Schlegel JD, et al. Biomechanical evaluation of the stability of thoracolumbar burst fractures. Spine. 1994;19:1731-1740.
Kostuik JP. Anterior fixation for fractures of the thoracic and lumbar spine with or without neurologic involvement. Clin Orthop Relat Res. 1984;189:103-115.
Kostuik JP. Anterior spinal cord decompression for lesions of the thoracic and lumbar spine: techniques, new methods of internal fixation, results. Spine. 1983;8:512-531.
McAfee PC, Zdeblick TA. Tumors of the thoracic and lumbar spine: surgical treatment via the anterior approach. J Spinal Disord. 1989;2:145-154.
McCormack T, Karaikovic E, Gaines RW. The load-sharing classification of spine fractures. Spine. 1994;19:1741-1744.
Overby MC, Rothman AS. Anterolateral decompression for metastatic epidural spinal cord tumors: results of a modified costotransversectomy approach. J Neurosurg. 1985;62:344-348.
Ozgur BM, Aryan HE, Pimenta L, et al. Extreme lateral interbody fusion: a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435-443.
Stauffer RN, Coventry MB. Anterior interbody lumbar spine fusion: analysis of Mayo Clinic series. J Bone Joint Surg Am. 1972;54:756-768.
Sundaresan N, Digiacinto GV, Hughes JE, et al. Treatment of neoplastic spinal cord compression: results of a prospective study. Neurosurgery. 1991;29:645-650.
Watkins R. Assessment of results and complications of anterior lumbar fusion. In: Lin P, Gill K, editors. Lumbar Interbody Fusion. Rockville, MD: Aspen; 1989:153-169.
Weinstein JN, McLain RF. Primary tumors of the spine. Spine. 1987;12:843-851.
Whang PG, Vaccaro AR. Thoracolumbar fractures: anterior decompression and interbody fusion. J Am Acad Orthop Surg. 2008;16:424-431.
Whang PG, Vaccaro AR, Poelstra KA, et al. The influence of fracture mechanism and morphology on the reliability and validity of two novel thoracolumbar injury classification systems. Spine. 2007;32:791-795.
Willen J, Lindahl S, Nordwall A. Unstable thoracolumbar fractures: a comparative clinical study of conservative treatment and Harrington instrumentation. Spine. 1985;10:111-122.
1 Malawer M, Delaney T. Treatment of metastatic cancer to bone. In: De Vita V, Hellman S, Rosenberg S, editors. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott-Raven; 1993:2225-2246.
2 Constans JP, de Divitiis E, Donzelli R, et al. Spinal metastases with neurological manifestations: review of 600 cases. J Neurosurg. 1983;59:111-118.
3 Boland PJ, Lane JM, Sundaresan N. Metastatic disease of the spine. Clin Orthop Relat Res. 1982;169:95-102.
4 Sundaresan N, Digiacinto GV, Hughes JE. Surgical treatment of spinal metastases. Clin Neurosurg. 1986;33:503-522.
5 Sundaresan N, Galicich JH, Bains MS, et al. Vertebral body resection in the treatment of cancer involving the spine. Cancer. 1984;53:1393-1396.
6 Sundaresan N, Galicich JH, Lane JM, et al. Treatment of neoplastic epidural cord compression by vertebral body resection and stabilization. J Neurosurg. 1985;63:676-684.
7 Boring C, Squires T, Tong T. Cancer statistics 1993. CA Cancer J Clin. 1993;43:7-26.
8 Ebersold M, Hitchon P, Duff J, et al. Primary bony spinal lesions. In: Benzel E, editor. Spine Surgery: Techniques, Complication Avoidance, and Management. Philadelphia: Churchill Livingstone; 1999:663-677.
9 Bach F, Larsen BH, Rohde K, et al. Metastatic spinal cord compression: occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir (Wien). 1990;107:37-43.
10 Jaeckle KA, Young DF, Foley KM. The natural history of lumbosacral plexopathy in cancer. Neurology. 1985;35:8-15.
11 Weinstein JN, McLain RF. Primary tumors of the spine. Spine. 1987;12:843-851.
12 Delaney T, Oldfield E. Spinal cord compression. In: Devita V, Hellman S, Rosenberg S, editors. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott-Raven; 1993:2118-2128.
13 Solini A, Paschero B, Orsini G, et al. The surgical treatment of metastatic tumours of the lumbar spine. Ital J Orthop Traumatol. 1985;11:427-442.
14 Cooper PR, Errico TJ, Martin R, et al. A systematic approach to spinal reconstruction after anterior decompression for neoplastic disease of the thoracic and lumbar spine. Neurosurgery. 1993;32:1-8.
15 Sundaresan N, Digiacinto GV, Hughes JE, et al. Treatment of neoplastic spinal cord compression: results of a prospective study. Neurosurgery. 1991;29:645-650.
16 Fidler MW. Anterior decompression and stabilisation of metastatic spinal fractures. J Bone Joint Surg Br. 1986;68:83-90.
17 Kostuik JP. Anterior spinal cord decompression for lesions of the thoracic and lumbar spine: techniques, new methods of internal fixation, results. Spine. 1983;8:512-531.
18 Onimus M, Schraub S, Bertin D, et al. Surgical treatment of vertebral metastasis. Spine. 1986;11:883-891.
19 Overby MC, Rothman AS. Anterolateral decompression for metastatic epidural spinal cord tumors: results of a modified costotransversectomy approach. J Neurosurg. 1985;62:344-348.
20 Siegal T. Surgical decompression of anterior and posterior malignant epidural tumors compressing the spinal cord: a prospective study. Neurosurgery. 1985;17:424-432.
21 Arbit E, Galicich JH. Vertebral body reconstruction with a modified Harrington rod distraction system for stabilization of the spine affected with metastatic disease. J Neurosurg. 1995;83:617-620.
22 McAfee PC, Zdeblick TA. Tumors of the thoracic and lumbar spine: surgical treatment via the anterior approach. J Spinal Disord. 1989;2:145-154.
23 Barcena A, Lobato RD, Rivas JJ, et al. Spinal metastatic disease: analysis of factors determining functional prognosis and the choice of treatment. Neurosurgery. 1984;15:820-827.
24 Tokuhashi Y, Matsuzaki H, Toriyama S, et al. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine. 1990;15:1110-1113.
25 An HS, Simpson JM, Ebraheim NA, et al. Low lumbar burst fractures: comparison between conservative and surgical treatments. Orthopedics. 1992;15:367-373.
26 Levine AM, Edwards CC. Low lumbar burst fractures: reduction and stabilization using the modular spine fixation system. Orthopedics. 1988;11:1427-1432.
27 Bohlman HH. Treatment of fractures and dislocations of the thoracic and lumbar spine. J Bone Joint Surg Am. 1985;67:165-169.
28 Brown G. Bone resorption in the canal following a thoracolumbar fracture with a displaced diaphyseal fragment. Iowa Orthop J. 1989;9:69-71.
29 Willen J, Lindahl S, Nordwall A. Unstable thoracolumbar fractures: a comparative clinical study of conservative treatment and Harrington instrumentation. Spine. 1985;10:111-122.
30 Dunn HK. Anterior spine stabilization and decompression for thoracolumbar injuries. Orthop Clin North Am. 1986;17:113-119.
31 Jacobs RR, Casey MP. Surgical management of thoracolumbar spinal injuries: general principles and controversial considerations. Clin Orthop Relat Res. 1984;189:22-35.
32 Denis F, Armstrong GW, Searls K, et al. Acute thoracolumbar burst fractures in the absence of neurologic deficit: a comparison between operative and nonoperative treatment. Clin Orthop Relat Res. 1984;189:142-149.
33 Ferguson RL, Allen BLJr. An algorithm for the treatment of unstable thoracolumbar fractures. Orthop Clin North Am. 1986;17:105-112.
34 Kostuik JP. Anterior fixation for fractures of the thoracic and lumbar spine with or without neurologic involvement. Clin Orthop Relat Res. 1984;189:103-115.
35 Krompinger WJ, Frederickson BE, Mino DE, et al. Conservative treatment of fractures of the thoracic and lumbar spine. Orthop Clin North Am. 1986;17:161-170.
36 DeWald RL. Burst fractures of the thoracic and lumbar spine. Clin Orthop Relat Res. 1984;189:150-161.
37 Weitzman G. Treatment of stable thoracolumbar spine compression fractures by early ambulation. Clin Orthop. 1971;76:116-122.
38 Roy-Camille R, Saillant G, Mazel C. Plating of thoracic, thoracolumbar, and lumbar injuries with pedicle screw plates. Orthop Clin North Am. 1986;17:147-159.
39 Denis F. Spinal instability as defined by the three-column spine concept in acute spinal trauma. Clin Orthop Relat Res. 1984;189:65-76.
40 Levine A. Classification of spinal injury. In: Levine A, Eismont F, Garfin S, et al, editors. Spine Trauma. Philadelphia: WB Saunders; 1998:123-132.
41 McAfee PC, Yuan HA, Fredrickson BE, et al. The value of computed tomography in thoracolumbar fractures: an analysis of one hundred consecutive cases and a new classification. J Bone Joint Surg Am. 1983;65:461-473.
42 Magerl F, Aebi M, Gertzbein SD, et al. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184-201.
43 McCormack T, Karaikovic E, Gaines RW. The load-sharing classification of spine fractures. Spine. 1994;19:1741-1744.
44 James KS, Wenger KH, Schlegel JD, et al. Biomechanical evaluation of the stability of thoracolumbar burst fractures. Spine. 1994;19:1731-1740.
45 Stauffer E. Thoracolumbar Spine Fractures Without Neurological Deficit. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1993.
46 Holdsworth F. Fractures, dislocations, and fracture-dislocations of the spine. J Bone Joint Surg Am. 1970;52:1534-1551.
47 Wang J, Delamarter R. Lumbar fractures of the spine. In: Capen D, Haye W, editors. Comprehensive Management of Spine Trauma. Philadelphia: Mosby; 1998:214-234.
48 Benzel E. Stability and instability of the spine. In: Benzel E, editor. Biomechanics of Spine Stabilization: Principles and Clinical Practice. New York: McGraw-Hill; 1995:25-38.
49 Oxland T, Kuslich S, Kohrs D, et al. The BAK interbody fusion system: biomechanical rationale and early clinical results. In: Margulies J, Floman Y, Farcy JC, Neuwirth MG, editors. Lumbosacral and Spinopelvic Fixation. Philadelphia: Lippincott-Raven; 1996:545-561.
50 Dickman CA. Internal fixation and fusion of the lumbar spine using threaded interbody cages. BNI Q. 1997;13:4-25.
51 Watkins R. Assessment of results and complications of anterior lumbar fusion. In: Lin P, Gill K, editors. Lumbar Interbody Fusion. Rockville, MD: Aspen; 1989:153-169.
52 Stauffer RN, Coventry MB. Anterior interbody lumbar spine fusion: analysis of Mayo Clinic series. J Bone Joint Surg Am. 1972;54:756-768.
53 Sacks S. Anterior interbody fusion of the lumbar spine: indications and results in 200 cases. Clin Orthop Relat Res. 1966;44:163-170.
54 Sacks S. Anterior interbody fusion of the lumbar spine. J Bone Joint Surg Br. 1965;47:211-223.
55 Leong J. Anterior interbody fusion. In: Lin P, Gill K, editors. Lumbar Interbody Fusion. Rockville, MD: Aspen; 1989:133-148.
56 Flynn JC, Hoque MA. Anterior fusion of the lumbar spine: end result study with long-term follow-up. J Bone Joint Surg Am. 1979;61:1143-1150.
57 Freebody D, Bendall R, Taylor RD. Anterior transperitoneal lumbar fusion. J Bone Joint Surg Br. 1971;53:617-627.
58 Crock HV. Anterior lumbar interbody fusion: indications for its use and notes on surgical technique. Clin Orthop Relat Res. 1982;165:157-163.
59 Cahill DW. Pyogenic infections of the spine. In: Menezes AH, Sonntag VKH, editors. Principles of Spinal Surgery. New York: McGraw-Hill; 1996:1453-1465.
60 Vincent K, Benson DR. Differential diagnosis and conservative treatment of infectious disease. In: Frymoyer J, editor. The Adult Spine: Principles and Practice. New York: Raven; 1991:763-785.
61 Zeidman S, Ducker T. Infectious complications of spine surgery. In: Benzel E, editor. Spine Surgery: Techniques, Complication Avoidance, and Management. New York: Churchill Livingstone; 1999:1445-1457.
62 Sridhar K, Ramamurthi B. Granulomatous fungal and parasitic infections of the spine. In: Menezes AH, Sonntag VKH, editors. Principles of Spinal Surgery. New York: McGraw-Hill; 1996:1467-1495.
63 Kirkaldy-Willis WH, Thomas TG. Anterior approaches in the diagnosis and treatment of infections of the vertebral bodies. J Bone Joint Surg Am. 1965;47:87-110.
64 Blumenthal SL, Baker J, Dossett A, et al. The role of anterior lumbar fusion for internal disc disruption. Spine. 1988;13:566-569.
65 Hodgson AR, Yau ACMC. Anterior approach to the spinal column. In: Apley AG, editor. Recent Advances in Orthopedics. Baltimore: Williams and Wilkins; 1964:289-326.
66 Dwyer AF, Newton NC, Sherwook AA. An anterior approach to scoliosis. Clin Orthop. 1969;62:192.
67 Perry J. Surgical approaches to the spine. In: Pierce N, Nichol V, editors. The Total Care of Spinal Cord Injuries. Boston: Little, Brown; 1977:53-79.
68 Watkins RG. Surgical Approaches to the Spine. New York: Springer; 2003.
69 Ozgur BM, Aryan HE, Pimenta L, et al. Extreme lateral interbody fusion: a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435-443.
70 Sasso RC, Kenneth Burkus J, LeHuec JC. Retrograde ejaculation after anterior lumbar interbody fusion: transperitoneal versus retroperitoneal exposure. Spine. 2003;28:1023-1026.
71 Benzel EH. Anterior cantilever beam fixation and related techniques. In: Benzel EH, editor. Biomechanics of Spine Stabilization: Principles and Clinical Practice. New York: McGraw-Hill; 1995:187-189.
72 Chou D, Lu DC, Weinstein P, et al. Adjacent-level vertebral body fractures after expandable cage reconstruction. Report of 4 cases. J Neurosurg Spine. 2008;8:584-588.
73 Aebi M, Thalgott JS, Webb J. AO ASIF Principles in Spine Surgery. Berlin: Springer-Verlag; 1998.
74 Evans JH. Biomechanics of lumbar fusion. Clin Orthop Relat Res. 1985;193:38-46.
75 Dickman CA, Maric Z. The biology of bone healing and techniques of spinal fusion. BNI Q. 1994;10:2-12.
76 Ray CD. Spinal interbody fusions: a review, featuring new generation techniques. Neurosurg Q. 1997;7:135-156.
77 Benzel EH. Spinal fusion. In: Benzel EH, editor. Biomechanics of Spine Stabilization: Principles and Clinical Practice. New York: McGraw-Hill; 1995:103-108.
78 Goel VK, Kim YE, Lim TH, et al. An analytical investigation of the mechanics of spinal instrumentation. Spine. 1988;13:1003-1011.
79 Faciszewski T, Winter RB, Lonstein JE, et al. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults: a review of 1223 procedures. Spine. 1995;20:1592-1599.
80 Rajaraman V, Vingan R, Roth P, et al. Visceral and vascular complications resulting from anterior lumbar interbody fusion. J Neurosurg. 1990;91:60-64.
81 Westfall SH, Akbarnia BA, Merenda JT, et al. Exposure of the anterior spine: technique, complications, and results in 85 patients. Am J Surg. 1987;154:700-704.
82 Baker JK, Reardon PR, Reardon MJ, et al. Vascular injury in anterior lumbar surgery. Spine. 1993;18:2227-2230.
83 Sicard GA, Reilly JM, Rubin BG, et al. Transabdominal versus retroperitoneal incision for abdominal aortic surgery: report of a prospective randomized trial. J Vasc Surg. 1995;21:174-181.
84 Inoue S, Watanabe T, Hirose A, et al. Anterior discectomy and interbody fusion for lumbar disc herniation: a review of 350 cases. Clin Orthop Relat Res. 1984;183:22-31.
85 Tiusanen H, Seitsalo S, Osterman K, et al. Retrograde ejaculation after anterior interbody lumbar fusion. Eur Spine J. 1995;4:339-342.
86 Johnson RM, McGuire EJ. Urogenital complications of anterior approaches to the lumbar spine. Clin Orthop Relat Res. 1981;154:114-118.