Chapter 69 Angioid Streaks
Introduction
The appearance of angioid streaks was initially described by Doyne in 1889 as irregular and radiating lines extending from the optic nerve to the peripheral retina found in an eye with retinal hemorrhages after blunt trauma.1 The term “angioid streaks” originated as the ophthalmoscopic appearance of the lines was similar to that of blood vessels.2 Histopathologic studies found that angioid streaks represent irregular dehiscences in the collagenous and elastic portion of Bruch’s membrane.3,4 Associations have been found between angioid streaks and systemic conditions such as pseudoxanthoma elasticum (Grönbald-Strandberg syndrome),5,6,7 osteitis deformans (Paget’s disease),5,8–10 blood dyscrasias such as sickle-cell anemia,5,11–13 fibrodysplasia hyperelastica (Ehlers–Danlos syndrome),5,14 and acromegaly.5,15 However, angioid streaks may also occur in patients without associated systemic disease.16 Patients with angioid streaks usually are asymptomatic unless complications such as macular choroidal neovascularization develop.5,16 In cases of macular involvement the prognosis is often poor, with most eyes progressing to legal blindness without treatment.5,6,16 Multiple therapeutic strategies have been used to treat choroidal neovascularization secondary to angioid streaks, including argon laser photocoagulation,17–19 transpupillary thermotherapy,20 photodynamic therapy,21,22 macular translocation surgery,23–25 intravitreal antivascular endothelial growth factor treatments with pegabtanib,26 bevacizumab,27–33 or ranibizumab,33–38 and combination therapy.39
Histopathology
Angioid streaks represent discrete irregular breaks in Bruch’s membrane, and are often associated with atrophic changes of the overlying retinal pigment epithelium (RPE) and calcific degeneration.4 Klein proposed a dual mechanism for the development of Bruch’s membrane, including (1) a primary abnormality in the fibers of Bruch’s membrane and (2) increased deposits of metal salts or an increasing tendency for their pathologic deposition.3 The deposition of calcium may cause Bruch’s membrane to be more brittle and to develop choroidal rupture.4 Recent immunohistochemical studies show significant calcium deposition and infiltration of vascularized tissue above the RPE from Bruch’s membrane in the area of choroidal neovascularization in an eye with angioid streaks.40
Tissue metalloproteinase, specifically MMP-9, was found in high concentrations in the excised Bruch’s membrane in an area of choroidal neovascularization in an eye with angioid streaks. MMP-9 is known to induce basement membrane destruction and angiogenesis.40
In the early stages, angioid streaks are partial breaks of the thickened and calcified Bruch’s membrane with thinning of the RPE, events that do not cause anatomic changes in the overlying retinal layers.41 Subsequently a full-thickness defect of the Bruch’s membrane may occur followed by atrophy of the choriocapillaris, RPE, and photoreceptors. Fibrovascular proliferation from the choroid may occur through the Bruch’s membrane break resulting in choroidal neovascularization and subsequent development of a disciform scar.4,10,41 This process usually results in slowly progressive macular changes and vision loss. Sudden vision loss can result, however, from a serous or hemorrhagic detachment around the areas of choroidal neovascularization.4,10 Sudden loss of central vision may occur following mild trauma leading to choroidal rupture and submacular hemorrhage resulting from the brittleness of Bruch’s membrane.4,10
Systemic associations
Angioid streaks have been most commonly associated with systemic conditions such as pseudoxanthoma elasticum (Grönbald–Strandberg syndrome),5–7 osteitis deformans (Paget’s disease),5,8–10 fibrodysplasia hyperelastica (Ehlers–Danlos syndrome),5,14 acromegaly,5,15 Marfan syndrome,6 and blood dyscrasias such as sickle-cell anemia,11,12 thalassemia,6 and spherocytosis.6 Angioid streaks have also been described in patients with the following conditions: alpha-beta-lipoproteinemia, acquired hemolytic anemia, hemochromatosis, hypertension, diabetes, hypercalcinosis, hyperphosphatemia, diffuse lipomatosis, Sturge–Weber syndrome, tuberous sclerosis, neurofibromatosis, microsomia, epilepsy, senile elastosis, cutaneous calcinosis, and trauma5,6,16 (Box 69.1).
Box 69.1
Systemic conditions associated with angioid streaks
In a large study examining associated systemic diagnosis in 50 patients with angioid streaks, half of the patients were found to have a related systemic condition.5 Seventeen of the 25 patients were diagnosed also with pseudoxanthoma elasticum (PXE), 5 patients with Paget’s disease, and 3 patients had sickle-cell disease.5 The remaining half of the patients with angioid streaks did not demonstrate associated systemic disease.5
The most common systemic association of angioid streaks is PXE, an inherited disorder associated with degeneration of the elastic fibers in the dermatologic, gastrointestinal, cardiovascular, and ocular tissues. PXE accounts for 59–87% of cases with angioid streaks.7 In PXE, the primary finding is elastic fiber degeneration in connective tissue, followed by a secondary calcium deposition.4,10 In addition to angioid streaks, eyes also demonstrate a so-called “peau d’orange” pigmentary change, reticular pigmentary dystrophy affecting the macula, atrophic lesions of the RPE, crystalline bodies, and optic disc drusen (in 21% of patients with PXE and angioid streaks).42 By 20 years after first diagnosis, angioid streaks develop in almost all patients with PXE.16
In patients with Paget’s disease, extensive Bruch’s membrane calcification and angioid streaks followed by choroidal neovascularization and disciform scarring may develp.9,10 About 10% of patients with advanced Paget’s disease develop angioid streaks.9,10
Similar significant calcium depositions at Bruch’s membrane have been identified using histochemical and electron microscopic studies in patients with sickle-cell hemoglobinopathies.13 The presence of iron–calcium complexes at the level of Bruch’s membrane was previously suggested as an etiology for angioid streaks in patients with hemoglobinopathy; however, histopathologic studies demonstrate no increased iron deposition at the Bruch’s membrane.42 Compared to PXE, a smaller proportion of patients develop choroidal neovascularization and subsequent visual loss.6
Ocular manifestations and clinical course
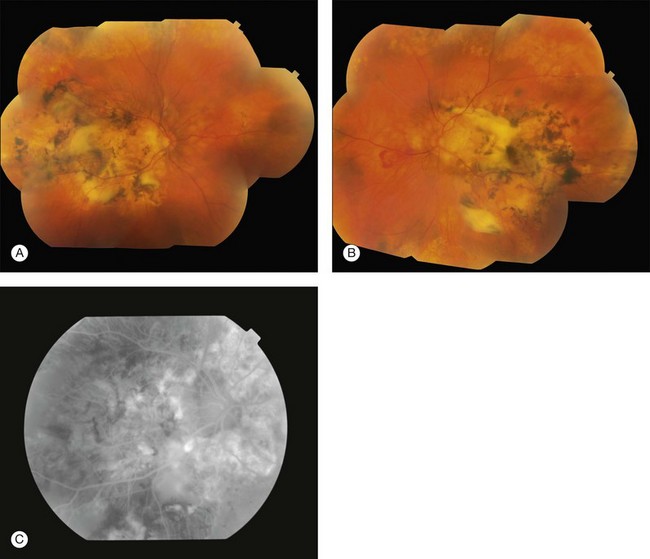
Angioid streaks usually originate from the optic nerve and may either radiate out or surround it concentrically and appear as irregular lines of varying width.6 The subretinal lines can range in diameter from 50 to 500 µm.16 The color of angioid streaks varies based on the fundus pigmentation and tends to be reddish in light-colored individuals and brown-colored in darker-pigmented individuals16 (see Fig. 69.1A–D).
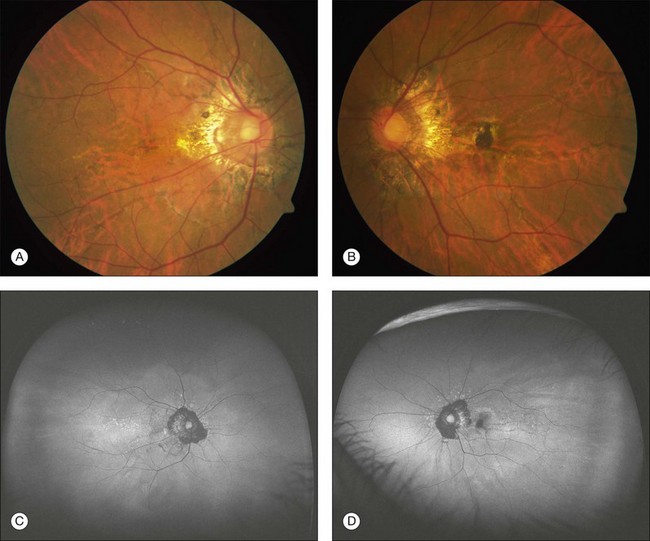
Fig. 69.1 A 63-year-old Asian woman with pseudoxanthoma elasticum and angioid streaks. Wide-field imaging of the right (A) and left (B) eyes shows angioid streaks (darker lines) radiating out from the optic disc. A hyperpigmented scar just nasal to the fovea developed after laser photocoagulation (A). The laser treatment was given prior to the use of intravitreal anti-VEGF therapy. Fundus autofluorescence imaging demonstrates the angioid streaks in the right (C) and left (D) eye. Spectral domain optical coherence tomography in this patient is shown in Fig. 69.3.
Angioid streaks have not been reported in newborns, and few cases have been described in individuals under 10 years of age.6 Angioid streaks remain over time and do not regress, and the streaks may increase in length and width over time.43 New streaks may form adjacent to old lesions. Over time, the adjacent RPE and choriocapillaris may develop atrophy.43
Most patients with angioid streaks are asymptomatic unless the macula is involved, with the development of traumatic rupture of the Bruch’s membrane or choroidal neovascularization (Figs 69.1B, 69.2A,B). If the macula is involved, patients may report metamorphopsia or blurred vision.
The most common and significant complication of angioid streaks is the development of choroidal neovascularization (CNV). Choroidal neovascularization is often bilateral and occurs in 72–86% of eyes with angioid streaks.16 CNV is usually bilateral but asymmetric, with an interval of approximately 18 months between the development of CNV in initial and fellow eye.7
Ocular imaging and diagnosis
Fluorescein angiography (FA)
Usually the diagnosis of angioid streaks is made on fundoscopic examination, but fluorescein angiography may be helpful to detect the streaks and associated choroidal neovascularization when the findings are subtle. Irregular hyperfluorescence of the angioid streaks occurs during early phase angiography followed by varying degrees of staining during the later phases.4 In some individuals with deeply pigmented choroidal tissue, the angioid streaks may be difficult to detect angiographically; whereas in lightly pigmented individuals, fluorescein angiography may aid in the identification of the angioid streaks before ophthalmoscopic detection4 (Fig. 69.2C).
Fundus autofluorescence (FAF)
Autofluorescence imaging uses light emission from lipofuscin in RPE cells and is considered to reflect RPE metabolic activity. Angioid streaks can show increased or decreased fundus autofluorescence. Autofluorescence often demonstrates RPE atrophy more extensive than that seen on fundus ophthalmoscopy or fluorescein angiography; therefore, FAF may be a useful noninvasive tool to monitor the progression of the RPE changes related to angioid streaks and choroidal neovascularization6 (see Figs 69.1C,D). FAF in eyes with angioid streaks has also been similar to the FAF images from eyes with pattern dystrophy. FAF demonstrated large areas of confluent hypoautofluorescence, demonstrating widespread loss of RPE cells in eyes with PXE.44
Indocyanine green angiography (ICGA)
A study incorporating the use of multiple modalities (FA, ICGA, FAF, and confocal near-infrared reflectance) in patients with angioid streaks and PXE has suggested a centrifugal spread of progressive calcification of Bruch’s membrane that begins at the posterior pole and progresses toward the retinal periphery.45 A central area of decreased fluorescence centered on the posterior pole on late-phase ICGA, while eccentric areas demonstrated a normal fluorescence on late-phase ICGA.45
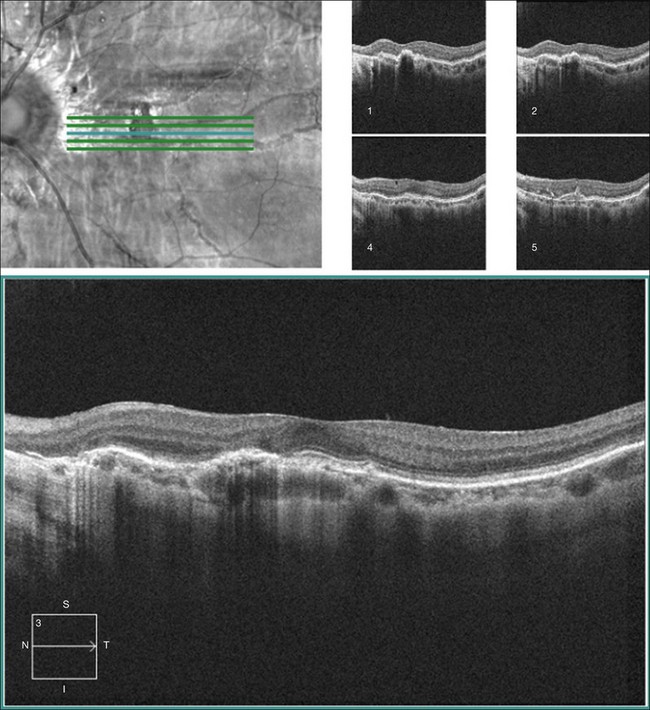
Spectral domain optical coherence tomography (SD-OCT)
In eyes with advanced fundus pathology, such as large areas of atrophy and fibrosis, the underlying Bruch’s membrane breaks cannot be detected on fundus photography, fluorescein angiography, or fundus autofluorescence imaging.46 SD-OCT can detect abnormalities in Bruch’s membrane as well as subretinal fibrosis and deposits that can be difficult to discern in areas of atrophy on FAF, FA, or ICGA46 (Fig. 69.3).
Therapy
There is no known prophylaxis except perhaps eye protection to reduce trauma. Treatment strategies are only directed to the eyes with choroidal neovascularization (CNV). Untreated CNV results in poor visual outcomes in the majority of published reports. One study found a final visual acuity of 20/640 in a group of 26 untreated eyes with either active CNV or disciform scar secondary to angioid streaks.19
Laser photocoagulation
Results from laser photocoagulation in the treatment of macular CNV demonstrate a high recurrence rate of up to 77% and poor overall visual outcomes.17–19 Thermal laser was one of the first therapies used for the treatment of macular CNV (see Fig. 69.1B). In one case series, the eyes treated with laser for macular CNV developed further CNV growth and vision loss.8 However, other studies suggested that laser treatment for extrafoveal CNV related to angioid streaks resulted in better visual outcomes than untreated eyes.17 Prophylactic treatment of angioid streaks prior to development of CNV is not recommended.42 Although the outcome following treatment is poor, there has been the sense that visual loss at least is somewhat delayed. Most retina specialists advise focal laser therapy if there is discrete extrafoveal CNV.
Transpupillary thermotherapy
Using a diode laser beam with 810 nm wavelength, transpupillary thermotherapy (TTT) may have better penetration to the choriocapillaris and may be less damaging to the RPE. TTT employs a diode with a lower threshold to avoid producing a thermal burn. However, a retrospective study investigating the use of TTT in treatment of subfoveal choroidal neovascularization found no significant long-term benefit in reducing the growth of the CNV or in visual improvement.20
Photodynamic therapy
Data from both retrospective and prospective case series of eyes treated with photodynamic therapy (PDT) demonstrate variability in visual outcomes after treatment.47 Several early reports investigating PDT use in eyes with angioid streaks suggest a reduction of CNV progression compared to natural history alone.21,47 A retrospective, placebo-controlled case series found a greater reduction in mean visual acuity over the mean follow-up period of 18 months in untreated (from 20/160 to 20/640) versus PDT-treated eyes (from 20/126 to 20/500).21 However, another study found mean visual acuity decreased after PDT treatment from 20/400 to 20/600.48 Extension of an initial study with two additional years of follow-up identified progressive decrease in visual acuity following PDT treatment.49
Macular translocation
Prior to the use of anti-vascular endothelial growth factor (VEGF) treatments for CNV, macular translocation was an option for CNV treatment. Translocation is a surgical technique used to move the macular neuroretina to lie on top of an area of RPE without previous choroidal neovascularization. Several techniques have been described, including a limited translocation, as well as a 360° translocation where the entire retina is rotated. Varying short-term visual acuity improvement has been reported in a few studies employing macular translocation for CNV related angioid streaks, but the number of eyes treated (less than 10) was limited.24,25
Anti-VEGF treatment
Laser photocoagulation, TTT, and PDT have not been as successful in reducing the degree of visual loss compared with the visual outcomes following anti-VEGF therapy. Treatments with anti-VEGF therapies such as with both bevacizumab27–33 and ranibizumab33–38 have demonstrated a marked reduction in the rate of visual acuity loss in the treated eyes compared with that in untreated eyes but again, follow-up is short and randomized trials lacking.
Bevacizumab
The majority of eyes with CNV secondary to angioid streaks treated with intravitreal bevacizumab demonstrated an improvement or stabilization in mean visual acuity on long-term follow-up ranging from 12 to 28 months.27–33 Several retrospective case series reported stabilization or improvement of visual acuity after bevacizumab treatment for CNV related to angioid streaks in 87–100% of eyes with a follow-up ranging from 12 to 28 months.27–31 Mean visual acuity improved by 3 or more lines in 44–62% of eyes by 12 months after bevacizumab treatment.28,30
In a study involving patients with angioid streaks secondary to PXE, the mean visual acuity improved from 20/80 to 20/40 with an average of 6.5 injections over a mean follow-up of 28 months.27 In the same study, eyes with early disease demonstrated better visual outcomes, with a mean visual acuity of 20/25 compared to a final mean visual acuity of 20/63 in eyes with advanced disease.27
Eyes with CNV were initially treated with intravitreal bevacizumab and were followed every 4–6 weeks.27–31 Retreatment with another bevacizumab injection was given if CNV activity was detected, such as decreased visual acuity, new or persistent leakage on FA, retinal hemorrhage, or sub- or intraretinal fluid was found on OCT.27,30
Multiple studies with at least 12-month follow-up reported a mean of approximately four bevacizumab injections (1.25–1.5 mg) were given over 12–18 months to treat the CNV secondary to angioid streaks.28,29,31 Recurrent CNV is common, occurring in 33% of eyes by 19 months in one case series.31 Not only can CNV recur at the same location, but new CNV can also develop in a different location requiring retreatment.31
Angiographic resolution of the CNV was found in 67% of the eyes by 19 months.31 At final follow-up between 12 and 19 months, several studies have shown a reduction of central retinal thickness by 67–103 µm after bevacizumab treatment.28,30
Despite a majority of eyes demonstrating stable or improved visual acuity at the final follow-up in studies using bevacizumab to treat CNV secondary to angioid streaks, 8–13% of eyes demonstrate further decreased visual acuity.27,30,31 In the eyes with visual loss after undergoing bevacizumab treatment, the visual decline is postulated to be related to atrophic macular changes and not from active choroidal neovascularization.27,30
Ranibizumab
Similar to the visual outcomes in bevacizumab trials, the majority of eyes treated with intravitreal ranibizumab demonstrated improved or stable visual outcomes at final follow-up, ranging from 3 months to 24 months.34–38 In several prospective and retrospective case series reports, final visual acuity outcomes were stable or improved in 66–93% of eyes treated with ranibizumab (0.3–0.5 mg).34,35,37,38 While the majority of eyes treated with intravitreal ranibizumab maintained or improved vision, 7–33% of eyes treated with ranibizumab lost vision by the end of the study.34–38
In two prospective studies, all eyes were given either three or four monthly loading doses of ranibizumab.37,38 Then an “as needed” treatment protocol was used: another ranibizumab injection was given if CNV activity was detected, such as decreased visual acuity, new or persistent leakage on FA, retinal hemorrhage, or sub- or intraretinal fluid was found on OCT. An average of 5–7 ranibizumab injections was given in two prospective trials with a follow-up of 14–16 months, respectively.37,38 One prospective trial found that 78% of eyes needed retreatment after the first three loading doses of ranibizumab.38 Angiographic resolution of the CNV after ranibizumab treatment was reported in 66% of eyes.35 On OCT testing, a mean decrease of 107 µm was shown in one prospective study at one year.37
Combination therapy
One prospective trial investigated the outcomes using combination reduced fluence PDT (25 J/cm2) and intravitreal ranibizumab (0.5 mg) for treatment-naïve eyes with CNV related to angioid streaks.39 At 12 months of follow-up, 9 of the 10 eyes demonstrated stable or improved vision, with 6 eyes showing visual acuity gains of two or greater lines. One eye had more than 3 lines of decreased vision at the end of the study.39 Given the small sample size of this study, further investigations involving combination therapies are needed before determining its added benefit over monotherapy with anti-VEGF treatment alone.
1 Doyne RW. Choroidal and retinal changes. The results of blows on the eyes. Trans Ophthalmol Soc UK. 1889;9:128.
2 Knapp H. On the formation of dark angioid streaks as unusual metamorphosis of retinal hemorrhage. Arch Ophthalmol. 1892;26:289–292.
3 Klein BA. Angioid streaks: a clinical and histopathologic study. Am J Ophthalmol. 1947;30:955–968.
4 Gass JDM. Pathogenesis of disciform detachment of the neuroepithelium. VI. Disciform detachment secondary to heredodegenerative, neoplastic and traumatic lesions of the choroid. Am J Ophthalmol. 1967;63:289–711.
5 Clarkson JG, Altman RD. Angioid streaks. Surv Ophthalmol. 1982;26:235–246.
6 Finger RP, Issa PC, Ladewig MS, et al. Pseudoxanthoma elasticum. Surv Ophthamol. 2009;54:272–285.
7 Connor PJ, Jr., Juergens JL, Perry HO, et al. Pseudoxanthoma elasticum and angioid streaks: A review of 106 cases. Am J Med. 1961;30:537–543.
8 Clarkson JG. Paget’s disease and angioid streaks: One complication less? Br J Ophthalmol. 1991;75:511.
9 Dabbs TR, Skjodt K. Prevalence of angioid streaks and other ocular complications of Paget’s disease of bone. Br J Ophthalmol. 1990;74:579–582.
10 Gass JDM, Clarkson JG. Angioid streaks and disciform macular detachment in Paget’s disease (osteitis deformans). Am J Ophthalmol. 1973;75:576–586.
11 Condon PI, Serjeant GR. Ocular findings in elderly cases of homozygous sickle-cell disease in Jamaica. Br J Ophthalmol. 1976;60:361–364.
12 Geeraets WJ, Guerry D, III. Angioid streaks and sickle-cell disease. Am J Ophthalmol. 1960;49:450–470.
13 Jampol LM, Acheson R, Eagle RC, Jr., et al. Calcification of Bruch’s membrane in angioid streaks with homozygous sickle cell disease. Arch Ophthalmol. 1987;105:93–98.
14 Green WR, Friedman-Kien A, Banfield WG. Angioid streaks in Ehlers–Danlos syndrome. Arch Ophthalmol. 1966;76:197–204.
15 Paton D. Angioid streaks and acromegaly. Am J Ophthalmol. 1963;56:841–842.
16 Georgalas I, Papconstantinou D, Koutsandrea C, et al. Angioid streaks, clinical course, complications, and current therapeutic management. Ther Clinical Risk Mgmt. 2009;5:81–89.
17 Gelisken O, Hendriskse F, Deutman AF. A long-term follow-up study of laser coagulation of neovascular membranes in angioid streaks. Am J Ophthalmol. 1988;105:299–303.
18 Lim JI, Bressler NM, Marsh MJ, et al. Laser treatment of choroidal neovascularization in patients with angioid streaks. Am J Ophthalmol. 1993;116:414–423.
19 Pece A, Avanza P, Galli L, et al. Laser photocoagulation of choroidal neovascularization in angioid streaks. Retina. 1997;17:12–16.
20 Ozdek S, Bozan E, Gurelik G, et al. Transpupillary thermotherapy for the treatment of choroidal neovascularization secondary to angioid streaks. Can J Ophthalmol. 2007;42:95–100.
21 Karacorlu M, Karacorlu S, Ozdemir H, et al. Photodynamic therapy with verteporfin for choroidal neovascularization in patients with angioid streaks. Am J Ophthalmol. 2002;134:360–366.
22 Menchini U, Virgili G, Introini U, et al. Outcomes of choroidal neovascularization in angioid streaks after photodynamic therapy. Retina. 2004;24:763–771.
23 Mennel S, Schmidt JC, Meyer CH. Therapeutic strategies in choroidal neovascularization secondary to angioid streaks. Am J Ophthalmol. 2003;136:580–582.
24 Roth DB, Estafanous M, Lewis H. Macular translocation for subfoveal choroidal neovascularization in angioid streaks. Am J Ophthalmol. 2001;131:390–392.
25 Thomas MA, Dickinson JD, Melberg NS, et al. Visual results after surgical removal of subfoveal choroidal neovascular membranes. Ophthalmology. 1994;101:1384–1396.
26 Cekic O, Gocmez E, Kocabora MS. Management of CNV in angioid streaks by intravitreal use of specific anti-VEGF aptamer (pegaptanib sodium): Long-term results. Curr Eye Res. 2011;36:492–495.
27 Finger RP, Issa PC, Schmitz-Valckenberg S, et al. Long-term effectiveness of intravitreal bevacizumab for choroidal neovascularization secondary to angioid streaks in pseudoxanthoma elasticum. Retina. 2011;10:1–11.
28 El Matri L, Kort F, Bouraoui R, et al. Intravitreal bevacizumab for the treatment of choroidal neovascularization secondary to angioid streaks: one year of follow-up. Acta Ophthalmol. 2011;89(7):641–646.
29 Teixeira A, Mattos T, Velletri R, et al. Clinical course of choroidal neovascularization secondary to angioid streaks treated with intravitreal bevacizumab. Ophthal Surg Lasers Imaging. 2010;41:546–549.
30 Wiegand TW, Rogers AH, McCabe F, et al. Intravitreal bevacizumab treatment of choroidal neovascularization in patients with angioid streaks. Br J Ophthalmol. 2009;93:47–51.
31 Sawa M, Gomi F, Tsujikawa M, et al. Long-term results of intravitreal bevacizumab injection for choroidal neovascularization secondary to angioid streaks. Am J Ophthalmol. 2009;148:584–590.e2.
32 Chang LK, Spaide RF, Brue C, et al. Bevacizumab treatment for subfoveal choroidal neovascularization from causes other than age-related macular degeneration. Arch Ophthalmol. 2008;126:941–945.
33 Myung J, Bhatnagar P, Spaide RF, et al. Long-term outcomes of intravitreal antivascular endothelial growth factor therapy for the management of choroidal neovascularization in pseudoxanthoma elasticum. Retina. 2010;30:748–755.
34 Carneiro AM, Silva RM, Veludo MJ, et al. Ranibizumab treatment for choriodal neovascularization from causes other than age-related macular degeneration and pathological myopia. Ophthalmologica. 2011;225:81–88.
35 Mimoun G, Tilleul J, Leys A, et al. Intravitreal ranibizumab for choroidal neovascularization in angioid streaks. Am J Ophthalmol. 2010;150:692–700.
36 Heier JS, Brown D, Ciulla T, et al. Ranibizumab for choroidal neovascularization secondary to causes other than age-related macular degeneration: a phase I clinical trial. Ophthalmology. 2011;118:111–118.
37 Ladas ID, Kotsolis AI, Ladas DS, et al. Intravitreal ranibizumab for macular choroidal neovascularization secondary to angioid streaks: one-year results of a prospective study. Retina. 2010;30:1185–1189.
38 Vadala M, Pece A, Cipolla S, et al. Angioid streak-related choroidal neovascularization treated by intravitreal ranibizumab. Retina. 2010;30:903–990.
39 Artunay O, Yuzbasioglu E, Rasier R, et al. Combination treatment with intravitreal injection of ranibizumab and reduced fluence photodynamic therapy for choroidal neovascularization secondary to angioid streaks: preliminary clinical results of 12-month follow-up. Retina. 2011;10:1–8.
40 Kazato Y, Shimada H, Nakashizuka H, et al. Immunohistochemical findings of a Bruch’s membrane defect and active choroidal neovascularization in angioid streaks. Jpn J Ophthalmol. 2011;54:172–174.
41 Dreyer R, Green WR. The pathology of angioid streaks: a study of twenty-one cases. Trans Pa Acad Ophthalmol Otolaryngol. 1978;31:158–167.
42 Gass HD. Stereoscopic atlas of macular diseases: Diagnosis and treatment, 4th ed. St Louis: Mosby; 1997. p. 118–25
43 Shilling JS, Black RK. Prognosis and therapy of angioid streaks. Trans. Ophthalmol Soc UK. 1975;95:301–306.
44 Sawa M, Ober MD, Freund KB, et al. Fundus autofluorescence in patients with pseudoxanthoma elasticum. Ophthalmology. 2006;113:814–820.
45 Issa PC, Finger RP, Gotting C, et al. Centrifugal fundus abnormalities in pseudoxanthoma elasticum. Ophthalmology. 2010;117:1406–1414.
46 Issa PC, Finger RP, Holz FG, et al. Multimodal imaging including spectral domain OCT and confocal near infrared reflectance for characterization of outer retinal pathology in pseudoxanthoma elasticum. IOVS. 2009;50:5913–5918.
47 Chan WM, Lim TH, Pece A, et al. Verteporfin PDT for non standard indications: a review of current literature. Graefes Arch Clin Exp Ophthalmol. 2010;248:613–626.
48 Shaikh S, Ruby AJ, Williams GA. Photodynamic therapy using verteporfin for choroidal neovascularization in angioid streaks. Am J Ophthalmol. 2003;136:1–6.
49 Browning AC, Amoaku WM, Chung AK, et al. Photodynamic therapy for angioid steaks. Ophthalmology. 2007;114:1592.