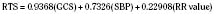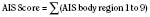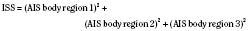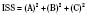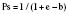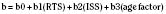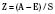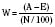CHAPTER 30 Anesthesia for the Pediatric Trauma Patient
Trauma is the forceful disruption of bodily homeostasis that affects physical, psychological, and family functioning, and it remains the number one pediatric public health problem worldwide (Jurkovich et al., 2004). Each year injuries kill more children in the United States than the combined number of pediatric deaths from cancer, congenital anomalies, heart and chronic respiratory diseases, influenza, pneumonia, septicemia, and cerebrovascular disease (Centers for Disease Control and Prevention [CDC], 2002). Over 6 million children are evaluated and treated annually in emergency departments for their injuries, and of these, 500,000 children require hospitalization. Approximately 92,000 children annually become permanently physically disabled as a result of their injuries (Baker et al., 1992; Department of Rehabilitation Medicine, 1995; Tuggle, 1998; CDC, 2002; National Center for Injury Control and Prevention, 2005). Many children who survive their injuries are burdened with lifelong physical limitations or disabilities. For every 400 pediatric injuries, 250 require treatment in the emergency room, 16 are admitted to the hospital, and 1 dies as a result of the injuries (National Center for Injury Control and Prevention, 2005). Trauma care for children includes prevention, treatment and resuscitation, and rehabilitation.
Risk of death from injury can occur during any of three critical times. The first occurs at the time of injury, the second is within the first few hours after trauma, and the third is some time later as a result of complications from the injuries sustained at the time of the traumatic event. Development of effective regional trauma systems and educational programs, such as the Advanced Trauma Life Support for Doctors course, has contributed significantly to the reduction in mortality during the second and third of these critical periods (Tuggle, 1998; Rogers et al., 1999). Unfortunately, however, over 70% of mortality from trauma occurs at the time of injury.
Epidemiology
In 2006, approximately 6 million children received treatment in emergency departments for nonfatal injuries, and of these over 500,000 children needed hospitalization (Child Trends Data Bank, 2005). Injuries can be classified as either intentional or unintentional; most children’s injuries are unintentional. Falls (32.4%) are the leading cause of nonfatal, unintentional injuries among hospitalized children 1 to 14 years of age, followed by injuries sustained as a result of being an occupant in a motor vehicle crash (12.3%) (National Center for Injury Control and Prevention, 2006). The rate of injury and the morbidity and mortality rates remain relatively stable until age 14, when they begin to increase substantially. Males are injured more commonly than females. Motor vehicle accidents are responsible for 75% of pediatric traumatic deaths (DiScala, 2002). Intracranial injuries are the cause of most pediatric traumatic deaths. Unintentional injuries are more prevalent among children who are male, poor, and black or Native American (Safe Kids USA, 2005). In 2004, there were 654,647 (715/100,000 population) nonfatal intentional injuries and 4738 (5.8/100,000 population) fatal intentional injuries in children 19 years and younger. Abuse (physical and sexual), followed by self harm were the major causes of intentional injuries (National Center for Injury Control and Prevention, 2005).
Injuries can be classified as either blunt or penetrating. Blunt injures far outnumber penetrating injuries (12:1), and whereas most are unintentional, up to 7% of injuries are a result of physical assault or abuse (DiScala, 2002). See related video online at www.expertconsult.com.
Pediatric trauma systems
Historical Development
In North America, improvements to trauma care began in the 1960s and 1970s (Trunkey, 1983; Haller, 1995; Mullins, 1999; Morrison et al., 2002). Pediatric trauma systems did not develop in isolation but in concert with adult care. Trauma care was advanced in both the Korean and Vietnam wars, with the military experiences of treating injured soldiers. A report by Howard (1966) highlighted the enormity of the injury problem and stressed its significance as a neglected public health problem. Furthermore, a series of “preventable death studies” was published that provided fodder for community and political support of coordinated care for injured patients. Funding for emergency medical services first became available in 1966 with the National Highway Safety Act, and further support followed with the Emergency Medical Services System Act in 1973 (Mullins, 1999). In 1968, Cook County hospital in Chicago (for adult care) and Kings County hospital in Brooklyn (for pediatrics) were recognized as the first specialized centers for civilian trauma care in the United States. In 1969, the University of Maryland and the Maryland State police developed a coordinated transport system for injured patients to preferentially take them to a hospital with specific interest in caring for injured patients (Cowley et al., 1973; Cowley and Scanlan, 1979). Illinois is credited as the first state to develop a comprehensive state-wide trauma system that included categorization of trauma hospitals and the establishment of a communication system and a trauma registry (Boyd et al., 1973). In 1984, the Department of Health and Human Services, in conjunction with the National Highway Traffic Safety Administration, began the Emergency Medical Services for Children Program in the United States. This funding has been a critical resource for developing programs and research to improve emergency service systems for children.
The American College of Surgeons Committee on Trauma (ACSCOT) has also strived to improve trauma care in North America. This has been accomplished through the publication of standards for trauma systems, the creation of educational courses for health care professionals, and the establishment of and verification programs to ensure published standards are met. In 1976, the first version of the Optimal Resources for Care of Seriously Injured was released (Committee on Trauma: American College of Surgeons, 1999). The most recent version of this publication (2006) is currently in use and has been retitled “Resources for the Optimal Care of Injured Patient.” It remains a dynamic and important document. This publication established criteria for defining levels of trauma centers (I through III) and trauma systems (I and II), and it defined resource requirements for prehospital care through discharge, as well as specific pediatric needs. Level I centers offer the widest range of services for the most severely injured patient, whereas level III centers allow for stabilization and triage. With respect to trauma system development, eight criteria were defined and have become accepted benchmarks for trauma care (Boxes 30-1 and 30-2). Recently, designation of a level IV trauma center has occurred in some states where the resources do not exist for a level III trauma center. Level IV trauma centers are able to provide initial evaluation, stabilization, preliminary diagnoses, and transportation to a center with a higher level of care. These centers may also provide surgery and critical care services (as defined in the scope of services of trauma care). A trained trauma nurse and physicians are available on the patients’ arrival to the emergency department.
Box 30-1 American College of Surgeons Trauma Center Levels and Descriptions
From MacKenzie EJ, Hoyt DB, Sacra JC, et al: National Inventory of Hospital Trauma Centers, JAMA 289(12):1515–1522, 2003.
Box 30-2 American College of Surgeons Criteria for Trauma System Development
From Adler P: Directorate for epidemiology, Washington, DC, 1994, U.S. Consumer Product Safety Commission.
Another valuable contribution by the ACSCOT is the Advanced Trauma Life Support course (ATLS). First released in 1979, the course is now taught all over the world (American College of Surgeons Committee on Trauma, 1997). The first pediatric chapter was introduced in 1983. Although initially designed for surgeons, a wide variety of health care professionals involved in trauma care participate today. The ATLS course provides a common language, framework, and approach to injured patients that facilitate communication between health care professionals in order to optimize and prioritize care. Regular recertification is mandated to keep physicians current with new advances in trauma management. Similar to other ACSCOT programs, the ATLS course is constantly evolving. The current version is the seventh edition.
Trauma System Components
A trauma system is not simply an isolated hospital that cares for injured patients but a broad coalition of participants that includes an integrated approach to trauma. This includes prevention, prehospital care, and hospital care through rehabilitation (Ehrlich et al., 2001, 2002).
A primary step in developing an effective trauma system is a needs assessment of the community that it serves. For example, rural trauma systems differ from those in urban settings—each have different mortality rates, injury patterns, geographic areas, available resources, and expertise (Rogers et al., 1999; Ehrlich et al., 2004). Community responsibility and involvement (particularly with injury prevention) are essential for political support and funding. Studies support the concept that community-based injury-prevention programs play a substantial role in reducing morbidity and mortality (Hulka et al., 1997; Nathens et al., 2000).
A second important consideration in developing a regional trauma system is to understand that all facilities have a role in treating injured patients. A tiered approach (e.g., Levels I through IV) with specific hospitals designated to care for the varied complexity of injured patients is essential. Level I hospitals are tertiary or quaternary centers, with levels II and III centers having fewer capabilities and resources. Level IV hospitals are distinct because of their remote nature (rural trauma). A list of recommended requirements for each level of care can be found in the ACSCOT publication Resources for the Optimal Care of the Injured patient: 2006 (Committee on Trauma: American College of Surgeons, 1999). A basic tenet and overarching requirement for this tiered system is a central communication (central command) structure and a well-developed triage system so that the most severely injured patients go first to the appropriately designated hospital. In addition, a trauma registry system is essential for outcomes analysis and quality-assurance programs.
Anesthesiologists play a variety of roles in trauma response. These roles span from assisting as primary responders in airway management and vascular access to coordinating operating-room resources to ensure that patients are treated in a timely and effective fashion. A pediatric anesthesiologist is an invaluable resource when treating injured children. A 1993 report from the Institute of Medicine identified specific pediatric emergency medical service deficiencies in rural states (Institute of Medicine, 1993), including the pediatric airway management, vascular access, and special resuscitation needs of injured children. Airway control is a primary tenet of trauma care, and proper endotracheal intubation (ETI) is recognized as a definitive method to achieve airway control. ETI, however, in injured children is required less often than in adults; hence, maintaining skills can be difficult for health care providers (Baker et al., 2009). Several studies have looked at airway management in pediatric trauma and examined significant aspects, including how success and complication rates of ETI vary by location (e.g., field, transferring hospital, or trauma center) and personnel, whether bag-mask ventilation is effective with respect to oxygenation and ventilation before ETI, and whether multiple attempts at intubation adversely affect patient care or outcomes (Cooper et al., 1992; Gausche et al., 2000; Glaeser, 2000; Ehrlich et al., 2004). These studies have demonstrated that field intubation success rates are poor. Successful pediatric intubations relate to the skill of the person performing the intubation, and anesthesiologists’ airway management skills are considered superior to those of all other health care professionals. Failed attempts at ETI appear to produce a spiral effect that results in multiple failed attempts and delayed transfer to definitive care. Complications resulting from ETI seem far more common when the intubation is attempted in the field and the risk of complication is exacerbated with each attempt. Increased complication rates do impact patient recovery, as shown by longer durations of hospitalization (Cooper et al., 1992; Gausche et al., 2000; Glaeser, 2000).
Trauma scoring systems
Triage Scores: Where Is the Injured Child Best Treated?
An ideal triage scoring system would have few data points, be easy to apply, have limited subjective assessments, and have a high sensitivity and specificity. Unfortunately, no triage score accomplishes all of these tasks, but each system does have strengths and limitations. For example, studies have shown that assessments made in the field by paramedics are less accurate and reliable for pediatric trauma patients when compared with adults (Engum, 2000). Alternatively, comparisons between adult and pediatric trauma triage scores have not shown that a pediatric specific trauma score provides a significant advantage over the original adult measure.
Glasgow Coma Scale
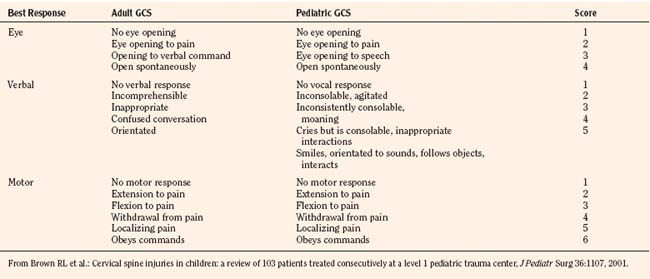
In 1974, Teasdale and Jennett first introduced the GCS. A lower score reflects a lower level of consciousness and therefore a potentially more serious head injury. Scores can range from 3 to a maximum of 15 (normal). The GCS measures three specific components of consciousness (eye movement, verbal, and motor responses) with higher scores (up to 5) given to the best response. A patient with a GCS of 13 to 14 is considered to have a mild head injury; 9 to 12 indicates a moderate injury; and a score of 8 or lower indicates a severe insult. Adaptations of the GCS for the pediatric population have occurred, and a Pediatric GCS is now widely used (Table 30-1).
GCS scores determined in the field are less predictive of outcome than those generated at a hospital (Meredith et al., 1995). When each individual component of the GCS is evaluated, the motor component is the strongest predictor of outcome.
Revised Trauma Score
The revised trauma score (RTS) is a physiologic-based triage score (Table 30-2). The RTS was derived from two earlier versions of a triage scores, the Triage Index and the Trauma Score (Champion et al., 1980, 1981, 1989). The RTS has three variables—respiratory rate, systolic blood pressure, and GCS. The RTS is the sum of each variable multiplied by a weighted coefficient.
| Clinical Measurement | Parameter | Score |
| Respiratory rate | 10−24 25−35 >35 <10 0 |
4 3 2 10 |
| Systolic blood pressure | >90 70−89 50−69 <50 0 |
4 3 2 1 0 |
| Glasgow Coma Scale | 14−15 11−13 8−10 5−7 3−4 |
4 3 2 1 0 |
* The Revised Trauma score is the sum of the weighted variables (see formula in text). The higher the score, the better the prognosis.
From Centers for Disease Control and Prevention: Traumatic brain injury in the United States: a report to Congress, Atlanta, 1999, Department of Health and Human Services National Center for Injury Control and Prevention.
where GCS is the Glasgow Coma Scale; SBP is systolic blood pressure; and RR is respiratory rate. These variables were determined to correlate statistically with survival and mortality. A higher RTS is associated with a better chance of survival. An injured patient with a RTS score of 11 or lower is recommended to be treated at a designated trauma center. Eichelberger established and developed pediatric coefficients for the RTS that was then validated in the pediatric population (Engum et al., 2000). The RTS is also recommended as one of the triage tools in the American College of Surgeons trauma guidelines, Resources for the Optimal Care of the Injured Patient (Committee on Trauma: American College of Surgeons, 1999).
Pediatric Trauma Score
The Pediatric Trauma Score (PTS) was first introduced by Tepas in 1987 (Tepas et al., 1987; Nayduch et al., 1991). Its design was thought to be more reflective of pediatric injuries (Table 30-3). Six variables comprise both physiologic and injury attributes. After a number for each variable is totaled, a score of 8 or lower is considered to be a marker for children requiring care at a designated trauma center. A limitation of the PTS is that some variables are subjectively scored, particularly the central nervous system (CNS) assessment. Therefore, there is a risk of interrater and intrarater variability. The PTS has been compared with the RTS with surprisingly few apparent differences or inherent advantages (Tepas et al., 1987, 1988; Nayduch et al., 1991).
| Clinical | Parameter | Score* |
| Weight (kg) | >20 10-19 <10 |
2 1 –1 |
| Airway | Normal Maintainable Unmaintainable |
2 1 –1 |
| Systolic blood pressure | >90 50-89 <50 |
2 1 –1 |
| Central nervous system | Awake Obtunded or loss of consciousness Coma or decerebrate |
2 1 –1 |
| Open wound | None Minor Major or penetrating |
2 1 –1 |
| Skeletal | None Closed fracture Open or multiple fractures |
2 1 –1 |
* A total score of 8 or less suggests care at a designated trauma center.
Data from Tepas JJ III, Mollitt DL, Talbert JL, et al: The pediatric trauma score as a predictor of injury severity in the injured child, J Pediatr Surg 22:14–18, 1987; Tobias JD, Ross AK: Intraosseous infusions: a review for the anesthesiologist with a focus on pediatric use, Anesth Analg 110:391–401, 2010.
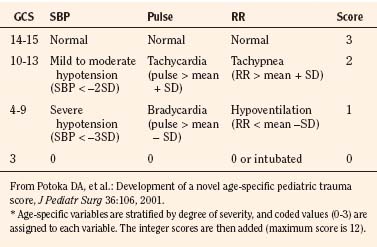
Age-Specific PTS
The most recent proposed triage score is the Age Specific PTS (Table 30-4). First described by Potoka et al. in 2001, the main addition is the use of age-specific physiologic variables. To date, long-term data are lacking and only a single study suggests that it is better than traditional adult scores; therefore, the clinical utility as a triage score still remains to be proven. This score is, however, a pediatric-specific tool that addresses areas where other adult scores become less accurate (e.g., extremes of age).
Abbreviated Injury Scale
The Abbreviated Injury Scale (AIS) emerged from the automotive industry in 1969 (JAMA, 1971; Association for the Advancement of Automotive Medicine, 1990). It was initially designed as an epidemiologic tool to describe motorvehicle crashes but has been adapted to all types of trauma (JAMA, 1971; Association for the Advancement of Automotive Medicine, 1990). Revised and updated several times over the years, the first version published was the AIS-90 (for the year 1990) in 1998 (Association for the Advancement of Automotive Medicine, 1990). This score evaluates nine body regions. A scale from 1 to 6 is used to define injuries (1, minor; 2, moderate; 3, serious; 4, severe; 5, critical; and 6, maximal.) The underlying premise is an association of injury with threat to life. A body region with a score of 6 is considered an unsurvivable injury. The scores are determined retrospectively and based primarily on ICD-9-CM codes. A significant amount of expertise is required to assign these ratings, and interrater and intrarater variability is common. To help limit this phenomenon, software has been developed to convert the ICD-9-CM codes directly to an AIS score. Unfortunately, ICD-9-CM coding itself is inherently variable (MacKenzie et al., 1985, 1989).
Injury Severity Score
The Injury Severity Score (ISS) is one of the most widely used scoring systems in the trauma literature. It correlates well with several important trauma outcomes such as mortality and duration of hospitalization. Developed in 1974, the ISS is a method of characterizing the trauma patient with multiple injuries (Baker et al., 1974; Baker and O’Neill, 1976). The ISS is based on the AIS, which describes the severity of injury to different body regions (see the previous section). For example:
Survival Probability: Trauma Score and Injury Severity Score
The Trauma Score and Injury Severity Score (TRISS) is not a score but rather a method of predicting mortality. It was first proposed in 1987 and combines the physiologic variables of the RTS with the anatomic severity-of-injury scores generated by the ISS (Boyd et al., 1987). The final result is that a probability of survival (Ps) is generated.
Where e is the base of the natural log 2.72183, and b is derived from the following formula:
The age factor is 0 for those younger than 55 years of age, and 1 for those who are 55 years of age. The variables b0, b1, b2, and b3 are blunt and penetrating trauma coefficients for adult and pediatric populations (Eichelberger et al., 1993).
TRISS is the most widely used predictor of survival in the trauma literature. As with other statistical processes, limitations exist. These are specifically noted in patients older than 55 years of age (extremes of age), and with trauma patients whose ISS is greater than 25. In fact, some authors suggest that because of the TRISS deficiencies, this method of calculating survival probabilities should be abandoned completely, particularly in urban centers (Cayten et al., 1991; Demetriades et al., 1998).
Application of the TRISS Results (W and Z Scores)
A common application of the TRISS and Ps is to compare practice-based outcomes with population-based outcomes. Typically, this comparison uses a group of national norms that were identified in the Major Trauma Outcomes Study (MTOS) (Champion et al., 1990b). The MTOS data set is derived from 160,000 hospitalized patients at 139 centers between 1982 and 1989. Children represented 11% of this population. From 80,544 adult patients in this data set, regression coefficients for predicting mortality were derived based on the revised trauma score and ISS (TRISS methodology). A single data set can be compared with the MTOS. A “Z score” is generated, whereby
W Scores
W is used when the Z score is found to be significant. W measures the statistical differences between the actual (A) and expected (E) survivors in a patient group. Sample size plays an important role in delineating the clinical significance of the difference between the actual and expected numbers of survivors (Taylor et al., 1986). Consider the following formula:
Both the W and Z scores are limited by the same factors that limit TRISS data.
A Severity Characterization of Trauma Score
A Severity Characterization of Trauma (ASCOT) score is an attempt to revise and fix the limitations found using TRISS methodology (i.e., severely injured patients and extremes of age) (Champion et al., 1990b). ASCOT uses individual components of the RTS (not totaled) and incorporates a modification of AIS entitled The Anatomic Profile. Furthermore, it excludes those patients with either very severe or nonserious injuries (a maximum AIS score of less than 2 and an RTS score of greater than 0; an AIS score of 6 and an RTS score equal to 0). The Anatomic Profile categorizes the AIS scores that are greater than 2 into three groups: head, brain, and spinal cord injuries; thorax or neck injuries; and all other serious injuries. A comparison of ASCOT vs. TRISS with a pediatric population did not provide more reliable or accurate scoring over the TRISS.
Primary and secondary surveys
The first priority is to save a life through identifying and treating all life-threatening illnesses and injuries. To understand and acquire the necessary clinical skills for the management of injured adults and children, it is best to become certified by completing the ATLS course designed by the American Colleges of Surgeons (Schall et al., 2002). The ATLS course is a well-recognized certification for trauma care and is now used worldwide to teach physicians and other health care providers. The ATLS course gives a common approach and language to the care of injured patients, thus allowing a framework for physicians and allied health personnel to communicate.
The initial management of the injured child can be divided into two phases. The first is the primary survey that incorporates the “ABCs” and where all life-threatening injuries are identified and treated. The second phase is the secondary survey, where other injuries that contribute significantly to illness and deaths are identified and treatment is instituted. The ABCs are defined in the following list, and the priorities of the primary survey are further detailed in Box 30-3.
Box 30-3 ABCs of Resuscitation
Data from Tepas JJ 3rd, Mollitt DL, Talbert JL, et al: The pediatric trauma score as a predictor of injury severity in the injured child, J Pediatr Surg 22:14–18, 1987; Tobias JD, Ross AK: Intraosseous infusions: a review for the anesthesiologist with a focus on pediatric use, Anesth Analg 110:391–401, 2010.
During the resuscitation of the injured child, many processes are conducted concurrently (Fig. 30-1). It is important to continually assess, intervene, and reassess within the priorities of the primary survey. The final components of the primary survey are the placement of a Foley catheter and gastric tube unless contraindicated. The contraindications to placing a Foley catheter include pelvic fracture and blood at the tip of the meatus.
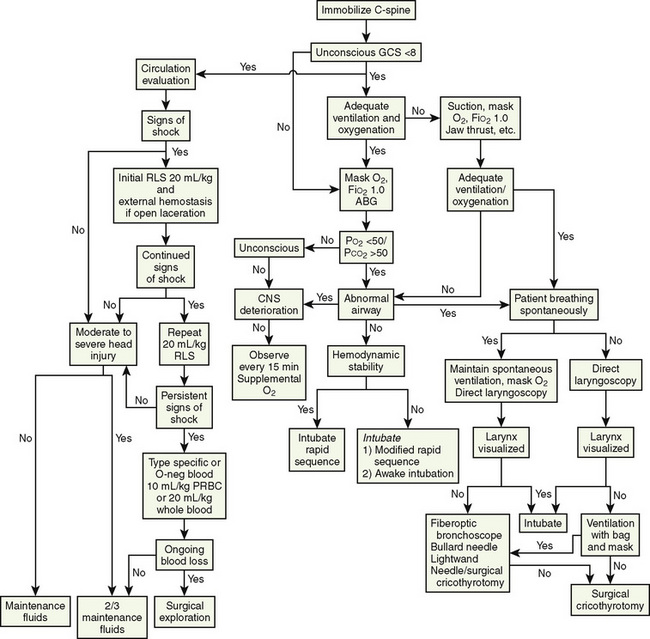
FIGURE 30-1 Guidelines for airway and cardiovascular assessment in the traumatized pediatric patient.
The secondary survey is comprised of a complete physical examination, patient history, laboratory tests, and radiologic imaging. This phase may be delayed or completed in the operating room in patients who require urgent interventions. Historical information about the patient and the event should be collected during the secondary survey. The acronym SAMPLE (standing for Symptoms, Allergies, Medications, Past illnesses, Last meal, Events, and Environment) may be helpful in guiding the trauma team. Definitive care occurs in the intensive care unit or operating room and often involves care by pediatric surgical subspecialists (e.g., neurosurgeons or orthopedic surgeons). Patients may also require transfer to comprehensive trauma centers during this phase (Krantz, 1996).
Airway
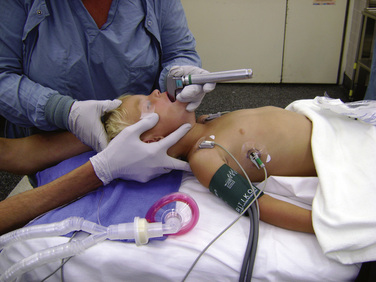
The initial management of the pediatric airway involves bag-valve-mask ventilation with a jaw-thrust maneuver. Intubation is indicated in patients with respiratory or cardiac compromise or an altered level of consciousness. All pediatric trauma patients should be considered to have a full stomach and possible cervical spine injury. See related video online at www.expertconsult.com. Because of this, the airway should be secured after a rapid-sequence induction (RSI) (that may include cricoid pressure) with manual inline stabilization and an oral ETI. Nasotracheal intubation may be suboptimal and difficult because of the small size and acute angle of the nasopharynx and the more anterior and cephalad position of the glottic opening.
Because of this, the airway should be secured after a rapid-sequence induction (RSI) (that may include cricoid pressure) with manual inline stabilization and an oral ETI. Nasotracheal intubation may be suboptimal and difficult because of the small size and acute angle of the nasopharynx and the more anterior and cephalad position of the glottic opening.
The RSI for pediatric trauma patients can be accomplished with an induction agent that is immediately followed by a muscle relaxant. Standard induction agents for trauma patients include etomidate (0.2 to 0.3 mg/kg) and ketamine (2 to 4 mg/kg) or the combination of fentanyl (2 to 3 mcg/kg), midazolam (0.05 to 0.1 mg/kg) with lidocaine (1 mg/kg). Sodium thiopental (STP) and propofol should be reserved for patients who are not hemodynamically unstable. STP is an ideal induction agent for patients with head trauma, provided they are not hypovolemic. Ketamine is relatively contraindicated in patients with increased intracranial pressure (ICP). Although etomidate provides hemodynamic stability in trauma patients who are hypovolemic, it may decrease survival in patients with sepsis secondary to adrenal suppression (Annane et al., 2002). Muscle relaxation can be achieved with rocuronium (0.8 to 1.2 mg/kg) or succinylcholine (1 to 1.5 mg/kg). Succinylcholine is contraindicated in crush injuries, long-bone fractures, and patients susceptible to malignant hyperthermia. In patients who are hemodynamically stable, the combination of propofol (4 mg/kg) and remifentanil (3 mcg/kg) can be used for rapid-sequence intubation and has an onset and offset similar to propofol and succinylcholine (1 mg/kg) (Crawford et al., 2005).
ETIs have been deemphasized in the prehospital setting, because they are often unsuccessful. Gausche and others (2000) reported a success rate of only 57%. All intubated trauma patients that come to the emergency department should have the placement of their endotracheal tubes (ETTs) confirmed with either end-tidal carbon dioxide (CO2) or direct laryngoscopy.
If initial intubation attempts are unsuccessful after an RSI, the patient should be ventilated with bag-mask ventilation. If a patient cannot be ventilated or if it is very difficult, a laryngeal mask airway (LMA) can be placed to facilitate ventilation and subsequent intubation. However, it must be recognized that the LMA does not protect the airway from aspiration and must be replaced by an ETT as soon as skilled personnel become available. The stomach should be decompressed with a nasogastric or orogastic tube after intubation, and a chest x-ray should be obtained to verify the ETT position (Fig. 30-2).
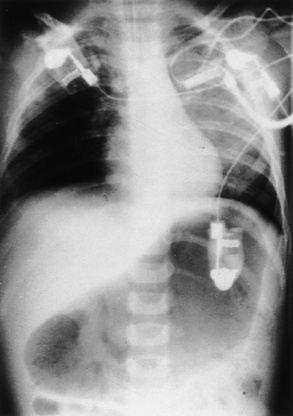
FIGURE 30-2 Gastric dilation often occurs after crying or positive-pressure ventilation by gas and mask.
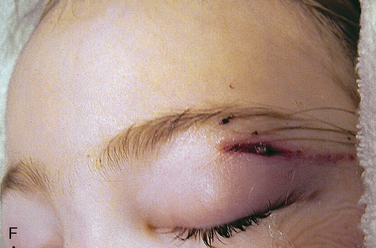
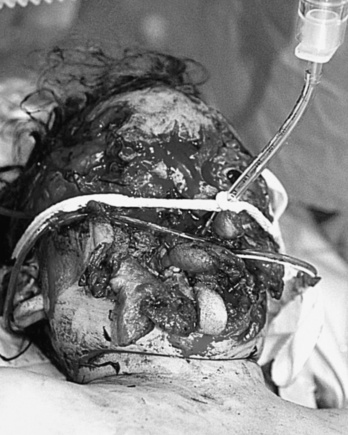
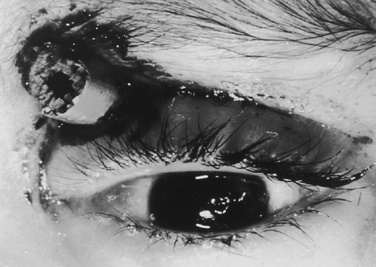
Depending on the nature of the underlying injury, securing the airway in a patient who has sustained multiple injuries or even isolated facial injuries can be extremely complicated, as illustrated in Figure 30-3. The management of such cases calls upon the resourcefulness and skills of the anesthesiologist and requires careful consideration of damage to surrounding structures such as major blood vessels and the airway structures themselves. The ability to maintain a patent airway via face mask, and the potential for an expanding hematoma that may subsequently compromise an airway that may be patent at the current time, must be anticipated. Additional considerations include the risks of increased ICP with concomitant head trauma, exacerbating an existing cervical spine injury, and aspiration during airway manipulation. The presence of rhinorrhea, otorrhea, or ecchymoses around the eyes should raise suspicion about a possible basilar skull fracture, and any instrumentation of the nasal passages, including passage of a nasal ETT or an N/G tube, should be avoided. Similarly, crepitus at the neck may herald the presence of a tracheal disruption, and intubation under direct vision using a fiberoptic scope should be considered to avoid false passage of the ETT. See related video online at www.expertconsult.com.
In cases in which airway difficulty is anticipated, it may be prudent to transport the child to the operating room with an anesthesiologist and otolaryngologist once the child has been stabilized hemodynamically and additional injuries have been ruled out. The airway may then be secured with preparations to perform an emergent tracheostomy in case of failed laryngoscopy. An inhalational induction may be tolerated by the patient who has been volume resuscitated. This permits direct laryngoscopy or flexible fiberoptic intubation while the patient is breathing spontaneously. In patients who have suffered loss of consciousness or head injury, there should be a high index of suspicion for cervical spine injury, even with the absence of radiologic evidence. When performing laryngoscopy, inline axial stabilization must be performed (Fig. 30-4). Inline axial stabilization is performed by an assistant during laryngoscopy. The assistant should place both arms on either side of the patient’s head while gripping the patient’s shoulders. The assistant’s function is to maintain the patient in a neutral position during laryngoscopy, avoiding flexion, extension, or rotation of the cervical spine. The use of muscle relaxants is best avoided until the airway is secured. If intravenous agents are required to induce anesthesia, it is preferable to use short-acting agents such as propofol and remifentanil that effectively blunt ICP responses to direct laryngoscopy yet permit return of spontaneous respiration in case of failed intubation.
Circulation and Access
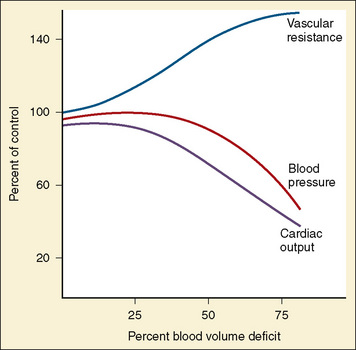
Children who sustain multiple injuries often arrive in hypovolemic or hemorrhagic shock that must be promptly recognized and treated. Unlike adults, children maintain an almost normal blood pressure until 25% to 35% of their circulating blood volume is lost (Fig. 30-5). This is likely because of their high sympathetic tone that causes peripheral vasoconstriction in an effort to maintain blood pressure in the face of a diminished blood volume. Therefore, tachycardia is an earlier sign of impending shock than hypotension. Additionally, signs of poor peripheral perfusion such as delayed capillary refill (more than 2 seconds), weak or thready pulses, mottling or cyanosis of the skin, and impaired consciousness are earlier indicators of shock than low blood pressure. The presence of hypotension as a result of hypovolemia should be considered an ominous sign that usually heralds impending cardiovascular collapse. Table 30-5 describes the stages of pediatric shock and clinical signs seen at these stages.
TABLE 30-5 Stages of Pediatric Blood Volume Loss (Shock) and Associated Clinical Signs
| Blood Volume Loss | Clinical Signs |
| <20% | CV: Tachycardia; weak, thready pulses Skin: Cool to touch, capillary refill 2-3 sec Renal: Slight decrease in urine output, increase in specific gravity CNS: Irritable, may be combative |
| 25% | CV: Tachycardia; weak, thready distal pulses Skin: Cold extremities, cyanosis, and mottling Renal: Decrease in urine output CNS: Confusion, lethargy |
| 40% | CV: Frank hypotension; tachycardia may progress to bradycardia Skin: Pale, cold Renal: No urine output CNS: Comatose |
CV, Cardiovascular; CNS, central nervous system.
From Rasmussen GE, Grandes CM: Blood, fluids, and electrolytes in the pediatric trauma patient, Int Anesthesiol Clin 32:79-101, 1994.
It is imperative to rapidly assess the pediatric trauma patient for signs of shock upon arrival in the trauma center and at regular intervals thereafter. The initial fluid bolus administered in the trauma setting is warmed isotonic crystalloid (lactated Ringer’s solution or normal saline) in a bolus of 20 mL/kg, IV (see Fig. 30-1). The pulse, capillary refill, and blood pressure are reassessed. A second bolus of 20 mL/kg is administered if there is no significant response or only a transient improvement in these parameters. A third crystalloid bolus may be given if necessary to maintain appropriate vital signs and circulation. Blood (10 mL/kg) should be administered next if additional fluid resuscitation is required. The need for blood transfusion initially is uncommon and usually signals surgical bleeding that may require an operation.
Pneumothorax is a common complication of blunt chest injury in children, with nearly one fourth of pneumothoraces under tension (Nakayama et al., 1992). Unilateral or bilateral tension pneumothoraces may produce hypotension and hypoxemia. The classic signs of tension pneumothorax are ipsilateral tympany, shift of the trachea to the contralateral side, and distended neck veins.
IO access is placed in the medial surface of the proximal tibia 1 to 3 cm below the tibial tuberosity or the distal femoral metaphysis. IO has been used as a lifesaving measure to establish short-term vascular access in critically ill or injured children (Fig. 30-6).
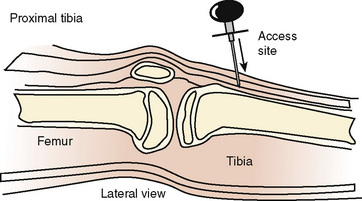
FIGURE 30-6 Appropriate placement of the IO infusion needle on the medial surface, distal to tibial tuberosity.
(From Ellemunter H: Intraosseous lines in preterm and full-term neonates, Arch Dis Child Fetal Neonatal Ed 80:74F, 1999.)
The use of IO infusions in the algorithm of trauma and cardiopulmonary resuscitation has evolved over the years and now takes a more prominent role for providing vascular access to children. The subject of IO has been reviewed by Tobias and Ross (2010).
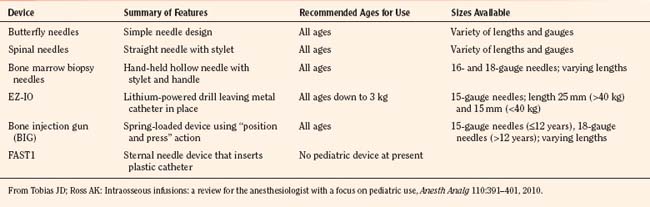
Access needles for IOs include manual devices such as butterfly needles, spinal needles, bone marrow biopsy needle such as Susfast (Cook Critical Care; Bloomington, Indiana) and the basic Jamshidi needle (Baxter Healthcare Corporation; Deerfield, Illinois). More recently, automated devices have been marketed (Fig. 30-7). The EZ-IO (Vidacare; San Antonio, Texas) functions like a battery-powered drill, with a beveled drill and a preset depth. It is designed to be used in the tibia. The Bone Injection Gun (BIG; Waismed; Kansas City, Missouri) is a spring-locked device also designed to be used in the tibia. It comes in a range of sizes for use in infants, small children, and adults. A device specifically designed for entry into the bone marrow of the sternum is the FAST1 (First Access for Shock and Trauma, PYN6 Medical Corporation; Vancouver, British Columbia). It is approved for patients ages 12 and older. Table 30-6 summarizes these devices. Details regarding instructions on the use of these devices can be viewed on the manufacturers’ websites.
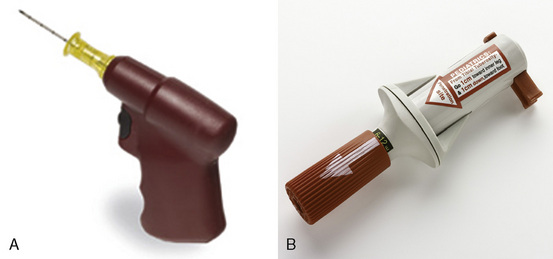
FIGURE 30-7 Automated devices. A, EZ-IO. B, Bone Injection Gun.
(A, Courtesy Vidacare, San Antonio, TX; B, Courtesy Waismed, Kansas City, Mo.)
A review of the use of IO access in pediatric trauma patients up to 10 years of age reported successful placement of access in 28 out of 32 attempts in the prehospital setting and in the trauma care center (Guy et al., 1993). In this study, IO access was placed successfully by paramedics, nurses, and physicians with only one incident of minor extravasation of fluid and no long-term complications in the survivors. The commonest complication of IO access is subperiosteal infiltration that in most cases resolves spontaneously without further problems. The most feared complication—osteomyelitis—occurs in 0.6% cases (Rosetti et al., 1985). Other rare complications include fractures and emboli. Although few complications have been reported with this technique, it must be recognized that the high mortality in patients who require IO access prevents the assessment of long-term complications.
Secondary Survey
Diagnostic Evaluation
The diagnostic evaluation of the injured child involves clinical examination supplemented by radiologic examinations and laboratory testing. Imaging plays a major role in the evaluation of the injured child (Vane, 2002). Improvements in imaging techniques have allowed progress in the nonoperative management of abdominal and thoracic trauma, supplanting exploratory laparotomy and diagnostic peritoneal lavage in many hemodynamically stable patients.
The most common cause of a distended abdomen in a child is swallowed air (see Fig. 30-2). Distention can be massive and can appear as if the child has an acute abdomen condition. Placement of a nasogastric tube is imperative. Often that alone improves both abdominal and respiratory status. Abdominal distention or tenderness despite nasogastric-tube decompression suggests a possible solid-organ or hollow-viscus injury. Hemodynamic instability with abdominal tenderness or distension mandates immediate surgical consultation and or operation. Ultrasound is increasingly being used in the emergent assessment of injured patients. Focused Assessment by Sonography in Trauma (FAST) is used to detect intraabdominal blood; however, the clinical significance in children of this finding and the management are unclear. FAST has largely replaced the diagnostic peritoneal lavage that has no role in the diagnosis of pediatric abdominal injury. CT scanning of the abdomen is still the most effective method to diagnosis solid-organ injuries and intraabdominal blood. FAST may not add much to the care, because most stable patients with or without blood loss are managed conservatively (Thourani et al., 1998; Miller et al., 2003; Soundappan et al., 2005). Unstable patients may require surgical intervention.
Because most injured children are generally healthy and take few or no medications, laboratory screening examinations are limited and focused. Children with minor injuries (e.g., upper extremity fractures) may require limited or no laboratory testing. In more significantly injured patients, laboratory testing may generally be safely limited to specific clinical indications rather than a generalized routine trauma panel (Chu et al., 1996). A complete blood count, blood gas, blood type and screen, and urinalysis are suggested for initial testing in significantly injured patients. Routine testing of liver functions, pancreatic enzymes and coagulation parameters is of limited value and should only be obtained when clinically indicated.
Anesthetic perioperative management
Children with multiple injuries often arrive with an unusual combination of anesthetic problems that present a challenge to the anesthesiologist. It must be emphasized that the likelihood of a successful outcome is greatly enhanced by the initial stabilization efforts that must include early initiation of critical-care management in the emergency department based on appropriate and rapid physical examination and diagnostic studies rather than an urgent rush to the operating room for emergency surgical interventions (Meyer, 1999). Respiratory stabilization and suitable hemodynamic support, including volume resuscitation can prevent further decompensation as well as the development of secondary injuries. This in turn requires a well-coordinated effort by all members of the health care team including the anesthesiologist, the emergency department physician, surgeons of the relevant specialties, respiratory therapists, and critical-care nurses. Only in rare instances is there little time for initial stabilization and a child must undergo emergency surgery to ensure a favorable outcome. In such cases there must be clear communication between the anesthesiologist and surgeon regarding the time available for resuscitation, securing vascular access, and placement of invasive monitoring catheters, keeping in mind the need for immediate surgical intervention.
Fasting Duration
It is common practice to consider all trauma patients at risk for aspiration regardless of the time of last oral intake. The rationale for this approach is that major injury, the presence of pain and anxiety, and the administration of opioid analgesics delay gastric emptying. Additionally, bag-and-mask ventilation at the scene of the accident or in the emergency department that leads to gastric distention and the use of oral contrast solutions for diagnostic imaging studies may further increase the risk for aspiration. Indeed, previous investigators have demonstrated that patients who come for emergency surgery are at five times the risk for aspiration compared with those who undergo elective surgery (Olsson et al., 1986). Other investigators reported a 17% incidence of vomiting and 3% incidence of aspiration in 60 children younger than 19 years of age who required emergency ETI after they sustained a severe traumatic injury (Nakayama et al., 1992). Interestingly, residual gastric volume has been previously found to have a greater correlation with the interval from oral intake to injury than with actual fasting interval (Bricker et al., 1989).
These data raise two questions regarding the management of anesthetic induction for the trauma patient: whether it is possible to predict the safe interval between oral intake, injury, and induction of anesthesia, whether imposing a fasting duration once the trauma has already occurred offers any benefit in terms of reduction in aspiration risk. Goodwin and Robinson (2000) surveyed 167 practicing anesthesiologists in the United Kingdom regarding their practice in three different scenarios after a forearm fracture in a child. Approximately one third of the respondents did not believe there was any benefit in delaying the procedure and would perform a RSI and ETI regardless of the fasting duration, whereas almost two thirds of the respondents would delay the procedure if it was not emergent and then use a LMA or face mask as they would for elective cases. Such variability in clinical practice related to the management of the trauma patient is likely because of the difficulty in predicting a safe interval between oral intake, injury, and induction of anesthesia with regard to aspiration risk. A conservative and practical approach would be to proceed with surgery when an operating room becomes available and do a RSI and intubation if airway difficulty is not anticipated.
Induction of Anesthesia
Anesthetic induction techniques should be individualized according to the nature of the injuries, whether the airway has been secured before arrival in the operating suite, anticipated airway difficulty, hemodynamic status of the patient, and the presence of ongoing hemorrhage. The child with head trauma merits special consideration because of the risk of increased ICP during induction of anesthesia. Selection of an induction technique in these patients must be made with the goal of avoiding secondary brain injury. IV-induction agents such as thiopental, propofol, or etomidate may be preferred because of their beneficial effects on ICP and cerebral oxygen consumption (CMRo2). On the other hand, a child with an anticipated difficult airway may be better managed with an inhaled route of induction so that spontaneous respiration is assured. Induction of anesthesia in a patient with dehydration or hypovolemia may lead to cardiovascular collapse. It is therefore imperative to have adequate IV access and rehydrate these patients before induction of anesthesia. A brief description of commonly used induction agents and pitfalls with the use of each in a child with trauma follows. Additional details about anesthetic agents are described in Chapters 7 and 22, Pharmacology of Pediatric Anesthesia and Anesthesia for Neurosurgery.
Propofol
Induction of anesthesia with propofol in healthy children is commonly associated with a significant (10% to 20%) decrease in mean arterial pressure (MAP) because of its direct relaxant effects on vascular smooth muscle that cause a reduction in systemic vascular resistance and preload (Aun et al., 1993). It should, therefore, be used with caution, if at all, in patients with depleted intravascular volume. Its beneficial effects of cerebral vasoconstriction, reduced CBF, and CMRo2 in patients with head trauma are offset to an extent by a reduction in CPP because of a decrease in systemic blood pressure. It has been further hypothesized that the decrease in CPP may lead to reflex cerebral vasodilation to maintain CBF, thereby also negating its beneficial effects in reducing ICP (Spitzfaden et al., 1999).
The literature evaluating the use of propofol in adult neurosurgery patients has yielded conflicting results. Previous studies have demonstrated that whereas propofol effectively lowered ICP in patients with elevated ICP after TBI and during cerebral aneurysm surgery, there was a significant reduction in overall CPP because of a greater decrease in MAP than ICP (Herregods et al., 1988; Ravussin et al., 1988; Pinaud et al., 1990). Other investigators reported no reduction in ICP with propofol sedation in adults with head trauma (Stewart et al., 1994).
Etomidate
Etomidate provides both hemodynamic stability as well as cerebral protection, making it the ideal anesthetic induction agent for emergency surgery in a child with multiple traumatic injuries. Although it does cause a direct myocardial depressant effect, it does so to a significantly lesser extent than equipotent doses of other induction agents, including thiopental, propofol, and ketamine (Stowe et al., 1992). Etomidate, however, maintains sympathetic outflow and produces no significant changes in blood pressure, making it the agent of choice in the hemodynamically unstable patient. Similar to thiopental and propofol, it is a cerebral vasoconstrictor and causes a reduction in ICP, CBF, and CMRo2. However, because MAP is maintained with etomidate, CPP is also maintained. Perhaps the only concern with its use has been adrenal suppression; however, this is believed to have questionable clinical significance with brief use (Crozier et al., 1987). A recent retrospective review reported successful fracture reduction in 52 of 53 patients who received etomidate alone or in combination with midazolam or opioids (Dickinson et al., 2001). This study found a low incidence of minor side effects, including nausea and vomiting, mild hypotension, and prolonged sedation in one patient each.
Ketamine
Ketamine is a dissociative anesthetic that is commonly selected for induction in children who are hypovolemic, because its sympathomimetic actions result in increases in blood pressure and heart rate. However, like the other induction agents described above, ketamine has direct myocardial depressant effects as well as direct vasodilatory effects. In fact, significant hypotension has been reported after ketamine administration in critically ill patients, likely as a result of its direct myocardial depressant effects predominating in the presence of depleted catecholamine stores (Waxman et al., 1980).
Ketamine, however, is a potent cerebral vasodilator and causes a marked increase in CBF. Although CMRo2 usually remains unchanged after ketamine administration, ICP may increase, especially in patients with intracranial pathologic conditions. However, data regarding the effects of ketamine on ICP remain inconclusive, with some studies demonstrating modest decreases in ICP after ketamine administration, particularly when they are administered concomitantly with other sedatives (Mayberg et al., 1995; Albanese et al., 1997). A recent controlled, randomized, double-blind trial found no differences in mean daily values of ICP, CPP, and the number of episodes of ICP elevations in patients with severe TBI who were sedated with ketamine and midazolam compared with those who were sedated with sufentanil and midazolam (Bourgoin et al., 2003). Yet, its cerebral vasodilatory effects preclude the use of ketamine as an induction agent in patients with head trauma.
Maintenance of Anesthesia
In a child with severe head trauma, efforts must be directed at preventing secondary brain injury and protecting the injured brain from further ischemic injury by selecting anesthetic techniques that maintain blood pressure while reducing ICP. All volatile anesthetics cause cerebral vasodilation, which has been correlated with increasing minimum alveolar concentration (MAC) in children (Vavilala and Lam, 2002). Isoflurane affects CBF and cerebral autoregulation to a lesser extent than halothane. Sevoflurane offers greater advantages in that CBF velocities do not increase significantly with less than 1 MAC, and cerebral pressure autoregulation is maintained up to 1.5 MAC sevoflurane (Gupta et al., 1997; Monkhoff et al., 2001). For these reasons, sevoflurane may be the preferred volatile anesthetic for the child with TBI, and it would be prudent to limit its use to 1 MAC.
Opioids (fentanyl, sufentanil, or remifentanil) are often administered as intermittent bolus doses or infusions to supplement volatile anesthetics, for postoperative analgesia, and as additional measures to lower ICP. However, increased ICP has been reported in an 11-year-old child with closed head injury after bolus doses of fentanyl that responded to hyperventilation and barbiturates (Tobias, 1994). In addition, recent studies in adults have reported a transient but significant increase in ICP accompanied by a decrease in MAP and CPP after bolus doses of morphine, fentanyl, sufentanil, and alfentanil (Albanese et al., 1999; de Nadal et al., 2000). The exact mechanism of these changes remains unknown; however, impaired cerebrovascular autoregulation and direct cerebral vasodilatory effects of opioids have been implicated. Such effects may have significant implications in the management of the child with traumatic head injuries; however, until additional data become available, the judicious use of opioid infusions with careful monitoring of hemodynamic parameters is recommended.
Fluid Resuscitation
Shock is defined as a metabolic demand that exceeds either oxygen supply or oxygen delivery (Rasmussen and Grandes, 1994). When a child who has sustained multiple injuries arrives for surgical intervention, the fluid status must be assessed quickly (before induction of anesthesia) based on a physical examination as well as a history of fluid resuscitation received before arrival in the operating suite. The anesthesiologist must be prepared to continue the resuscitation in case of ongoing blood loss or third spacing of fluid. The goals of fluid resuscitation should be to maintain normovolemia as well as the osmolar and oncotic pressures in the intravascular space. Isotonic crystalloid solutions such as lactated Ringer’s solution or normal saline are most commonly used in the initial stages of resuscitation. Hypertonic saline solutions have also been used in this setting, based on the premise that they increase serum osmolality and thereby maintain intravascular volume for longer periods and with smaller volumes administered than isotonic solutions (Rasmussen and Grandes, 1994). However, the data that support these arguments are inconclusive and further work in this area is needed. The decision to administer glucose-containing solutions must be based on serial blood-glucose values (Sharma et al., 2009). This is of greatest importance in the presence of head trauma, because elevated blood-glucose levels have been found to correlate significantly with indicators of the severity of brain injury and poor neurologic outcomes in children with severe brain injuries (Michaud et al., 1991).
Colloid solutions such as 5% albumin and hydroxyethyl starch have also been used for fluid resuscitation. Hydroxyethyl starch may exacerbate existing coagulopathy by interfering with platelet function, decreasing fibrinogen activity, and interfering with factor VIII. It is therefore unsuitable for the pediatric trauma patient. The purported benefits of colloid solutions include their ability to increase colloid oncotic pressure and prolonged maintenance of intravascular volume and smaller volumes required compared with crystalloid solutions (Rasmussen and Grandes, 1994). For these reasons, colloids may also be beneficial in children with head trauma because the smaller volume of fluids administered may reduce the likelihood of cerebral edema. One of the major concerns with the use of colloids has been the cost. In most patients who require massive fluid resuscitation, the cost of using colloids to supplement crystalloids may be justified. The Saline vs. Albumin Fluid Evaluation (SAFE) study, a randomized, controlled trial conducted in 16 intensive care units in Australia and New Zealand, concluded that albumin and saline should be considered clinically equivalent treatments for volume resuscitation in intensive care patients (Finfer et al., 2004). Further discussion of crystalloid and colloid use in pediatrics has been reviewed by Bailey et al. (2010).
Blood Product Transfusion
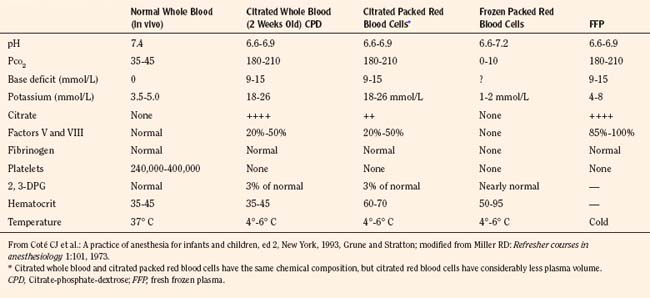
The primary purposes for transfusion of blood products in a pediatric trauma patient are to maintain oxygen delivery and to ensure hemostasis (see Chapter 36, Systemic Disorders). Packed red blood cells are required when oxygen-carrying capacity is inadequate to meet tissue demands and metabolic rate. Losses of up to 40% of blood volume can usually be replaced with isotonic crystalloid solutions or colloids without physiologic signs of inadequate oxygen delivery (Solheim and Wesenberg, 2001). When estimated blood volume losses exceed 40%, the decision to transfuse blood should be based on an overall assessment of the patient, including the hemodynamic status, the extent of ongoing blood loss and the underlying comorbidity. Some children may require blood transfusion with blood-volume losses of less than 40% if the blood loss has been rapid or if they have significant underlying medical conditions such as congenital cyanotic heart disease or blood dyscrasias. Although there can be no fixed numeric transfusion trigger in all trauma patients, Box 30-4 presents formulas that may be used as general guidelines to calculate allowable blood losses (Rasmussen and Grandes, 1994). Blood banks in most centers supply blood components rather than whole blood. The primary advantage of component therapy is more efficient and cost-effective use of resources by eliminating the transfusion of unnecessary components and making components from a single blood donation available to several patients. It also permits improved preservation of individual components (Table 30-7).
Box 30-4 Formulas to Use as a General Guideline to Calculate Allowable Blood Loss
* The ABL, in milliliters, must be multiplied by 3 if replacement is by crystalloid and replaced, 1:1 if blood is to be used.
From Rasmussen GE, Grandes CM: Blood, fluids, and electrolytes in the pediatric trauma patient, Int Anesthesiol Clin 32:79, 1994.
Fresh Frozen Plasma
Fresh frozen plasma (FFP) must be separated from whole blood within 6 to 8 hours of collection. It generally takes approximately 45 minutes to thaw, because it is stored at –18° C, and it must be used within 24 hours once it has been thawed. FFP provides factors II, V, VIII, IX, X, XI, and antithrombin III. In general, FFP should be transfused when clotting studies become abnormal with a prolonged prothrombin time (PT) or activated partial thromboplastin time (APTT). Nonsurgical bleeding in children who receive more than one blood volume of PRBCs often require FFP because of factors V and VIII deficiency. The recommended initial dose of FFP is 10 to 15 mL/kg (see Chapter 36, Systemic Disorders, Table 36-25).
Platelets
Platelets are prepared by centrifugation and recentrifugation of fresh whole blood. Dilutional thrombocytopenia is the most likely cause of nonsurgical or microvascular bleeding after massive blood transfusion, and usually platelets are required before FFP for this condition. To raise the platelet count by approximately 20,000, 0.1 units/kg of platelets are needed. Because platelet counts of 50,000 are adequate to achieve surgical hemostasis, doses in excess of 0.2 units/kg are rarely required (see Chapter 36, Systemic Disorders, Table 36-25).
Cryoprecipitate
Cryoprecipitate that is produced by refreezing the insoluble portion of plasma is rich in factor VIII and fibrinogen. The residual component from 1 unit of FFP yields 100 units of cryoprecipitate. The primary indications for cryoprecipitate in the trauma patient are bleeding abnormalities after massive transfusion, disseminated intravascular coagulation (DIC), and decreased fibrinogen levels. The recommended initial dose of cryoprecipitate is 0.1 units/kg (see Chapter 36, Systemic Disorders, Table 36-25).
Massive Blood Replacement
Massive blood replacement is defined as the administration of one blood volume or more within 24 hours. It causes a number of physiologic derangements that can be detrimental in the child with multiple injuries, including coagulation defects, electrolyte and acid-base abnormalities, and hypothermia (see Chapter 36, Systemic Disorders). Dilutional thrombocytopenia and clotting factor deficiencies have been primarily implicated in the etiology of nonsurgical bleeding after massive blood transfusion. However, mathematical models have demonstrated that a third of the patient’s own blood remains after a single blood volume exchange, thereby retaining sufficient platelets and clotting factors to permit hemostasis (Marsaglia and Thomas, 1971). Therefore, other factors, such as incompatibility of transfused blood and DIC, have also been implicated in the etiology of nonsurgical bleeding in the trauma patient.
The ratio of fresh frozen plasma to PRBCs may be an important determinant of outcome in trauma patients who receive massive transfusions. Sperry and others (2008) demonstrated in adults that during massive transfusions, an FFP/PRBC ratio of greater than or equal to 1:1.5 decreased the number of transfusions and one-day mortality more than did an FFP/PRBC ratio of less than 1:1.5. This means that those patients who received close-to-equal volumes of FFP and PRBCs had decreased 24-hour mortality rates over those who received significantly more PRBCs than FFP, although patients receiving larger quantities of FFP had a higher incidence of ARDS. The likely explanation for the reduction in mortality is the early correction of coagulopathy and reduction in hemorrhage.
Monitoring the coagulation status is vital in patients who require massive transfusions. Hypothermia, acidosis, and hemodilution with hypofibrinogenemia further exacerbate any coagulopathy. Massive transfusions decrease fibrinogen, decrease platelets, and dilute coagulation factors. Intraoperative evaluation of the bleeding patient can be assessed by classic tests (PT, PTT, and platelet count). However, the coagulation system, including clot formation and clot dissolution, can also be assessed by thromboelastography. Thromboelastography is a global measure of hemostasis and is further described in Chapter 36, Systemic Disorders.
Factor VIIA
Recombinant Factor VIIA (rFVIIA) is currently approved by the FDA for treatment and prevention of bleeding disorders in patients with hemophilia A or B who have developed inhibitors to factor VIII or factor IX, respectively (Novo Nordisk, 2005). However, rFVIIa is increasingly being used as an off-label drug for patients, both children and adults, when conventional therapies have failed to control bleeding after trauma. A retrospective case series published in Pediatrics in 2009 concluded that administration of rFVIIa to bleeding surgical trauma patients is associated with a significant decrease in blood-product administration, with low associated mortality and adverse events (Alten et al., 2009).
Specific injuries
Head Injuries
TBI is the leading cause of morbidity and mortality resulting from trauma in children (Langlois et al., 2006). A TBI is caused by a blow or jolt to the head or a penetrating head injury that disrupts the normal function of the brain. Severe TBI often includes an extended period of unconsciousness or amnesia after the injury and is associated with the poorest outcomes (Adelson et al., 2006). Among children ages 0 to 19 years, TBI results in an estimated 7441 deaths, 62,000 hospitalizations, and 564,000 emergency-department visits annually (CDC, 1999). The leading causes of TBI in children are falls (39%) and motor-vehicle crashes (11%) (Fig. 30-8) (National Center for Injury Control and Prevention, 2005).
Most preventable deaths and deficits after pediatric head injury are secondary to subsequent complications, including diffuse brain swelling and resultant elevated ICP, which are independent predictors of mortality (Jurkovich et al., 2004). Poor CPP, often related to elevated ICP and trauma related systemic hypotension, are also known to worsen outcome. See related video online at www.expertconsult.com.
Problems that result from TBI, such as diminished cognition and memory loss, are often not visible (Langlois et al., 2006). TBI can cause a wide range of functional changes that affect thinking, sensation, language, and emotion (CDC, 2003). TBI can also cause epilepsy and increase the risk for conditions that become more prevalent with age, such as Alzheimer’s or Parkinson’s disease (CDC, 2003). The impact of a TBI not only affects the child but the whole family. The two age groups at highest risk for TBI are children between the ages of 0 and 4 years and those between 15 and 19 years old. Males are 1.5 times more likely to sustain a TBI, and African Americans have the highest death rate from TBI. The outcomes and the treatment of children with a severe TBI remain a great challenge. The CDC estimates that at least 5.3 million Americans, approximately 2% of the U.S. population, currently have a long-term or lifelong requirement for assistance with daily living activities as a result of a TBI (Langlois et al., 2006). Each year, 56,000 children are discharged home with a permanent disability from a TBI, whereas another 5000 require intensive inpatient rehabilitation facilities. The economic burden of head injury to patients is substantial—estimated to be $56.3 billion, with the highest rate of injuries reported among the lower socioeconomic classes. TBI is the most common reason a child requires intensive care or develops a significant life-long disability.
There are important differences between a TBI in a child compared with a TBI in an adult (Luerssen, 2006). Because of the smaller body mass of children, the energy received from an injury (e.g.,. fall) results in a greater force applied per unit body area and to a body with less fat and less connective tissue that might absorb or diminish the energy. The brain of a child is also anatomically different from that of an adult; the subarachnoid space is smaller and offers less protection because there is less buoyancy. Thus, head momentum is more likely to impart parenchymal structural damage. Additionally, the brain is proportionately larger to the rest of the body and therefore a bigger target for injury. Children are particularly susceptible to the effects of secondary brain injury that may be produced by hypovolemia with reduced cerebral profusion, hypoxia, seizures, or hyperthermia. Alternatively, the young child with open fontanels and mobile cranial suture lines is more tolerant of an expanding intracranial mass lesion. Overall, children with a TBI have better outcomes than adults with a TBI.
Monitoring of ICP and CBF are essential in the head injured patient. ICP can be measured with intraventricular catheters, subarachnoid bolts, and epidural sensors. CBF can be assessed by Doppler, and brain metabolic demands can be evaluated with the use of internal jugular bulb catheters and near-infrared measurements of mixed venous oxygen saturation Sv-o2 (see Chapter 22, Anesthesia for Neurosurgery). In general, efforts are made to insure adequate venous drainage (30-degree, head-up position), adequate oxygenation, avoidance of hypotension, and maintenance of slight hypocarbia (partial pressure of CO2 [Paco2] of 35 to 38).
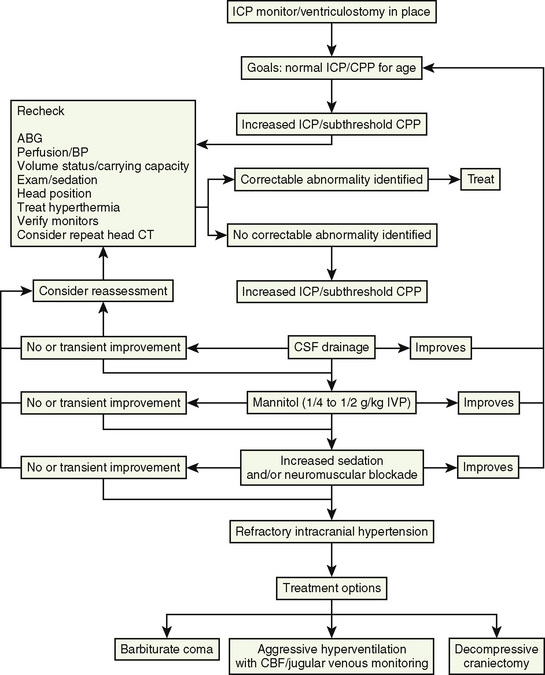
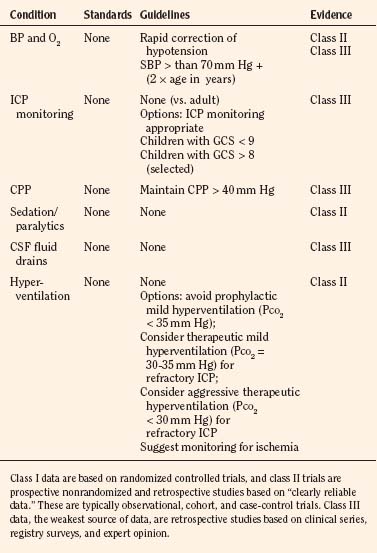
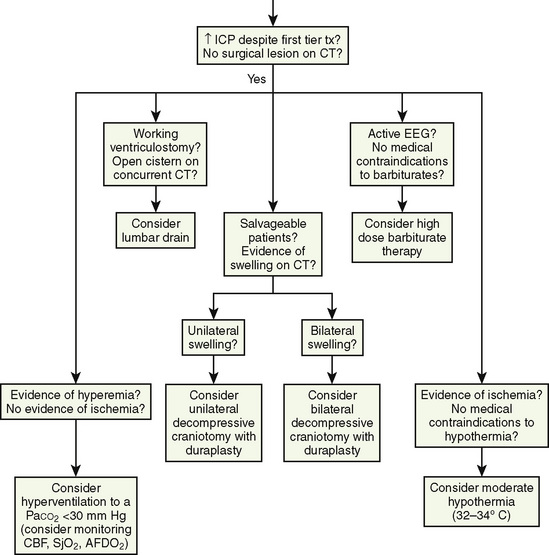
At present, there is insufficient evidence-based medicine to provide standards of care in children with head injuries. However, a number of interventions have become mainstay as guidelines for treatment of the pediatric patient with head injuries. Most of these guidelines emanate from data in adults (Krantz, 1996; Gupta et al., 1997). An algorithm for the treatment of intracranial hypertension in children is shown in Figure 30-9.
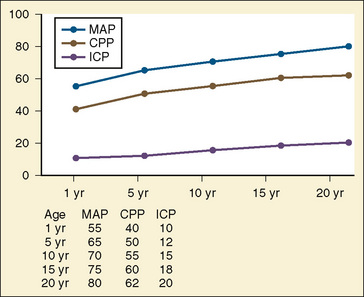
Primary and secondary brain injuries are the major variables affecting outcomes. Primary injury describes the immediate disruption of neuronal, axonal, and supportive structures and vascular tissue and is essentially untreatable except by prevention (Luerssen, 2006). If the primary injury is not fatal, it triggers a cascade of intracellular and extracellular biochemical changes that can augment and accelerate the injury. This is known as secondary injury. Secondary injury produces new damage to the tissue at the primary injury site as well as in other areas of the brain (Luerssen, 2006). For example, hypermetabolic responses related to neuronal tissue injury occur that may outstrip local or regional substrate supply. Ischemia is the final pathway that produces brain-tissue damage and poor clinical outcomes; it is caused by hypoxia, hypotension, seizures, hyperglycemia, and hyperthermia. Despite a lack of systematic evidence, there has been a trend toward improved outcomes by aggressively treating ischemia. The key features of such therapy are support of MAP, reduction of ICP to ensure CPP, and surgery for compressive lesions (such as epidural hematoma) (Alberico et al., 1987; Downard et al., 2000). Figure 30-10 depicts changes in MAP, CPP, and ICP with age. In addition to basic interventions of normal-to-slight hypocapnea, adequate perfusion pressure and oxygenation, the use of hyperosmolar therapy and hypothermia to treat increased ICP has been examined. Table 30-8 lists some of the commonly used interventions (excluding hyperosmolar therapy, discussed in the next section) and the strength of supporting data.
Hyperosmolar Therapy
In areas where the blood brain barrier (BBB) is disrupted, the flow of proteins and electrolytes across the membrane is facilitated. Hydrostatic pressure becomes the dominant driving force for fluid movement from the intravascular space to brain tissue (Klatzo, 1967; Harukuni et al., 2002). This leads to brain swelling with an increase in ICP, a decrease in CPP, cerebral hypoxia, and secondary brain injury.
The beneficial effects of osmotherapy on ICP are thought to result from brain shrinkage after the shift of water out of brain parenchyma. This has been confirmed in animal studies where osmotherapy after brain injury led to shrinkage of normal, but not injured, brain tissue (Wisner et al., 1990; Shackford et al., 1992).
Mannitol and hypertonic saline (3% normal saline) are the two most commonly administered osmotic agents. Mannitol is used in 70% of pediatric intensive care units (two class III studies); however, it has not been subjected to controlled clinical trials vs. placebo or other osmolar agents in children (Wakai et al., 2007). Mannitol lowers cerebral blood volume and ICP by reducing blood viscosity while maintaining CBF. It osmotically dehydrates the brain parenchyma to decrease intracranial volume and pressure, and therapy can be titrated to a serum osmolality of 320 mOsm/L. Although it has been the predominant osmotherapeutic drug for the past four decades, mannitol has several limitations. Hyperosmolality is a common problem, and a serum osmolarity greater than 320 mOsmol/L is associated with adverse renal and CNS effects (Dorman et al., 1990; Roberts et al., 2003). The osmotic diuresis that accompanies mannitol administration may lead to hypotension, especially in hypovolemic patients. Although controversial, accumulation of mannitol in cerebral tissue may lead to a rebound phenomenon and increased ICP. A 2007 Cochrane Database review evaluated mannitol for acute TBI in adults. Only four studies met the eligibility criteria. The review concluded that compared with pentobarbital, mannitol may have a beneficial effect on mortality in patients with raised ICP, but mannitol may have a detrimental effect on mortality when compared with hypertonic saline (Wakai et al., 2007).
Hypertonic saline (3% normal saline) has a similar mechanism of action as mannitol with the added theoretic benefits of restoration of normal cellular resting membrane potential and cell volume, stimulation of atrial naturietic peptide release, inhibition of inflammation, and enhancement of cardiac output (Moss and Gould, 1988; McManus and Soriano, 1998; Dickinson et al., 2001). It has also been suggested that it may help patients who are refractory to mannitol infusions. The serum osmolarity may be allowed to rise as high as 360 mOsm/L. Proposed beneficial effects of hypertonic saline in TBI may result because the permeability of the BBB to sodium is low (Betz, 1983). Hypertonic saline produces an osmotic gradient between the intravascular and intracellular/interstitial compartments, leading to shrinkage of brain tissue (where BBB is intact) and therefore a reduction in ICP; the reflection coefficient (selectivity of the BBB to a particular substance) of NaCl is more than that of mannitol, making it potentially a more effective osmotic drug (Fenstermacher and Johnson, 1966). Hypertonic saline also augments volume resuscitation and increases circulating blood volume, MAP, and CPP, and it restores the neuronal membrane potential, maintains the BBB integrity, and modulates of the inflammatory response by reducing adhesion of leukocytes to endothelium (Schmoker et al., 1992; Hartl et al., 2002). The detrimental effects of hypertonic saline include central pontine myelinolysis, coagulopathies, excessive intravascular volume, and electrolyte abnormalities. Electrolyte abnormalities are common. Careful monitoring is required, because hyperkalemia and natriuresis may develop after intravascular administration. Hypertonic saline also tends to reduce the plasma strong-ion difference, and a nonanion-gap metabolic acidosis may result (Bruegger et al., 2005). A number of studies suggest that hypertonic saline may be more effective than mannitol in reducing ICP and have a longer duration of action (Berger et al., 1994; Mirski et al., 2000). Whether this leads to improved outcomes is not known. Clinical studies in children using 3% normal saline are limited (Berger et al., 2004; Vialet et al., 2003).
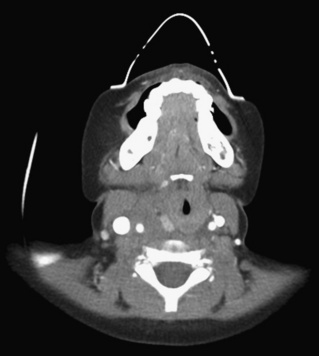
Cervical Spine Injuries
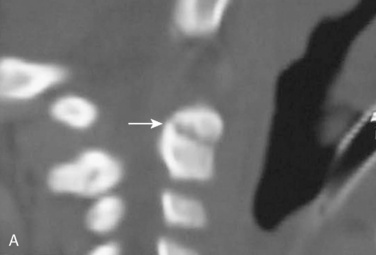
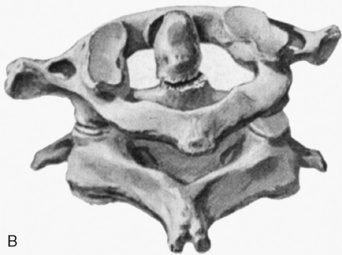
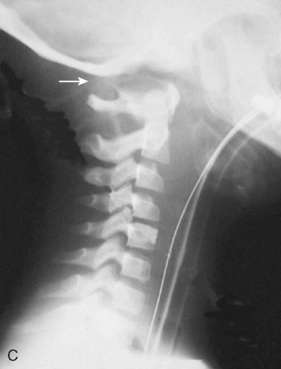
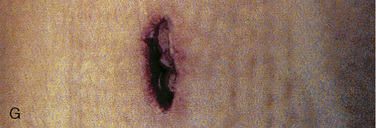
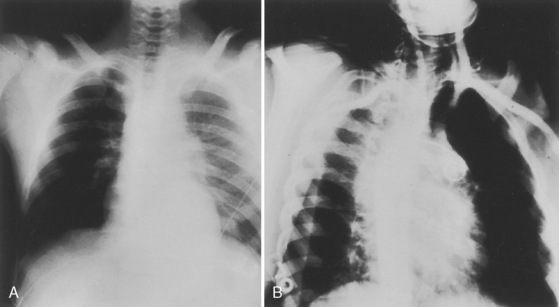
Cervical spine injuries remain one of the most devastating consequences of trauma. The incidence of cervical spine injury in pediatric trauma patients is low (1% to 2%), but the morbidity and mortality of these injuries are substantial. Kokoska et al. (2001), in a 5-year review from the National Pediatric Trauma Registry, reported a 1.6% incidence (408 out of 24,740, 17% mortality) of blunt cervical spine injuries. Injury patterns were different by age with children less than 10 years of age having a higher incidence of C1-C4 injuries vs. C5-C7 injuries (85% vs. 57%; p < .01) (Kokoska et al., 2001). Brown et al. (2001) reviewed the experience of 103 pediatric cervical spine injury patients over 9 years and found an 18% mortality rate. Subluxation of cervical vertebrae and odontoid fractures are more common in children (Fig. 30-11). See related video online at www.expertconsult.com. Distinguishing between a true subluxation and a pseudosubluxation in a young child can be challenging (Table 30-9). In 40% of children younger than 7 years of age, pseudosubluxation-anterior displacement of C2 on C3 less than 3 mm is a normal variant. Pseudosubluxation becomes more prominent when a child is supine (i.e., on a backboard). Another normal variant is an increased distance between the dens and the anterior arch of C1 found in 20% of children (Bohn et al., 1990). The combination of a child’s proportionately large head, elastic interspinous ligaments, and horizontal position of the vertebrae is thought to explain both the pattern of injury and the normal variations (Bohn et al., 1990).
Distinguishing between a true subluxation and a pseudosubluxation in a young child can be challenging (Table 30-9). In 40% of children younger than 7 years of age, pseudosubluxation-anterior displacement of C2 on C3 less than 3 mm is a normal variant. Pseudosubluxation becomes more prominent when a child is supine (i.e., on a backboard). Another normal variant is an increased distance between the dens and the anterior arch of C1 found in 20% of children (Bohn et al., 1990). The combination of a child’s proportionately large head, elastic interspinous ligaments, and horizontal position of the vertebrae is thought to explain both the pattern of injury and the normal variations (Bohn et al., 1990).
TABLE 30-9 Upper Normal Limits of Cervical Spine Measurements in Adults and Children
| Adults | Children | |
| Predental space | 2.5 mm | 4 to 5 mm |
| C2-3 override (flexion) | 3 mm | 4 to 5 mm |
| Prevertebral space | 7 mm | 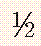 to to 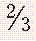 AP distance (extension) vertebral body AP distance (extension) vertebral body |
From Eichelberger MR: Pediatric trauma prevention, acute care, rehabilitation, St. Louis, 1993, Mosby.
One method to reduce excess and unnecessary imaging is to use Clinical Decision Rules (CDRs). CDRs are developed to reduce the uncertainty of medical decision-making by standardizing the collection and interpretation of clinical data (Laupacis et al., 1997; Stiell and Wells, 1999; McGinn et al., 2000).
Studies have shown that although CCR and NLC criteria may reduce the need for cervical spine imaging in children 10 years old and younger, the criteria are not sensitive or specific enough to be used as currently designed (Viccellio et al., 2001; Stiell et al., 2003; Ehrlich et al., 2009). Neither clinical decision rule is performed at a high enough level to be used with confidence.
A second issue pertaining to cervical spine clearance revolves around determining which imaging modality to use to assess the cervical spine. Physical examination in alert patients has been proven to be reliable; however, controversy remains as to which imaging studies to perform on pediatric CSI patients when the patient has altered mental status or is intubated (Marion et al., 2008). Most data for the diagnosis of cervical spine injures have been derived from the adult literature. Griffen et al. described a retrospective study of 3,018 blunt trauma patients of whom 1199 (40%) were at risk for CSI (Griffen et al., 2003). Plain radiographs (3 views) and a CT cervical spine were performed. Of 116 patients with spinal injuries, there were 41 patients whose injuries were missed by plain films, but diagnosed by CT scan. More significantly, all of the 41 patients with missed injuries required treatment. A prospective study from Schenarts et al. (2001) compared the use of cervical-spine CT for the upper cervical spine (C1-C3) to plain films for adult trauma patients with altered mental status. Plain films identified only 54% of the injuries to the upper cervical spine in their series, compared with 96% diagnosed with CT scan. In contrast to the previous study, a different study found that three injuries seen on plain films were missed on CT, including one atlantooccipital dislocation in a patient with quadriplegia and two subluxations (Schmoker et al., 1992).
Rana et al. (2009) recently examined their experience with cervical-spine imaging using either CT or conventional films in cervical-spine clearance with intubated children. Plain radiographs missed injuries and had a significantly lower sensitivity compared with CT scan (61% vs. 100%). In addition, all patients who remained intubated for 24 hours received flexion and extension views, but no new injuries were detected. This result is supported in adult trauma literature; Insko et al. (2002) reviewed the use of flexion-extension films and demonstrated that 30% of the patients were unable to flex and extend adequately, leading to further imaging with CT or MRI. They concluded that these films are of limited diagnostic utility in the acute setting for evaluation of the cervical spine at a Level I pediatric trauma center (Insko et al., 2002; Rana et al., 2009).
Radiation exposure from CT scans is an important concern and driving force for the rational use of imaging in children. Adelgais et al. (2004) examined this issue in a prospective study reviewing the differences of radiation exposure between conventional radiographs and CT. In this study, the patients receiving CT had 1.25 times the effective radiation dose compared with plain films. However, the comparison groups were markedly different; the patients undergoing CT scan were significantly more ill than the patients who only received plain films. The CT group had higher numbers of abnormal head CT (41% vs. 20%), intubation (20% vs. 2%), and transfer to intensive care or operating rooms (46% vs. 23%), compared with the group only receiving plain films. Keenan et al. (2001) examined this issue from another perspective by reviewing the number of excess radiographs required to clear the cervical spine of injury compared with the use of CT scan. Patients who underwent CT of the neck on initial evaluation had significantly fewer repeated radiographs than patients without cervical-spine CT (mean, 2.1 ± 2.6 vs. 3.6 ± 2.7 repeated radiographs, p = 0.04). The amount of radiation exposure was also determined, and the authors concluded that the children with initial CT did receive a higher effective dose (p < 0.001); however, patients with a GCS of less than 8 received equivalent doses compared with those not undergoing early cervical-spine CT (p=0.15) (Keenan et al., 2001). Although radiation exposure is increased in patients undergoing CT imaging, the actual increased risk of cancer above the natural background rate is low at 0.25% (Bier, 1990).
Child Abuse
The CDC defines child maltreatment as any act of commission (abuse) or omission (neglect) by a parent or other caregiver that results in harm, potential for harm, or threat of harm to a child. Unfortunately, such harm perpetrated on children permeates society. Even worse, child abuse remains to a great extent concealed or inadequately appreciated. Many experts believe that the number of annual referrals filed probably represent less than one third of actual cases; in fact, some sources describe as much as a tenfold difference between victim or parental self-reports and official estimates of incidence (Coté et al., 2009; Gilbert et al., 2009). Whether participating in the resuscitation and stabilization of a victim of acute pediatric trauma or assisting in the serial procedures required of more chronic medical conditions, anesthesiologists should step back and view their pediatric patients in a larger context that includes the possibility of child maltreatment.
Epidemiology
The U.S. Department of Health and Human Services collects and publishes data on child abuse in an annual report (U.S. Department of Health and Human Services, 2006). Of the estimated 3.2 million referrals made to Children’s Protective Services (CPS) in 2007, 61.7% progressed to investigation or assessment. Approximately 25% of these investigations indicated or substantiated child maltreatment, leading to an estimated 794,000 children being counted as victims of abuse or neglect. The most recognized forms of maltreatment are generally categorized as physical abuse, sexual abuse, psychological or emotional abuse, and neglect. During 2007, 59% of the victims suffered from neglect, whereas 11% were physically abused, 8% were sexually abused, and 4% were psychologically maltreated. Maltreatment is inversely related to age, as victimization is most prevalent among children under 1 year of age.
Perhaps even more disturbing is the high recidivism rate for perpetrators, because maltreatment often develops into a repetitive pattern of offense. In one study, 75% of victims younger than 24 months of age demonstrated evidence or history of previous trauma or injury (Kellogg, 2007). Children who are prior victims are 96% more likely to experience a repetition of abuse compared with those who are not previous victims. These trends are also inversely related to age, as adolescents from 16 to 21 years old are 51% less likely to experience recurrence compared with infants (U.S. Department of Health and Human Services, 2006).
Clearly, the most tragic outcome associated with maltreatment is a child’s death. Such cases often go unrecognized and unreported because of poor coordination and communication between various agencies, as well as a lack of standardized terminology and investigation techniques. Nevertheless, in 2007, 1760 children’s deaths were attributed to maltreatment. Again, the most fragile and defenseless children endure the greatest risk. Children younger than 1 year old accounted for 42% of fatalities, and 76% of the deaths attributed to maltreatment were associated with children under 4 years old (U.S. Department of Health and Human Services, 2006).
Many of these children have already been seen by various branches of the health care system, but the practice of pediatric anesthesiology provides a further level of screening for some of the most at-risk children. Children with disabilities, chronic illnesses, or special needs are at an increased risk for maltreatment and make up 8% of all child abuse victims (U.S. Department of Health and Human Services, 2006). It has been observed that compared with children who are not disabled, children with disabilities are 3.76 times more likely to be neglected, 3.79 times more likely to be physically abused, and 3.14 times more likely to be sexually abused (Hibbard and Desch, 2007).
Although child abuse affects children of all ages, genders, cultures, and socioeconomic strata, certain children are statistically predisposed to increased risk. Some of the indicative characteristics include the child’s age (infants and preschoolers) and whether the child was born premature, is developmentally delayed, possesses special health needs, or succumbs to behavioral problems. Also, given that greater than 80% of documented abuse cases are perpetrated by parents or caregivers, these victims’ home or care environments demonstrate other clear risk factors (Box 30-5) (Hornor, 2005; Kellogg, 2007). Obviously, bias should be avoided, because these risk factors in isolation do not indicate child abuse. Such characteristics should instead aid and guide further investigation as well as prevention strategies and treatment plans.
It must be noted that children may be naturally prone to injury as they test the limits of their autonomy. Most childhood injuries are not the result of maltreatment; hence, common sense should apply when considering each child’s ability to sustain observed bruising or trauma (Fig. 30-12). The same injury in a mobile 4-year-old child may be diagnostic of physical abuse in an infant. Likewise, outside of infancy, superficial injuries or bruising should be consistent with the hazards of daily living activities. Bruising to skin over bony prominences (e.g., shins, knees, forehead, chin, and forearms) is more consistent with normal activities, whereas injuries to protected, padded areas (e.g., neck, abdomen, buttocks, genitalia, cheeks, and ears) should always be suspect.
Anesthesiologists are perfectly positioned to routinely complete a full-body survey in the course of each patient’s positioning in the operating room and for anesthesia. The key is to recognize a pattern or constellation of physical findings that may point to possible abuse or neglect. Some compelling or indicative findings are noted in Table 30-10. Complete documentation of injuries, including diagrams and photographs, is paramount to facilitate peer review as well as CPS and law enforcement intervention and court proceedings.
TABLE 30-10 Physical Examination Findings Associated with Maltreatment
| General | Notes |
| Extensive dental caries | Examples of general neglect |
| Severe diaper dermatitis | |
| Derelict wound care | |
| General uncleanliness | |
| Failure to thrive (may manifest in nutritional neglect) | |
| Anxiety and reluctance when the child is asked about abuse | |
| Skin | In groups or patterns, especially around soft, protected, padded areas |
| Bruises | |
| Bites | |
| Lacerations | |
| Burns |
Management Issues
Laws mandating the report of suspected abuse and neglect exist in all 50 states. Proof is not required before reporting, and good-faith reporting has immunity in some form or another (Kellogg, 2007).
In most tertiary care or academic centers, assistance may be readily available. However, in other health care facilities, there may not be any familiarity with these processes, so providers should solicit assistance from hospital social workers, administrators, or regional tertiary care centers. In the absence of any other source or after normal business hours, law enforcement and CPS are available to provide information and assure child safety. The U.S. Department of Justice also furnishes applicable information (http://www.ojp.usdoj.gov/ovc/help/ca.htm) and advertises a child-abuse hotline (1-800-4-A-Child) for referral information.
Facial Trauma
Although severe facial trauma in children is a relatively uncommon event, it does occur, and it can make ETI challenging if not impossible (Fig. 30-13) (Zerfowski and Bremerich, 1998). Blood, secretions, hematomas, damaged tissues, or dentition may all obstruct the airway during respiration, ventilation, direct laryngoscopy, and fiberoptic intubation. Facial injuries can be categorized (in order of decreasing incidence) as soft-tissue injuries, dental injuries, and facial fractures. As depicted in Figure 30-14, the incidence of these injuries varies with the age of the child. See related video online at www.expertconsult.com. In all instances, injuries are more common in males than females. Facial trauma in children under 5 years of age is less severe because of the protective environment supported by parental supervision (Kaban, 1993). However, Shaikh and Worrall (2002) found that 42% of the pediatric population sampled with facial injuries were younger than 5 years of age, where soft-tissue injuries were more common. The vast majority of these injuries were caused by falls, where insecurity of motion and lack of coordination prevented victims from shielding their faces or turning their heads. In such cases, the injury is then focused on a relatively small area of the face, from the nose to the mentum, which is referred to as the falling zone (Zerfowski and Bremerich, 1998). The rates of soft-tissue injuries increase in adolescence. This trend may be explained by more aggressive, risky behavior that may be related to alcohol consumption or sports activities. Compared with soft-tissue injuries, the incidence of dental injuries is diminished throughout childhood. It should nevertheless be noted that accidental aspiration of teeth before or during resuscitation can complicate care and necessitate removal via bronchoscopy. Facial fractures were the least common form of pediatric facial trauma. Facial fractures accounted for only 8% of patients in the study by Zerfowski and Bremerich (1998). Nasal fractures are the most common facial fractures in children. Anderson (1995) noted that nasal fractures accounted for over 50% of all facial fractures, followed by mandibular fractures and lastly maxillary fractures.
In all instances, injuries are more common in males than females. Facial trauma in children under 5 years of age is less severe because of the protective environment supported by parental supervision (Kaban, 1993). However, Shaikh and Worrall (2002) found that 42% of the pediatric population sampled with facial injuries were younger than 5 years of age, where soft-tissue injuries were more common. The vast majority of these injuries were caused by falls, where insecurity of motion and lack of coordination prevented victims from shielding their faces or turning their heads. In such cases, the injury is then focused on a relatively small area of the face, from the nose to the mentum, which is referred to as the falling zone (Zerfowski and Bremerich, 1998). The rates of soft-tissue injuries increase in adolescence. This trend may be explained by more aggressive, risky behavior that may be related to alcohol consumption or sports activities. Compared with soft-tissue injuries, the incidence of dental injuries is diminished throughout childhood. It should nevertheless be noted that accidental aspiration of teeth before or during resuscitation can complicate care and necessitate removal via bronchoscopy. Facial fractures were the least common form of pediatric facial trauma. Facial fractures accounted for only 8% of patients in the study by Zerfowski and Bremerich (1998). Nasal fractures are the most common facial fractures in children. Anderson (1995) noted that nasal fractures accounted for over 50% of all facial fractures, followed by mandibular fractures and lastly maxillary fractures.
FIGURE 30-14 Type of injuries per year of age, demonstrated separately for soft-tissue injuries, dental trauma, and fractures.
(From Ferfowski M, Bremerich A: Facial trauma in children and adolescents, Clin Oral Invest 2:120, 1998.)
Another form of pediatric facial injury that can pose a challenge to anesthesiologists is laceration or impalement of oropharyngeal structures or tissues. It is not uncommon for children to fall with foreign objects in their mouths (e.g., pencils or toothbrushes), sometimes sustaining abrasions or lacerations to the soft palate or oropharynx. Most of such injuries are mild and spontaneously heal without intervention (Hellmann et al., 1993). However, some injuries raise concerns of vascular involvement and require angiographic studies before surgical extraction of the foreign body (Fig. 30-15). Carotid artery thrombosis and stroke have been reported after blunt pharyngeal trauma (Moriarty et al., 1997).
Extrusion of eye contents through a full-thickness–penetrating ocular laceration may result in permanent loss of vision (Fig. 30-16). Intraoperative anesthetic management of open globe injuries is discussed further in Chapter 27, Anesthesia for Ophthalmic Surgery.
Dog Bites
Dog-bite victims represent another common instance of facial injury in children. In a study by McHeik et al. (2000), the majority of children suffering dog bites (68%) were younger than 5 years of age. It was believed that the increased rate of dog bites in this age group was as a result of young children’s lack of caution and comprehension regarding animals, coupled with the fact that their heads and necks are on the same level as that of a dog. A study by Bernardo et al. (2002) revealed that younger children (younger than 6 years old) commonly sustained dog bites from their family dogs in their own homes and confirmed that these injuries typically involve facial structures. The goals of immediate surgical repair are to diminish scarring and decrease the rate of wound infection.
Soft-Tissue Neck Trauma
Soft tissue injuries to the neck can occur in children. As mentioned earlier in the chapter, blunt trauma is much more common than penetrating trauma in the pediatric patient. Although rare, penetrating trauma can occur in this area also. Atta and Walker (1998) divided the neck into three anatomic zones. Zone I is the area between the thoracic outlet and the cricoid cartilage and contains the proximal common carotid, vertebral, and subclavian arteries, the trachea, the esophagus, the thoracic duct, and the thymus (Fig. 30-17). Zone II is between the cricoid cartilage and the angle of the mandible. Contents include the internal and external carotid arteries, jugular veins, pharynx, larynx, esophagus, recurrent laryngeal nerve, spinal cord, trachea, thyroid, and parathyroids. Zone III is between the angle of the mandible and the base of the skull. Its vascular structures include extracranial carotid and vertebral arteries and the uppermost segments of the jugular veins. Zone I injuries have the worst prognosis with regard to morbidity and mortality. Zone II injuries are the most prevalent but have the best prognosis because of their accessibility (Levy and Gruber, 2008).
Chest Trauma
Pulmonary contusions are the most common thoracic injury in children (Vane, 2002; Miller et al., 2003; Soundappan et al., 2005). Because most of the bony support or rib cage in a child is cartilaginous, significant forces may be transmitted to the lung and mediastinum without external signs. Pulmonary contusions may signal a simple isolated lung injury that includes pneumothorax, hemothorax, and rib fractures. Life-threatening injuries that impair ventilation include open or tension pneumothorax, flail chest, or injuries to the trachea or bronchi. A thoracotomy is rarely required, although chest tubes have been placed in up to 50% of reported case series. Injuries to the heart, diaphragm, great vessels, bronchi, and esophagus are rare. A chest tube stabilizes most life-threatening pulmonary lesions (e.g., tension pneumothorax). An important consideration when managing pulmonary trauma in children is that when a child suffers a significant pulmonary injury the mortality rate increases tenfold (Chu et al., 1996; Thourani et al., 1998; Soundappan et al., 2005). Tension pneumothorax is an urgent yet manageable complication of chest injury that produces diminished breath sounds, tracheal deviation, hypotension, and increased ventilation pressure. It occurs because of accumulation of needed air in the pleural space under pressure. An area in the injured lung acts as a one-way valve, leaking air into the pleural space. The leaked air becomes trapped in the pleural space, and each successive breath introduces more air under progressively increasing pressure. This impairs ventilation and venous return to the heart. It should be treated immediately with decompression by bore-IV catheter insertion in the midclavicular second intercostal space and followed by chest tube placement.
Another consequence of a cartilaginous rib cage is that rib fractures and flail chest are less common in children than adults; however, significant intrathoracic injury, such as pulmonary contusion, may occur in the absence of a rib fracture. Flail chest occurs when four or more ribs are each fractured in two places by blunt trauma. The floating fracture segments produce paradoxical movement of the chest wall so that spontaneous ventilation is impaired. Any underlying pulmonary contusion further impairs gas exchange or ventilation. Although penetrating chest wounds in children are rare, as in an adult they can be immediately life threatening (Fig. 30-18). Hemothorax can occur when intercostal vessels or lung parenchyma are injured. It is important to have a high index of suspicion because blood in the pleural space can be asymptomatic; however, a hemothorax can cause significant hemodynamic compromise, because each hemithorax can hold 40% of a pediatric patient’s blood volume (Grisoni and Volsko, 2001).
Abdominal Trauma
A rapid deceleration or shearing injury (e.g., motor vehicle crash) is the mechanism most commonly associated with abdominal injuries. Solid organs (e.g., the liver, spleen, and kidneys) are the most commonly injured, followed by the hollow visceral organs (Baker et al., 1992; Soundappan et al., 2005; Adelson et al., 2006; Langlois et al., 2006). Among these visceral injuries, the third and fourth parts of the duodenum, terminal ileum, and sigmoid colon are most at risk to injury because of their relative fixation. Death from abdominal injuries is usually secondary to hemorrhage. Injuries to the great vessels, genitourinary tract, pancreas, and pelvis are rare and account for proportionately fewer deaths. The body of the pancreas as it crosses the vertebrae column is the most susceptible portion of the pancreas injured by blunt force or compression (e.g., from a bicycle’s handle bar or seat belt) (Fig. 30-19).
The management of solid-organ injuries in children has evolved over several years from an operative to a nonoperative approach (CDC, 1999). Success rates for nonoperative management of liver, spleen, and kidney trauma now exceed 90%. The relatively new use of interventional radiology techniques has further decreased the need for emergency surgery in children with solid abdominal injuries (CDC, 2003; Jankowitz and Adelson, 2006; Wakai et al., 2007). Furthermore, evidence-based guidelines now exist for critical care, radiographic procedures, follow-up care, and return to activity (CDC, 1999). The extent of the injury is scored on a scale from 1 to 5, based on radiographic findings, with a higher number representing increasing severity. However, the decision to operate is solely determined by the clinical situation. The only indication for operative intervention is hemodynamic instability—not the radiographic stage.
A common mechanism of injury in children is motor-vehicle crashes. The lap-belt complex is a pattern of injury commonly encountered after a motor-vehicle crash (Fig. 30-20). The lap-belt complex describes a pattern of injury to intestinal viscera and its mesentery, with concomitant fracture, dislocation, or subluxation of the thoracic, lumbar, sacral spine, or the iliac wing. It was first described by Garrett and Braunstein (1962) to denote distinctive pattern of injury resulting from the use of lap-style safety belts (Luerssen, 2006). The lap-belt complex is characterized by soft-tissue injury, intraabdominal injury, and orthopedic injuries. The soft-tissue injury may be a slight bruise, ecchymosis, or a degloving injury with fascial dehiscence. The abdominal injury may be a solid organ, hollow viscus hematoma or perforation, mesenteric bleeding, or a bladder or aortic injury. Orthopedic injuries may include fractures, dislocation, or subluxation of the lumbar, sacral, thoracic spine or the iliac wing.
Lawnmower Related Injuries
Lawnmowers cause more than 9400 injuries annually in the United States in children younger than 18 years of age, with almost one quarter of these injuries occurring in children younger than 5 years of age (U.S. Consumer Product Commission, 1990–1999). The age distribution is bimodal, with peaks at 2 and 15 years, probably representing injuries to bystanders vs. operators. Over 7% of children who incur mower-related injuries require hospitalization, a higher rate than injuries incurred from any other consumer product (U.S. Consumer Product Commission, 1990–1999). Riding mowers, commonly used for lawn and field maintenance in this country, are more powerful and complex to operate than walk-behind mowers. They carry a much higher risk of injury and possible death than walk-behind mowers (more than three times higher) (Adler, 1994). In 1993, a U.S. Consumer Product Safety Commission report identified four mechanisms of injury caused by riding mowers: loss of mower stability, blade contact, layout and function of the mower controls, and running over or backing over young children (Adler, 1993). Back-over injuries occur approximately twice as often as run-over injuries, with about 85% of these injuries occurring in children between 15 months and 10 years of age. Typically, this occurs when the child is playing in the area, following the mower, or falls off the back of the mower (Deppa, 1994). The type of injuries children incur from lawnmowers range from lacerations (41%) to amputations and avulsions (7%) (U.S. Consumer Product Commission, 1990–1999). Aside from initial stabilization and treatment, children with these injuries may require repeated anesthetics for wound débridement, reductions of bony fractures, skin grafts, or reconstructive surgery. A child with a lower extremity injury may benefit from combined epidural and general anesthesia, with continued epidural analgesia in the postoperative period. Because of infection, consideration for antibiotic prophylaxis and tetanus must be considered. In addition, emotional trauma both to the patient and family is considerable. The following list shows some of the recommendations of the American Academy of Pediatrics Committee on Injury and Poison Prevention (Smith, 2001):
Skeletal Injuries
Musculoskeletal injuries are rarely life threatening, except when they are associated with ongoing severe hemorrhage. Still, they are a leading cause of morbidity and long-term disability, and if not managed appropriately and in a timely manner, long-term sequelae may result, including limb deformities, permanent neurologic and joint dysfunction, and loss of limb viability. See related video online at www.expertconsult.com. Additional considerations in children include premature growth arrest and potential for growth-plate injuries. In the child with multiple injuries, it is important to identify the priorities of treatment. Control of bleeding as a result of skeletal injuries should occur as part of the primary survey. Once life-threatening injuries such as head and chest injuries are addressed and initially stabilized, the extent of skeletal injuries should be carefully assessed based on symptoms, physical examination, and radiography. Appropriate initial measures include functional bracing or splinting to alleviate pain and immobilization of bone fragments to prevent further injury of adjacent neurovascular structures, allow safe transport of the patient, and minimize impairment of limb function. In patients with adequate alignment of fracture segments, this may be the only necessary treatment. Other interventions that may be needed in case of displaced fractures include external traction, external fixation, and internal fixation.
Additional considerations in children include premature growth arrest and potential for growth-plate injuries. In the child with multiple injuries, it is important to identify the priorities of treatment. Control of bleeding as a result of skeletal injuries should occur as part of the primary survey. Once life-threatening injuries such as head and chest injuries are addressed and initially stabilized, the extent of skeletal injuries should be carefully assessed based on symptoms, physical examination, and radiography. Appropriate initial measures include functional bracing or splinting to alleviate pain and immobilization of bone fragments to prevent further injury of adjacent neurovascular structures, allow safe transport of the patient, and minimize impairment of limb function. In patients with adequate alignment of fracture segments, this may be the only necessary treatment. Other interventions that may be needed in case of displaced fractures include external traction, external fixation, and internal fixation.
Urgent or emergent surgical interventions are usually indicated in the case of complex or displaced fractures associated with vascular damage and potential for limb ischemia, neurologic dysfunction, open fractures, joint dislocations that cannot be reduced, and compartment syndromes (Musgrave and Mendelson, 2002). Vascular injuries in conjunction with limb fractures are, fortunately, rare in children. Although the majority of vascular injuries are associated with supracondylar distal humerus fractures, they may also occur in conjunction with fractures of the distal femur, proximal tibia, displaced pelvic fractures, and knee dislocations. Children with suspected vascular injuries may require an angiogram to delineate the extent of the injury and to determine the need for revascularization. In the case of open fractures, wound irrigation and débridement with extensive removal of contaminated and necrotic tissue are required. Children with open fractures often require repeated débridement under general anesthesia every 48 to 72 hours until all of the devitalized tissue has been removed. Pain out of proportion to the extent of the injury should raise concern about compartment syndrome. In these cases, emergent fasciotomies of all involved compartments is indicated because significant muscle necrosis can occur if intracompartment pressures exceed 30 mm Hg for longer than 8 hours (Musgrave and Mendelson, 2002).
Fractures of the femur in children most commonly involve the femoral shaft or the distal femoral physis. Femoral-shaft fractures can result in significant blood loss from the fracture segments, and such blood loss may not be readily recognized because the blood accumulates in the large thigh compartments. Serial hematocrits should be obtained in children with femur fractures, and they should be adequately volume resuscitated before the induction of anesthesia to avoid cardiovascular collapse on induction. A high index of suspicion for compartment syndrome should be maintained, particularly in the patient with concomitant head trauma who may be difficult to evaluate. Children with distal femur fractures are at risk of arterial injury and compartment syndrome; attention should be focused on the neurovascular status of the limb (Musgrave and Mendelson, 2002).
The issue of early vs. delayed stabilization of femur fractures in children with closed head injuries remains contentious. Interpretation of the existing literature is confounded by variability in study design, small sample size of patients studied, differences in severity of head injury in the early vs. late treatment groups, and variable definitions of early vs. late treatment. Previous studies in adults have found that early fixation significantly reduced the incidence of severe pulmonary complications, including respiratory distress syndrome, pneumonia, and pulmonary emboli (Bone et al., 1989; Behrman et al., 1990). Pediatric studies found that early stabilization did not lessen the risk of pulmonary complications in the child with multiple injuries. A retrospective study by Hedequist and others (1999) identified 25 children with femur fractures and CNS injury (GCS score of 8). Seven of these children underwent early stabilization, of whom 4 (57%) experienced a respiratory complication compared with 8 complications out of 18 (44%) who underwent delayed fixation. In another retrospective study in children, Mendelson and others (2001) reported twice the number of respiratory complications in the late treatment group (4 out of 13 vs. 2 out of 12, p = ns). This difference may be clinically significant, but the groups were dissimilar in that children in the late-treatment group had a higher incidence of increased ICP. Other purported benefits of early fixation include early patient mobilization, shorter hospital and intensive care unit stays, improved predictability of fracture outcome, and decreased costs.
Proponents of late stabilization argue that minor hemodynamic changes, including shifts in blood pressure and volume status, may potentiate secondary brain injury and lead to adverse neurologic outcomes. Previous investigators have reported a greater incidence of intraoperative hypotension and hypoxemia and lower mean GCS scores in patients undergoing early fracture stabilization (Jaicks et al., 1997; Townsend et al., 1998). Other studies have found no relation between timing of fracture fixation and head trauma outcome (McKee et al., 1997; Kalb et al., 1998; Mendelson et al., 2001). The Committee on Trauma of the American College of Surgeons has recommended that femoral fractures be treated early provided hemodynamic stability has been achieved (Burgess and Cates, 1993). The appropriate timing of fixation for a femur fracture in a child with a head injury remains open to further investigation. Until more definitive data become available, it may be prudent to delay operative fixation of femur fractures until stabilization of hemodynamic and neurologic status are achieved. Also, adequate volume resuscitation and careful monitoring of end-organ perfusion and pressure, including blood pressure and ICP monitoring, should be strongly considered to guide intraoperative interventions that reduce the risk of secondary brain injury.
Another potential complication of long-bone fractures is compartment syndrome. Acute compartment syndrome can be both a limb- and life-threatening surgical emergency. Compartment syndrome is defined as a condition where the circulation of blood through tissue is compromised within a closed space by increased pressure within that space (Matsen, 1975). Fractures that are most commonly associated with compartment syndrome are of the tibia and forearm (Namias, 2007). The classic signs and symptoms of compartment syndrome are the seven “Ps”: pain, pallor, parasthesias, paralysis, pulselessness, pressure, and poikilothermia. Patients exhibit pain out of proportion to the clinical situation. Treatment should include elevation of the extremity, removal of restrictive dressings, and maintenance of normotension. Definitive surgical management of compartment in the extremity is fasciotomy (Fig. 30-21).
Summary
For questions and answers on topics in this chapter, go to “Chapter Questions” at www.expertconsult.com.
Adelgais K.M., Grossman D.C., Langer S.G., et al. Use of helical computed tomography for imaging the pediatric cervical spine. Acad Emerg Med. 2004;11:228-236.
Adelson P.D., Buxton N., Appletoin R. Prognosis and recovery of pediatric head injury. In: Oldham K., editor. Pediatric surgery and urology: long term outcomes. Cambridge: Cambridge University Press; 2006:926-935.
Adler P.. Directorate for Epidemiology. U.S. Consumer Product Safety Commission. 1994. editor: Washington DC
Adler P.. Directorate for Epidemiology. U.S. Consumer Product Safety Commission. 1993. editor: Washington DC
Albanese J., Arnaud S., Rey M., et al. Ketamine decreases intracranial pressure and electroencephalographic activity in traumatic brain injury patients during propofol sedation. Anesthesiology. 1997;87:1328-1334.
Albanese J., Viviand X., Potie F., et al. Sufentanil, fentanyl, and alfentanil in head trauma patients: a study on cerebral hemodynamics. Crit Care Med. 1999;27:407-411.
Alberico A.M., Ward J.D., Choi S.C., et al. Outcome after severe head injury: relationship to mass lesions, diffuse injury, and ICP course in pediatric and adult patients. J Neurosurg. 1987;67:648-656.
Alten J.A., Benner K., Green K., et al. Pediatric off-label use of recombinant factor VIIa. Pediatrics. 2009;123:1066-1072.
American Academy of Pediatrics Section on Orthopaedics, American Academy of Pediatrics Committee on Pediatric Emergency Medicine, American Academy of Pediatrics Section on Critical Care, et al. Management of pediatric trauma. Pediatrics. 2008;121(4):849-854.
American College of Surgeons Committee on Trauma. Advanced life support for doctors, ed 6. Chicago: American College of Surgeons, 1997.
Anderson P.J. Fractures of the facial skeleton in children. Injury. 1995;26:47-50.
Annane D., Sebille V., Charpentier C., et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock.[see comment][erratum appears in JAMA. 2008 Oct 8;300:141652 Note: Chaumet-Riffaut, Philippe [corrected to Chaumet-Riffaud, Philippe]]. JAMA. 2002;288:862-871.
Association for the Advancement of Automotive Medicine. The abbreviated injury scale. 1990.
Atta H.M., Walker M.L. Penetrating neck trauma: lack of universal reporting guidelines. Am Surg. 1998;64:222-225.
Aun C.S., Sung R.Y., O’Meara M.E., et al. Cardiovascular effects of i.v. induction in children: comparison between propofol and thiopentone. Br J Anaesth. 1993;70:647-653.
Bailey A.G., McNaull P.P., Jooste E., et al. Perioperative crystalloid and colloid fluid management in children: where are we and how did we get here? Anesth Analg. 2010;110(2):375-390.
Baker S.P., O’Neil B., Ginburg M.J., et al. The injury fact book. ed 2. 1992. New York
Baker S.P., O’Neill B. The injury severity score: an update. J Trauma. 1976;16:882-885.
Baker S.P., O’Neill B., Haddon W.Jr, et al. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187-196.
Baker T.W., King W., Soto W., et al. The efficacy of pediatric advanced life support training emergency medical service providers. Pediatr Emerg Care. 25(8), 2009.
Behrman S.W., Fabian T.C., Kudsk K.A., et al. Improved outcome with femur fractures: early vs. delayed fixation. J Trauma. 1990;30(7):792-797.
Berger S., Schurer L., Hartl R., et al. 7.2% NaCl/10% dextran 60 versus 20% mannitol for treatment of intracranial hypertension. Acta Neurochir Suppl (Wien). 1994;60:494-498.
Bernardo L.M., Gardner M.J., Rosenfield R.L., et al. A comparison of dog bite injuries in younger and older children treated in a pediatric emergency department. Pediatr Emerg Care. 2002;18:247-249.
Betz A.L. Sodium transport in capillaries isolated from rat brain. J Neurochem. 1983;41:1150-1157.
Bier V., Committee on the Biological Effects of Ionizing Radiations. Health effects of exposure at low levels of ionizing radiation. Washington DC: National Academy Press, 1990.
Bohn D., Armstrong D., Becker L., et al. Cervical spine injuries in children. J Trauma. 1990;30:463-469.
Bone L.B., Anders M.J., Rohrbacher B.J. Treatment of femoral fractures in the multiply injured patient with thoracic injury. Clin Orthop Relat Res. 1998;347:57-61.
Bourgoin A., Albanese J., Wereszczynski N., et al. Safety of sedation with ketamine in severe head injury patients: comparison with sufentanil. Crit Care Med. 2003;31:711-717.
Boyd C.R., Tolson M.A., Copes W.S. Evaluating trauma care: the TRISS method. Trauma Score and the Injury Severity Score. J Trauma. 1987;27:370-378.
Boyd D.R., Dunea M.M., Flashner B.A. The Illinois plan for a statewide system of trauma centers. J Trauma. 1973;13:24-31.
Bricker S.R., McLuckie A., Nightingale D.A. Gastric aspirates after trauma in children. Anaesthesia. 1989;44:721-724.
Brown R.L., Brunn M.A., Garcia V.F. Cervical spine injuries in children: a review of 103 patients treated consecutively at a level 1 pediatric trauma center. J Pediatr Surg. 2001;36:1107-1114.
Bruegger D., Bauer A., Rehm M., et al. Effect of hypertonic saline dextran on acid-base balance in patients undergoing surgery of abdominal aortic aneurysm. Crit Care Med. 2005;33:556-563.
Burgess R.C., Cates H. Deformities of the forearm in patients who have multiple cartilaginous exostosis. J Bone Joint Surg Am. 1993;75:13-18.
Cayten C.G., Stahl W.M., Murphy J.G., et al. Limitations of the TRISS method for interhospital comparisons: a multihospital study. J Trauma. 1991;31:471-481. discussion 481–472
Centers for Disease Control and Prevention: Injury prevention and control data and statistics (WISQARS). Available http://www.cdc.gov/injury/wisqars/nonfatal.htm accessed Non-Fatal Injury Data for 2006 on October 20, 2010
Centers for Disease Control and Prevention. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to prevent a serious public health problem. Services DoHaH. 2003. editor: Atlanta
Centers for Disease Control and Prevention. The injury fact book. 2002.
Centers for Disease Control and Prevention. Traumatic Brain Injury in the United States a report to Congress. In Department of Health and Human Services. Atlanta: National Center for Injury Control and Prevention; 1999. editor
Champion H.R., Copes W.S., Sacco W.J., et al. A new characterization of injury severity. J Trauma. 1990;30:539-545. discussion 545–536
Champion H.R., Copes W.S., Sacco W.J., et al. The Major Trauma Outcome Study: establishing national norms for trauma care. J Trauma. 1990;30:1356-1365.
Champion H.R., Sacco W.J., Carnazzo A.J., et al. Trauma score. Crit Care Med. 1981;9:672-676.
Champion H.R., Sacco W.J., Copes W.S., et al. A revision of the Trauma Score. J Trauma. 1989;29:623-629.
Champion H.R., Sacco W.J., Hannan D.S., et al. Assessment of injury severity: the triage index. Crit Care Med. 1980;8:201-208.
Chu U.B., Clevenger F.W., Imami E.R., et al. The impact of selective laboratory evaluation on utilization of laboratory resources and patient care in a level-I trauma center. Am J Surg. 1996;172:558-562. discussion 562–553
Committee on Trauma: American College of Surgeons. Resources for optimal care of the injured patient. ed 3. 1999.
Cooper A., Barlow B., Davidson L., et al. Epidemiology of pediatric trauma: importance of population-based statistics. J Pediatr Surg. 1992;27:149-153. discussion 153–144
Coté C.J., Lerman J., Todres I.D. A practice of anesthesia for infants and children, ed 4. Philadelphia, PA: Saunders, 2009.
Cowley R.A., Hudson F., Scanlan E., et al. An economical and proved helicopter program for transporting the emergency critically ill and injured patient in Maryland. J Trauma. 1973;13:1029-1038.
Cowley R.A., Scanlan E. University trauma center: operation, design and staffing. Am Surg. 1979;45:79-85.
Crawford M.W., Hayes J., Tan J.M. Dose-response of remifentanil for tracheal intubation in infants. Anesth Analg. 2005;100:1599-1604.
Crozier T.A., Beck D., Schlaeger M., et al. Endocrinological changes following etomidate, midazolam, or methohexital for minor surgery. Anesthesiology. 1987;66:628-635.
de Nadal M., Munar F., Poca M.A., et al. Cerebral hemodynamic effects of morphine and fentanyl in patients with severe head injury: absence of correlation to cerebral autoregulation. Anesthesiology. 2000;92:11-19.
Demetriades D., Chan L.S., Velmahos G., et al. TRISS methodology in trauma: the need for alternatives. Br J Surg. 1998;85:379-384.
Department of Rehabiliatation Medicine Biannual Report of the National Pediatric Trauma Registry. Department of Rehabiliation Medicine. Tufts University/New England Medical Center, 1995. editor
Deppa R., editor. Directorate for Engineering Sciences, in U.S. Consumer Product Safety Commission. 1994. Washington DC
Dickinson R., Singer A.J., Carrion W. Etomidate for pediatric sedation prior to fracture reduction. Acad Emerg Med. 2001;8:74-77.
DiScala C. National Pediatric Trauma Registry Annual Report. Boston: Tufts University Rehabilitation and Childhood Trauma Research and Training Center, 2002.
Dorman H.R., Sondheimer J.H., Cadnapaphornchai P. Mannitol-induced acute renal failure. Medicine (Baltimore). 1990;69:153-159.
Downard C., Hulka F., Mullins R.J., et al. Relationship of cerebral perfusion pressure and survival in pediatric brain-injured patients. J Trauma. 2000;49:654-658. discussion 658–659
Ehrlich P.F., Helmkamp J.C., Seidman P.A., et al. Endotracheal intubation in a rural pediatric population. J Pediatr Surg. 2004;39(9):376-380.
Ehrlich P.F., Ortega J., Mucha P.Jr. The need for a statewide trauma system. WV Med J. 2001;98:66-68.
Ehrlich P.F., Rockwell S., Kincaid S., et al. American College of Surgeons, Committee on Trauma Verification Review: does it really make a difference? J Trauma. 2002;53:811-816.
Ehrlich P.F., Seidman P.S., Atallah O., et al. Endotracheal intubations in rural pediatric trauma patients. J Pediatr Surg. 2004;39:1376-1380.
Ehrlich P.F., Wee C., Drongowski R., et al. Canadian C-spine rule and the national emergency X-radiography utilization low-risk criteria for C-spine radiography in young trauma patients. J Pediatr Surg. 2009;44:987-991.
Eichelberger M.R., Champion H.R., Sacco W.J., et al. Pediatric coefficients for TRISS analysis. J Trauma. 1993;34:319-322.
Engum S.A., Mitchell M.K., Scherer L.R., et al. Prehospital triage in the injured pediatric patient. J Pediatr Surg. 2000;35:82-87.
Fenstermacher J.D., Johnson J.A. Filtration and reflection coefficients of the rabbit blood-brain barrier. Am J Physiol. 1966;211:341-346.
Finfer S., Norton R., Bellomo R., et al. The SAFE study: saline vs. albumin for fluid resuscitation in the critically ill. [retraction in Mayr W, Prowse C. Vox Sang. 2004 Aug;87:2142; PMID: 15355508] Vox Sang. 2004;87(Suppl 2):123-131.
Garrett J.W., Braunstein P.W. The seat belt syndrome. J Trauma. 1962;2:220-238.
Gausche M., Lewis R.J., Stratton S.J., et al. Effect of out-of-hospital pediatric endotracheal intubation on survival and neurological outcome: a controlled clinical trial. JAMA. 2000;283:783-790.
Gelderman M.P., Vostal J.G. Current and future cellular transfusion products. Clin Lab Med. 2010;30(2):443-452.
Gilbert R., Widom C.S., Browne K., et al. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373:68-81.
Glaeser P. Out-of-hospital intubation of children. JAMA. 2000;283:797-798.
Goodwin M.W., Robinson K.N. A pragmatic approach to fasting in paediatric trauma? Paediatr Anaesth. 2000;10:452-453.
Griffen M.M., Frykberg E.R., Kerwin A.J., et al. Radiographic clearance of blunt cervical spine injury: plain radiograph or computed tomography scan? J Trauma. 2003;55:222-226. discussion 226–227
Grisoni E.R., Volsko T.A. Thoracic injuries in children. Respir Care Clin N Am. 2001;7(1):25-38.
Gupta S., Heath K., Matta B.F. Effect of incremental doses of sevoflurane on cerebral pressure autoregulation in humans. Br J Anaesth. 1997;79:469-472.
Guy J., Haley K., Zuspan S.J. Use of intraosseous infusion in the pediatric trauma patient. J Pediatr Surg. 1993;28:158-161.
Haller J.A.Jr. Life-threatening injuries in children: what have we learned and what are the challenges? Bull Am Coll Surg. 1995;80:8-18. 43
Harukuni I., Kirsch J.R., Bhardwaj A. Cerebral resuscitation: role of osmotherapy. J Anesth. 2002;16:229-237.
Hedequist D., Starr A.J., Wilson P., et al. Early versus delayed stabilization of pediatric femur fractures: analysis of 387 patients. J Orthop Trauma. 1999;13:490-493.
Hellmann J.R., Shott S.R., Gootee M.J. Impalement injuries of the palate in children: review of 131 cases. Int J Pediatr Otorhinolaryngol. 1993;26:157-163.
Herregods L., Verbeke J., Rolly G., et al. Effect of propofol on elevated intracranial pressure. Preliminary results. Anaesthesia. 1988;43(Suppl):107-109.
Hibbard R.A., Desch L.W. Maltreatment of children with disabilities. Pediatrics. 2007;119:1018-1025.
Hornor G. Physical abuse: recognition and reporting. J Pediatr Health Care. 2005;19:4-11.
Howard J.M. Accidental death and disability: the neglected disease of modern society. 1966. Washington DC
Hulka F., Mullins R.J., Mann N.C., et al. Influence of a statewide trauma system on pediatric hospitalization and outcome. J Trauma. 1997;42:514-519.
Insko E.K., Gracias V.H., Gupta R., et al. Utility of flexion and extension radiographs of the cervical spine in the acute evaluation of blunt trauma. J Trauma. 2002;53:426-429.
Institute of Medicine. Emergency medical services for children. 1993. Washington DC
Jaicks R.R., Cohn S.M., Moller B.A. Early fracture fixation may be deleterious after head injury. J Trauma. 1997;42(1):1-5.
Jankowitz B.T., Adelson P.D. Pediatric traumatic brain injury: past, present and future. Dev Neurosci. 2006;28:264-275.
Jurkovich G.J., Rivara F.P., Johansen J.M., et al. Centers for Disease Control and Prevention injury research agenda: identification of acute care research topics of interest to the Centers for disease Control and Prevention-National Center for Injury Prevention and Control. J Trauma. 2004;56:1166-1170.
Kaban L.B. Diagnosis and treatment of fractures of the facial bones in children 1943–1993. J Oral Maxillofac Surg. 1993;51:722-729.
Kalb D.C., Ney A.L., Rodriguez J.L., et al. Assessment of the relationship between timing of fixation of the fracture and secondary brain injury in patients with multiple trauma. Surgery. 1998;124(4):739-744.
Keenan H.T., Hollingshead M.C., Chung C.J., et al. Using CT of the cervical spine for early evaluation of pediatric patients with head trauma. Am J Roentgenol. 2001;177:1405-1409.
Kellogg N.D. Evaluation of suspected child physical abuse. Pediatrics. 2007;119:1232-1241.
Klatzo I. Presidental address: neuropathological aspects of brain edema. J Neuropathol Exp Neurol. 1967;26:1-14.
Kokoska E.R., Keller M.S., Rallo M.C., et al. Characteristics of pediatric cervical spine injuries. J Pediatr Surg. 2001;36:100-105.
Krantz B.. Trauma. D.V. Feliciano, K.L. Mattox. Appleton & Lang; 1996:123-129.
Langlois J.A., Rutland-Brown W., Thomas K., editors. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths, in Centers for Disease Control and Prevention. 2006. Atlanta
Laupacis A., Sekar N., Stiell I.G. Clinical prediction rules: a review and suggested modifications of methodological standards. JAMA. 1997;277:488-494.
Levy D., Gruber B. Neck trauma. 2008.http://emedicine.medscape.com/article/827223-overview. vol
Luerssen T.G. Central nervous system injuries. Philadelphia: Mosby, 2006.
MacKenzie E.J., Shapiro S., Eastham J.N. The Abbreviated Injury Scale and Injury Severity Score: levels of inter- and intrarater reliability. Med Care. 1985;23:823-835.
MacKenzie E.J., Steinwachs D.M., Shankar B. Classifying trauma severity based on hospital discharge diagnoses: validation of an ICD-9CM to AIS-85 conversion table. Med Care. 1989;27:412-422.
Marion D.W., Domeier R., Dunham C.M., et al. Practice management guidelines for identifying cervical spine injuries after trauma. 2008.http://www.EAST.org/tpg/chap3u.pdf.
Marsaglia G., Thomas E.D. Mathematical consideration of cross circulation and exchange transfusion. Transfusion. 1971;11:216-219.
Matsen F.A.3rd. Compartmental syndrome: an unified concept. Clin Orthop Relat Res. 1975:8-14. need abbreviation
Mayberg T.S., Lam A.M., Matta B.F., et al. Ketamine does not increase cerebral blood flow velocity or intracranial pressure during isoflurane/nitrous oxide anesthesia in patients undergoing craniotomy. Anesth Analg. 1995;81:84-89.
McGinn T.G., Guyatt G.H., Wyer P.C., et al. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA. 2000;284:79-84.
McHeik J.N., Vergnes P., Bondonny J.M. Treatment of facial dog bite injuries in children: a retrospective study. J Pediatr Surg. 2000;35:580-583.
McKee M.D., Schemitsch E.H., Vincent L.O., et al. The effect of a femoral fracture on concomitant closed head injury in patients with multiple injuries. J Trauma. 1997;42(6):1041-1045.
McManus M.L., Soriano S.G. Rebound swelling of astroglial cells exposed to hypertonic mannitol. Anesthesiology. 1998;88:1586-1591.
Melillo E.P., Hawkins D.J., Lynch L., et al. Difficult airway management of a child impaled through the neck. Paediatr Anaesth. 2001;11:615-617.
Mendelson S.A., Dominick T.S., Tyler-Kabara E., et al. Early versus late femoral fracture stabilization in multiply injured pediatric patients with closed head injury. J Pediatr Orthop. 2001;21(5):594-599.
Meredith W., Rutledge R., Hansen A.R., et al. Field triage of trauma patients based upon the ability to follow commands: a study in 29,573 injured patients. J Trauma. 1995;38:129-135.
Meyer P.G. Critical care management of severe paediatric trauma: a challenge for anaesthesiologists. Paediatr Anaesth. 1999;9:373-376.
Michaud L.J., Rivara F.P., Longstreth W.T.Jr, et al. Elevated initial blood glucose levels and poor outcome following severe brain injuries in children. J Trauma. 1991;31:1356-1362.
Miller M.T., Pasquale M.D., Bromberg W.J., et al. Not so FAST. J Trauma. 2003;54:52-59. discussion 59–60
Mirski A.M., Denchev I.D., Schnitzer S.M., et al. Comparison between hypertonic saline and mannitol in the reduction of elevated intracranial pressure in a rodent model of acute cerebral injury. J Neurosurg Anesthesiol. 2000;12:334-344.
Monkhoff M., Schwarz U., Gerber A., et al. The effects of sevoflurane and halothane anesthesia on cerebral blood flow velocity in children. Anesth Analg. 2001;92:891-896.
Moriarty K.P., Harris B.H., Benitez-Marchand K. Carotid artery thrombosis and stroke after blunt pharyngeal injury. J Trauma. 1997;42:541-543.
Morrison W., Wright J.L., Paidas C.N. Pediatric trauma systems. Crit Care Med. 2002;30:S448-456.
Moss G.S., Gould S.A. Plasma expanders: an update. Am J Surg. 1988;155:425-434.
Mullins R.J. A historical perspective of trauma system development in the United States. J Trauma. 1999;47:S8-14.
Musgrave D.S., Mendelson S.A. Pediatric orthopedic trauma: principles in management. Crit Care Med. 2002;30:S431-S443.
Nakayama D.K., Waggoner T., Venkataraman S.T., et al. The use of drugs in emergency airway management in pediatric trauma. Ann Surg. 1992;216:205-211.
Namias N. Advances in burn care. Curr Opin Crit Care. 2007;13:405-410.
Nathens A.B., Jurkovich G.J., Rivara F.P., et al. Effectiveness of state trauma systems in reducing injury-related mortality: a national evaluation. J Trauma. 2000;48:25-30. discussion 30–21
National Center for Injury Control and Prevention (CDC). WFIMRx. 2006.http://webappa.cdc.gov/sasweb/ncipc/mortrate.html.
National Center for Injury Control and Prevention. W, TM Web-based Injury Statistics Query and Reporting System. 2005.http://www.cdc.gov/ncipc/wisqars/. [1999–2005]
Nayduch D.A., Moylan J., Rutledge R., et al. Comparison of the ability of adult and pediatric trauma scores to predict pediatric outcome following major trauma. J Trauma. 1991;31:452-457. discussion 457–458
NovoSeven. Coagulation factor VIIa [recombinant]) pagkage insert. Princeton, NJ: Novo Nordisk, 2005.
Olsson G.L., Hallen B., Hambraeus-Jonzon K. Aspiration during anaesthesia: a computer-aided study of 185,358 anaesthetics. Acta Anaesthesiol Scand. 1986;30:84-92.
Pinaud M., Lelausque J.N., Chetanneau A., et al. Effects of propofol on cerebral hemodynamics and metabolism in patients with brain trauma. Anesthesiology. 1990;73:404-409.
Potoka D.A., Schall L.C., Ford H.R. Development of a novel age-specific pediatric trauma score. J Pediatr Surg. 2001;36:106-112.
Rana A.R., Drongowski R., Breckner G., et al. Traumatic cervical spine injuries: characteristics of missed injuries. J Pediatr Surg. 2009;44:151-155. discussion 155
Rasmussen G.E., Grandes C.M. Blood, fluids, and electrolytes in the pediatric trauma patient. Int Anesthesiol Clin. 1994;32:79-101.
Rating the severity of tissue damage I: the abbreviated scale. JAMA. 1971;215:277-280.
Ravussin P., Guinard J.P., Ralley F., et al. Effect of propofol on cerebrospinal fluid pressure and cerebral perfusion pressure in patients undergoing craniotomy. Anaesthesia. 1988;43(Suppl):37-41.
Roberts I., Schierhout G., Wakai A. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev. 2003. CD001049
Rogers F.B., Shackford S.R., Osler T.M., et al. Rural trauma: the challenge for the next decade. J Trauma. 1999;47:802-821.
Rosetti V.A., Thompson B.M., Miller J., et al. Intraosseous infusion: an alternative route of pediatric intravascular access. Ann Emerg Med. 1985;14:885-888.
Ross S.E., Leipold C., Terregino C., et al. Efficacy of the motor component of the Glasgow Coma Scale in trauma triage. J Trauma. 1998;45:42-44.
Safe Kids USA. Injury Facts: Childhood injury, in USA SK. 2005. editor Washington, D.C.
Schall L.C., Potoka D.A., Ford H.R. A new method for estimating probability of survival in pediatric patients using revised TRISS methodology based on age-adjusted weights. J Trauma. 2002;52:235-241.
Schenarts P.J., Diaz J., Kaiser C., et al. Prospective comparison of admission computed tomographic scan and plain films of the upper cervical spine in trauma patients with altered mental status. J Trauma. 2001;51:663-668. discussion 668–669
Schmoker J.D., Shackford S.R., Wald S.L., et al. An analysis of the relationship between fluid and sodium administration and intracranial pressure after head injury. J Trauma. 1992;33:476-481.
Shackford S.R., Zhuang J., Schmoker J. Intravenous fluid tonicity: effect on intracranial pressure, cerebral blood flow, and cerebral oxygen delivery in focal brain injury. J Neurosurg. 1992;76:91-98.
Shaikh Z.S., Worrall S.F. Epidemiology of facial trauma in a sample of patients aged 1–18 years. Injury. 2002;33:669-671.
Sharma D., Jelacic J., Chennuri R., et al. Incidence and risk factors for perioperative hyperglycemia in children with traumatic brain injury. Anesth Analg. 2009;108(1):81-89.
Smith G.A. Technical report: lawn mower-related injuries to children. Pediatrics. 2001;107:E106.
Solheim B.G., Wesenberg F. Rational use of blood products. Eur J Cancer. 2001;37:2421-2425. discussion 2425–2427
Soundappan S.V., Holland A.J., Cass D.T., et al. Diagnostic accuracy of surgeon-performed focused abdominal sonography (FAST) in blunt paediatric trauma. Injury. 2005;36:970-975.
Sperry J.L., Ochoa J.B., Gunn S.R., et al. An FFP:PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65(5):986-993.
Spitzfaden A.C., Jimenez D.F., Tobias J.D. Propofol for sedation and control of intracranial pressure in children. Pediatr Neurosurg. 1999;31:194-200.
Stewart L., Bullock R., Rafferty C., et al. Propofol sedation in severe head injury fails to control high ICP, but reduces brain metabolism. Acta Neurochir Suppl (Wien). 1994;60:544-546.
Stiell I.G., Clement C.M., McKnight R.D., et al. The Canadian C-spine rule versus the NEXUS low-risk criteria in patients with trauma. N Engl J Med. 2003;349:2510-2518.
Stiell I.G., Wells G.A. Methodologic standards for the development of clinical decision rules in emergency medicine. Ann Emerg Med. 1999;33:437-447.
Stowe D.F., Bosnjak Z.J., Kampine J.P. Comparison of etomidate, ketamine, midazolam, propofol, and thiopental on function and metabolism of isolated hearts. Anesth Analg. 1992;74:547-558.
Taylor M.S., Sacco W.J., Champion H.R. On the power of a method for comparing survival of trauma patients to a standard survival curve. Comput Biol Med. 1986;16:1-6.
Teasdale G., Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;304(7872):81-84.
Tepas J.J.3rd, Patel J.C., DiScala C., et al. Relationship of trauma patient volume to outcome experience: can a relationship be defined? J Trauma. 1998;44(5):827-830.
Tepas J.J.3rd, Mollitt D.L., Talbert J.L., et al. The pediatric trauma score as a predictor of injury severity in the injured child. J Pediatr Surg. 1987;22:14-18.
Tepas J.J.3rd, Ramenofsky M.L., Mollitt D.L., et al. The Pediatric Trauma Score as a predictor of injury severity: an objective assessment. J Trauma. 1988;28:425-429.
Thourani V.H., Pettitt B.J., Schmidt J.A., et al. Validation of surgeon-performed emergency abdominal ultrasonography in pediatric trauma patients. J Pediatr Surg. 1998;33:322-328.
Tobias J.D. Increased intracranial pressure after fentanyl administration in a child with closed head trauma. Pediatr Emerg Care. 1994;10:89-90.
Tobias J.D., Ross A.K. Intraosseous infusions: a review for the anesthesiologist with a focus on pediatric use. Anesth Analg. 2010;110:391-401.
Townsend R.N., Lheureau T., Protech J., et al. Timing fracture repair in patients with severe brain injury (Glasgow Coma Scale score >9). J Trauma. 1998;44(6):977-982.
Trunkey D.D. Trauma. Sci Am. 1983;249:28-45. NEED ABBREV
Tuggle D. Resources for optimal care of the injured patient. ed 4. 1999. in, ed 4th., 1998
U.S. Consumer Product Commission. National Electronic Injury Surveillance System Database. 1990–1999. Bethesda
U.S. Department of Health and Human Services Administration on Children Youth, and, Families. Office G.P., editor. Child Maltreatment. 2006. Washington, DC
Vane D.W. Imaging of the injured child: important questions answered quickly and correctly. Surg Clin North Am. 2002;82:315-323.
Vavilala M.S., Lam A.M. Perioperative considerations in pediatric traumatic brain injury. Int Anesthesiol Clin. 2002;40:69-87.
Vialet R., Albanese J., Thomachot L., et al. Isovolume hypertonic solutes (sodium chloride or mannitol) in the treatment of refractory posttraumatic intracranial hypertension: 2 mL/kg 7.5% saline is more effective than 2 mL/kg 20% mannitol. Crit Care Med. 2003;31:1683-1687.
Viccellio P., Simon H., Pressman B.D., et al. A prospective multicenter study of cervical spine injury in children. Pediatrics. 2001;108:E20.
Wakai A., Roberts I., Schierhout G. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev. 2007. CD001049
Waxman K., Shoemaker W.C., Lippmann M. Cardiovascular effects of anesthetic induction with ketamine. Anesth Analg. 1980;59:355-358.
Wisner D.H., Schuster L., Quinn C. Hypertonic saline resuscitation of head injury: effects on cerebral water content. J Trauma. 1990;30:75-78.
Zerfowski M., Bremerich A. Facial trauma in children and adolescents. Clin Oral Investig. 1998;2:120-124.