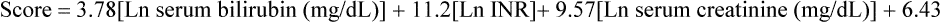Anesthesia for the patient undergoing liver transplantation
Preoperative evaluation
Table 176-1 lists some of the relevant physiologic consequences of liver failure and the consequences that may occur during liver transplantation. Prior to presenting for transplantation, candidates are screened for comorbid cardiopulmonary conditions: a resting echocardiogram assesses cardiac function and allows estimation of pulmonary artery pressures. A bubble test (injection of agitated saline while monitoring for echo-contrast in the right side of heart chambers) can also be performed; delayed appearance of the contrast agent suggests that the patient may have hepatopulmonary syndrome. If the patient has risk factors for coronary artery disease (∼40%-50% of adult patients), noninvasive testing is frequently performed, often by dobutamine stress echocardiography, because patients with significant coronary artery disease have poor peritransplant outcomes.
Table 176-1
Pathophysiologic Changes Associated with Liver Failure
| Organ System | Change | Consequence(s) |
| Cardiovascular | Hyperdynamic circulation (high cardiac output, low SVR) Portal hypertension Ascites Pulmonary hypertension |
Hypotension Varices, splenomegaly Bleeding (dilated vessels, thrombocytopenia) Fluid shifts after drainage High perioperative mortality rate (>80%) if severe |
| Respiratory | Respiratory alkalosis Restrictive physiology (ascites with or without pleural effusion) Hepatopulmonary syndrome (intrapulmonary shunting) |
Atelectasis; reduced compliance Hypoxemia |
| Hematologic | Decreased factor synthesis Thrombocytopenia Anemia |
Bleeding potential |
| CNS | Hepatic encephalopathy Cerebral edema (in fulminant failure) |
Delayed awakening Raised ICP; consider ICP monitoring |
| Renal | Hepatorenal syndrome Hyponatremia |
Renal failure—volume and electrolyte management concerns Possibility of CPM if corrected intraoperatively |
The severity of end-stage liver disease is assessed by calculating the MELD (Model of End-stage Liver Disease) score, which incorporates the patient’s serum bilirubin, serum creatinine, and the international normalized ratio for prothrombin time to predict survival (Box 176-1). A higher MELD score is associated with more severe liver failure; higher MELD scores are also predictive of a greater rate of intraoperative blood product transfusion and need for vasopressors.
Intraoperative management
Anesthesia
Transplantation procedure
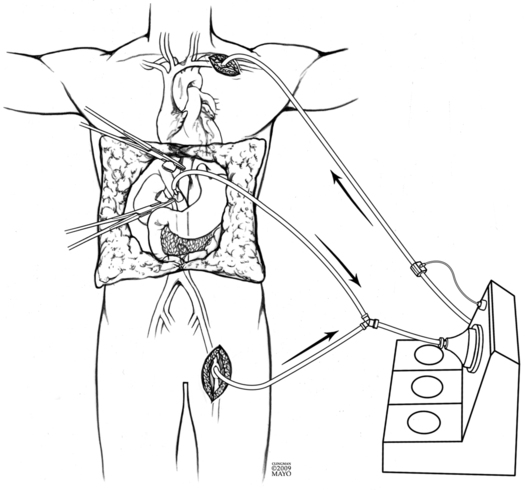
Initial dissection and hepatectomy can result in significant blood loss from friable dilated vessels in the abdominal wall, in the abdomen, and around the liver. Excision of the liver involves mobilization and then clamping and dividing the hepatic vasculature (hepatic artery, portal vein, and IVC). Venovenous bypass is occasionally used depending on surgeons’ experience and preference to overcome the loss of venous return to the heart, the lack of which can cause cardiovascular collapse. Cannulas are placed in the portal and femoral veins; blood drains by gravity to a centrifugal pump, which then returns the blood to the upper body via a large-bore cannula (Figure 176-1). An alternative surgical approach, and one more commonly used, is the “piggyback” technique, in which the surgeon separates the liver from the IVC using a side bite of the IVC (i.e., partial IVC occlusion), allowing some IVC flow to continue during surgery. With this approach, portal venous return is still lost.