CHAPTER 23 Anesthesia for General Abdominal, Thoracic, Urologic, and Bariatric Surgery
For the most part, anesthetic considerations for pediatric general surgery are similar to those for adults. Inhalation anesthesia supplemented with muscle relaxants can provide adequate operating conditions. Nitrous oxide should be avoided in the presence of a bowel obstruction and when one-lung anesthesia may render the patient hypoxemic. When aspiration of gastric contents is a major concern, either rapid-sequence induction or awake intubation should be performed. Because children about to undergo urgent emergency surgery frequently have fluid and electrolyte imbalances as well as underlying hemodynamic instability, a thorough preoperative assessment of the patient is essential. Anesthetic agents are selected to render the patient unconscious, but, in addition, regional anesthesia is used to provide the child with perioperative pain relief. The details of the regional techniques of caudal, lumbar epidural anesthesia, ilioinguinal/iliohypogastric nerve block, penile nerve block, and intercostal nerve block are discussed in Chapter 16, Regional Anesthesia. The planned operative approach influences anesthetic management. As the frontiers of minimally invasive surgery expand, these new techniques can markedly influence the patient’s cardiorespiratory stability and, consequently, the choice of anesthetic agents.
Video endoscopy
With the development of smaller instruments, the technological progress in video imaging, and the increasing experience among pediatric surgeons, video endoscopic surgery is being performed for a growing number of pediatric surgical indications. Benefits of video laparoscopy and thoracoscopy include small incisions and scars, reduced surgical intervention and postoperative pain, earlier return of bowel function, and more rapid recovery (Box 23-1) (Reddick and Olsen, 1989; Soper et al., 1992; Rogers et al., 1992; Rodgers, 1993; Soper et al., 1994; Steiner et al., 1994; Sawyers, 1996; Hunter, 1997; Danelli et al., 2002). Fiberoptic endoscopes that can be passed through a needle are now manufactured, and digital video signals can be electronically modified to yield sharp, detailed, color images with a minimum light intensity. Digital cameras are designed to maintain an image in an upright orientation regardless of how the telescope is rotated. They are also equipped with an optical or a digital zoom to magnify the image or give the illusion of moving the telescope closer to the object of interest. The smallest of telescopes use fiberoptics and are less than 2 mm in diameter. Two-millimeter disposable ports, mounted on a Veress needle, are used for introduction of these small instruments. Larger instruments and ports are used in larger patients and for more complex cases.
General abdominal surgery
Abdominal and thoracic pathologic conditions requiring surgical intervention can be caused by metabolic or endocrine disturbances, tumors, inflammatory processes, or embryologic disorders. Box 23-2 lists abdominal conditions commonly encountered in pediatric general surgery.
Laparoscopy
The laparoscopic procedures that can be performed in infants and children are virtually unlimited. A list of operations currently being performed is shown in Box 23-3. As surgeons gain more experience with laparoscopic surgery, the time required to complete these operations decreases (Fig. 23-1). The safety and efficacy of commonly performed laparoscopic procedures compared with alternative approaches (e.g., endoscopic, open surgical techniques) have been compared (Mattei, 2007; Chertin et al., 2007; Perger et al., 2009).
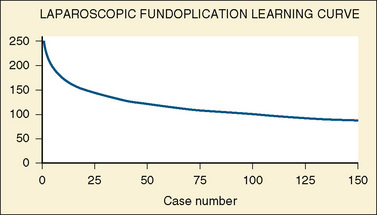
FIGURE 23-1 The learning curve for laparoscopic fundoplication.
(Adapted from Georgeson KE, Inge TH, Albanese CT: Laparoscopically assisted anorectal pull-through for high imperforate anus: a new technique, J Pediatr Surg 35:927, 2000.)
Laparoscopic gastrostomy involves placement of an umbilical port and a left subcostal cannula (the future site of the gastrostomy). The stomach is pulled to the abdominal wall and the gastrostomy is performed using the Seldinger technique (Fig. 23-2). Operative time is approximately 30 minutes (Tomicic et al., 2002). The risks may be lower than in percutaneous endoscopic gastrostomy in small children, because the procedure is done under direct vision. There is less trauma than with open surgery, and feedings are initiated within 24 hours. Laparoscopic fundoplication for the treatment of gastroesophageal reflux disease is associated with a complication and recurrence rate comparable with or less than that for open surgery (Esposito et al., 2000).
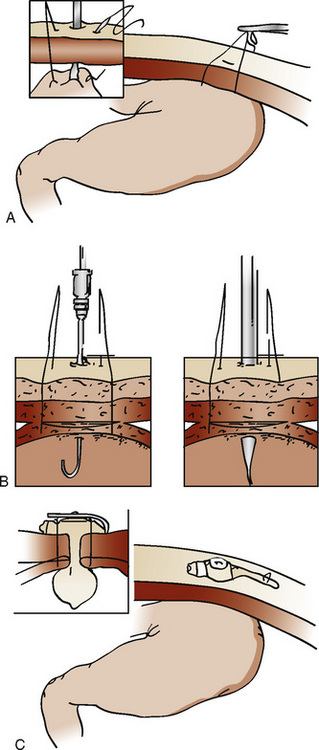
FIGURE 23-2 Laparoscopic gastrostomy. The stomach is entered and pulled up to the anterior abdominal wall (A) and is sutured in place (B). The gastrostomy tube is then placed (C). See related video online at www.expertconsult.com.
The laparoscopic treatment of appendicitis in children has been controversial, particularly in complicated cases (e.g., gangrene, perforation). Experience indicates, however, that laparoscopic appendectomy is not associated with an increased risk compared with open surgery, even in the presence of perforation (Meguerditchian et al., 2002). The incidence of wound infections and intraabdominal abscesses may be less in laparoscopic than in open appendectomy (Paya et al., 2000). Surgical times are comparable, and postoperative pain and length of hospital stay are diminished (Canty et al., 2000; Lintula et al., 2001). Comparable results have been reported for laparoscopic cholecystectomy (Esposito et al., 2001) and laparoscopic splenectomy in pediatric patients (Danielson et al., 2000; Park et al., 2000). Infants undergoing laparoscopic pyloromyotomy have shorter times to enteral feeding and hospital discharge (Hall et al., 2009). Diagnostic laparoscopy and laparoscope-guided cholangiography are being used in the evaluation of neonatal conjugated hyperbilirubinemia, avoiding the need for laparotomy and operative cholangiography (Hay et al., 2000).
The role of laparoscopy in the treatment of solid neoplasms is evolving. Indications include biopsy of suspected malignancies, staging or determination of resectability, second-look procedures to help determine response to chemotherapy, and diagnosis of recurrent or metastatic disease (Sailhamer et al., 2003). Laparoscopic tumor ablation or curative resection may have a role in selected cases. Open surgery may be required when complete resection of the intact specimen with delineation of surgical margins is part of the protocol design in patients enrolled in multicenter studies. Although advocates of laparoscopic surgery maintain that it can reduce hospital costs, promote earlier patient discharge, produce less postoperative pain, improve cosmetic results, and allow patients a more rapid return to full activity, evidence for this is questionable so far (Rangel et al., 2003).
Anesthetic Considerations
Although regional anesthesia may be used alone in older children, general anesthesia is nearly always used for laparoscopic procedures. The use of the laryngeal mask airway (LMA) has been described in adults undergoing laparoscopy. The reliability of the standard LMA to provide adequate gas exchange during positive pressure ventilation is controversial (Maltby et al., 2000; Lu et al., 2002). More favorable ventilation and a reduction in inadvertent gastric insufflation have been reported with the LMA-ProSeal (Laryngeal Mask Company Limited, Henley on Thames, UK) (Maltby et al., 2002). The pediatric ProSeal LMA has been shown to be effective for short laparoscopic procedures (Sinha et al., 2007). In infants and children, however, endotracheal tube (ETT) placement remains the standard.
A variety of general anesthetic techniques have been used for laparoscopic surgery. Regional anesthesia is not commonly used as an adjunct to general anesthesia in pediatric patients unless the laparoscopy is converted to an open procedure. The use of nitrous oxide is controversial. Concerns have been raised that nitrous oxide may cause bowel distention, compromising visibility and exposure during surgery (Eger and Saidman, 1965; Cunningham and Brull, 1993). In addition, nitrous oxide may exacerbate the already increased incidence of nausea and vomiting after laparoscopy (Lonie and Harper, 1986; Divatia et al., 1996; Tramer et al., 1996), although the findings of several studies have failed to confirm these effects of nitrous oxide (Taylor et al., 1992; Jensen et al., 1993). However, nitrous oxide can also support combustion. Because of its antiemetic effect, propofol has been recommended for maintenance of anesthesia during laparoscopy (Martin et al., 1993; Song et al., 1998). The combination of propofol and remifentanil has been advocated because the patient emerges rapidly and without an increase in postoperative nausea and vomiting compared with the use of inhalation anesthesia (Grundmann et al., 2001). Because of the increased incidence of postoperative nausea and vomiting associated with laparoscopy, prophylactic treatment with antiemetics and histamine blockers (droperidol, metoclopramide) have been commonly used. Orogastric suctioning at the end of the operation may also help reduce the risk for postoperative nausea and vomiting.
For most surgical procedures, postoperative pain is reduced with laparoscopy compared with open surgery, and postoperative analgesia can usually be achieved with intravenous (IV) and oral agents. Although diminished compared with open surgery, pain after laparoscopic surgery is associated with the incision, visceral manipulation, irritation and traction of nerves, vascular traction and injury, the presence of residual gas in the abdomen, and inflammatory mediators (Alexander, 1997). Pain is frequently localized to the back or shoulder. Laparoscopic repair has been associated with more postoperative pain than open hernia repair (Koivusalo et al., 2009).
A variety of approaches to prevent and treat pain after laparoscopy have been described. Bupivacaine infiltration at incision sites before skin incision has been shown to decrease postoperative pain (Kato et al., 2000; Moiniche et al., 2000). Bupivacaine infiltration has been found to be superior to IV fentanyl or tenoxicam in reducing postoperative pain (Salman et al., 2000). Low-dosage intrathecal morphine and bupivacaine also decrease postoperative pain (Motamed et al., 2000). Intraperitoneal local anesthetic instillation and mesosalpinx block may diminish postoperative pain after laparoscopy and may be beneficial in reducing postoperative shoulder pain (Kiliç et al., 1996). Intraperitoneal instillation of both bupivacaine and meperidine has been shown to be more efficacious than the combination of intraperitoneal bupivacaine and intramuscular meperidine (Colbert et al., 2000).
Caution must be used to avoid toxic plasma concentrations of local anesthetics due to systemic absorption in infants and children, however. Perioperative acetaminophen, nonsteroidal antiinflammatory agents, and other nonopioid analgesics should be used in combination with opioids as needed for postoperative analgesia. Clonidine has been shown to reduce the requirement for postoperative opioids, and it has the advantage of decreasing the tachycardia associated with pneumoperitoneum (Yu et al., 2003).
Physiologic changes during laparoscopic surgery are related to positioning (Trendelenburg, reverse Trendelenburg), increased abdominal pressure resulting from gas insufflation, and increased arterial CO2 tension associated with insufflation. The magnitude of physiologic changes associated with laparoscopic surgery is influenced by the patient’s age, underlying myocardial function, and anesthetic agents. The reverse Trendelenburg position may cause hypotension, especially in the anesthetized patient with intravascular hypovolemia. The Trendelenburg position causes cephalad displacement of the diaphragm, restricting lung excursion and posing a risk for endobronchial intubation. In addition, central venous pressure and heart rate increase, and systemic arterial pressures and cardiac output decrease (Hirvonen et al., 1995). The pulmonary effects depend on the patient’s age, weight, pulmonary function, extent of Trendelenburg position, anesthetic agents, and ventilation technique (Sprung et al., 2002). Atelectasis and a decrease in functional residual capacity and pulmonary compliance may be observed. Ventilation/perfusion mismatch may result in decreased arterial oxygen tension. Neuromuscular blockade, endotracheal intubation, and positive pressure ventilation may help to reduce the pulmonary effects of the Trendelenburg position. As long as intraabdominal pressure is kept below 15 mm Hg, oxygen saturation can generally be maintained during position changes and pneumoperitoneum despite adverse changes in respiratory mechanics (Sprung et al., 2003). Significant hypercarbia may occur despite adjustments in mechanical ventilation, especially in infants.
Both pneumoperitoneum and the Trendelenburg position reduce femoral venous flow, increasing the risk for thrombotic complications (Rosen et al., 2000). Cardiovascular instability associated with laparoscopy has also been attributed to hypercarbia-induced arrhythmias, venous gas embolus, compression of the vena cava, pneumothorax, and pneumomediastinum (Lalwani and Aliason, 2009). Insufflation to an intraabdominal pressure of 12 mm Hg can cause septal hypokinesis and left ventricular wall motion abnormalities (Hoymork et al., 2003; Huettemann et al., 2003). The increase in intraabdominal pressure associated with gas insufflation results in increased intrathoracic pressure and increased pulmonary and systemic vascular resistances and decreased cardiac output (Hirvonen et al., 1995, 2000). Arterial blood pressure may be decreased, maintained, or even elevated by an increase in systemic vascular resistance. Reduction in splanchnic, hepatic, and renal blood flow and increases in the plasma concentrations of catecholamines, cortisol, prolactin, growth hormone, and glucose levels have been reported with CO2 pneumoperitoneum (Hashikura et al., 1994; Mikami et al., 1998; Ishizuka et al., 2000).
A new technique, known as gasless laparoscopy, eliminates the risks of pneumoperitoneum by using mechanical retraction (Canestrelli et al., 1999). Reduced visualization is associated with this technique, but its application to pediatrics remains uncertain (Lukban et al., 2000).
Inguinal Herniorrhaphy and Umbilical Herniorrhaphy
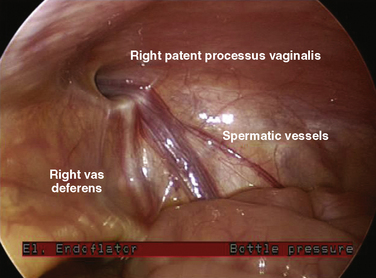
The surgical technique for this procedure is well described (Rowe and Lloyd, 1986). Laparoscopic techniques have also been described (Lobe and Schropp, 1992; Lee and Liang, 2002; Schier et al., 2002), as well as needleoscopic techniques (Prasad et al., 2003). The overall complication rate after an elective hernia repair is about 2%, and it increases to 14% after operations for incarcerated hernia. A major surgical issue in patients with a unilateral inguinal hernia is whether the contralateral side should be explored, thereby subjecting the patient to possible unnecessary damage to the contralateral vas deferens and spermatic cord. In a number of studies, a patent contralateral processus vaginalis occurs about 60% of the time. However, this patency appears to be age related, with the highest rate occurring in infants (63%), and with the incidence decreasing until 2 years of age, when it appears to plateau at 41% (Rowe and Lloyd, 1986). Despite the high incidence of patent processus vaginalis, the incidence of contralateral hernias is about 15%. The development of a contralateral hernia is also age dependent. If the initial hernia developed in the first year of life, there is a fourfold greater chance that a contralateral hernia will develop compared with children whose initial hernia manifested after 1 year of age. In girls with unilateral inguinal hernias, the incidence of positive explorations for contralateral hernias is 60%. Consequently, girls almost always undergo contralateral exploration. Laparoscopy without a separate incision has been advocated to examine the contralateral side for a patent process vaginalis when the ipsilateral hernia sac is of sufficient width to allow passage of a laparoscope (Yerkes et al., 1998) (Fig. 23-3).
The following discussion pertains to elective, uncomplicated hernias. Anesthesia can be induced by mask inhalation of volatile agents or by IV or rectal technique. Endotracheal intubation is usually unnecessary for herniorrhaphy, except in infants younger than 1 year, in whom it may be difficult to maintain an adequate airway with bag and mask ventilation without distending the stomach. However, the use of the LMA in these patients may make tracheal intubation unnecessary. The patient must be well anesthetized when the spermatic cord is being manipulated. Inadequate depth of anesthesia at this stage can result in laryngospasm or bradycardia. Caudal epidural anesthesia or ilioinguinal/iliohypogastric nerve block can be quite effective, both in providing postoperative pain relief and in diminishing the intraoperative anesthetic requirements (Markham et al., 1986). Premature infants have a particularly high incidence of inguinal hernias. In these infants, for whom an inhalation anesthetic may have increased risks, spinal anesthesia (Harnik et al., 1986) and caudal epidural (Spear et al., 1988) anesthesia have been used successfully to avoid general anesthesia and endotracheal intubation (Williams et al., 2006).
Orchiopexy
The objectives of repair for undescended testicles are to alter the course of the spermatic artery from the renal pedicle to the internal ring to the external ring, and to create in its place a direct line from the renal pedicle to the scrotum. However, the surgical approach to patients with undescended testes is not uniform (Hinman, 1987; Heiss and Shandling, 1992). The general approach to patients with a nonpalpable testis is inguinal exploration. If neither the testis nor proof of its absence is found, the lower posterolateral surface of the peritoneal cavity is explored. When the testis is found, it is either removed or surgically placed in the scrotum. This can be accomplished by a staged orchiopexy, autotransplantation of the testis, or Fowler-Stephens procedure. The Fowler-Stephens approach takes advantage of the vascular arcades between the deferential and spermatic arteries in the cord. Because of this collateral blood flow, high ligation of the testicular vessels can preserve the testicular blood supply and provide the surgeon with mobility in bringing the testicle down into the scrotum. The Fowler-Stephens approach has undergone modification and is now generally done in two stages. The first stage involves clipping the spermatic vessel, whereas the second stage, performed months later, involves the formal orchiopexy. With the advent of laparoscopic surgery, both stages of the Fowler-Stephens approach can be done with the aid of a laparoscope (Atlas and Stone, 1992; Bogaert et al., 1993).
Surgery for Pyloric Stenosis
Pyloric stenosis is one of the most common gastrointestinal abnormalities appearing in the first 6 months of life. This disorder has a polygenic mode of inheritance and occurs four times more commonly in males and more frequently in white infants. The frequency of this disorder ranges from 1.4 to 8.8 per 1000 live births (Zeidan et al., 1988; Dubé et al., 1990; Saunders and Williams, 1990; Bissonnette and Sullivan, 1991; Murtagh et al., 1992). Some controversy exists regarding the associated risk for pyloric stenosis with the maternal postnatal exposure to macrolides (Louik et al., 2002; Sorensen et al., 2003). Pyloric stenosis has been associated with cleft palate and esophageal reflux.
The cardinal features of pyloric stenosis are projectile vomiting, visible peristalsis, and a hypochloremic, hypokalemic, metabolic alkalosis. Although hypokalemia is a frequent finding, Schwartz and colleagues (2003) reported in a retrospective chart review that 36% of patients with pyloric stenosis were noted to have hyperkalemia. Nonbilious vomiting is the classic presenting symptom and generally occurs between 2 and 8 weeks of age. Jaundice occurs in less than 5% of patients and is thought to be associated with caloric deprivation and hepatic glucuronyl transferase deficiency. The jaundice resolves after successful treatment. Diagnosis is made by palpation of an olive-sized mass in the upper abdomen and is frequently confirmed by radiographic studies. Although false-positive studies are rare, false-negative findings can occur in up to 19% of the ultrasound examinations and in 10% of the contrast studies. Preoperative ultrasound measurements of the pylorus correlate well with intraoperative surgical measurements (Muramori et al., 2007).
The pathologic condition involves gross thickening of the circular muscles of the pylorus, resulting in a gradual obstruction of the gastric outlets. Vanderwinden and coworkers (1992) noted a deficiency of nitric oxide synthetase in the muscle layers of infants with pyloric stenosis. The pathophysiology of pyloric stenosis frequently leads to hypovolemia and a hypochloremic metabolic alkalosis.
Winters (1973) outlined the pathophysiology that leads to hypochloremic, hypokalemic, metabolic alkalosis. In pyloric stenosis, persistent vomiting results in a loss of gastric juices rich in hydrogen and chloride ions and, to a lesser extent, sodium and potassium ions. Because the obstruction is at the level of the pylorus, the vomitus does not contain the usual alkaline secretions of the small intestine; the patient develops a metabolic alkalosis. As an increased bicarbonate load is presented to the kidneys, the resorptive capacity of the proximal tubule is overwhelmed, and an increased amount of NaHCO3 and water is delivered to the distal tubule. Because NaHCO3 cannot be reabsorbed in the distal tubule, aldosterone secretion occurs. Increased aldosterone increases sodium reabsorption and kaliuresis. Potassium loss is further exacerbated by potassium being exchanged in the tubule for hydrogen in an effort to maintain normal plasma pH.
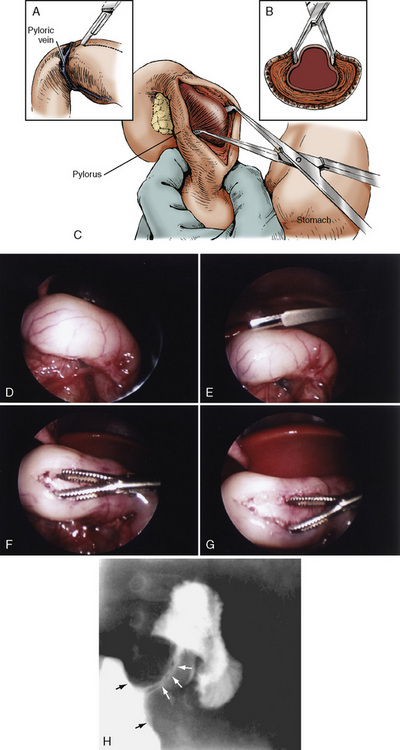
Surgical pyloromyotomy, a relatively simple procedure in the hands of skilled pediatric surgeons, is curative (Fig. 23-4). The operative mortality rates of 10% has declined to less than 0.5%. The surgery can be performed either as an open procedure or laparoscopically. In a comparative study, Campbell and colleagues (2002) noted that laparoscopic pyloromyotomy has become the dominant approach. See related video online at www.expertconsult.com Hall and coworkers (2009) have shown that laparoscopically performed procedures have shorter times for patients to achieve full enteral feeds and faster hospital discharge times than patients having open pyloromyotomy. However, laparoscopic pyloromyotomy is associated with an increased rate of complications, higher hospital charges, and a reduction in the general surgical resident’s operating experience (Campbell et al., 2002). Pyloromyotomy for pyloric stenosis is not a medical emergency that requires immediate surgical intervention. The major anesthetic considerations are recognizing and treating dehydration and acid-base abnormalities before beginning anesthesia. In addition, the patient is at risk for aspirating gastric contents.
See related video online at www.expertconsult.com Hall and coworkers (2009) have shown that laparoscopically performed procedures have shorter times for patients to achieve full enteral feeds and faster hospital discharge times than patients having open pyloromyotomy. However, laparoscopic pyloromyotomy is associated with an increased rate of complications, higher hospital charges, and a reduction in the general surgical resident’s operating experience (Campbell et al., 2002). Pyloromyotomy for pyloric stenosis is not a medical emergency that requires immediate surgical intervention. The major anesthetic considerations are recognizing and treating dehydration and acid-base abnormalities before beginning anesthesia. In addition, the patient is at risk for aspirating gastric contents.
The initial therapeutic approach is aimed at repletion of intravascular volume and correction of electrolyte and acid-base abnormalities (e.g., 5% dextrose in 0.45% NaCl with 40 mmol/L of potassium infused at 3 L/m2 per 24 hours). Most children respond to therapy within 12 to 48 hours, after which surgical correction can proceed in a nonemergent manner. The use of cimetidine has also been shown to rapidly normalize the metabolic alkalosis in patients with hypertrophic pyloric stenosis (Banieghbal, 2009).
Once the child is satisfactorily hydrated and after the appropriate monitors (precordial stethoscope, electrocardiogram, pulse oximeter, and blood pressure cuff) are placed, the infant is ready for induction of anesthesia. The obstructed pylorus and associated vomiting increase the possibility of aspirating gastric contents during induction of anesthesia. A thorough evacuation of stomach contents through a nasogastric or orogastric tube, with proper preoxygenation and monitoring, greatly reduces the chance of regurgitation during induction, although it does not completely eliminate the possibility of aspiration (Cook-Sather et al., 1997). Infants with pyloric stenosis are thus considered by some anesthesiologists to have a status equivalent to that of infants with a full stomach; therefore, a rapid-sequence induction is preferred to secure the airway and minimize the risks of aspiration (Dierdorf and Krishna, 1981; Battersby et al., 1984). On the other hand, mask inhalation induction preceded by careful emptying of the stomach has been used safely in several pediatric centers (MacDonald et al., 1987). In a prospective nonrandomized observational study of 76 infants with pyloric stenosis, Cook-Sather and colleagues (1998) compared three techniques: awake intubation, rapid-sequence intubation, and modified rapid-sequence intubation (ventilation through cricoid pressure). In this study, awake intubation was not superior to anesthetized, paralyzed intubations. Awake intubation prevented neither bradycardia nor oxygen desaturations.
After induction and intubation of the trachea, a nasogastric or an orogastric tube is reinserted and left in place during the operative procedure. This allows the surgeon to test the integrity of the pyloric mucosa after pyloromyotomy. A small volume of air is injected down the nasogastric tube, and the surgeon manipulates the air bubble into the duodenum and occludes the bowel lumen both proximal and distal to the incision. Mucosal perforation is indicated if there is air leakage. After the operation, which usually requires less than 30 minutes, the effects of any nondepolarizing muscle relaxant are reversed. The infant can then be safely extubated when fully awake and with intact protective airway reflexes. Some believe that opioid analgesia is seldom necessary (Battersby et al., 1984) and may predispose patients to a prolonged emergence from anesthesia (MacDonald et al., 1987). It is not unusual to encounter lethargy or drowsiness in these infants in the immediate postoperative period. Respiratory depression has been noted to occur postoperatively and is possibly related to cerebrospinal fluid pH and hyperventilation (Andropoulos et al., 1994). Rare occurrences of hypoglycemia, apnea, convulsions, and cardiac arrest in the early postoperative period have also been cited. These events have been ascribed to the cessation of IV glucose infusions and the depletion of liver glycogen (Shumake, 1975). Infants usually begin oral feedings 8 hours after the procedure. The choice of maintenance anesthetic agent for infants with pyloric stenosis has been studied (Wolf et al., 1996; Chipps et al., 1999; Davis et al., 2001; Galinkin et al., 2001).
Wolf and coworkers (1996) found that clinical postoperative apnea occurred in 3 of 11 infants anesthetized with isoflurane, and in none of the nine infants anesthetized with desflurane. In a multicenter study comparing halothane and remifentanil, where both drugs were administered to similar clinical endpoints, remifentanil was not associated with postoperative respiratory depression. In this study, all infants received both preoperative and postoperative pneumograms, and remifentanil (as opposed to halothane) was not associated with new pneumogram abnormalities in the postoperative period (Davis et al., 2001; Galinkin et al., 2001).
Wilms Tumor Procedures
Wilms tumor is the most common childhood abdominal malignancy, occurring with an incidence, consistent throughout the world, of 5.0 to 7.8 per 1 million children less than 15 years of age. Wilms tumor accounts for about 6% of all malignancies in childhood. The incidence is equal in the two genders. The peak age at diagnosis is between 1 and 3 years. Wilms tumor occurs bilaterally in 5% of patients. Patients with Wilms tumor frequently have associated anomalies (aniridia, 1%; hemihypertrophy, 2%; genitourinary abnormalities, 5%; ectopic and solitary kidneys [horseshoe kidneys, ureteral duplications, hypospadias]). Other associated conditions include Beckwith-Wiedemann syndrome and neurofibromatosis. The signs and symptoms associated with Wilms tumor are variable. The most frequent finding is an increasing abdominal girth with a palpable abdominal mass (85%). Hypertension occurs in 60% of patients, and hematuria is present in 10% to 25%. Acquired von Willebrand’s syndrome with Wilms tumor occurs infrequently (Baxter et al., 2009).
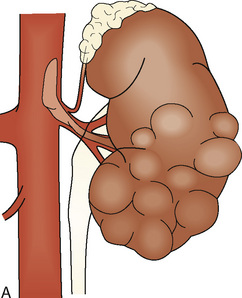
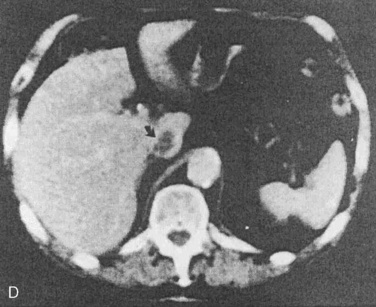
Wilms tumor is generally located in the upper or lower renal pole. It may involve the renal vein (10% of cases) and extend up the vena cava to the right atrium (5% of cases) (Fig. 23-5). Prognosis for patients with the disease is related to its staging (Table 23-1). Patients with favorable staging have an 80% to 90% chance of cure, whereas patients with metastasis have a 50% chance of long-term survival. Risk factors for local recurrence of Wilms tumor include an advanced local stage (involvement of the paraaortic lymph nodes), unfavorable histology, and spillage of tumor at the time of resection (Shamberger et al., 1999). Overexpression of HER2, an oncoprotein, has been shown to be a good predictor of overall survival and longer recurrence-free survival (Ragab et al., 2010). Therapy for Wilms tumor includes surgery, chemotherapy, and radiotherapy. Depending on the size of the tumor and the staging, chemotherapy may be started either before or after surgery. Chemotherapy generally involves vincristine, actinomycin, and anthracycline (doxorubicin).
| Stage | Description |
| I | Tumor limited to the kidneys and excised |
| II | Tumor extending beyond the kidneys, but completely excised |
| III | Residual nonhematogenous tumor confined to the abdomen |
| IV | Hematogenous metastases Lymph node involvement |
| V | Bilateral renal involvement at diagnosis |
Preoperative evaluation of the patient is related to the presence of metastases and the patient’s cardiopulmonary function. If the patient has had prior chemotherapy with Adriamycin, cardiac function should be assessed by echocardiography (see Chapter 4, Cardiovascular Physiology, and Chapter 36, Systemic Disorders). Serum electrolyte levels should be assessed if there is a history of vomiting. Renal dysfunction is unusual even in patients with bilateral Wilms tumor. Although the surgical approach is generally an open incision, laparoscopic nephrectomy has been reported for the unilateral removal of tumor (Barber et al., 2009; Duarte et al., 2009). Anesthetic considerations revolve around the issue of abdominal distention with delayed gastric emptying and potentially large intraoperative blood losses. Abdominal distention may place the patient at risk for aspiration of gastric contents, so full-stomach precautions should be taken at the induction of anesthesia. Intraoperative blood loss can be a significant factor because of the tumor’s location and possible involvement of the renal vein and vena cava (Cristofani et al., 2007). Two large-bore IV catheters are recommended. Because of the possibility that the vena cava may be cross-clamped (either to be explored for extension of tumor or to control hemorrhage), the large-bore catheters should be inserted above the diaphragm.
Neuroblastoma Procedures
Neuroblastoma, the most common extracranial solid tumor of childhood, involves the postganglionic sympathetic nervous system. Fifty percent of tumors arise in the adrenals, 30% occur below the diaphragm, and 20% occur in cervical or thoracic sites. Neuroblastoma accounts for 8% to 10% of pediatric cancer malignancies. The median age at presentation is 22 months, with 37% of patients presenting at less than 1 year of age, and 51% of new patients being less than 4 years of age. Clinical presentation may be related to the primary tumor, to its metastasis, or to the associated paraneoplastic syndromes (Table 23-2). Metastases from neuroblastoma occur in lymph nodes, liver, cortical bone, bone marrow, orbits, and skin. The paraneoplastic syndromes can exhibit hypertension secondary to catecholamine release, or kidney displacement with renal artery stretching and renin-angiotensin stimulus. The gastrointestinal symptoms (diarrhea, flushing, abdominal distention) are attributed to vasoactive intestinal peptides (VIPs), whereas the etiology of opsoclonus and ataxia is unclear.
| Primary tumor | Abdominal mass or pain Respiratory distress or dysphagia Vocal cord paralysis Bowel or bladder dysfunction Horner’s syndrome Heterochromia of iris on affected side Incidental finding on chest radiograph |
| Metastatic disease | Hepatomegaly Lymphadenopathy Bone pain Periorbital ecchymoses Subcutaneous nodules Marrow replacement with anemia, fever, or bruising from low blood counts Systemic illness Failure to thrive Fever of unknown origin |
| Paraneoplastic syndromes | Vasoactive intestinal peptide syndrome (chronic watery diarrhea and abdominal distention) Opsoclonus-myoclonus or cerebellar ataxia syndrome Excessive catecholamine syndrome (hypertension, headaches, flushing, sweating, tachycardia, palpitations) |
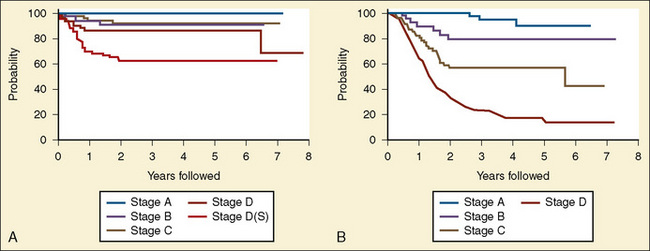
Tumor prognosis has been related to age at presentation, extent of disease (staging), degree of tumor differentiation, amount of catecholamine metabolites, serum ferritin level, lactate dehydrogenase level, neuron-specific enolase level, serum lymphocyte count, ganglioside presence, N-myc amplification, deletion of chromosome 1p, additional copies of chromosome 17q, and TRKA expression (Smith et al., 1989; Hiyama et al., 1991; Berthold et al., 1992; Eckschlager, 1992; Murakami et al., 1992; Qualman et al., 1992; Shuster et al., 1992; Haase et al., 1999). However, patient age and tumor stage are the two most important independent variables (Fig. 23-6). The Evans staging system uses tumor location, lymph node involvement, and presence of metastases, whereas the Pediatric Oncology Group system emphasizes tumor resectability and identification of residual disease to predict survival and treatment.
Treatment involves surgical resection and chemotherapy. Although neuroblastoma is radiosensitive, 45% of patients present with metastasis, so the use of radiation is sometimes limited in primary therapy. In a series of adrenal neuroblastomas less than 6 cm not associated with adjacent vessel or organ involvement, DeLagausie and colleagues (2003) reported successful tumor removal with laparoscopic techniques. The anesthetic considerations depend on the planned surgical procedure, the location and size of the tumor, and the metabolic effects of the tumor. Electrolyte imbalance may result from vomiting and diarrhea caused by excessive production of VIP. Despite the production of catecholamines, significant hypertension has been reported in 9% to 30% of patients (Weinblatt et al., 1983; Haberkern et al., 1992).
Intraoperatively, blood loss and third-space fluid losses can accompany the resection of tumor. Haberkern and coworkers (1992) noted in a retrospective review that 45% of patients had hypotension after the tumor excision, whereas fewer than 3% of the patients had cardiovascular signs of increased catecholamine release during tumor resection. Although in patients with mediastinal neuroblastoma, airway complications are rare because of the tumor’s location in the posterior mediastinum, airway compromise can occur, and evidence of airway compression by the tumor should be evaluated before starting the anesthetic induction. IV or inhalational inductions may be performed. Both volatile agents and opioids have been safely used along with combined regional and general anesthetic techniques (Haberkern et al., 1992).
Anti-Gastroesophageal Reflux Procedures
Gastroesophageal reflux (GER) involves a dysfunction of the esophageal sphincter mechanism that allows gastric contents to return into the esophagus. Thus, GER may place an anesthetized patient at risk for aspiration. The clinical spectrum of GER can range from patients who are completely asymptomatic to patients with severe esophagitis, esophageal bleeding, esophageal stricture, malnutrition, and respiratory compromise. In the pediatric population, GER can be physiologic, secondary to immature maturation of the lower esophageal sphincter mechanism; however, this aspect of GER generally resolves by 15 months of age. GER is also seen in children who are neurologically compromised, as well as in patients who have survived diaphragmatic hernias, tracheoesophageal fistula, and esophageal atresia repairs (Barry and Auldist, 1974). GER has also been noted in about 10% of patients who have undergone successful treatment of pyloric stenosis.
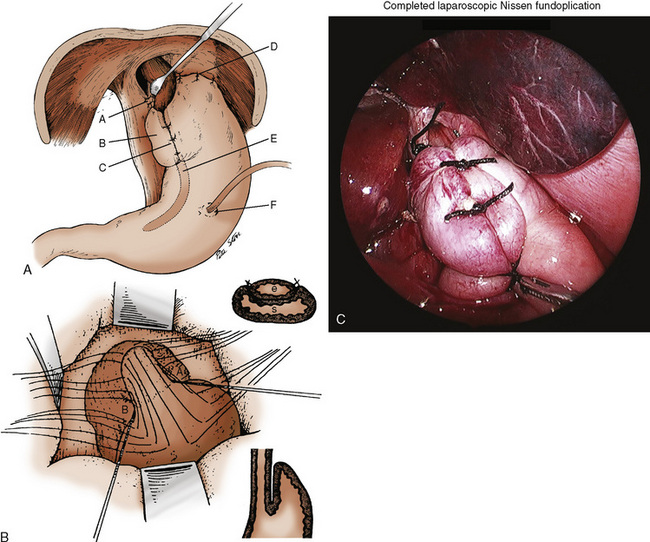
Surgical procedures are aimed at establishing an intraabdominal segment of esophagus and creating a physiologic angle of His (Fig. 23-7). The two common procedures are the 360-degree fundoplication of Nissen and the partial wrap of Thal-Nissen. To avoid the gas bloat syndrome (aerophagia, gastric distention, inability to belch or vomit) associated with Nissen fundoplications, the Thal-Nissen partial wrap is frequently used.
 The surgical procedure can be performed as either an open or a laparoscopic procedure (Randolph, 1983; Georgeson, 1993; Rothenberg, 1998; Bourne et al., 2003; Esposito et al., 2003; Steyaert et al., 2003). See related video online at www.expertconsult.com. The failure rates of the surgical approaches appear to be similar (Lopez et al., 2008), and laparoscopic fundoplications can be performed in children who have had previous open procedures (Barsness et al., 2009). In children undergoing laparoscopic antireflux procedures, the physiologic perturbations of pneumoperitoneum, increased intraabdominal pressure, and the associated absorption of carbon dioxide need to be considered.
The surgical procedure can be performed as either an open or a laparoscopic procedure (Randolph, 1983; Georgeson, 1993; Rothenberg, 1998; Bourne et al., 2003; Esposito et al., 2003; Steyaert et al., 2003). See related video online at www.expertconsult.com. The failure rates of the surgical approaches appear to be similar (Lopez et al., 2008), and laparoscopic fundoplications can be performed in children who have had previous open procedures (Barsness et al., 2009). In children undergoing laparoscopic antireflux procedures, the physiologic perturbations of pneumoperitoneum, increased intraabdominal pressure, and the associated absorption of carbon dioxide need to be considered.
Surgical success rates approach 95% in pediatric patients with normal neurologic development, but in children who are neurologically impaired, morbidity and mortality remain high. It is important to determine if the underlying symptoms of these patients result from GER as opposed to nasopharyngeal incoordination or esophageal or antral dysmotility; (Martinez et al., 1992; Smith et al., 1992). The presence of GER places the patient at risk for aspiration during induction of anesthesia. Preoperative preparation with H2-blockers and motility drugs should be continued. A rapid-sequence induction should be used if a difficult airway is not anticipated. At least one large-bore IV catheter should be placed, although fluid and blood losses are minimal. However, pneumothorax, lacerated spleen, puncture, or compression of the vena cava or aorta, and lacerated hepatic veins can occur.
Other anesthesia concerns in patients with GER are the degree of neurologic and respiratory compromise of the patient. Because these children frequently have seizure disorders, preoperative concern should be directed at proper anticonvulsant therapy. Oral anticonvulsants generally cannot be administered for 48 to 72 hours in the postoperative period. Consequently, patients requiring carbamazepine and valproic acid need alternative medicines so that breakthrough seizures do not occur. In addition, a significant number of patients with neurocompromise may also have ventricular-peritoneal shunts. However, the Nissen fundoplication does not appear to increase the risk for shunt infection to more than the risk of placing a shunt (Bui et al., 2007).
For children with severe respiratory compromise or neuromuscular disease, postoperative ventilatory support may be necessary. In children without significant preoperative pulmonary compromise, extubation may be delayed after surgery and supplemental oxygen given as needed. After surgery, the use of antireflux medication decreased. Within 1 year of surgery, 75% were reported on antireflux medication (Lee et al., 2008).
Surgery For Biliary Atresia
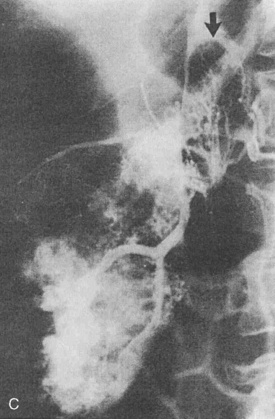
Biliary atresia, characterized by a lack of gross patency of the extrahepatic bile duct, occurs in 1:15,000 live births (Shim et al., 1974). In 10% to 15% of patients, other abnormalities associated with embryologic development are seen, including absent inferior vena cava, intestinal malrotation, polysplenia, and preduodenal portal vein (Lilly and Chandra, 1974). Although biliary atresia is often considered a congenital lesion, it has dynamic properties as well. In microscopic studies of the biliary anatomy obtained from patients at 2 and 4 months of age, the histologic results suggest that biliary structures gradually disappear and are replaced by fibrous tissue. In addition, the success rate for the palliative surgical procedure has been reported as 50% in infants operated on before 4 months of age and 80% in those undergoing surgery before 2 months of age (Ohi et al., 1985). In a study by Serinet and colleagues (2009), among 695 patients who underwent the Kasai procedure, the 2-, 5-, 10-, and 15-year survival rates were 57.1, 32.9, 32.4, and 28.5%, respectively. Increased age at the time of Kasai procedure has been associated with decreased survival rates (Serinet et al., 2009).
Kasai and coworkers (1989), in a review of 245 patients undergoing corrective procedures over a 35-year period, noted that 10-year survival was 74% in infants operated on before 60 days of life. However, Tan and colleagues (1994) have questioned whether earlier corrective surgery is associated with ductal patency. In a series of 205 patients, they noted that survival may be more closely related to the severity of intrahepatic biliary cholangiopathy.
In a 27-year review of 81 patients with biliary atresia, Wildhaber and coworkers (2003) noted that direct bilirubin of less than 2.0, the absence of bridging liver fibrosis, and the number of cholangitis episodes were predictive factors in the success of the Kasai portoenterostomy. Popovic and colleagues (2003) noted that cholinesterase levels can be a useful index of liver function (protein synthesis) early after the Kasai procedure and is independent of albumin synthesis.
Clinically, biliary atresia presents in infants from 1 to 6 weeks of age. About 50% are anicteric until the second or third week of life. The diagnosis of biliary atresia is confirmed either by liver biopsy or by exploratory laparotomy. Surgical palliation for biliary atresia involves hepatic portoenterostomy (Kasai procedure) (Fig. 23-8).
Complications of the surgical repair and from the underlying disease state include cholangitis, portal hypertension, and fat-soluble vitamin deficiency (Kasai et al., 1975). For the anesthesiologist, these complications take on greater significance in patients who return to the operating room for further surgical revision of biliary drainage, treatment of intraabdominal sepsis, or relief of an intestinal obstruction. Because these complications occur frequently and because end-stage liver disease can follow the Kasai procedure, the role of liver transplantation as a primary treatment of biliary atresia has been raised. Kasai and coworkers (1989) suggested that liver transplantation as a primary form of treatment may be indicated for patients older than 3 months with an enlarged, hard liver. Laurent and colleagues (1990) noted that although Kasai’s operation does improve the prognosis of biliary atresia, it is not a definitive cure, and 80% of these patients become candidates for liver transplantation.
Anesthetic management for a Kasai procedure (Kasai, 1974) in patients with biliary atresia follows the basic principles of pediatric anesthesia. In infants in whom venous access is already present, induction is achieved with a hypnotic agent, such as propofol (2 to 3 mg/kg), and a muscle relaxant (cisatracurium, 0.2 mg/kg). In infants without an IV catheter in place, inhalation induction is performed with oxygen, nitrous oxide, and sevoflurane. Once the child is adequately anesthetized, an IV catheter is inserted and a muscle relaxant is administered to facilitate endotracheal intubation and to decrease the concentration of potent inhalation anesthetic. After induction, anesthesia is maintained with an oxygen-air-isoflurane mixture along with IV opioids. Because of bowel distention, nitrous oxide is avoided.
Gelman and coworkers (1984) have shown that hepatic blood flow and oxygen supply are better maintained during isoflurane than during halothane anesthesia. Consequently, isoflurane in an oxygen and air mixture is most commonly administered to patients undergoing surgery for biliary atresia.
Prevention of hypothermia is a major concern for the anesthesiologist. The large surface-to-volume ratio of infants, their relative lack of insulation tissue, coupled with the cold operating room, exposure of body cavities to low environmental temperatures, infusions of cold fluid, and ventilation with dry gases, all increase the potential for hypothermia during surgery. Consequently, great effort must be applied both before and during surgery to protect against heat loss. Methods of preventing heat loss are discussed in Chapter 6, Thermoregulation: Physiology and Perioperative Disturbances.
In infants with biliary atresia undergoing the Kasai procedure, if major fluid shifts have not occurred, blood loss has been minimal, and the patient has remained warm, all efforts are made to reverse the muscle relaxation and extubate the trachea at the end of the procedure. In children with other organ system failures (specifically sepsis, cholangitis, or pneumonia), those who are cold at the end of the procedure (<35° C), or those who have undergone transfusion of more than one blood volume, extubation is delayed until warmth and hemodynamic stability are restored. In these children, postoperative recovery and monitoring are carried out in an intensive care setting. The long-term (5-, 10-, and 20-year) survival rates for 80 children after a Kasai procedure, without transplantation, were 63%, 54%, and 44%, respectively. By age 20, almost half had cirrhosis and its sequelae (Shinkai et al., 2009).
Liver Tumor Procedures
Liver tumors in children are uncommon, but 72% of pediatric primary hepatic tumors and 1.1% of all childhood tumors are malignant. Of these malignant tumors, hepatoblastoma and hepatocarcinoma are the predominant tissue types (Table 23-3). Hepatoblastoma commonly affects white boys less than 2 years of age. An abdominal mass that has increased in size is usually the presenting symptom. Anemia, jaundice, and ascites are infrequent findings. Liver function test results are frequently normal. From 2% to 3% of patients may have an associated hemihypertrophy (Geiser et al., 1970). Hepatoblastoma has also been associated with isosexual precocity as a result of the liver’s ectopic gonadotropic production and the Beckwith-Wiedemann syndrome (Sotelo-Avita et al., 1976), polyposis coli, Wilms tumor, and fetal alcohol syndrome. An increased incidence of hepatoblastoma has been associated with low birth weight (Ikeda et al 1997; Feusner et al., 1998) (Box 23-4). Between 1973 and 1997, the rate of hepatoblastoma increased. This is in contrast to the decreased incidence observed for hepatocarcinoma (Darbari et al., 2003).
| Tumor Type | Tumor | Cases Reported |
| Hepatomas | Hepatoblastoma | 129 |
| Hepatocellular carcinoma | 98 | |
| Other | Mixed mesenchymal tumor | 9 |
| Rhabdomyosarcoma | 6 | |
| Angiosarcoma | 4 | |
| Undifferentiated sarcoma | 3 | |
| Teratocarcinoma | 1 | |
| Cholangiosarcoma | 1 | |
| Malignant histiocytoma | 1 |
From Exelby PR, Filler RM, Grosfeld JL: Liver tumors in children in the particular reference to hepatoblastoma and hepatocellular carcinoma: American Academy of Pediatrics Surgical Section Survey—1974, J Pediatr Surg 10:329, 1975.
Box 23-4 Clinical Antecedents of Hepatoblastoma and Hepatocellular Carcinoma in Childhood
From Finegold MJ, Egler RA, Goss JA, et al: Liver tumors: pediatric population, Liver Transpl 14:1545, 2008.
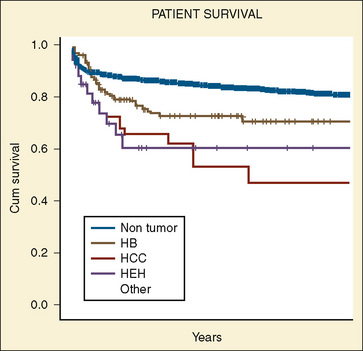
Hepatoblastomas are derived from primitive epithelial parenchyma and are classified by the predominant epithelial component. Among the variants are fetal, embryonal, macrotrabecular, and anaplastic. Survival is related to the histology and completeness of the surgical resection (Table 23-4). Fetal histology has a greater than 90% survival rate, compared with a 50% survival rate for embryonal histology. A few survivors have anaplastic histology. Patients with resectable stages I and II hepatoblastomas have better survival (95%) than patients with unresectable stage III (69%) or metastatic disease (37%). For patients with resectable disease, a 5-year survival rate is 83%, as opposed to 41% for those who had residual tumor after surgery (Finegold et al., 2008) (see Box 23-4). Complete surgical resection offers the only chance for cure in patients with hepatocellular carcinoma, because the tumor is chemoresistant. In patients requiring a liver transplant, the 1-, 5-, and 10-year survival rates are 86%, 67%, and 58%, respectively (Fig. 23-9).
TABLE 23-4 Children’s Oncology Group Staging for Hepatoblastoma
| Stage | Comments |
| Stage I | Complete resection Favorable histology |
| Purely fetal histology with a low mitotic index | |
| Stage II | Gross total resection with microscopic residuals or with preoperative or intra- operative rupture |
| State III | Unresectable tumors as determined by the attending surgeon partially resected tumors with macroscopic residual, or any tumor with lymph node involvement |
| Stage IV | Measurable metastatic disease to lungs or other organs |
From Finegold MJ, Egler RA, Goss JA, et al: Liver tumors: pediatric population, Liver Transpl 14:1545, 2008.
Liver transplantation has been used as both a primary surgical therapy and a rescue for incomplete resection or recurrence of disease. Patients who were rescued had a 30% disease-free survival compared with 82% in the primary treatment group (Otte et al., 2004).
Hepatocellular carcinoma appears to have two age peaks: one at less than 4 years of age and the other between 12 and 15 years of age (Leventhal, 1987). As in adults with hepatocellular carcinoma, the prognosis of survival in children with the disease is 5% to 10%. Typically, patients with hepatocellular carcinoma have the systemic symptoms of weight loss, jaundice, fever, and lethargy. As opposed to adults with this tumor, only about 5% of children have associated cirrhosis (Jones, 1960). Other associated diseases include von Gierke’s disease, type I glycogenesis, cystinosis, extrahepatic biliary atresia, α1-antitrypsin deficiency, hypoplasia of intrahepatic bile ducts, Wilson’s disease, giant cell hepatitis, and Solo’s syndrome (Zangeneh et al., 1969; Palmer and Wolfe, 1976; Weinberg et al., 1976; Dehner, 1978) (see Box 23-4). Complete surgical resection offers the only chance for a cure for patients with hepatocellular carcinoma, because the tumor is chemoresistant. In patients requiring a liver transplant, the 1-, 5-, and 10-year patient survival rates are 86%, 67%, and 58%, respectively (see Fig. 23-9).
Anesthetic management for pediatric patients undergoing hepatic lobe resection or tumor resection involves the same principles as for patients with biliary atresia. Efforts at maintaining adequate alveolar ventilation, temperature homeostasis, cardiovascular stability, and fluid management have been described. Some patients receive adjunct chemotherapy before surgery. The chemotherapeutic protocol must be reviewed. Frequently, Adriamycin, an anthracycline, is a major component of hepatic cancer chemotherapy and has been associated with a dosage-dependent irreversible cardiomyopathy. All patients receiving anthracycline chemotherapy should be studied by history, physical examination, electrocardiography, chest radiography, and echocardiography to further evaluate signs and symptoms of cardiac toxicity and cardiac reserve (see Chapters 4 and 36, Cardiovascular Physiology and Systemic Disorders).
Hirschsprung’s Disease Procedures
The basic pathology underlying congenital megacolon, or Hirschsprung’s disease, is aganglionosis, or total absence of ganglion cells, in the intrinsic nerve supply of the bowel. The aganglionic area extends proximally from the anal sphincter and involves varying lengths of colon. The normal nerve supply, consisting of Auerbach’s plexuses and Meissner’s plexuses, which together form the myenteric nerve complex of the bowel, usually becomes an increasingly diffuse network in the descending and terminal portions of the bowel. Absence of the ganglion cells, which occurs in approximately 1 in 10,000 infants, causes a condition resembling spasm in the area without ganglion cells, and the normal bowel proximal to the spastic portion undergoes tremendous distention, with retention of feces and intestinal obstruction or, in less serious cases, prolonged bouts of constipation. Although the cause of Hirschsprung’s disease is unknown, defects in nonadrenergic, noncholinergic innervations may prevent relaxation of the aganglionic segment. Bealer and colleagues (1994) demonstrated that nitric oxide synthetase is deficient in the aganglionic colon of patients with Hirschsprung’s disease, and that this deficiency may prevent smooth muscle relaxation of the aganglionic segment. On a molecular level, mutations in the RET protooncogene have been found (Sancandi et al., 2000).
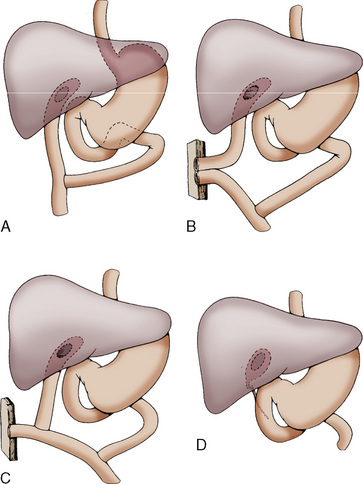
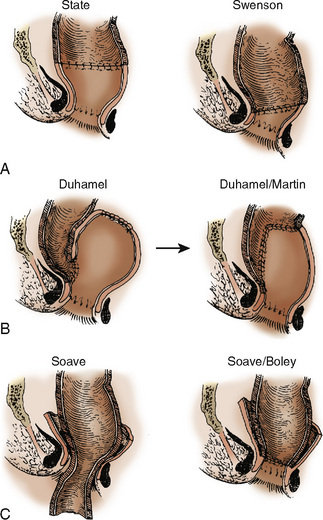
In 10% to 20% of patients with Hirschsprung’s disease, it is clinically present at birth. Symptomatic infants have delayed passage of meconium, irritability, failure to thrive, and abdominal distention. Older children may present with constipation, fecal soiling, and diarrhea. The major complication from Hirschsprung’s disease is acute enterocolitis, a potentially life-threatening event. Diagnosis of Hirschsprung’s disease is made by radiographic examination and confirmed by suction biopsy specimens from the rectum. Surgery is usually a two-stage procedure, with the initial procedure being a colostomy. The definitive surgical procedure is generally performed when the child is older (>1 year). The surgical approaches are varied and over time have undergone modifications (Fig. 23-10). Attention has focused on the use of a one-stage transanal endorectal pull-through approach (Elhalaby et al., 2004). Each surgical technique has its own associated intraoperative and perioperative complications, but enterocolitis, wound dehiscence, anastomotic leakage, intestinal obstruction, and fecal soiling are common to all. All of these surgical procedures aim either to excise or to bypass the aganglionic portion of the bowel and to free and advance the remaining normal portion of bowel toward the rectum. These procedures are often long (6 to 8 hours) and involve surgical explorations through the perineum and abdomen.
Laparoscopic surgery has also been used in the treatment of Hirschsprung’s disease (Jona et al., 1998; Wulkan and Georgeson, 1998; Georgeson et al., 1999). The minimally invasive assisted pull-through technique is generally used for patients when the aganglionic segment is confined to the rectum sigmoid or proximal left colon (Georgeson, 2002). Anesthetic concerns for patients with Hirschsprung’s disease are similar to those for any child having surgery. Maintaining body temperature and providing appropriate fluid therapy (for replacement of large third-space losses) are the major challenges for the anesthesiologist.
Anesthesia induction can be either by inhalation or IV means. Because of the surgical bowel manipulation and the relatively obstructive nature of the underlying disease, nitrous oxide is discontinued after induction, and anesthesia is maintained with a mixture of air, oxygen, and potent inhalation agent. Long-term follow-up of patients with Hirschsprung’s disease suggests that 25% of patients will require reoperation, and that 19% to 25% of patients will develop enterocolitis (Fortuna et al., 1996).
Surgery For Appendicitis
Acute appendicitis is a common condition in children. Although the mortality from acute appendicitis is rare (case-fatality ratio, 0.3%) (Addiss et al., 1990), morbidity (peritonitis, abscess formation, wound infection) is related to the state of the appendix at the time of the operation. The highest incidence of appendicitis is found in patients aged 10 to 19 years. The incidence of perforation of the appendix in children appears to be about 30% to 45%, but in preschool children and infants it can be as high as 80%. The incidence of appendicitis appears to be affected by race and gender. The incidence of appendicitis is 1.4 to 1.6 times greater in whites than nonwhites, whereas the perforation rate for nonwhites is 22% compared with 18% for whites (Addiss et al., 1990).
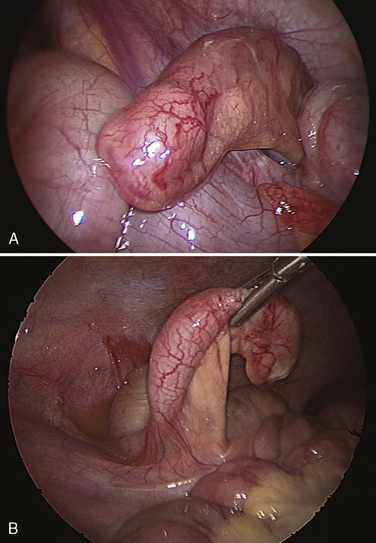
The pathophysiology of appendicitis is thought to be related to an obstruction of the lumen of the appendix, with subsequent bacterial overgrowth and distention of the appendix (Fig. 23-11). In untreated cases, distention and overgrowth lead to gangrene and rupture. Although there is some urgency in making the diagnosis and surgically removing the appendix, the operation is never so urgent that a proper review of the patient’s medical history and physical assessment cannot be performed.
Once the child has been adequately volume-resuscitated and a normal airway is anticipated, an IV rapid-sequence induction with cricoid pressure is performed. Anesthetic technique (inhalation agents versus opioid-based techniques) may depend on the surgical technique used to remove the appendix. Although frequently the appendix is removed by laparotomy, the role of laparoscopy (especially in female patients) in both diagnosis and management is increasing (Gilchrist et al., 1992; Kuster and Gilroy, 1992; Olsen et al., 1993). See related video online at www.expertconsult.com In a study by  McAnena and coworkers (1992), the median postoperative hospitalization stay was one half and the rate of wound infection one third in patients undergoing laparoscopic appendectomy compared with patients undergoing open appendectomy. In a study involving 30 pediatric hospitals, Newman and colleagues (2003) noted significant variability in practice and resource utilization among the institutions. In addition, the length of stay did not differ between those patients who underwent an open or a laparoscopic appendectomy.
McAnena and coworkers (1992), the median postoperative hospitalization stay was one half and the rate of wound infection one third in patients undergoing laparoscopic appendectomy compared with patients undergoing open appendectomy. In a study involving 30 pediatric hospitals, Newman and colleagues (2003) noted significant variability in practice and resource utilization among the institutions. In addition, the length of stay did not differ between those patients who underwent an open or a laparoscopic appendectomy.
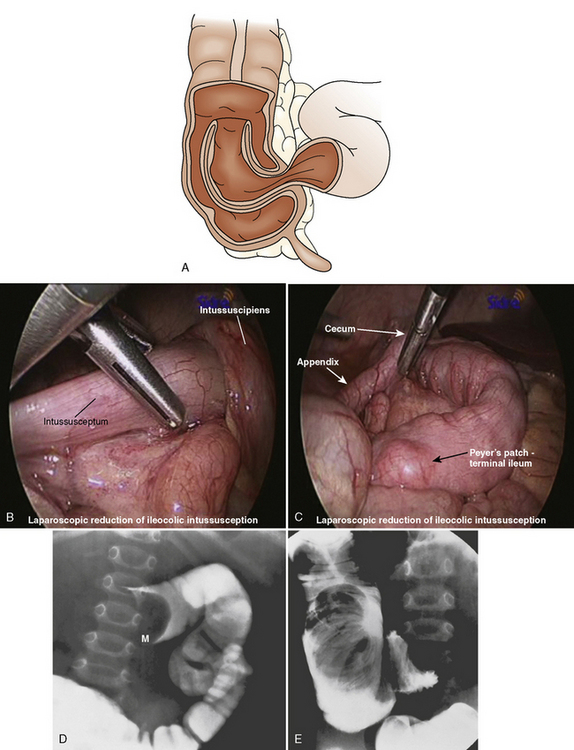
Intussusception Repair
Intussusception is the invagination or telescoping of one portion of the intestine into another (Fig. 23-12). It tends to occur more frequently in males than females. Over 50% of cases occur in children less than 1 year of age, and less than 10% of cases occur in children older than 5 years. Ninety percent of cases have idiopathic causes—frequently seen in children less than 1 year of age. Older children are more likely to have Meckel’s diverticulum, intestinal polyps, lymphoma, adhesions, trauma, hemolytic uremic syndrome, or ectopic pancreatic nodule as an etiology. Intussusception has also been reported postoperatively and after blunt abdominal trauma (Komadina and Smrkolj, 1998; Linke et al., 1998). In addition, an association between rotavirus vaccine and intussusception has been reported (Zanardi et al., 2001).
The clinical presentation of acute intussusception involves sudden paroxysms of abdominal pain, bloody stools, and an abdominal mass, although in one series of patients, 18% of the children had painless intussusception (Hutchison et al., 1980). In a review of 14 published reports, Losek (1993) noted that bloody stools were present in 42% of patients. Occult blood was noted in 43% and abdominal masses were present in 62% of patients. Intussusception can also manifest with neurologic findings (lethargy, apnea, seizures, hypotonia, opisthotonus) similar to a picture of septic encephalopathy (Conway, 1993). Other symptoms and signs may include diarrhea, vomiting, fever, and dehydration. In other children, intussusception can be a chronic entity that may mimic gastroenteritis (Shekhawat et al., 1992), whereas in neonates, intussusception may mimic necrotizing enterocolitis (Price et al., 1993).
About 90% of intussusceptions are ileocolic, and the remainder are ileoileal and colocolic. Treatment for intussusception involves the administration of appropriate fluids to combat dehydration, and radiologic or surgical attempts to reduce the invaginated bowel. Hydrostatic enemas with barium or air have been reported to be successful in 80% of patients. However, enemas are contraindicated in patients with evidence of peritonitis, shock, and intestinal perforation. Surgical laparotomy with manual reduction or resection (or both) as well as laparoscopic approaches have been described for surgical management in patients with unsuccessful radiologic reduction and in patients with signs of intestinal perforation, peritonitis, and shock (Hay et al., 1999).
General thoracic surgery
Thoracic surgery in children is performed for a wide variety of congenital, neoplastic, infectious, and traumatic lesions (Box 23-5). The patient may be a few hours old with a congenital cystic adenomatoid malformation (CCAM) and life-threatening respiratory distress or an adolescent with an asymptomatic mediastinal tumor. Regardless of age or disease, four principles are common to all patients undergoing general anesthesia for thoracic surgery, as follows:
A thorough preoperative evaluation is essential in caring for the pediatric patient scheduled for thoracic surgery. Appropriate imaging and laboratory studies should be performed preoperatively according to the lesion involved. Guidelines for fasting, choice of premedication, and preparation of the operating room are used as for other infants and children scheduled for major surgery. After induction of anesthesia, placement of an IV catheter, and tracheal intubation, arterial catheterization should be performed for most patients undergoing thoracotomy as well as for those with severe lung disease having thoracoscopic surgery. This facilitates monitoring of arterial blood pressure during manipulation of the lungs and mediastinum, as well as monitoring of arterial blood gas tensions during single lung ventilation (SLV). For thoracoscopic procedures of relatively short duration in patients without severe lung disease, the insertion of an arterial catheter is not required (Rao et al., 1981). Placement of a central venous catheter is generally not indicated if peripheral IV access is adequate for projected fluid and blood administration.
Inhaled anesthetic agents are commonly administered in 100% O2 during maintenance of anesthesia. Isoflurane may be preferred because there is less attenuation of hypoxic pulmonary vasoconstriction (HPV) than with other inhaled agents, although this has not been studied in children (Benumof et al., 1987). Nitrous oxide is avoided. Use of IV opioids may facilitate a decrease in the concentration of inhaled anesthetics used and thereby limit impairment of hypoxic pulmonary vasoconstriction. Alternatively, total IV anesthesia may be used with a variety of agents. The combination of general anesthesia with regional anesthesia and postoperative analgesia is particularly desirable for thoracotomy, but it may also be beneficial for thoracoscopic procedures, especially when thoracostomy tube drainage, a source of significant postoperative pain, is used after surgery. A variety of regional anesthetic techniques have been described for intraoperative anesthesia and postoperative analgesia, including intercostal and paravertebral blocks, intrapleural infusions, and epidural anesthesia (see Chapter 16, Regional Anesthesia).
In awake patients, except for young infants, ventilation is normally distributed preferentially to dependent regions of the lung, so that there is a gradient of increasing ventilation from the most nondependent to the most dependent lung segments. Because of gravitational effects, perfusion normally follows a similar distribution, with increased blood flow to dependent lung segments; therefore, ventilation and perfusion are normally well matched. However, controlled ventilation under general anesthesia with decreased functional residual capacity and absent diaphragmatic contractions results in a reverse distribution of ventilation (see Chapter 3, Respiratory Physiology in Infants and Children). During thoracic surgery, these and other factors act to increase ventilation/perfusion (V/Q) mismatch. Compression of the dependent lung in the lateral decubitus position may cause atelectasis. Surgical retraction, SLV, or both result in collapse of the operative lung. Hypoxic pulmonary vasoconstriction (HPV), which acts to divert blood flow away from underventilated lung regions, thereby minimizing V/Q mismatch, may be diminished by the use of inhaled anesthetic agents and other vasodilating drugs. These factors apply similarly to infants, children, and adults. The overall effect of the lateral decubitus position on V/Q mismatch, however, differs in infants compared with older children and adults.
In adults with unilateral lung disease, oxygenation is optimal when the patient is placed in the lateral decubitus position with the healthy lung dependent (down) and the diseased lung nondependent (up) (Bachland et al., 1975; Remolina et al., 1981). Presumably, this is related to an increase in blood flow to the dependent, healthy lung and a decrease in blood flow to the nondependent, diseased lung as a result of the hydrostatic pressure (i.e., gravitational) gradient between the two lungs. This phenomenon promotes V/Q matching in the adult patient undergoing thoracic surgery in the lateral decubitus position.
In infants with unilateral lung disease, however, oxygenation is better with the healthy lung up (Heaf et al., 1983). Several factors account for this discrepancy between adults and infants. Infants have a soft, easily compressible rib cage that cannot fully support the underlying lung. Functional residual capacity, especially in the lower lung, is closer to or at residual volume, making airway closure likely to occur in the dependent lung even during tidal breathing (Mansell et al., 1972).
Finally, the infant’s increased oxygen requirement, coupled with a small functional residual capacity, predisposes to hypoxemia. Infants normally consume 6 to 8 mL of O2/kg per minute, compared with a normal O2 consumption in adults of 2 to 3 mL/kg per minute (Dawes, 1973). For these reasons, infants are at increased risk for significant oxygen desaturation during surgery in the lateral decubitus position.
Thoracoscopy
During the past decade, the use of video-assisted thoracoscopic surgery has dramatically increased in both adults and children (see Video Endoscopy, p. 745). As with laparoscopy, reported advantages of thoracoscopy include smaller chest incisions, reduced postoperative pain, and more rapid postoperative recovery compared with thoracotomy (Weatherford et al., 1995; Angelillo Mackinlay et al., 1996 Mouroux et al., 1997).
Thoracoscopic surgery is being used extensively for pleural débridement in patients with empyema, lung biopsy, and wedge resections for interstitial lung disease, mediastinal masses, and metastatic lesions. More extensive pulmonary resections, including segmentectomy and lobectomy, have been performed for lung abscess, bullous disease, sequestrations, lobar emphysema, CCAM, and neoplasms. Thoracoscopic procedures used in infants and children are listed in Box 23-6.
Thoracoscopy can be performed while both lungs are being ventilated using CO2 insufflation and a retractor to displace lung tissue in the operative field. However, SLV is extremely desirable during thoracoscopy, because lung deflation improves visualization of thoracic contents and may reduce lung injury caused by the retractors (Benumof, 1995).
Surgery for Chest Wall Deformities
Pectus excavatum (funnel chest) (Fig. 23-13) and the less common pectus carinatum (pigeon breast) deformities are congenital abnormalities of the sternum, ribs, and costal cartilages. These deformities are usually minimal at birth but progress with age. A higher incidence of both deformities occurs in children with Marfan syndrome or congenital heart disease, and in families in which other children have the defect (Shamberger and Welch, 1987; Robicsek and Lobato, 2000). These children often appear asymptomatic but occasionally have cardiac or pulmonary abnormalities related to the deformity (Malek et al., 2003). Patients with pectus excavatum generally present with normal or modestly reduced forced vital capacity and total lung capacity and, in severe cases, V/Q mismatch. The heart is displaced to the left and compressed, lending to arrhythmias, right-axis deviation on electrocardiogram, a functional murmur, and reduced stroke volume—most noticeable in the standing position and during exercise, explaining the mild exercise intolerance experienced by some patients. The cardiac and pulmonary abnormalities are in most instances benign and may worsen as the child ages but may be improved by surgical repair. There also is an increased incidence of mitral valve prolapse in patients with pectus deformities (Shamberger et al., 1987).
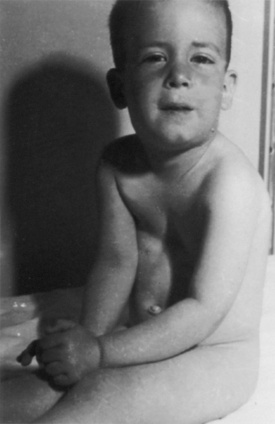
FIGURE 23-13 Pectus excavatum deformity becomes most obvious when the child is in the sitting position.
Classic operative repair involves extrapleural excision of the sternocostal cartilages and mobilization of the sternum and ribs. The most common complications of operative repair are pneumothorax, flail chest, and postoperative atelectasis; blood loss is usually minimal to moderate. Intraoperative monitoring includes temperature, blood pressure, pulse, heart and breath sounds, airway pressure, and oxygen saturation or tension. Capnography is also useful, but arterial catheterization is needed only if there is a specific indication. General anesthesia with controlled ventilation is the method of choice, with no agents specifically indicated or contraindicated because of the operation itself. Oxygen by face mask is administered in the recovery room, but it is usually not needed after the child fully awakens. Although patient-controlled analgesia is commonly used for postoperative analgesia, both intercostal nerve blocks and thoracic epidural analgesia have become increasingly popular for children undergoing pectus repair (Robicsek, 2000).
A thoracic epidural catheter provides more reliable analgesia to the operative area than a lumbar epidural that has been threaded a great distance. However, thoracic epidural catheters are not as easy to insert as lumbar catheters, and many practitioners are not comfortable with their routine use. Although a technique using electrocardiographic guidance and insertion from the caudal space has been described, it is not widely used (Tsui et al., 2002). An additional issue with the thoracic catheters is the safety of their insertion under general anesthesia (Horlocker, 2003). Although some children allow insertion before induction (McBride et al., 1996), many younger children are not likely to remain cooperative for the procedure, mandating insertion after induction (Hammer, 2002; Birmingham et al., 2003). Moreover, several centers have actively and successfully used thoracic epidural techniques in anesthetized children for thoracic and cardiac procedures without complications related to insertion after induction (Cassady, 2000; Birmingham, 2003). Solutions of both bupivacaine with fentanyl and fentanyl alone have been used successfully, including in the patient-controlled mode for appropriately mature children (Caudle et al., 1993; Birmingham et al., 2003) (see Chapter 16, Regional Anesthesia).
Another approach is to use a minimally invasive technique in which the costal cartilages are preserved and the sternum is elevated with a bar. Under direct vision and through a thorascope, a transmediastinal tunnel is created and a prebent bar is passed behind the sternum with the convex side down. The bar is then rotated 180 degrees to elevate the sternum (Fig. 23-14) (Nuss et al., 1998; Nuss, 2002). Borowitz and coworkers (2003) have shown that static pulmonary function and ventilatory response to exercise was normal both before and after surgery, thereby suggesting that placement of the bar does not result in an increased chest wall restriction. In addition, Lawson and colleagues (2003) noted that the surgical repair of the pectus excavatum after the Nuss procedure had a positive impact on the patient’s physical and emotional well-being. Complications of this minimally invasive approach include atelectasis, subcutaneous emphysema, pericardial and pleural effusions, myocardial perforation, diaphragmatic perforation, and dislocation of the stabilizing bar (Willekes et al., 1999; Hebra et al., 2000; Molik et al., 2001; Moss, 2001; Hosie et al., 2002; Uemura et al., 2003). Postoperative pain after the Nuss procedure is significant. Thoracic epidural analgesia for 2 to 3 days, followed by oral opioid and nonsteroidal antiinflammatory drug therapy, is appropriate.
Thoracotomy, Lobectomy, and Pneumonectomy
Surgical Lesion
If a space-occupying lesion is present, the patient is examined for signs of decreased cardiac output, diminished lung volume and reserve, and airway compression (Keon, 1981). History focuses not only on general exercise tolerance but also on signs of intermittent airway obstruction (stridor, cyanosis, or wheezing). Physical examination includes checking for a shift in the trachea, asymmetric chest movement, wheezing, and any signs of respiratory distress. Laboratory assessment should include a chest radiograph, but additional studies such as tomography, angiography, or computed tomography often provide more exact data about vascular or airway compression and compromise. It is crucial to determine the extent of airway compression and physiologic compromise, because impairment may worsen with induction of anesthesia as sympathetic and muscular tones are reduced.
If the intrathoracic lesion is a primary or metastatic tumor, the history concentrates on previous treatment (Baldeyrou et al., 1984). Previous treatment for the tumor, especially chemotherapy and radiation, is important to know about. Special attention is given to anthracycline (cardiac toxicity), bleomycin (pulmonary toxicity), and steroid (adrenal suppression) therapy. If there is any question about functional disability caused by this treatment, consultation with the child’s oncologist is useful. Anemia, thrombocytopenia, and malnutrition are common in these patients and should be improved before surgery (Beattie, 1984). A special consideration is the immunocompromised patient with an unknown pulmonary infiltrate. This is usually assumed to be an opportunistic infection, but because it may represent metastasis, a biopsy is occasionally requested. These patients are often in poor general condition and may require postoperative ventilatory support, especially if they had only marginal compensation before surgery (Imoke et al., 1983; Prober et al., 1984).
Preparation
Preparation for surgery starts with a discussion of the proposed anesthetic with the parents and, if appropriate, with the child. The anesthetic plan, including monitoring, possible complications, and potential for postoperative ventilation, is discussed. It is best to delay surgery until any infection or bronchospasm has been brought under optimal medical control with antibiotics, chest physiotherapy, and bronchodilators, as needed (Sutton et al., 1983). It may be difficult or impossible to eradicate infections or bronchospasm completely in destructive lesions such as bronchiectasis. If this is the case, it is acceptable to proceed after reasonable medical therapy has optimized the patient’s status so that no further improvement is anticipated.
Monitoring
At a minimum, thoracotomy requires monitoring of inspired oxygen, blood pressure, heart and breath sounds, airway pressure, and temperature, as well as an electrocardiogram. Oxygen saturation by pulse oximeter or, less commonly, by transcutaneous oxygen tension (Po2) monitor (Harnick et al., 1983) is vital for detection of sudden changes in oxygenation from lung compression or kinking of the airway. Capnography is particularly useful for detecting sudden changes in effective ventilation. Arterial cannulation for pressure and arterial blood samples is useful and is needed if extensive blood loss or resection of lung tissue is expected or if the child is already critically ill. Percutaneous arterial cannulas (24 gauge in neonates, 22 gauge in children up to 8 to 10 years of age, and 20 gauge in preadolescents and older) can be inserted in children and should be used whenever indicated. Central venous monitoring is used less commonly but can be helpful for guiding extensive volume replacement. Urinary drainage is a consideration for particularly long procedures.
Positioning
Positioning of the patient has often been used to minimize spillage of lung contents because double-lumen tubes are impractical in smaller patients (Conlan et al., 1986). Suction through the ETT may not be adequate to control the large quantities of pus freed during surgical manipulation. The prone and lateral positions are the most commonly used. Positioning can cause significant ventilatory changes in children. Functional residual capacity (FRC) decreases during general anesthesia (Motoyama et al., 1982), and it falls dramatically once the pleura is opened (Larsson et al., 1987). The practical problems of dislodgment of the ETT with movement and adequate padding in these positions are especially important in children. Open-celled foam with adhesive backing (Reston; 3M, St. Paul, MN) can be applied to the thorax, pelvic rim, and other pressure points to minimize the effects of positioning. Also, the tube position must be rechecked each time the patient is moved.
Anesthesia
Single-Lung Ventilation Techniques
Single-Lung Ventilation Using a Single-Lumen Endotracheal Tube
The simplest means of providing SLV is to intentionally intubate the ipsilateral main stem bronchus with a conventional single-lumen ETT (Baraka, 1987; Kubota et al., 1987; Rowe et al., 1994). See related video online at www.expertconsult.com. When the left bronchus is to be intubated, the bevel of the ETT is rotated 180 degrees and the patient’s head is turned to the right (Bloch, 1986; Kubota et al., 1987). The ETT is advanced into the bronchus until breath sounds on the operative side disappear. A fiberoptic bronchoscope (FOB) may be passed through or alongside the ETT to confirm or guide placement (Watson et al., 1982). When a cuffed ETT is used, the distance from the tip of the tube to the distal cuff must be shorter than the length of the bronchus so that the ETT does not occlude the upper lobe bronchus (Lammers et al., 1997) (Fig. 23-15). This technique is simple and requires no special equipment other than a FOB. This may be the preferred technique of SLV in emergency situations such as airway hemorrhage or contralateral tension pneumothorax.
When the left bronchus is to be intubated, the bevel of the ETT is rotated 180 degrees and the patient’s head is turned to the right (Bloch, 1986; Kubota et al., 1987). The ETT is advanced into the bronchus until breath sounds on the operative side disappear. A fiberoptic bronchoscope (FOB) may be passed through or alongside the ETT to confirm or guide placement (Watson et al., 1982). When a cuffed ETT is used, the distance from the tip of the tube to the distal cuff must be shorter than the length of the bronchus so that the ETT does not occlude the upper lobe bronchus (Lammers et al., 1997) (Fig. 23-15). This technique is simple and requires no special equipment other than a FOB. This may be the preferred technique of SLV in emergency situations such as airway hemorrhage or contralateral tension pneumothorax.
Single-Lung Ventilation Using a Balloon-Tipped Bronchial Blocker
A Fogarty embolectomy catheter or an end-hole, balloon-wedge catheter may be used for bronchial blockade to provide SLV (Fig. 23-16) (Ginsberg, 1981; Lin and Hackel, 1994; Hammer et al., 1996; Turner et al., 1997). Placement of a Fogarty catheter is facilitated by bending the tip of its stylet toward the bronchus on the operative side. A FOB is used to reposition the catheter and confirm appropriate placement. When an end-hole catheter is placed outside the ETT, the bronchus on the operative side is initially intubated with an ETT. A guidewire is then advanced into that bronchus through the ETT. The ETT is removed and the blocker is advanced over the guidewire into the bronchus. An ETT is then reinserted into the trachea alongside the blocker catheter. The catheter balloon is positioned in the proximal main stem bronchus under fiberoptic visual guidance. With an inflated blocker balloon, the airway is completely sealed, providing more-predictable lung collapse and better operating conditions than with an ETT in the contralateral bronchus.
A potential problem with this technique is dislodgment of the blocker balloon into the trachea. The inflated balloon then blocks ventilation to both lungs or prevents collapse of the operated lung, or both. The balloons of most catheters currently used for bronchial blockade have low-volume, high-pressure properties, and overdistention can damage or even rupture the airway (Borchardt et al., 1998). One study, however, reported that bronchial blocker cuffs produced lower cuff-to-tracheal pressures than double-lumen tubes (Guyton et al., 1997). When closed-tip bronchial blockers are used, the operative lung cannot be suctioned, and continuous positive airway pressure (CPAP) cannot be provided to the operative lung if needed.
When a bronchial blocker is placed outside the ETT, care must be taken to avoid injury caused by compression and resultant ischemia of the tracheal mucosa. The sum of the catheter diameter and the outer diameter of the ETT should not exceed the tracheal diameter. Diameters for pediatric ETTs are shown in Table 23-5.
| Inner Diameter (mm) | Outer Diameter* (mm) |
| 3.0 | 4.3 |
| 3.5 | 4.9 |
| 4.0 | 5.5 |
| 4.5 | 6.2 |
| 5.0 | 6.8 |
| 5.5 | 7.5 |
| 6.0 | 8.2 |
| 6.5 | 8.9 |
| 7.0 | 9.6 |
* Cuffed tubes add approximately 0.5 mm to the outer diameter.
From Sheridan Tracheal Tubes, Kendall Healthcare, Mansfield, Mass.
Adapters have been used that facilitate ventilation during placement of a bronchial blocker through an indwelling ETT (Arndt et al., 1999; Takahashi et al., 2000). For use in children, a 5-F endobronchial blocker designed with a multiport adapter and FOB has been described (Cook Critical Care, Bloomington, IN) (Hammer, 2001; Hammer et al., 2002). The balloon is elliptical, so it conforms to the bronchial lumen when inflated. The blocker catheter has a maximal outer diameter of 2.5 mm (including the deflated balloon), a central lumen with a diameter of 0.7 mm, and a distal balloon with a capacity of 3 mL. The balloon has a length of 1.0 cm, corresponding to the length of the right main stem bronchus in children approximately 2 years of age (Scammon, 1923). The blocker is placed coaxially through a dedicated port in the adapter, which also has a port for passage of an FOB and ports for connection to the anesthesia breathing circuit and ETT (Fig. 23-17). The FOB port has a plastic sealing cap, whereas the blocker port has a Tuohy-Borst connector, which locks the catheter in place and maintains an airtight seal. Because oxygen can be administered during passage of the blocker and FOB, the risk for hypoxemia during blocker placement is diminished, and repositioning of the blocker may be performed with fiberoptic guidance during surgery (Bird et al., 2007).
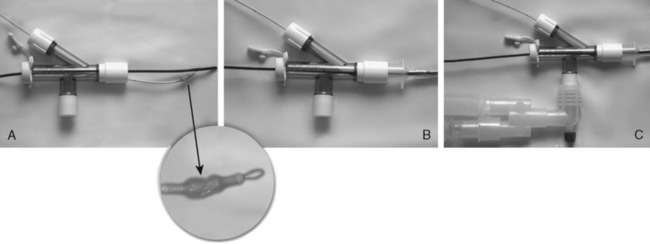
(From Hammer GB: Selective bronchial blockade in small infants, Anesth Analg 93(6):1624–1625, 2001.)
When placement of a bronchial blocker inside the ETT is guided by an FOB, both the blocker catheter and the FOB must pass through the indwelling ETT. The inner diameter of the ETT through which the catheter and FOB are to be placed must be larger than the sum of the outer diameters of the catheter and the FOB. The 5-F blocker catheter and an FOB with a 2.2-mm diameter, for example, may be inserted through an ETT as small as 5.0 mm in inner diameter. For children with an indwelling ETT smaller than 5.0 mm in inner diameter, a blocker catheter can be positioned under fluoroscopy (Fig. 23-18).
Single-Lung Ventilation Using a Univent Tube
The Univent tube (Fuji Systems Corporation, Tokyo, Japan) is a single-lumen ETT with a second lumen containing a small blocker catheter that can be advanced into a bronchus (Fig. 23-19) (Kamaya and Krishna, 1985; Kawande, 1987; Gayes, 1993). A balloon located at the distal end of this small tube serves as a blocker. Univent tubes require a FOB for successful placement. Univent tubes are now available in sizes as small as 3.5- and 4.5-mm inner diameter for use in children older than 6 years (Table 23-6) (Hammer et al., 1998). Because the blocker tube is firmly attached to the main ETT, displacement of the Univent blocker balloon is less likely than when other blocker techniques are used. The blocker of the 4.5-mm Univent tube has a small lumen, which allows egress of gas and can be used to insufflate oxygen or to suction the operated lung.
| Inner Diameter (mm) | Outer Diameter* (mm) |
| 3.5 | 7.5/8.0 |
| 4.5 | 8.5/9.0 |
| 6.0 | 10.0/11.0 |
| 6.5 | 10.5/11.5 |
| 7.0 | 11.0/12.0 |
| 7.5 | 11.5/12.5 |
| 8.0 | 12.0/13.0 |
| 8.5 | 12.5/13.5 |
| 9.0 | 13.0/14.0 |
A disadvantage of the Univent tube is the large amount of cross-sectional area occupied by the blocker channel, especially in the smaller-sized tubes. Smaller Univent tubes have a disproportionately high resistance to gas flow (Slinger and Lesiuk, 1998). The Univent tube’s blocker balloon has low-volume, high-pressure characteristics, so mucosal injury can occur during normal inflation (Benumof et al., 1992; Kelley et al., 1992).
Single-Lung Ventilation Using a Double-Lumen Tube
All double-lumen tubes (DLTs) are essentially two tracheal tubes of unequal length, molded longitudinally together. The shorter tube ends in the trachea, and the longer tube, in the bronchus. Marraro (1994) described a bilumen tube for infants (Pawar and Marraro, 2005). DLTs for older children and adults have cuffs located on the tracheal and bronchial lumens. The tracheal cuff, when inflated, allows positive pressure ventilation. The inflated bronchial cuff allows ventilation to be diverted to either or both lungs and protects each lung from contamination from the contralateral side.
Conventional plastic DLTs, once available only in adult sizes (35-F, 37-F, 39-F, and 41-F), are now available in smaller sizes (Table 23-7). The smallest cuffed DLT is 26-F (Rusch, Duluth, GA) and may be used in children as young as 8 years. DLTs are also available in sizes 28-F and 32-F (Mallinckrodt Medical, St. Louis, MO), suitable for children 10 years of age and older.
DLTs are inserted in children using the same technique used in adults (Brodsky and Mark, 1983). The tip of the tube is inserted just past the vocal cords and the stylet is withdrawn. The DLT is rotated 90 degrees to the appropriate side and then advanced into the bronchus. In the adult population, the depth of insertion is directly related to the height of the patient (Brodsky et al., 1996). No equivalent measurements are yet available in children. If fiberoptic bronchoscopy is to be used to confirm tube placement, an FOB with a small diameter and sufficient length must be available (Slinger, 1989).
A DLT offers the advantage of ease of insertion and the ability to suction and oxygenate the operative lung with CPAP. Left DLTs are preferred to right DLTs because of the shorter length of the right main bronchus (Benumof et al., 1987). Right DLTs are more difficult to accurately position because of the greater risk for right upper lobe obstruction. DLTs are safe and easy to use. There are very few reports of airway damage from DLTs in adults, and none in children. Their high-volume, low-pressure cuffs should not damage the airway if they are not overinflated with air or distended with nitrous oxide while in place. Guidelines for selecting appropriate tubes (or catheters) for SLV in children are shown in Table 23–7. There is significant variability in overall size and airway dimensions among children, particularly among teenagers, and these recommendations are based on average values for airway dimensions. Larger DLTs may be safely used in adult-size teenagers.
Postoperative Care
Tracheal extubation at the completion of surgery is often possible after simple subsegmental resection or lobectomy. However, the patient’s underlying cardiopulmonary reserve, the course of the surgery, and the expected postoperative course may preclude extubation. Although postoperative pain can cause significant splinting, intercostal or epidural blocks, coupled with judicious parenteral opioids, can minimize the discomfort (see Chapters 15 and 16, Pain Management and Regional Anesthesia). Whether in the operating room or in the intensive care area, before extubation the patient must be awake, breathing well, able to cough and maintain an airway, and able to maintain acceptable oxygenation with no more than 40% inspired oxygen. A chest radiograph should be obtained as soon as possible after surgery to detect any significant pneumothorax or atelectasis. Atelectasis is common and usually responds to humidity, encouragement to cough, CPAP, and if necessary, endotracheal suction.
The expected postoperative course depends on both the surgical procedure and the underlying diseases. After simple lobectomy, most children develop normally and have normal exercise tolerance (McBride et al., 1980). Children who have undergone pneumonectomy may have more problems (Buhain and Brody, 1973). With time, overinflation of the remaining lung occurs, with a demonstrable decrease in vital capacity. These children may have significant exercise intolerance for a prolonged period after surgery.