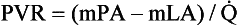Anatomic and Physiologic Aspects of the Pulmonary Vasculature
Before diseases of the pulmonary vasculature are considered in Chapters 13 and 14, this chapter discusses a few of the general anatomic and physiologic aspects of the pulmonary vessels. Included in the discussion on physiology are several topics relating to hemodynamics of the pulmonary circulation, as well as a brief consideration of some nonrespiratory metabolic functions of the pulmonary circulation.
Anatomy
In contrast to the systemic arteries, which carry blood from the left ventricle to the rest of the body, the pulmonary arteries, which carry blood from the right ventricle into the lungs, are relatively low-pressure, thin-walled vessels. Under normal circumstances, the mean pressure within the main pulmonary arteries is roughly one sixth the pressure in the aorta. The pulmonary trunk, which carries the outflow from the right ventricle, divides almost immediately into the right and left main pulmonary arteries, which subsequently divide into smaller branches. Throughout these progressive divisions, the pulmonary arteries and their branches travel with companion airways, following closely the course of the progressively dividing bronchial tree. By the time the vessels are considered arterioles, the outer diameter is less than approximately 0.1 mm. An important feature of the smaller pulmonary arteries is the presence of smooth muscle within the walls that is responsible for the vasoconstrictive response to various stimuli, particularly hypoxia, which allows for matching of perfusion to well-ventilated lung units. (See Chapter 1 for discussion of 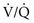 mismatch.)
mismatch.)
The pulmonary capillaries form an extensive network of communicating channels coursing through alveolar walls. Rather than being described as a series of separate vessels, the capillary system has been described as a continuous meshwork or sheet bounded by alveolar walls on each side and interrupted by “posts” of connective tissue, akin to the appearance of an underground parking garage. The capillaries are in close proximity to alveolar gas, separated only by alveolar epithelial cells and a small amount of interstitium present in some regions of the alveolar wall (see Figs. 8-1 and 8-2). Overall, the capillary surface area is approximately 125 m2 and represents approximately 85% of the available alveolar surface area. The design of this capillary system is extraordinarily well suited to the requirements of gas exchange, inasmuch as it contains an enormous effective surface area of contact between pulmonary capillaries and alveolar gas.
Physiology
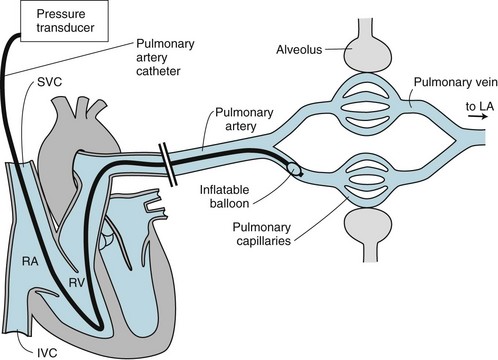
Left atrial pressure is difficult to measure directly. However, a special catheter designed for this purpose, called a pulmonary artery balloon occlusion catheter or Swan-Ganz catheter, has been used widely in clinical application for such pressure measurements (Fig. 12-1). This catheter is inserted into a large vein (usually in the neck or groin) and passed through the right heart into a pulmonary artery. The catheter tip is equipped with a tiny soft balloon that lodges in a segmental pulmonary artery and temporarily blocks flow to the segment. After a short period of equilibration, because there is no blood flow passing the catheter tip, the pressure measured at the tip of the catheter reflects the pressure “downstream” in the pulmonary veins and left atrium.
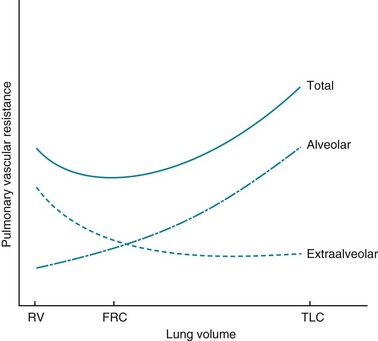
Another factor that affects PVR is lung volume. In discussing the nature of this effect, it is useful to distinguish two categories of pulmonary vessels on the basis of their size and location. One category, called alveolar vessels, includes the capillary network coursing through alveolar walls. When alveoli are expanded and lung volume is raised, these vessels are compressed within the stretched alveolar walls, and their contribution to PVR is increased. In contrast, when alveoli are emptied and lung volume is lowered, the resistance of these alveolar vessels is diminished. The other category consists of the larger vessels called extraalveolar vessels. They are not compressed by air-filled alveoli. The supporting structure that surrounds the walls of these vessels has attachments to alveolar walls, and the elastic recoil of the alveolar walls provides radial traction to keep these vessels open. This concept is similar to the concept discussed in Chapter 6 concerning the effect of alveolar wall attachments on airway diameter (see Fig. 6-6). When lung volume is increased, elastic recoil of the alveolar walls is increased, and the extraalveolar vessels become larger. When lung volume is decreased, the resistance of the extraalveolar vessels increases. This differential effect of lung volume on the resistance of alveolar versus extraalveolar vessels is shown in Figure 12-2. The total PVR is least at the normal resting expiratory position of the lung (i.e., at functional residual capacity).
Distribution of Pulmonary Blood Flow
The relatively low pressures in the pulmonary arteries have important implications regarding the way blood flow is distributed in the lung. When a person is in the upright position, blood going to the upper zones of the lung is flowing against gravity and must be under sufficient pressure in the pulmonary artery to make this antigravitational journey. Because the top of the lung is approximately 15 cm above the level of the main pulmonary arteries, a pressure of 15 cm H2O is required to achieve perfusion of the apices. The mPA of 15 mm Hg (approximately 19 cm H2O) is normally just sufficient to achieve flow to this region. In contrast, flow to the lower lung zones—that is, below the level of the main pulmonary arteries—is assisted by gravity. Therefore, in the upright individual, gravity provides a normal gradient of blood flow from the apex to the base of the lung, with the base receiving substantially greater flow than the apex (see Fig. 1-4). As discussed in Chapter 1, this distribution of blood flow in the lung has major implications regarding the manner in which ventilation and perfusion are matched.
The three-zone model for describing the determinants of pulmonary blood flow discussed in Chapter 1 actually is more complicated now that a zone 4 has been recognized. In this zone, which occupies the base of the lung at low lung volumes, blood flow progressively diminishes as the most dependent region of the lung is approached. To explain why a zone 4 exists, we must return to the concept of extraalveolar pulmonary vessels. At the lung bases, the weight of the lung results in decreased alveolar volume, accompanied by distortion and compression of extraalveolar vessels. As a result, the resistance of the extraalveolar vessels increases considerably, the total vascular resistance in this zone increases, and blood flow diminishes.
The distribution of blood flow in the lung can be measured with radioactive isotopes. A perfusion scan is a particularly useful technique that involves intravenous injection of radiolabeled particles, specifically macroaggregates of albumin, that are of sufficient size to lodge in the pulmonary capillaries. An external counter over the lung records the distribution of lodged particles and hence the distribution of blood flow to the lung. This technique, when performed in the upright individual, not only confirms the expected gradient of blood flow in the lung but also detects regions of decreased or absent perfusion in disease states such as pulmonary embolism (see Chapter 3).
Other Aspects of Pulmonary Vascular Physiology
A variety of other factors that influence pulmonary vascular tone are being increasingly recognized. Autonomic innervation of the pulmonary arterial system is present but not extensive. Sympathetic and parasympathetic stimulation have the expected opposing effects, causing vasoconstriction and vasodilation, respectively. Humoral stimuli altering vascular tone are numerous; examples include histamine and the prostaglandin products of arachidonic acid metabolism. Most recently, interest has focused on two molecules mentioned earlier in the discussion of hypoxic vasoconstriction, each of which is known to have important effects on the pulmonary vasculature: nitric oxide (a potent vasodilator) and endothelin (a potent vasoconstrictor). Recognition of the role of these vasoactive compounds in the pathophysiology of disease states involving the pulmonary vasculature has led to development of drugs targeting these actions that have therapeutic benefits in patients with pulmonary arterial hypertension (see Chapter 13).
Another important aspect of pulmonary vascular physiology relates to fluid movement from pulmonary capillaries into the interstitium of the alveolar wall. Because of the importance of abnormalities in fluid transport across the capillaries in acute respiratory distress syndrome and respiratory failure, this topic is discussed specifically in Chapter 28.
Browning, EA, Chatterjee, S, Fisher, AB. Stop the flow: a paradigm for cell signaling mediated by reactive oxygen species in the pulmonary endothelium. Annu Rev Physiol. 2012;74:403–424.
Fishman, AP. A century of pulmonary hemodynamics. Am J Respir Crit Care Med. 2004;170:109–113.
Keith, IM. The role of endogenous lung neuropeptides in regulation of the pulmonary circulation. Physiol Rev. 2000;49:519–537.
Mazza, E, Taichman, DB. Functions and control of the pulmonary circulation. In: Mandel J, Taichman DB, eds. Pulmonary vascular disease. Philadelphia: Saunders Elsevier, 2006.
Sommer, N, Dietrich, A, Schermuly, RT, et al. Regulation of hypoxic pulmonary vasoconstriction: basic mechanisms. Eur Respir J. 2008;32:1639–1651.
Stenmark, KR, Fagan, KA, Frid, MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691.
Weibel, ER. The pathway for oxygen: structure and function in the mammalian respiratory system. Cambridge, MA: Harvard University Press; 1984.
Weir, EK, Lopez-Barneo, J, Buckler, KJ, et al. Acute oxygen-sensing mechanisms. N Engl J Med. 2005;353:2042–2055.
Weir, EK, Cabrera, JA, Mahapatra, S, et al. The role of ion channels in hypoxic pulmonary vasoconstriction. Adv Exp Med Biol. 2010;661:3–14.
West, JB. Respiratory physiology—the essentials, ed 9. Baltimore: Lippincott Williams & Wilkins; 2012.

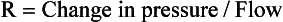
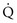 (Equation 12-2). Thus:
(Equation 12-2). Thus: