Anatomic and Physiologic Aspects of Neural, Muscular, and Chest Wall Interactions with the Lungs
This chapter focuses on the anatomic and physiologic features of the controlling system and the respiratory muscles to provide background for the discussion in Chapters 18 and 19. Those chapters discuss disorders affecting respiratory control, respiratory musculature, and the chest wall. Although much of the physiology and many of the clinical problems discussed here and in the next two chapters do not directly involve the lungs, they are so closely intertwined with respiratory function and dysfunction that they are appropriately considered in a textbook of pulmonary disease.
Respiratory Control
Organization of Respiratory Control
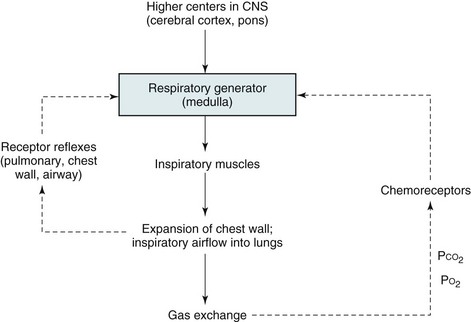
The basic organization of the respiratory control system is shown in Figure 17-1. Crucial to this system is the CNS “generator.” Signals that originate from the generator travel down the spinal cord to the various respiratory muscles. The inspiratory muscles, the most important of which is the diaphragm, respond to the signals by contracting and initiating inspiration. This process is described later in more detail under Respiratory Muscles.
Chemoreceptors
Maintenance of arterial blood gases is the ultimate goal of ventilatory control, and an important feedback loop adjusts respiratory center output if blood gases are not maintained at the appropriate level (see Fig. 17-1). Elevation of PCO2 (hypercapnia) and depression of PO2 (hypoxemia) both are capable of stimulating ventilation. In each case, one or more chemoreceptors detect alterations in PCO2 or PO2 and accordingly vary their input to the medullary respiratory center.
The primary sensor for CO2 is located near the ventrolateral surface of the medulla and is called the central chemoreceptor. Even though it is located in the medulla, the central chemoreceptor is clearly separate from the medullary respiratory center and should not be confused with it. The central chemoreceptor does not appear to respond directly to blood PCO2 but rather to the pH of the extracellular fluid (ECF) surrounding the chemoreceptor. The pH level, in turn, is determined by the level of hydrogen (H+) and bicarbonate (HCO3−) ions as well as PCO2 (see Appendix C for further discussion). The blood-brain barrier, the permeability properties of which influence the composition of cerebrospinal fluid (CSF) and brain ECF, prevents free movement of either H+ or HCO3− from blood to brain ECF, whereas CO2 passes freely. The feedback loop for changes in PCO2 can be summarized as follows:

Ventilatory Response to Hypercapnia and Hypoxia
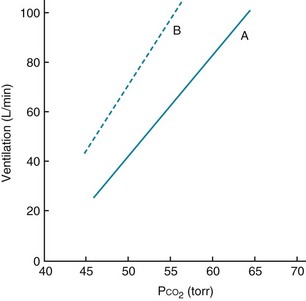
When arterial PO2 is held constant, ventilation increases by approximately 3 L/min for each millimeter of mercury rise in arterial PCO2 in adults. This relatively linear response, the magnitude of which varies considerably among individuals, is shown in Figure 17-2, which also shows that the response to increments in PCO2 also depends on PO2. At a lower PO2, the response to hypercapnia is heightened.
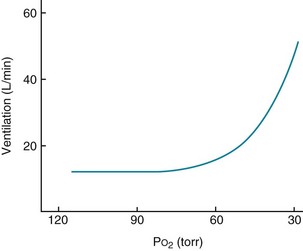
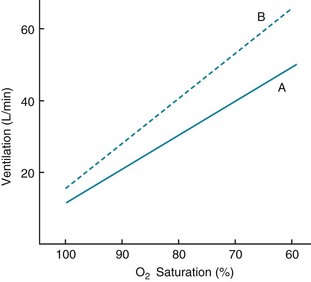
With hypoxemia, the same linear relationship does not exist between alterations in partial pressure and ventilation. Rather, the ventilatory response is relatively small until PO2 falls to approximately 60 mm Hg, below which the rise in ventilation is much more dramatic (Fig. 17-3). The curvilinear relationship between PO2 and ventilation can be made linear if ventilation is plotted against O2 saturation instead of partial pressure (Fig. 17-4). However, despite the linear relationship between ventilation and O2 saturation, it is the partial pressure of O2, not the content or saturation, that is sensed by the chemoreceptor.
PCO2 also has an effect on a patient’s response to hypoxia. The sensitivity to hypoxia is increased as PCO2 is raised and is decreased as PCO2 is lowered (see Fig. 17-4). This feature is important to consider when testing for responsiveness to hypoxia. As the patient hyperventilates in response to a low PO2, PCO2 drops, and ventilation is stimulated less than it would be if PCO2 were unchanged. Therefore, PCO2 should be kept constant so that the condition for testing actually is “isocapnic” hypoxia.
Respiratory Muscles
The purpose of signals emanating from the respiratory generator is to initiate inspiratory muscle activity. Although the primary inspiratory muscle is the diaphragm, other muscle groups contribute to optimal movement of the chest wall under a variety of conditions and needs. Notable among these other inspiratory muscle groups are the scalene and parasternal intercostal muscles, which display inspiratory activity even during normal quiet breathing. The so-called accessory muscles of inspiration (e.g., sternocleidomastoid and trapezius muscles) are not normally used during quiet inspiration but can be recruited when necessary, either when diaphragm function is impaired or when ventilation is significantly increased. Another set of intercostal muscles, the external intercostal muscles, are also inspiratory muscles, but their overall importance during inspiration is less clear. Finally, additional muscles coordinate upper airway activity during inspiration. Proper functioning of these muscles maintains patency of the upper airway, whereas dysfunction may be important in the pathogenesis of certain clinical disorders associated with upper airway obstruction, such as obstructive sleep apnea (see Chapter 18).
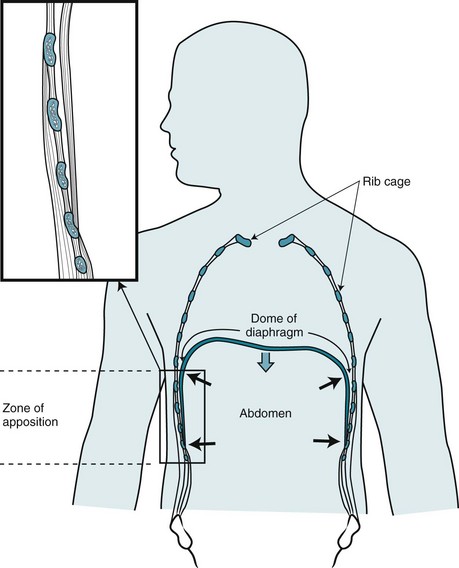
During inspiration, the diaphragm contracts and its muscle fibers shorten. To understand the effect of this contraction, consider the configuration of the diaphragm within the chest. At its lateral aspect, the diaphragm is adjacent to the inner part of the lower rib cage. This portion of the chest wall and the diaphragm is known as the zone of apposition (Fig. 17-5). In this region, the muscle fibers of the diaphragm are oriented vertically. When the diaphragm contracts, shortening of these vertically oriented fibers diminishes the zone of apposition and causes the more medial dome of the diaphragm to descend. At the same time, by pushing abdominal contents downward, diaphragmatic contraction increases not only intraabdominal pressure but also the lateral pressure on the lower rib cage transmitted through the apposed diaphragm. The effect of diaphragmatic contraction is thus to lift the lower ribs and expand the lower chest wall at the same time the abdominal wall moves outward. The external intercostal muscles, located between the ribs, also contract during inspiration, contributing as well to the lower rib cage being lifted and rotated outward.
In summary, normal operation of the respiratory apparatus depends on a signal generated by the respiratory center and eventually translated into an efficient pattern of respiratory muscle contraction. Although feedback and control systems ensure optimal functioning of this system, this finely coordinated mechanism may fail in numerous ways. Chapters 18 and 19 examine clinically important dysfunction occurring at various levels of this complex system.
Bianchi, AL, Denavit-Saubié, M, Champagnat, J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45.
Burton, MD, Kazemi, H. Neurotransmitters in central respiratory control. Respir Physiol. 2000;122:111–121.
Calverley, PMA. Control of breathing. In: Hughes JMB, Pride NB, eds. Lung function tests: physiological principles and clinical applications. London: WB Saunders; 2000:107–120.
Caruana-Montaldo, B, Gleeson, K, Zwillich, CW. The control of breathing in clinical practice. Chest. 2000;117:205–225.
Duffin, J, Hung, S. Respiratory rhythm generation. Can Anaesth Soc J. 1985;32:124–137.
Garcia, AJ, 3rd., Zanella, S, Koch, H, et al. Chapter 3–networks within networks: the neuronal control of breathing. Prog Brain Res. 2011;188:31–50.
Goridis, C, Dubreuil, V, Thoby-Brisson, M, et al. Phox2b, congenital central hypoventilation syndrome and the control of respiration. Semin Cell Dev Biol. 2010;21:814–822.
Hedemark, LL, Kronenberg, RS. Chemical regulation of respiration. Chest. 1982;82:488–494.
Schwartzstein, RM, Parker, MJ. The controller: directing the orchestra. In: Schwartzstein RM, Parker MJ, eds. Respiratory physiology: a clinical approach. Philadelphia: Lippincott Williams & Wilkins; 2006:126–148.
Semenza, GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547.
von Euler, C. Neural organization and rhythm generation. In: Crystal RG, West JB, Weibel ER, et al, eds. The lung: scientific foundations. ed 2. Philadelphia: Lippincott-Raven; 1997:1711–1724.
West, JB. Respiratory physiology—the essentials, ed 9. Philadelphia: Lippincott Williams & Wilkins; 2012.
American Thoracic Society and European Respiratory Society. ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624.
Derenne, J-P, Macklem, PT, Roussos, C. The respiratory muscles: mechanics, control, and pathophysiology. Am Rev Respir Dis. 1978;118:119–133.
Derenne, J-P, Macklem, PT, Roussos, C. The respiratory muscles: mechanics, control, and pathophysiology, part 2. Am Rev Respir Dis. 1978;118:373–390.
Derenne, J-P, Macklem, PT, Roussos, C. The respiratory muscles: mechanics, control, and pathophysiology, part 3. Am Rev Respir Dis. 1978;118:581–601.
De Troyer, A. The mechanism of inspiratory expansion of the rib cage. J Lab Clin Med. 1989;114:97–104.
De Troyer, A, Estenne, M. Functional anatomy of the respiratory muscles. Clin Chest Med. 1988;9:175–193.
Epstein, SK. An overview of respiratory muscle function. Clin Chest Med. 1994;15:619–639.
Guenter, CA, Whitelaw, WA. The role of diaphragm function in disease. Arch Intern Med. 1979;139:806–808.
Macklem, PT. Respiratory muscles: the vital pump. Chest. 1980;78:753–758.
Moxham, J. Respiratory muscle testing. Monaldi Arch Chest Dis. 1996;51:483–488.
Polkey, MI, Moxham, J. Terminology and testing of respiratory muscle dysfunction. Monaldi Arch Chest Dis. 1999;54:514–519.
Ratnovsky, A, Elad, D, Halpern, P. Mechanics of respiratory muscles. Respir Physiol Neurobiol. 2008;163:82–89.
Roussos, C, Macklem, PT. The respiratory muscles. N Engl J Med. 1982;307:786–797.
Schwartzstein, RM, Parker, MJ. Statics: snapshots of the ventilatory pump. In: Schwartzstein RM, Parker MJ, eds. Respiratory physiology: a clinical approach. Philadelphia: Lippincott Williams and Wilkins; 2006:34–60.