Airway Pharmacology
After reading this chapter you will be able to:
 Analyze three phases that constitute the course of drug action from dose to effect.
Analyze three phases that constitute the course of drug action from dose to effect.
 Describe classes of drugs that are delivered via the aerosol route.
Describe classes of drugs that are delivered via the aerosol route.
 Compare mode of action, indications, and adverse effects that characterize each major class of aerosolized drug.
Compare mode of action, indications, and adverse effects that characterize each major class of aerosolized drug.
 Compare available aerosol formulations, brand names, and dosages for each specific drug class.
Compare available aerosol formulations, brand names, and dosages for each specific drug class.
 Select the appropriate drug class for a specific patient or clinical situation.
Select the appropriate drug class for a specific patient or clinical situation.
The primary focus of respiratory care pharmacology is the delivery of bronchoactive inhaled aerosols to the respiratory tract for the diagnosis and treatment of pulmonary diseases. Although other drug classes are used in respiratory care, discussion in this chapter is limited to bronchoactive inhaled aerosols. Other drug classes are reviewed in pharmacology texts.1,2
Principles Of Pharmacology
Drug Administration Phase
The drug administration phase describes the method by which a drug dose is made available to the body. Administering drugs directly to the respiratory tract uses the inhalation route, and the dose form is an aerosol of liquid solutions, suspensions, or dry powders. The most commonly used devices to administer orally or nasally inhaled aerosols are the metered dose inhaler (MDI), the small volume nebulizer (SVN), and the dry powder inhaler (DPI). Reservoir devices, including holding chambers with one-way inspiratory valves and simple, nonvalved spacer devices, are often added to MDIs to reduce the need for complex hand-breathing coordination and to reduce oropharyngeal impaction of the aerosol drug (see Chapter 36).
The advantages of treatment of the respiratory tract with inhaled aerosols are as follows:
• Aerosol doses are usually smaller than doses for systemic administration.
• Onset of drug action is rapid.
Disadvantages of the delivery of inhaled aerosols in treating respiratory disease include the number of variables affecting the delivered dose and lack of adequate knowledge of device performance and use among patients and caregivers.3
Pharmacodynamic Phase
| Signaling Mechanism | Example |
| Mediation by G protein (guanine nucleotide)–linked receptors | Beta-adrenergic agonists, antimuscarinic agents |
| Attachment to intracellular receptors by lipid-soluble drugs | Corticosteroids |
The mechanisms of drug action are briefly described for each class of bronchoactive drug.
Airway Receptors and Neural Control of the Lung
• Adrenergic (adrenomimetic): Drug that stimulates a receptor responding to norepinephrine or epinephrine
• Antiadrenergic: Drug that blocks a receptor for norepinephrine or epinephrine
• Cholinergic (cholinomimetic): Drug that stimulates a receptor for acetylcholine
• Anticholinergic: Drug that blocks a receptor for acetylcholine
• Muscarinic: Drug that stimulates acetylcholine receptors specifically at parasympathetic nerve–ending sites
Because cholinergic receptors exist at autonomic ganglia and at the myoneural junction in skeletal muscle, the terms muscarinic and antimuscarinic distinguish cholinergic agents whose action is limited to parasympathetic sites. Neostigmine is a cholinergic (indirect-acting) drug that increases receptor stimulation at both the myoneural junction and the parasympathetic sites. By contrast, atropine is an antimuscarinic agent, which blocks the action of acetylcholine only at the parasympathetic sites. Table 32-1 summarizes receptors and their effects for the cardiopulmonary system. A more detailed description of the autonomic nervous system and receptor subtypes is provided by Katzung and colleagues.2
TABLE 32-1
Airway Receptors and Their Effects in the Cardiopulmonary System*
| Location | Receptor | Effect |
| Heart | Beta-1-adrenergic | Increased rate, force |
| M2-cholinergic | Decreased rate | |
| Bronchiolar smooth muscle | Beta-2-adrenergic | Bronchodilation |
| M3-cholinergic | Bronchoconstriction | |
| Pulmonary blood vessels | Alpha-1-adrenergic | Vasoconstriction |
| Beta-2-adrenergic | Vasodilation | |
| M3-cholinergic | Vasodilation | |
| Bronchial blood vessels | Alpha-1-adrenergic | Vasoconstriction |
| Beta-2-adrenergic | Vasodilation | |
| Submucosal glands | Alpha-1-adrenergic | Increased fluid, mucin |
| Beta-2-adrenergic | Increased fluid, mucin | |
| M3-cholinergic | Exocytosis, secretion |
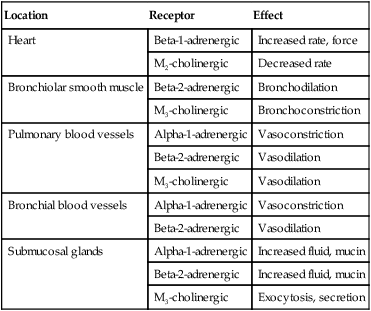
M2, M3, Subtypes of muscarinic (M) cholinergic receptors.
*Adrenergic and muscarinic cholinergic receptor subtypes are indicated.
Adrenergic Bronchodilators
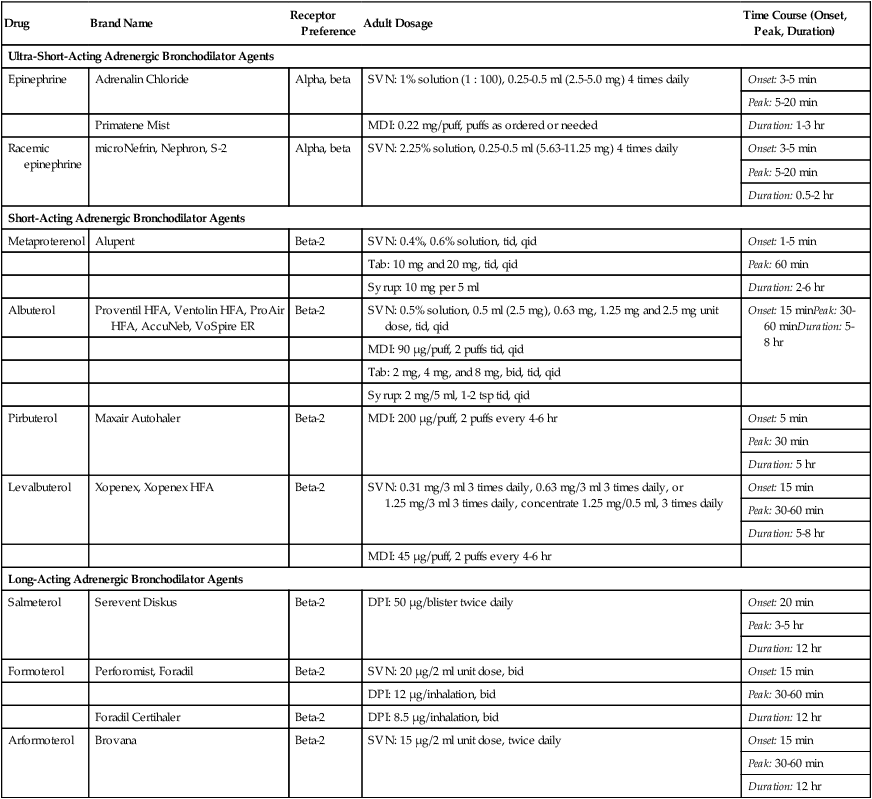
Adrenergic bronchodilators represent the largest group of drugs among the aerosolized agents used for oral inhalation. Table 32-2 lists bronchodilators in this group, with their aerosol formulations, selected brand names, and dosages.
TABLE 32-2
Adrenergic Bronchodilator Agents Available in the United States
| Drug | Brand Name | Receptor Preference | Adult Dosage | Time Course (Onset, Peak, Duration) |
| Ultra-Short-Acting Adrenergic Bronchodilator Agents | ||||
| Epinephrine | Adrenalin Chloride | Alpha, beta | SVN: 1% solution (1 : 100), 0.25-0.5 ml (2.5-5.0 mg) 4 times daily | Onset: 3-5 min |
| Peak: 5-20 min | ||||
| Duration: 1-3 hr | ||||
| Primatene Mist | MDI: 0.22 mg/puff, puffs as ordered or needed | |||
| Racemic epinephrine | microNefrin, Nephron, S-2 | Alpha, beta | SVN: 2.25% solution, 0.25-0.5 ml (5.63-11.25 mg) 4 times daily | Onset: 3-5 min |
| Peak: 5-20 min | ||||
| Duration: 0.5-2 hr | ||||
| Short-Acting Adrenergic Bronchodilator Agents | ||||
| Metaproterenol | Alupent | Beta-2 | SVN: 0.4%, 0.6% solution, tid, qid | Onset: 1-5 min |
| Tab: 10 mg and 20 mg, tid, qid | Peak: 60 min | |||
| Syrup: 10 mg per 5 ml | Duration: 2-6 hr | |||
| Albuterol | Proventil HFA, Ventolin HFA, ProAir HFA, AccuNeb, VoSpire ER | Beta-2 | SVN: 0.5% solution, 0.5 ml (2.5 mg), 0.63 mg, 1.25 mg and 2.5 mg unit dose, tid, qid | Onset: 15 minPeak: 30-60 minDuration: 5-8 hr |
| MDI: 90 µg/puff, 2 puffs tid, qid | ||||
| Tab: 2 mg, 4 mg, and 8 mg, bid, tid, qid | ||||
| Syrup: 2 mg/5 ml, 1-2 tsp tid, qid | ||||
| Pirbuterol | Maxair Autohaler | Beta-2 | MDI: 200 µg/puff, 2 puffs every 4-6 hr | Onset: 5 min |
| Peak: 30 min | ||||
| Duration: 5 hr | ||||
| Levalbuterol | Xopenex, Xopenex HFA | Beta-2 | SVN: 0.31 mg/3 ml 3 times daily, 0.63 mg/3 ml 3 times daily, or 1.25 mg/3 ml 3 times daily, concentrate 1.25 mg/0.5 ml, 3 times daily | Onset: 15 min |
| Peak: 30-60 min | ||||
| Duration: 5-8 hr | ||||
| MDI: 45 µg/puff, 2 puffs every 4-6 hr | ||||
| Long-Acting Adrenergic Bronchodilator Agents | ||||
| Salmeterol | Serevent Diskus | Beta-2 | DPI: 50 µg/blister twice daily | Onset: 20 min |
| Peak: 3-5 hr | ||||
| Duration: 12 hr | ||||
| Formoterol | Perforomist, Foradil | Beta-2 | SVN: 20 µg/2 ml unit dose, bid | Onset: 15 min |
| DPI: 12 µg/inhalation, bid | Peak: 30-60 min | |||
| Foradil Certihaler | Beta-2 | DPI: 8.5 µg/inhalation, bid | Duration: 12 hr | |
| Arformoterol | Brovana | Beta-2 | SVN: 15 µg/2 ml unit dose, twice daily | Onset: 15 min |
| Peak: 30-60 min | ||||
| Duration: 12 hr | ||||
Indications for Use
Indication for Short-Acting Agents
Short-acting beta-2 agonists, such as albuterol and levalbuterol, are indicated for relief of acute reversible airflow obstruction in asthma or other obstructive airway diseases. Short-acting agents are termed rescue agents in the 2007 National Asthma Education and Prevention Program Expert Panel III (NAEPP EPR III) guidelines.6
Mode of Action and Effects
• Alpha-receptor stimulation: Causes vasoconstriction and a vasopressor effect (increased blood pressure)
• Beta-1-receptor stimulation: Causes increased heart rate and myocardial contractility
• Beta-2-receptor stimulation: Relaxes bronchial smooth muscle, stimulates mucociliary activity, and has some inhibitory action on inflammatory mediator release
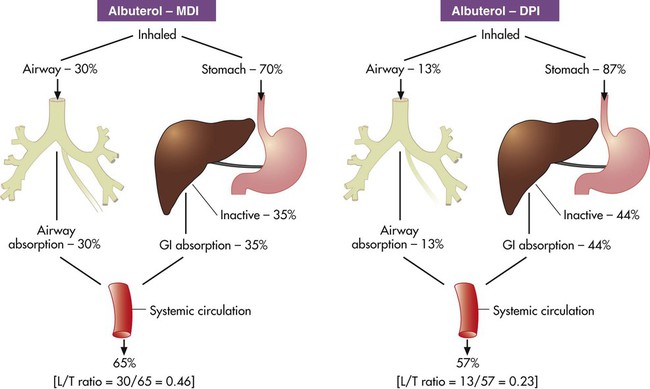
Bronchodilation, through stimulation of beta-2 receptors, is the desired therapeutic effect. Both alpha-adrenergic and beta-adrenergic receptors are G protein–linked receptors. Figure 32-2 illustrates the mode of action for relaxation of airway smooth muscle when a beta-2 receptor is stimulated. The nature of the beta receptor and its activity is presented in detail in a review by Barnes.7
Adrenergic Bronchodilator Agents
Adrenergic bronchodilator agents represent the evolution of a drug class. Although all of these agents are adrenergic agonists, the differences among individual agents are due to their receptor preference (alpha-adrenergic, beta-1-adrenergic, beta-2-adrenergic) and their different pharmacokinetics as listed in Table 32-2. These differences determine the optimal clinical application of individual agents, as discussed subsequently. The adrenergic bronchodilators form three subgroups.
Short-Acting Noncatecholamine Agents
Single-Isomer Beta Agonists
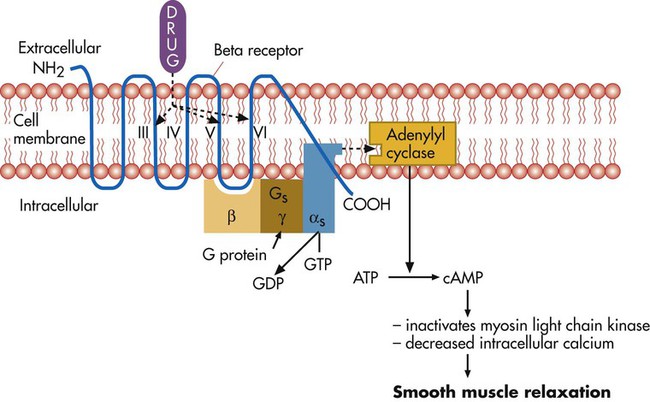
Levalbuterol is approved as a single-isomer beta-2-selective agonist. Previous inhaled formulations of adrenergic bronchodilators all were synthetic racemic mixtures, containing both the (R)-isomer and the (S)-isomer in equal amounts. Levalbuterol is the pure (R)-isomer of racemic albuterol. Both stereoisomers of albuterol are shown in Figure 32-3 with the single-isomer (R-isomer) form of levalbuterol. Although the (S)-isomer is physiologically inactive on adrenergic receptors, there is accumulating evidence that the (S)-isomer is not completely inactive. Box 32-1 lists some of the physiologic effects of (S)-albuterol noted in the literature.8–14 The effects noted antagonize the bronchodilating effects of the (R)-isomer and promote bronchoconstriction. In addition, the (S)-isomer is more slowly metabolized than the (R)-isomer.
Levalbuterol is available as a nebulization solution in three strengths: 0.31 mg/3 ml, 0.63 mg/3 ml, and 1.25 mg/3 ml. As a result of mixing of other inhaled agents, levalbuterol is also available as a concentrate of 1.25 mg/0.5 ml and an MDI. In a study by Nelson and associates,15 the 0.63-mg dose was found to be comparable to the 2.5-mg racemic albuterol dose in onset and duration. Side effects of tremor and heart rate changes were less with the single-isomer formulation. The 1.25-mg dose showed a higher peak effect on forced expiratory volume in 1 second (FEV1) with an 8-hour duration compared with racemic albuterol. Side effects with this dose were equivalent to the side effects seen with racemic albuterol. An equivalent clinical response was seen with one-fourth of the racemic dose (0.63 mg) using the pure isomer, although the racemic mixture contains 1.25 mg of the (R)-isomer (half of the total 2.5-mg dose). A detailed review is available of levalbuterol and differences between the (R)-isomer and (S)-isomer of albuterol.16
Long-Acting Adrenergic Bronchodilators
The release of salmeterol offered the first long-acting adrenergic bronchodilator in the United States. In contrast to previous agents, the duration of action of salmeterol is about 12 hours. The pharmacokinetics of salmeterol makes it suitable for maintenance therapy, in particular, with nocturnal asthma. However, it is not well suited for relief of acute airflow obstruction or bronchospasm because its onset is longer than 20 minutes, with a peak effect occurring by 3 to 5 hours. Although this agent is a beta-2 agonist, its exact mode of action differs from previous beta-2 agonists, allowing persistent receptor stimulation over a prolonged period of hours. A more detailed discussion of the action of salmeterol can be found in a review by Johnson and colleagues.17
Formoterol is a second long-acting, beta-2-specific agent and is approved for general clinical use in the United States. The duration of effect is approximately 12 hours, but in contrast to salmeterol, the onset of action and peak effect of formoterol are rapid and similar to albuterol.18 Nonetheless, patients should be cautioned about the risk of accumulation and toxicity if formoterol is used as a rescue agent in the same way that shorter acting beta agonists, such as albuterol, are used. As with salmeterol, the extensive side chain or tail makes formoterol more lipophilic than shorter acting bronchodilators and is the basis for its longer duration of effect.
A new novel once-daily, long-acting beta-agonist, indacaterol, was approved in the United States in 2011 under the brand name of Arcapta Neohaler. It has been used mainly to treat asthma, and additional indications for COPD are being examined. Indacaterol is also being studied in combination with tiotropium bromide with successful preliminary outcomes.1
Adverse Effects
The implication of beta-2-adrenergic agonists in deaths from asthma—termed the asthma paradox or the beta agonist controversy—remains debated.19 There is evidence of loss of a bronchoprotective effect with use of beta agonists, and patients should be cautioned to avoid asthma triggers.20 The increased prevalence of asthma in general remains a troublesome and unresolved issue.
Assessment of Bronchodilator Therapy
• Monitor flow rates using bedside peak flowmeters, portable spirometry, or laboratory reports of pulmonary function before and after bronchodilator studies to assess reversibility of airflow obstruction.
• Assess arterial blood gases or pulse oximetry saturation, as needed, for acute states with asthma or COPD to monitor changes in ventilation and gas exchange (oxygenation).
• Note the effect of beta agonists on blood glucose (increase) and K+ (decrease) laboratory values, if using high doses, such as with continuous nebulization or emergency department treatments.
• In the long-term, monitor pulmonary function studies of lung volumes, capacities, and flows.
• Instruct asthmatic patients in the use and interpretation of disposable peak flowmeters to assess severity of asthmatic episodes and provide an action plan for treatment modification.
• Emphasize in patient education that beta agonists do not treat underlying inflammation and do not prevent progression of asthma, and additional antiinflammatory treatment or more aggressive medical therapy may be needed if there is a poor response to the rescue beta agonist.
• Instruct and then verify correct use of aerosol delivery device (SVN, MDI, reservoir, DPI).
• Instruct patients in use, assembly, and especially cleaning of aerosol inhalation devices.
The following actions are suggested to evaluate patient response to long-acting beta agonists:
• Assess ongoing lung function, including predose FEV1 over time and variability in peak expiratory flows.
• Assess amount of rescue beta agonist use and nocturnal symptoms.
• Assess number of exacerbations, unscheduled clinic visits, and hospitalizations.
• Assess days of absence from school or work because of symptoms.
• Assess ability to reduce the dose of concomitant inhaled corticosteroids.
• Long-acting beta-2 agonists are not to be used without a controller medication (i.e., corticosteroid).
• Long-acting beta-2 agonists should not be used by patients who are controlled on low-dose or medium-dose inhaled corticosteroids.
• Long-acting beta-2 agonists should be used only if patients are not controlled with agents such as inhaled corticosteroids.
• Long-acting beta-2 agonists should be for short-term use only. A long-acting beta-2 agonist should be discontinued when asthma is controlled.
• Children should use a long-acting beta-2 agonist only in conjunction with a corticosteroid. The use of a combination product is needed to increase adherence.
Anticholinergic Bronchodilators
Indications for Use
Ipratropium bromide and tiotropium bromide are the only inhaled anticholinergic bronchodilators available in the United States. Table 32-3 lists the dosage forms and pharmacokinetics of ipratropium and tiotropium. Generally, anticholinergic agents have been found to be as effective as beta agonists in airflow improvement in COPD but less so in asthma. A nasal formulation of ipratropium is also available for relief of allergic and nonallergic perennial rhinitis, including the common cold.21
TABLE 32-3
Inhaled Anticholinergic Bronchodilator Agents*
| Drug | Brand Name | Adult Dosage | Time Course (Onset, Peak, Duration) |
| Ipratropium bromide | Atrovent HFA | HFA MDI: 17 µg/puff, 2 puffs 4 times daily | Onset: 15 min |
| SVN: 0.02% solution (0.2 mg/ml), 500 µg 3-4 times daily | Peak: 1-2 hr | ||
| Nasal spray: 21 µg or 40 µg, 2 sprays per nostril 2-4 times daily (dosage varies) | Duration: 4-6 hr | ||
| Ipratropium bromide and albuterol | Combivent | MDI: Ipratropium 18 µg/puff and albuterol 90 µg/puff, 2 puffs 4 times daily | Onset: 15 min |
| Peak: 1-2 hr | |||
| Duration: 4-6 hr | |||
| DuoNeb | SVN: Ipratropium 0.5 mg and albuterol 2.5 mg | ||
| Tiotropium bromide | Spiriva | DPI: 18 µg/inhalation, 1 inhalation daily (1 capsule) | Onset: 30 min |
| Peak: 3 hr | |||
| Duration: 24 hr |
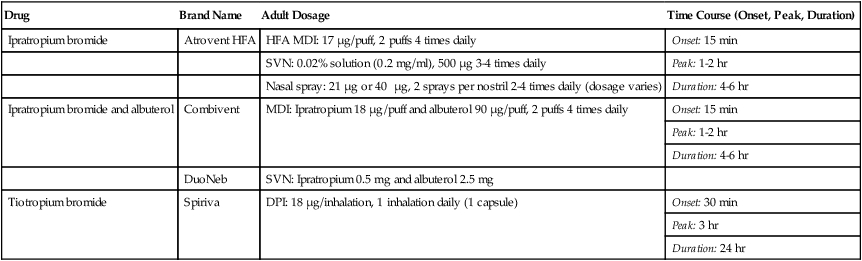
*A holding chamber is recommended with MDI administration to prevent accidental eye exposure.
Mode of Action
As antimuscarinic agents, ipratropium and tiotropium act as competitive antagonists for acetylcholine at muscarinic receptors on airway smooth muscle. Part of the airflow obstruction in COPD may be due to vagally mediated, reflex cholinergic stimulation. Airway irritation and inflammation stimulate afferent sensory C-fibers in the airway, which synapse with efferent vagal (cholinergic) fibers to the airway and mucous glands. The muscarinic receptor subtype on smooth muscle and submucosal mucous glands is the M3 receptor, which is a G protein–linked receptor. The effect of acetylcholine, the usual neurotransmitter, on the muscarinic (M3) receptors on airway smooth muscle is bronchoconstriction. The M1 receptor at the ganglionic junction enhances cholinergic nerve transmission. The M2 receptor is an autoreceptor inhibiting further release of acetylcholine so that blockade can increase acetylcholine release and may offset the bronchodilating effect of antimuscarinics.22
Tiotropium exhibits receptor subtype selectivity for M1 and M3 receptors. The drug binds to all three muscarinic receptors (M1, M2, and M3) but dissociates much more slowly than ipratropium from the M1 and M3 receptors; this results in a selectivity of action on M1 and M3 receptors. In patients with COPD, tiotropium provides a bronchodilating effect for 24 hours with an adequate dose.22 Inhalation of a single dose gives a peak plasma level within 5 minutes, with a rapid decline to very low levels within 1 hour. The site of action of anticholinergic agents in reversing cholinergic-induced airflow obstruction is shown in Figure 32-4.
Adverse Effects
Ipratropium bromide and tiotropium bromide are fully ionized compounds that are not well absorbed and distributed throughout the body, whereas atropine sulfate is a tertiary ammonium compound that is easily absorbed into the bloodstream. As a result, atropine produces many systemic side effects when inhaled, even though it is delivered locally to the lung. Side effects include the local topical effect of dry mouth, pupillary dilation, lens paralysis, increased intraocular pressure, increased heart rate, urinary retention, and altered mental state. Because of its many side effects and the availability of ionized compounds such as ipratropium, the use of atropine sulfate by nebulization is not recommended. In contrast, the side effects of inhaled anticholinergics are largely limited to its local site of action (Box 32-2).
Mucus-Controlling Agents
The two agents approved in the United States for oral inhalation with an effect on mucus are N-acetyl-cysteine (NAC) and dornase alfa. Both agents are mucolytic, although their modes of action differ. Table 32-4 lists these agents, their formulations, dosages, and bland aqueous aerosols. A review by Rubin23 provides additional detail.
TABLE 32-4
Mucoactive Agents Available for Aerosol Administration
| Drug | Brand Name | Adult Dosage | Use |
| N–Acetylcysteine 10% | Mucomyst | SVN: 3-5 ml | Bronchitis, efficacy not proven |
| N–Acetylcysteine 20% | Mucomyst | SVN: 3-5 ml | Bronchitis, efficacy not proven |
| Dornase alfa | Pulmozyme | SVN: 2.5 mg/ampule, 1 ampule daily* | CF |
| Aqueous aerosols: water, saline (0.45%, 0.9%, 5%-10%) | NA | SVN: 3-5 ml, as ordered | Sputum induction, secretion mobilization |
| USN: 3-5 ml, as ordered |
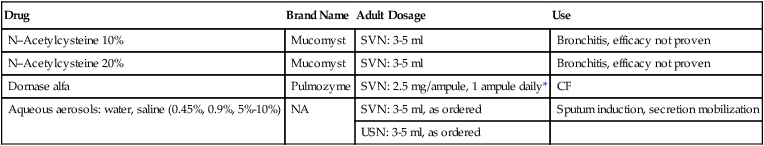
*Use recommended nebulizer system (see package insert). Approved nebulizers include Hudson T Updraft II, Marquest II with Pulmo-Aide compressor, or PARI LC Jet Plus with PARI Inhaler Boy compressor.
N-Acetyl Cysteine
Indications for Use
NAC is indicated to reduce accumulation of airway secretions, with concomitant improvement in pulmonary function and gas exchange and prevention of recurrent respiratory infection and airway damage. Diseases of excessive viscous mucus secretions and poor airway clearance include COPD, acute tracheobronchitis, and bronchiectasis. NAC also is used to treat or prevent liver damage that can occur when a patient takes an overdose of acetaminophen.24 Despite excellent in vitro mucolytic activity and a long history of use, no data clearly show that oral or aerosolized NAC is effective therapy for treating any lung disease.25 This situation may be partially due to NAC selectively depolymerizing the essential mucin polymer structure and leaving the pathologic polymers of DNA and F-actin intact in respiratory secretions.
Mode of Action
The mucus macromolecule consists of a polypeptide (protein) chain of amino acids, to which carbohydrate side chains are attached. There is internal cross-linking between strands with disulfide ( S
S S
S ) bonds and hydrogen bonds.26 NAC acts as a classic mucolytic to reduce the viscosity of mucus by substituting its own sulfhydryl group for the disulfide group in mucus, breaking a portion of the bond forming the gel structure. The drug is effective in reducing viscosity and can be helpful by direct bronchial instillation during bronchoscopy to remove mucus plugs.
) bonds and hydrogen bonds.26 NAC acts as a classic mucolytic to reduce the viscosity of mucus by substituting its own sulfhydryl group for the disulfide group in mucus, breaking a portion of the bond forming the gel structure. The drug is effective in reducing viscosity and can be helpful by direct bronchial instillation during bronchoscopy to remove mucus plugs.
Side Effects
Other side effects that can occur include the following:
• Airway obstruction secondary to rapid liquefaction of secretions
• Disagreeable odor secondary to hydrogen sulfide
• Incompatibility with certain antibiotics (sodium ampicillin, amphotericin B, erythromycin, tetracyclines, and aminoglycosides) if mixed in solution
• Increased concentration and toxicity of nebulizer solution toward end of treatment
• Reactivity of acetylcysteine with rubber, copper, iron, and cork
Dornase Alfa
Dornase alfa (Pulmozyme) is a genetically engineered clone of the natural human pancreatic DNase enzyme, which can digest extracellular DNA material. It is a peptide mucolytic and can reduce extracellular DNA and F-actin polymers. It is occasionally referred to as rhDNase (recombinant human DNase). It is designated as an orphan drug. Administration and dosage are given in Table 32-4.
Other Mucoactive Agents
Bland aerosols of water, including distilled water and normotonic, hypertonic, and hypotonic saline, have traditionally been nebulized to improve mobilization of secretions in respiratory disease states. The mucus gel layer is relatively resistant to the addition or removal of water after it is formed. Bland aerosols have been found to increase secretion clearance and sputum production and cause productive coughing.29 The effect is probably a vagally mediated reflex production of cough and mucus secretion. Bland aerosols are more properly considered expectorants rather than mucolytic agents. Clinicians must be alert to the possibility of bronchospasm with nonisotonic solutions, in particular, in patients with hyperreactive airways.
Assessment of Mucoactive Drug Therapy
During Treatment and Short-Term
• Teach and then verify correct use of aerosol nebulization system, including cleaning.
• Assess therapy based on indication for drug: mucolysis and improved clearance of secretions.
• Monitor airflow changes or adverse effects such as a decrease in FEV1.
• Assess the patient’s breathing pattern and rate.
• Assess the patient’s subjective reaction to treatment (changes in breathing effort or pattern).
• Discontinue therapy if the patient experiences adverse reactions.
General Contraindications
Gastroesophageal reflux and inability of the patient to protect the airway are risk factors for postural drainage that should be considered if postural drainage is necessary with mucoactive therapy. Mucoactive agents should be discontinued if there is evidence of clinical deterioration. Patients with acute bronchitis or exacerbation of chronic disease (CF, COPD) may be less responsive to mucoactive therapy, possibly secondary to infection and muscular weakness, which can reduce airflow-dependent mechanisms further.26
Inhaled Corticosteroids
Corticosteroids are endogenous hormones produced in the adrenal cortex, which regulate basic metabolic functions in the body and exert an antiinflammatory effect.30 The use of aerosolized corticosteroids is reviewed in this section. All corticosteroids used to treat asthma and COPD are glucocorticoids.
Indications and Purposes
The two general formulations of aerosolized glucocorticoids are orally inhaled and intranasal aerosol preparations. Orally inhaled preparations are listed in Table 32-5. The primary use of orally inhaled corticosteroids is for antiinflammatory maintenance therapy of persistent asthma6 and severe COPD.31 The use of intranasal steroids is for control of seasonal allergic or nonallergic rhinitis. Most agents in Table 32-5 are available as intranasal preparations, with the exception of the combination drugs.
TABLE 32-5
Corticosteroids and Combination Products Available by Aerosol for Oral Inhalation*
| Drug | Brand Name | Formulation and Dosage |
| Beclomethasone dipropionate HFA | QVAR | MDI: 40 and 80 µg/puff |
| Adults and children ≥12 yr: 40-80 µg twice daily† or 40-160 µg twice daily‡ | ||
| Children ≥5 yr: 40-80 µg twice daily | ||
| Ciclesonide | Alvesco | MDI: 40 µg/puff and 80 µg/puff |
| Adults and children ≥12 yr: 80-160 µg twice daily† or 80-320 µg twice daily‡ | ||
| Flunisolide hemihydrate HFA | AeroSpan | MDI: 80 µg/puff |
| Adults and children ≥12 yr: 2 puffs twice daily, adults no more than 4 puffs daily§ | ||
| Children 6-11 yr: 1 puff daily, no more than 2 puffs daily | ||
| Fluticasone propionate | Flovent HFA | MDI: 44, 110, and 220 µg/puff |
| Adults and children ≥12 yr: 88 µg twice daily†, 88-220 µg twice daily‡, or 880 µg twice daily§ | ||
| Children 4-11 yr: 88 µg twice daily¶ | ||
| Flovent Diskus | DPI: 50, 100, and 250 µg | |
| Adults and children ≥12 yr: 100 µg twice daily†, 100-250 µg twice daily‡, 1000 µg twice daily§ | ||
| Children 4-11 yr: 50 µg twice daily | ||
| Budesonide | Pulmicort Flexhaler | DPI: 90 µg/actuation and 180 µg/actuation |
| Adults and children ≥12 yr: 180-360 µg bid†, 180-360 µg bid‡, 360-720 µg bid§ | ||
| Children ≥6 yr: 180-360 µg bid | ||
| Pulmicort Respules | SVN: 0.25 mg/2 ml, 0.5 mg/2 ml, 1 mg/2 ml | |
| Children 1-8 yr: 0.5-mg total dose given once daily or twice daily in divided doses†,‡ 1 mg given as 0.5 mg twice daily or once daily§ | ||
| Mometasone furoate | Asmanex Twisthaler | DPI: 220 µg/actuation; or 110 µg actuation |
| Adults and children ≥12 yr: 220-440 µg daily†, 220-440 µg daily‡, 440-880 µg daily§; children 4-11 yrs: 110-220 µg daily | ||
| Fluticasone propionate/salmeterol | Advair Diskus | DPI: 100 µg fluticasone/50 µg salmeterol, 250 µg fluticasone/50 µg salmeterol, or 500 µg fluticasone/50 µg salmeterol |
| Advair HFA | Adults and children ≥12 yr: 100 µg fluticasone/50 µg salmeterol, 1 inhalation twice daily, about 12 hr apart (starting dose if not currently taking inhaled corticosteroids) | |
| Maximal recommended dose 500 µg fluticasone/50 µg salmeterol twice daily | ||
| Children ≥4 yr: 100 µg fluticasone/50 µg salmeterol, 1 inhalation twice daily, about 12 hr apart (for patients who are symptomatic while taking an inhaled corticosteroid)§ | ||
| MDI: 45 µg fluticasone/21 µg salmeterol, 115 µg fluticasone/21 µg salmeterol, or 230 µg fluticasone/21 µg salmeterol§ | ||
| Adults and children ≥12 yr: 2 inhalations twice daily, about 12 hr apart | ||
| Budesonide/formoterol fumarate HFA | Symbicort | MDI: 80 µg budesonide/4.5 µg formoterol and 160 µg budesonide/4.5 µg formoterol twice dailyAdults and children ≥12yr: 320 µg budesonide/9 µg formoterol; or 160 µg budesonide/9 µg formoterol twice daily |
| Mometasone furoate/formoterol fumarate HFA | Dulera | MDI: 100 µg mometasone/5 µg formoterol and 200 µg mometasone/5 µg formoterol |
| Adults and children ≥12 yr: If previously on medium dose of corticosteroids, ≤400 µg mometasone/20 µg formoterol daily; if previously on high dose of corticosteroid, ≤800 µg mometasone/20 µg formoterol daily |
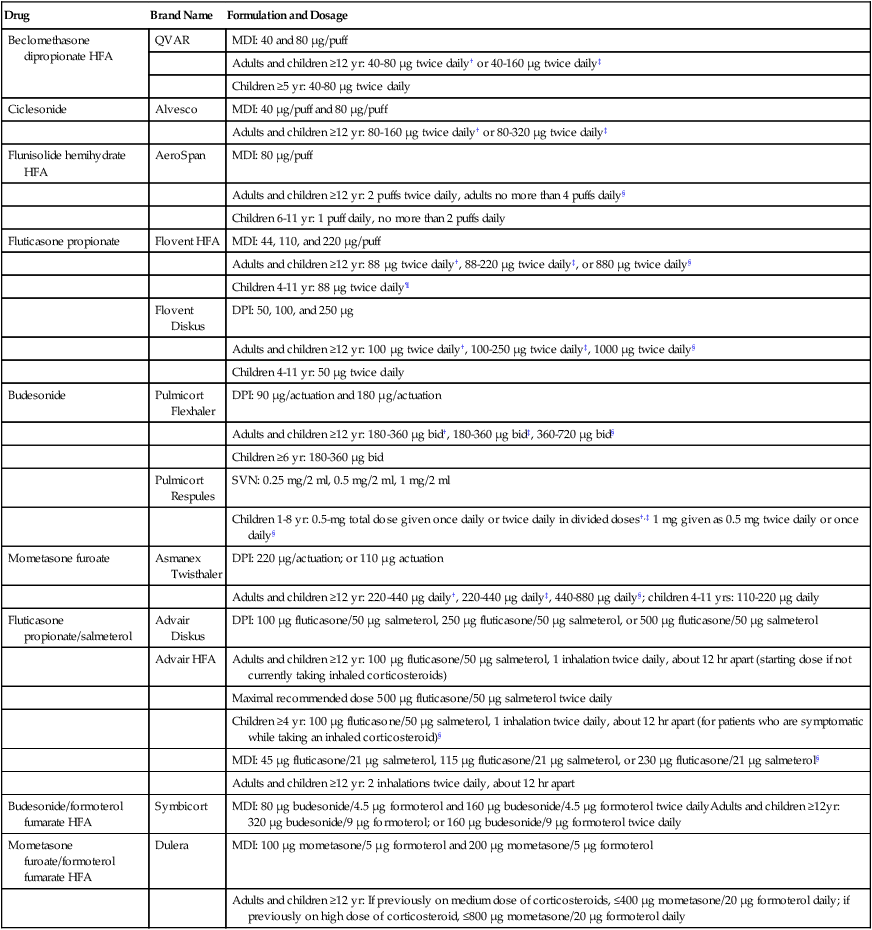
*Individual agents are discussed in the text. Detailed information about each agent should be obtained from the manufacturer’s drug insert.
†Recommended starting dose if taking only bronchodilators.
‡Recommended starting dose if previously taking inhaled corticosteroids.
§Recommended starting dose if previously taking oral corticosteroids.
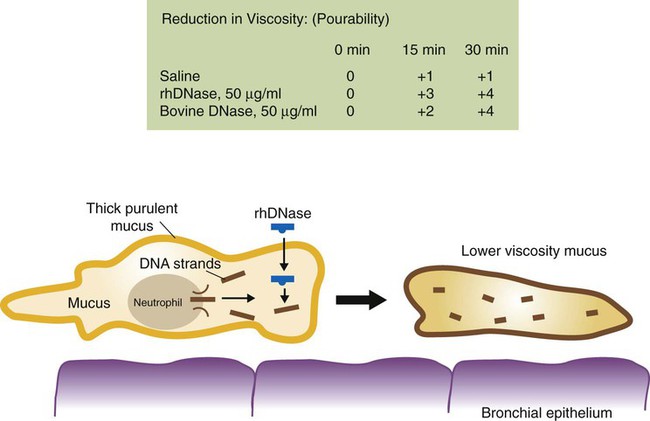
Mode of Action
Glucocorticoids are lipid-soluble drugs that act on intracellular receptors. The complex action of steroids is illustrated in Figure 32-6.32–34 Because steroid action involves modification of cell transcription, full antiinflammatory effects require hours to days. It is important for patients to understand that inhalation of an aerosolized steroid does not provide immediate relief as with an adrenergic bronchodilator. However, daily compliance with the inhaled medication is essential to controlling the inflammation of asthma. Oral corticosteroids may be needed initially to clear the airway or as “burst” therapy to control asthma exacerbations.
Adverse Effects
The type and severity of side effects seen with inhaled aerosolized corticosteroids are much less than with systemic use, as with other classes of aerosolized drugs. Box 32-3 lists systemic and local effects that can occur with inhaled steroids. The systemic effect of adrenal suppression is not usually seen with inhaled doses less than 800 mcg/day in adults or less than 400 mcg/day in children. Use of a reservoir device should be routine with inhaled steroids to prevent a swallowed portion adding to the systemic effect and to prevent the local effects of oral candidiasis and dysphonia. Growth retardation with use of inhaled steroids in asthma is controversial. Some investigators found no growth suppression even with high-dose inhaled steroids. Allen and colleagues35 published a comprehensive review of inhaled steroids.
Assessment of Drug Therapy
The basic actions to evaluate an aerosol drug treatment should be followed (see section on Assessment of Bronchodilator Therapy). As with other drug therapy, the indications for this class of drug should be present. The 2007 NAEPP and 2010 Global Initiative on Obstructive Lung Disease (GOLD) COPD guidelines are recommended for guidance.6,31 In addition, with inhaled corticosteroids, the following actions are suggested:
• Verify that the patient understands that a corticosteroid is a controller agent and is different from a rescue bronchodilator (relieving agent); assess the patient’s understanding of the need for consistent use of an inhaled corticosteroid (compliance with therapy).
• Instruct the patient in the use of a peak flowmeter to monitor baseline peak expiratory flow (PEF) and changes. Verify that there is a specific action plan, based on symptoms and PEF results. The patient should understand when to contact a physician with deterioration in PEF or exacerbation of symptoms.
Long-Term
• Assess severity of symptoms (coughing, wheezing, nocturnal awakenings, symptoms during exertion; use of rescue bronchodilator; number of exacerbations; missed work or school days; and pulmonary function), and modify level or dosage as recommended by NAEPP and GOLD guidelines.6,31
• Assess for the presence of side effects with inhaled steroid therapy (oral thrush, hoarseness or voice changes, cough or wheezing with MDI use); use a reservoir (preferably a holding chamber) with MDI use, and verify correct technique.
Nonsteroidal Antiasthma Drugs
Nonsteroidal antiinflammatory drugs constitute a growing class of drugs in the treatment of asthma. These include mast cell stabilizers (cromolyn sodium); antileukotrienes, also termed leukotriene modifiers (zafirlukast, zileuton, montelukast); and a new class, monoclonal antibodies or anti-IgE agents (omalizumab). Antileukotrienes are administered orally, and the monoclonal antibody agent omalizumab is given parenterally, but these are included as bronchoactive drugs. Table 32-6 lists pharmaceutical details for each agent.
TABLE 32-6
Nonsteroidal Antiasthma Medications*
| Generic Drug | Brand Name | Formulation and Dosage |
| Mast Cell Stabilizer | ||
| Cromolyn sodium | SVN: 20 mg/ampule or 20 mg/2 ml | |
| Adults and children ≥2 yr: 20 mg inhaled 4 times daily | ||
| NasalCrom | Spray: 40 mg/ml (4%) (5.2 mg per actuation) | |
| Adults and children ≥2 yr: 1 spray each nostril, 3-6 times daily every 4-6 hr | ||
| Gastrocrom | Oral concentrate: 100 mg/5 ml | |
| Adults and children ≥13 yr: 2 ampules 4 times daily, 30 min before meals and at bedtime | ||
| Children 2-12 yr: 1 ampule 4 time daily, 30 min before meals and at bedtime | ||
| Antileukotrienes | ||
| Zafirlukast | Accolate | Tablets: 10 and 20 mg |
| Adults and children ≥12 yr: 20 mg twice daily, without food | ||
| Children 5-11 yr: 10 mg twice daily | ||
| Montelukast | Singulair | Tablets: 10 mg and 4-mg and 5-mg cherry-flavored chewable; 4-mg packet of granules |
| Adults and children ≥15 yr: 1 10-mg tablet daily | ||
| Children 6-14 yr: 1 5-mg chewable tablet daily | ||
| Children 2-5 yr: 1 4-mg chewable tablet or 1 4-mg packet of granules daily 6-23 mo: 1 4-mg packet of granules daily | ||
| Zileuton | Zyflo; Zyflo CR | Tablets: 600 mg |
| Adults and children ≥12 yr: 1 600-mg tablet 4 times per day; CR, 2 tablets twice daily, within 1 hr of morning and evening meals | ||
| Monoclonal Antibody | ||
| Omalizumab | Xolair | Adults and children ≥12 yr: subcutaneous injection every 4 wk; dose dependent on weight and serum IgE level |
*Detailed prescribing information should be obtained from the manufacturer’s package insert.
Indication for Use
The general indication for clinical use of nonsteroidal antiasthma agents is prophylactic management (control) of persistent asthma (Step 2 or greater asthma, using the classification in the 2007 NAEPP guidelines6). Step 2 asthma is defined as more than 2 days/week with but not daily symptoms and more than 2 nights/month with awakenings and FEV1 of 80% or greater. Step 3 asthma is defined as daily symptoms and 3 to 4 nights/month with awakening and FEV1 greater than 60% but less than 80%. Step 4 and above asthma is defined as symptoms throughout the day and night awakenings more than once a week and FEV1 less than 60%. For children older than 12 years and adults, omalizumab is available for use in asthma above step 4.36
The following are qualifications to the general indications for use of these agents:
• Cromolyn sodium and antileukotrienes are typically recommended as alternatives to introducing inhaled corticosteroids in Step 2 and Step 3 asthma.
• Cromolyn sodium and montelukast in particular are often used in infants and young children as alternatives to inhaled corticosteroids in Step 2 asthma because of their safety profiles.
• Antileukotrienes can be useful in combination with inhaled steroids to reduce the dose of the steroid and are listed as alternatives in Step 2 through Step 4 asthma.
• The monoclonal antibody omalizumab is available for consideration in the appropriate population.37
All of the nonsteroidal antiasthma drugs described in this chapter are controllers, not relievers, and are used in asthma requiring antiinflammatory drug therapy (Box 32-4).
Mode of Action
Cromolyn sodium acts by inhibiting the degranulation of mast cells in response to allergic and nonallergic stimuli. This inhibition prevents release of histamine and other mediators of inflammation. These mediators cause bronchospasm and trigger an increasing cascade of further mediator release and inflammatory cell activity in the airway.38
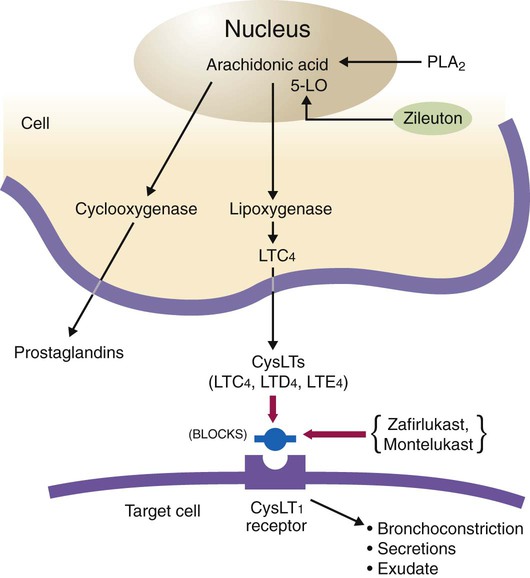
Zafirlukast and montelukast act as leukotriene receptor antagonists and are selective competitive antagonists of leukotriene receptors LTD4 and LTE4. Leukotrienes such as LTC4, LTD4, and LTE4 (previously known as SRS-A) stimulate leukotriene receptors termed CysLT1 to cause bronchoconstriction, mucus secretion, vascular permeability, and plasma exudation into the airway. The mode of action is shown in Figure 32-7. The drug inhibits asthma reactions induced by exercise, cold air, allergens, and aspirin.39
Zileuton inhibits the 5-lipoxygenase enzyme that catalyzes the formation of leukotrienes from arachidonic acid (see Figure 32-7).40 Omalizumab is a recombinant DNA–derived humanized antibody that binds to IgE. The agent inhibits the attachment of IgE to mast cells and basophils, reducing the release of chemical mediators of the allergic response.41
Adverse Effects
A potential adverse effect with any nonsteroidal antiasthma drug is inappropriate use. These agents are not bronchodilators and offer no benefit for acute airway obstruction in asthma. Using the NAEPP terminology, all of these agents are controllers rather than relievers.6
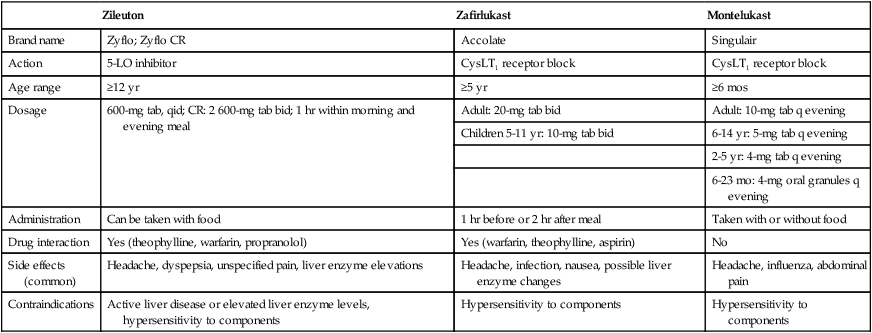
Table 32-7 summarizes information and comparative features of the three antileukotriene agents, including drug interactions, common side effects, and contraindications. The most common adverse reactions seen with omalizumab include injection site reaction, viral infections, respiratory tract infections, headache, sinusitis, and pharyngitis.
TABLE 32-7
Summary of Comparative Features of Three Available Antileukotriene Agents
| Zileuton | Zafirlukast | Montelukast | |
| Brand name | Zyflo; Zyflo CR | Accolate | Singulair |
| Action | 5-LO inhibitor | CysLT1 receptor block | CysLT1 receptor block |
| Age range | ≥12 yr | ≥5 yr | ≥6 mos |
| Dosage | 600-mg tab, qid; CR: 2 600-mg tab bid; 1 hr within morning and evening meal | Adult: 20-mg tab bid | Adult: 10-mg tab q evening |
| Children 5-11 yr: 10-mg tab bid | 6-14 yr: 5-mg tab q evening | ||
| 2-5 yr: 4-mg tab q evening | |||
| 6-23 mo: 4-mg oral granules q evening | |||
| Administration | Can be taken with food | 1 hr before or 2 hr after meal | Taken with or without food |
| Drug interaction | Yes (theophylline, warfarin, propranolol) | Yes (warfarin, theophylline, aspirin) | No |
| Side effects (common) | Headache, dyspepsia, unspecified pain, liver enzyme elevations | Headache, infection, nausea, possible liver enzyme changes | Headache, influenza, abdominal pain |
| Contraindications | Active liver disease or elevated liver enzyme levels, hypersensitivity to components | Hypersensitivity to components | Hypersensitivity to components |
Assessment of Drug Therapy
• Verify that the patient understands that nonsteroidal antiasthma agents are controller drugs and their difference from rescue bronchodilators (relieving agents); assess the patient’s understanding of the need for consistent use of these agents (compliance with therapy).
• Instruct the patient in use of a peak flowmeter to monitor baseline PEF and changes. Verify that there is a specific action plan, based on symptoms and PEF results. The patient should be clear on when to contact a physician with deterioration in PEF or exacerbation of symptoms.
Long-Term
• Assess severity of symptoms (coughing, wheezing, nocturnal awakenings, symptoms during exertion); use of rescue medication; number of exacerbations; missed work or school days; pulmonary function), and modify level of asthma therapy (up or down, as described in the 2007 NAEPP EPR III guidelines for step therapy).
• Assess for the presence of side effects with nonsteroidal antiasthma agents; refer to the particular agent and its side effects (listed previously).
Aerosolized Antiinfective Agents
Multiple aerosolized antiinfective agents are available. Some agents may be used less often than others in respiratory therapy. The antiinfective agents pentamidine, ribavirin, inhaled tobramycin, and zanamivir are briefly outlined here. Drug formulations and dosages are given in Table 32-8.
TABLE 32-8
| Drug | Brand Name | Formulation and Dosage | Clinical Use |
| Pentamidine isethionate | NebuPent | 300 mg powder in 6 ml sterile water; 300 mg once every 4 wk | PCP prophylaxis |
| Ribavirin | Virazole | 6 g powder in 300 ml sterile water (20-mg/ml solution); given every 12-18 hr/day for 3-7 days by SPAG nebulizer | RSV |
| Tobramycin | TOBI | 300-mg/5-ml ampule; adults and children ≥6 years: 300 mg bid, 28 days on/28 days off drug | P. aeruginosa infection in CF |
| Aztreonam | Cayston | 75 mg/1 ml; adults and children ≥7 yr: 75 mg tid, 28 days on/28 days off drug | P. aeruginosa infection in CF |
| Zanamivir | Relenza | DPI: 5 mg/inhalation; adults ≥5 years: 2 inhalations (1 5-mg blister per inhalation) bid, 12 hr apart for 5 days | Influenza |
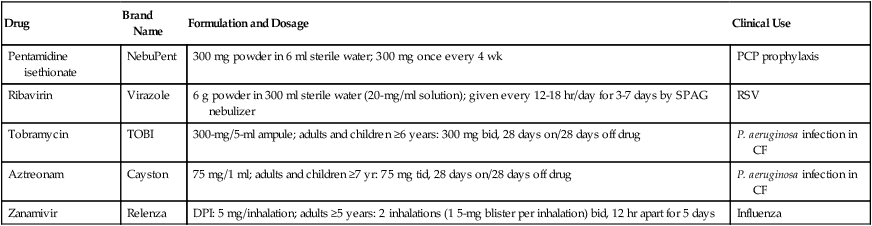
*Details on use and administration should be obtained from manufacturer’s drug insert material before use.
Pentamidine Isethionate
Indication for Use
Comparisons of the efficacy of aerosolized pentamidine with oral trimethoprim-sulfamethoxazole (TMP-SMX), together with reports of serious adverse effects with aerosolized pentamidine, led to a reevaluation of aerosol therapy with pentamidine for prophylaxis of PCP. General recommendations for prophylaxis of PCP were published by the U.S. Centers for Disease Control and Prevention (CDC) in for HIV-positive children42 and adults.43 In the 2009 CDC recommendations, oral TMP-SMX was preferred for prophylaxis of PCP as long as adverse side effects from TMP-SMX were absent or acceptable.43 Aerosolized pentamidine is recommended as an alternative therapy for prophylaxis of PCP if TMP-SMX cannot be tolerated.
Assessment
• If coughing and bronchospasm are present, provide a short-acting beta agonist or an anticholinergic bronchodilator such as ipratropium if present with inhaled pentamidine.
• Monitor for occurrence rate of PCP and rate of long-term hospitalizations.
• Monitor for presence of side effects (shortness of breath, possible pneumothorax, conjunctivitis, rash, neutropenia, dysglycemia) or appearance of extrapulmonary P. jiroveci infection.
• Evaluate need for prior use of a bronchodilator if symptoms of bronchospasm or coughing occur after inhalation of pentamidine.
Ribavirin
Ribavirin (Virazole) is an antiviral agent used in the treatment of severe lower respiratory tract infections caused by respiratory syncytial virus (RSV). RSV is a common seasonal respiratory infection in infants and young children, which is usually self-limiting. The cost-effectiveness of ribavirin continues to be debated. Recommendations for use of the drug were published in a statement by the American Academy of Pediatrics.44 Administration of the aerosol requires use of a special large-reservoir nebulizer called a small particle aerosol generator (SPAG). The mode of action of ribavirin is ascribed to the similarity of the drug to guanosine, a natural nucleoside. Substitution of ribavirin for the natural nucleoside interrupts the viral replication process in the host cell.
Assessment
• Monitor signs of improvement in RSV infection severity, including vital signs, respiratory pattern and work of breathing (clinically), level of fractional inspired oxygen (FiO2) needed, level of ventilatory support, arterial blood gases, body temperature, and other indicators of pulmonary gas exchange.
• Monitor the patient for evidence of side effects, such as deterioration in lung function, bronchospasm, occlusion of endotracheal tube (if present), cardiovascular instability, skin irritation from the aerosol drug, and equipment malfunction related to drug residue.
Inhaled Tobramycin
Patients with CF have chronic respiratory infection with Pseudomonas aeruginosa and other microorganisms. Such chronic infection causes recurrent acute respiratory infections and deterioration of lung function. With the exception of the quinoline derivatives such as ciprofloxacin, antibiotics such as the aminoglycosides (e.g., tobramycin), which are effective against Pseudomonas organisms, have poor lung bioavailability when taken orally. Consequently, these antibiotics must be given either intravenously or by inhalation. The aminoglycoside tobramycin has been approved for inhaled administration (TOBI) and is intended to manage chronic infection with P. aeruginosa in patients with CF. Goals of therapy are to treat or prevent early colonization with P. aeruginosa and maintain present lung function or reduce the rate of deterioration. The emergence of bacterial resistance was not seen in clinical trials with inhaled tobramycin.45
Adverse Effects
Side effects with parenteral aminoglycosides include possible auditory and vestibular damage with potential for deafness and nephrotoxicity. Other possible effects are listed in Box 32-5. Side effects observed since the introduction of inhaled tobramycin have been minimal and include voice alteration and tinnitus in a small percentage of patients. Risk for more serious side effects with tobramycin, whether by inhaled or parenteral routes, increases with the use of other aminoglycosides, in the presence of poor renal function and dehydration, with preexisting neuromuscular impairment, or with use of other ototoxic drugs.
The following precautions are suggested with use of inhaled tobramycin:
• Inhaled tobramycin should be used with caution in patients with preexisting renal, auditory, vestibular, or neuromuscular dysfunction.
• Tobramycin solution should not be mixed with beta-lactam antibiotics (penicillins, cephalosporins) because of admixture incompatibility, and mixing with other drugs in general is discouraged.
• Nebulization of antibiotics during hospitalization should be performed under conditions of containment, as previously described for pentamidine and ribavirin, to prevent environmental saturation and development of resistant organisms in the hospital.
• Aminoglycosides can cause fetal harm if administered to pregnant women; exposure to ambient aerosol drug should be avoided by women who are pregnant or trying to become pregnant.
• Local airway irritation resulting in cough and bronchospasm with decreased ventilatory flow rates is possible with inhaled antibiotics and seems to be related to the osmolality of the solution.46,47 Peak flow rates and chest auscultation should be used before and after treatments to evaluate airway changes. Pretreatment with a beta agonist may be needed.
• Allergic reactions in the patient, staff, or family should be considered if exposure to the aerosolized drug is not controlled. The use of a nebulizing system with a scavenging filter, one-way valves, and thumb control could reduce ambient contamination with the drug, as previously described.
In clinical trials, inhaled tobramycin was administered using the PARI LC Plus nebulizer with a DeVilbiss Pulmo-Aide compressor. Other nebulizer systems must be tested to ensure adequate drug output and particle size because antibiotic solutions differ in viscosity from the aqueous bronchodilator solutions used in common disposable nebulizers. Studies have reported that not all nebulizer-compressor systems perform adequately with antibiotic solutions, and higher flow rates of 10 to 12 L/min may be needed with nebulizers.48,49
Assessment
• Verify that the patient understands that nebulized tobramycin should be given after other CF therapies, including other inhaled drugs.
• Check whether the patient has renal, auditory, vestibular, or neuromuscular problems or is taking other aminoglycosides or ototoxic drugs. Consider whether tobramycin should be used for the patient based on severity of preexisting or concomitant risk factors.
• Monitor lung function to note improvement in FEV1.
• Assess rate of hospitalization before and after institution of inhaled tobramycin.
• Assess need for IV antipseudomonal therapy.
• Assess improvement in weight.
• Monitor for occurrence of side effects, such as tinnitus or voice alteration; have the patient rinse and expectorate after aerosol treatments.
• Evaluate for changes in hearing or renal function during use of inhaled tobramycin.
Inhaled Aztreonam
Aztreonam was approved in December 1986 by the FDA as a monobactam, a synthetic bactericidal antibiotic; it is given as an IV solution. Inhaled aztreonam (Cayston) was approved in 2010 to improve pulmonary symptoms in patients with CF colonized with P. aeruginosa.50 Inhaled aztreonam is not indicated for patients younger than 7 years old or patients with Burkholderia cepacia infection. This agent has been studied only in patients with FEV1 greater than 25% or less than 75% of predicted. The agent is delivered by itself using the Altera Nebulizer System.
Colistimethate Sodium
Colistimethate sodium (colistin) is an antibiotic used to treat sensitive strains of gram-negative bacilli, particularly P. aeruginosa. Colistimethate sodium is available as an inhaled formulation in Europe as Promixin; this agent is not approved for inhalation by the FDA. However, nebulization of the parenteral formulation is commonly used in patients with CF. Falagas and colleagues51 published a review of IV and aerosolized colistimethate sodium.
Inhaled Zanamivir
Mode of Action
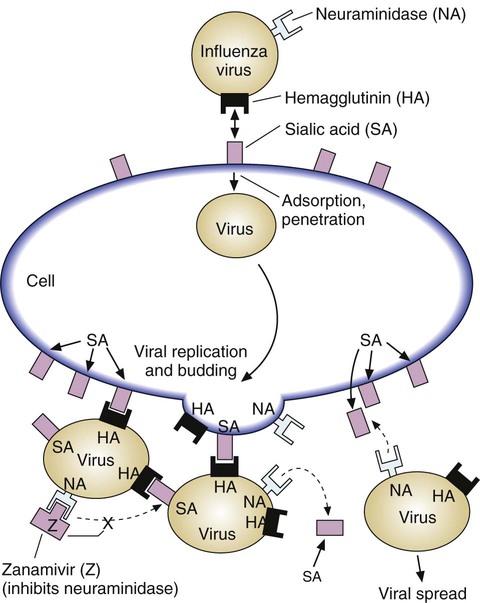
The influenza virus attaches to respiratory tract cells by binding of viral surface hemagglutinin to the cell’s surface molecule of sialic acid (Figure 32-8). The viral particle also has an enzyme, neuraminidase, on its surface. When replicated viral particles are released from the host cell after infection, the viral neuraminidase cleaves the sialic acid on both the host cell surface and other viral particle surfaces so that mature virus can be released and spread. Without neuraminidase, influenza virus would clump together and to the host cell, preventing spread. Zanamivir and oseltamivir combine with the surface neuraminidase, preventing its action and the spread of viral particles.
Adverse Effects
Several adverse effects can occur with inhaled zanamivir:
• Bronchospasm and deterioration in lung function, especially in patients with COPD or asthma
• Possible undertreatment of bacterial infection masquerading as a viral infection or a secondary bacterial infection in the presence of influenza
• Allergic reactions, as may occur with any drug
• Adverse reactions, such as diarrhea, nausea, vomiting, bronchitis, cough, sinusitis, dizziness, and headaches
Because of the effect on lung function in patients with respiratory disease and reports of adverse reactions, revised labeling for the drug carries a warning that zanamivir is not generally recommended for patients with underlying airways disease.52 However, other studies have determined that high-risk patients such as patients with asthma and COPD were not affected by the use of zanamivir.53
Clinical Efficacy
In studies of clinical efficacy, the use of zanamivir resulted in shortening of the median time to alleviation of symptoms by 1 day. In subjects who began treatment within 30 hours of illness, the median time to alleviation of symptoms was reduced by approximately 3 days.54 Zanamivir is not approved for prophylaxis of influenza, although some data suggest a preventive effect in patients exposed to influenza virus.53 Cost-versus-efficacy issues revolve around the modest reduction in symptoms and inability to confirm the presence of influenza quickly, easily, and inexpensively as the basis for the drug treatment.
Assessment
• Assess improvement in influenza symptoms, including fever reduction, less myalgia and headache, reduced coughing and sore throat, and less systemic fatigue.
• Monitor for airway irritation and symptoms of bronchospasm, especially during initial use of the dry powder aerosol. Provide a short-acting beta agonist if needed or if the patient is at risk for airway reactivity (COPD, asthma).
Inhaled Pulmonary Vasodilators
The use of nitric oxide gas to treat neonates with persistent pulmonary hypertension is approved by the FDA and is discussed in detail in Chapter 38. In addition to this medical gas, inhaled medications are being tested and used to treat pulmonary hypertension. Several such agents are being studied, including epoprostenol (Flolan) and alprostadil (Prostin VR Pediatric); however, only two, iloprost, and treprostenil, are approved by the FDA for widespread use. Siobal55 published a review of aerosolized prostacyclins and nitric oxide.
Nitric Oxide
Indications for Use
As described in more detail in Chapter 38, nitric oxide (INOmax) is indicated in the treatment of neonates (>34 weeks’ gestational age) with hypoxic respiratory failure.56 The patient should have evidence of pulmonary hypertension in which nitric oxide would improve oxygenation and decrease the need for extracorporeal membrane oxygenation. Off-label uses include reducing pulmonary artery pressure in the neonate.57
Iloprost
Indications for Use
Iloprost (Ventavis) inhalation is indicated for the treatment of pulmonary hypertension.59 Iloprost inhalation is administered with the I-neb nebulizer.
Treprostinil
Indication for Use
Treprostinil (Tyvaso) is indicated for the treatment of pulmonary arterial hypertension to increase walking distance in patients with New York Heart Association class III symptoms.60 It is administered using the Tyvaso Inhalation System, which is an ultrasonic, pulsed-delivery device.


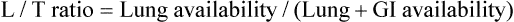
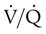 ) ratio (decrease in PaO2/SpO2)
) ratio (decrease in PaO2/SpO2)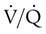 with inhalation of the aerosol is not clinically significant and reverses quickly. Bronchospasm resulting from chlorofluorocarbon propellants can be prevented by changing to newer hydrofluoroalkane-propelled MDIs or a different aerosol delivery form.
with inhalation of the aerosol is not clinically significant and reverses quickly. Bronchospasm resulting from chlorofluorocarbon propellants can be prevented by changing to newer hydrofluoroalkane-propelled MDIs or a different aerosol delivery form.
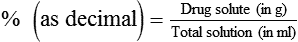
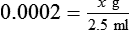
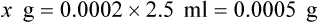
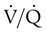 mismatching.
mismatching.