Acute Coronary Syndromes and Acute Myocardial Infarction
Definitions
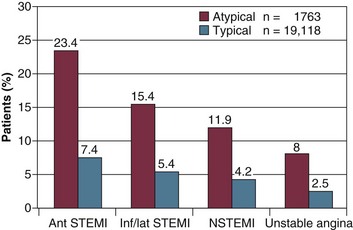
Acute coronary syndrome (ACS) refers to “any constellation of clinical symptoms that are compatible with acute myocardial ischemia.”1 Therefore, the ACS spectrum encompasses unstable angina (UA), non–ST segment elevation myocardial infarction (NSTEMI), and ST segment elevation myocardial infarction (STEMI). The presence or absence in the blood of either troponin or the MB fraction of creatine kinase (CK-MB) determines the distinction between a diagnosis of either UA or myocardial infarction (MI). (For convenience, these and other relevant abbreviations are listed in Table 30.1.) An expert consensus document titled the “Third Universal Definition of Myocardial Infarction” was published in 2012 by a joint task force of the European Society of Cardiology, the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA), and the World Heart Federation.2 The clinical diagnosis of an acute MI was defined as a rise or fall of cardiac biomarkers (preferably troponin) with at least one value above the 99th percentile of the upper reference limit (URL) together with evidence of myocardial ischemia with at least one of the following: symptoms of ischemia, ECG changes indicative of new ischemia (new ST-T changes or new left bundle branch block), development of pathologic Q waves in the electrocardiogram (ECG), or imaging evidence of new loss of viable myocardium or new regional wall motion abnormality.2 Definitions also exist for the diagnosis of an acute MI in three other circumstances: sudden death, after percutaneous coronary intervention (PCI), and after coronary artery bypass graft (CABG) surgery.2
Table 30.1
Cardiac Critical Care Abbreviations
| ACC | American College of Cardiology |
| ACCF | American College of Cardiology Foundation |
| ACE | angiotensin-converting enzyme |
| ACS | acute coronary syndrome |
| ACT | activated clotting time |
| AF | atrial fibrillation |
| AHA | American Heart Association |
| APSAC | anisoylated plasminogen-streptokinase activator complex |
| aPTT | activated partial thromboplastin time |
| ARB | angiotensin receptor blocker |
| AVB | atrioventricular block |
| BMS | bare metal stent |
| BNP | brain natriuretic peptide |
| CABG | coronary artery bypass grafting |
| CAD | coronary artery disease |
| CHF | congestive heart failure |
| CI | confidence interval |
| CK-MB | MB* fraction of creatine kinase |
| CT | computed tomography |
| DES | drug-eluting stent |
| ECG | electrocardiogram |
| EF | ejection fraction |
| ESC | European Society of Cardiology |
| GP | glycoprotein |
| HDL | high-density lipoprotein |
| IABP | intra-aortic balloon pump |
| ICH | intracranial hemorrhage |
| ICU | intensive care unit |
| ICD | implantable cardioverter-defibrillator |
| INR | international normalized ratio |
| IRA | infarct-related artery |
| LAD | left anterior descending (artery) |
| LBBB | left bundle branch block |
| LDL | low-density lipoprotein |
| LMWH | low-molecular-weight heparin |
| LVEF | left ventricular ejection fraction |
| MI | myocardial infarction |
| MR | mitral regurgitation |
| MRI | magnetic resonance imaging |
| NNT | number needed to treat |
| NO | nitric oxide |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| NSTEMI | non-ST segment elevation myocardial infarction |
| NTG | nitroglycerin |
| PA | pulmonary artery |
| PCI | percutaneous coronary intervention |
| PCWP | pulmonary capillary wedge pressure |
| RCA | right coronary artery |
| rPA | reteplase |
| RVMI | right ventricular myocardial infarction |
| SK | streptokinase |
| STEMI | ST segment elevation myocardial infarction |
| tPA | tissue plasminogen activator |
| UA | unstable angina |
| UFH | unfractionated heparin |
| VF | ventricular fibrillation |
| VSR | ventricular septal rupture |
| VT | ventricular tachycardia |
The increased sensitivity of troponin compared with CK-MB and the new criteria for the diagnosis of acute MI dictate that many patients who were classified as having UA by the old criteria are now given a diagnosis of acute MI. Among 1851 patients who were enrolled in a prospective study, 538 patients received a diagnosis of acute MI based on dynamic changes in troponin T, compared with only 427 patients when CK-MB was used to diagnose acute MI, representing a 41% increase.3 A retrospective analysis of 2181 patients with suspected ACS and no ST segment elevation found that the prevalence of acute MI ranged from 9.7% to 22% based on differing troponin-based definitions, compared with 7.8% based on CK-MB alone.4 Meier and colleagues5 studied 493 consecutive patients with suspected ACS. Of those, 224 patients had elevated CK-MB, and an additional 51 patients had normal CK-MB but elevated troponin I. The latter group was characterized by a greater incidence of comorbid conditions and higher 6-month mortality. Among 29,357 patients with non–ST segment elevation ACS who were enrolled in a registry called “Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines?” (CRUSADE), 18% of patients were CK-MB negative and troponin positive.6 The risk of in-hospital death was significantly increased among troponin-positive patients regardless of CK-MB status.
The Global Registry of Acute Coronary Events (GRACE) is a prospective observational registry of 26,267 patients with ACS who were admitted to 106 hospitals in 14 countries.7 (A list of eponyms in use for various cardiac registries and drug trials is provided in Table 30.2.) Among the 10,719 patients (10.4%) with both CK-MB and troponin data, 1110 patients without elevation of CK-MB were diagnosed with acute MI by virtue of elevated troponin. Patients who were troponin negative had similar 6-month mortality regardless of CK-MB status, but patients who were CK-MB negative and troponin positive had a twofold greater hospital case-fatality rate.
Table 30.2
Cardiac Drug Trial and Registry Eponyms
| 4S | Scandinavian Simvastatin Survival Study |
| ACUITY | Acute Catheterization and Urgent Intervention Triage Strategy |
| AIMI | AngioJet Rheolytic Thrombectomy in Patients Undergoing Primary Angioplasty for Acute Myocardial Infarction |
| AIRE | Acute Infarction Ramipril Efficacy |
| APRICOT | Antithrombotics in the Prevention of Reocclusion in Coronary Thrombolysis |
| ASPECT-2 | Antithrombotics in the Secondary Prevention of Events in Coronary Thrombosis-2 |
| ASSENT | Assessment of Safety and Efficacy of a New Thrombolytic |
| BHAT | Beta-Blocker Heart Attack Trial |
| CADILLAC | Controlled Abciximab and Device Investigation to Lower Angioplasty Complications |
| CAPRICORN | Carvedilol Post-Infarct Survival Control in LV Dysfunction |
| CARE | Cholesterol and Recurrent Events Trial |
| CAST | Cardiac Arrhythmia Suppression Trial |
| CLARITY | Clopidogrel as Adjunctive Reperfusion Therapy |
| COMMIT | Clopidogrel and Metoprolol in Myocardial Infarction Trial |
| CREATE | Clinical Trial of Reviparin and Metabolic Modulation in Acute Myocardial Infarction Treatment Evaluation |
| CRISP AMI | Counterpulsation to Reduce Infarct Size Pre-PCI Acute Myocardial Infarction |
| CRUSADE | Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines |
| CURE | Clopidogrel in Unstable Angina to Prevent Recurrent Events |
| DANAMI | Danish Multicenter Randomized Study on Fibrinolytic Therapy versus Acute Coronary Angioplasty for Acute Myocardial Infarction |
| DAVIT-II | Danish Verapamil Infarction Trial |
| Early ACS | The Early Glycoprotein IIb/IIIa Inhibition in Non-ST-Segment Elevation Acute Coronary Syndrome |
| EPHESUS | Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study |
| EXTRACT | Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment |
| FRISC | Fast Revascularization during Instability in Coronary Artery Disease |
| GISSI | Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico |
| GRACE | Global Registry of Acute Coronary Events |
| GUSTO | Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries |
| HINT | Holland Interuniversity Nifedipine/Metoprolol Trial |
| HORIZONS-AMI | Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction |
| ICTUS | Invasive versus Conservative Treatment in Unstable Coronary Syndromes |
| ISIS | International Study of Infarct Survival |
| LATE | Late Assessment of Thrombolytic Efficacy |
| LIPID | Long-Term Intervention with Pravastatin in Ischemic Disease Trial |
| MDPIT | Multicenter Diltiazem Postinfarction Trial |
| MERLIN | Middlesbrough Early Revascularization to Limit Infarction |
| MILIS | Multicenter Investigation of the Limitation of Infarct Size |
| MITI | Myocardial Infarction Triage and Intervention |
| NRMI | National Registry of MI |
| OASIS | Organization for the Assessment of Strategies for Ischemic Syndromes |
| OAT | Occluded Artery Trial |
| OPTIMAAL | Optimal Trial in Myocardial Infarction with the Angiotensin II Antagonist Losartan |
| PAMI | Primary Angioplasty in Myocardial Infarction |
| PAMI-II | Second Primary Angioplasty in Myocardial Infarction |
| PLATO | Study of Platelet Inhibition and Patient Outcomes |
| PROVE IT | Pravastatin or Atorvastatin Evaluation and Infection Therapy |
| PURSUIT | Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy |
| REACT | Rescue Angioplasty versus Conservative Treatment or Repeat Thrombolysis |
| RITA-3 | Randomized Intervention Trial of Unstable Angina |
| SAVE | Survival and Ventricular Enlargement |
| SHOCK | Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock? |
| SMILE | Survival of Myocardial Infarction Long-Term Evaluation |
| SWORD | Survival With Oral d-Sotalol |
| TACTICS | Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy |
| TIMACS | Timing of Intervention in Acute Coronary Syndrome |
| TIMI | Thrombolysis in Myocardial Infarction |
| TRACE | Trandolapril Cardiac Evaluation |
| TRANSFER-AMI | Trial of Routine Angioplasty and Stenting after Fibrinolysis to Enhance Reperfusion in Acute Myocardial Infarction |
| TRITON-TIMI 38 | Trial to Assess Improvements in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38 |
| VALIANT | Valsartan in Acute Myocardial Infarction |
| VANQWISH | Veterans Affairs Non-Q Wave Infarction Strategies in Hospital |
| WARIS II | Warfarin, Aspirin, Reinfarction Study |
The advent of highly sensitive troponin assays has contributed to further changes in the detection of acute MI in patients with myocardial ischemia.8–10 Bonaca and colleagues8 conducted a prospective study of the prognostic value of a sensitive assay for cardiac troponin I in 4513 patients with non–ST segment elevation ACS who were enrolled in a randomized trial of ranolazine versus placebo. Patients with low-level increases of serum troponin I (0.04 mcg/L to < 0.1 mcg/L) had a significantly higher risk of death at 12 months (6.4% versus 2.4%, p = 0.005). Another study found that implementation of a sensitive assay for troponin I in patients with suspected ACS increased the detection of MI by 29%, identified patients who were at the highest risk of recurrent MI and death, and was associated with improved clinical management that resulted in fewer deaths and admissions with recurrent MI.9
ST Segment Elevation Myocardial Infarction
Clinical Manifestations
Clinical History
The initial differentiation of ACS from other causes of chest pain is based on the chest pain history, physical examination, presence of risk factors for CAD, and the ECG. Certain chest pain characteristics are associated with decreased or increased likelihoods of ACS.11 The feature that was found to be associated with the highest risk of a diagnosis of ACS is radiation of pain to one or both shoulders or arms. A prospective study of patients who presented to an emergency department for evaluation of chest pain determined that pain relief by nitroglycerin is not a useful indicator of the presence or absence of ACS.12 Nitroglycerin relieved chest pain in 35% of patients with active CAD, compared with 41% of patients without active CAD (p > 0.2).12
Another literature review included 15 studies published from 1989 to 2002 that identified symptoms of ACS.13 Chest pain was the most common symptom among both men and women, but atypical symptoms were common, especially among women. Compared with men, women with ACS were significantly more likely to report back and jaw pain, nausea, vomiting, dyspnea, indigestion, and palpitations. A significant fraction of patients with acute MI do not complain of chest pain at the time of presentation.14–16 A total of 1674 U.S. hospitals contributed patients to the National Registry of Myocardial Infarction (NRMI) 2.14 Among 434,877 patients with confirmed MI who were enrolled in the NRMI-2 registry between June 1994 and March 1998, 142,445 (33%) did not have chest pain at the time of presentation to the hospital. There were several notable differences between the groups who presented with and without chest pain. Only 23% of patients without chest pain had ST segment elevation on the initial ECG, compared with 47% of the patients with chest pain. The group of patients who presented without chest pain was older (74 versus 67 years) and had a higher proportion of women (49% versus 38%). A subsequent analysis of 1,143,513 patients who were enrolled in the registry found that the proportion of patients who presented without chest pain was significantly higher for women than men (42% versus 30.7%; p < 0.001).16 The gender differences in clinical presentation without chest pain diminished with increasing age.16
The absence of chest pain has a major impact on hospital management and outcomes, even among patients who present with ST segment elevation.14–16 A report from the GRACE registry compared 6385 patients with STEMI and typical symptoms with 541 patients whose presenting symptoms did not include chest pain.15 Patients without chest pain were significantly less likely to receive reperfusion therapy (i.e., fibrinolysis or primary PCI, β-blockers), and aspirin. Perhaps as a consequence of undertreatment, hospital mortality was significantly greater among patients with STEMI and no chest pain than among patients with chest pain (18.7% versus 6.3%; p > 0.001) (Fig. 30.1).
There is a longstanding belief that diabetes is associated with silent myocardial ischemia and painless MI due to autonomic neuropathy. Nevertheless, in the NRMI 2 registry, only 33% of the patients with painless MI had diabetes mellitus, and in the GRACE registry, only 32% of patients with painless ACS had diabetes.14,15
Physical Examination
The initial physical examination provides important prognostic information in patients with acute MI. Killip and Kimball published their classic study in 1967.17 Among 250 patients with acute MI, 81 patients (33%) had no heart failure (Killip class I), 96 (38%) had mild heart failure (Killip class II), 26 (10%) had pulmonary edema (Killip class III), and 47 (19%) had cardiogenic shock (Killip class IV). Respective mortality rates were 6%, 17%, 38%, and 81%. Although the overall mortality for acute MI has decreased since 1967, the Killip class on admission remains a powerful predictor of outcome among patients treated with reperfusion therapy.18,19 DeGeare and coworkers19 performed an analysis of 2654 patients with acute MI who were enrolled in three primary angioplasty trials. Patients in Killip class IV were excluded. Increasing Killip class was associated with an increased need for intra-aortic balloon counterpulsation and a greater incidence of renal failure, major arrhythmias, and major bleeding. After controlling for confounding variables, the Killip class on admission remained a multivariate predictor of both in-hospital and 6-month mortality.
Diagnostic Approach
The American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines has published detailed recommendations for the diagnosis and management of patients with ACS.1,20–24 Conditions for which there is evidence, general agreement, or both that a given procedure or treatment is useful and effective are categorized as Class I1,20 (not to be confused with Killip class I). Conditions for which there is conflicting evidence or divergence of opinion are categorized as Class II. The weight of evidence or opinion is in favor of usefulness or efficacy for Class IIa conditions, whereas usefulness or efficacy is less well established for Class IIb conditions. Class III conditions are those for which there is evidence or general agreement that a procedure/treatment is not useful or effective and may be harmful in some cases.
Electrocardiogram
The ACC/AHA Guidelines for the Management of Patients with ST-Elevation Myocardial Infarction include three Class I indications for an ECG.20 The first is that all patients with chest discomfort or other symptoms suggestive of STEMI should have a 12-lead ECG within 10 minutes of arrival in the emergency department (and it should be interpreted by an experienced physician). The second is that serial ECGs should be performed at intervals of 5 to 10 minutes in patients with a nondiagnostic initial ECG if the patient remains symptomatic and there is a high clinical suspicion of STEMI. The third is that right-sided ECG leads should be obtained to screen for right ventricular MI in patients with inferior STEMI.
The electrocardiographic diagnosis of acute MI in the presence of a left bundle branch block (LBBB) is problematic.25,26 Angiographic studies have demonstrated a low prevalence of acute MI among patients with a new LBBB.27–30 Analysis of the Mayo Clinic’s primary PCI database found that only 12 of 36 patients with a new LBBB and clinical symptoms suspicious for an MI met troponin criteria for an MI, resulting in emergency activation of the cardiac catheterization laboratory for a false-positive diagnosis of acute MI in two thirds of patients with a new LBBB.28
Sgarbossa and associates31 devised an algorithm for the diagnosis of acute MI in patients with LBBB that used three electrocardiographic criteria: ≥ 1 mm ST segment elevation concordant with the QRS complex; ≥ 1 mm ST segment depression in leads V1, V2, or V3; and ≥ 5 mm ST segment elevation discordant with the QRS complex. Several subsequent studies investigated the utility of the so-called Sgarbossa criteria.26,29 A study that enrolled 83 patients with LBBB and symptoms suggestive of acute MI found that the ECG algorithm based on the Sgarbossa criteria had a sensitivity of only 10%.26
Patients with an acute MI who present with an LBBB that is new or of indeterminate age have a worse prognosis than patients without an LBBB. A bundle branch block was present on the admission electrocardiogram in 4% of the patients with a suspected acute MI who were included in a meta-analysis of nine trials that randomized 58,600 patients to either a control group or fibrinolytic therapy.32 Mortality at 35 days for patients who were randomized to the control groups was greater among patients who presented with a bundle branch block (23.6%), compared with patients who had ST segment elevation in the anterior leads (16.9%) or inferior leads (8.4%), or ST segment depression 13.8%).32 Fibrinolytic therapy was associated with a 25% reduction in mortality among patients who presented with a bundle branch block on ECG.32 The publication did not provide information regarding the age of the bundle branch block or whether the meta-analysis was limited to patients with an LBBB or also included patients with a right bundle branch block.32 Among 3053 patients with an acute MI who were enrolled in the Primary Angioplasty in Myocardial Infarction (PAMI) trials, an LBBB was an independent predictor of in-hospital death (odds ratio 5.53; 95% confidence interval [CI] 1.89 to 16.1; p = 0.002).33
Patients with LBBB and acute MI frequently have no chest pain at the time of presentation. Chest pain was not reported by 47% of the 29,585 patients with LBBB and acute MI who were enrolled in the National Registry of Myocardial (NRMI) 2 registry.34 Patients who presented without chest pain were less likely to receive aspirin or a β-blocker and four times less likely to receive reperfusion therapy (odds ratio 0.25).34 This may explain why patients with acute MI and LBBB who presented without chest pain had a 47% greater in-hospital mortality rate than patients who presented with chest pain (27% versus 18%; p < 0.001).34
A new or presumably new LBBB in patients with symptom onset within the prior 12 hours was designated as a Class I indication for either fibrinolytic therapy or primary percutaneous coronary intervention (PCI) in the guidelines for the management of acute MI that were issued by the American College of Cardiology/American Heart Association Task Force on Practice Guidelines in 2004.20 According to the most recent revision of the practice guidelines for ST elevation MI, a new or presumably new LBBB “should not be considered diagnostic of acute MI in isolation.”24 The risks of fibrinolytic therapy may be increased in patients with LBBB due to older age and a higher prevalence of hypertension. Therefore, the most prudent strategy in patients with LBBB and a suspected acute MI may be immediate coronary angiography, both to diagnose an acute coronary artery occlusion and to eliminate the risk of fibrinolytic therapy in patients who cannot benefit from it (i.e., patients with a non–ST segment elevation MI or a non-cardiac condition). A more detailed discussion of the management of patients with LBBB and suspected MI can be found in an excellent review article that was published in 2012.25
The ECG provides additional important information in patients with acute MI. Patients with acute inferior STEMI who have ST segment depression in the precordial leads have larger infarctions, more complications post-MI, and a higher mortality rate than patients without precordial ST segment depression.35 The presence of Q waves in the infarct territory on the initial ECG is an independent predictor of greater 30-day mortality irrespective of the infarct location or time between symptom onset and administration of fibrinolytic therapy.36 Nevertheless, substantial myocardial salvage is possible despite Q waves on the initial ECG.37
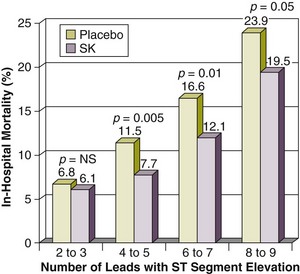
The electrocardiographic leads with ST segment elevation have been correlated with occlusions of the left anterior descending (LAD), left circumflex, or right coronary arteries.38,39 The number of leads with ST segment elevation before reperfusion therapy and the degree of resolution of ST segment elevation after either fibrinolytic therapy or primary angioplasty confer useful prognostic information. The Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI) trial investigators reported that in-hospital mortality was directly related to the number of leads with ST segment elevation for both the patients treated with streptokinase and the control group.40 Treatment with streptokinase significantly reduced in-hospital mortality among patients with ST segment elevation in four or more leads, but not among patients with ST segment elevation that was confined to two or three leads40 (Fig. 30.2). Early ST segment recovery is associated with improved infarct zone wall motion41 and greater myocardial salvage as assessed by technetium-99m sestamibi scintigraphy.42 Also, resolution of ST segment elevation within 90 minutes after either primary angioplasty or fibrinolytic therapy identifies patients with lower mortality at 30 days and 1 year and 5 years after STEMI.43–46 Continuous ECG monitoring is customary for the detection of arrhythmias and conduction abnormalities.
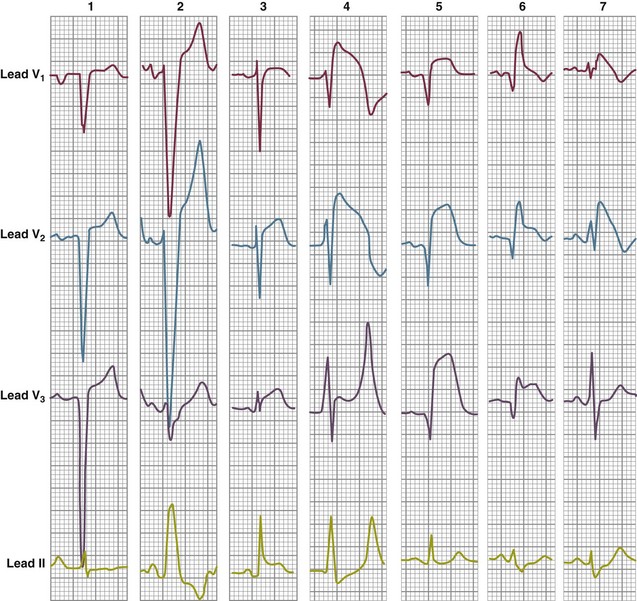
It is important to recognize that ST segment elevation occurs in numerous conditions other than acute MI.47 The list includes left ventricular hypertrophy, LBBB, acute pericarditis, hyperkalemia, Brugada syndrome, pulmonary embolism, and left ventricular apical ballooning syndrome (takotsubo cardiomyopathy)47,48 (Fig. 30.3).
Cardiac Enzymes
Myocardial necrosis is accompanied by the release of several biochemical markers in circulating blood, including creatine kinase, myoglobin, troponins T and I, and lactate dehydrogenase. As noted previously, a typical rise and gradual fall of troponin or more rapid rise and fall of CK-MB are required to diagnose an acute, evolving, or recent MI.2 The ACC/AHA guidelines, however, stress that decisions such as initiation of reperfusion therapy for patients with ST segment elevation and symptoms of STEMI should not be delayed until the results of serum cardiac biomarkers are available.49
Although troponin has become the preferred biomarker for myocardial necrosis, numerous other causes of an elevated troponin have been recognized, and several may be associated with chest pain or ST segment elevation.50 An elevated troponin in patients with pulmonary embolism is associated with right ventricular dysfunction and an increased risk of hypotension and death.51–53 Elevated cardiac troponin also has been reported in patients with acute pericarditis, and patients with ST segment elevation were more likely to have an elevated troponin.54
Echocardiography
Echocardiography may be a useful diagnostic tool under a variety of circumstances. A transesophageal echocardiogram may be useful to differentiate STEMI from aortic dissection. Both transthoracic and transesophageal echocardiography are useful in patients with congestive heart failure (CHF) or hypotension to evaluate left and right ventricular function, to rule out cardiac tamponade, and to diagnose ventricular septal rupture or mitral regurgitation (MR). Mitral regurgitation is frequent among patients with uncomplicated MI. Color Doppler echocardiography was performed within 48 hours of admission in a series of 417 consecutive patients with acute MI.55 Mild mitral regurgitation was present in 121 patients (29%), moderate mitral regurgitation in 21 (5%), and severe mitral regurgitation in 4 (1%).55 Patients with any mitral regurgitation had higher 30-day and 1-year mortality rates, and mitral regurgitation was independently associated with increased 1-year mortality.55 Echocardiography performed within 30 days after acute MI revealed mitral regurgitation in 50% of a cohort of 773 patients.56 Cardiac auscultation did not detect a murmur in 54% of patients with mild and 31% of patients with moderate or severe mitral regurgitation.56 Among 30-day survivors of an MI, during a mean follow-up period of 4.7 years moderate or severe mitral regurgitation detected by echocardiography within 30 days of MI was associated with a 55% increase in the relative risk (RR) of death independent of age, gender, left ventricular ejection fraction (EF), and Killip class.56
Hemodynamic Monitoring
The value of pulmonary artery catheterization in critically ill patients has been questioned.57 The evidence base regarding the impact of pulmonary artery catheters on outcome in patients with acute MI is limited to retrospective studies because pulmonary artery catheterization in patients with acute MI has not been evaluated in a prospective, randomized, controlled trial.58–60 Cohen and associates60 performed a retrospective analysis of pulmonary artery catheterization in patients with ACS who were enrolled in two large international randomized clinical trials, Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries (GUSTO) Ilb and GUSTO III. The study compared the outcomes in 735 patients who received PA catheters with those in 25,702 patients who did not. Except for patients with cardiogenic shock, mortality at 30 days was significantly greater among patients who received pulmonary artery catheters, both before and after adjustment for baseline differences and subsequent events that may have prompted insertion of a pulmonary artery catheter.
According to the ACC/AHA Guidelines, the Class I indications for pulmonary artery catheter monitoring are (1) progressive hypotension that either is unresponsive to fluid administration or is developing in a patient in whom fluid administration is contraindicated and (2) a suspected mechanical complication, such as a VSD or papillary muscle rupture, if an echocardiogram has not been performed.20 Intra-arterial pressure monitoring is recommended for patients with systolic blood pressure less than 80 mm Hg, patients with cardiogenic shock, and patients receiving vasopressor and inotropic drugs.20
Diagnostic Cardiac Catheterization and Coronary Angiography
Right heart catheterization and contrast ventriculography can provide useful diagnostic information in patients with suspected acute MI. Measurement of right heart pressures is useful in patients with suspected right ventricular MI and in patients with hypotension. Measurement of the oxygen content of blood in the right atrium and pulmonary artery is useful in patients with a suspected VSD. A contrast left ventriculogram provides an assessment of regional and global left ventricular function and the competence of the mitral valve. Left ventriculography was performed during the index cardiac catheterization in 1976 (95%) of 2082 patients with acute MI who were enrolled in the Controlled Abciximab and Device Investigation to Lower Angioplasty Complications (CADILLAC) trial.61 Mild mitral regurgitation was present in 192 patients (9.7%), and moderate or severe mitral regurgitation was present in 58 patients (2.9%). Mitral regurgitation was not detected by physical examination in 50% of a cohort of 50 patients with acute MI and moderately severe or severe mitral regurgitation that was demonstrated by left ventriculography.62
Numerous studies have addressed the role of routine early angioplasty after fibrinolytic therapy.63–68 A prospective cohort study of 21,912 patients with a first acute MI concluded that revascularization within 14 days of the acute MI was associated with a significant reduction in 1-year mortality (RR 0.47; 95% CI 0.37 to 0.60; p < 0.001).63 Several randomized trials have shown beneficial effects of a routine invasive strategy immediately after fibrinolysis,65 within 24 hours after fibrinolysis,66 and 1 to 6 weeks after acute MI.67 The most recent trials have provided support for the practice of routine early angioplasty after fibrinolytic therapy.68–70 The Trial of Routine Angioplasty and Stenting after Fibrinolysis to Enhance Reperfusion in Acute Myocardial Infarction (TRANSFER-AMI) enrolled 1059 patients with a “high-risk” STEMI who received fibrinolytic therapy at centers that did not have the capability to perform PCI.68 The patients were randomized to either “standard” treatment (including rescue PCI or delayed angiography) or immediate transfer to another hospital for PCI within 6 hours after fibrinolysis. The primary end point—the composite of death, reinfarction, recurrent ischemia, new or worsening congestive heart failure, or cardiogenic shock within 30 days—occurred in 11% of the patients who were assigned to routine early PCI, compared with 17.2% of the patients who were assigned to standard treatment (RR 0.64; 95% CI 0.47 to 0.87; p = 0.004). Borgia and colleagues69 performed a meta-analysis of seven randomized, controlled trials that compared routine early PCI after successful fibrinolysis with PCI only for patients without evidence of reperfusion (rescue PCI). After a follow-up period of 30 days routine early PCI after successful fibrinolysis reduced the rates of reinfarction (odds ratio 0.55; 95% CI 0.36 to 0.82; p = 0.003), the combined end point of death and reinfarction (odds ratio 0.65; 95% CI 0.49 to 0.88; p = 0.004), and recurrent ischemia (odds ratio 0.25; 95% CI 0.13 to 0.49; p < 0.001). The benefits of early PCI persisted after 6 to 12 months of follow-up. D’Souza and associates70 performed a meta-analysis of eight randomized trials that compared routine early PCI with ischemia-driven PCI after fibrinolysis in patients with STEMI. PCI within 24 hours after fibrinolytic therapy was associated with less re-infarction and recurrent ischemia.
The 2004 and 2007 Focused Update of the ACC/AHA Guidelines for the management of patients with STEMI include five Class I recommendations, two Class IIa recommendations, and one Class IIb recommendation for coronary angiography in patients with acute MI20,21 (Box 30.1). Coronary angiography is recommended in survivors of STEMI who are candidates for revascularization therapy with spontaneous ischemia, intermediate-risk or high-risk findings on noninvasive testing, hemodynamic or electrical instability, mechanical defects, prior revascularization, or high-risk clinical features.
Approach to Management
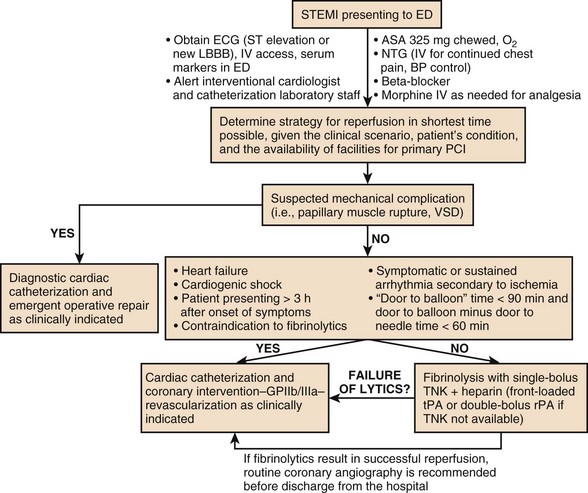
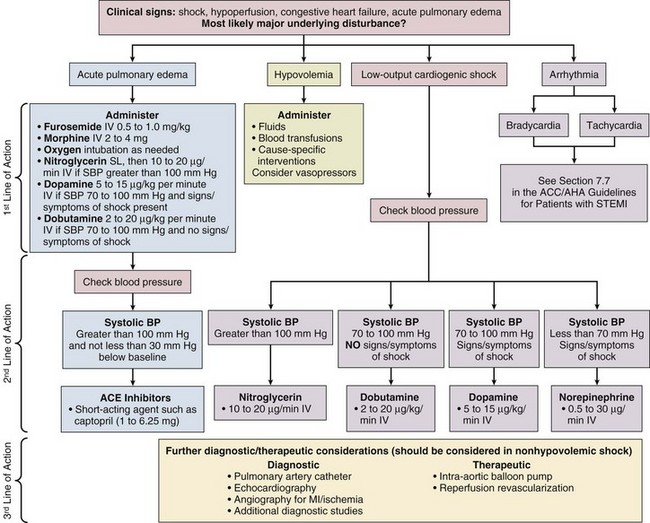
Figure 30.4 presents an algorithm for the treatment of acute STEMI.
Oxygen
The effects of supplemental oxygen on ischemic injury was studied by Madias and colleagues.71 Seventeen patients with acute anterior MI who were not in cardiogenic shock underwent precordial ST segment mapping before and after inhalation of 100% oxygen for 1 hour. The mean arterial partial pressure of oxygen increased from 70 mm Hg on room air to 278 mm Hg during oxygen inhalation. During oxygen inhalation there was a 16% reduction in the sum of all ST segment elevation, with reversion to baseline after oxygen was discontinued. Two hundred patients with suspected acute MI were enrolled in a double-blind, randomized trial of supplemental oxygen versus compressed air.72 No apparent benefit was observed for oxygen therapy, and the mortality rate was higher in the oxygen group than in the control group (9/80 versus 3/77; p = NS). A meta-analysis of 3 trials that enrolled 387 patients found that the pooled relative risk of death for patients who were treated with oxygen compared to air was 2.88 (95% CI 0.88 to 9.83).21 Hyperoxia during inhalation of high concentrations of oxygen causes an increase in coronary vascular resistance and a decrease in coronary blood flow.73
Although supplemental oxygen is routinely administered to patients with STEMI, according to the ACC/AHA guidelines the only Class I indication for this intervention is an arterial oxygen saturation less than 90%.20 The guidelines also include a Class IIa indication: “It is reasonable to administer supplemental oxygen to all patients with uncomplicated STEMI during the first 6 hours.”20 Randomized, controlled trials of oxygen therapy in patients with STEMI are planned.74
Analgesia and Sedation
Relief of pain is an important goal in patients with acute MI. The 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients with ST-Elevation Myocardial Infarction included several recommendations regarding analgesic drugs.21 According to the guidelines, morphine sulfate is the analgesic of choice for the management of pain associated with STEMI, and pain associated with STEMI is a Class I indication for intravenous morphine.21 There are no published randomized trials of morphine therapy in patients with acute MI. An analysis of the CRUSADE registry, however, revealed that use of morphine was associated with a 50% higher mortality in patients with NSTEMI even after risk adjustment.75 One proposed mechanism of morphine’s adverse effect is opioid-induced cortisol deficiency.76 Until additional data become available, it may be prudent to limit the use of morphine to patients with persistent pain despite treatment with nitrates and a β-adrenergic antagonist.
The abundant evidence that nonsteroidal anti-inflammatory drugs (NSAIDs) have adverse effects in patients with cardiovascular disease has been reviewed in great detail in multiple publications, including a scientific statement from the American Heart Association and a meta-analysis of 31 trials that enrolled 116,429 patients.77–79 A Danish study of 58,432 patients who were hospitalized for a first-time acute MI between 1995 and 2002 found that treatment with either a selective cyclooxgyenase-2 inhibitor or a nonselective NSAID after discharge from the hospital significantly increased the risk of death.80 A subsequent study by the same investigators found that even short-term treatment with NSAIDs was associated with an increased risk of death and recurrent MI in patients with a prior MI.81 One possible explanation for the adverse cardiovascular effects of NSAIDs is inhibition of the clinical benefits of aspirin. An analysis of the Physicians’ Health Study concluded that there was greater than a twofold increased risk of a first MI among healthy male U.S. physicians who were randomized to aspirin and also took other NSAIDs on > 60 days per year.82 An important pharmacologic study demonstrated that inhibition of platelet aggregation by aspirin was blocked when ibuprofen was administered before aspirin.83 The 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients with ST-Elevation Myocardial Infarction included two new recommendations regarding both nonselective and cyclooxygenase-2 selective NSAIDs: a Class I recommendation that patients routinely taking NSAIDs (except for aspirin) before a STEMI should have those agents discontinued at the time of presentation with STEMI; and a Class III recommendation that NSAIDs (except for aspirin) “should not be administered during hospitalization for STEMI because of the increased risk of mortality, reinfarction, hypertension, heart failure, and myocardial rupture associated with their use.”21
Nitrates
The ability of sublingual or intravenous nitroglycerin to relieve chest pain in patients with acute MI is well documented.84 The beneficial physiologic effects of nitrates include vasodilation of peripheral arteries and veins, causing reductions in pulmonary capillary wedge pressure (PCWP), mean arterial pressure, and peripheral vascular resistance, thereby decreasing left ventricular preload and afterload and myocardial oxygen demand.85 Also, vasodilation of the coronary arteries may improve myocardial oxygen supply, especially in patients with a component of coronary spasm.86 Severe hypotension and bradycardia have been observed after administration of either sublingual or intravenous nitroglycerin in patients with acute MI.87 Patients with right ventricular MI may experience severe hypotension during administration of nitroglycerin because adequate right ventricular preload is required to maintain cardiac output. Nitroglycerin is contraindicated in patients who have taken phosphodiesterase inhibitors because they potentiate nitroglycerin-induced hypotension.88
There are two Class I indications for nitroglycerin in patients with STEMI. Sublingual nitroglycerin (0.4 mg) every 5 minutes for a total of three doses is recommended for relief of ischemic discomfort.20 Intravenous nitroglycerin is indicated for relief of ongoing ischemic discomfort, control of hypertension, or management of pulmonary congestion.20 It has been proposed that intravenous nitroglycerin may limit myocardial infarct size and expansion.89 Two large clinical trials, however, were unable to demonstrate significant improvements in mortality by the prolonged administration of nitroglycerin after acute MI.90,91 The GISSI-3 trial enrolled 19,394 patients with acute MI.90 Patients who were randomized to treatment with nitroglycerin received intravenous nitroglycerin for 24 hours, followed by transdermal nitroglycerin for 6 weeks.90 Nitroglycerin did not reduce the 6-week rate of death or clinical heart failure. The Fourth International Study of Infarct Survival (ISIS-4) enrolled 58,050 patients with suspected acute MI in a 2 × 2 × 2 factorial study that included randomization to isosorbide mononitrate 60 mg daily or placebo for 28 days.91 No significant effect of nitroglycerin on mortality was found after 5 weeks or 1 year.
Several studies have investigated the effect of another nitrate, nitroprusside, on hemodynamics and outcome in patients with acute MI.92–95 Intravenous nitroprusside reduced PCWP and increased cardiac index in patients with acute MI.92,93 A comparison of intravenous nitroprusside with intravenous nitroglycerin in 10 patients with acute anterior MI demonstrated that ST segment elevation increased during infusion of nitroprusside, whereas it decreased during infusion of nitroglycerin.92 Experimental data indicate that nitroprusside may exacerbate myocardial ischemia or injury by redistribution of myocardial blood flow from ischemic to nonischemic zones.92 A Veterans Administration Cooperative Study enrolled 812 patients with acute MI and a PCWP greater than 12 mm Hg in a double-blind, randomized trial of nitroprusside infused for 48 hours.95 Compared with the placebo group, mortality at 13 weeks was increased by nitroprusside in patients whose infusions started within 9 hours of the onset of pain. A smaller European trial randomized 328 patients with acute MI to infusion of nitroprusside or 5% glucose.94 The trial was terminated when 1-week mortality in the control group was significantly greater than in the nitroprusside group (10.9% versus 3.1%; p < 0.05). The use of nitroprusside in patients with acute MI should probably be reserved for patients with severe hypertension that is unresponsive to treatment with intravenous nitroglycerin.
Aspirin
The Second International Study of Infarct Survival (ISIS-2) provided definitive evidence that aspirin reduces mortality in patients with acute MI.96 The study used a 2 × 2 factorial design to randomize 17,187 patients to four treatment groups: streptokinase, aspirin 160 mg daily for 1 month, both, or neither. Aspirin reduced the rate of in-hospital reinfarction both in the patients who received streptokinase and in the patients who did not receive fibrinolytic therapy. At 35 days, the vascular-cause mortality rate was 9.4% among the patients in the aspirin treatment group patients, compared with 11.8% among those in the placebo group, representing a 23% reduction (p < 0.00001). Aspirin also significantly reduced all-cause mortality. Also, the combination of aspirin and streptokinase reduced mortality more than did either agent alone. The effects of the initial dose of aspirin on short-term outcomes after fibrinolytic therapy were tested by analyzing the outcomes of 48,422 patients with STEMI who were enrolled in two large clinical trials, Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) I and GUSTO III.97 Compared with an initial dose of 162 mg, an initial dose of 325 mg was associated with a significant increase in moderate or severe bleeding in-hospital, but the rates of reinfarction and death at 24 hours, 7 days, and 30 days were not significantly different.97 Nevertheless, the ACC/AHA Practice Guidelines for the Management of Patients with STEMI recommend that patients who present with acute STEMI who have not taken aspirin should receive 162 to 325 mg of non-enteric-coated aspirin, and the aspirin tablets should be chewed.20 Also, the 2011 update of the guidelines for PCI include a Class IIa recommendation that “After PCI, it is reasonable to use 81 mg of aspirin per day in preference to higher maintenance doses.”98
Reocclusion of a patent infarct artery after successful fibrinolytic therapy is associated with higher in-hospital mortality, reduced event-free survival after hospital discharge, and long-term impairment of regional and global left ventricular function.99–102 There are conflicting opinions regarding aspirin’s effect on reocclusion of an infarct artery.103,104 The Antithrombotics in the Prevention of Reocclusion in Coronary Thrombolysis (APRICOT) study randomized 300 patients with an open infarct artery within 48 hours after fibrinolysis to three treatment groups: aspirin 325 mg daily, warfarin, or placebo.105 Cardiac catheterization was performed 3 months later in 248 patients. The reocclusion rates were not significantly different: 32% (24/74) with placebo, 30% (24/81) with warfarin, and 25% (23/93) with aspirin. A pooled analysis of published studies estimated that the incidence of reocclusion after streptokinase or tissue plasminogen activator (tPA) is approximately 11% with aspirin, compared with 25% without aspirin.103
Inhibitors of the Platelet P2Y12 Receptor
The combination of aspirin with inhibitors of the platelet P2Y12 receptor has been shown to be superior to aspirin alone in patients with a STEMI.106–108 The Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY) study enrolled 3491 patients who received fibrinolytic therapy for STEMI and randomized them to receive clopidogrel 75 mg daily or placebo in a double-blind fashion.106 Coronary angiography performed at a median of 84 hours after randomization in each group demonstrated an occluded IRA in 18.4% of the placebo group patients, compared with 11.7% of the clopidogrel group patients (p < 0.001). PCI was performed during the index hospitalization in 1863 (53.4%) of the patients who were enrolled in the CLARITY trial.108 The combined incidence of cardiovascular death, recurrent MI, or stroke from PCI to 30 days after randomization was significantly lower among patients who were treated with clopidogrel and aspirin compared with the patients who received aspirin alone (3.6% versus 6.2%; adjusted odds ratio 0.54; 95% CI 0.35 to 0.85; p = 0.008).108 The Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT) randomized 45,852 patients with suspected acute MI to receive treatment with aspirin 162 mg daily plus clopidogrel 75 mg daily or placebo.107 The in-hospital mortality rate was significantly lower for the clopidogrel group than for the placebo group (7.5% versus 8.1%; p = 0.03). The CLARITY study used a clopidogrel loading dose of 300 mg; the COMMIT study did not employ a loading dose.
A multivariate-weighted logistic regression analysis of the outcomes of 8429 STEMI patients who were enrolled in 26 randomized clinical trials concluded that pretreatment with a loading dose of clopidogrel before primary PCI was an independent predictor of coronary artery patency before PCI (odds ratio 1.51; 95% CI 1.31 to 1.74; p < 0.0001) and decreased mortality after PCI (odds ratio 0.57; 95% CI 0.38 to 0.85; p = 0.0055).109 Among patients with STEMI who were enrolled in the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial and underwent primary PCI, a clopidogrel loading dose of 600 mg (n = 2158), compared with 300 mg (n = 1153) was associated with significantly lower 30-day rates of mortality, reinfarction, and stent thrombosis, and was an independent predictor of freedom from major adverse cardiac events at 30 days.110
Prasugrel, another inhibitor of the platelet P2Y12 receptor, was studied in patients with ACS, including patients with STEMI, in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction (TRITON-TIMI) 38.111,112 The trial randomized 3534 patients with STEMI who were undergoing either primary PCI (PCI within 12 hours of symptom onset) or secondary PCI (PCI between 12 hours and 14 days after symptom onset) to either prasugrel (60 mg loading dose and 10 mg/day maintenance dose) or clopidogrel (300 mg loading dose and 75 mg maintenance dose) for 6 to 15 months.112 The primary end point of cardiovascular death, nonfatal MI, or nonfatal stroke, was significantly less frequent at both 30 days and 15 months among patients who were randomized to prasugrel compared with patients who were randomized to clopidogrel.112
Ticagrelor, an inhibitor of the platelet P2Y12 receptor, was studied in patients with ACS, including patients with STEMI, in the Study of Platelet Inhibition and Patient Outcomes (PLATO).113,114 The trial randomized 7544 patients with STEMI who were undergoing primary PCI to either ticagrelor (180 mg loading dose and 90 mg twice daily maintenance dose) or clopidogrel (300 mg loading dose and 75 mg maintenance dose) for 6 to 12 months.114 Compared with clopidogrel, treatment with ticagrelor reduced several secondary end points, including MI (hazard ratio 0.80; p = 0.03), total mortality (hazard ratio 0.82; p = 0.05), and definite stent thrombosis (hazard ratio 0.66; p = 0.03); major bleeding was not significantly different (hazard ratio 0.98; p = 0.76).114
The 2007 and 2009 focused updates of the ACC/AHA Practice Guidelines for the Management of Patients with STEMI include several new recommendations regarding antiplatelet therapy (Box 30.2).21,22 The Class I recommendations include the following: (1) clopidogrel 75 mg/day orally should be added to aspirin in patients with STEMI regardless of whether they undergo reperfusion with fibrinolytic therapy or do not receive reperfusion therapy. Treatment with clopidogrel should continue for at least 14 days. (2) A loading dose of a P2Y12 inhibitor is recommended for STEMI patients for whom PCI is planned. The options include clopidogrel, prasugrel, and ticagrelor. Prasugrel is contraindicated in patients with a history of TIA or stroke and active pathologic bleeding. Also, prasugrel should not be administered to patients older than age 75 because of an increased risk of fatal and intracranial bleeding. Finally, the maintenance dose of prasugrel should be reduced to 5 mg daily in patients who weigh less than 60 kg.
Anticoagulant Therapy
The rationale for anticoagulant therapy in patients with STEMI includes promotion of infarct artery patency, and prevention of deep vein thrombosis, pulmonary embolism, left ventricular mural thrombus, and cerebral embolism. Left ventricular mural thrombus formation after acute MI occurs more commonly after anterior than nonanterior wall MI and is associated with an increased risk of systemic embolization.115,116 Data conflict regarding the incidence of left ventricular thrombus in patients who receive reperfusion therapy. The GISSI-2 study, in which all patients received fibrinolytic therapy, observed left ventricular thrombi in 51 of 180 consecutive patients with a first anterior acute MI who underwent serial echocardiography within 48 hours after the onset of symptoms and before hospital discharge.117 Another study, however, detected left ventricular thrombi in only 6.4% of patients with acute anterior MI who underwent echocardiography on days 1, 14, and 90 after MI.118 A double-blind, randomized trial compared a 10-day course of high-dose subcutaneous UFH (12,500 units every 12 hours) with low-dose subcutaneous UFH (5000 units every 12 hours) in the prevention of left ventricular thrombus in 221 patients with acute anterior MI who did not receive fibrinolytic therapy.119 Echocardiography 10 days after MI demonstrated left ventricular thrombi in 10 of 95 patients (11%) in the high-dose group and in 28 of 88 patients (32%) in the low-dose group (p = 0.0004). A meta-analysis of seven studies that enrolled 270 patients suggests that systemic anticoagulation in patients with mural thrombi reduces embolic complications.115
Clinical trials have evaluated both subcutaneous and intravenous unfractionated heparin (UFH) in patients with acute MI who were treated with various fibrinolytic agents. Randomized, controlled clinical trials have shown that adjunctive therapy with intravenous UFH increases the patency of the IRA after administration of tPA.120,121 A meta-analysis that included 68,000 patients who were enrolled in randomized trials that compared UFH plus aspirin with aspirin alone showed that only 5 lives were saved per 1000 patients who received UFH in addition to streptokinase.122 The meta-analysis was heavily influenced by two studies, GISSI-2123 and ISIS-3,124,125 that enrolled 62,067 patients who were randomly assigned to receive fibrinolytic therapy plus either aspirin alone or aspirin plus subcutaneous UFH. Another meta-analysis was limited to six randomized controlled trials that enrolled 1735 patients who received either intravenous UFH or no heparin after fibrinolytic therapy.126 The analysis found that the addition of intravenous UFH to tPA or streptokinase had insignificant effects on mortality and reinfarction, but the risk of bleeding was significantly increased.126
Several randomized clinical trials127–131 and meta-analyses132,133 have been performed to compare low-molecular-weight heparin (LMWH) with placebo or UFH as adjuncts to fibrinolytic therapy in patients with STEMI. A meta-analysis of 16,943 patients who were enrolled in four randomized trials revealed that the end points of death or reinfarction at 7 days and at 30 days were significantly reduced by LMWH compared with placebo.133 A meta-analysis of 7098 patients who were enrolled in six randomized trials revealed that LMWH, compared with UFH, reduced the rates of reinfarction during hospitalization and at 30 days, but the rates of death were not significantly different.133 Neither meta-analysis included a subsequent trial, Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment (ExTRACT)-TIMI 25, that compared enoxaparin, an LMWH, with UFH in patients with STEMI who received fibrinolytic therapy.129,130 The study was a double-blind, randomized comparison of enoxaparin given subcutaneously twice daily until hospital discharge versus intravenous UFH for 48 hours in 20,506 patients with STEMI. A fibrinolytic agent was received by 99.7% of the patients: 55% received alteplase, 20% received streptokinase, 19% received tenecteplase, and 5.5% received reteplase. The primary end point, death or nonfatal recurrent MI through 30 days, occurred in 12% of patients in the UFH group and in 9.9% of patients in the enoxaparin group (p < 0.001). The rates of major bleeding at 30 days were 1.4% in the UFH group and 2.1% in the enoxaparin group (p < 0.001), but the rates of intracranial hemorrhage were not significantly different (UFH 0.7%, enoxaparin 0.8%; p = 0.14). The enoxaparin strategy significantly reduced the risk of nonfatal MI at 1 year (5.7% versus 6.8%; hazard ratio 0.82; 95% CI 0.73 to 0.92; p < 0.001).130 One of the mechanisms underlying the benefit of low-molecular-weight heparin compared with unfractionated heparin may be improved patency of the infarct artery after fibrinolytic therapy.131
Fondaparinux, a synthetic pentasaccharide, is a factor Xa inhibitor that binds antithrombin and inhibits factor Xa. The Organization for the Assessment of Strategies for Ischemic Syndromes (OASIS) conducted two trials to evaluate fondaparinux in patients with ACS134 and STEMI.135,136 The OASIS-6 trial was a randomized, double-blind comparison of fondaparinux 2.5 mg daily or control from days 3 through 9 in 12,092 patients with STEMI.135 Forty-five percent of the patients received fibrinolytic therapy (streptokinase in 73%), 28.9% underwent primary PCI, and 23.7% did not receive any reperfusion therapy. The primary efficacy outcome, death or reinfarction at 30 days, was significantly lower in the fondaparinux group than in the control group (9.7% versus 11.2%; hazard ratio 0.86; 95% CI 0.77 to 0.96; p = 0.008). Also, fondaparinux significantly reduced the rates of death at day 9, day 30, and the end of the study (3 to 6 months). Significant heterogeneity in the effect of fondaparinux was observed in relation to the reperfusion strategy, with benefit observed in patients who received no reperfusion therapy or a fibrinolytic agent, but not in patients who underwent primary PCI. The rate of severe bleeding was not increased by fondaparinux.
A subgroup analysis of the OASIS-6 results was performed to compare the effects of fondaparinux with usual care (i.e., UFH or placebo) in patients with STEMI who did not receive any reperfusion therapy.136 Fondaparinux significantly reduced the composite end point of death or recurrent MI, without an increase in severe bleeding or stroke, compared with UFH or placebo.136
The dose of fondaparinux must be adjusted in patients with renal insufficiency, but adjustment for body weight is not necessary. The anticoagulant effect of the drug cannot be monitored by conventional clotting tests, such as the activated clotting time or partial thromboplastin time. Also, the relatively long half-life of fondaparinux, 17 to 21 hours, conceivably may be viewed as an impediment to early sheath removal and ambulation after cardiac catheterization. Because of the risk of catheter thrombosis, fondaparinux should not be used as the sole anticoagulant during PCI, and an additional anticoagulant with antifactor IIa activity should be administered.21
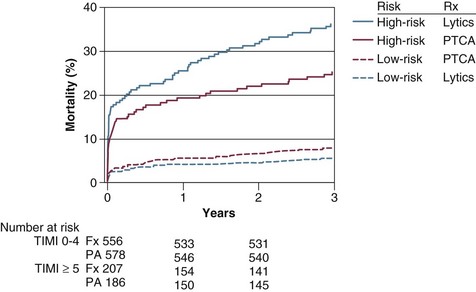
Bivalirudin, a direct thrombin inhibitor, was compared with UFH plus glycoprotein IIb/IIIa inhibitors in 3602 patients with STEMI undergoing primary PCI in the HORIZONS-AMI trial.137–139 Compared with UFH plus glycoprotein IIb/IIIa inhibitors, anticoagulation with bivalirudin was associated with a reduced rate of net adverse clinical events and major bleeding at 30 days and 1 year.137,138 Also, treatment with bivalirudin was associated with both cardiac and all-cause mortality rates that were significantly lower after 30 days and 1 year.137,138 Among the 477 patients who were classified as “high risk,” the mortality rates at 1 year were 8.4% among patients treated with bivalirudin, compared with 15.9% among patients treated with UFH plus a glycoprotein IIb/IIIa inhibitor (p = 0.01).139
According to the updated recommendations for the use of anticoagulants as ancillary therapy to reperfusion therapy that were published in 2013,24 “patients undergoing reperfusion with fibrinolytics should receive anticoagulant therapy for a minimum of 48 hours and preferably for the duration of the index hospitalization, up to 8 days or until revascularization if performed (regimens other than UFH are recommended if anticoagulant therapy is given for more than 48 hours because of the risk of heparin-induced thrombocytopenia with prolonged UFH treatment).” An activated partial thromboplastin time (aPTT) greater than 70 seconds during treatment with UFH was shown to be associated with a higher risk of death, stroke, and bleeding among patients who were enrolled in the GUSTO-1 trial.140 Therefore, the ACC/AHA guidelines recommend adjustment of the dose of UFH to maintain an aPTT of 50 to 70 seconds. Also, the platelet count should be monitored daily during treatment with UFH because there is a 3% incidence of heparin-induced thrombocytopenia.141 See Box 30.3 for recommendations regarding the doses of UFH, enoxaparin, fondaparinux, and bivalirudin.
Fibrinolytic Therapy
The dependence of myocardial necrosis on the duration of coronary occlusion was demonstrated using a canine model of MI.142 The landmark angiographic study performed by DeWood and coworkers143 confirmed the presence of coronary artery thrombi in patients with STEMI. Although these experimental and clinical observations provided a rationale for fibrinolytic therapy, the initial studies of fibrinolytic agents for acute MI preceded both findings. According to one review of the literature, the first reported use of fibrinolytic therapy for acute MI was in 1958.144 By 1979, several multicenter studies of intravenous streptokinase had been performed, but the benefit of reperfusion therapy remained unproven, in part because the trial designs were flawed.145,146
Effect of Fibrinolysis on Survival
Four well-designed, multicenter randomized trials established that three fibrinolytic agents—streptokinase,96,147 anisoylated plasminogen-streptokinase activator complex (APSAC) (i.e., anistreplase),148 and tPA149—each reduced short-term and long-term mortality in patients with acute MI. The Fibrinolytic Therapy Trialists’ Collaborative Group analyzed nine trials that randomized a total of 58,600 patients with suspected acute MI to a fibrinolytic therapy group or a control group.150 The absolute risk of death increased with age, but absolute reductions in mortality were comparable among younger and older patients up to 75 years of age. Several trials had upper age limits for enrollment. The remaining trials enrolled 5788 patients 75 years or older and found no significant effect of fibrinolytic therapy on mortality at 35 days (25.3% for the control patients versus 24.3% for patients who received fibrinolytic therapy).150
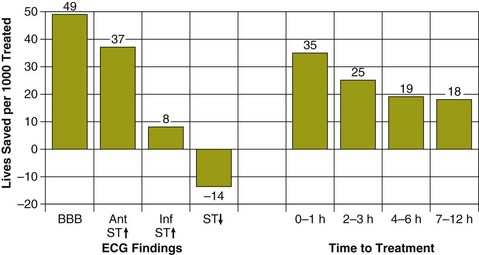
The baseline ECG findings and the elapsed time between the onset of symptoms and the initiation of treatment were significant determinants of the impact of fibrinolytic therapy on mortality at 35 days150 (Fig. 30.5). The greatest reduction in mortality was observed in patients who presented with either bundle branch block (BBB) (control 23.6% versus fibrinolytic 18.7%) or ST segment elevation in the anterior leads (control 16.9% versus fibrinolytic 13.2%).150 Fibrinolytic therapy increased mortality among patients with ST segment depression on the baseline ECG.
A linear relationship between the absolute reduction in mortality and the delay from symptom onset to randomization was found among the 45,000 patients who presented with ST elevation or BBB on the ECG.150 Fibrinolytic therapy significantly reduced mortality even among patients who received treatment 7 to 12 hours after the onset of symptoms, but patients who received treatment within the first hour after the onset of their symptoms received the greatest benefit. Patients who receive fibrinolytic therapy within the first hour after symptom onset have the greatest proportional mortality reduction,151 as well as the highest incidence of so-called aborted MI, defined as maximal creatine kinase level up to twice the upper limit of normal and typical evolution of ECG changes.152,153 A multicenter trial of fibrinolytic therapy reported that the baseline-adjusted mortality was significantly lower among the 13.3% of patients who had an aborted MI than among those who did not.152
Persistent occlusion of the IRA after acute MI is associated with left ventricular remodeling, resulting in increased left ventricular end-systolic volume, a major predictor of survival after acute MI.154,155 Some evidence suggests that reperfusion later than 6 hours after the onset of symptoms has a favorable effect on ventricular remodeling, with less ventricular dilation observed after successful reperfusion than after no reperfusion therapy.156,157 Several clinical trials have investigated the effects of fibrinolytic therapy on clinical events in patients who received treatment more than 6 hours after the onset of symptoms. A South American multicenter trial randomized 2080 patients within 7 to 12 hours after the onset of symptoms to receive streptokinase or placebo and found no significant difference in mortality rates in-hospital, after 35 days, and after 1 year.158 The Late Assessment of Thrombolytic Efficacy (LATE) study randomized 5711 patients who presented with suspected acute MI between 6 and 24 hours after the onset of symptoms to receive tPA or placebo.159 Treatment with tPA significantly reduced mortality among patients who received treatment within 12 hours of symptom onset: the 35-day mortality rate was 8.9% for the tPA group versus 11.97% for placebo, representing a relative reduction of 25.6% (95% CI 6.3% to 45%; p = 0.0229). Mortality at 35 days was not significantly reduced by the administration of tPA to patients who received treatment 12 to 24 hours after symptom onset.
The major causes of delayed fibrinolytic therapy for acute MI are failure of patients to seek medical care160,161 and delays in administration of fibrinolytic therapy.162,163 A retrospective review of data for 2409 patients hospitalized with acute MI in Minnesota in 1992 and 1993 reported that 40% of the patients delayed presentation to the hospital more than 6 hours after the onset of symptoms.160 The ACC/AHA Practice Guidelines set a goal of initiating fibrinolytic therapy within 30 minutes of contact with the medical system.20 Among 68,430 patients with STEMI who received fibrinolytic therapy and were enrolled in the NRMI-3 and NRMI-4 registries, only 46% of patients received a fibrinolytic drug within 30 minutes of arrival.163 There was no significant improvement in the so-called door-to-needle time in the 1015 participating hospitals from 1999 to 2002.163 A more recent study of 3219 patients with STEMI who received fibrinolytic therapy in 178 hospitals between 2007 and 2008 found that the “door-to-needle” time was ≤ 30 minutes in only 44.5% of patients.164 Female gender and age > 75 were associated with longer door-to-needle times.164 Prehospital administration of fibrinolytic therapy has been investigated as one approach to reducing the delay between symptom onset and reperfusion.165–167 A meta-analysis of six randomized trials that compared prehospital with in-hospital fibrinolytic therapy for acute MI found that the time to fibrinolytic therapy and all-cause in-hospital mortality were significantly reduced by the prehospital administration of fibrinolytic drugs.165
Coronary Artery Patency after Fibrinolytic Therapy
Early angiographic studies investigated the rates of coronary reperfusion after intracoronary168–170 or intravenous administration of fibrinolytic agents.171 The Thrombolysis in Myocardial Infarction (TIMI) Study Group devised a grading system of coronary patency that has been adopted widely171 (Box 30.4). Fibrinolysis was judged to be successful if an IRA that was occluded (TIMI grade 0 or 1) before treatment improved to either partial perfusion (TIMI grade 2) or complete perfusion (TIMI grade 3) 90 minutes after the fibrinolytic therapy began.171 The first TIMI trial revealed that only 31% of occluded arteries were patent (TIMI grade 2 or 3) 90 minutes after intravenous streptokinase, compared with a 62% patency rate after a 3-hour intravenous infusion of tPA (p < 0.001).172 Subsequent studies that examined the relationship between the TIMI grade flow and clinical outcome concluded that TIMI grade 3 flow, but not TIMI grade 2 flow, improves both in-hospital and long-term mortality after acute MI.173,174 Therefore, the criteria for evaluating fibrinolytic therapy were revised, and TIMI grade 2 flow is no longer considered a successful outcome.175
The GUSTO-I trial randomized 41,021 patients to four fibrinolytic strategies: streptokinase plus subcutaneous UFH, streptokinase plus intravenous UFH, accelerated tPA plus intravenous UFH, or a combination of streptokinase and tPA plus intravenous UFH.176 Thirty-day mortality was lowest for the accelerated tPA-UFH regimen, 6.3%. A substudy of GUSTO-I included 2431 patients who underwent coronary angiography to assess patency of the IRA.177,178 TIMI grade 3 flow was achieved 90 minutes after initiation of fibrinolytic therapy in 54% (157/292) of patients in the accelerated tPA-UFH group, compared with 31% of patients who received streptokinase plus UFH (176/576). Analysis of the relationship between patency at 90 minutes and mortality at 30 days regardless of treatment assignment revealed a significant difference between the mortality rate associated with grade 3 flow and the mortality associated with grade 0 or 1 flow (4.4% versus 8.9%; p = 0.009).
The relationship between time to treatment and the mortality reduction by fibrinolytic therapy may be a reflection of several factors. One is that earlier reperfusion achieves greater myocardial salvage.179 Another factor is that time to treatment may influence the patency rate 90 minutes after administration of certain fibrinolytic drugs.180 Patency of the IRA 90 minutes after administration of a nonfibrin-specific fibrinolytic drug, such as streptokinase, anistreplase, or urokinase, is lower when patients are first treated beyond 3 hours after the onset of symptoms than when the drugs are administered within 3 hours after onset.172,180–182 After treatment with tPA or reteplase (rPA), fibrin-specific fibrinolytic agents, the rates of TIMI grade 3 flow are similar for patients who received treatment within 3 hours or at 3 hours or later after the onset of symptoms.172,181,182 The time-dependent reperfusion efficacy is reflected by the rates of in-hospital mortality. A retrospective analysis of six angiographic trials that included 1174 patients found that in-hospital mortality among patients who received nonfibrin-specific drugs was twofold greater for patients treated more than 3 hours after symptom onset compared with patients treated within 3 hours.182 Among patients who received tPA or rPA, in-hospital mortality did not differ for patients treated within 3 hours of symptom onset or later than 3 hours after symptom onset.
More sophisticated methodologies for assessing myocardial reperfusion have been devised, such as the TIMI frame count and TIMI myocardial perfusion grade.183–185 Application of these methods demonstrated that even among patients with TIMI grade 3 flow after fibrinolytic therapy, clinical outcomes and survival are related to the speed of epicardial flow and the state of myocardial perfusion.183,184 Therefore, a major goal of research has been to determine whether combinations of fibrinolytic and antiplatelet drugs might enhance myocardial reperfusion and achieve further reductions in mortality. Compared with full-dose tPA or rPA, a combination of a reduced dose of either tPA or rPA plus abciximab, a platelet glycoprotein IIb/IIIa (GP IIb/IIIa) inhibitor, was found to increase the rates of TIMI 3 flow at 60 and 90 minutes after administration.186,187 Unfortunately, a difference in 30-day mortality between standard-dose rPA and half-dose rPA plus full-dose abciximab was not demonstrated by a large clinical trial, GUSTO-V, that enrolled 16,588 patients with evolving STEMI.188
Complications of Fibrinolytic Therapy
Intracranial hemorrhage and other hemorrhagic complications are the major risks associated with the administration of fibrinolytic therapy.189,190 The NRMI-2 database accrued 71,073 patients who received tPA for acute MI from June 1, 1994, to September 30, 1996. Intracranial hemorrhage was confirmed by computed tomography (CT) or magnetic resonance imaging (MRI) in 625 patients (0.88%).190 In-hospital mortality was 53%, and 25.3% of patients with intracranial hemorrhage who survived to hospital discharge had neurologic deficits. A multivariate analysis identified several risk factors that were significantly associated with an increased risk of intracranial hemorrhage: older age, female gender, systolic blood pressure greater than 140 mm Hg, diastolic blood pressure greater than 100 mm Hg, and history of stroke. An aPTT longer than 70 seconds was associated with an increased risk of hemorrhagic stroke in the GUSTO-I trial.122 Bolus administration of fibrinolytic agents may be associated with an increased risk of intracranial hemorrhage compared with infusion.191,192 Although phase II trials indicated a statistically nonsignificant reduction in the risk of intracranial hemorrhage, meta-analysis of phase III trials revealed a statistically significant 25% increase in the risk of intracranial hemorrhage with bolus fibrinolytic therapy.192 According to the ACC/AHA guidelines, “The occurrence of a change in neurological status during or after reperfusion therapy, particularly within the first 24 hours after initiation of treatment, is considered to be due to intracranial hemorrhage until proven otherwise.”20 When intracranial hemorrhage is suspected, an emergency CT scan should be performed, and fibrinolytic, antiplatelet, and anticoagulant therapies should be discontinued until the diagnosis is ruled out. Cryoprecipitate or fresh frozen plasma should be given to replenish coagulation factors.20 Protamine should be administered to patients who are receiving UFH. Neurosurgery to evacuate parenchymal hemorrhages or subdural hematomas may improve outcome.193
Among 40,903 patients enrolled in the GUSTO-I trial, 1.2% suffered severe bleeding, defined as bleeding that caused hemodynamic compromise that required treatment, and 11.4% experienced moderate hemorrhage, defined as bleeding that required transfusion but did not lead to hemodynamic compromise requiring intervention.189 The most common sources of moderate and severe bleeding were procedure related. The rate of moderate or severe bleeding was 6% among patients who underwent no procedures, compared with 17% among patients who underwent coronary angiography, 43% among patients who received a PA catheter, and 50% among patients who received an intra-aortic balloon pump (IABP) or underwent coronary artery bypass surgery. Older age, lower body weight, and female sex were the three strongest independent predictors of hemorrhage. The risk of noncerebral bleeding was greater after streptokinase than after tPA, but the risk of intracranial hemorrhage was greater after tPA.
Patient Selection
The 2013 ACCF/AHA Guidelines for STEMI include one Class I indication for fibrinolytic therapy: in the absence of contraindications, fibrinolytic therapy should be given to patients with STEMI and onset of ischemic symptoms within the previous 12 hours when it is anticipated that primary PCI cannot be performed within 120 minutes of first medical contact.24 The 2013 STEMI Guidelines also include one Class IIa recommendation for fibrinolytic therapy: in the absence of contraindications and when PCI is not available, fibrinolytic therapy is reasonable for patients with STEMI if there is clinical or ECG evidence of ongoing ischemia within 12 to 24 hours of symptom onset and a large area of myocardium at risk or hemodynamic instability.24 There is a long list of absolute and relative contraindications to fibrinolytic therapy (Box 30.5). Special attention should be paid to factors that may increase the risk of intracranial hemorrhage, such as a history of such hemorrhage, recent closed head or facial trauma, uncontrolled hypertension, or ischemic stroke within the previous 3 months. PCI is preferable to fibrinolytic therapy in patients with an increased risk of intracranial hemorrhage. Active menstrual bleeding should not be considered a contraindication to fibrinolytic therapy.194,195 The GUSTO-I trial included 12 menstruating women who received fibrinolytic therapy, 2 of whom required a transfusion for moderate vaginal bleeding.195 Nontraumatic cardiopulmonary resuscitation also should not be considered a contraindication to fibrinolytic therapy.194,196
Increasing age is a risk factor for death and other adverse events after either primary PCI or fibrinolytic therapy for STEMI.197 The risk of intracranial hemorrhage after fibrinolytic therapy also increases with advancing age.190,198 Data are conflicting regarding the benefit or lack of benefit of fibrinolytic therapy in patients with STEMI who are older than 75. One analysis of a Medicare database that included 2673 patients aged 76 to 86 found that fibrinolytic therapy conferred a survival disadvantage, with a hazard ratio of 1.38 for 30-day mortality.199 Fibrinolytic therapy was associated with a 13% reduction in the composite of 1-year mortality and cerebral bleeding in a cohort of 6891 patients 75 years and older with a first STEMI who were enrolled in a Swedish registry.200 A study performed in the Netherlands randomized 87 patients with acute MI who were older than 75 to primary PCI or streptokinase.201 The primary composite end point of death, reinfarction, or stroke at 30 days occurred in 4 (9%) patients in the PCI group, compared with 12 (29%) in the streptokinase group (RR 4.3, 95% CI 1.2 to 20; p = 0.01). After 1 year, mortality was significantly greater for the streptokinase group than for the PCI group (29% versus 11%; RR 3.4, 95% CI 1.0 to 13.5; p = 0.03). One caveat regarding the study is that the mean time from hospital admission to first balloon inflation was 59 ± 19 minutes (range 33 to 120 minutes)—considerably shorter than door-to-balloon times in the United States.
Many patients with acute MI have contraindications to fibrinolytic therapy or do not meet eligibility criteria for fibrinolytic therapy.202,203 Contraindications such as recent surgery, trauma, or gastrointestinal bleeding would be relatively frequent in patients who develop an acute MI while already hospitalized for another illness. Analysis of patients with STEMI who were enrolled in the NRMI-2, -3, and -4 databases suggested that immediate mechanical reperfusion using either PCI or coronary artery bypass surgery reduced the risk of in-hospital death among patients with contraindications to fibrinolytic therapy.204
Percutaneous Coronary Intervention
Dr. Andreas Gruntzig performed the first balloon angioplasty of a coronary artery in 1977.205 Dr. Peter Rentrop reported his initial experience with PCI for acute MI in 1979.206,207 O’Neill and colleagues208 published a randomized trial of PCI compared with intracoronary streptokinase for acute MI in 1986. The most recent meta-analysis identified 23 trials that randomly assigned a total of 7739 patients with STEMI to receive intravenous fibrinolytic therapy or undergo primary PCI, defined as PCI without previous or concomitant fibrinolytic therapy.209 Numerous other randomized trials have been performed to investigate several other applications of PCI in patients with acute MI. Rescue PCI refers to PCI that is performed after unsuccessful fibrinolytic therapy. After successful fibrinolysis, PCI may be performed immediately, on a routine, deferred basis, or in a selective fashion (e.g., to treat inducible ischemia).
Primary Percutaneous Intervention
Myocardial salvage and long-term mortality are correlated with both the TIMI grade flow and the myocardial blush grade achieved after primary PCI for STEMI.210–213 TIMI grade 3 flow is achieved in a high percentage of patients who undergo primary PCI for STEMI. The largest clinical trial of PCI compared with fibrinolytic therapy for acute MI was the Danish Multicenter Randomized Study on Fibrinolytic Therapy versus Acute Coronary Angioplasty in Acute Myocardial Infarction (DANAMI-2) study.214–216 Immediate angiography was performed in 777 of the 790 (98%) patients who were randomized to undergo PCI. The initial angiogram showed TIMI grade 0 or 1 flow in 68% of patients, and grade 3 flow in 18%. PCI was attempted in 706 patients, resulting in postprocedural flow of TIMI grade 3 in 82%, grade 2 in 16%, and grade 0 or 1 in 2%. The Zwolle Myocardial Infarction Study Group reported the outcome of 1702 patients who underwent PCI for STEMI.217 Successful PCI, defined as TIMI grade 3 flow and a residual lumen diameter less than 50%, was achieved more often during routine hours (8 a.m. to 6 p.m.) than during off-hours (6 p.m. to 8 a.m.) (96.2% versus 93.1%; p < 0.01).
Despite the presence of TIMI grade 3 flow, the myocardial blush grade, an indicator of myocardial perfusion, is abnormal in a majority of patients who undergo primary PCI for STEMI.211,212 Among a cohort of 777 patients who underwent primary PCI for STEMI, normal myocardial blush (grade 3) was achieved in only 148 patients (19%), whereas 236 patients (30%) had blush grade 0 or 1.211 Multivariate analysis showed that myocardial blush grade was an independent predictor of long-term mortality, with mortality after follow-up for 1.9 ± 1.7 years of 3% for grade 3, 6% for grade 2, and 23% for grade 0 or 1 myocardial blush (p < 0.0001).211
Distal embolization of thrombus218 and microvascular “no reflow”219 are two of the mechanisms of impaired myocardial perfusion after primary PCI. Distal embolization was observed in 27 of 178 patients (15%) who underwent primary PCI for STEMI.218 Patients with distal embolization had lower left ventricular EFs at discharge from the hospital and higher long-term mortality. Microvascular obstruction detected by MRI is a prognostic marker for cardiovascular events after acute MI, even after controlling for infarct size.219 Numerous clinical trials have been performed to investigate various mechanical methods of protecting the coronary microcirculation during PCI for ACS or acute STEMI.220–231 Two different distal embolic protection devices, one that consists of a distal balloon occlusion and aspiration system and another that employs a filter, failed to improve myocardial reperfusion, reduce infarct size, or improve event-free survival in patients with acute MI.224–226 A meta-analysis of 12 clinical studies concluded that the distal embolic protection devices had no significant effect on mortality.229
Use of the X-Sizer thrombectomy catheter (ev3, Inc., Plymouth, Minnesota) before coronary angioplasty or stenting appears to reduce distal embolization and improve epicardial flow, myocardial blush, and resolution of ST segment elevation, especially in patients with angiographic evidence of intraluminal thrombus.220–222 The AngioJet Rheolytic Thrombectomy catheter (Possis Medical, Inc., Minneapolis, Minnesota) was evaluated in a multicenter, randomized study called the AngioJet Rheolytic Thrombectomy in Patients Undergoing Primary Angioplasty for Acute Myocardial Infarction (AIMI) study.223 The AIMI trial randomized 480 patients within 12 hours of symptom onset of STEMI to PCI alone or PCI with adjunctive rheolytic thrombectomy. No significant differences were observed in myocardial perfusion blush or resolution of ST segment elevation. Infarct size measured by myocardial perfusion imaging was greater in the thrombectomy group, and major adverse cardiac events were more frequent in the thrombectomy group (6.7% versus 1.7%; p = 0.01).223 A meta-analysis of five randomized trials concluded that adjunctive mechanical thrombectomy with either the AngioJet catheter or the X-Sizer catheter was associated with increased mortality compared with PCI alone (5.3% versus 2.8%; p = 0.05) in patient with acute MI.229
At least 16 clinical trials have been performed to test the effect of aspiration thrombectomy on clinical outcomes in patients with STEMI. The largest trial, the Thrombus Aspiration during Percutaneous Coronary Intervention in Acute Myocardial Infarction Study (TAPAS), was an open trial with a blinded evaluation of end points that randomized 1071 patients to either conventional PCI or thrombus aspiration using a 6 French aspiration catheter during PCI.227,228 Thrombus aspiration resulted in improved myocardial blush grade and resolution of ST-segment elevation, and lower 1-year mortality.227,228 Three of the four meta-analyses of randomized trials have concluded that adjunctive manual aspiration thrombectomy improves mortality in patients undergoing primary PCI for STEMI.229–232 The 2009 Focused Updates of the ACC/AHA Guidelines for the Management of Patients with ST-Elevation Myocardial Infarction included a Class IIa recommendation for aspiration thrombectomy during PCI for STEMI.22
Abciximab, a GP IIb/IIIa inhibitor, has been reported to improve recovery of microvascular perfusion after PCI for acute MI.233 An intracoronary bolus of abciximab reduced infarct size at 30 days by a small but significant amount in patients who underwent primary PCI for STEMI due to occlusion of the proximal or middle segments of the left anterior descending coronary artery.234 On the basis of observations from small studies, intracoronary vasodilators, such as adenosine, frequently are employed to treat “no reflow” after PCI.235–237
Proponents of primary PCI and fibrinolytic therapy for acute MI have written excellent reviews of the advantages and disadvantages of both reperfusion therapies.238,239 The evidence base supporting primary PCI for STEMI includes single-center series, multicenter randomized trials, large registries, and several meta-analyses. The Myocardial Infarction Triage and Intervention (MITI) Project Registry described a cohort of patients with acute MI who either underwent primary angioplasty (1050 patients) or received fibrinolytic therapy (2095 patients) at 19 hospitals in Seattle, Washington, between 1988 and 1994.240 No significant difference in mortality was observed during hospitalization or long-term follow-up between the two groups. The MITI Registry included patients who received streptokinase, whereas a subsequent NRMI-2 report that was limited to patients who received tPA also found that in-hospital outcomes were similar for both methods of reperfusion.241 Another cohort study of 20,683 Medicare beneficiaries with acute MI concluded that 30-day and 1-year mortality rates were lower among patients who underwent primary angioplasty than among patients who received fibrinolytic therapy.242
The DANAMI-2 trial did not show a significant difference between PCI and fibrinolytic therapy in the rates of death or stroke at 30 days, but the rate of reinfarction at 30 days was significantly lower: 1.6% for the PCI group compared with 6.3% for the fibrinolysis group (p < 0.001).214 Although the 3-year mortality was not significantly different among low-risk patients, defined as a TIMI score of 0 to 4, the 3-year mortality among high-risk patients, defined as a TIMI score ≥ 5, was significantly lower for the patients who underwent PCI compared with the patients who received fibrinolytic therapy.215 Published meta-analyses of PCI versus fibrinolytic therapy included DANAMI-2 and 22 other randomized trials.209,243 The trials were rather heterogeneous in design: stents were used in 12 trials, GP IIb/IIIa inhibitors were used in 8 trials, and 5 trials compared fibrinolytic therapy with PCI performed after transfer from a referral hospital to a hospital that provides invasive cardiac services. The rates of short-term death, nonfatal reinfarction, stroke, and the combined end point of death, nonfatal reinfarction, and stroke were lower for PCI than fibrinolytic therapy. Another analysis pooled the individual patient 6-month follow-up data from 11 randomized trials of PCI versus fibrinolytic therapy for acute MI.244 At 6 months, the mortality rates were 6.2% for PCI and 8.2% for fibrinolysis (RR 0.73; CI 0.55 to 0.98; p = 0.04). A meta-analysis of 11 randomized trials that enrolled 4320 patients with STEMI found that long-term mortality (≥ 1 year) was 24% lower among patients treated with primary PCI compared with patients treated with fibrinolytic therapy.243 A meta-analysis of 12 observational studies that enrolled 54,571 patients with STEMI found that long-term mortality was not significantly reduced by primary PCI compared with fibrinolytic therapy.243
Analysis of patients enrolled in NRMI-2 showed that the in-hospital mortality was 28% lower among patients who underwent primary PCI at hospitals with the highest volume than among those who had PCI at hospitals with the lowest volume (adjusted RR 0.72; CI 0.60 to 0.87; p < 0.001).245 Among 463 hospitals that performed primary PCI for STEMI and participated in NRMI-4, the hospitals with the greatest relative utilization of primary PCI, versus fibrinolytic therapy, for reperfusion had shorter door-to-balloon times and lower in-hospital mortality rates.246 These data may have provided some of the rationale for the ACC/AHA Practice Guidelines regarding the performance of PCI for STEMI.20 PCI should be performed in a cardiac catheterization laboratory that performs more than 200 PCI procedures per year, including at least 36 cases of primary PCI for STEMI. The operator should perform more than 75 PCI procedures per year.
Both the extent of myocardial salvage179,247 and the mortality benefit150,248,249 of fibrinolytic therapy and primary PCI are inversely related to the time elapsed between symptom onset and treatment. Among a cohort of 1791 patients with STEMI treated with primary PCI, the relative risk (RR) of death at 1 year increased by 7.5% for each 30-minute delay.250 Several studies have analyzed the relationship between mortality and the so-called door-to-balloon time, defined as the duration of time between arrival at the hospital and the first balloon inflation.249,251–253 Although some studies found that in-hospital mortality was not related to the door-to-balloon time,251 most studies have shown that both in-hospital and late mortality are higher when door-to-balloon time is longer.249,252,253 Among 2082 patients with acute MI who were enrolled in the CADILLAC trial, door-to-balloon time was an independent predictor of 1-year mortality in patients who presented within 2 hours after the onset of symptoms (n = 965; hazard ratio 1.24; 95% CI 1.05 to 1.46; p = 0.013), but not in patients who presented later than 2 hours (n = 944; hazard ratio 0.88; 95% CI 0.67 to 1.15; p = 0.33).254
The ACC/AHA Practice Guidelines set a goal of balloon inflation within 90 minutes of presentation, but observational studies indicate that this goal is seldom achieved.163,255 Among 33,647 patients with STEMI who underwent primary PCI between 1999 and 2002 and were enrolled in the NRMI-3 and NRMI-4 registries, only 35% of patients received treatment within 90 minutes of arrival.163 Krumholz and colleagues256 analyzed the door-to-balloon times reported by hospitals to the Centers for Medicare & Medicaid Services from January 1, 2005, through September 30, 2010. Door-to-balloon time decreased from a median of 96 minutes in 2005 to a median of 64 minutes during the first three quarters of 2010.256 The percentage of patients who had door-to-balloon times < 90 minutes increased from 44.2% to 91.4%.256
The time of day and day of week had significant effects on door-to-balloon times among 33,647 patients with STEMI who underwent primary PCI between 1999 and 2002 and were enrolled in the NRMI-3 and NRMI-4 registries.257 Fifty-four percent of patients who underwent primary PCI were treated during off-hours (weekdays, 5 p.m. to 7 a.m. and weekends). Door-to-balloon times exceeded 90 minutes in 74% of patients who underwent PCI during off-hours, compared with 53% of patients treated during regular hours (weekdays, 7 a.m. to 5 p.m.) (p < 0.001). Treatment delays are far greater among patients who are transferred to another hospital to undergo primary PCI.255 Among 4278 patients who underwent interhospital transfer for primary PCI during the period 1999 to 2002, the median total door-to-balloon time was 180 minutes.255 Only 4.2% of patients underwent PCI within the benchmark of 90 minutes.255 Analysis of 23 randomized trials that compared primary PCI with fibrinolytic therapy for STEMI indicated that PCI affords a mortality advantage only if the door-to-balloon time exceeds the door-to-needle time by less than 1 hour.258
Several approaches have been suggested to reduce the delay between symptom onset and reperfusion in patients treated by primary PCI. One approach that has been tested is performance of primary PCI at hospitals that have cardiac catheterization laboratories but lack on-site cardiac surgery.259,260 One trial randomized patients with STEMI to undergo primary PCI (n = 225) or receive accelerated tPA (n = 226) at 11 community hospitals without on-site cardiac surgery.260 The composite end point of death, recurrent MI, and stroke was significantly lower among patients treated with primary PCI than among those who received tPA, both 6 weeks after MI (10.7% versus 17.7%; p = 0.03) and 6 months after MI (12.4% versus 19.9%; p = 0.03). A subsequent study by the NRMI investigators compared the outcomes of 58,821 patients with STEMI who presented to 214 hospitals with on-site cardiac surgery with the outcomes of patients who presented to 52 hospitals without on-site cardiac surgery.261 The patients who presented to hospitals without on-site cardiac surgery were less likely to receive guideline-recommended medications within 24 hours, and they were less likely to undergo acute reperfusion therapy, but there was no difference in mortality among patients who underwent primary PCI.261 A meta-analysis of 11 studies that included 124,074 patients who underwent primary PCI for STEMI found that the in-hospital mortality for patients at hospitals without on-site cardiac surgery was not different from hospitals having on-site surgery (odds ratio, 0.96; 95% CI 0.88 to 1.05).262 Another proposed strategy to reduce the delay between symptom onset and reperfusion is the diversion of patients with acute MI to a primary PCI hospital, instead of the current practice of transporting patients to the nearest emergency department.238 A survey of 365 hospitals identified six strategies that were significantly associated with a faster door-to-balloon time.263 The use of prehospital ECGs to diagnose and triage patients with a suspected STEMI is associated with a greater use of reperfusion therapy and shorter door-to-needle and door-to-balloon times.264
Late reocclusion after successful primary angioplasty is associated with decreased long-term survival.265 The CADILLAC trial randomized 2082 patients with acute MI to undergo percutaneous transluminal coronary angioplasty (PTCA) or stenting.266 Although stenting did not improve the myocardial blush score,267 the angiographic rates of reocclusion of the IRA at 7 months was 5.7% after coronary stenting, compared with 11.3% after PTCA.266
At least 15 randomized clinical trials and at least 5 observational studies, plus numerous meta-analyses of those studies, have been performed to compare drug-eluting stents (DES) with bare metal stents (BMS) in patients with STEMI. The largest randomized trial enrolled 3006 patients with STEMI and found that compared with BMS, paclitaxel-eluting stents reduced angiographic evidence of restenosis and repeat revascularization for recurrent ischemia, without increasing the risk of stent thrombosis or death at 12 months.268 Among 7217 patients who underwent PCI with stenting for acute MI in Massachusetts between April 1, 2003, and September 30, 2004, treatment with DES, compared with BMS, was associated with decreased rates of 2-year mortality and need for repeat revascularization.269 On the other hand, the multinational GRACE registry reported that mortality from either 6 months to 2 years or from 1 to 2 years was significantly greater among 5093 STEMI patients who received DES, compared with BMS.270 A meta-analysis of five observational studies found that STEMI patients treated with DES had a significantly lower mortality compared with patients who received BMS (odds ratio 0.65; 95% CI 0.53 to 0.80; p < 0.001).271 A meta-analysis of 15 randomized, controlled trials that enrolled a total of 7867 STEMI patients concluded that compared with a BMS, a DES was associated with a reduction in target vessel revascularization, but an increased risk of very late stent thrombosis (> 1 year after PCI).272 The apparent increased risk of very late stent thrombosis is consistent with both post-mortem and in vivo evidence of higher rates of incomplete stent apposition and uncovered stent struts among STEMI patients treated with DES compared with BMS.273,274 Nevertheless, the 2009 Focused Updates of the ACC/AHA STEMI and PCI guidelines include a Class IIa recommendation that “It is reasonable to use a DES as an alternative to a BMS for primary PCI in STEMI.”22
Rescue Percutaneous Coronary Intervention
Compared with TIMI grade 3 flow, TIMI grade 0 or 1 flow at 90 minutes after fibrinolytic therapy is associated with worse left ventricular function and increased mortality rates.177,178 Compared with complete resolution of ST segment elevation, incomplete resolution of ST segment elevation after fibrinolytic therapy is associated with larger infarct size and greater short-term and long-term mortality.42,44,275 Therefore, various angiographic or electrocardiographic criteria have been employed to define unsuccessful fibrinolysis and rescue PCI. A report from the TIMI 10B and TIMI 14 trials of fibrinolytic therapy defined rescue PCI as PCI performed between 90 and 150 minutes after the start of therapy for patients with TIMI 0 or 1 flow 90 minutes after the start of therapy.276 The Middlesbrough Early Revascularization to Limit Infarction (MERLIN) trial defined failed fibrinolytic therapy as failure of the ST segment elevation in the worst lead to have resolved by 50% 60 minutes after the onset of fibrinolytic therapy.277 The Rescue Angioplasty versus Conservative Treatment or Repeat Thrombolysis (REACT) trial’s definition of rescue PCI was PCI performed within 12 hours after failed fibrinolytic therapy, defined as an ECG obtained 90 minutes after the start of fibrinolytic therapy that showed < 50% resolution of the ST segment in the lead showing the greatest ST segment elevation.278 Thus, many of the patients who underwent PCI in the REACT trial would meet the TIMI group’s definition of either adjunctive PCI, defined as PCI for patients with TIMI grade 2 or 3 flow, or delayed PCI, defined as PCI longer than 150 minutes after fibrinolytic therapy, rather than rescue PCI as defined by the TIMI group and other investigators.276 Among patients enrolled in the TIMI 10B and 14 trials, the rate of TIMI grade 3 flow was significantly greater after adjunctive PCI than after rescue PCI (89% versus 78%, p = 0.001),276 which might account for the REACT trial’s 98% (106 of 108 patients) success rate for rescue PCI.278
There is conflicting information regarding the impact of rescue PCI on mortality. Among 150 patients who were enrolled in the TIMI 10B trial and had TIMI 0 or 1 flow 90 minutes after fibrinolytic therapy, 2-year mortality was significantly less among the patients who underwent rescue PCI (n = 120) than among those who did not (n = 30) (p = 0.03).279 The randomized trials that compared rescue PCI with conservative therapy were insufficiently powered to detect an effect on mortality. Although differences in trial design and the definition of rescue PCI make it somewhat difficult to compare the results of various trials, at least two meta-analyses of the randomized trials have been published.280,281 A pooled analysis of the short-term mortality (in-hospital or 30-day) among 942 patients who were enrolled in five randomized trials revealed that the risk of death was 36% lower among patients who were randomized to PCI (RR 0.64, 95% CI 0.41 to 1.00, p = 0.048).280 Another meta-analysis included six trials that randomized 908 patients to rescue PCI or conservative therapy.281 Rescue PCI was not associated with a reduction in all-cause mortality at 6 months (RR 0.69; 95% CI 0.46 to 01.05), but it was associated with significant reductions in the risk of heart failure and reinfarction, and an increased risk of stroke and minor bleeding.281
Perhaps as a result of the varying definitions used to define rescue PCI, the term rescue PCI was not employed in the 2009 Focused Updates of the STEMI and PCI practice guidelines.22 Based on the results of the TRANSFER-AMI study, the guidelines recommend that high-risk STEMI patients who receive fibrinolytic therapy at a non-PCI-capable facility should be transferred immediately to a PCI-capable facility for diagnostic cardiac catheterization and PCI if appropriate. High-risk patients are defined as ≥ 2 mm of ST segment elevation in two anterior leads, or ≥ 1 mm ST segment elevation in inferior leads with at least one of the following: systolic blood pressure < 100 mm Hg, heart rate > 100/min, Killip class II or III, ≥ 2 mm of ST segment depression in the anterior leads, or ≥ 1 mm of ST segment elevation in right-sided lead V4 indicative of right ventricular involvement.22
Selection of Reperfusion Strategy
There is evidence that reperfusion therapy is underutilized in the United States.282 The NRMI-2 registry included 84,663 patients with STEMI who presented to the hospital with diagnostic ECG changes on the initial ECG within 6 hours after symptom onset, and without contraindications to fibrinolytic therapy.282 Despite their eligibility to receive reperfusion therapy, 24% received none (i.e., neither fibrinolytic therapy nor PCI). Age older than 75 years, female gender, lack of chest pain at presentation, and LBBB were independent predictors of failure to receive reperfusion therapy. Among patients enrolled in the NRMI-2 registry, patients in Killip class II or III were less likely to receive reperfusion therapy than patients in Killip class I.283
Analysis of the NRMI-2 registry suggested that the risk of in-hospital death was reduced more by primary PCI than by fibrinolytic therapy in patients with CHF.283 Thus, the ACC/AHA Practice Guidelines recommend primary PCI for patients with severe CHF or pulmonary edema (Killip class III) when the onset of symptoms is within 12 hours.20 Primary PCI is considered reasonable (a Class IIa recommendation) for patients who present with severe CHF, persistent ischemic symptoms, or hemodynamic or electrical instability 12 to 24 hours after symptom onset.20 As discussed subsequently, cardiogenic shock within 36 hours of acute MI is considered an indication for primary PCI.
PCI may be preferable to fibrinolytic therapy in patients with acute MI who are classified as high risk by virtue of a TIMI risk score of 5 or higher.215 Among 1527 patients who were enrolled in the DANAMI-2 trial, no difference in mortality was observed between low-risk patients (TIMI score 0 to 4) who underwent primary PCI and those who received fibrinolytic therapy (8% versus 5.6%; p = 0.11) (Fig. 30.6).215 The 3-year mortality rate was significantly lower in high-risk patients who underwent PCI than in patients who received fibrinolytic therapy (25.3% versus 36.2%; p = 0.02) (see Fig. 30.6).215
Acute MI in patients who have undergone previous CABG surgery frequently is due to thrombotic occlusion of saphenous vein bypass grafts, rather than occlusion of native coronary arteries.284,285 Data are limited regarding the efficacy of intravenous fibrinolytic therapy in patients with previous CABG surgery, but in one small study, angiography revealed extensive residual thrombus in the presumed culprit vein grafts.284 The Second Primary Angioplasty in Myocardial Infarction Trial (PAMI-2) included 58 patients with previous surgery who had either STEMI or NSTEMI.285 The infarct-related vessel was a native coronary artery in 26 patients (45%) and a bypass graft in 32 patients (55%), including 31 saphenous vein grafts and 1 internal mammary artery graft. PCI was attempted in 72% of the bypass grafts, resulting in TIMI grade 3 flow in only 70.2% of the grafts, compared with 94.3% of native coronary arteries in patients without previous CABG surgery.
Patients presenting more than 12 hours after symptom onset are not considered candidates for fibrinolytic therapy but may benefit from primary PCI.286 A trial that compared primary PCI with conservative therapy included 365 patients with acute STEMI between 12 and 48 hours after symptom onset.286 Left ventricular infarct size measured by technetium Tc 99m sestamibi imaging 5 to 10 days after randomization was significantly smaller in patients managed using invasive strategies than in patients managed conservatively (8% versus 13%; p < 0.001). The Occluded Artery Trial (OAT) randomized 2166 stable patients with one- or two-vessel CAD and a total occlusion of the IRA 3 to 28 days after MI to medical therapy or PCI with stenting.287 PCI did not reduce the occurrence of death, reinfarction, or CHF. Consequently, the following new Class III recommendation was included in the 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients with STEMI: “PCI of a totally occluded infarct artery greater than 24 hours after STEMI is not recommended in asymptomatic patients with one- or two-vessel disease if they are hemodynamically and electrically stable and do not have evidence of severe ischemia.”21
Platelet Glycoprotein Ilb/IIIa Receptor Antagonists
Numerous clinical trials have investigated the role of platelet GP IIb/IIIa inhibitors in patients with acute STEMI, either in conjunction with fibrinolytic agents or primary PCI.288,289 One rationale of including a GP IIb/IIIa inhibitor in either pharmacologic or mechanical reperfusion strategies is that platelet inhibition may improve myocardial perfusion and enhance salvage of ischemic muscle by reducing distal embolization of platelet aggregates. Another proposed rationale is to achieve coronary artery patency using lower doses of fibrinolytic drugs. Although the addition of abciximab to either tPA290 or rPA187 has been shown to improve angiographic indices of myocardial reperfusion, combination therapy with abciximab and a fibrinolytic drug has not been shown to reduce mortality compared with fibrinolytic therapy alone. A trial that randomized 16,588 patients with acute STEMI to treatment with either standard-dose rPA or half-dose rPA plus full-dose abciximab showed no differences in either 30-day or 1-year mortality.291 A meta-analysis of three fibrinolytic trials that included 23,166 patients who were randomized to receive either abciximab plus half-dose rPA or tenecteplase (TNK) versus full-dose rPA or TNK found that abciximab was associated with a significant reduction in the 30-day rate of reinfarction (2.3% versus 3.6%; p < 0.001), but 30-day mortality was 5.8% for both groups.288 Therefore, there is no Class I indication for a combination of fibrinolytic agents with GP IIb/IIIa inhibitors in the ACC/AHA Practice Guidelines.20
Although at least 18 randomized trials have been performed to evaluate GP IIb/IIIa inhibitors in patients with acute STEMI who undergo primary PCI, it has been difficult to draw definitive conclusions for several reasons.289 First, three different GP IIb/IIIa inhibitors have been studied, abciximab and the so-called small molecule inhibitors eptifibatide and tirofiban. Second, the agents have been studied in conjunction with various other antiplatelet, anticoagulant, and fibrinolytic regimens. Third, the timing of drug administration has varied from prehospital therapy to periprocedural treatment. Fourth, the trials have been underpowered to evaluate the effects on mortality.
The largest trial, HORIZONS-AMI, compared the combination of UFH and a GP IIb/IIIa inhibitor, either abciximab or eptifibatide, with bivalirudin in 3602 patients with STEMI who were treated with primary PCI.137–139 Compared with UFH plus a GP IIb/IIIa inhibitor, treatment with bivalirudin was associated with a reduced rate of major bleeding and, among patients who were classified as “high risk,” a lower 1-year mortality rate.138,139
A meta-analysis of 16 trials that enrolled a total of 10,085 patients with STEMI who underwent primary PCI concluded that GP IIb/IIIa inhibitors did not reduce the rates of mortality or reinfarction at 30 days.289 A meta-analysis of 5 randomized trials (n = 2138 patients) that compared abciximab with the small-molecule GP IIb/IIIa inhibitors in STEMI patients undergoing primary PCI found no differences in outcome.292 A meta-analysis that included 1662 patients who were enrolled in 11 randomized trials that compared early versus late administration of GP IIb/IIIa inhibitors before primary PCI concluded that early administration of abciximab improved survival compared with late administration.293 A registry that enrolled 1086 patients who received abciximab also found that early administration of abciximab before transfer for PCI, compared with late administration, was associated with lower 1-year mortality among patients with a TIMI risk score ≥ 3.294 A meta-analysis of 8 randomized trials that compared intracoronary with intravenous administration of GP IIb/IIIa inhibitors during primary PCI for STEMI found that intracoronary administration was associated with improved post-PCI blood flow and reduced mortality at 30 days.295 The INFUSE-AMI trial, however, compared the effects of intracoronary abciximab with no abciximab in patients undergoing primary PCI for an anterior STEMI, and there was no significant difference in infarct size at 30 days measured by MRI.234
Based on these data, the 2009 focused update of the ACC/AHA Guidelines for the Management of Patients with STEMI included no Class I recommendations and two Class II recommendations regarding the use of GP IIb/IIIa receptor antagonists in patients with STEMI.22 A Class IIa recommendation is “It is reasonable to start treatment with glycoprotein IIb/IIIa receptor antagonists at the time of primary PCI (with or without stenting) in selected patients with STEMI.” A Class IIb recommendation is “The usefulness of glycoprotein IIb/IIIa receptor antagonists (as part of a preparatory pharmacological strategy for patients with STEMI before their arrival in the cardiac catheterization laboratory for angiography and PCI) is uncertain.”
β-Blockers
β-Adrenergic blockers exert both antiarrhythmic and anti-ischemic effects. Experimental studies have shown that β-blockers increase the ventricular fibrillation threshold in ischemic myocardium,296 and randomized clinical trials have demonstrated that early administration of intravenous followed by oral metoprolol reduces the incidence of ventricular fibrillation in patients with acute STEMI.297,298
Experimental studies have shown that β-blockers can limit the extent of myocardial infarction during coronary occlusion because they reduce heart rate, systemic arterial pressure, and myocardial contractility, thereby decreasing myocardial oxygen demand. Clinical data are conflicting, however, regarding the effects of β-blockers on infarct size in patients, with several studies showing reduced infarct size299,300 and at least one showing no reduction.301
Numerous clinical trials have been performed to examine the effects of early or delayed β-blockade on short-term and long-term clinical outcomes in patients with acute MI. Both atenolol302 and metoprolol303 administered by intravenous infusion followed by oral administration reduced mortality in patients who did not receive fibrinolytic therapy. A pooled analysis of 27 randomized trials indicated that early β-blockade reduced mortality by 13% in the first week, and the mortality reduction benefit was greatest in the first 2 days.304
Subsequent trials have examined the impact of early intravenous β-blockade on the outcome of patients treated with fibrinolytic agents for acute STEMI.305,306 In the TIMI II-B study, 1434 patients who received intravenous tPA for acute STEMI were randomized to immediate or deferred β-blockade.305 The deferred blockade group received oral metoprolol beginning on day 6, whereas the immediate blockade group received intravenous metoprolol within 2 hours of initiation of tPA, followed by oral metoprolol. The incidence of reinfarction (2.7% versus 5.1%, p = 0.02) and recurrent chest pain (18.8% versus 24.1%, p < 0.02) at 6 days was lower in the immediate group. The GUSTO-I trial protocol recommended that patients without hypotension, bradycardia, or heart failure receive intravenous atenolol as soon as possible after enrollment, followed by oral atenolol daily.306 Although adjusted 30-day mortality was significantly lower in patients who received atenolol, intravenous atenolol was associated with greater mortality compared with oral treatment alone (odds ratio 1.3; 95% CI 1.0 to 1.5; p = 0.02). Also, administration of intravenous atenolol was associated with increased risks of heart failure, shock, recurrent ischemia, and need for a pacemaker. The Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT) randomized patients with suspected acute MI to treatment with metoprolol (up to 15 mg intravenously, followed by 200 mg/day orally; n = 22,929) or placebo (n = 22,923).298 Treatment was discontinued either at discharge from the hospital or on day 28 of the hospital stay; 93% of the patients had STEMI, and approximately 54% of the patients received a fibrinolytic agent. The risk of reinfarction during treatment was 18% lower among patients who received metoprolol (2% versus 2.5%; p = 0.001). The overall in-hospital mortality rates were 7.7% in the metoprolol treatment group and 7.8% in the placebo group (odds ratio 0.99; 95% CI 0.92 to 1.05; p = 0.69). Allocation to metoprolol was associated with a significant 22% reduction in death attributed to arrhythmia (1.7% versus 2.2%; p = 0.0002), but there was a 29% increase in death attributed to cardiogenic shock among the metoprolol treatment group (2.2% versus 1.7%; p = 0.0002).
At least 32 randomized trials including nearly 27,000 patients have been conducted to determine the effect of β-blockade on long-term survival after acute MI, and several meta-analyses have been published.304,307,308 The Norwegian Multicenter Study Group randomized 1884 patients to receive double-blind treatment with either oral timolol or placebo beginning 7 to 28 days after acute MI.309 The cumulative mortality rate at 33 months was 39% lower in the timolol group than in the placebo group (10.6% versus 17.5%; p = 0.0005), and the sudden-death rate at 33 months was reduced by 45% (7.7% versus 13.9%; p = 0.0001). After continued follow-up for up to 6 years, a significant difference in mortality was maintained.310 The Beta-Blocker Heart Attack Trial (BHAT) demonstrated that treatment with propranolol beginning 5 to 21 days after acute MI also reduced mortality during an average follow-up period of 25 months.311 A pooled analysis of 31 long-term trials found that β-blocker therapy was associated with a 23% reduction in the odds of death (95% CI 15 to 31).308 According to that analysis, the calculated number of patients needed to treat (NNT) for 2 years with a β-blocker to avoid one death is 42, which is less than the calculated NNT for antiplatelet therapy, which is 153.308
The Carvedilol Post-Infarct Survival Control in LV Dysfunction (CAPRICORN) study is an important trial that was not available for inclusion in the pooled analyses discussed earlier.312 The CAPRICORN study randomized 1959 patients with a left ventricular EF 40% or lower to carvedilol or placebo beginning 3 to 21 days after acute MI. Forty-six percent of the patients had received reperfusion therapy, and 97% had received an angiotensin-converting enzyme (ACE) inhibitor for at least 48 hours before randomization. After an average follow-up period of 1.3 years, a 23% reduction was found for all-cause mortality (12% versus 15%; hazard ratio 0.77; 95% CI 0.60 to 0.98; p = 0.031), identical to that reported in a meta-analysis of previous randomized trials.308 The CAPRICORN trial supports the conclusion that β-blockade reduces mortality after acute MI even among patients who receive reperfusion therapy and ACE inhibitors for left ventricular dysfunction.
The 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients with ST-Elevation Myocardial Infarction includes several modifications of the 2004 guidelines and a new Class III recommendation.21 The three Class I recommendations regarding oral β-blocker therapy are as follows: (1) oral β-blocker therapy should be initiated in the first 24 hours for patients who do not have any of the following: (a) signs of heart failure, (b) evidence of a low output state, (c) increased risk for cardiogenic shock (age > 70 years, systolic blood pressure < 120 mm Hg, sinus tachycardia > 110 bpm or heart rate < 60 bpm, and increased time since onset of symptoms of STEMI), or (d) other relative contraindications to β-blockade (PR interval > 0.24 seconds, second- or third-degree heart block, active asthma, or reactive airway disease); (2) patients with early contraindications within the first 24 hours of STEMI should be reevaluated for candidacy for β-blocker therapy as secondary prevention; and (3) patients with moderate or severe LV failure should receive β-blocker therapy as secondary prevention with a gradual titration scheme. There are Class IIa and III recommendations regarding intravenous β-blockade in patients with STEMI. The Class IIa recommendation states that it is reasonable to administer an IV β-blocker at the time of presentation to STEMI patients who are hypertensive and who do not have any of the following: (1) signs of heart failure, (2) evidence of low output state, (3) increased risk for cardiogenic shock (discussed earlier), or (4) other relative contraindications to β-blockade (discussed earlier). The Class III recommendations are that IV β-blockers should not be administered to STEMI patients who have any of the following: (1) signs of heart failure, (2) evidence of a low output state, (3) increased risk for cardiogenic shock, or (4) other relative contraindications to β-blockade.
Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers
Acute MI triggers neurohormonal activation that is characterized by elevated plasma renin activity and aldosterone, and plasma renin activity was found to be an independent predictor of cardiovascular mortality among patients who were enrolled in the Survival and Ventricular Enlargement (SAVE) trial.313 ACE inhibitors have been shown to attenuate left ventricular enlargement after acute MI.314 Multiple clinical trials have demonstrated that ACE inhibitors reduce mortality after acute MI. The GISSI-3 trial randomized 19,394 patients with acute MI, with or without ST segment elevation, to an oral lisinopril treatment group or an open control group within 24 hours of symptom onset.90 Seventy-one percent of the patients received fibrinolytic therapy. Six-week mortality was 11% lower among patients in the lisinopril treatment group than it was for those in the control group (6.3% versus 7.1%; odds ratio 0.88; 95% CI 0.79 to 0.99). The ISIS-4 trial randomized 58,050 patients with acute MI to receive oral captopril or placebo within 24 hours of symptom onset.91 Seventy-nine percent of the patients had ST segment elevation on the initial ECG, and 70% of eligible patients received fibrinolytic therapy, predominantly with streptokinase. Five-week mortality was 7% lower among patients who received captopril than among those in the control group (7.19% versus 7.69%; 95% CI 1 to 13; p = 0.02). The individual patient data from ISIS-4 and GISSI-3 were combined with the data from two other large trials, creating a database of 98,496 patients, who were randomized to ACE inhibitor treatment or control groups during the acute phase (0 to 36 hours) of acute MI.315 Thirty-day mortality was 7% lower among patients who received an ACE inhibitor (7.1% versus 7.6%; 95% CI 2 to 11; p = 0.004). The absolute benefit of ACE inhibitor therapy was greater in patients with anterior MI.
Three additional trials have investigated the efficacy of ACE inhibitors in patients with left ventricular dysfunction or CHF: the SAVE study,316 the Acute Infarction Ramipril Efficacy (AIRE) study,317 and the Trandolapril Cardiac Evaluation (TRACE) study.318 The SAVE study enrolled 2231 patients with an acute MI, no overt CHF, and a left ventricular EF 40% or lower as measured by radionuclide ventriculography.316 The patients were randomized to double-blind treatment with captopril or placebo 3 to 16 days after acute MI. After an average follow-up period of 42 months, all-cause mortality was reduced by 19% (20% versus 25%; 95% CI 3 to 32; p = 0.019). The risk reduction was 22% among patients treated with fibrinolytic therapy (33% of the patients) compared with 17% among patients who were not. Captopril reduced the risk of recurrent MI by 25% (95% CI 5 to 40; p = 0.015).319 The AIRE study enrolled 2006 patients with an acute MI and clinical or radiologic evidence of CHF.317 The patients were randomized to double-blind treatment with ramipril or placebo beginning 3 to 10 days after acute MI. After average follow-ups of 15 months, all-cause mortality was reduced by 27% (17% versus 23%; 95% CI 11 to 40; p = 0.002). The TRACE study enrolled 1749 patients with an acute MI and an echocardiographic left ventricular EF 35% or lower.318 The patients were randomized to double-blind treatment with trandolapril or placebo beginning 3 to 7 days after acute MI. The relative risk (RR) of death from any cause in the trandolapril group, as compared with the control group, was 0.78 (95% CI 0.67 to 0.91; p = 0.001). After follow-up for a minimum of 6 years, the life expectancy of patients was 4.6 years for patients who received placebo versus 6.2 years for those who were treated with trandolapril, a median increase of 15.3 months.320 A pooled analysis of the data from individual patients who were enrolled in the SAVE, AIRE, and TRACE trials concluded that the mortality rate after a median treatment duration of 31 months was reduced from 29.1% in control patients to 23.4% in the ACE-inhibitor group (odds ratio 0.74; 95% CI 0.66 to 0.83; p < 0.0001).321
The Register of Information and Knowledge about Swedish Heart Intensive Care Admissions (RIKS-HIA) enrolled 105,225 patients with acute MI who were not treated with ACE inhibitors on admission.322 The association between treatment with an ACE inhibitor at discharge from the hospital and the outcome at 1 year was evaluated using Cox regression analyses adjusted for medications at discharge and the propensity score. Among the entire cohort, treatment with an ACE inhibitor was associated with a 24% reduction in mortality (RR 0.76; 95% CI 0.73 to 0.80), but among patients without heart failure, a significant benefit was observed only in patients with renal dysfunction.
Two randomized clinical trials compared captopril with an angiotensin receptor blocker (ARB) in high-risk acute MI patients: the Optimal Trial in Myocardial Infarction with the Angiotensin II Antagonist Losartan (OPTIMAAL)323 and the Valsartan in Acute Myocardial Infarction Trial (VALIANT).324 The OPTIMAAL trial enrolled 5477 patients with acute STEMI who met any of the following entry criteria: symptoms or signs of CHF, left ventricular EF less than 35%, or new anterior Q waves. Fifty-four percent of the patients received fibrinolytic agents. The patients were randomized to double-blind treatment with either losartan, titrated to a target dose of 50 mg daily, or captopril, titrated to a target dose of 50 mg three times daily, within 10 days of symptom onset. After an average follow-up period of 2.7 years, no significant difference in all-cause mortality was found between the losartan and the captopril treatment groups (18% versus 16%; RR 1.13; 95% CI 0.99 to 1.28; p = 0.07). The prespecified criterion for noninferiority was not satisfied. The VALIANT trial enrolled 14,703 patients with acute MI complicated by clinical or radiographic signs of CHF or reduced left ventricular EF (≤ 35% by echocardiography or contrast ventriculography or ≤ 40% by radionuclide ventriculography), or both. Approximately 50% of the patients underwent reperfusion therapy; 35%, fibrinolytic therapy; and 15%, primary PCI. Within 10 days after the acute MI, the patients were randomly assigned to three treatment groups: valsartan monotherapy, captopril monotherapy, or the combination of valsartan and captopril. After an average follow-up period of 24.7 months, all-cause mortality was not significantly different for the three groups: valsartan 19.9%, captopril 19.5%, and the combination 19.3%. The investigators concluded that valsartan is at least as effective as captopril, because the criterion for noninferiority of valsartan relative to captopril was met.
Both aldosterone and angiotensin II, a potent stimulus of adrenal aldosterone production, are increased in patients with CHF despite chronic treatment with an ACE inhibitor. Aldosterone exerts numerous adverse cardiovascular effects, including increased myocardial collagen deposition and fibrosis and cardiomyocyte apoptosis.325 Eplerenone, a selective aldosterone blocker, was studied in a multicenter, international, randomized, double-blind, placebo-controlled trial called the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS).326 The EPHESUS investigators enrolled 6632 patients with acute MI complicated by left ventricular dysfunction (EF less than 40%) plus either CHF or diabetes.326 The patients were randomized to double-blind treatment with eplerenone or placebo beginning 3 to 14 days after acute MI. At the time of enrollment, 86% of the patients were taking an ACE inhibitor or ARB, and 75% were taking a β-blocker. During a mean follow-up period of 16 months, significantly fewer deaths occurred in the eplerenone group (478 of 3319 patients) than in the placebo group (554 of 3313 patients) (14.4% versus 16.7%; RR 0.85; 95% CI 0.75 to 0.96; p = 0.008). A reduction in the rate of sudden death from cardiac causes (RR 0.79; 95% CI 0.64 to 0.97; p = 0.03) also was observed. Serious hyperkalemia, defined as a serum potassium of 6 mmol/L or more, occurred in 5.5% of patients in the eplerenone group versus 3.9% of those in the placebo group (p = 0.002).
The ACC/AHA Practice Guidelines include the following Class I recommendations regarding inhibitors of the renin-angiotensin-aldosterone system: (1) ACE inhibitors should be started and continued indefinitely in all patients recovering from STEMI with LVEF less than or equal to 40% and for those with hypertension, diabetes, or chronic kidney disease, unless otherwise contraindicated; (2) ACE inhibitors should be started and continued indefinitely in patients recovering from STEMI who are not lower risk (lower risk defined as those with normal LVEF in whom cardiovascular risk factors are well controlled and revascularization has been performed), unless contraindicated; (3) the use of angiotensin receptor blockers is recommended in patients who are intolerant of ACE inhibitors and have heart failure or have had an MI with LVEF less than or equal to 40%; (4) it is beneficial to use angiotensin receptor blocker therapy in other patients who are ACE-inhibitor intolerant and have hypertension; (5) the use of aldosterone blockade in post-MI patients without significant renal dysfunction (serum creatinine less than 2.5 mg/dL in men and less than 2 mg/dL in women) or hyperkalemia (serum potassium ≥ 5 mEq/L) is recommended in patients who are already receiving therapeutic doses of an ACE inhibitor and β-blocker, have an LVEF of less than or equal to 40%, and have either diabetes or heart failure.21 Intravenous ACE inhibitors should not be given within 24 hours of an acute MI because of the risk of hypotension.
Antiarrhythmic Drugs
Both atrial and ventricular arrhythmias are common in patients with acute MI. The incidence of atrial fibrillation was 10.4% among 40,891 patients who were enrolled in the GUSTO-I trial.327 Patients in whom atrial fibrillation developed after admission were more likely to have a stroke or die within 30 days after acute MI. Among patients enrolled in the TRACE study, which enrolled patients with an acute MI and a left ventricular EF less than 35%, atrial fibrillation occurred in 21% of patients and was associated with a 50% increase in adjusted mortality.328 Intravenous β-adrenergic blockade is the preferred therapy for patients with sustained atrial fibrillation or atrial flutter that is not associated with hemodynamic compromise, whereas sustained atrial fibrillation or flutter that is associated with hemodynamic compromise is an indication for synchronized cardioversion. Intravenous amiodarone is indicated for treatment of atrial fibrillation that does not respond to electrical cardioversion or recurs after cardioversion. Sustained atrial fibrillation should be treated with anticoagulants.
The incidence of primary ventricular fibrillation, defined as that occurring within 48 hours of acute MI and in the absence of cardiogenic shock or severe CHF, was 4.7% among a cohort of 5020 patients hospitalized for an uncomplicated acute MI in Worcester, Massachusetts, during 11 1-year periods between 1975 and 1997.329 The incidence of primary ventricular fibrillation in the GISSI-1 trial was not significantly different in the streptokinase and control groups (2.73% versus 2.93%; RR 0.93; 95% CI 0.75 to 1.15).330 A meta-analysis of 15 randomized trials of fibrinolytic therapy for acute MI confirmed that the likelihood of this arrhythmia is not altered by fibrinolytic therapy, with an incidence of ventricular fibrillation during the first hospital day of 2.99% for both the fibrinolytic treatment and placebo groups.331 There is evidence, however, that fibrinolytic therapy exerts a protective effect against secondary ventricular fibrillation, defined as ventricular fibrillation in patients with acute MI complicated by CHF or shock.331,332 Thus, the meta-analysis of 15 fibrinolytic trials found that the odds ratio for the development of ventricular fibrillation at any time during hospitalization in the fibrinolytic treatment group was 0.83 (95% CI 0.76 to 0.90; p < 0.0001).331
Primary ventricular fibrillation is an independent predictor of in-hospital mortality whether it occurs early (up to 4 hours) or late (after 4 to 48 hours) after the onset of acute MI.333 Among 9720 patients with a first STEMI who were enrolled in the GISSI-2 fibrinolytic trial, 356 of the 7755 patients who were in Killip class I at entry developed primary ventricular fibrillation.333 Early primary ventricular fibrillation occurred in 302 patients (3.7%) and late primary ventricular fibrillation occurred in 54 patients (0.6%); 226 patients had ventricular fibrillation within 1 hour of the onset of acute MI symptoms. In-hospital mortality rates were 13% among patients with late primary ventricular fibrillation (RR 3.80; 95% CI 1.80 to 8.02) and 7% among patients with early primary ventricular fibrillation (RR 2.00; 95% CI 1.29 to 3.12), compared with 4% among patients in Killip class I on admission in whom ventricular fibrillation did not develop. The in-hospital mortality rate associated with primary ventricular fibrillation was much higher in the Worcester Heart Attack Study, with an overall case-fatality rate of 44%, although improved survival was observed in patients who had primary ventricular fibrillation in the 1990s.329
Among 11,712 patients enrolled in the GISSI-1 study, secondary ventricular fibrillation occurred in 311 patients (2.7%).332 The incidence of secondary ventricular fibrillation within 24 hours of acute MI was similar among the patients treated with streptokinase and the control group, whereas streptokinase halved the frequency of secondary ventricular fibrillation later than 24 hours after admission (27/5860 versus 60/5852; RR 0.45; 95% CI 0.29 to 0.70). The protective effect of streptokinase was even greater among patients who were treated within 3 hours of symptom onset (9/3016 versus 39/3078; RR 0.23; 95% CI 0.12 to 0.45). In-hospital mortality was higher among patients with secondary ventricular fibrillation: 27.1% versus 17.3% for patients in Killip class II (RR 1.77; 95% CI 1.28 to 2.45) and 48.1% versus 35.3% for patients in Killip class III (RR 1.70; 95% CI 0.95 to 3.02). Secondary ventricular fibrillation did not affect in-hospital mortality among the patients in Killip class IV (67.9% with secondary ventricular fibrillation versus 71.9% without secondary ventricular fibrillation; RR 0.83; 95% CI 0.44 to 1.55).
Although primary and secondary ventricular fibrillation both are associated with an increased risk of in-hospital death, patients who survive to be discharged from the hospital after experiencing either type have a good prognosis. Among the patients who were enrolled in the GISSI-1 and GISSI-2 trials and had nonfatal primary ventricular fibrillation, 6-month mortality after hospital discharge among patients with the primary type was not significantly different from that in patients who did not have this arrhythmia.333,334 In GISSI-2, patients who survived the hospital phase of an acute MI complicated by secondary ventricular fibrillation had a 1-year mortality of 10.4%, compared with 13.9% among patients who did not have secondary ventricular fibrillation (20/193 versus 322/2319; RR 0.72; 95% CI 0.44 to 1.15).332
Partly on the basis of the results of one double-blind, randomized study,335 an editorial published in 1978 concluded that there was justification for routine prophylactic administration of lidocaine to all patients with acute MI to prevent primary ventricular fibrillation.336 Subsequent studies of prophylactic lidocaine in acute MI, including more than 20 randomized trials and at least four meta-analyses, concluded that lidocaine reduces the incidence of ventricular fibrillation but increases mortality.337 Therefore, according to the ACC/AHA Practice Guidelines that were published in 2004, prophylactic antiarrhythmic therapy is not recommended with use of fibrinolytic agents.20 Also, the routine use of lidocaine is not indicated for suppression of isolated ventricular premature beats, couplets, runs of accelerated idioventricular rhythm, and nonsustained ventricular tachycardia. The guidelines favor intravenous amiodarone for treatment of sustained monomorphic ventricular tachycardia.
Although ventricular ectopy is a marker of an increased risk of sudden death in survivors of acute MI, trials of chronic, oral antiarrhythmic drugs have not shown beneficial effects. The Cardiac Arrhythmia Suppression Trial (CAST) I and CAST II found that treatment with the class IC drugs encainide and flecainide, or the class IA drug moricizine, increased mortality in survivors of acute MI.338,339 d-Sotalol, a potassium channel blocker, also increased mortality among survivors of MI in the Survival with Oral d-Sotalol (SWORD) randomized trial.340 Clinical trials of amiodarone, a class III antiarrhythmic drug, suggested that it may reduce the incidence of ventricular fibrillation or arrhythmic death among survivors of acute MI.341,342
The implantable cardioverter-defibrillator (ICD) has become the standard of care for the prevention of sudden death in survivors of acute MI.343 There are two Class I indications for an ICD after STEMI: (1) an ICD is indicated for patients with ventricular fibrillation or hemodynamically significant sustained ventricular tachycardia later than 2 days after STEMI, provided that the arrhythmia is not judged to be due to transient or reversible ischemia or reinfarction. (2) An ICD is indicated for patients without spontaneous ventricular fibrillation or sustained ventricular tachycardia more than 48 hours after STEMI whose STEMI occurred at least 1 month previously, who have a left ventricular EF between 31% and 40%, demonstrated additional evidence of electrical instability (e.g., nonsustained ventricular tachycardia), and who have inducible ventricular fibrillation or sustained ventricular tachycardia on electrophysiology testing.20
Calcium Antagonists
No Class I indications are provided for calcium channel blockers in patients with acute MI because neither individual clinical trials nor analyses of the pooled results of multiple trials showed a reduction in mortality after acute MI.307,344,345 A randomized study that compared diltiazem with placebo starting 3 to 15 days after acute MI found that diltiazem therapy was associated with an increased risk of cardiac events in patients with radiographic evidence of pulmonary congestion.346 The same trial concluded that diltiazem increases the risk of late-onset CHF in patients with a left ventricular EF less than 40%.347 Thus, there are two Class III recommendations for calcium channel blockers in acute MI: (1) Diltiazem and verapamil are contraindicated in patients with STEMI and associated systolic left ventricular dysfunction and CHF. (2) Nifedipine (immediate-release form) is contraindicated in the treatment of STEMI because of the reflex sympathetic activation, tachycardia, and hypotension associated with its use.20
Lipid-Lowering Agents
Numerous trials have evaluated the effects of statins on coronary events in patients with acute and chronic CAD, but none of the trials restricted enrollment to patients with STEMI or provided subgroup analyses of the outcomes among patients with STEMI before enrollment. The Scandinavian Simvastatin Survival Study (4S) was the first clinical trial to demonstrate significant reductions in total mortality and coronary heart disease mortality by treatment with lipid-lowering therapy.348 Seventy-nine percent of the patients had a history of MI. After a median follow-up period of 5.4 years, total mortality was 12% in the placebo group versus 8% in the patients who were randomly assigned to receive simvastatin (RR 0.70; 95% CI 0.58 to 0.85; p = 0.0003). Also, there was a 37% reduction in the risk of undergoing a myocardial revascularization procedure. The beneficial effects of statin therapy in patients with a history of MI were confirmed by two studies that randomized patients to receive pravastatin 40 mg daily or placebo: the Cholesterol and Recurrent Events (CARE) trial349 and the Long-Term Intervention with Pravastatin in Ischemic Disease (LIPID) trial.350 All patients who were enrolled in the CARE trial had a history of MI between 3 and 20 months before enrollment, and 64% of the patients enrolled in the LIPID trial had a history of MI between 3 and 36 months before enrollment.
A diagnosis of hyperlipidemia cannot be excluded during the first week after an acute MI because both total cholesterol and low-density lipoprotein (LDL) cholesterol decrease significantly during the first week after an acute MI.351 There are numerous Class I recommendations for lipid management in patients who have had a STEMI. Although the current Class I guidelines recommend a target LDL cholesterol level less than 100 mg/dL, future guidelines may reflect data that support more aggressive treatment to achieve the goal of an LDL cholesterol level of less than 70 mg/dL.352–354
Intra-aortic Balloon Counterpulsation
Several randomized trials have evaluated the utility of intra-aortic balloon counterpulsation in high-risk patients with acute MI not complicated by cardiogenic shock. The Second Primary Angioplasty in Myocardial Infarction (PAMI-II) investigators randomized 436 patients with a high-risk acute MI to insertion of an intra-aortic balloon pump (IABP) or no IABP after primary PCI.355 The rates of reocclusion of the IRA, reinfarction, and mortality were not significantly different, and there was a higher incidence of stroke among patients who received an IABP (2.4% versus 0%; p = 0.03). Also, there was no enhancement of myocardial recovery at the time of hospital discharge or 6 weeks after discharge. The Counterpulsation to Reduce Infarct Size Pre-PCI Acute Myocardial Infarction (CRISP AMI) trial was a randomized trial to determine whether insertion of an IABP before primary PCI reduces infarct size in patients with an acute anterior STEMI without cardiogenic shock.356 The mean infarct size, measured by cardiac magnetic resonance imaging 3 to 5 days after PCI was 42.1% of the left ventricle in the IABP group compared with 37.5% of the left ventricle in the control group (p = 0.06). Thus, the IABP has not been shown to be beneficial in patients with high-risk or anterior acute MI who undergo primary PCI. The use of the IABP in patients with cardiogenic shock is discussed in a subsequent section of this chapter and in the chapter on cardiogenic shock.
Coronary Artery Bypass Graft Surgery
Randomized trials have not been performed to compare surgical reperfusion for acute MI with either conservative medical therapy or reperfusion by means of fibrinolytic therapy or PCI. A nonrandomized study compared the outcomes in 200 patients managed conservatively with those in 187 patients who underwent surgical reperfusion between 1972 and 1976.357 In-hospital mortality was 5.8% among patients who underwent CABG surgery, compared with 11.5% among patients who did not undergo myocardial reperfusion (p < 0.08). Among the patients who underwent coronary bypass grafting within 6 hours from the onset of symptoms of acute MI, in-hospital mortality was only 2% (2/100), compared with 10.3% (9/87) among the patients who underwent surgery more than 6 hours after the onset of symptoms.
Although registry data indicate that CABG surgery within 24 hours of an acute STEMI is associated with a marked increase of in-hospital mortality,358 emergency surgical revascularization should be considered in certain subsets of patients. Emergency CABG surgery is a reasonable option in patients with cardiogenic shock and coronary anatomy poorly suited for PCI (e.g., severe stenosis of the left main coronary artery). Among patients enrolled in the Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock? (SHOCK) trial, survival rates were similar for CABG surgery and for PCI.359 The 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery includes four Class I, two Class IIa, and two Class III recommendations regarding CABG in patients with acute MI360 (Box 30.6).
Mortality after CABG surgery remains elevated during the first week after an acute MI.358 Therefore, the 2004 ACC/AHA Practice Guidelines for the Management of Patients with ST-Elevation Myocardial Infarction recommended that patients who have been stabilized after STEMI and who have incurred a significant fall in LV function should have their surgery delayed to allow for myocardial recovery.20
An additional reason to delay surgery in stable patients is to permit recovery of platelet function in patients who have received a P2Y12 inhibitor. Red blood cell transfusion of 4 units or more after CABG was an independent predictor of 1-year mortality among 1491 patients with ACS who underwent CABG.361 There are conflicting data regarding the impact of P2Y12 inhibitors on the risk of bleeding and transfusion after CABG. One retrospective analysis of 596 patients with ACS who underwent CABG found that exposure to clopidogrel within 5 days of surgery increased the risk of major bleeding and reoperation.362 Among 1539 patients with ACS who were enrolled in the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial and underwent CABG before discharge, CABG within 5 days of the last clopidogrel dose was not associated with higher rates of transfusion or major bleeding compared with the rates among patients who did not receive any clopidogrel before CABG.363 Administration of clopidogrel within 5 days before CABG was associated with a modestly increased risk of red blood cell transfusion but was not significantly associated with reoperation for bleeding among 332 patients who were enrolled in the Duke Databank between January 1999 and December 2003.364 According to the 2011 ACCF/AHA Guideline for CABG, clopidogrel and ticagrelor should be discontinued for at least 5 days before surgery and prasugrel for at least 7 days to limit blood transfusions, while clopidogrel and ticagrelor should be discontinued for at least 24 hours in patients referred for urgent CABG.360 Finally, the guideline includes a Class I recommendation that aspirin (100 mg to 325 mg) should be administered to CABG patients preoperatively.360
Management of Complications
Pericarditis and Pericardial Tamponade
Pericarditis, defined as the detection of a pericardial friction rub, was diagnosed in 20% (141/703) of patients who were enrolled in the Multicenter Investigation of the Limitation of Infarct Size (MILIS).365 The frequency of pericarditis was higher in patients with Q wave MI than in those with non–Q wave MI (25% versus 9%; p < 0.001). Pericarditis was associated with a lower admission left ventricular EF (42% versus 48%; p < 0.001) and a higher incidence of CHF (47% versus 26%; p < 0.001). Pericarditis was accompanied by pleuritic or positional chest pain in 70% of patients. Diagnostic electrocardiographic changes usually are absent in patients with infarction-associated pericarditis.366 A prospective study of 423 patients with acute MI found that only 1 of the 31 patients with pericardial friction rubs had diagnostic ST segment changes.366
The GISSI investigators reported the frequency of pericardial involvement in patients who were enrolled in the GISSI-1 (n = 11,806) and GISSI-2 (n = 12,381) trials.367 The incidence of pericardial involvement was lower among patients who received fibrinolytic therapy than among patients in the control groups (6.7% versus 12%). Earlier treatment with fibrinolytic therapy was associated with a lower risk of pericardial involvement. Although pericardial involvement was associated with a higher long-term mortality, it was not an independent prognostic factor because it was strongly associated with the extent of infarction as determined by ECG, peak creatine kinase, and echocardiography.
The ACC/AHA Practice Guidelines recommend aspirin for treatment of pericarditis after STEMI.20 Colchicine or acetaminophen is recommended for patients who do not respond to aspirin. Corticosteroids and nonsteroidal anti-inflammatory drugs are discouraged because of an increased risk of scar thinning and infarct expansion. Finally, anticoagulation should be discontinued if a pericardial effusion is detected.
Hemorrhagic pericarditis and free wall rupture are two potential mechanisms of cardiac tamponade after acute MI. Among 102,060 patients with STEMI who were enrolled in seven randomized clinical trials and received fibrinolytic therapy, cardiac tamponade developed in 1018 patients (1%).368 Among the patients with tamponade, 153 also had a ventricular septal rupture or acute mitral regurgitation, and 865 had isolated cardiac tamponade. The adjusted 30-day mortality among 7-day survivors with tamponade was significantly increased (hazard ratio 7.9; 95% CI 4.7 to 13.5; p < 0.0001). Pericardial tamponade accounted for 1.4% of patients with cardiogenic shock among 1422 patients with acute MI who were enrolled in either the SHOCK registry or the randomized trial.369
Recurrent Ischemia or Infarction
Recurrent infarction (reinfarction) after an initial STEMI is relatively uncommon, but it often is an end point for clinical trials, because reinfarction is associated with increased morbidity and mortality.370,371 Symptomatic recurrent MI during the index hospitalization occurred in 4.2% (836/20,101) of patients who were enrolled in four clinical trials of various fibrinolytic agents.371 Recurrent MI occurred a median of 2.2 days after the initial MI and was associated with increased mortality rates at both 30 days (16.4% versus 6.2%; p < 0.001) and 2 years (hazard ratio 2.11, p < 0.001). In-hospital reinfarction occurred in 4.3% of patients (2258/55,911) a median of 3.8 days after fibrinolytic therapy in the GUSTO I and GUSTO III trials.370 The rates of reinfarction were 4.3% for alteplase, 4.5% for reteplase, and 4.1% for streptokinase (p = 0.55). Patients with in-hospital reinfarction had higher mortality at 30 days (11.3% versus 3.5%; odds ratio 3.5; p < 0.001) and from 30 days to 1 year (4.7% versus 3.2%; hazard ratio 1.5; p < 0.001).370 Compared with patients who did not have reinfarction, patients with reinfarction had higher rates of CHF (31.9% versus 13.9%) and cardiogenic shock (15.9% versus 2.4%).
The frequency of recurrent ischemia or reinfarction after an initial STEMI depends on the modality of reperfusion and the adjunctive therapy employed. One of the purported advantages of primary PCI over fibrinolytic therapy is a decreased rate of reinfarction. The 30-day rates of reinfarction in the DANAMI-2 trial were 6.3% (49/782) among patients who were randomized to fibrinolytic therapy compared with 1.6% (13/790) among patients who were assigned to the angioplasty group (p < 0.001).214 In a meta-analysis of 13 randomized trials of primary angioplasty versus fibrin-specific agents, the frequency of nonfatal reinfarction was 3% (74/2753) among patients randomized to angioplasty, compared with 6% (172/2757) among patients assigned to fibrinolytic therapy (odds ratio 0.42; 95% CI 0.31 to 0.55).209
There is evidence that coronary revascularization reduces the risk of reinfarction after fibrinolytic therapy. Among patients who were enrolled in four clinical trials of various fibrinolytic drugs, PCI or CABG surgery was performed during the index hospitalization in 26.1% (5238/20,039) of the patients and was associated with lower rates of both in-hospital recurrent MI (1.4% versus 4.7%, p < 0.001) and 2-year mortality.371
A variety of antithrombotic and antiplatelet therapies have been shown to reduce the risk of reinfarction after STEMI. In a trial that randomized 20,506 patients with STEMI to either enoxaparin or UFH as adjunctive treatment after fibrinolytic therapy, the rates of reinfarction at 30 days were 3% for the enoxaparin group (309/10,256) compared with 4.5% for the UFH group (458/10,223) (RR 0.67; 95% CI 0.58 to 0.77; p < 0.001).131 A meta-analysis of 11 clinical trials found that administration of abciximab was associated with a reduction in the 30-day reinfarction rates among patients who underwent primary angioplasty (1% versus 1.9%; odds ratio 0.56; 95% CI 0.33 to 0.94; p = 0.03) and among patients who received fibrinolytic therapy (2.3% versus 3.6%; odds ratio 0.64; 95% CI 0.54 to 0.75; p < 0.001).288
Numerous clinical trials have investigated the effect of warfarin alone or in combination with aspirin on the risk of reinfarction and other events in patients with ACS, but most of the studies were not restricted to patients with STEMI, the target international normalized ratio (INR) has varied, and conflicting results have emerged.372,373 The Warfarin Re-Infarction Study randomized 1214 patients to receive placebo or warfarin (target INR 2.8 to 4.8) after an average interval between the index MI and enrollment of 27 days.374 Approximately 70% of patients had Q waves on the baseline ECG, most patients did not receive reperfusion therapy, and all patients were advised not to take aspirin or other antiplatelet drugs. During an average treatment period of 37 months, the rate of reinfarction was significantly reduced by warfarin compared with placebo (82/607 versus 124/607; RR 34%; 95% CI 19% to 54%; p = 0.0007).
The Antithrombotics in the Secondary Prevention of Events in Coronary Thrombosis-2 (ASPECT-2) study randomized 999 patients to receive one of three antithrombotic regimens: aspirin 80 mg daily, warfarin (target INR 3.0 to 4.0), or the combination of aspirin 80 mg daily and warfarin (target INR 2.0 to 2.5).375 Patients were enrolled within 8 weeks of hospitalization for either UA (13%) or Q wave or non–Q wave MI. During a median follow-up period of 12 months, the primary composite end point of MI, stroke, or death was significantly less frequent for the two groups that received warfarin, but warfarin did not reduce the risk of MI, and it increased the risk of both major and minor bleeding.
The APRICOT-2 trial randomized 308 patients with a patent IRA within 48 hours after fibrinolytic therapy to receive either aspirin alone (80 mg daily) or aspirin plus warfarin for 3 months (target INR 2.0 to 3.0).376 The rate of reinfarction during 3 months of follow-up was 2% (3/135) for combination therapy, compared with 8% (11/139) for aspirin alone (p < 0.05). The Warfarin, Aspirin, Reinfarction Study (WARIS II) randomly assigned 3630 patients who were hospitalized for an acute MI to one of three treatment groups: warfarin alone (with a target INR of 2.8 to 4.2), aspirin alone (160 mg daily), or the combination of aspirin 75 mg daily and warfarin (target INR 2.0 to 2.5).377 Fibrinolytic drugs were administered to 53% to 55% of the patients in each group. During a mean observation period of 4 years, the rates of reinfarction were significantly less in both groups of patients who received warfarin: 9.7% (117/1206) for aspirin alone, 7.4% (90/1216) for warfarin alone (RR 0.74; 95% CI 0.55 to 0.998; p = 0.03), and 5.7% (69/1208) for aspirin plus warfarin (RR 0.56; 95% CI 0.41 to 0.78; p < 0.001). Another study that used a target INR of 1.5 to 2.5 found that the addition of warfarin to aspirin 81 mg daily did not reduce the rate of reinfarction compared with aspirin monotherapy (162 mg daily).378 The combination of aspirin 80 mg daily with low, fixed-dose warfarin (1 mg or 3 mg) was not superior to aspirin 160 mg daily in patients with a recent STEMI or NSTEMI.379 Thus, the clinical trials suggest that warfarin is superior to placebo, that the combination of aspirin and warfarin is superior to aspirin alone if the target INR is sufficiently high, and that the risk of major bleeding is increased by adding warfarin to aspirin. Also, the published data should not be extrapolated to patients who receive dual antiplatelet therapy (aspirin plus a thienopyridine) after either fibrinolytic therapy or coronary artery stenting, because most of the patients who were enrolled in the warfarin trials did not receive reperfusion therapy or a thienopyridine.
In the ISIS-2 trial, aspirin reduced the rate of in-hospital reinfarction both in the patients who received streptokinase and in those who did not receive fibrinolytic therapy. Higher platelet counts were associated with an increased risk of reinfarction among patients with STEMI for whom treatment consisted of aspirin plus a fibrinolytic drug.380 The addition of clopidogrel to aspirin abolishes the increased risk of reinfarction as the platelet count increases.380 In the CLARITY study, the rate of recurrent MI after fibrinolytic therapy was 4.1% among patients treated with clopidogrel and aspirin, compared with 5.9% among patients who received placebo and aspirin (representing a 31% reduction in odds).106
Clinical trials have demonstrated that β-blockers, ACE inhibitors, and statins also reduce the risk of reinfarction after acute MI, although most of the trials enrolled patients with both STEMI and NSTEMI, and subgroup analyses of outcomes in patients with STEMI were not published. Compared with metoprolol started 6 days after tPA for acute STEMI, metoprolol started within 2 hours of tPA was associated with lower rates of reinfarction (5.1% versus 2.7%; p = 0.02) and recurrent chest pain (24.1% versus 18.8%; p < 0.02) at 6 days.305 The Norwegian Multicenter Study Group randomly assigned 1884 patients to double-blind treatment groups, to receive either oral timolol or placebo, beginning 7 to 28 days after acute MI.309 The cumulative reinfarction rate at 33 months was 28% lower in the timolol group than in the placebo group (14.4% versus 20.1%; p = 0.0006). The CAPRICORN study randomized 1959 patients with a left ventricular EF of 40% or less to receive carvedilol or placebo beginning 3 to 21 days after acute MI. Forty-six percent of the patients had received reperfusion therapy and 97% had received an ACE inhibitor for at least 48 hours before beginning the study treatment. After an average follow-up period of 1.3 years, the rate of nonfatal MI was significantly lower in the carvedilol group than in the placebo group (3% versus 6%; hazard ratio 0.59; 95% CI 0.39 to 0.90; p = 0.014).
The GISSI 3 trial did not show an effect of lisinopril on the rate of reinfarction after 6 weeks.90 Although the AIRE317 and TRACE318 trials failed to show a significant effect of ramipril or trandolapril on the long-term risk of reinfarction, the SAVE study316,319 did observe a significant decrease in the reinfarction rate among patients who were randomly selected to receive captopril 3 to 16 days after acute MI. After an average follow-up period of 42 months, captopril reduced the risk of recurrent MI by 25% (95% CI 5% to 40%; p = 0.015).319 Thus, ACE inhibitors may not reduce the short-term risk of reinfarction after acute MI, but they may decrease the long-term risk of reinfarction.
Numerous trials have evaluated the effects of statins on coronary events in patients with acute and chronic CAD, but none of the trials restricted enrollment to patients with STEMI and many of the trials pooled the data of patients with UA, NSTEMI, and STEMI. All patients who were enrolled in the CARE trial had a history of MI between 3 and 20 months before randomization; 61% were enrolled after a Q wave MI.349 Although the mean interval from MI to enrollment was 10 months, during a median follow-up period of 5 years there was a significantly lower rate of nonfatal MI among patients who received pravastatin compared with patients who received placebo (6.5% versus 8.3%; RR 23%; 95% CI 4% to 39%; p = 0.02).349
Data are conflicting regarding the benefit of initiating statin therapy within 14 days of the onset of ACS. At least two meta-analyses of relevant randomized controlled trials have been published.381,382 One analysis of 12 randomized trials concluded that statin therapy initiated within 14 days of hospital admission does not reduce the risk of death, MI, or stroke during the first 4 months after ACS.381 Another meta-analysis of 13 randomized trials concluded that early statin therapy reduces death and cardiovascular events after 4 months of treatment.382 A prospective cohort study using data from the Swedish Registry of Cardiac Intensive Care concluded that initiation of statin therapy before discharge was associated with a 25% reduction in 1-year mortality in hospital survivors of acute MI.383
Among a cohort of 2301 patients who suffered reinfarction after administration of fibrinolytic therapy in the GUSTO I and Assessment of Safety and Efficacy of a New Thrombolytic 2 (ASSENT 2) clinical trials, reinfarction was treated with repeat fibrinolysis (n = 864), with revascularization (n = 525), or conservatively (n = 835).384 After adjustment for baseline characteristics, the 30-day mortality was significantly greater in the conservative group, 28%, compared with the repeat fibrinolysis group, 11% (odds ratio 2.2; 95% CI 1.5 to 3.1; p < 0.001) or the revascularization group, 11% (odds ratio 2.2; 95% CI 1.4 to 3.3; p < 0.0001). No significant difference was observed between the revascularization and repeat fibrinolysis groups.
The ACC/AHA Practice Guidelines provide several recommendations regarding the management of recurrent ischemia and reinfarction.20 They recommend escalation of medical therapy with nitrates, β-blockers, and intravenous anticoagulation. Insertion of an IABP should be considered in patients with hemodynamic instability, poor left ventricular function, or a large area of myocardium at risk. Recurrent ischemic-type chest discomfort is a Class I indication for coronary angiography and PCI or CABG surgery in patients who are considered candidates for revascularization. There is a Class IIa recommendation for readministration of fibrinolytic therapy to patients with ischemic-type chest discomfort and recurrent ST segment elevation who are not considered candidates for revascularization or for whom coronary angiography and PCI cannot be implemented within 60 minutes of the onset of recurrent ischemia. A Class III recommendation regarding streptokinase states that it should not be readministered to patients who received a non-fibrin-specific fibrinolytic agent more than 5 days previously.
Congestive Heart Failure
Wu and colleagues283 described the outcomes for patients with STEMI who were enrolled in the NRMI-2 database and had CHF on admission (Killip class II or III). A total of 36,303 of 190,518 patients with AMI (19.1%) had CHF on admission; 70.6% were in Killip class II and 29.4% were in Killip class III. Patients who presented with CHF were less likely to receive fibrinolytic therapy or undergo primary PTCA. CHF on admission was a strong independent predictor of in-hospital death (adjusted odds ratio 1.68; 95% CI 1.62 to 1.75).
Hasdai and associates385 combined the data from four large randomized trials of fibrinolytic therapy for STEMI to describe the incidence, timing, and consequences of mild to moderate CHF in patients with STEMI. Excluding patients with cardiogenic shock, 17,949 of 61,041 (29.4%) patients had mild to moderate CHF. Among the cohort with mild to moderate CHF, 8.7% had CHF only at baseline, 57.6% had CHF only after admission, and 33.7% had CHF at baseline and after admission. The incidence of death was similar for patients without CHF and patients with CHF at baseline that resolved after admission. Patients with CHF that persisted from baseline or developed after admission had a four times greater risk of death at 30 days (8% versus 2%).
There is evidence that patients with STEMI complicated by CHF benefit from early revascularization. Analysis of an Israeli database compared the outcomes of 629 patients with STEMI who presented in Killip class II or III CHF.386 Mortality at 6 months was lower among patients who underwent PTCA or CABG surgery within 30 days compared with patients who were managed noninvasively (11.6% versus 27.4%; odds ratio 0.40; 95% CI 0.24 to 0.64; p < 0.0001). Analysis of the NRMI-2 registry suggested that the risk of in-hospital death was reduced more by primary PCI than by fibrinolytic therapy in patients with CHF.283 Thus, the ACC/AHA Practice Guidelines recommend primary PCI for patients with severe CHF or pulmonary edema (Killip class III) when the onset of symptoms is within 12 hours.20 Primary PCI is considered reasonable (a Class IIa recommendation) for patients who present with severe CHF, persistent ischemic symptoms, or hemodynamic or electrical instability 12 to 24 hours after symptom onset.20
It is important to recognize that CHF also is an important prognostic factor in patients with UA or NSTEMI.387 Among a cohort of 13,707 patients with a confirmed diagnosis of ACS without prior CHF or cardiogenic shock at the time of presentation to the hospital, CHF (Killip class II or III) was present at hospital admission in 1778 patients (13%), and CHF developed later during hospitalization in an additional 869 patients (6.3%).387 The incidence of CHF was similar in patients with STEMI (15.6%) or NSTEMI (15.7%) but less frequent in patients with UA (8.2%). CHF at the time of admission was associated with a fourfold increase in crude in-hospital mortality rates across all three ACS subsets. The cumulative 6-month mortality rate was greater among patients in whom CHF developed during hospitalization (25.3%) than among patients who had CHF at admission (20.7%) or patients who did not have CHF (5.9%).
ACE inhibitors, eplerenone, and β-adrenergic antagonists are believed to improve the long-term survival of patients with MI complicated by CHF.388 The AIRE study showed that ramipril, initiated 3 to 10 days after acute MI, reduced all-cause mortality by 27% in patients with either STEMI or NSTEMI and clinical or radiologic evidence of CHF.317 The EPHESUS study randomized patients with either STEMI or NSTEMI and left ventricular EF less than 40%, to eplerenone or placebo beginning 3 to 14 days after the acute MI.326 Ninety percent of the patients had CHF, documented by the presence of pulmonary rales, a third heart sound, or evidence of pulmonary venous congestion on the chest radiograph. Concomitant medications included β-blockers in 75% of patients and an ACE inhibitor or angiotensin receptor blocker in 86%. At 30 days after randomization, eplerenone reduced the risk of all-cause mortality by 31% (3.2% versus 4.6%; RR 0.69; 95% CI 0.54 to 0.89; p = 0.004).389 During a mean follow-up period of 16 months, the all-cause mortality rate was 14.4% in the eplerenone group and 16.7% in the placebo group (RR 0.85; 95% CI 0.75 to 0.96; p = 0.008).326
BHAT, a study that randomized patients to receive propranolol or placebo 5 to 21 days after acute MI, included 710 patients who had a history of CHF before enrollment.390 After an average follow-up period of 25 months, propranolol reduced total mortality by 27% and sudden death by 47%. A retrospective analysis of the AIRE study was performed to determine the effects of β-blockade on the outcomes for patients with acute MI complicated by CHF.391 β-Blocker treatment was an independent predictor of reduced risk of total mortality (hazard ratio 0.66; 95% CI 0.48 to 0.90). The CAPRICORN study showed that carvedilol, started 3 to 21 days after MI, reduced all-cause mortality by 33% in patients with a left ventricular EF of 40% or less.312 Unfortunately, patients with acute MI complicated by CHF are less likely to receive a β-blocker than are patients without CHF.392
On the basis of the foregoing evidence, the ACC/AHA Practice Guidelines include eight Class I recommendations for patients with STEMI complicated by pulmonary congestion20: (1) an arterial oxygen saturation greater than 90% should be maintained using supplemental oxygen. (2) Morphine sulfate should be given. (3) Patients with a systolic blood pressure 100 mm Hg or higher should receive an ACE inhibitor, beginning with titration of a low dose of a short-acting drug such as captopril. (4) Patients with a systolic blood pressure of 100 mm Hg or higher should receive nitrates. (5) A loop diuretic should be administered to patients with volume overload. (6) Although β-blockade should be initiated before hospital discharge, β-blockers should not be administered acutely to patients with “frank cardiac failure evidenced by pulmonary congestion or signs of a low-output state.” (7) Patients already receiving an ACE inhibitor who have a left ventricular EF less than 40% and either symptomatic CHF or diabetes should receive long-term aldosterone blockade unless hyperkalemia (serum potassium greater than 5 mEq/L) or significant renal dysfunction (serum creatinine greater than 2.5 mg/dL in men or greater than 2 mg/dL in women) is present. (8) Echocardiography should be performed urgently to evaluate left and right ventricular function and to exclude a mechanical complication.
In view of the fact that the relevant clinical trials included patients with both STEMI and NSTEMI, it is logical that treatment of NSTEMI patients with CHF should conform to the practice guidelines as discussed for STEMI patients with CHF. Figure 30.7 presents an algorithm for the emergency management of MI complicated by CHF or hypotension.
Right Ventricular Dysfunction and Infarction
Among 416 patients with acute MI who were enrolled in an echocardiographic substudy of the SAVE trial, right ventricular function was an independent predictor of mortality and the development of CHF.393 The odds of cardiovascular mortality increased 16% for each 5% decrease in the percentage change in right ventricular cavity area from end diastole to end systole.
Occlusion of the right coronary artery (RCA) proximal to the acute marginal branches is the most frequent cause of right ventricular infarction, but occlusion of the LAD or a dominant left circumflex coronary artery also may result in right ventricular MI. Although autopsy studies have shown that anterior MI may be associated with a right ventricular infarction, right ventricular MI that is associated with hemodynamic compromise most commonly occurs in patients with an inferior MI because perfusion of the right ventricle occurs predominantly via the right ventricular branches of the RCA.394,395 In a series of 125 patients with acute inferior MI who underwent emergency coronary angiography, echocardiography performed before coronary reperfusion demonstrated ischemic dysfunction of the right ventricle in 53 (42%) patients.396 The RCA was the IRA in all patients with right ventricular MI, and depressed flow in the right ventricular branches was evident in each case. Right ventricular branch flow was preserved in patients without right ventricular MI.
Patients with inferior MI complicated by right ventricular infarction have an increased risk of major complications, including death, cardiogenic shock, and ventricular arrhythmias.397 Cardiogenic shock during hospitalization occurred in 6.9% of a series of 491 patients with inferior MI complicated by right ventricular MI.397 Patients with right ventricular infarction complicated by cardiogenic shock do not have a better prognosis than patients with cardiogenic shock associated with left ventricular failure.398 Among a cohort of 1129 patients with acute inferior MI, there was no difference in left ventricular infarct size or function between patients with (n = 491) and patients without (n = 638) right ventricular MI, indicating that the increased risk of right ventricular MI is due to right ventricular dysfunction rather than greater left ventricular injury.397 The impact of right ventricular MI on prognosis may depend on the patient’s age.399 Among a series of 798 consecutive patients with acute inferior MI, 296 (37%) satisfied electrocardiographic or echocardiographic criteria for right ventricular infarction.399 Major complications (45% versus 19%, p < 0.0001) and in-hospital death (22% versus 6%, p < 0.0001) occurred more often in patients with than in those without right ventricular MI. The diagnosis of right ventricular MI increased the mortality risk in patients aged 65 or greater, but not among younger patients.
Although numerous electrocardiographic signs of right ventricular MI have been described, ST segment elevation in lead V4R is the most reliable electrocardiographic indicator of this form of MI.400 Zehender and associates401 studied the diagnostic and prognostic value of ST segment elevation in V4R in a series of 200 consecutive patients with acute inferior MI. ST segment elevation in lead V4R was present on the initial ECG in 107 patients (54%). Based on the results of autopsy, coronary angiography, right ventriculography, nuclear scan, or invasive hemodynamic data, ST segment elevation in V4R had 88% sensitivity, 78% specificity, and 83% diagnostic accuracy for right ventricular MI. ST segment elevation in V4R was associated with an in-hospital mortality of 31%, compared with 6% among patients without ST segment elevation in V4R (p < 0.001). Multivariate analysis of clinical data confirmed that 0.1 mV or greater of ST segment elevation in V4R was a strong independent predictor of in-hospital death (RR 7.7; 95% CI 2.6 to 23) and major complications (RR 4.7; 95% CI 2.4 to 9).
The triad of hypotension, clear lung fields, and elevated jugular venous pressure should raise a suspicion of right ventricular MI in patients with inferior STEMI, but the triad has a sensitivity of less than 25%.402 The hemodynamic criteria that have been used to diagnose right ventricular MI are right atrial pressure greater than 10 mm Hg and equal or nearly equal to the pulmonary capillary wedge pressure, or a noncompliant pattern in the right atrium.403 According to the ACC/AHA Practice Guidelines for STEMI, inferior STEMI with hemodynamic compromise is a Class I indication for recording lead V4R and an echocardiogram to screen for right ventricular MI.20 Echocardiographic signs of this disorder include right ventricular dilation, right ventricular asynergy, and abnormal interventricular septal motion.403 Echocardiography also is valuable to exclude pericardial tamponade because both right ventricular MI and pericardial tamponade may manifest with hypotension and elevated jugular venous pressure.404
The ACC/AHA guidelines emphasize the importance of maintenance of right ventricular preload, reduction of right ventricular afterload, inotropic support of the right ventricle, maintenance of atrioventricular synchrony, and early reperfusion of the IRA.20 Volume loading plus dobutamine, but not volume loading alone, has been shown to improve cardiac index in patients with acute right ventricular infarction.405 Systemic vasodilators are poorly tolerated, and hypotension after administration of sublingual nitroglycerin is a common event in patients with right ventricular MI. Short-term inhalation of nitric oxide, a selective vasodilator of the pulmonary circulation, improved cardiac index by 24% in a series of 13 patients with right ventricular infarction and cardiogenic shock.406 In patients with right ventricular MI complicated by cardiogenic shock and refractory low cardiac output and hypotension, insertion of a percutaneous ventricular assist device should be considered as a therapeutic option.407
The status of right atrial function is an important determinant of the hemodynamic consequences of right ventricular MI.408 Hemodynamic compromise may result if the atrial contribution to ventricular filling is lost in patients with right ventricular MI. Therefore, high-grade atrioventricular block, other bradyarrhythmias, and atrial fibrillation are common causes of hypotension in patients with right ventricular MI. Ventricular pacing may not increase cardiac output in such patients, although atrial pacing and atrioventricular sequential pacing have been shown to improve cardiac output.409,410 Atrial fibrillation associated with hemodynamic compromise is an indication for electrical cardioversion.
The elevated right atrial pressure in patients with right ventricular MI may cause refractory hypoxemia as a result of increased right-to-left shunting in patients with an atrial septal defect or patent foramen ovale.406,411 Inhalation of NO reduced right-to-left shunting by 56% in a series of three patients with right ventricular MI.406 Right-to-left shunting in patients with right ventricular MI also can be treated by percutaneous closure of the patent foramen ovale.411
Some evidence indicates that reperfusion of the IRA improves right ventricular function and clinical outcome in patients with MI. Successful PCI of an occluded RCA in patients with right ventricular infarction has been associated with improved right ventricular wall motion within 1 hour412 and a reduction in right atrial pressure within 8 hours.413 Three to 5 days after successful PCI, right ventricular function was normal in 95% of patients.412 In a study of the data for 49 patients with shock and right ventricular MI who were enrolled in the SHOCK trial registry, the in-hospital mortality rate was found to be 65.2% among patients who did not undergo revascularization, compared with 42.3% among patients who underwent PCI or CABG surgery.398
Mechanical Causes of Congestive Heart Failure or Low Cardiac Output
Mitral Regurgitation
Among 1976 patients with acute MI who were not in cardiogenic shock and underwent cardiac catheterization within 12 hours of symptom onset, left ventriculography demonstrated mild mitral regurgitation in 192 patients (9.7%) and moderate or severe mitral regurgitation in 58 patients (2.9%).61 By multivariate analysis, mild mitral regurgitation and moderate or severe mitral regurgitation were the two strongest independent predictors of 1-year mortality. The hazard ratios were 2.40 (95% CI 1.31 to 4.42; p = 0.005) for mild and 2.82 (95% CI 1.34 to 5.92; p = 0.006) for moderate or severe mitral regurgitation. The 1-year mortality rates were 2.9% for patients with no mitral regurgitation (n = 1726), 8.5% for patients with mild mitral regurgitation (n = 192), and 20.8% for patients with moderate or severe mitral regurgitation (n = 58).
Acute severe mitral regurgitation accounted for 6.9% of patients with cardiogenic shock among 1422 patients with acute MI who were enrolled in either the SHOCK registry or the randomized trial.369 The median time from the onset of MI to shock was 12.8 hours.414 In a postmortem series of 20 cases of papillary muscle rupture, the posteromedial papillary muscle was ruptured in 16 patients and the anterolateral papillary muscle was ruptured in four patients.415 The greater tendency of the posteromedial papillary muscle to rupture is reflected by the distribution of the IRA in a series of 98 patients with acute mitral regurgitation and cardiogenic shock who were enrolled in the SHOCK studies. The location of the index MI was anterior in 34% of patients and nonanterior in 66%.414
The diagnosis of acute severe mitral regurgitation should be suspected in patients with acute onset of pulmonary edema or hypotension. The absence of a loud murmur does not exclude severe mitral regurgitation. The diagnosis can be confirmed by transthoracic or transesophageal echocardiography. The treatment of acute severe mitral regurgitation should include inotropic support, afterload reduction, an IABP, and emergency mitral valve surgery, but mortality is high despite surgical treatment. Among the patients with acute severe mitral regurgitation and cardiogenic shock who were enrolled in the SHOCK registry or the randomized trial, the in-hospital mortality rate was 40% among 43 patients who underwent valve surgery, compared with 71% among 51 patients who did not.414
Ventricular Septal Rupture
Among 41,021 patients with STEMI who were enrolled in the GUSTO-I trial, the incidence of ventricular septal rupture was only 0.2% (84/41,021 patients).416 Acute ventricular septal rupture accounted for 3.9% of patients with cardiogenic shock among 1422 patients with acute MI who were enrolled in either the SHOCK registry or randomized trial.369
The clinical manifestations of ventricular septal rupture may include chest pain, dyspnea, and hypotension. A harsh holosystolic murmur may be audible. Cardiogenic shock occurred in 67% of patients in the GUSTO-I trial in whom ventricular septal rupture developed.416 The diagnosis of ventricular septal rupture can be confirmed by Doppler echocardiography or right heart catheterization to measure the oxygen saturation in the right atrium, right ventricle, and pulmonary artery. The median time from MI to diagnosis of ventricular septal rupture was 16 hours among a series of patients with ventricular septal rupture and cardiogenic shock.417 The electrocardiographic location of the MI was inferior in 26 patients, anterior in 22, both anterior and inferior in 3, and apico-lateral in 1 patient.417 Only 35 of 55 patients underwent coronary angiography, and the IRA was identified in only 26 patients: the RCA in 12, the LAD in 11, and the left circumflex coronary artery in 3 cases.
The location of the IRA may be an important determinant of survival in patients with ventricular septal rupture.416,418 Among a series of 25 patients with this diagnosis, mortality was greater among patients with inferior MI than among patients with anterior MI.418 At least two factors may explain the differential outcome of patients with inferior and anterior MI complicated by ventricular septal rupture. First, the right ventricular volume overload caused by the left-to-right shunt may be less tolerated in the presence of ischemia or infarction of the right ventricle, both of which are more common with an inferior MI than an anterior MI. Second, histopathologic studies have shown that complex septal defects that are more difficult to repair surgically are more common in patients with an inferior MI.419
An IABP has been shown to decrease the shunt and increase systemic cardiac output in patients with ventricular septal rupture.420 Therefore, vasodilator therapy and an IABP often are used to stabilize patients before surgical repair of the rupture. The overall in-hospital survival rate among patients with ventricular septal rupture in the SHOCK registry was only 13% (7/55).417 Although six of the seven survivors underwent surgical repair, mortality was 81% (25/31) in the group of patients who underwent surgery. In the GUSTO-I trial, patients whose rupture was repaired surgically had better 30-day mortality (47%) than patients who received medical treatment (94%).416 Percutaneous closure of acute ventricular septal rupture may be an option in the future.
Left Ventricular Free Wall Rupture
Rupture of the left ventricular free wall may manifest in any of several ways: pericardial tamponade with acute hemodynamic collapse and immediate death, gradual onset of tamponade and hypotension, or subacute formation of a pseudoaneurysm.421 Although a 6% rate of cardiac rupture among patients with acute MI often is quoted, reports suggest that the rate probably is lower, at least among patients who receive reperfusion therapy. A total of 65 (1.7%) cases of cardiac rupture occurred among 3759 patients with STEMI who received fibrinolytic therapy and were randomized to receive either adjunctive heparin or hirudin.422 The prevalence of cardiac rupture or pericardial tamponade was 2.3% (28/1190) among patients with cardiogenic shock in the SHOCK registry; 13 patients had both rupture and pericardial tamponade, 9 had tamponade alone, and 6 had rupture alone.423
It has been suggested that the incidence of cardiac rupture may be lower after primary PCI than after fibrinolytic therapy. The overall incidence of left ventricular free wall rupture was 2.5% (n = 34) among 1375 patients with STEMI who underwent primary PCI (55.4%) or fibrinolytic therapy (44.6%).424 In a multivariate analysis, primary PCI was independently associated with a lower incidence of rupture, but no significant difference was observed in the incidence of rupture after primary PCI or fibrinolytic therapy (1.8% versus 3.3%; p = 0.686).
The timing of reperfusion therapy may affect both the risk and the timing of free wall rupture. Death from cardiac rupture appears to occur earlier in patients given fibrinolytic therapy than among patients who do not undergo reperfusion therapy.425 Honan and associates426 analyzed the relationship between the risk of cardiac rupture and the timing of fibrinolytic therapy for 58 cases of cardiac rupture among 1638 patients who were enrolled in four randomized trials that compared intravenous streptokinase with no fibrinolytic therapy (in the control group). The odds ratio of cardiac rupture increased significantly with increasing delay in the time to treatment. Regression analysis suggested that treatment within 7 hours after symptom onset reduces the risk of cardiac rupture, whereas treatment later than 17 hours after symptom onset increases the risk of cardiac rupture.426 Thus, it was hypothesized that early fibrinolytic therapy reduces the risk of rupture by reducing the extent of myocardial necrosis, whereas late fibrinolytic therapy increases the risk of rupture by promoting hemorrhagic infarction.
The antemortem diagnosis of free wall rupture, pericardial tamponade, and left ventricular pseudoaneurysm usually is confirmed by an echocardiogram. An echocardiogram was obtained in 20 of the 28 patients in the SHOCK registry who had rupture or pericardial tamponade. A pericardial effusion was observed in 15 (75%) and a myocardial tear was detected in 39%.423 Six patients underwent pericardiocentesis alone, and 21 had surgical repair of the rupture. The in-hospital survival rate was 39.3%.
Cardiac Arrhythmias and Heart Block
Both atrial and ventricular arrhythmias are common in patients with acute MI. The incidence of atrial fibrillation was 10.4% among 40,891 patients who were enrolled in the GUSTO-I trial.327 Among patients enrolled in the TRACE study, which enrolled patients with an acute MI and a left ventricular EF less than 35%, atrial fibrillation occurred in 21% of patients and was associated with a 50% increase in the adjusted mortality.328 Management of atrial arrhythmias is discussed in another chapter.
The likelihood of primary ventricular fibrillation (occurring within 48 hours of acute MI and in the absence of cardiogenic shock or severe CHF) is not altered by fibrinolytic therapy, with an incidence of ventricular fibrillation during the first hospital day of 2.99% for both the fibrinolytic and placebo groups.331 Some evidence, however, suggests that fibrinolytic therapy exerts a protective effect against secondary ventricular fibrillation (occurring in patients with acute MI complicated by CHF or shock).331,332 Management of ventricular arrhythmias is discussed in Chapter 32.
The incidence of second-degree or third-degree AV block was 6.9% among 75,993 patients with STEMI who received fibrinolytic therapy and were enrolled in a database that combined four randomized clinical trials.427 Inferior MI was the strongest independent predictor of AV block (odds ratio 3.3; 95% CI 3.1 to 3.5). In comparison with patients without AV block, adjusted mortality was greater at 30 days, 6 months, and 1 year among patients with AV block. The adjusted mortality odds ratios at 1 year were 2.4 (95% CI 2.2 to 2.6) for patients with AV block and inferior MI and 3.3 (95% CI 3.0 to 3.7) for patients with AV block and anterior MI.