Acute bacterial infections and bacterial abscesses
ACUTE BACTERIAL MENINGITIS
MACROSCOPIC APPEARANCES
The brain is swollen and congested. Hemorrhagic infarcts are common and may be extensive (Fig. 15.1), and a purulent exudate is seen over the cerebral hemispheres or the base of the brain or both (Fig. 15.2). The ventricles may appear compressed by the swollen cerebral hemispheres, or distended if the aqueduct has been obstructed by purulent material. There may be coexistent cerebral abscesses, particularly in association with Citrobacter diversus or Proteus mirabilis meningitis.
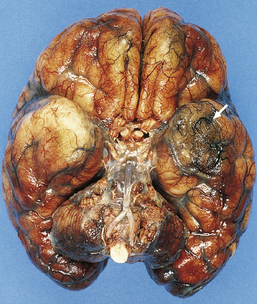
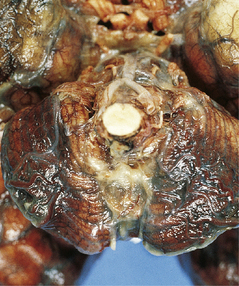
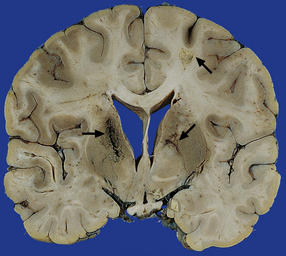
15.1 Neonatal meningitis caused by group B streptococcus.
There is focal left temporal infarction (arrow) with thrombosis of the overlying meningeal blood vessels. Elsewhere the meninges are markedly congested and there is blood in the subarachnoid space. (Courtesy of Dr Helen Porter, Bristol Children’s Hospital, UK.)
MICROSCOPIC APPEARANCES
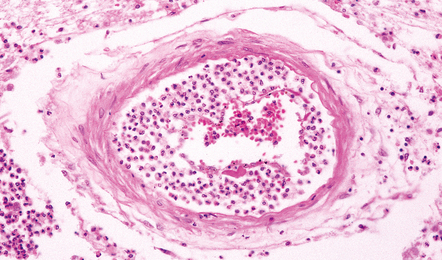
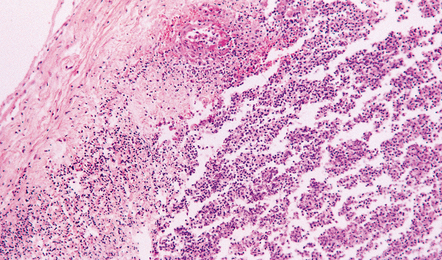
Inflammatory cells tend to infiltrate the walls of leptomeningeal and cortical arteries and veins (Fig. 15.3). Intimal accumulations of inflammatory cells may be a prominent feature. Venous thrombosis and infarction are often evident (Figs 15.1, 15.3) and may be extensive.
COMPLICATIONS
Complications of acute meningitis are:
 Obstructive (non-communicating) or communicating hydrocephalus (see Chapter 5).
Obstructive (non-communicating) or communicating hydrocephalus (see Chapter 5).
 Cavitation of cerebral gray (Fig. 15.4) and white matter.
Cavitation of cerebral gray (Fig. 15.4) and white matter.
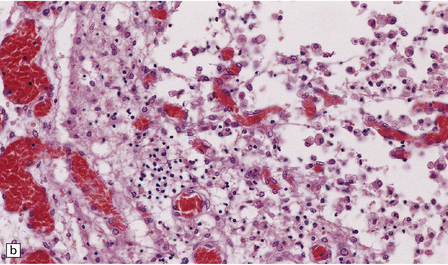
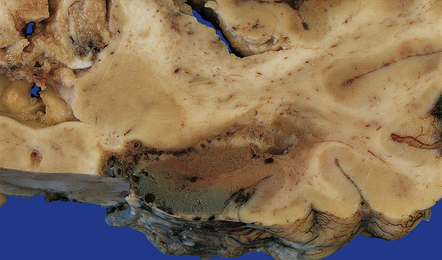
15.4 Neonatal bacterial meningitis.
(a) Extensive superficial cavitation of cerebral cortex in a case of neonatal group B streptococcal meningitis. (b) At higher magnification the cortex is seen to consist of cystic spaces containing newly formed blood vessels, foamy macrophages, and scattered lymphocytes.
ACUTE BACTERIAL MENINGITIS IN CHILDREN AND ADULTS
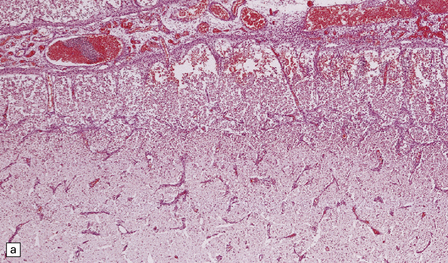
The brain is swollen and the leptomeninges are congested. A purulent exudate is seen in the subarachnoid space over the cerebral hemispheres and the base of the brain (Fig. 15.5a), and accumulating in the basal cisterns and cerebral sulci. The exudate associated with pneumococcal infections is often particularly prominent over the cerebral convexities (Fig. 15.5b). There may be little or no macroscopically discernible exudate in patients dying very acutely or those who have been partially treated with antibiotics. Sectioning of the brain may reveal edematous white matter and compressed ventricles or ventricular enlargement due to obstructive hydrocephalus. There is usually purulent fluid within the ventricles. The ventricular exudate may be pronounced in patients with ventricular shunt infection (Fig. 15.6), in whom the leptomeninges appear remarkably clear. Occasionally, the coagulopathy associated with meningococcal septicemia is complicated by ventricular hemorrhage. Small infarcts may result from thrombosis of penetrating arteries (Fig. 15.7). Venous thrombosis and hemorrhagic infarcts are less common than in neonatal meningitis.
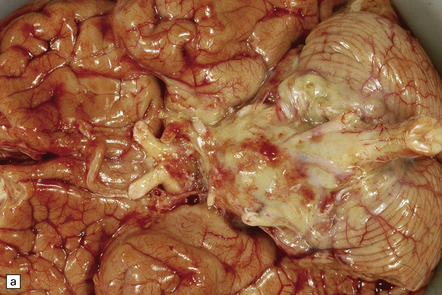
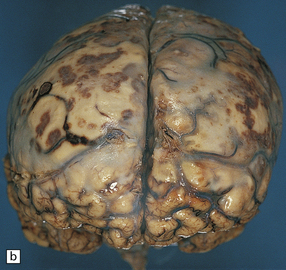
15.5 Bacterial meningitis.
(a) A thick basal exudate is evident, encasing the cranial nerves. (b) In this case of pneumococcal meningitis, the brain is swollen with a purulent exudate over the cerebral convexities and foci of infarction in the underlying cortex.
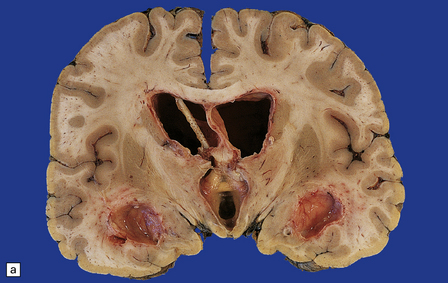
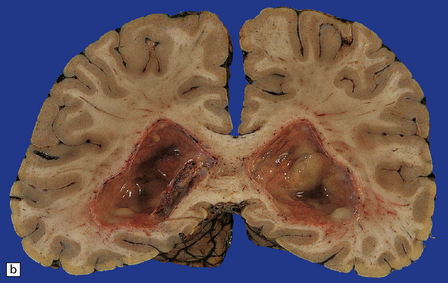
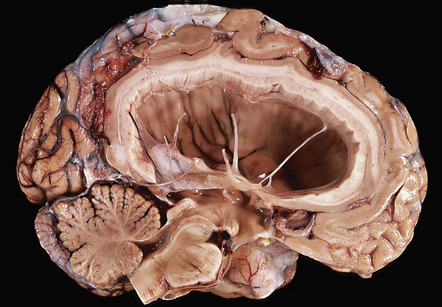
15.6 Ventriculitis due to Staphylococcus aureus shunt infection.
(a) The shunt tubing is visible in one of the lateral ventricles. The ventricles are dilated and their lining markedly congested. (b) The purulent exudate is well seen in the occipital horns.
MICROSCOPIC APPEARANCES
During the first few days, the subarachnoid (Fig. 15.8) and ventricular (Fig. 15.9) exudate contains large numbers of neutrophils and necrotic debris. Intracellular and extracellular bacteria can usually be demonstrated. The exudate extends along perivascular spaces into the cerebral cortex (Fig. 15.10), cerebellum, brain stem, and spinal cord. There is usually purulent material in the choroid plexus (Fig. 15.11). Perivascular inflammation and fibrinoid necrosis of blood vessels may be seen in the periventricular white matter (Figs 15.9, 15.12). With time, the numbers of lymphocytes and macrophages increase, and by the end of the first week these predominate. There is usually some proliferation of fibroblasts.
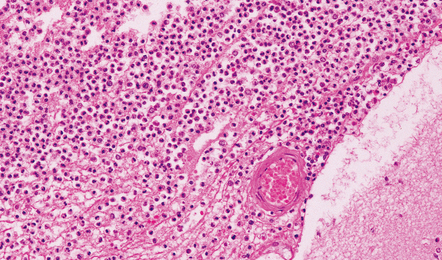
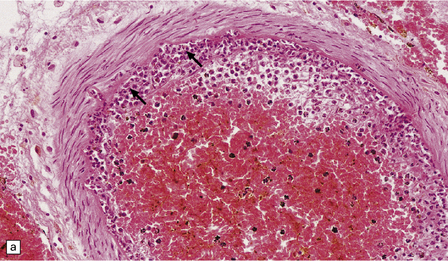
15.8 Meningeal exudate in acute purulent meningitis.
The exudate includes numerous neutrophils and strands of fibrin.
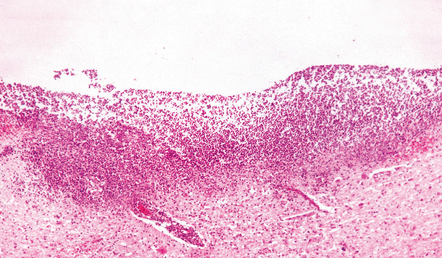
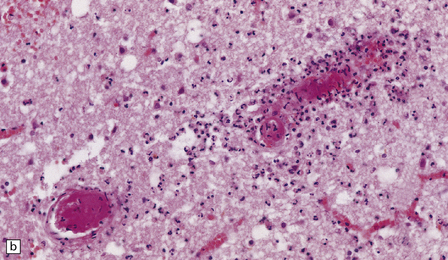
15.9 Purulent ventricular exudate.
An inflammatory infiltrate is present in the ventricles and the periventricular white matter.
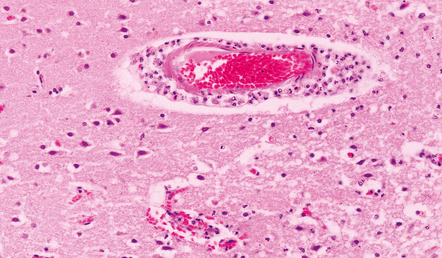
15.10 Acute purulent meningitis.
Perivascular spread of inflammatory cells in the superficial cerebral cortex is a common finding.
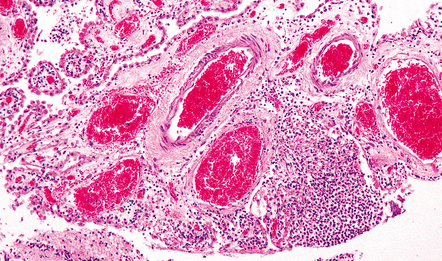
15.11 Listeria monocytogenes meningitis.
An infiltrate of inflammatory cells is present in the choroid plexus.
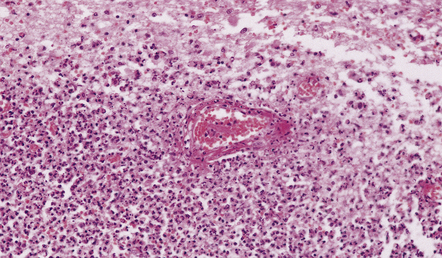
15.12 Acute purulent meningitis.
Fibrinoid necrosis of a small vein within an acute inflammatory cell infiltrate in the periventricular white matter.
Inflammatory cells tend to infiltrate the walls of leptomeningeal and cortical arteries and veins and may accumulate in the intima (Fig. 15.13). Thrombosis of small meningeal and cortical blood vessels (Fig. 15.14) with associated focal infarction is relatively common in cases coming to necropsy (Figs 15.5b, 15.7, 15.14). Meningitis due to B. anthracis is often hemorrhagic (Fig. 15.15).
COMPLICATIONS
As noted above, these include:
 Cerebral edema, which may be marked.
Cerebral edema, which may be marked.
 Arterial and venous infarcts (Fig. 15.16).
Arterial and venous infarcts (Fig. 15.16).
BRAIN ABSCESS
MACROSCOPIC APPEARANCES
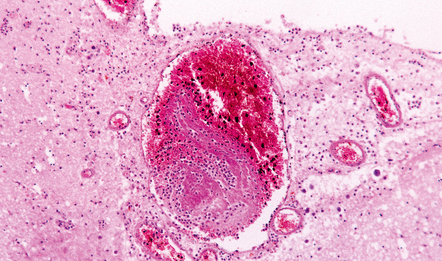
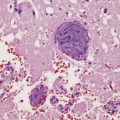
As would be expected, abscesses caused by direct spread of local infection tend to be located in the frontal (Fig. 15.18) or temporal lobes or the anterior parietal region, adjacent to the source of infection. Less commonly, otogenic infection may be complicated by a cerebellar abscess. Abscesses caused by hematogenous spread of infection tend to occur at junctions between gray and white matter and are often multiple (Fig. 15.19). They may be situated in any part of the brain, although the perfusion territory of the middle cerebral arteries is usually involved. Listeria monocytogenes has a predilection for the brain stem and tends to cause a purulent rhombencephalitis (Fig. 15.20), with or without an associated meningitis.
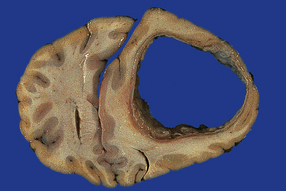
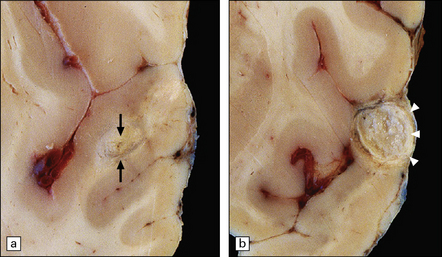
15.18 Brain abscess due to direct spread of infection.
Frontal abscess complicating chronic frontal sinus infection.
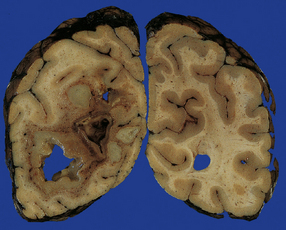
15.19 Hematogenous brain abscesses.
Multiple abscesses in a patient with a cardiac ventricular septal defect complicated by pulmonary hypertension and right-to-left shunting (Eisenmenger syndrome).
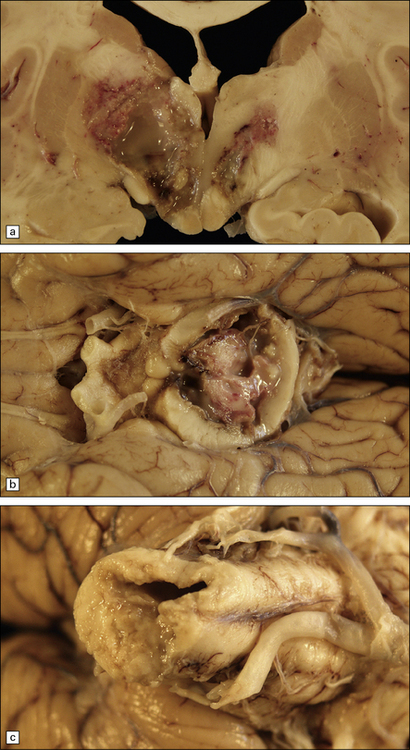
15.20 Purulent encephalitis caused by Listeria monocytogenes.
In this case, the encephalitis involved the basal ganglia and hypothalamus (a) as well as the midbrain (b), pons and medulla (c).
The earliest stage of focal cerebritis appears macroscopically as an ill-defined region of hyperemia with surrounding white matter edema. It takes about 1–3 weeks for an abscess with a visible capsule and purulent center to become macroscopically discernible. Over weeks and months, the abscess enlarges by expanding into the white matter. The capsule gradually thickens, although this occurs to a greater extent in the subcortical region than in the deep white matter, where the capsule tends to remain relatively thin. Abscesses caused by hematogenous spread of infection tend to be less well encapsulated than those caused by local spread. Abscesses occasionally rupture into the ventricular system, causing a purulent ventriculitis, or into the leptomeninges (Fig. 15.21).
MICROSCOPIC APPEARANCES
The evolution of a cerebral abscess can be subdivided into the following four stages:
 Focal suppurative encephalitis (days 1–2).
Focal suppurative encephalitis (days 1–2).
 Focal suppurative encephalitis with confluent central necrosis (days 2–7).
Focal suppurative encephalitis with confluent central necrosis (days 2–7).
Focal suppurative encephalitis lasts 1–2 days and is characterized by swelling of endothelial cells, and perivascular and parenchymal infiltration by neutrophils (Figs 15.22, 15.23). Small foci of necrosis develop rapidly. Only occasional mononuclear inflammatory cells are present at this stage.
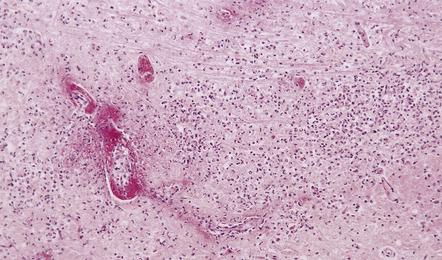
15.22 Focal suppurative brain stem meningoencephalitis due to Listeria monocytogenes infection.
Fibrinoid vascular necrosis is a common finding in Listeria meningoencephalitis, which tends particularly to involve the brain stem (rhombencephalitis).
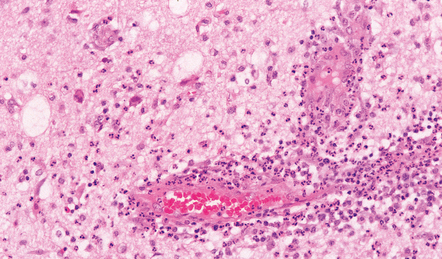
15.23 Acute cerebritis.
The inflamed brain tissue was adjacent to a focus of middle ear and petrous temporal bone infection.
Focal suppurative encephalitis with confluent central necrosis is characterized by adjacent foci of necrosis, which soon enlarge and become confluent (Fig. 15.24). By day 3 or 4, foamy macrophages are much more numerous and the infiltrate includes lymphocytes and some plasma cells. Bacterial brain abscesses associated with septicemia in immunocompromised patients may show extensive necrosis with little or no encapsulation (Fig. 15.25). Multiple, poorly encapsulated cerebral microabscesses are a frequent feature of septicemia due to Staph. aureus, particularly in the immunosuppressed, elderly or debilitated.
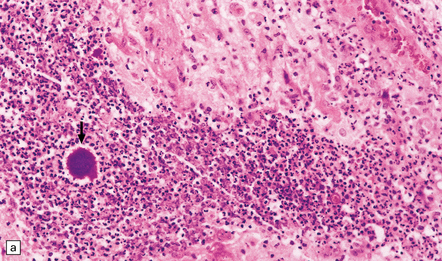
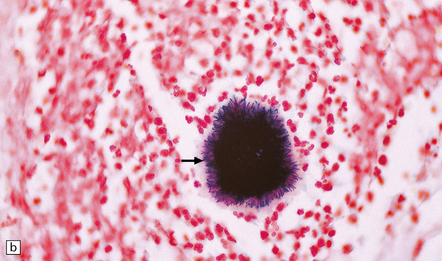
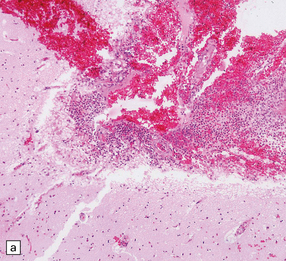
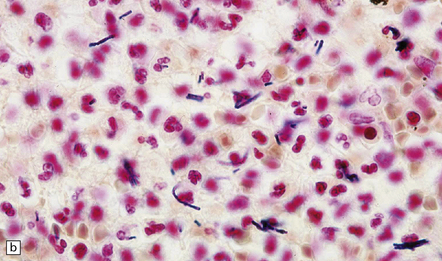
15.24 Focal suppurative encephalitis with confluent necrosis due to Actinomyces israelii infection.
(a) and (b) The characteristic ‘sulfur’ granules (arrow in a) are composed of numerous filamentous Gram-positive bacteria (arrow in b). Established Actinomyces abscesses tend to be multiloculated.
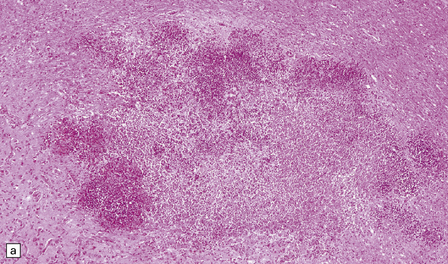
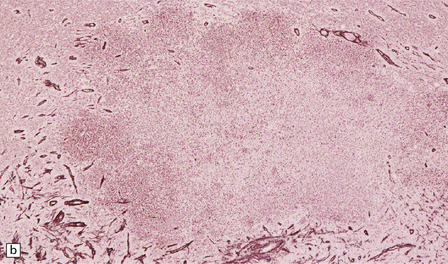
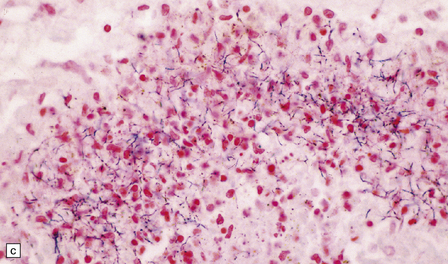
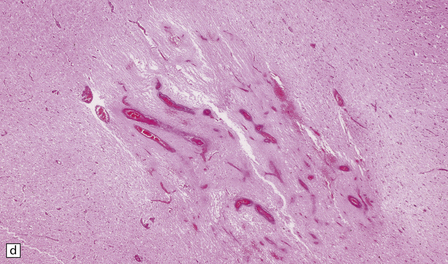
15.25 Bacterial brain abscesses in immunocompromised patients.
(a) Nocardia abscess in a bone marrow transplant recipient. (b) Reticulin impregnation of an adjacent section shows minimal encapsulation. (c) Gram-positive, beaded, filamentous Nocardia bacilli. (d) Focal cerebral necrosis complicating Pseudomonas aeruginosa septicemia. Note the minimal tissue reaction.
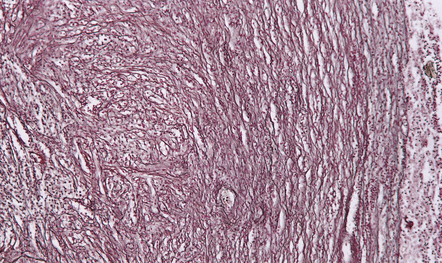
Early encapsulation is seen by days 5–7: an early granulation tissue response is evident around the margin of the necrotic tissue, with newly formed capillaries and scattered fibroblasts (Fig. 15.26). Over the next few days, the fibroblasts proliferate and deposit reticulin (Fig. 15.27). The surrounding brain tissue is edematous and contains swollen reactive astrocytes. Parenchymal blood vessels have plump endothelial cells and tend to be surrounded by small aggregates of lymphocytes.
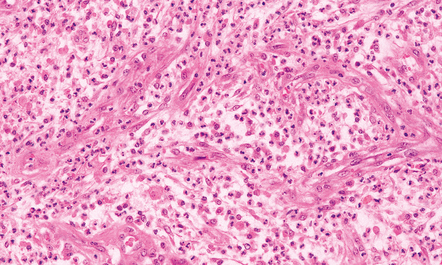
15.26 Early encapsulation of a cerebral abscess, with newly formed capillaries and scattered fibroblasts.
The inflammatory infiltrate includes macrophages and lymphocytes as well as neutrophils.
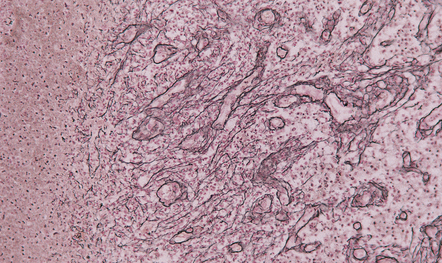
15.27 Deposition of reticulin in the wall of a 1–2-week-old cerebral abscess.
Some of the reticulin is separate from blood vessels and reflects infiltration by fibroblasts.
 A surrounding rim of granulation tissue within which are numerous neutrophils, foamy macrophages, and scattered lymphocytes.
A surrounding rim of granulation tissue within which are numerous neutrophils, foamy macrophages, and scattered lymphocytes.
 A capsule composed of multiple concentric layers of fibroblasts and collagen (Fig. 15.28), and variable numbers of inflammatory cells, mainly lymphocytes and macrophages. The capsule is penetrated by small blood vessels, most of which are radially oriented, in contrast to the fibroblasts and collagen, especially in the outer part of the capsule.
A capsule composed of multiple concentric layers of fibroblasts and collagen (Fig. 15.28), and variable numbers of inflammatory cells, mainly lymphocytes and macrophages. The capsule is penetrated by small blood vessels, most of which are radially oriented, in contrast to the fibroblasts and collagen, especially in the outer part of the capsule.
Progressive collagen deposition over subsequent weeks thickens the capsule. The thickness varies at different points, tending to be greater on the cortical than on the ventricular aspect (Fig. 15.29). Occasionally, the infection in a chronic abscess resolves in the absence of treatment, leaving a dense fibrous capsule lined by sparse macrophages and amorphous material.
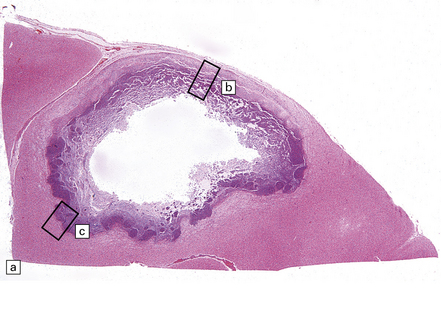
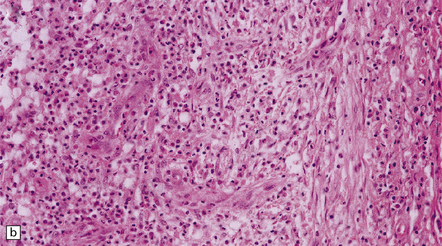
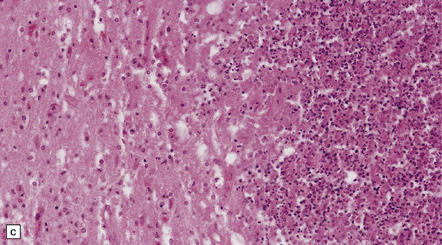
15.29 Varying thickness of the wall of an established cerebral abscess.
(a) Established cerebral abscess at the junction of cortex and white matter. (b) Higher magnification view of the subcortical superficial part of the abscess wall (marked in a). A thick zone of granulation tissue is covered by circumferential layers of collagen and fibroblasts. (c) On the deep surface of the same abscess (marked in a), the wall is poorly defined, with scarcely any separation of the contents of the abscess and the adjacent brain tissue.
SUBDURAL EMPYEMA
MACROSCOPIC AND MICROSCOPIC FEATURES
In most cases, an empyema is situated above the tentorium (Fig. 15.30), occasionally adjacent to the falx cerebri. Empyema occurs less commonly in the posterior fossa or, rarely, in the spinal canal (Fig. 15.30). Subdural empyema consists of pus surrounded by granulation tissue and a mixed inflammatory infiltrate.
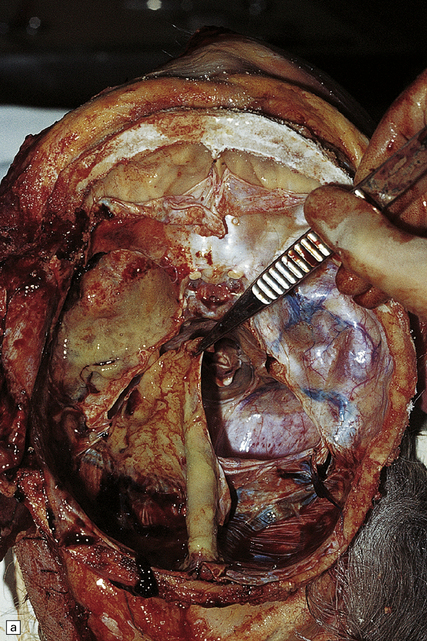
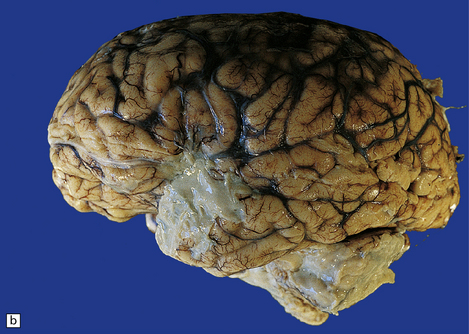
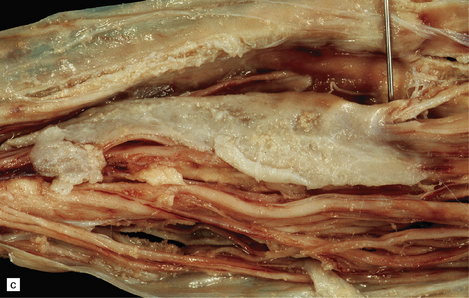
15.30 Subdural empyema.
(a) This supratentorial infection of hematogenous origin complicated a congenital heart defect with a right-to-left shunt. Purulent material covers the left side of the tentorium cerebelli and the dura mater in the left middle cranial fossa. (b) In this case, the empyema involved the middle and posterior cranial fossae. Staphylococcus aureus was isolated from this empyema and also from a psoas abscess in the same patient. (c) Subdural empyema surrounding the cauda equina.
EPIDURAL ABSCESS
MACROSCOPIC AND MICROSCOPIC APPEARANCES
A spinal epidural abscess usually extends over several vertebral levels (Fig. 15.31). Intracranial epidural abscesses tend to be biconvex in shape, sharply delimited by the skull and the displaced dura.

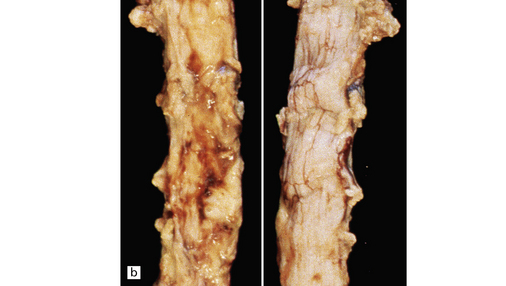
15.31 Spinal epidural abscess complicating vertebral osteomyelitis.
(a) A midsagittal section through the vertebral column reveals yellow discoloration and softening of several adjacent cervical vertebral bodies and some of the intervening discs. (b) There is purulent material on the ventral aspect of the dura (left panel) of the cervical cord.
Histology reveals extradural purulent material (Fig. 15.32) surrounded by inflamed granulation tissue. Osteomyelitis involving the vertebrae adjacent to a spinal epidural abscess may also be evident.
REFERENCES
Bartt, R. Listeria and atypical presentations of Listeria in the central nervous system. Semin Neurol.. 2000;20:361–373.
Cho, H.K., Lee, H., Kang, J.H., et al. The causative organisms of bacterial meningitis in Korean children in 1996–2005. J Korean Med Sci.. 2010;25:895–899.
Goodkin, H.P., Harper, M.B., Pomeroy, S.L. Intracerebral abscess in children: historical trends at Children’s Hospital Boston. Pediatrics.. 2004;113:1765–1770.
Kim, K.S. Pathogenesis of bacterial meningitis: from bacteraemia to neuronal injury. Nat Rev Neurosci.. 2003;4:376–385.
Kim, K.S. Acute bacterial meningitis in infants and children. Lancet Infect Dis.. 2010;10:32–42.
Koedel, U., Klein, M., Pfister, H.W. New understandings on the pathophysiology of bacterial meningitis. Curr Opin Infect Dis.. 2010;23:217–223.
Neuman, H.B., Wald, E.R. Bacterial meningitis in childhood at the Children’s Hospital of Pittsburgh: 1988–1998. Clin Pediatr (Phila).. 2001;40:595–600.
Roos, K.L. Acute bacterial meningitis. Semin Neurol.. 2000;20:293–306.
Theodoridou, M.N., Vasilopoulou, V.A., Atsali, E.E., et al. Meningitis registry of hospitalized cases in children: epidemiological patterns of acute bacterial meningitis throughout a 32-year period. BMC Infect Dis.. 2007;7:101.
Britt, R.H., Enzmann, D.R., Yeager, A.S. Neuropathological and computerized tomographic findings in experimental brain abscess. J Neurosurg.. 1981;55:590–603.
Calfee, D.P., Wispelwey, B. Brain abscess, subdural empyema, and intracranial epidural abscess. Curr Infect Dis Rep.. 1999;1:166–171.
Calfee, D.P., Wispelwey, B. Brain abscess. Semin Neurol.. 2000;20:353–360.
Carpenter, J., Stapleton, S., Holliman, R. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis.. 2007;26:1–11.
Kao, P.T., Tseng, H.K., Liu, C.P., et al. Brain abscess: clinical analysis of 53 cases. J Microbiol Immunol Infect.. 2003;36:129–136.
Lee, C.G., Kang, S.H., Kim, Y.J., et al. Brain abscess in Korean children: A 15-year single center study. Korean J Pediatr.. 2010;53:648–652.
Manzar, N., Manzar, B., Kumar, R., et al. The study of etiologic and demographic characteristics of intracranial brain abscess: a consecutive case series study from Pakistan. World Neurosurg.. 2011;76:195–200. [discussion 79–83].
Pendlebury, W.W., Perl, D.P., Munoz, D.G. Multiple microabscesses in the central nervous system: a clinicopathologic study. J Neuropathol Exp Neurol.. 1989;48:290–300.
Saez-Llorens, X. Brain abscess in children. Semin Pediatr Infect Dis.. 2003;14:108–114.
Tsou, T.P., Lee, P.I., Lu, C.Y., et al. Microbiology and epidemiology of brain abscess and subdural empyema in a medical center: a 10-year experience. J Microbiol Immunol Infect.. 2009;42:405–412.