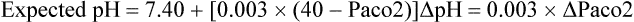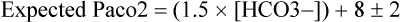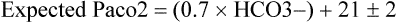Acid-base status
Terminology
Normal values of pH, defined as the negative logarithm of the hydrogen ion concentration [H+] (expressed in extracellular fluids in nanoequivalents per liter), range from 7.35 to 7.45. Changes in pH are inversely related to changes in [H+]: a 20% increase in [H+] decreases the pH by 0.1; conversely, a 20% decrease in [H+] increases the pH by a 0.1 (Table 47-1).
Table 47-1
pH for Given Hydrogen Ion Concentrations
| pH | [H+] (nEq/L) |
| 7.0 | 100 |
| 7.1 | 80 |
| 7.2 | 64 |
| 7.3 | 50 |
| 7.4 | 40 |
| 7.5 | 30 |
| 7.6 | 24 |
| 7.7 | 20 |
< ?xml:namespace prefix = "mml" />
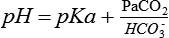
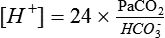
Tight control of the pH requires a fairly constant PaCO2/HCO3− ratio, which allows one to check the validity of an arterial blood gas (ABG) sample (Table 47-2).
Table 47-2
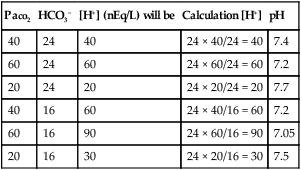
Examples of the Use of Henderson-Hasselbalch Equation to Calculate H+ Concentration with a Known HCO3− and PaCO2
| PaCO2 | HCO3− | [H+] (nEq/L) will be | Calculation [H+] | pH |
| 40 | 24 | 40 | 24 × 40/24 = 40 | 7.4 |
| 60 | 24 | 60 | 24 × 60/24 = 60 | 7.2 |
| 20 | 24 | 20 | 24 × 20/24 = 20 | 7.7 |
| 40 | 16 | 60 | 24 × 40/16 = 60 | 7.2 |
| 60 | 16 | 90 | 24 × 60/16 = 90 | 7.05 |
| 20 | 16 | 30 | 24 × 20/16 = 30 | 7.5 |
Compensatory responses
Every primary acid-base disorder should have an appropriate compensatory response. A primary respiratory (ventilatory) disorder induces a renal response—reabsorption of HCO3− in the proximal tubules is altered to compensate for the change in pH induced by the change in PaCO2. This process is slow, occurring over 24 to 36 h. A primary metabolic disorder, on the other hand, induces a much faster respiratory compensation, in which ventilation changes to increase or decrease PaCO2 (Table 47-3). The absence of compensation in a timely manner would probably indicate the presence of a secondary disturbance.
Table 47-3
Primary Change and Compensatory Response for the Primary Acid-Base Disorders
| Primary Disorder | Primary Change | Compensatory Response* |
| Respiratory acidosis | ↑ PaCO2 | ↑ HCO3− |
| Respiratory alkalosis | ↓ PaCO2 | ↓ HCO3− |
| Metabolic acidosis | ↓ HCO3− | ↓ PaCO2 |
| Metabolic alkalosis | ↑ HCO3− | ↑ PaCO2 |
*Homeostatic response in an attempt to maintain constant PaCO2/HCO3− ratio.
Assessment of acid-base disorders secondary to respiratory mechanics
Acute respiratory acidosis prior to onset of renal compensation:
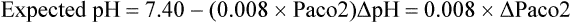
Acute respiratory alkalosis prior to onset of renal compensation:
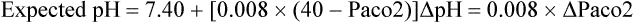
Chronic respiratory acidosis, renal compensation fully developed:
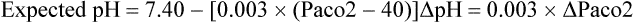
Chronic respiratory alkalosis, renal compensation fully developed: