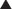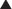Chapter 17 Immune Modifiers
Systemic Steroids
MOA (Mechanism of Action)
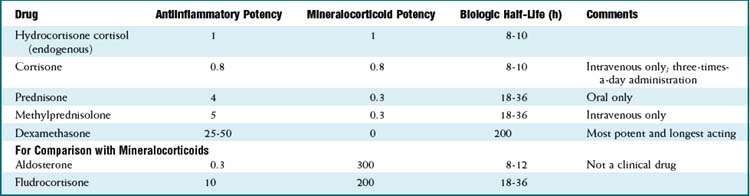
 Cortisol is the endogenous glucocorticoid and is synthesized from cholesterol. The hypothalamus secretes corticotropin-releasing hormone (CRH), which stimulates the anterior pituitary to release adrenocorticotropic hormone (ACTH), which acts on the adrenal glands to produce cortisol.
Cortisol is the endogenous glucocorticoid and is synthesized from cholesterol. The hypothalamus secretes corticotropin-releasing hormone (CRH), which stimulates the anterior pituitary to release adrenocorticotropic hormone (ACTH), which acts on the adrenal glands to produce cortisol. Cortisol acts in the nucleus of the cell; therefore it must first diffuse through the cell membrane. It is bound in the cytoplasm with heat shock protein and other proteins and transported into the nucleus.
Cortisol acts in the nucleus of the cell; therefore it must first diffuse through the cell membrane. It is bound in the cytoplasm with heat shock protein and other proteins and transported into the nucleus. Steroid receptors located in the nucleus are called glucocorticoid receptor elements. They regulate transcription of DNA by acting on promoters, which are specific DNA sites where RNA polymerase binds and starts transcription.
Steroid receptors located in the nucleus are called glucocorticoid receptor elements. They regulate transcription of DNA by acting on promoters, which are specific DNA sites where RNA polymerase binds and starts transcription. Steroids exert their actions in a broad range of tissues, and therefore there are many effects of glucocorticoids:
Steroids exert their actions in a broad range of tissues, and therefore there are many effects of glucocorticoids:
 Catabolism
Catabolism
Pharmacokinetics
Side Effects
 Weight gain and severe swelling, particularly in the face; caused, in part, by the mineralocorticoid effects
Weight gain and severe swelling, particularly in the face; caused, in part, by the mineralocorticoid effects Psychiatric symptoms including depression, mania, and psychosis; other types of cognitive dysfunction can also occur, including euphoria, insomnia, mental confusion
Psychiatric symptoms including depression, mania, and psychosis; other types of cognitive dysfunction can also occur, including euphoria, insomnia, mental confusion Cushing’s syndrome is a collection of signs from endogenous overproduction of cortisol. A cushingoid appearance can be produced from iatrogenic exogenous steroid:
Cushing’s syndrome is a collection of signs from endogenous overproduction of cortisol. A cushingoid appearance can be produced from iatrogenic exogenous steroid:
 Adrenal suppression: Because of negative feedback loops, exogenous glucocorticoids will suppress CRH and ACTH. Rapidly terminating exogenous glucocorticoids after prolonged exposure will result in hypoadrenalism. Steroids must therefore be tapered (administration of smaller and smaller doses) before being stopped if they have been administered for more than about 1 or 2 weeks.
Adrenal suppression: Because of negative feedback loops, exogenous glucocorticoids will suppress CRH and ACTH. Rapidly terminating exogenous glucocorticoids after prolonged exposure will result in hypoadrenalism. Steroids must therefore be tapered (administration of smaller and smaller doses) before being stopped if they have been administered for more than about 1 or 2 weeks.Important Notes
 The baseline secretion of cortisol in the body is 10 to 20 mg. In periods of stress (illness, trauma, inflammation, infection), the secretion increases.
The baseline secretion of cortisol in the body is 10 to 20 mg. In periods of stress (illness, trauma, inflammation, infection), the secretion increases. Glucocorticoids must be differentiated from mineralocorticoids; the prototype mineralocorticoid is aldosterone, also a steroid, which acts primarily on the kidney to regulate water and electrolyte homeostasis.
Glucocorticoids must be differentiated from mineralocorticoids; the prototype mineralocorticoid is aldosterone, also a steroid, which acts primarily on the kidney to regulate water and electrolyte homeostasis. One significant side effect of glucocorticoids is an increased white blood count (driven by an increase in neutrophils). Increased neutrophil counts are more commonly caused by infection; because steroids increase the risk of infection, it is sometimes clinically difficult to differentiate whether the increased white count is the result of a new infection (which would make the steroids relatively contraindicated) or the result of a direct effect of the steroid. The rise in white cell count can be quite dramatic when caused by steroid effect alone.
One significant side effect of glucocorticoids is an increased white blood count (driven by an increase in neutrophils). Increased neutrophil counts are more commonly caused by infection; because steroids increase the risk of infection, it is sometimes clinically difficult to differentiate whether the increased white count is the result of a new infection (which would make the steroids relatively contraindicated) or the result of a direct effect of the steroid. The rise in white cell count can be quite dramatic when caused by steroid effect alone. Steroids and nonsteroidal antiinflammatory drugs (NSAIDs) are commonly coadministered for inflammatory conditions that result in pain (e.g., RA). The risk of gastrointestinal (GI) bleeding with steroids or NSAIDs alone is 2 times and 4 times (respectively) higher than baseline, but when these agents are taken together, the risk is 12 times higher than baseline.
Steroids and nonsteroidal antiinflammatory drugs (NSAIDs) are commonly coadministered for inflammatory conditions that result in pain (e.g., RA). The risk of gastrointestinal (GI) bleeding with steroids or NSAIDs alone is 2 times and 4 times (respectively) higher than baseline, but when these agents are taken together, the risk is 12 times higher than baseline. Because of the long list of side effects, steroids are not ideal for long-term high-dose administration. In diseases in which prolonged and substantial immunosuppression is required, steroid-sparing immunosuppressants are administered so that doses of steroids can be reduced or the steroids can be completely discontinued.
Because of the long list of side effects, steroids are not ideal for long-term high-dose administration. In diseases in which prolonged and substantial immunosuppression is required, steroid-sparing immunosuppressants are administered so that doses of steroids can be reduced or the steroids can be completely discontinued.Advanced
 Prophylactic antibiotics against Pneumocystis pneumonia (also called Pneumocystis jiroveci pneumonia or Pneumocystis carinii pneumonia [PCP]) are administrated to patients on long-term moderate- to high-dose steroids because of the risk of acquiring this infection when immunosuppressed. Human immunodeficiency virus (HIV) infection is the most common risk factor for PCP
Prophylactic antibiotics against Pneumocystis pneumonia (also called Pneumocystis jiroveci pneumonia or Pneumocystis carinii pneumonia [PCP]) are administrated to patients on long-term moderate- to high-dose steroids because of the risk of acquiring this infection when immunosuppressed. Human immunodeficiency virus (HIV) infection is the most common risk factor for PCP In addition to being a risk factor for PCP, steroids are also coadministered to patients being treated for PCP because the antibiotic-mediated destruction of the pathogen generates a strong inflammatory response in the lungs, making the respiratory condition much worse. Steroids blunt this response.
In addition to being a risk factor for PCP, steroids are also coadministered to patients being treated for PCP because the antibiotic-mediated destruction of the pathogen generates a strong inflammatory response in the lungs, making the respiratory condition much worse. Steroids blunt this response.Evidence
 Systemic steroids and adult asthma: A systematic review in 2009 concluded that the available studies were frequently underpowered. However, some general conclusions and recommendations were made: steroids administered in the emergency department reduce hospitalizations; steroids accelerate improvements in lung function; there was no benefit in using doses larger than 50 to 100 mg prednisone equivalent; and no benefit was seen when steroids were administered for longer than 5 to 10 days total.
Systemic steroids and adult asthma: A systematic review in 2009 concluded that the available studies were frequently underpowered. However, some general conclusions and recommendations were made: steroids administered in the emergency department reduce hospitalizations; steroids accelerate improvements in lung function; there was no benefit in using doses larger than 50 to 100 mg prednisone equivalent; and no benefit was seen when steroids were administered for longer than 5 to 10 days total. Systemic steroids and RA: A Cochrane review in 2007 (15 studies, N = 1414 patients) examined radiological progression of disease and concluded that the proportion of benefit gained by glucocorticoids in reducing the progression of erosions from an average of all the studies over 1 year was 67.2% (confidence interval [CI] 48.9%, 85.4%) and over 2 years was 61.3% (CI 46.5%, 76.1%). Furthermore, this benefit was achieved in patients who were (mostly) already receiving disease-modifying antirheumatic drug (DMARD) treatment. It therefore represents a gain over and above any benefits from DMARDs alone.
Systemic steroids and RA: A Cochrane review in 2007 (15 studies, N = 1414 patients) examined radiological progression of disease and concluded that the proportion of benefit gained by glucocorticoids in reducing the progression of erosions from an average of all the studies over 1 year was 67.2% (confidence interval [CI] 48.9%, 85.4%) and over 2 years was 61.3% (CI 46.5%, 76.1%). Furthermore, this benefit was achieved in patients who were (mostly) already receiving disease-modifying antirheumatic drug (DMARD) treatment. It therefore represents a gain over and above any benefits from DMARDs alone.Introduction to Monoclonal Antibodies
Newer technologies are enabling the animal (usually mouse) portion of the antibody to be less and less, so that the resulting antibody is mostly human and therefore not destroyed by the patient’s own immune system for being a foreign antibody by human antimurine antibodies (HAMAs). Chimeric (human-mouse combination) antibodies contain fewer mouse regions than full mouse antibodies. Humanization involves replacing most of the mouse antibody with equivalent human regions while keeping only the variable, antigen-specific regions intact. Humanized mAbs have more human regions than chimeric mAbs do. Finally, fully human mAbs that contain no mouse regions are now being created (Figure 17-1).
Fusion Proteins
Use of Monoclonal Antibodies
The list of uses for mAbs is growing, but the major categories are as follows:
 Cancer: Cancer cells express unique antigens that can be directly targeted for destruction. Furthermore, there are growth factors that stimulate cancer cell growth that can also be inhibited.
Cancer: Cancer cells express unique antigens that can be directly targeted for destruction. Furthermore, there are growth factors that stimulate cancer cell growth that can also be inhibited. Immunosuppressants:
Immunosuppressants:
Naming Monoclonal Antibodies
Targets
Notes
Table 17-2 will not be fully inclusive by the time it is published because of the rapid growth of this area of medicine. Some drugs listed may not yet be approved for use.
| Name | Type | Target |
|---|---|---|
| Rituximab | Chimeric | CD20 on B lymphocytes |
| Ocrelizumab | Humanized | CD20 on B lymphocytes |
| Ofatumumab | Human | CD20 on B lymphocytes |
| Tositumomab | Mouse |
EGF, epidermal growth factor; HER2, human epidermal growth factor receptor 2; 131I, iodine-131; IgE, immunoglobulin E; IL, interleukin; 111In, indium-111; PSA, prostate-specific antigen; TNF, tumor necrosis factor; 90Y, yttrium-90.
B-Cell Biologics
Description
B-cell biologics specifically target B-cell lymphocytes for either destruction or suppression.
MOA (Mechanism of Action)
 Drugs that target B cells cause either destruction of the cells or interference with their ability to mount an immune response.
Drugs that target B cells cause either destruction of the cells or interference with their ability to mount an immune response. As a B cell–depleting agent (destruction of B cells):
As a B cell–depleting agent (destruction of B cells):
 Accelerated destruction by other anticancer drugs has also been observed when used in combination with CD20 therapy.
Accelerated destruction by other anticancer drugs has also been observed when used in combination with CD20 therapy. As an immunomodulator (inhibitor of B cells):
As an immunomodulator (inhibitor of B cells):
 B-cell depletion (through the cytotoxic actions described previously) is an effective method of blunting a pathologic inflammatory response.
B-cell depletion (through the cytotoxic actions described previously) is an effective method of blunting a pathologic inflammatory response. B cells play a pivotal role in the development and progression of many autoimmune diseases. B cells must go through a series of steps before they become immunologically active, and many of these steps require cytokine binding and stimulation. Therefore blocking these cytokines can result in inhibition of the following B cell functions:
B cells play a pivotal role in the development and progression of many autoimmune diseases. B cells must go through a series of steps before they become immunologically active, and many of these steps require cytokine binding and stimulation. Therefore blocking these cytokines can result in inhibition of the following B cell functions:
 Bound to radioactive ligands: When bound to radioactive ligands, the mAb delivers targeted radiotherapy to the target cells and destroys the cells through close contact with the radioactive ligand.
Bound to radioactive ligands: When bound to radioactive ligands, the mAb delivers targeted radiotherapy to the target cells and destroys the cells through close contact with the radioactive ligand.Pharmacokinetics
 The half-life of rituximab can be variable when treating tumor. The drug will become bound and removed from circulation when a high number of CD20 receptors are present, effectively decreasing the drug concentration and half-life. Therefore at the start of cancer therapy the half-life is around 75 hours, whereas at the end of therapy it can be as long as 200 hours.
The half-life of rituximab can be variable when treating tumor. The drug will become bound and removed from circulation when a high number of CD20 receptors are present, effectively decreasing the drug concentration and half-life. Therefore at the start of cancer therapy the half-life is around 75 hours, whereas at the end of therapy it can be as long as 200 hours.Indications
Contraindications
Side Effects
Early
Important Notes
 CD20 antigen is not expressed by either plasma cells or B-lymphoid stem cells; therefore rituximab does not reduce total immunoglobulin (Ig) serum concentrations.
CD20 antigen is not expressed by either plasma cells or B-lymphoid stem cells; therefore rituximab does not reduce total immunoglobulin (Ig) serum concentrations.Advanced
 B cell–depleting and B cell–nondepleting strategies have both been used for treatment of autoimmune diseases.
B cell–depleting and B cell–nondepleting strategies have both been used for treatment of autoimmune diseases. Future therapies:
Future therapies:
 BAFF (B-cell activating factor), also called BlyS (B-lymphocyte stimulator), prolongs the survival of B cells, stimulates maturation, and promotes survival of autoreactive B. Anti-BAFF antibodies (belimumab) or BAFF receptor fusion proteins (briobacept) are being investigated for treating RA and SLE.
BAFF (B-cell activating factor), also called BlyS (B-lymphocyte stimulator), prolongs the survival of B cells, stimulates maturation, and promotes survival of autoreactive B. Anti-BAFF antibodies (belimumab) or BAFF receptor fusion proteins (briobacept) are being investigated for treating RA and SLE.T-Cell Biologics
Description
T-cell biologics specifically target T-cell lymphocytes for either destruction or suppression.
MOA (Mechanism of Action)
 The roles and activities of T cells are extremely diverse and complicated. Their contributions to disease are currently incompletely understood, and this section is a very simplified explanation of a very complicated system. This area of medicine is changing quickly, as is the understanding of these disease processes.
The roles and activities of T cells are extremely diverse and complicated. Their contributions to disease are currently incompletely understood, and this section is a very simplified explanation of a very complicated system. This area of medicine is changing quickly, as is the understanding of these disease processes.CD3
 CD3 is the defining marker for T cells. Therefore, all T cells express CD3. As a result, drugs that target CD3 have the potential to influence all subtypes of T cells and are therefore nonspecific. They will influence proinflammatory as well as antiinflammatory T cells and also target activated and nonactivated T cells.
CD3 is the defining marker for T cells. Therefore, all T cells express CD3. As a result, drugs that target CD3 have the potential to influence all subtypes of T cells and are therefore nonspecific. They will influence proinflammatory as well as antiinflammatory T cells and also target activated and nonactivated T cells. A strong inflammatory reaction, cytokine-release syndrome, often occurs with the first dose of OKT3. It can be severe, resulting in a range of signs and symptoms from fever to tachycardia to meningitis (noninfectious) and adult respiratory distress syndrome (ARDS). This reaction occurs because of a massive synchronized T-cell activation.
A strong inflammatory reaction, cytokine-release syndrome, often occurs with the first dose of OKT3. It can be severe, resulting in a range of signs and symptoms from fever to tachycardia to meningitis (noninfectious) and adult respiratory distress syndrome (ARDS). This reaction occurs because of a massive synchronized T-cell activation.
CD25 (IL-2 Receptor)
Autoimmune Diseases
 Increased CD25 (IL-2 receptor) expression has been demonstrated in many autoimmune diseases. Suppression or antagonism of IL-2 has resulted in reduced inflammation in many of these diseases.
Increased CD25 (IL-2 receptor) expression has been demonstrated in many autoimmune diseases. Suppression or antagonism of IL-2 has resulted in reduced inflammation in many of these diseases. Important source of apparent conflict:
Important source of apparent conflict:
 Regulatory T cells (Treg) are very important in the control of self-tolerance. They play a vital role in preventing the immune system from attacking a person’s own tissues and cells. They express CD25 and require IL-2 for their growth and survival; however, they suppress the immune system.
Regulatory T cells (Treg) are very important in the control of self-tolerance. They play a vital role in preventing the immune system from attacking a person’s own tissues and cells. They express CD25 and require IL-2 for their growth and survival; however, they suppress the immune system. Autoreactive T cells are defined as T cells that attack self antigens (those that are present in the body), and these attacks are a major contributor to autoimmune diseases.
Autoreactive T cells are defined as T cells that attack self antigens (those that are present in the body), and these attacks are a major contributor to autoimmune diseases.CD28 (CTLA-4)
Autoimmune Diseases
CD11a (LFA-1)
 CD11a is important for interactions between the integrin LFA-1 and cell adhesion molecule intercellular adhesion molecule 1 (ICAM-1). Integrins are proteins that facilitate the attachment of cells to other cells or to extracellular matrices. Once attached, the cell can transmigrate from the blood into the tissue, across the endothelial barrier.
CD11a is important for interactions between the integrin LFA-1 and cell adhesion molecule intercellular adhesion molecule 1 (ICAM-1). Integrins are proteins that facilitate the attachment of cells to other cells or to extracellular matrices. Once attached, the cell can transmigrate from the blood into the tissue, across the endothelial barrier.Indications
Side Effects
 With all classes of drugs, the risk of immunosuppression leading to infection is an ever-present side effect.
With all classes of drugs, the risk of immunosuppression leading to infection is an ever-present side effect. Infusion reactions: Nausea, vomiting, diarrhea, hypotension, shortness of breath, and headache, to name a few, can all occur.
Infusion reactions: Nausea, vomiting, diarrhea, hypotension, shortness of breath, and headache, to name a few, can all occur.CD3
 With OKT3 only, cytokine release syndrome: a massive inflammatory response caused by binding to the T cells combined with Fc receptor cross-linking.
With OKT3 only, cytokine release syndrome: a massive inflammatory response caused by binding to the T cells combined with Fc receptor cross-linking.
 The syndrome is attributed to increased serum levels of cytokines, particularly the production of TNF.
The syndrome is attributed to increased serum levels of cytokines, particularly the production of TNF. Symptoms usually are worst with the first dose; frequency and severity decrease with subsequent doses.
Symptoms usually are worst with the first dose; frequency and severity decrease with subsequent doses.Evidence
Abatacept and Rheumatoid Arthritis
 A Cochrane review in 2009 (seven studies, 2908 patients) found that patients treated with abatacept, compared with placebo, were more likely (relative risk [RR] 2.21) to achieve clinical benefit as measured by the ACR50, an index of pain, disability, and number of affected joints. The number needed to treat (NNT) was 5. The risk of side effects was low (RR 1.05) compared with placebo, and the harms were assessed to be not significant except for increased infections assessed at 12 months.
A Cochrane review in 2009 (seven studies, 2908 patients) found that patients treated with abatacept, compared with placebo, were more likely (relative risk [RR] 2.21) to achieve clinical benefit as measured by the ACR50, an index of pain, disability, and number of affected joints. The number needed to treat (NNT) was 5. The risk of side effects was low (RR 1.05) compared with placebo, and the harms were assessed to be not significant except for increased infections assessed at 12 months.Mixed Biologics
MOA (Mechanism of Action)
CD52 (CAMPATH-1)
 CD52 is present on both B- and T-cell lymphocytes, normal neutrophils, and most B- and T-cell lymphomas.
CD52 is present on both B- and T-cell lymphocytes, normal neutrophils, and most B- and T-cell lymphomas. CD52 is not expressed on CD34+ lymphocytes (which is a marker for hematopoietic progenitor cells), so hematopoietic progenitor cells are not targeted with CD52 therapy. This is important because targeting very early stem cells will result in destruction of multiple cell lines, which would be undesirable.
CD52 is not expressed on CD34+ lymphocytes (which is a marker for hematopoietic progenitor cells), so hematopoietic progenitor cells are not targeted with CD52 therapy. This is important because targeting very early stem cells will result in destruction of multiple cell lines, which would be undesirable. This results in extensive lympholysis (destruction of lymphocytes) by inducing apoptosis and produces prolonged T- and B-cell depletion
This results in extensive lympholysis (destruction of lymphocytes) by inducing apoptosis and produces prolonged T- and B-cell depletionIL-6 Receptor
 Overproduction of IL-6 is thought to play an important role in the pathogenesis of some autoimmune diseases such as RA. IL-6 is elevated in patients with RA, and IL-6 levels correlate with disease severity
Overproduction of IL-6 is thought to play an important role in the pathogenesis of some autoimmune diseases such as RA. IL-6 is elevated in patients with RA, and IL-6 levels correlate with disease severitySide Effects
Tumor Necrosis Factor (TNF)–α Inhibitors
MOA (Mechanism of Action)
 TNF-α is mostly produced by macrophages but is also produced by other inflammatory cells. It binds to TNF receptors (called TNFRs), which are present on many different cells of the immune system.
TNF-α is mostly produced by macrophages but is also produced by other inflammatory cells. It binds to TNF receptors (called TNFRs), which are present on many different cells of the immune system. It promotes inflammation and is believed to play important roles in both inflammatory reactions to infections and also, when present in abnormally high levels, in the pathogenesis of autoimmune diseases. It has been called the master regulator of the immune system.
It promotes inflammation and is believed to play important roles in both inflammatory reactions to infections and also, when present in abnormally high levels, in the pathogenesis of autoimmune diseases. It has been called the master regulator of the immune system. The inflammatory cascades are very complicated. TNF-α is believed to exert its proinflammatory actions by promoting:
The inflammatory cascades are very complicated. TNF-α is believed to exert its proinflammatory actions by promoting:
 The acute phase response, which occurs early in the inflammatory response and is mediated in part by the release of other proinflammatory cytokines, including IL-1, IL-6, and IL-8, resulting in fever, loss of appetite, vasodilation, and tachycardia
The acute phase response, which occurs early in the inflammatory response and is mediated in part by the release of other proinflammatory cytokines, including IL-1, IL-6, and IL-8, resulting in fever, loss of appetite, vasodilation, and tachycardiaContraindications
Side Effects
 Acute inflammatory reaction: Because of the ability of TNF to influence the immune system, extensive inflammatory reactions can occur within 24 hours (usually within 6 hours) after administration. They are characterized by fever, hypotension, tachycardia, itching, chest pain, and shortness of breath. These reactions are relatively common (10% of the time), but only rarely are they severe enough to discontinue treatment.
Acute inflammatory reaction: Because of the ability of TNF to influence the immune system, extensive inflammatory reactions can occur within 24 hours (usually within 6 hours) after administration. They are characterized by fever, hypotension, tachycardia, itching, chest pain, and shortness of breath. These reactions are relatively common (10% of the time), but only rarely are they severe enough to discontinue treatment. Delayed inflammatory reaction: Signs and symptoms that occur within 2 weeks after the injection are probably mediated by antibodies to the drug. Symptoms of these reactions include fever, rash, urticaria (itching), myalgia (muscle pain), arthralgia (joint pain), jaw tightness, and edema.
Delayed inflammatory reaction: Signs and symptoms that occur within 2 weeks after the injection are probably mediated by antibodies to the drug. Symptoms of these reactions include fever, rash, urticaria (itching), myalgia (muscle pain), arthralgia (joint pain), jaw tightness, and edema. Infection: Because of the immune suppression, common bacterial and also opportunistic infections are more common in patients receiving TNF suppression:
Infection: Because of the immune suppression, common bacterial and also opportunistic infections are more common in patients receiving TNF suppression:
Important Notes
 Anti-TNF therapy for RA is considered a biologic DMARD, which, as previously noted, stands for disease-modifying antirheumatic drug. DMARDs slow the progression of joint destruction. Use of DMARDs is in contrast to “symptom-only” therapy, which would include analgesics (such as acetaminophen or NSAIDs), in that symptom-only therapy does not slow the progression of the disease.
Anti-TNF therapy for RA is considered a biologic DMARD, which, as previously noted, stands for disease-modifying antirheumatic drug. DMARDs slow the progression of joint destruction. Use of DMARDs is in contrast to “symptom-only” therapy, which would include analgesics (such as acetaminophen or NSAIDs), in that symptom-only therapy does not slow the progression of the disease. Antibodies to the antibody (ATA) can occur. In this situation, the patient’s immune system recognizes the mAb as a foreign antigen and generates antibodies against it, thus neutralizing it. This reaction is less common with mAb types that are more similar to human antibodies: human (3%) < humanized < chimeric (10%).
Antibodies to the antibody (ATA) can occur. In this situation, the patient’s immune system recognizes the mAb as a foreign antigen and generates antibodies against it, thus neutralizing it. This reaction is less common with mAb types that are more similar to human antibodies: human (3%) < humanized < chimeric (10%).Advanced
 TNF is active when three monomers bind together into a trimeric unit and bind to two different receptors: TNFR1 and TNFR2. These receptors are also called p55 and p75, respectively.
TNF is active when three monomers bind together into a trimeric unit and bind to two different receptors: TNFR1 and TNFR2. These receptors are also called p55 and p75, respectively. In contrast to infliximab and adalimumab, certolizumab does not contain an Fc portion and therefore does not induce complement activation, antibody-dependent cellular cytotoxicity, or apoptosis. Remember that the Fab portion is the antigen-specific region and the Fc region is important for determining the type of response that occurs after the Fab portion binds to the antigen.
In contrast to infliximab and adalimumab, certolizumab does not contain an Fc portion and therefore does not induce complement activation, antibody-dependent cellular cytotoxicity, or apoptosis. Remember that the Fab portion is the antigen-specific region and the Fc region is important for determining the type of response that occurs after the Fab portion binds to the antigen.
Evidence
Rheumatoid Arthritis
Monotherapy versus Methotrexate, and Monotherapy versus Combination Therapy
 A 2008 systematic review of DMARD therapy for RA concluded that anti-TNF monotherapy was similar in efficacy to treatment with methotrexate alone, whereas the combination of an anti-TNF agent with methotrexate reduced disease activity more and slowed radiographic progression to a greater extent than did anti-TNF monotherapy or methotrexate alone. These findings were similar to those of a Cochrane review in 2009 that examined all biologic DMARDs in the treatment of RA.
A 2008 systematic review of DMARD therapy for RA concluded that anti-TNF monotherapy was similar in efficacy to treatment with methotrexate alone, whereas the combination of an anti-TNF agent with methotrexate reduced disease activity more and slowed radiographic progression to a greater extent than did anti-TNF monotherapy or methotrexate alone. These findings were similar to those of a Cochrane review in 2009 that examined all biologic DMARDs in the treatment of RA.Infliximab (with or without Methotrexate) versus Placebo (Plus Methotrexate)
 A Cochrane review in 2002 (two trials, 529 patients) found that after 6 months, response rates were significantly improved with all infliximab doses compared with controls. The NNT with infliximab to achieve an American College of Rheumatology (ACR) 20, 50, or 70 response (these are different measures of response based on disability, pain, and number of affected joints) in patients with refractory RA under specialist care ranged from 2.94 to 3.33 for ACR 20, up to 5.88 to 12.5 for ACR 70. Withdrawals because of adverse events and withdrawals for other reasons were not statistically significantly different in patients receiving infliximab than in controls.
A Cochrane review in 2002 (two trials, 529 patients) found that after 6 months, response rates were significantly improved with all infliximab doses compared with controls. The NNT with infliximab to achieve an American College of Rheumatology (ACR) 20, 50, or 70 response (these are different measures of response based on disability, pain, and number of affected joints) in patients with refractory RA under specialist care ranged from 2.94 to 3.33 for ACR 20, up to 5.88 to 12.5 for ACR 70. Withdrawals because of adverse events and withdrawals for other reasons were not statistically significantly different in patients receiving infliximab than in controls.FYI
 TNF-α was initially called cachexin and was described in 1975 for its ability to lyse tumors in animal models (giving rise to the name tumor necrosis factor).
TNF-α was initially called cachexin and was described in 1975 for its ability to lyse tumors in animal models (giving rise to the name tumor necrosis factor).Antimetabolites
MOA (Mechanism of Action)
 Purines and pyrimidines are the two types of bases used in DNA and RNA. Purines are guanine (G) and adenine (A); pyrimidines are cytosine (C), thymine (T), and uracil (U). Uracil (U) is found only in RNA, whereas thymine (T) is only in DNA. The bases are all attached to a single phosphate group, making them monophosphates.
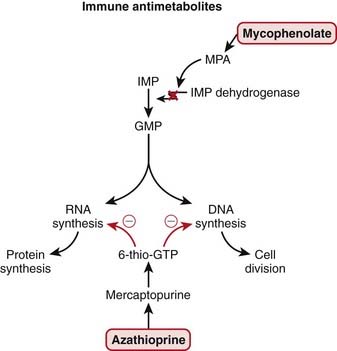
Purines and pyrimidines are the two types of bases used in DNA and RNA. Purines are guanine (G) and adenine (A); pyrimidines are cytosine (C), thymine (T), and uracil (U). Uracil (U) is found only in RNA, whereas thymine (T) is only in DNA. The bases are all attached to a single phosphate group, making them monophosphates. Inosine monophosphate (IMP), the precursor to guanine monophosphate (GMP), is converted by IMP dehydrogenase.
Inosine monophosphate (IMP), the precursor to guanine monophosphate (GMP), is converted by IMP dehydrogenase. Mycophenolate is hydrolyzed to mycophenolic acid (MPA), which inhibits IMP dehydrogenase and thereby prevents GMP production. GMP is a required base for both DNA and RNA, and without it, DNA and RNA synthesis is reduced (Figure 17-2).
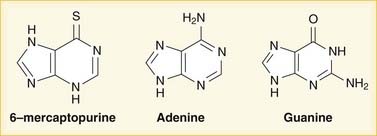
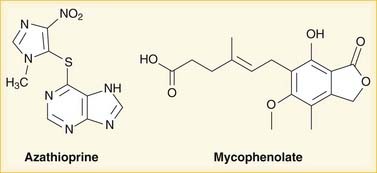
Mycophenolate is hydrolyzed to mycophenolic acid (MPA), which inhibits IMP dehydrogenase and thereby prevents GMP production. GMP is a required base for both DNA and RNA, and without it, DNA and RNA synthesis is reduced (Figure 17-2). Azathioprine is converted to mercaptopurine (itself a drug), which is then metabolized through more steps to 6-thio-GTP; 6-thio-GTP can be incorporated into DNA and RNA, but because it is not a normal base, it halts further DNA and RNA synthesis. It is called a fraudulent nucleoside.
Azathioprine is converted to mercaptopurine (itself a drug), which is then metabolized through more steps to 6-thio-GTP; 6-thio-GTP can be incorporated into DNA and RNA, but because it is not a normal base, it halts further DNA and RNA synthesis. It is called a fraudulent nucleoside. Regardless of the exact mechanism of action on DNA and RNA:
Regardless of the exact mechanism of action on DNA and RNA:
 Reduced DNA synthesis results in reduced proliferation of cells. As a general rule, rapidly dividing cells are more sensitive to drugs that influence cell division. These include the following:
Reduced DNA synthesis results in reduced proliferation of cells. As a general rule, rapidly dividing cells are more sensitive to drugs that influence cell division. These include the following:
 Two features of antimetabolites that make them a little more specific to inhibiting the immune system include:
Two features of antimetabolites that make them a little more specific to inhibiting the immune system include:
Pharmacokinetics
Azathioprine
 Azathioprine is metabolized quickly (half-life of 10 minutes) but has many active metabolites; therefore blood level measurements of the parent drug do not provide useful information.
Azathioprine is metabolized quickly (half-life of 10 minutes) but has many active metabolites; therefore blood level measurements of the parent drug do not provide useful information. Azathioprine is metabolized by the liver to mercaptopurine; mercaptopurine metabolism occurs via a couple different enzymes, one of which is xanthine oxidase, an enzyme that is important in the disease gout; allopurinol, a xanthine oxidase inhibitor used to treat gout, results in dramatically increased levels of active metabolites. This is an important drug interaction.
Azathioprine is metabolized by the liver to mercaptopurine; mercaptopurine metabolism occurs via a couple different enzymes, one of which is xanthine oxidase, an enzyme that is important in the disease gout; allopurinol, a xanthine oxidase inhibitor used to treat gout, results in dramatically increased levels of active metabolites. This is an important drug interaction.
 Another important enzyme that metabolizes mercaptopurine is thiopurine methyltransferase (TPMT); the TPMT activity rate is very important because TPMT catalyzes a reaction to produce an inactive metabolite and is therefore felt to be a strong predictor for myelosuppression and hepatotoxicity. Patients with low TPMT activity develop higher levels of 6-thio-GTP and are therefore more susceptible to acute bone marrow suppression and liver damage. TPMT activity is genetically determined, and tests for TPMT activity levels are available.
Another important enzyme that metabolizes mercaptopurine is thiopurine methyltransferase (TPMT); the TPMT activity rate is very important because TPMT catalyzes a reaction to produce an inactive metabolite and is therefore felt to be a strong predictor for myelosuppression and hepatotoxicity. Patients with low TPMT activity develop higher levels of 6-thio-GTP and are therefore more susceptible to acute bone marrow suppression and liver damage. TPMT activity is genetically determined, and tests for TPMT activity levels are available. Timing of efficacy: It can take a couple of months before azathioprine exerts its effects. This is thought to be related to the long time required to generate intracellular levels of 6-thio-GTP.
Timing of efficacy: It can take a couple of months before azathioprine exerts its effects. This is thought to be related to the long time required to generate intracellular levels of 6-thio-GTP.Side Effects
Important Notes
 In treatment of autoimmune diseases, steroids are generally the first-line immunosuppressants employed because they act quickly; however, there are many side effects related to prolonged steroid use, and therefore nonsteroid immunosuppressants are generally preferred for long-term immunosuppression. Antimetabolites are used therefore for long-term “steroid-sparing” immunosuppression.
In treatment of autoimmune diseases, steroids are generally the first-line immunosuppressants employed because they act quickly; however, there are many side effects related to prolonged steroid use, and therefore nonsteroid immunosuppressants are generally preferred for long-term immunosuppression. Antimetabolites are used therefore for long-term “steroid-sparing” immunosuppression. Antimetabolites exert their immunosuppressant effects over a period of weeks, which is a slower onset than that of steroids, which become effective in about a day.
Antimetabolites exert their immunosuppressant effects over a period of weeks, which is a slower onset than that of steroids, which become effective in about a day.Advanced
 Azathioprine is converted to mercaptopurine; however, azathioprine is felt to be a more effective immunosuppressant than mercaptopurine because of increased intracellular uptake. Intracellular levels of the metabolite 6-thio-GTP need to be high for the drugs to be effective, and azathioprine appears to be taken up specifically by lymphocytes.
Azathioprine is converted to mercaptopurine; however, azathioprine is felt to be a more effective immunosuppressant than mercaptopurine because of increased intracellular uptake. Intracellular levels of the metabolite 6-thio-GTP need to be high for the drugs to be effective, and azathioprine appears to be taken up specifically by lymphocytes.Calcineurin Inhibitors
MOA (Mechanism of Action)
 These drugs act on T cells (a type of lymphocyte). T cells are involved in cell-mediated immunity (as opposed to humoral immunity, which is mediated by B lymphocytes).
These drugs act on T cells (a type of lymphocyte). T cells are involved in cell-mediated immunity (as opposed to humoral immunity, which is mediated by B lymphocytes). Calcineurin is a protein phosphatase responsible for the transcription of IL-2, an important immune system cytokine. Calcineurin inhibitors stop the production of IL-2.
Calcineurin is a protein phosphatase responsible for the transcription of IL-2, an important immune system cytokine. Calcineurin inhibitors stop the production of IL-2. IL-2 enables the activation of inactive T cells. It also plays a role in proliferation and differentiation of T cells (resulting in immunologic memory).
IL-2 enables the activation of inactive T cells. It also plays a role in proliferation and differentiation of T cells (resulting in immunologic memory). A protein called nuclear factor of activated T cells (NFAT) binds T cell DNA and stimulates production of IL-2. NFAT is activated through dephosphorylation by calcineurin (Figure 17-5).
A protein called nuclear factor of activated T cells (NFAT) binds T cell DNA and stimulates production of IL-2. NFAT is activated through dephosphorylation by calcineurin (Figure 17-5). Through the inhibition of IL-2 production and thus T cell activation, proliferation, and differentiation, calcineurin inhibitors are immunosuppressants.
Through the inhibition of IL-2 production and thus T cell activation, proliferation, and differentiation, calcineurin inhibitors are immunosuppressants. Calcineurin inhibitors are not very effective at blunting the activity of T cells that have already been activated. They are more effective at preventing the immune response before it starts. For example, if acute rejection of a transplanted organ has already started, other immunosuppressants are required for effective treatment.
Calcineurin inhibitors are not very effective at blunting the activity of T cells that have already been activated. They are more effective at preventing the immune response before it starts. For example, if acute rejection of a transplanted organ has already started, other immunosuppressants are required for effective treatment.Pharmacokinetics
 Cyclosporine has a narrow therapeutic index. Levels must be frequently measured to help reduce the probability of side effects.
Cyclosporine has a narrow therapeutic index. Levels must be frequently measured to help reduce the probability of side effects.Indications
Side Effects
Other
 Nephrotoxicity: Kidney damage is a common problem with administration of cyclosporine. This is the most important side effect. It is possibly mediated through renal arteriolar vasoconstriction. Renal damage often limits administration of cyclosporine.
Nephrotoxicity: Kidney damage is a common problem with administration of cyclosporine. This is the most important side effect. It is possibly mediated through renal arteriolar vasoconstriction. Renal damage often limits administration of cyclosporine.Important Notes
 In patients with kidney transplants (a population of patients who regularly receive calcineurin inhibitors), it can be diagnostically difficult to determine if declining renal function is a result of rejection (which would be managed by increasing the dose or adding immunosuppressive drugs) or of toxicity of the immunosuppressive drugs (which would be managed by lowering the dose or stopping the drugs).
In patients with kidney transplants (a population of patients who regularly receive calcineurin inhibitors), it can be diagnostically difficult to determine if declining renal function is a result of rejection (which would be managed by increasing the dose or adding immunosuppressive drugs) or of toxicity of the immunosuppressive drugs (which would be managed by lowering the dose or stopping the drugs).Advanced
 Less common side effects include the following:
Less common side effects include the following:
 Haemolytic uremic syndrome is characterized by microangiopathic anemia, thrombocytopenia, and acute renal failure. Simply thought of, it is a process whereby platelets are consumed into intravascular aggregates, causing a meshwork of clots that lyse red blood cells and the floating intracellular debris plugs up the glomeruli and tubules, resulting in kidney failure.
Haemolytic uremic syndrome is characterized by microangiopathic anemia, thrombocytopenia, and acute renal failure. Simply thought of, it is a process whereby platelets are consumed into intravascular aggregates, causing a meshwork of clots that lyse red blood cells and the floating intracellular debris plugs up the glomeruli and tubules, resulting in kidney failure. Posterior reversible encephalopathy syndrome is an uncommon side effect with cyclosporine that is also seen with eclampsia and in the setting of severe hypertension (two conditions of extreme vasoconstriction). On brain imaging studies, there is widespread edema that predominates in the parietal and occipital regions (posterior regions). There is also clinical evidence of neurotoxicity (dysfunction).
Posterior reversible encephalopathy syndrome is an uncommon side effect with cyclosporine that is also seen with eclampsia and in the setting of severe hypertension (two conditions of extreme vasoconstriction). On brain imaging studies, there is widespread edema that predominates in the parietal and occipital regions (posterior regions). There is also clinical evidence of neurotoxicity (dysfunction).FYI
 Tacrolimus is in the same chemical family as macrolide antibiotics, but it is never used as an antibiotic.
Tacrolimus is in the same chemical family as macrolide antibiotics, but it is never used as an antibiotic. The IL-2 receptor is also called CD25. CD stands for cluster of differentiation and reflects a nomenclature system for defining cell surface markers on white blood cells (and now for other cells, too). There are more than 300 different CDs. The number gives no indication of the function or structure of the CD, and there is an enormous range of CDs. CD4 and CD8 are probably the most widely recognized CDs.
The IL-2 receptor is also called CD25. CD stands for cluster of differentiation and reflects a nomenclature system for defining cell surface markers on white blood cells (and now for other cells, too). There are more than 300 different CDs. The number gives no indication of the function or structure of the CD, and there is an enormous range of CDs. CD4 and CD8 are probably the most widely recognized CDs. Cyclosporine is not water soluble and requires bile production to be intact for oral administration. In patients with liver transplant who might have impaired bile production, the absorption of cyclosporine would be unpredictable. It was modified into a microemulsion in the 1990s, which solved this problem.
Cyclosporine is not water soluble and requires bile production to be intact for oral administration. In patients with liver transplant who might have impaired bile production, the absorption of cyclosporine would be unpredictable. It was modified into a microemulsion in the 1990s, which solved this problem.Target of Rapamycin (mTOR) Inhibitors
MOA (Mechanism of Action)
 mTOR (mammalian target of rapamycin) is a serine-threonine protein kinase activated by several growth factors after receptor binding.
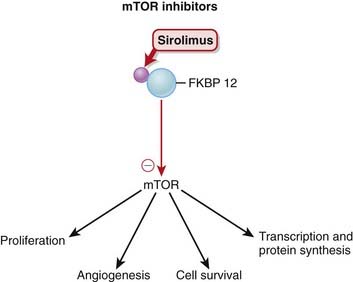
mTOR (mammalian target of rapamycin) is a serine-threonine protein kinase activated by several growth factors after receptor binding. Sirolimus binds a protein called FKBP 12 (FKBP stands for FK506 binding protein) in the cytoplasm. The drug-protein complex then binds and inhibits mTOR (Figure 17-6).
Sirolimus binds a protein called FKBP 12 (FKBP stands for FK506 binding protein) in the cytoplasm. The drug-protein complex then binds and inhibits mTOR (Figure 17-6). mTOR regulates important functions of the cell, including proliferation, angiogenesis (blood vessel formation), cell survival, protein synthesis, and transcription. It is recognized as a key point in many cellular functions.
mTOR regulates important functions of the cell, including proliferation, angiogenesis (blood vessel formation), cell survival, protein synthesis, and transcription. It is recognized as a key point in many cellular functions. Through this mechanism, mTOR inhibitors:
Through this mechanism, mTOR inhibitors:
Pharmacokinetics
 Sirolimus and everolimus are administered orally, whereas temsirolimus is administered intravenously.
Sirolimus and everolimus are administered orally, whereas temsirolimus is administered intravenously.Side Effects
Important Notes
 Sirolimus is not nephrotoxic, a very significant advantage over calcineurin inhibitors, especially when being used in the setting of renal transplant, when every effort is made to protect the new transplanted kidney. Calcineurin inhibitors, which are also used in renal transplant patients, are nephrotoxic.
Sirolimus is not nephrotoxic, a very significant advantage over calcineurin inhibitors, especially when being used in the setting of renal transplant, when every effort is made to protect the new transplanted kidney. Calcineurin inhibitors, which are also used in renal transplant patients, are nephrotoxic.Advanced
 Sirolimus first binds FKBP (FK506 binding protein), the protein that FK506 (tacrolimus) binds to. However, the drug-protein complex when bound to sirolimus acts differently than when bound to tacrolimus. Therefore the two drugs act on the same protein in the body but in a different way.
Sirolimus first binds FKBP (FK506 binding protein), the protein that FK506 (tacrolimus) binds to. However, the drug-protein complex when bound to sirolimus acts differently than when bound to tacrolimus. Therefore the two drugs act on the same protein in the body but in a different way. Among rapamycin, cyclosporine, and tacrolimus (immunosuppressants for transplant rejection), rapamycin has the strongest antiangiogenic activity.
Among rapamycin, cyclosporine, and tacrolimus (immunosuppressants for transplant rejection), rapamycin has the strongest antiangiogenic activity.
 Drug interaction: Although sirolimus is not nephrotoxic alone, coadministration with a calcineurin inhibitor (another drug used for renal transplants) results in greater kidney damage than with a calcineurin inhibitor alone. Therefore there is some interaction with calcineurin inhibitors that potentiates the renal damage induced by the calcineurin inhibitor, and the two drugs should not be coadministered.
Drug interaction: Although sirolimus is not nephrotoxic alone, coadministration with a calcineurin inhibitor (another drug used for renal transplants) results in greater kidney damage than with a calcineurin inhibitor alone. Therefore there is some interaction with calcineurin inhibitors that potentiates the renal damage induced by the calcineurin inhibitor, and the two drugs should not be coadministered.FYI
 Important nomenclature:
Important nomenclature:
 Sirolimus is classified as a macrolide (antibiotic). However, it was initially developed as an antifungal and does have antifungal properties. It is important to note that when an infection is being treated, a strong immune system is desirable; sirolimus is an immunosuppressive and therefore is used clinically in a way, ironically, that is the polar opposite of the use for which it was originally designed.
Sirolimus is classified as a macrolide (antibiotic). However, it was initially developed as an antifungal and does have antifungal properties. It is important to note that when an infection is being treated, a strong immune system is desirable; sirolimus is an immunosuppressive and therefore is used clinically in a way, ironically, that is the polar opposite of the use for which it was originally designed.Activated Protein C
Description
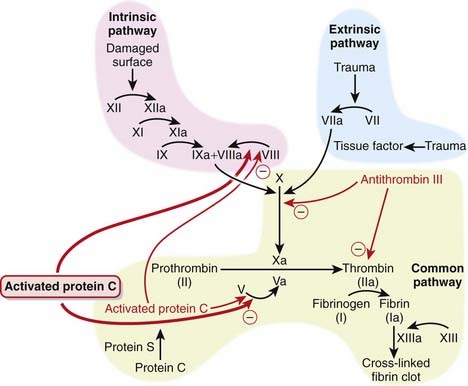
Activated protein C is part of the coagulation cascade but is used as an antiinflammatory.
MOA (Mechanism of Action)
 Therefore, selectively inhibiting the coagulation cascade has the potential to inhibit the inflammatory response, and this is the basis for using activated protein C in severe inflammatory states known as severe sepsis or septic shock.
Therefore, selectively inhibiting the coagulation cascade has the potential to inhibit the inflammatory response, and this is the basis for using activated protein C in severe inflammatory states known as severe sepsis or septic shock. Protein C is a natural anticoagulant that is a vitamin K–dependant protein synthesized by the liver. It becomes activated and works in conjunction with protein S to inhibit coagulation by:
Protein C is a natural anticoagulant that is a vitamin K–dependant protein synthesized by the liver. It becomes activated and works in conjunction with protein S to inhibit coagulation by:
 In addition to its anticoagulant properties, activated protein C has also been shown to demonstrate the following antiinflammatory effects:
In addition to its anticoagulant properties, activated protein C has also been shown to demonstrate the following antiinflammatory effects:
Pharmacokinetics
 Activated protein C is metabolized by plasma proteases: 80% is eliminated within 13 minutes, and 98% is cleared within 2 hours. This is important because patients with septic shock sometimes require surgery, and activated protein C needs to be discontinued 2 hours before surgery. Furthermore, if bleeding occurs while a patient is on activated protein C, then the anticoagulant effect can be reversed within 2 hours.
Activated protein C is metabolized by plasma proteases: 80% is eliminated within 13 minutes, and 98% is cleared within 2 hours. This is important because patients with septic shock sometimes require surgery, and activated protein C needs to be discontinued 2 hours before surgery. Furthermore, if bleeding occurs while a patient is on activated protein C, then the anticoagulant effect can be reversed within 2 hours.Evidence
 In two large RCTs (the PROWESS and ADDRESS studies) comparing activated protein C with placebo, a mortality benefit (absolute risk reduction of 6.1%) was demonstrated in patients who were very sick (APACHE score greater than 25) and treated with activated protein C. However, there was no mortality benefit in patients who were less sick (APACHE score less than 25).
In two large RCTs (the PROWESS and ADDRESS studies) comparing activated protein C with placebo, a mortality benefit (absolute risk reduction of 6.1%) was demonstrated in patients who were very sick (APACHE score greater than 25) and treated with activated protein C. However, there was no mortality benefit in patients who were less sick (APACHE score less than 25).FYI
 APACHE stands for Acute Physiology and Chronic Health Evaluation, and the APACHE scoring system is used to describe severity of illness of patients admitted to the intensive care unit. The score ranges from 0 to 71 and is based on 17 physiologic parameters such as heart rate, white blood count, and Pco2.
APACHE stands for Acute Physiology and Chronic Health Evaluation, and the APACHE scoring system is used to describe severity of illness of patients admitted to the intensive care unit. The score ranges from 0 to 71 and is based on 17 physiologic parameters such as heart rate, white blood count, and Pco2. Primitive organisms use coagulation as an immune response; to produce clot around the infection and isolate it from the rest of the circulation. This helps to defend against the advancement of the infection.
Primitive organisms use coagulation as an immune response; to produce clot around the infection and isolate it from the rest of the circulation. This helps to defend against the advancement of the infection.Glatiramoids
MOA (Mechanism of Action)
 The key pathophysiologic features of MS include mononuclear cell infiltration, demyelination, and scarring (gliosis) of the central nervous sytem, leading to significant neurologic deficits.
The key pathophysiologic features of MS include mononuclear cell infiltration, demyelination, and scarring (gliosis) of the central nervous sytem, leading to significant neurologic deficits. The actions of glatiramer in the treatment of MS are not well understood and are believed to be mediated by multiple mechanisms, with the common theme of having an immunomodulating effect.
The actions of glatiramer in the treatment of MS are not well understood and are believed to be mediated by multiple mechanisms, with the common theme of having an immunomodulating effect. Glatiramer competes for binding to major histocompatibility complex (MHC) class II molecules on APCs.
Glatiramer competes for binding to major histocompatibility complex (MHC) class II molecules on APCs.Side Effects
 Chest pain has been observed during clinical trials; its mechanism is unknown. The pain is transient and does not appear to be clinically harmful.
Chest pain has been observed during clinical trials; its mechanism is unknown. The pain is transient and does not appear to be clinically harmful.Important Notes
 CIS is characterized by a single demyelinating event, an attack that is suggestive of MS. These attacks include typical MS symptoms, such as sudden loss of vision. CIS is a recognized precursor to development of MS, although not all patients with CIS will go on to develop this disease. This has made it difficult to determine whether CIS patients should be initiated on this very expensive therapy, which includes daily injections, for an indeterminate amount of time.
CIS is characterized by a single demyelinating event, an attack that is suggestive of MS. These attacks include typical MS symptoms, such as sudden loss of vision. CIS is a recognized precursor to development of MS, although not all patients with CIS will go on to develop this disease. This has made it difficult to determine whether CIS patients should be initiated on this very expensive therapy, which includes daily injections, for an indeterminate amount of time. The other main agents used to treat MS are the interferons (IFNs) IFN alfa-1a and IFN beta-1b. These agents also have to be injected, and their main side effect is a flulike syndrome.
The other main agents used to treat MS are the interferons (IFNs) IFN alfa-1a and IFN beta-1b. These agents also have to be injected, and their main side effect is a flulike syndrome.Advanced
 A new class of drugs has emerged in the fight against MS. The integrin inhibitors prevent the movement of lymphocytes from blood vessels into the brain. α4β1 Integrin is expressed on the surface of activated lymphocytes and acts on a receptor on the luminal surface of vascular endothelium, vascular cell adhesion molecule (VCAM).
A new class of drugs has emerged in the fight against MS. The integrin inhibitors prevent the movement of lymphocytes from blood vessels into the brain. α4β1 Integrin is expressed on the surface of activated lymphocytes and acts on a receptor on the luminal surface of vascular endothelium, vascular cell adhesion molecule (VCAM). This interaction between α4β1 integrin and VCAM results in adherence of the activated lymphocyte to the vascular wall, which induces the lymphocyte to secrete proteases that enable the lymphocyte to transmigrate across the vascular endothelium and gain access to tissue.
This interaction between α4β1 integrin and VCAM results in adherence of the activated lymphocyte to the vascular wall, which induces the lymphocyte to secrete proteases that enable the lymphocyte to transmigrate across the vascular endothelium and gain access to tissue. Natalizumab binds to α4β1 integrin, thus interfering with the interaction between it and VCAM, resulting in the inhibition of lymphocyte adherence and preventing transmigration of activated lymphocytes into tissue.
Natalizumab binds to α4β1 integrin, thus interfering with the interaction between it and VCAM, resulting in the inhibition of lymphocyte adherence and preventing transmigration of activated lymphocytes into tissue.FYI
 Protiramer, the second glatiramoid, is currently being evaluated in clinical trials for treatment of MS.
Protiramer, the second glatiramoid, is currently being evaluated in clinical trials for treatment of MS. A random sequence of amino acids is often referred to as a polymer by biochemists, in the same way that a polymer in chemistry is a sequence of molecules. Glatiramer is actually a polypeptide consisting of a random sequence of four amino acids: glutamate, lysine, alanine, and tyrosine, hence the name glatiramer.
A random sequence of amino acids is often referred to as a polymer by biochemists, in the same way that a polymer in chemistry is a sequence of molecules. Glatiramer is actually a polypeptide consisting of a random sequence of four amino acids: glutamate, lysine, alanine, and tyrosine, hence the name glatiramer.