Chapter 101 Whole-organ pancreas and pancreatic islet transplantation
Overview
Type 1 diabetes mellitus, formerly known as juvenile diabetes, is characterized by hyperglycemia as a result of the nearly complete destruction of the insulin-producing β-cells of the pancreatic islets of Langerhans. The loss of β-cells is the result of a T-cell–mediated autoimmune attack that typically occurs during childhood or early adolescence. Insulin replacement can lead to acceptable control of blood glucose levels; however, affected individuals are subject to the development of various secondary complications that include cardiac disease, stroke, retinopathy and blindness, nephropathy and renal failure, peripheral and autonomic neuropathy, and amputation (Atkinson & Eisenroth, 2001). Although tight glycemic control has been shown to decrease the number of diabetes-related secondary complications, it also has been shown to lead to an increased number of dangerous hypoglycemic episodes (Diabetes Control and Complications Trial [DCCT] Research Group, 1993).
Whole-Organ Pancreas Transplantation
History and Early Results
On December 20, 1893, P. Watson Williams grafted three pieces of a sheep pancreas into the subcutaneous tissues of a diabetic child, who died 3 days later of unrelenting diabetic ketoacidosis (Williams, 1894). This first attempt to treat diabetes with transplantation, although unsuccessful, preceded decades of animal experimentation, in which investigators developed the methods necessary to perform a vascularized pancreas transplant; pancreas transplantation was subsequently used as a model to study diabetes and glucose homeostasis.
The first clinical vascularized pancreas transplantation was performed on December 17, 1966, by William Kelly and Richard Lillehei at the University of Minnesota. The patient had temporary insulin independence but eventually required graft removal and ultimately died of postoperative complications (Kelly et al, 1967). The subsequent early experience with pancreas transplantation at Minnesota and at a few other centers was characterized by some technical success, but no graft functioned beyond 1 year, and the enthusiasm for this procedure dwindled.
Recipient Operation
In two areas of pancreas transplantation, current-day techniques differ: in the drainage of exocrine secretions and the venous drainage of the graft. Exocrine drainage is performed either via the intestinal tract or via the urinary tract. Throughout most of the 1980s and 1990s, drainage of the pancreatic secretions into the recipient bladder was the most common form of exocrine drainage. This technique is convenient for monitoring organ function by measurement of amylase levels in the urine; however, problems with hematuria, cystitis, bicarbonate loss, and dehydration are all associated with bladder drainage. These complications necessitate surgical revision to enteric drainage in up to 20% of bladder-drained pancreas recipients (Stratta, 2005). Based on these issues, and on the lower rejection rates observed with newer immunosuppressive medications, the majority of transplant centers now perform enteric drainage of the exocrine secretions. This enteric drainage is either directly into a loop of jejunum in a side-to-side fashion or into a Roux-en-Y limb of jejunum.
The venous drainage of the graft is either to the systemic circulation, via an iliac vein or the inferior vena cava, or to the portal circulation. Portal venous drainage has the theoretic advantage of delivering insulin in a more physiologic manner, because insulin undergoes a “first pass” through the liver, and the hyperinsulinemia that results from systemic drainage is avoided (Gaber et al, 1995). There is also an immunologic advantage of portal drainage that has been observed in several experimental studies, in which the delivery of foreign antigen via the portal system results in diminished immune responses. Despite these potential advantages, no demonstrable difference has been found in outcomes between human transplants drained by the portal vein and those drained by the systemic vein, and systemic venous drainage is how the majority of pancreas transplantations are performed.
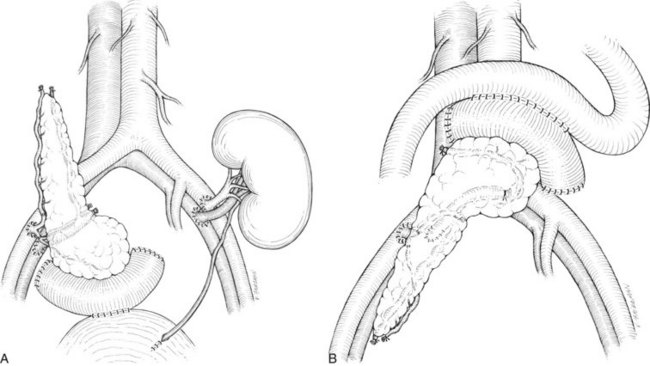
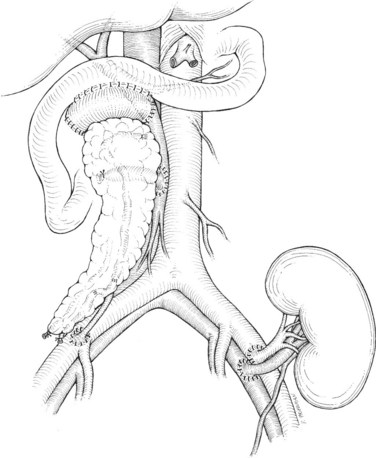
There are two common locations in the abdomen where the transplant is placed based on the type of venous drainage planned: either in the pelvis, most commonly the right side, for systemic venous drainage or in the midabdomen for portal venous drainage. When the graft is placed in the pelvis, the donor portal vein is anastomosed to the external iliac vein, the common iliac vein, or the inferior vena cava. In this pelvic position, the graft is oriented with the duodenum in an inferior position, if bladder drainage is planned (Fig. 101.1A), or with the duodenum in either the superior (Fig. 101.1B) or inferior position if enteric drainage is planned. Alternatively, for portal venous drainage, the pancreas is placed in the mid-abdomen below the transverse colon with the duodenum oriented superiorly. The portal vein of the pancreas is anastomosed to a major branch of the superior mesenteric vein, found in the small intestine mesentery, in an end-to-side fashion (Fig. 101.2). Enteric drainage for exocrine secretions must be used with the portal venous drainage technique. With either venous drainage technique, the donor arterial conduit to the pancreas graft is anastomosed in an end-to-side manner to the recipient common or external iliac artery.
Complications
The major complications following pancreas transplantation are often technical in nature. Pancreas graft thrombosis, arterial or venous, is more frequent after pancreas transplantation than after other solid-organ transplants, and the reported incidence is approximately 10%. Thrombosis usually occurs within the first week following transplantation and likely reflects the relatively low blood flow through the organ. In most instances of thrombosis, graft removal is necessary (Humar et al, 2000). Early pancreatitis occurs in 10% to 20% of cases and is largely a reflection of ischemic damage to the gland during preservation and reperfusion injury. Hyperamylasemia and graft edema are characteristic, and graft pancreatitis is usually treated nonsurgically with octreotide. Leakage at the site of pancreatic exocrine drainage is another early complication, with management often dictated by the method of drainage. Bladder-drained transplants with a small leak at the duodenocystostomy can often be managed by Foley catheter drainage of the bladder, allowing the site of leakage to heal over time. Enteric-drained transplants with a leak at the duodenojejunostomy will often result in peritonitis and usually require operative intervention to control the leak.
Results
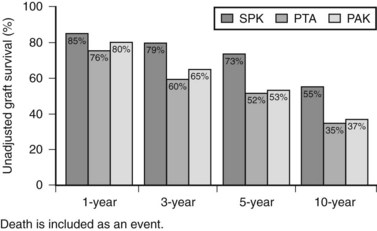
Patient and graft survival following pancreas transplantation have improved significantly in recent years. Depending upon the type of transplantation—SPK, PAK, or PTA—patient survival is approximately 96% to 98% at 1 year and 85% to 89% at 5 years after transplantation. Graft survival varies according to the type of transplantation performed (Fig. 101.3). For SPK recipients, 1-, 5-, and 10-year pancreas graft survival rates are 85%, 73%, and 55%, respectively. For PAK recipients, the respective rates are 80%, 53%, and 37%. Finally, for PTA recipients, rates of pancreas graft survival are 76%, 52%, and 35%, respectively (Axelrod et al, 2010).
The impact of a successful pancreas transplantation on the complications associated with diabetes mellitus is debated. Because the successful transplantation restores euglycemia and normal hemoglobin A1c levels, most proponents argue that diabetic complications should cease and perhaps reverse. Neuropathy appears to stabilize and even slowly improve following pancreas transplantation, whereas retinopathy progression is slowed after several years of graft function. The development of diabetic nephropathy in the transplanted kidney of SPK and early PAK recipients appears to be prevented by successful pancreas transplantation. In PTA recipients, diabetic nephropathy appears to stabilize following transplantation (Fioretto et al, 1998); however, the renal benefit is likely outweighed by the detriment to renal function caused by the immunosuppressive agents, specifically tacrolimus and cyclosporine.
Risk/Benefit Considerations
One analysis evaluated the survival benefit of pancreas transplantation by examining a large transplant database to compare the survival of pancreas recipients versus those patients listed for transplantation but who did not receive a transplant. Using this study design, the two patient groups were ensured to be as similar as possible. Because the indications, risks, and benefits differ, depending on whether the patient had underlying renal failure, the analysis was stratified based on whether the patient was listed for or transplanted with an SPK, PAK, or PTA (Venstrom et al, 2003). This analysis revealed a marked survival benefit for those patients who received a combined pancreas and kidney transplant; a 57% reduction in mortality rate was observed over 4 years compared with similar patients who remained on the waiting list.
In contrast to the clear survival benefit of SPK transplantation, the PAK and PTA recipients experienced an increased mortality following transplantation. During the 4-year follow-up period, PAK recipients experienced a 42% increase in mortality rate, and PTA recipients experienced a 57% increase in mortality rate compared with the comparable group of patients who remained on the waiting list. A similar analysis was reported that challenged the survival disadvantage of PAK and PTA (Gruessner et al, 2004a). Their analysis found again, as expected, that the SPK procedure conferred a marked survival advantage; however, unlike the Venstrom study, no survival disadvantage was evident in PAK or PTA transplant recipients.
These results substantiate the marked clinical benefit observed when caring for patients who undergo the SPK procedure (Rayhill et al, 2000). Importantly, the relative contribution of the transplanted kidney versus the transplanted pancreas to overall patient survival has not been differentiated in a controlled study. This issue is significant given the marked patient survival benefit that has been attributed to kidney transplantation (Wolfe et al, 1999).
A recent retrospective database analysis lends important insight into the role that pancreas graft function contributes towards overall patient survival (Weiss et al, 2009). This analysis examined outcomes in individuals on the SPK waiting list who underwent either an SPK or transplantation of the kidney alone. Recipients of an SPK transplant who had pancreas allograft function 1 year after transplantation had significantly greater patient survival at 7 years (89%) compared with SPK recipients who lost their pancreas transplant within the first year after transplantation (74%) or to those recipients that underwent a kidney transplant alone from either a living donor (80%) or a deceased donor (65%).
Although the survival benefit for SPK recipients is clear, the survival benefit for PAK and PTA recipients has not been conclusively demonstrated (Sutherland et al, 2009). The same analysis by Weiss demonstrates improved patient survival at 5 years for diabetic recipients who underwent a PAK compared with kidney transplantation alone, although this did not reach statistical significance and was subject to selection bias. A recent single-center report demonstrates excellent outcomes 3 years after PAK, in which both patient and graft survival are similar to SPK recipients at that center (Fridell et al, 2009). In this report, PAK recipients had a 90% pancreas allograft, 92% kidney allograft, and a 92% patient survival 3 years after PAK transplantation, compared with 83% pancreas allograft, 86% kidney allograft, and 88% patient survival in SPK recipients. If these excellent PAK outcomes persist with further follow-up and can be replicated at other centers, it is reasonable to assume that a survival benefit to PAK could be demonstrated in future data analyses.
Pancreatic Islet Transplantation
History and Early Results
The original descriptions in rodents of successful islet isolation (Lacy & Kostianovsky, 1967) and subsequent transplantation (Ballinger & Lacy, 1972) caused great excitement in the medical community, in the hope that this type of therapy might be applied to patients with type 1 diabetes. Although the ability to obtain normal glucose control in diabetic rodents with an islet transplant was described decades ago (Reckard et al, 1973), translating this success to humans has been more difficult. Various centers worldwide have attempted human islet transplantation, starting in 1974, with 445 recipients receiving islets between 1974 and 2000. The vast majority of these recipients also received a kidney transplant, either prior to or at the same time as the islet transplant. Analysis of the reported cases between 1990 and 2000 demonstrated that only 19% of patients were off insulin for more than 1 week, and at 1-year follow-up, only 11% of recipients were insulin independent (Brendel et al, 2001).
These discouraging results were attributed to a number of factors, including the possibility that recurrent autoimmunity was causing progressive islet damage following transplantation, because the diabetes in these recipients was the autoimmune form. Support for this hypothesis came from a series of patients at the University of Pittsburgh who underwent upper abdominal exenteration, including total pancreatectomy, followed by liver and islet allotransplantation. In this group of 11 patients, who did not have autoimmune diabetes, 6 (55%) exhibited sustained insulin independence, a success rate far greater than what had been achieved in type 1 diabetic recipients (Carroll et al, 1995).
Islet Autotransplantation
Although the overall experience is limited, the success rate in recipients of autotransplantation has been far greater than in those who received allogeneic islets for the treatment of type 1 diabetes. The largest reported series is from the University of Minnesota, where between the years 1977 and 2007, 173 patients with chronic pancreatitis underwent total, near total (95%), or completion pancreatectomy with islet autotransplantation (Sutherland et al, 2008). These investigators achieved a 65% rate of graft function and a 32% rate of insulin independence, rates of success that correlate closely with the average yield of islets from the removed pancreatic tissue following digestion and processing. This group previously reported higher rates of successful islet autotransplantation in patients who had no prior surgery, or only a prior Whipple procedure, whereas those that had a prior Puestow procedure or distal pancreatectomy had lower chances of success (Gruessner et al, 2004b). Similar results have been reported by another group in a series of 22 patients, where 68% of patients showed acceptable graft function, and 41% were insulin independent (Rodriguez Rilo et al, 2003). Interestingly, both of these groups infused unpurified pancreatic digests, where the islets had not undergone the additional purification step performed during deceased-donor pancreas processing for allotransplantation.
The Edmonton Protocol and Beyond
Interest in islet transplantation was piqued by the Edmonton investigators reporting that, for the first time, diabetes in patients could be consistently reversed by transplantation of isolated pancreatic islets (Shapiro et al, 2000). The Edmonton approach relied on a novel immunosuppression regimen that completely avoided steroids and that utilized a unique combination of induction therapy with an anti–interleukin (IL)-2 receptor antibody and maintenance therapy with sirolimus and tacrolimus. The rationale for this combination was based on the desire to avoid agents with known β-cell toxicity. Although this particular drug combination was likely an important factor in the trial’s success, perhaps of even greater impact was the large number of islets that patients received; these investigators infused a larger number of islets than were administered in prior islet transplantation trials, using two or three infusions per patient, acquired from multiple deceased donors.
Whereas previous trials in the late 1990s had often achieved partial success with single infusions of islets, as evidenced by a reduced insulin requirement and increased C-peptide levels, in no trial had the further step been taken to retransplant such patients to increase the net islet mass (Hering & Ricordi, 1999). In the Edmonton series, patients who showed evidence of partial success from the first infusion received an additional infusion, sometimes two, until they had accumulated a sufficient islet mass to gain insulin independence; in general, this occurred when a threshold of 8000 to 10,000 islets per kilogram of recipient body weight had been infused.
After the landmark report by the Edmonton investigators, almost 500 recipients received islet transplants at one of approximately 40 centers worldwide (Harlan et al, 2009). In the first concerted effort to replicate the provocative results of the Edmonton Protocol, a multicenter trial sponsored by the Immune Tolerance Network enlisted 10 centers in North America and Europe to each perform four islet transplants using the Edmonton regimen (Shapiro et al, 2006). Despite a diligent attempt to ensure uniformity in technique between centers, the rate of success varied dramatically, depending on the extent of experience at the transplanting site: at the three most experienced centers, reversal of hyperglycemia was routinely achieved; however, less experienced teams gained insulin independence in only a fraction of cases (approximately 20%). Thus, although these results confirmed the efficacy of Edmonton’s approach, they also revealed the exacting nature of the technique and demonstrated the inherent difficulty in replicating an identical protocol at all centers.
A number of single-center reports have now validated the Edmonton Protocol (Goss et al, 2002; Markmann et al, 2003), and although this protocol represents a tremendous leap forward for the field, the Edmonton results also delineate the major problem that remains: the need for islets procured from multiple deceased donors to gain insulin independence in a single recipient. This requirement markedly increases costs and represents a distinct disadvantage when compared with whole-organ transplantation; however, it should be remembered that, thus far, the pancreata utilized for isolated islet transplantation have only been organs that were unsuitable for whole-organ transplantation. It seems reasonable to assume that these organs are inferior to those used in whole-organ transplantation, and that improved results may be achieved if islet transplantation candidates had access to the best organs.
The need for such a large number of islets to achieve insulin independence in clinical transplantation was unexpected in light of the finding that as little as 10% of the total islet mass can maintain normoglycemia following partial pancreatectomy. Because the total islet mass in normal individuals is estimated at 1 to 2 million, it would follow that transplanting only 200,000 islets should regain normoglycemia in the majority of diabetic recipients; instead, accumulating evidence from the islet transplant studies by Edmonton and others suggest that for an average individual, 600 to 700,000 islets are required. The nearly two-thirds loss of potency after transplantation has not been fully explained in patients, but experimental data from small animal studies indicates that the majority of transplanted islets fail to engraft (Davalli et al, 1995). Understanding and overcoming the loss of islet mass during the engraftment phase has been an important focus of recent islet transplantation clinical trials.
Some centers have reported improved success at gaining insulin independence with islets from a single donor by refining the Edmonton approach (Hering et al, 2004, 2005; Markmann et al, 2003). The most successful reports are from the University of Minnesota, where investigators have used potent induction immunosuppression and high-quality islets. In their first study, four of six consecutive patients were rendered insulin independent with single-donor islet infusions using induction therapy with the anti-CD3 monoclonal antibody hOKT3γ1 (alanine-alanine) (Hering et al, 2004). This was combined with the selection of relatively larger donors and somewhat smaller recipients with low daily insulin requirements, thus maximizing the islet mass delivered per recipient body weight.
An even more impressive result was reported by the same group using polyclonal antithymocyte globulin (Thymoglobulin) for induction therapy; in a series of 10 consecutive patients treated with Thymoglobulin induction therapy, 9 were rendered insulin independent with a single infusion of islets (Hering et al, 2005). The 1 patient who did not become insulin independent still had a marked reduction in daily insulin requirement. Collectively, these studies suggest that insulin independence can be achieved consistently with single-donor islet infusions under the correct circumstances. Overcoming the single-donor hurdle will help in the establishment of isolated islet transplantation as a more widely applicable therapy for patients with type 1 diabetes.
A second, more daunting obstacle is the realization that the durability of function after islet transplantation is less than whole-organ pancreas grafts. Whereas it is clear that very experienced islet centers can gain insulin independence in most islet recipients, and that short-term (1-year) insulin-free survival may be close to that seen with whole-organ transplants, the intermediate (2- to 4-year) insulin-free survival is less encouraging. In a report by Edmonton investigators, with follow-up on patients in their ongoing series transplants of islets alone (Ryan et al, 2005), fewer than 25% of patients remained free from exogenous insulin administration at 3 years (Fig. 101.4B). Although a somewhat disappointing result, more encouraging was the observation that more than 85% of these patients had evidence of some islet graft function by C-peptide measurement (Fig. 101.4A). Similar findings of declining islet function over time have been reported by the multicenter Collaborative Islet Transplant Registry: of the recipients who achieved insulin independence, approximately 50% were back on insulin 2 years later (Alejandro et al, 2008).
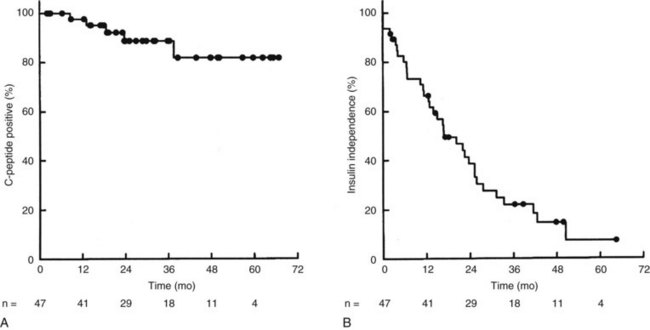
FIGURE 101.4 Islet graft survival rates following isolated islet transplantation at the University of Alberta, Edmonton (Ryan et al, 2005). A, Survival analysis for islet function as documented by C-peptide secretion over time, demonstrating 85% graft survival at 3 years. B, Survival analysis for insulin independence over time, demonstrating that fewer than 25% of patients are free from exogenous insulin therapy at 3 years.
(From Ryan EA, et al, 2005: Five-year follow-up after clinical islet transplantation. Diabetes 54:2060-2069. Copyright 2005 American Diabetes Association.)
Despite the eventual need for most recipients to resume doses of insulin, the partial graft function that persists is relevant, because many patients who were transplanted with the indication of hypoglycemia unawareness remain cured of this devastating complication. It is also evident that a partially functioning graft can significantly improve glycemic control as measured by serial hemoglobin A1c determinations. A small group of islet recipients who have resumed insulin therapy have been given supplemental islet infusions several years after the initial islet transplant and have regained durable insulin independence in most cases (Koh et al, 2010).
The mechanisms responsible for the gradual loss of islet function remain unclear. One hypothesis is that inadequate control of the deleterious alloimmune and autoimmune responses compromise islet function over time. The absence of a reliable measure of an antiislet immune reaction and the difficulty in obtaining biopsy tissue for pathologic diagnosis contribute to the complexity in clarifying this issue. One study reported durable insulin independence (>3 years) in islet recipients when more potent induction immunosuppression was used, compared with what was used in the Edmonton Protocol (Bellin et al, 2008). An alternative explanation is that the currently used immunosuppressive agents are toxic to the islets over time. This explanation may fit better with the pace of graft dysfunction and the fact that graft function often stabilizes without antirejection therapy. In addition, studies have documented very high immunosuppressive drug levels in the portal circulation, an expected consequence of the drugs being administered orally (Desai et al, 2003). The role of immune responses and medication toxicity to the loss of islet graft function should be clarified by the application of newer immunosuppressive agents in the next generation of islet transplantation studies.
Complications and Risk/Benefit Considerations
The demonstration that the procedure is relatively safe for the recipient has been important in the early trials of isolated islet transplantation. The most feared potential complications include bleeding and portal vein thrombosis. The Edmonton experience in 65 patients demonstrates that bleeding occurred in more than 20% of recipients, most of whom required either transfusion or surgical intervention. Main portal vein thrombosis, the most potentially troublesome complication of portal islet infusion, has fortunately been rare (<1%); however, in the Edmonton experience, 7% of recipients had evidence of segmental portal vein thrombus that resolved without sequelae (Ryan et al, 2005).
The risk of bleeding and thrombosis may be related in part to the technique used to perform the infusion. The procedure can be performed by an interventional radiologist, gaining access to the portal vein via a percutaneously placed transhepatic catheter and releasing the islets into the portal system (Froud et al, 2004). Alternatively, the procedure can be performed via a small laparotomy to gain access to a small mesenteric vein, through which a catheter can be advanced into the main portal vein for islet infusion (Gaber et al, 2004). The small vein is then ligated, eliminating the potential for hemorrhage. Thus the operative approach allows anticoagulation to be administered without the risk of bleeding from a puncture site in the hepatic parenchyma, although it usually requires a general anesthetic.
The relative safety advantage of islet transplantation over whole-organ pancreas transplantation was clearly evident in a comparison of the two approaches (Frank et al, 2004). In this study, major complications that included bleeding that required transfusion and reoperation were more common in the whole-organ group, although a number of minor complications were frequent in the islet transplant group. Some of these minor complications included immunosuppression-related toxicity (mouth ulcerations, edema), periportal hepatic steatosis, and mild liver function test abnormalities. A comparison of the major advantages and disadvantages of whole-organ pancreas and isolated islet transplantation can be seen in Table 101.1.
Table 101.1 Comparison of Whole Pancreas and Islet Transplantation
| Whole Pancreas Transplantation | Islet Transplantation | |
|---|---|---|
| Indication | Diabetic patients with renal failure (92% of cases) | Diabetic patients with hypoglycemic unawareness |
| Availability | Widely available | Research protocols only |
| Procedure | Major abdominal operation under general anesthesia | Percutaneous or “mini-lap” approach |
| Metabolic control | Normal | Good on a diabetic diet |
| Longevity of transplant | 79% function at 3 years (SPK) | 85% function at 3 years but <25% insulin free |
| Effect on secondary complications of diabetes | Stabilization and improvement | Unknown |
SPK, simultaneous pancreas and kidney transplantation
One of the most common and concerning potential complications of islet transplantation using the Edmonton protocol is calcineurin inhibitor–associated nephrotoxicity. Chronic renal failure in the setting of nonrenal solid-organ transplantation is well described, with an approximate 20% incidence at 10 years (Ojo et al, 2003). Similar results in islet transplant recipients would represent a particularly morbid complication given the well-established detrimental survival impact of renal failure in the diabetic population (Allen & Walker, 2003). A recent report of renal function in islet transplant recipients indicates that renal function remained stable in an appropriately selected and managed cohort of patients despite their being placed on potentially nephrotoxic immunosuppressive medications (Leitao et al, 2009). This study demonstrates that renal function can be adequately preserved in islet recipients under the correct circumstances.
Because of the side effects of chronic immunosuppression, the benefit of isolated islet transplantation, like that of isolated pancreas transplantation, may be for only select individuals. For this reason, most islet trials to date have enrolled type 1 diabetic participants with the most labile and dangerous form of the disease, specifically, diabetics attended by frequent episodes of hypoglycemia unawareness; patients are thought to be at greatest risk of severe morbidity or death from these events. Islet transplantation, even if only partially successful, has been universally found to be highly effective in reducing the frequency of these hypoglycemic episodes (Alejandro et al, 2008).
An alternative strategy has been to select recipients who are already on immunosuppressive therapy to support another organ transplant, such as a kidney (Kaufman et al, 2002). In this situation, the additional risk to the recipient relates mainly to the islet infusion procedure itself, which should be quite safe. In this setting, recipients usually lack severe hypoglycemia unawareness, although glucose control is usually suboptimal, it can be expected to improve following islet transplantation. An additional major concern in patients receiving an islet after kidney (IAK) transplantation is the function of the lifesaving renal allograft that is already in place. The experience to date suggests that IAK transplantation can be performed without jeopardizing the kidney graft. An important study in this area, although not conducted in a randomized manner, found evidence that IAK transplantation is not only safe, but it likely has a beneficial impact on renal allograft function (Fiorina et al, 2003). They studied 36 IAK recipients and stratified them based on the success of the islet transplant at 1 year, defined by continued C-peptide production. There were 24 successful cases and 12 unsuccessful cases; the successful IAK recipients demonstrated superior renal graft survival up to 7 years after islet transplantation, and the unsuccessful islet recipients developed increased microalbuminuria over time, likely reflecting diabetic damage in the transplanted kidney.
In addition, successful IAK recipients had improved cardiovascular function in terms of ejection fraction and degree of diastolic dysfunction when compared with kidney-only recipients (Fiorina et al, 2005). Finally, an improvement in patient survival was noted in successful IAK recipients compared with recipients with failed IAK transplantation and with diabetic recipients who had undergone kidney transplantation alone. A major caveat to these findings is that they are derived from a small number of IAK patients, therefore they must be substantiated in larger cohorts. In addition, these results are not based on recipients randomized to either treatment arm, thus any benefit in secondary complications attributable to IAK transplantation is subject to selection bias and requires further investigation in properly controlled studies.
Future Directions in Islet Transplantation
There are many areas of active investigation in clinical and experimental islet transplantation, including an important set of collaborative studies being supported by the National Institutes of Health. These trials have the opportunity to secure a place for islet transplantation as a standard therapeutic modality. For isolated islet transplantation to gain equal footing with or to surpass whole-organ pancreas transplantation as the preferred therapy for patients with diabetes, a number of conditions will need to be met. First, improvements must occur so that reversal of diabetes is readily accomplished with the islets from a single donor in the majority of cases. This will require not only advances in isolation techniques that allow greater recovery of healthy islet tissue, it will also require a greater understanding of the events early after transplantation that are responsible for engraftment of only a fraction of the delivered islet mass (Harlan et al, 2009). Second, clarification is needed of the immunologic and physiologic mechanisms that contribute to islet dysfunction over time that lead to the need for reinstitution of low doses of exogenous insulin in a majority of islet transplant subjects.
Finally, should the hurdles mentioned above be overcome, the reliance on deceased donors as the sole source of islets will be insufficient to treat the large number of patients with type 1 diabetes who could benefit from islet transplantation. Living donors are one alternate source of islet tissue as shown in a report of living donor islet transplantation from Japan (Matsumoto et al, 2005); however, the potential for donor morbidity makes this approach highly controversial, until the long-term durability and efficacy of islet transplantation has been firmly established. Ultimately, derivation of β-cells from xenogeneic or stem cell sources promises to provide a limitless supply of transplantable β-cells. The ongoing trials of islet transplantation will provide a critical foundation for these future therapies by defining the optimal site for implantation, the best means to monitor survival, and the most conducive immunosuppression that avoids autoimmune and alloimmune damage without pharmacologic β-cell toxicity.
Alejandro R, et al. 2008 update from the Collaborative Islet Transplant Registry. Transplantation. 2008;86:1783-1788.
Allen KV, Walker JD. Microalbuminuria and mortality in long-duration type 1 diabetes. Diabetes Care. 2003;26:2389-2391.
Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221-229.
Axelrod DA, et al. Kidney and pancreas transplantation in the United States, 1999-2008: the changing face of living donation. Am J Transplant. 2010;10:987-1002.
Ballinger WF, Lacy PE. Transplantation of intact pancreatic islets in rats. Surgery. 1972;72:175-177.
Bellin MD, et al. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am J Transplant. 2008;8:2463-2470.
Brendel MD, et al. International islet transplant registry newsletter #9. ITR Newsletter. 2001;8:1-20.
Carroll PB, et al. Long-term (>3-year) insulin independence in a patient with pancreatic islet cell transplantation following upper abdominal exenteration and liver replacement for fibrolamellar hepatocellular carcinoma. Transplantation. 1995;59:875-879.
Davalli AM, et al. A selective decrease in the beta cell mass of human islets transplanted into diabetic nude mice. Transplantation. 1995;59:817-820.
Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
Desai NM, et al. Elevated portal vein drug levels of sirolimus and tacrolimus in islet transplant recipients: local immunosuppression or islet toxicity? Transplantation. 2003;76:1623-1625.
Fioretto P, et al. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339:115-117.
Fiorina P, et al. Islet transplantation is associated with improvement of renal function among uremic patients with type I diabetes mellitus and kidney transplants. J Am Soc Nephrol. 2003;14:2150-2158.
Fiorina P, et al. Islet transplantation is associated with an improvement of cardiovascular function in type 1 diabetic kidney transplant patients. Diabetes Care. 2005;28:1358-1365.
Frank A, et al. Transplantation for type 1 diabetes: comparison of vascularized whole-organ pancreas with isolated pancreatic islets. Ann Surg. 2004;240:631-643.
Fridell JA, et al. The case for pancreas after kidney transplantation. Clin Transplant. 2009;23:447-453.
Froud T, et al. Use of D-Stat to prevent bleeding following percutaneous transhepatic intraportal islet transplantation. Cell Transplant. 2004;13:55-59.
Gaber AO, et al. Results of pancreas transplantation with portal venous and enteric drainage. Ann Surg. 1995;221:613-624.
Gaber AO, et al. Insulin independence achieved using the transmesenteric approach to the portal vein for islet transplantation. Transplantation. 2004;77:309-311.
Goss JA, et al. Achievement of insulin independence in three consecutive type-1 diabetic patients via pancreatic islet transplantation using islets isolated at a remote islet isolation center. Transplantation. 2002;74:1761-1766.
Gruessner RWG, Sutherland DER, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant. 2004;4:2018-2026.
Gruessner RWG, et al. Transplant options for patients undergoing total pancreatectomy for chronic pancreatitis. J Am Coll Surg. 2004;198:559-569.
Harlan DM, et al. Current advances and travails in islet transplantation. Diabetes. 2009;58:2177-2184.
Hering BJ, Ricordi C. Islet transplantation for patients with type 1 diabetes: results, research priorities and reasons for optimism. Graft. 1999;2:12-27.
Hering BJ, et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant. 2004;4:390-401.
Hering BJ, et al. Single donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293:830-835.
Humar A, et al. Decreased surgical risks of pancreas transplantation in the modern era. Ann Surg. 2000;231:269-275.
Kaufman DB, et al. Sequential kidney/islet transplantation using prednisone-free immunosuppression. Am J Transplant. 2002;2:674-677.
Kelly WD, et al. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery. 1967;61:827-837.
Koh A, et al. Supplemental islet infusions restore insulin independence after graft dysfunction in islet transplant recipients. Transplantation. 2010;89:361-365.
Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16:35-39.
Leitao CB, et al. Stable renal function after islet transplantation: importance of patient selection and aggressive clinical management. Transplantation. 2009;87:681-688.
Markmann JF, et al. Insulin independence following isolated islet transplantation and single islet infusions. Ann Surg. 2003;237:741-749.
Matsumoto S, et al. Insulin independence after living-donor distal pancreatectomy and islet allotransplantation. Lancet. 2005;365:1642-1644.
Ojo AO, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931-940.
Rayhill SC, et al. Simultaneous pancreas-kidney transplantation and living related donor renal transplantation in patients with diabetes: is there a difference in survival? Ann Surg. 2000;231:417-423.
Reckard CR, Ziegler MM, Barker CF. Physiological and immunological consequences of transplanting isolated pancreatic islets. Surgery. 1973;74:91-99.
Rodriguez Rilo HL, et al. Total pancreatectomy and autologous islet cell transplantation as a means to treat severe chronic pancreatitis. J Gastrointest Surg. 2003;7:978-989.
Ryan EA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060-2069.
Shapiro AM, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318-1330.
Shapiro AMJ, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230-238.
Stratta RJ. Surgical nuances in pancreas transplantation. Transplant Proc. 2005;37:1291-1293.
Sutherland DER, Gruessner AC, Radosevich DM. Kidney or kidney-pancreas transplant for the uremic diabetic? Nat Rev Nephrol. 2009;5:554-556.
Sutherland DER, et al. Islet autotransplant outcomes after total pancreatectomy: a contrast to islet allograft outcomes. Transplantation. 2008;86:1799-1802.
Venstrom JM, et al. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA. 2003;290:2817-2823.
Weiss AS, Smits G, Wiseman AC. Twelve-month pancreas graft function significantly influences survival following simultaneous pancreas-kidney transplantation. Clin J Am Soc Nephrol. 2009;4:988-995.
Williams PW. Notes on diabetes treated with extract and by grafts of sheep’s pancreas. Br Med J. 1894;2:1303-1304.
Wolfe RA, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725-1730.