Chapter 32 Water and Electrolyte Disturbances
Disturbances in water and electrolytes are common in the intensive care unit (ICU). In the first section of this chapter the physiology of water balance, including the distribution of intravenous fluids and the effects of cardiopulmonary bypass (CPB), are briefly reviewed. In the second section, clinical abnormalities of water and electrolytes are discussed. Common terms are explained in Table 32-1. Relevant physiology discussed in Chapter 1 is referred to throughout.
Table 32-1 Definitions of Terms
| Osmosis | The movement of water caused by a concentration difference in the water on each side of a semipermeable membrane |
| Osmotic pressure | The hydrostatic pressure that must be applied to stop osmosis |
| Osmole | The number of molecules present in 1 g of a substance, multiplied by its molecular weight (of undissociated solute) |
| Performing this calculation allows the concentration of a solute to be expressed in terms of the number of molecules. For example, if 60 g urea ([NH2]2CO, molecular weight = 60 g) is dissolved in 1 kg of water, the osmolality of the solution is 1 osmole per kg. For a substance that fully dissociates (e.g., NaCl), if the molecular weight (in grams) is dissolved in 1 kg of solute, the concentration is 2 osmoles per kg. | |
| Osmolality | The number of osmoles per kilogram of water |
| Osmolarity | The number of osmoles per (total) liter of solution |
| When solute is dissolved in solvent, the volume is greater than the volume of the solvent alone. | |
| In dilute solutions with small solute molecules, osmolarity is (approximately) equal to osmolality. | |
| In concentrated solutions of large solvent molecules, osmolality is greater than osmolarity. | |
| Tonicity | The “effective” osmolality |
| Solutes that are distributed evenly across semipermeable membranes do not exert osmotic pressure. | |
| Solutes that are restricted in their movement across compartments are “effective osmoles,” and tonicity describes their concentration. |
PHYSIOLOGY
Body Water: Distribution, Constituents, and Movement
The intracellular and interstitial (extracellular) compartments are separated by cellular membranes. Water distributes freely between these two compartments according to the osmotic pressure difference across cellular membranes. At equilibrium, the number of osmotically active particles inside and outside cells is equal (about 285 mOsm/l). However, the constituents of the intracellular and extracellular fluids are quite different (Table 32-2). Some substances diffuse freely across cell membranes (e.g., oxygen, carbon dioxide, urea) and are therefore at the same concentration inside and outside cells. The movement of other substances (notably ions and glucose) across cell membranes is tightly controlled by selective permeability and active transport, which results in different concentrations inside and outside cells (see Table 32-2). The major cation in the extracellular fluid is sodium, and under normal circumstances it is the primary determinant of plasma osmolarity. The major anions in the extracellular fluid are chloride and bicarbonate. Because ions carry positive and negative charges, the active transport and selective permeability of ions allows variable charge separation to occur across the cell membrane, leading to the formation of resting and active membrane potentials (see Chapter 1).
Table 32-2 Composition of Intracellular and Extracellular Fluid
| Electrolyte | Intracellular Fluid (mmol/l) | Extracellular Fluid* (mmol/l) |
|---|---|---|
| Sodium | 10 | 140 |
| Potassium | 155 | 3.8 |
| Chloride | 3 | 108 |
| Bicarbonate | 10 | 26 |
| Calcium (ionized) | <0.01 | 1.2 |
| Magnesium | 10 | 0.8 |
| Phosphate | 105 | 1.0 |
* Because the plasma contains highly negatively charged protein molecules, minor differences exist between the electrolyte content of the interstitial fluid and the plasma.
In addition to diffusion and osmosis, there is mass movement (filtration) of fluid between the capillary and the interstitium based on the balance of Starling forces across the capillary wall. The Starling forces are composed of the hydrostatic and the oncotic pressure gradients between the capillary lumen and the interstitial space (see Equation 1-12). The balance of the Starling forces varies among capillary beds, but overall there is a net loss of fluid from plasma to the interstitium. Filtered fluid is returned to the plasma through the lymphatic system.
Intravenous Fluids
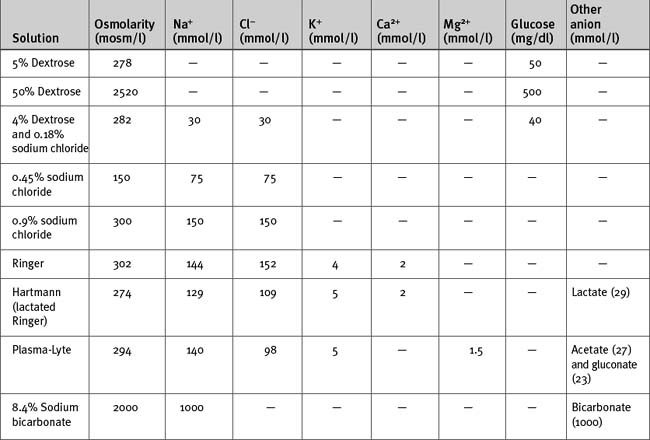
Intravenous fluids may be crystalloids (Table 32-3) or colloids. They are used mainly for treating hypovolemia (resuscitation fluid) and for replacement of obligatory daily losses (maintenance fluid). They are administered intravenously (i.e., into the plasma space) and, depending on their constituents and tonicity, the water is distributed to a variable extent throughout the extracellular and intracellular compartments.
Crystalloids
The anion in sodium-based crystalloids is predominantly chloride. Chloride is the only anion in 0.9% sodium chloride and Ringer solution and is therefore present in supraphysiologic concentrations. Large volumes of these solutions can cause a hyperchloremic metabolic acidosis (see Chapter 31). More physiologic concentrations of chloride are present in buffered, balanced electrolyte solutions such as Hartmann (lactated Ringer) and Plasma-Lyte. Because bicarbonate is not stable in solution for long periods, these solutions contain lactate (Hartmann) or acetate and gluconate (Plasma-Lyte) as the nonchloride anion.
Hypotonic sodium solutions reduce the tonicity of the extracellular fluid and therefore, by osmosis, some water will be distributed to the intracellular space. For instance, with 0.45% sodium chloride, which has an osmolarity of 150 mOsm/l, about half of the water will be distributed to the intracellular compartment. In contrast, hypertonic sodium solutions increase the tonicity of the extracellular fluid, and “drag” water from the intracellular compartment by osmosis. Thus, the extracellular compartment will expand by a volume greater than the volume administered. Hypotonic and hypertonic solutions are used in special circumstances. For instance, 0.45% sodium chloride is used to replace hypotonic renal losses during the polyuric phase of acute renal failure, whereas hypertonic sodium chloride is used to reduce intracranial pressure in patients with cerebral edema. Rapid administration of large volumes of hypotonic solutions (e.g., pure water) into a peripheral vein (where flow may be sluggish) may cause osmotically mediated swelling—and potential lysis—of red blood cells. Ideally these solutions should be administered slowly via a central line.
Colloids
Colloid solutions contain large, oncotically active molecules in a base solution of either 0.9% sodium chloride or a buffered, balanced electrolyte solution. Colloid molecules are too big to traverse gap junctions, so more of the water in these solutions tends to be retained within the plasma space. Theoretically, assuming a plasma volume of 3 liters in a 70-kg patient, 1 liter of an isooncotic colloid solution increases plasma volume by 1 liter, which is 4 to 5 times the plasma volume expansion achieved by the same volume of an isotonic sodium-based crystalloid. However, with critical illness, the vascular endothelium “leaks,” allowing colloid molecules to pass into the interstitium, where they exert an osmotic pressure effect. This probably explains the observation that when resuscitating critically ill patients with 0.9% sodium chloride only 1.3 times the volume is required (not four times as predicted) compared with 4% albumin to achieve the same hemodynamic end points.1
Commonly used natural colloids include albumin and fresh-frozen plasma. Albumin is available as 4%, 5%, and 20% preparations. Both 4% and 5% solutions are approximately isooncotic with plasma; 20% albumin is hyperoncotic and therefore expands the plasma volume by about four times its volume. Commonly used artificial colloids include modified gelatins (e.g., Gelofusine) and hydroxyethyl starch compounds. Hydroxyethyl starch comprises a family of colloids that are categorized on the basis of their average size into high (>400 kD), medium (200 kD), and low (70 kD) molecular weight preparations.2 Two commonly used hydroxyethyl starches are hetastarch (average molecular weight 480 kD) and pentastarch (average molecular weight 200 kD). By comparison, Gelofusine has an average molecular weight of about 35 kD. Most artificial colloids are isooncotic or slightly hyperoncotic. The plasma half-time of artificial colloids varies among preparations but is typically on the order of 4 to 6 hours. Thus, the colloid effect has largely dissipated by 24 hours. Large volumes of hydroxyethyl starch can cause impaired hemostasis, mainly because of reduced effectiveness of the factor VIII/von Willebrand factor complex (i.e., acquired von Willebrand disease).2 High molecular weight compounds such as hetastarch appear to cause greater impairment of hemostasis than do medium-sized compounds such as pentastarch.3,4 Impaired hemostasis may also occur with gelatin-based colloids.5 To avoid hemostatic problems, the dosages of these artificial colloids should be kept below 20 ml/kg. All artificial colloids can cause allergic (including anaphylactic) reactions.
Crystalloids Versus Colloids for Fluid Resuscitation
There has been a great deal of debate over many decades on the pros and cons of crystalloids versus colloids for fluid resuscitation. Some ICUs use predominantly colloids, others predominantly crystalloids. Colloids are popular in Europe, whereas crystalloids are popular in North America. However, as long as fluids are administered to appropriate physiologic end points the choice between a colloid and a crystalloid is not important.1,6 In view of the additional cost and potential for adverse events with artificial colloids, a primarily crystalloid-based fluid regime seems preferable.
In 1998, a metaanalysis found an apparent excess mortality rate associated with the use of albumin in critically ill patients.7 This and a subsequent analysis by the same group8 created a storm of controversy and led to the publication of other metaanalyses that did not support the original finding.9,10 The issue of the safety of albumin was resolved in 2004 with the publication of a prospective, randomized trial involving nearly 7000 patients that found no difference in mortality rates between patients resuscitated with 4% albumin and those given 0.9% sodium chloride.1
Regulation of the Osmolarity and Volume of the Extracellular Compartment
Regulation of Water Balance
In addition to increased osmolarity, antidiuretic hormone secretion is also stimulated by hypotension and hypovolemia. In the presence of two competing pathophysiologic states—hypovolemia and low tonicity— circulating volume is defended over tonicity.
Regulation of Sodium Balance
Reduced circulating volume (e.g., due to hemorrhage) leads to the renal retention of sodium via the renin-angiotensin-aldosterone system (see Chapter 1). A change in the volume of the plasma is also sensed by arterial baroreceptors and atrial stretch receptors. This leads to a reduction in the release of the natriuretic peptides, which also promotes a reduction in renal sodium loss. Both of these mechanisms cause an increase in plasma osmolarity which, under the influence of antidiuretic hormone, promotes water retention. Thus, sodium and water are retained together and the volume of the extracellular fluid is determined by sodium balance.
Effects of Cardiopulmonary Bypass and Cardiac Surgery
Patients undergoing CPB have an obligatory fluid load related to the bypass circuit prime volume—typically a total of 4 ml/kg of crystalloid, colloid, or both. In addition, several liters of colloid or crystalloid fluids may be given during the intraoperative and the early postoperative periods. This occurs for a number of reasons. First, fluid is needed to replace urinary and blood losses. Second, in patients with impaired cardiac function, fluid may be given to optimize cardiac performance. Third, third-space fluid loss occurs. Third-space loss is abnormal filtrative loss from the plasma to the interstitium. It occurs due to the perturbations in the Starling forces that accompany cardiac surgery. In particular, the systemic inflammatory response to CPB can result in arteriolar vasodilatation and leakiness of the capillary endothelium, which increases fluid filtration from plasma to the interstitium (see Chapters 1 and Chapter 2).
These effects—increased fluid administration, third-space losses, and excessive salt and water retention—cause a significant increase in total extracellular fluid volume during the early postoperative period. Weight gains of 7.4% on postoperative day 1 and of 6.6% on day 2 have been described following routine cardiac surgery.11 Third-space losses are far greater in critically ill patients with endothelial dysfunction.
CLINICAL ABNORMALITIES OF WATER AND ELECTROLYTES
Disorders of Sodium and Water Balance
Abnormal Extracellular Volume
Abnormalities of extracellular volume include abnormalities of plasma volume and total extracellular volume. Often the two compartments change together; thus, increased plasma volume usually coexists with expansion of the entire extracellular compartment. This is the case with heart failure. However, it is possible to have low plasma volume (i.e., hypovolemia) despite an expanded extracellular space. Hypovolemia is discussed in Chapter 20.
Expansion of the extracellular volume occurs because of abnormal sodium retention or excessive sodium administration. It causes systemic edema but not necessarily pulmonary edema. In cardiac surgery patients, systemic edema typically occurs in two situations: (1) with severe cardiac dysfunction, in which case plasma (circulating) volume is high; and (2) with marked systemic inflammation, in which case plasma volume may be low or normal.
Disorders of Sodium Concentration: Hyponatremia and Hypernatremia
In the cardiothoracic ICU, hyponatremia usually occurs in association with a low plasma osmolarity and represents a relative excess of water, whereas hypernatremia occurs in association with a high plasma osmolarity and represents a relative lack of water. The common causes of hyponatremia and hypernatremia are listed in Table 32-4 and Table 32-5, respectively.
| Water excess with normal or increased extracellular fluid volume |
| Excess administration of free water (e.g., intravenous |
| 5% dextrose) (SIADH) |
| • Surgery |
| • Nausea |
| • Acute respiratory failure |
| • Neoplasm |
| • Drugs (corticosteroids, opioids, major tranquillizers) |
| Renal failure |
| Heart failure |
| Hepatic failure |
| Hypothyroidism |
| Water excess with reduced extracellular fluid volume |
| Diuretics |
| Adrenal insufficiency |
| Hyperglycemia |
SIADH, syndrome of inappropriate antidiuretic hormone secretion. Note: All causes of hyponatremia are associated with reduced plasma osmolarity except hyperglycemia; see text for details.
Table 32-5 Causes of Hypernatremia
| Sodium excess (high extracellular fluid volume) |
| Administration of isotonic or hypertonic sodium chloride |
| Administration of sodium bicarbonate |
| Water loss (low extracellular fluid volume) |
| Polyuric phase of acute renal failure |
| High gastric or large bowel fluid losses |
| Sweating |
| Diuretics (loop, osmotic) |
| Diabetes insipidus: nephrogenic or central (see text for details) |
Note: All causes are associated with increased plasma osmolarity.
Hyponatremia.
Mild hyponatremia does not produce symptoms, but an acute fall in plasma sodium below 125 mmol/l can cause cerebral edema, which manifests as lethargy, headache, nausea, confusion, and eventually obtundation and seizures. The most common cause of hyponatremia in postoperative patients is excessive administration of low-sodium crystalloid solutions (i.e., 5% dextrose, dextrose-sodium chloride) in combination with the syndrome of inappropriate antidiuretic hormone (SIADH) secretion. Some degree of SIADH occurs in all postoperative patients due to the effects of surgical stress, pain, nausea, and drugs (morphine, corticosteroids). Postoperative SIADH is usually mild, but severe hyponatremia can develop due to SIADH resulting from other causes (e.g., certain infections and tumors). Urinary sodium tends to be higher with pure SIADH (>20 to 40 mmol/l) than with pure water intoxication (<10 mmol/l). Chronic treatment with diuretics can also cause hyponatremia if diuretic-induced sodium and water losses are replaced with sodium-poor fluid. This is often the situation in chronic diuretic use. Severe hypovolemia also induces antidiuretic hormone secretion because the body attempts to defend volume over tonicity, which further exacerbates hyponatremia.
For severe acute hyponatremia, hypertonic sodium chloride may be administered. However, rapid correction of hyponatremia can lead to acute pontine demyelination, which manifests as paraparesis, dysarthria, seizures, coma, and death. This risk is greatest with the rapid correction of severe, chronic hyponatremia. In patients with mild or no symptoms and severe hyponatremia, the plasma sodium concentration should be increased by no more than 8 mmol/l/day (0.3 mmol/l/hr).12 In patients with severe symptoms of hyponatremia (confusion, obtundation, and seizures), a maximum rate of correction of 1.5 to 2 mmol/l/hr for 3 to 4 hours is acceptable until symptoms abate; this is followed by minimal further increase in the ensuing 20 hours to ensure that acute pontine demyelination is not precipitated. However, should severe symptoms persist (e.g., coma, seizures) a rise of more than 8 mmol/l/day may be justified.12,13
Hypernatremia.
An important cause of hypernatremia is diabetes insipidus. Diabetes insipidus involves either a failure of production of antidiuretic hormone (central diabetes insipidus) or lack of renal responsiveness to antidiuretic hormone (nephrogenic diabetes insipidus). Central diabetes insipidus may occur in patients with neurologic injury or brain death (see Chapter 38). Nephrogenic diabetes insipidus may occur due to loss of the hypertonic medullary interstitium (see Chapter 1). This is the cause of the polyuria that accompanies renal failure and diuretic administration. Nephrogenic diabetes insipidus may also occur because of reduced responsiveness of the distal nephron to antidiuretic hormone; causes of this state include drugs (e.g., amphotericin B, aminoglycosides, and lithium), hypokalemia, and hypercalcemia. Patients with diabetes insipidus produce large volumes of very dilute urine (urinary osmolality <200 mosmol/l) which, if not replaced, causes hypovolemia.
The treatment of hypernatremia depends on the cause and on the patient’s intravascular volume status. With hypovolemia, initial resuscitation should include a balanced crystalloid solution such as Plasma-Lyte. Subsequent treatment should be directed at the relative water deficit. For ongoing losses of sodium-poor fluid, administration of half-normal sodium chloride (0.45% sodium chloride) or free water is appropriate. Half-normal sodium chloride is particularly useful in the management of the polyuric phase of acute renal failure. Because 0.45% sodium chloride is hypotonic, it should be given slowly or administered into a central vein to avoid hemolysis. Free water may be given enterally (as tap water added to enteral feeds) or intravenously as 5% dextrose. A dosage of between 50 and 150 ml/hr is appropriate in most circumstances. Treatment of central diabetes insipidus is described in Chapter 38.
Abnormalities of Potassium, Magnesium, Calcium, and Phosphate
Potassium
Virtually all of the potassium filtered at the glomerulus is reabsorbed, and renal control of potassium balance is determined by secretion in the distal nephron. The main determinants of potassium secretion are the plasma potassium concentration and aldosterone activity. Aldosterone causes the renal reabsorption of sodium in exchange for either potassium or hydrogen ions (see Chapter 31). Thus, mineralocorticoid excess (e.g., treatment with corticosteroids) can cause hypervolemia, hypokalemia, and metabolic alkalosis. The causes of hypokalemia and hyperkalemia in the cardiothoracic ICU are listed in Table 32-6 and Table 32-7, respectively.
| Increased urinary loss |
| Diuretics (loop, thiazide, osmotic) |
| Polyuria secondary to hypothermic CPB |
| Mineralocorticoid excess (e.g., Cushing syndrome, Conn syndrome, treatment with corticosteroids, renal artery stenosis) |
| Hypomagnesemia |
| Other drugs (e.g., amphotericin B) |
| Renal tubular acidosis (types 1 and 2) |
| Increased gastrointestinal loss |
| High nasogastric aspirates |
| Vomiting |
| Diarrhea or fistula loss |
| Potassium movement into cells |
| Alkalemia |
| Insulin |
| β2-adrenoceptor agonists (epinephrine, albuterol, isoproterenol) |
| Methylxanthines (aminophylline) |
| Periodic paralysis (hypokalemic form) |
| Other losses |
| Renal replacement therapy |
| Sweating |
CPB, cardiopulmonary bypass.
Table 32-7 Causes of Hyperkalemia
| Artefact (blood trauma during sampling) |
| Decreased potassium excretion |
| Chronic renal failure |
| Acute renal failure |
| Potassium-sparing diuretics |
| ACE inhibitors and angiotensin receptor blockers |
| Nonsteroidal antiinflammatory drugs |
| Cyclosporin (aldosterone resistance) |
| Adrenal dysfunction (Addison disease) |
| Renal tubular acidosis (type 4) |
| Periodic paralysis (hyperkalemic form) |
| Potassium movement out of cells |
| Cessation of insulin treatment |
| β blockers |
| Suxamethonium |
| Acidemia |
| Tissue necrosis or reperfusion |
| Digoxin toxicity |
| Rhabdomyolysis |
| Increased intake |
| Overcorrection of hypokalemia |
ACE, angiotensin-converting enzyme.
Hypokalemia.
Hypokalemia is very common during the early postoperative period and is associated with a range of cardiac arrhythmias, including atrial and ventricular premature beats, atrial fibrillation, and torsades de pointes ventricular tachycardia. On the resting electrocardiogram (ECG), hypokalemia produces characteristic changes related to delayed ventricular repolarization. Initially there is reduced amplitude of the T wave and an increase in the U wave; later there may be ST segment depression or T wave inversion with a marked increase in the U wave and prolongation of the QU interval.
Once patients have been extubated and are eating, oral potassium supplements may be given if required. In addition to hypokalemia, potassium supplementation may also be indicated for the treatment of metabolic alkalosis (see Chapter 31).
Hyperkalemia.
Unless caused by excessive potassium supplementation, hyperkalemia is uncommon in the first few hours following cardiac surgery. It usually develops in the context of acute renal dysfunction or abrupt cessation of insulin therapy. Even moderate (acute) renal dysfunction can precipitate life-threatening hyperkalemia when patients are treated with angiotensin-converting enzyme inhibitors, potassium-sparing diuretics, or β blockers or when insulin is stopped. In certain situations, massive hyperkalemia can occur with the administration of suxamethonium (see Table 4-6). Artefactual hyperkalemia may occur due to mechanical trauma to red blood cells during blood sampling. This cause should always be considered when the blood sample is difficult to obtain or no other cause is likely.
Treatment options for hyperkalemia and their mechanisms of action are listed in Table 32-8. For life-threatening hyperkalemia (i.e., an acute rise in potassium above 7 mmol/l), the following interventions are appropriate: (1) intravenous calcium chloride 5 mg/kg (0.035 mmol/kg); (2) intravenous sodium bicarbonate (50 mmol, given separately from calcium); (3) intravenous glucose (50 ml 50% dextrose) and insulin (15 units of a rapidly acting formulation); (4) modest hyperventilation (in intubated patients). For less severe forms of hyperkalemia (i.e., potassium >6.0 to 6.5 mmol/l), continuous nebulized albuterol (5 mg administered until the potassium falls below 6 mmol/l) combined with intravenous furosemide (40 mg) is safe and effective. If there are ECG changes indicative of hyperkalemia, calcium chloride should be given. Most treatments listed in Table 32-8 increase cellular uptake of potassium and therefore are only temporizing measures. Increased potassium removal is facilitated by diuretics, cation-exchange resins, and renal replacement therapy (see Chapter 33); if hyperkalemia is rapidly progressive and occurs in the context of acute renal failure, early institution of renal replacement therapy is indicated.
| Stabilization of the myocardium |
| Calcium |
| Increased potassium entry into cells |
| β2-adrenoceptor agonists (e.g., albuterol) |
| Insulin (with glucose) |
| Sodium bicarbonate |
| Modest hyperventilation/avoidance of hypercarbia |
| Removal of potassium |
| Loop diuretics |
| Cation-exchange resins (calcium resonium) |
| Renal replacement therapy |
Magnesium
The normal plasma magnesium concentration is 0.75 to 1.0 mmol/l (1.8 to 2.4 mg/dl). The majority of the magnesium present in the body is in cells and bone, with only 1% in the extracellular fluid. Magnesium balance is regulated primarily by renal excretion under the influence of parathyroid hormone and vitamin D. Diuretics, metabolic acidosis, volume expansion, and catecholamines all enhance renal magnesium loss. Thus, hypomagnesemia is common during the postoperative period. Hypomagnesemia can also occur with high gastrointestinal fluid losses, pancreatitis, and parenteral nutrition. Hypomagnesemia predisposes to cardiac arrhythmias both directly and by potentiating renal potassium loss. The ECG findings in hypomagnesemia are similar to those in hypokalemia. The use of magnesium as an antiarrhythmic drug is described in Chapters 3 and Chapter 21. Occasionally, very high plasma magnesium levels (2 to 3 mmol/l) are required for arrhythmia suppression. Hypermagnesemia typically occurs secondary to renal failure or drug administration. Side effects of hypermagnesemia include vasodilation, bradycardia, atrioventricular block, weakness, and marked augmentation of the effect of neuromuscular blocking drugs.
Calcium
Ionized hypocalcemia is common in the cardiothoracic ICU, and the causes are listed in Table 32-9. CPB is associated with heparin and protamine use and with major fluid shifts, all of which can cause hypocalcemia. Large-volume blood transfusion can cause hypocalcemia due to the binding of calcium by citrate. Alkalosis increases the binding of calcium to albumin, which results in a normal total calcium and ionized hypocalcemia. Alkalosis is an important cause of severe symptomatic ionized hypocalcemia. Sodium bicarbonate, used to treat non-anion-gap metabolic acidosis (see Chapter 31), can bind with calcium to cause ionized hypocalcemia. The reduced elimination of phosphate in chronic renal failure leads to hyperphosphatemia. This causes the formation of insoluble calcium phosphate salts, resulting in hypocalcemia (see Chapter 33). Hypomagnesemia inhibits secretion of parathyroid hormone, which causes secondary hypocalcemia. In this situation, the treatment is magnesium, not calcium.
| Binding with other substances |
| Alkalosis |
| Massive transfusion |
| Pancreatitis |
| Hyperphosphatemia |
| Drugs |
| Protamine |
| Heparin |
| Aminoglycosides |
| Phenytoin |
| Other |
| Sepsis |
| Hypomagnesemia (inhibition of parathyroid hormone secretion) |
| Cardiopulmonary bypass |
| Renal failure (due to hyperphosphatemia and low vitamin D) |
| Chronic liver disease |
Hypercalcemia is very uncommon in cardiac surgery patients and usually results from overtreatment of hypocalcemia. The most common cause of hypercalcemia is malignancy, particularly involving bone; occasionally, hypercalcemia occurs in patients with non-small cell lung cancer; it is caused by the ectopic secretion of parathyroid hormone (see Table 12-1).
Phosphorus
Inorganic phosphorus (PO42) is found predominantly inside cells, where it plays an important role in intermediary metabolism. The normal plasma concentration is 0.70 to 1.50 mmol/l (2.2 to 4.6 mg/dl). Hyperphosphatemia is usually due to renal failure or tissue necrosis. It may be an early marker of mesenteric ischemia (see Chapter 34). The main consequence of hyperphosphatemia is ionized hypocalcemia. Hypophosphatemia may arise because of high phosphate loss (due mainly to renal replacement therapy) or increased cellular uptake. Increased cellular uptake of phosphate occurs with (1) glucose loading (e.g., in parenteral nutrition)—particularly after a period of starvation (See Chapter 34); (2) treatment with β2-adrenoreceptor agonists; (3) respiratory alkalosis; (4) sepsis. Aluminum-containing antacids (e.g., sucralfate) inhibit the gastrointestinal absorption of phosphate and can cause hypophosphatemia. (Hyperphosphatemia due to renal failure may be treated with aluminum hydroxide gels so as to prevent bone disease.) The consequences of hypophosphatemia are unclear. It is usually clinically silent but, if severe, may contribute to muscle weakness and cardiac dysfunction. Treatment is with intravenous supplementation, either as sodium or potassium phosphate.
1 Finfer S, Bellomo R, Boyce N, et al. A comparison of albumin and sodium chloride for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247-2256.
2 Treib J, Baron JF, Grauer MT, et al. An international view of hydroxyethyl starches. Intens Care Med. 1999;25:258-268.
3 Roche AM, James MF, Bennett-Guerrero E, et al. A head-to-head comparison of the in vitro coagulation effects of sodium chloride-based and balanced electrolyte crystalloid and colloid intravenous fluids. Anesth Analg. 2006;102:1274-1279.
4 Strauss RG, Pennell BJ, Stump DC. A randomized, blinded trial comparing the hemostatic effects of pentastarch versus hetastarch. Transfusion. 2002;42:27-36.
5 Niemi TT, Suojaranta-Ylinen RT, Kukkonen SI, et al. Gelatin and hydroxyethyl starch, but not albumin, impair hemostasis after cardiac surgery. Anesth Analg. 2006;102:998-1006.
6 Roberts I, Alderson P, Bunn F, et al. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database of Systematic Reviews. 2004:CD 000567.
7 Cochrane Injuries Group Albumin Reviewers. Human albumin administration in critically ill patients: systematic review of randomised controlled trials. Cochrane Injuries Group Albumin Reviewers. BMJ. 1998;317:235-240.
8 Bunn F, Lefebvre C, Li Wan Po A, et al. Human albumin solution for resuscitation and volume expansion in critically ill patients. The Albumin Reviewers. Cochrane Database of Systematic Reviews. 2000:CD 001208.
9 Wilkes MM, Navickis RJ. Patient survival after human albumin administration: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2001;135:64-149.
10 Vincent JL, Navickis RJ, Wilkes MM. Morbidity in hospitalized patients receiving human albumin: a meta-analysis of randomized, controlled trials. Crit Care Med. 2004;32:2029-2038.
11 Larson SL, Schimmel CH, Shott S, et al. Influence of fast-track anesthetic technique on cardiovascular infusions and weight gain. J Cardiothorac Vasc Anesth. 1999;13:424-430.
12 Adrogue HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342:1581-1589.
13 Adrogue HJ. Consequences of inadequate management of hyponatremia. Am J Nephrol. 2005;25:240-249.