Chapter 30 Warming devices
Background
Physical principles
Heat generation
In the human body, the generation of energy is by chemical reaction and its quantity determined by the substrates and products of the reaction. Combustion of glucose and protein produces 4.1 kcal/kg, whereas fat provides 9.3 kcal/kg. Although heat generated in this way depends on the level of activity/metabolism, which is reduced under anaesthesia by 15–40%, most core hypothermia is the result of altered distribution of body heat rather than alterations in the balance of heat production and dissipation.
Devices used to prevent perioperative hypothermia
Passive devices
Although ordinary blankets, bedding and clothes prevent heat loss to some extent, they are not appropriate in the setting of the operating theatre where higher standards of cleanliness are required. The first products specifically designed for this setting were called ‘space’ blankets. These are made from a lightweight non-permeable material incorporating a reflective layer that reduces the patient’s radiant heat losses. The non-permeable element provides insulation from the operating theatre environment and reduces the convective heat losses. Their effectiveness is partly based on the high emissivity of heat from the human body. They also have the advantage that they meet the safety standards of ‘Flammable Fabrics’ Acts. However, for the majority of procedures, insulation alone is insufficient in preventing heat losses during anaesthesia, surgical preparation and subsequent surgery. There is, therefore, the need to provide heat from an external source.
Active devices
Circulating water devices
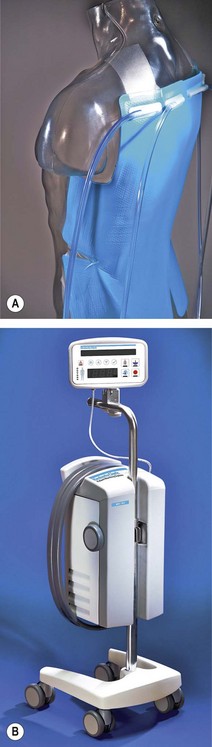
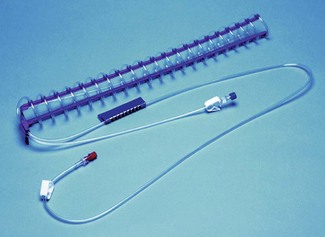
Newer devices overcome these problems by circulating the water through special garments or pads. They include the Kimberly-Clark Patient Warming System (Figs 30.1A and B), which uses adhesive ‘energy transfer’ pads with micro-channels for circulating water that can be applied to the back, thighs, chest, or any combination of the three, depending on the site of surgery. Another modern system is the Allon circulating-water garment. This conductive heating garment is divided into separate segments for arms and thighs, which allows clinicians to cover different body surfaces depending on the site of surgery. Perhaps unsurprisingly, given the different thermal characteristics of water and air, both the above have both been shown to be more efficient at warming volunteers than forced-air devices (see below).
Carbon fibre and polymer devices
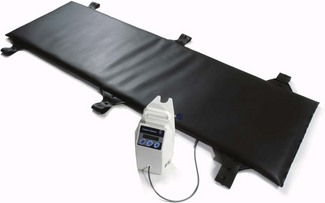
Carbon fibre heating mattresses consist of electrically conductive bundles of this material that criss-cross the device in much the same way as the wire element in electric blankets. When an electric current is passed through the device the resistance of the material causes the mattress to heat up. However, the biggest problem with these is that it is difficult for such systems to deliver uniform heating characteristics, with the consequent risk of burns to a patient. This is because the area of heating surface may be inadequate and the hardness of the bundles means that these require some form of pressure relief material on top which attenuates the warming performance.
The working components are encapsulated in a latex-free cover, with welded seams, which means that the mattress can be cleaned in the same way as an operating table (Fig. 30.2).
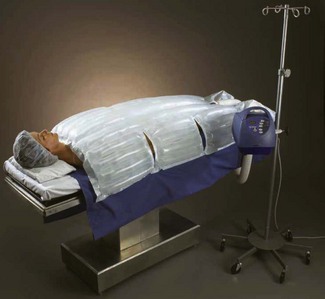
Forced-air warming blankets
These have revolutionized patient warming. Broadly speaking a large volume of air is blown over a 450–1400 W electrical element which warms it to 35–46°C. This is then passed through a ‘quilted’ blanket that covers the patient (Fig. 30.3). The power consumption is around 850 W for the lower powered devices and up to 1500 W for the more powerful ones (i.e. a factor of 10–20 greater than the carbon polymer mattresses). There is a significant variability in the performance of the different types of forced air heating devices (Table 30.1).
Table 30.1 Efficacy of forced air warming systems with full body blankets in a laboratory simulation. Heat exchange coefficients (h), mean temperature gradients at a calculated surface temperature of 32°C (ΔT at 32°C), 34°C (ΔT at 34°C), 36°C (ΔT at 36°C) and 38°C (ΔT at 38°C) and the resulting heat exchange between the full body blanket and the manikin
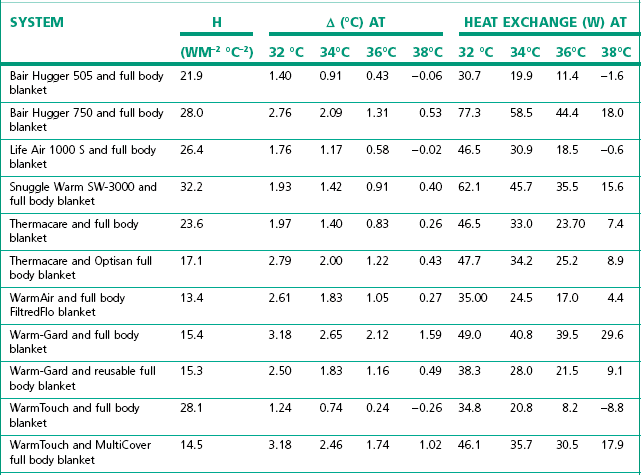
(From Brauer A, English MJ, Steinmetz N, et al. Efficacy of forced-air warming systems with full body blankets. Can J Anesth 2007;54:34–41, with permission.)
Various different blankets have been developed in order to maximize the surface area covered during different surgical procedures and exposures; including now forced warm air mattresses for positioning underneath the patient. With improving technology, the heating devices themselves can be much smaller and so it has been possible to develop special jackets with portable heaters that can be used to keep patients warm throughout the perioperative period.
Radiant heaters
Radiant heaters are electric heaters that generate heat using infrared radiation in the same way that the sun heats the Earth (Fig. 30.4). The infrared spectrum has a wavelength of 0.7–10 µm. Non-industrial heaters use the medium part of the spectrum (approx. 1.5–5.6 µm), typically utilizing the range 2–4 µm. Radiant heat transfer, unlike conduction and convection, requires no intermediate conductor or convector, as infrared energy, like light, passes directly from the source to the receiver. The rate of heat transfer depends on the emissivity of the source, the absorptivity of the receiver, the difference between their absolute temperatures (raised to the fourth power), and the distance between them.
The element on a typical neonatal unit typically consumes 600 W power. They can either generate heat to a set air temperature or, via a feedback mechanism, to a set skin temperature. The danger with the latter is that if the sensor falls off it will read air temperature, which will run the risk of overheating of the patient.
Other devices
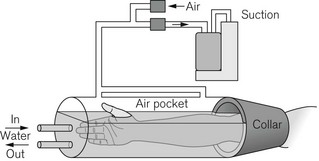
Locally applied warm water and pulsating negative pressure
With appropriate methodology it is feasible to warm the whole patient with very localized heat application. One study has shown the device illustrated in Fig. 30.5 to be more effective than forced-air warming used during laparotomy10.
Devices used to warm intravenous fluids
Fluid warmers can be broadly classified into forced air, metal plate, circulating water and infrared devices. There are also high-flow and low-flow versions with large variations in performance especially at higher flow rates (Tables 30.2 and 30.3).
Table 30.2 Simulated clinical evaluation of four standard fluid warming devices to measure output temperature and flow rates (mean and range)*
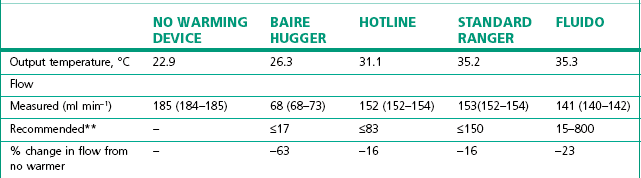
** Manufacturer’s recommended maximum flow rates are given for delivery of fluid at 37°C.11
(From Turner M, Hodzovic I, Mapleson WW. Simulated clinical evaluation of four fluid warming devices. Anaesthesia. 2006;61:571–5, with permission.)
Table 30.3 Simulated clinical evaluation of four standard fluid warming devices to measure output temperature and flow rates (mean and range)*
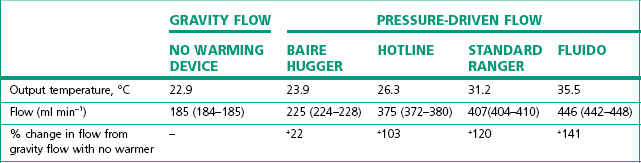
* With giving set pressurised to 300 mmHg compared to no warming device with gravity flow (all through a standard IV set and 14 G cannula).11
(From Turner M, Hodzovic I, Mapleson WW. Simulated clinical evaluation of four fluid warming devices. Anaesthesia. 2006;61:571–5, with permission.)
Forced-air/coil warmers
As can be seen from the entry for the Baire Hugger (Fig. 30.6) device in Table 30.2, these are the least effective and consist simply of a coil placed inside the hose of a forced air warming mattress. Their poor performance can be explained by the different thermal capacities of air and water (see above). In this case air, which has a low capacity, is being used to heat fluid which has a high capacity.
Plate warmers
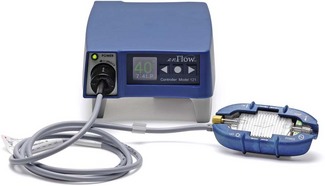
In these devices the fluid passes through a special cartridge that brings it into indirect contact with an electrically heated metal plate. The temperature of the plate is set to 40°C (rather than 37°C) to compensate for the loss of heat over the length of line between the warmer and the patient. Small warmer units are also available that can be placed close to the patient’s infusion site (Fig. 30.7). The water channel of the cartridge is made from plastic with one side bonded to an aluminium-serrated plate. The latter is placed in direct contact with the heater element and is responsible for the transfer of heat to fluid passing through the plastic channels in the cartridge.
Infrared flow compensated fluid warmers
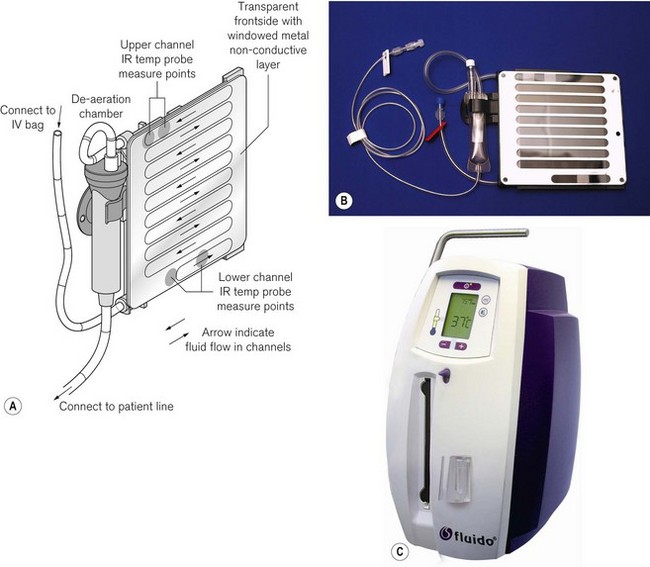
The Fluido system (Fig. 30.8) is a dry fluid warming system. Fluid channelled through a rigid cassette is heated by four infrared lamps with a maximum output of 1200 W.
High-flow fluid warmers
The ‘Level One’ (Fig. 30.9) system from Smiths Medical uses heated water at 42°C and an aluminium counter current heat exchanger to deliver flow rates up to a claimed 1400 ml min−1. Two rigid housings for the bags of infusate are pressurised to 300 mmHg by an in built compressor to deliver an uninterrupted flow. The maximum effective flow rate for fluids at 10°C is approximately half of that for fluids stored at 20°C. These devices have been inadvertently charged with bags partly containing air and have delivered fatal air emboli into patients. Although newer versions have an air detector, this can be bypassed. There is also an upgrade available for the older machines, but the risk of air embolus remains significant with any system that uses a pressurised infusion bag.
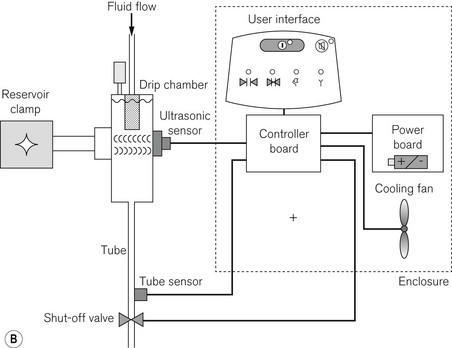
The Fluido infra red warmer (see above) can also be used as a high flow device. The casette can be fitted with two infusion lines that are inserted into IV fluid bags pressurised by pneumatic chambers fed by a compressor. The AirGuard component (Fig. 30.10) protects against air embolism. If the fluid in the chamber falls below a fixed level a valve will close the supply tubing, to prevent the infusion of air. In addition, an infrared tube sensor continuously monitors the presence of this tubing to ensure that it is fixed correctly into the shut-off valve. The complete system is mounted on a drip stand (Fig. 30.11).
1 Rajagopalan S, Mascha E, Na J, Sessler DI. The Effects of Mild Perioperative Hypothermia on Blood Loss and Transfusion Requirement. Anesthesiology. 2008;108(1):71–77.
2 Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996 May 9;334(19):1209–1215.
3 Frank SM, Fleisher LA, Breslow MJ, Higgins MS, Olson KF, Kelly S, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA. 1997;277(14):1127–1134. 1997 April 9
4 Scott EM, Leaper DJ, Clark M, Kelly PJ. Effects of warming therapy on pressure ulcers–a randomized trial. AORN J. 2001 May;73(5):921–927. 9–33, 36–8
5 Lenhardt R, Marker E, Goll V, Tschernich H, Kurz A, Sessler DI, et al. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology. 1997 Dec;87(6):1318–1323.
6 NICE. Perioperative hypothermia (inadvertent): The management of inadvertent perioperative hypothermia in adults. London: National Institute for Health and Clinical Excellence; 2008. Contract No.: Document Number
7 Camus Y, Delva E, Cohen S, Lienhart A. The effects of warming intravenous fluids on intraoperative hypothermia and postoperative shivering during prolonged abdominal surgery. Acta Anaesthesiol Scand. 1996 Aug;40(7):779–782.
8 Gelman S. Venous Function and Central Venous Pressure: A Physiologic Story. Anesthesiology. 2008;108(4):735–748.
9 Bräuer A, English MJ, Steinmetz N, Lorenz N, Perl T, Weyland W, Quintel M. Efficacy of forced-air warming systems with full body blankets. Can J Anaesth. 2007;54(1):34–41.
10 Rein EB, Filtvedt M, Walloe L, Raeder JC. Hypothermia during laparotomy can be prevented by locally applied warm water and pulsating negative pressure. Br J Anaesth. 2007 Mar;98(3):331–336.
11 Turner M, Hodzovic I, Mapleson WW. Simulated clinical evaluation of four fluid warming devices. Anaesthesia. 2006;61(6):571–575.