Chapter 124 Vitreous, Retinal, and Choroidal Biopsy
Introduction
The diagnosis of posterior segment infectious or inflammatory disease is based primarily on clinical examination. In the majority of cases, the slit-lamp biomicroscopic and ophthalmoscopic appearance of the vitreous, retina, and choroid is diagnostic of a specific disorder, especially when combined with the findings from ancillary tests such as fluorescein angiography, ocular echography, and radiologic and serologic tests.1
However, not all patients have the characteristic clinical features or classic appearance of a specific disease, and the etiology of their disorder can be a diagnostic dilemma. The inability to make a specific diagnosis has been reported in up to 33% of patients who present with intraocular inflammation.2 Moreover, some infectious and malignant processes are manifested first, or only, in the eye, in which case the diagnostic yield from systemic testing is low. This is often the situation in patients with atypical malignancy in the eye, chronic low-grade endophthalmitis after cataract surgery, intraocular inflammation of atypical viral, bacterial, or fungal origin, and some types of progressive retinitis, choroiditis, and pigment epitheliopathies. In these patients, particularly if they are immunocompromised or if one of the possible diagnoses is malignancy, the disease process may threaten not only vision but also life. Intraocular biopsy provides affected ocular tissue for culture, examination, and other tests to help with therapeutic decision-making.3 In most of these cases, therapeutic intervention will be either antimicrobial, antineoplastic, or anti-inflammatory.4,5
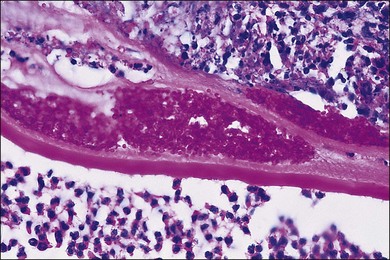
A number of factors in recent years have brought about an increased demand for tissue diagnosis in posterior segment inflammatory disease. One of these is the rise in the number of individuals who have been iatrogenically immunosuppressed as a result of systemic disease, malignancy, or organ transplantation. In addition, the frequent use of intravitreal steroids has also led to the report of opportunistic ocular infections in immunocompetent individuals.6 The clinical presentation of ocular inflammatory disease may be variable and atypical in these immunocompromised patients; the possible etiologic agents are numerous and diverse, and serologic testing is frequently misleading. Another factor increasing the indication for intraocular biopsy is the technologic advance of modern cataract surgery. With phacoemulsification and in-the-bag intraocular lens placement, every year millions of patients are left with most of their capsular bags intact, forming small potential reservoirs in which, rarely, sequestered organisms of normally low-grade pathogenicity can multiply. Clinicians are confronted with a number of patients whose chronic, smoldering, postoperative endophthalmitis is due to organisms such as Candida parapsilosis or Propionibacterium acnes (Figs 124.1–124.5), which cannot be adequately treated until a tissue diagnosis is made. In addition, ongoing advances in laboratory testing continue to improve the diagnostic yield from biopsied material. For example, polymerase chain reaction (PCR) testing, a biochemical technique that allows for the detection of infinitesimal amounts of specific nucleic acids, now permits identification of genetic components of infectious organisms. Finally, advances in vitreoretinal surgical instrumentation and increasing use of 25- and 23-gauge (G) transconjunctival pars plana vitrectomy7 have allowed for possibly safer access and acquisition of ocular tissue for analyses. Table 124.1 lists common indications for ocular biopsy.
| Biopsy site | Diagnostic dilemma |
|---|---|
| Vitreous | Endophthalmitis (bacterial or fungal) |
| Intraocular lymphoma | |
| Retinitis with associated vitritis | |
| Retinal | Retinitis (atypical or not responsive to empiric therapy) |
| Choroidal | Tumor |
| Choroiditis (atypical or not responsive to empiric therapy |
The efficacy of ocular biopsy depends partly on the expertise of the surgeon who obtains the specimen and the laboratory personnel who analyze the specimen(s). Before embarking on surgery, harvesting and handling of the specimen should be carefully mapped out in advance. The pathologist should be familiar with ophthalmic disease processes, aware of the diagnoses being considered, and involved at the outset in planning how the specimen will be handled from the moment it is taken – a step that is critical to the success of the procedure.4,8,9
The pathologist’s available investigative methods include, among others, light microscopy, electron microscopy, immunohistochemistry, and PCR. PCR can be used to identify unique DNA and RNA sequences specifically from infectious agents and, occasionally, from other disease processes, such as proliferating monoclonal B lymphocytes in ocular malignant lymphoma.9–13 The laboratories responsible for culture and identification of bacterial, fungal, and viral disease should receive the specimen as soon as possible. It should be plated on a variety of specific media and maintained under the correct (aerobic, anaerobic, temperature) conditions. Plates may need to be specially treated or held for extra time to reveal growth from some of the more fastidious organisms.
Vitreous biopsy
With the evolution of modern vitrectomy techniques, including 23G, 25G, and 27G vitrectomy, removal of vitreous from the eye has become a common and relatively safe procedure.14 A number of different goals are achieved with diagnostic vitrectomy5,15 In vitritis with a possible neoplastic, infectious, or inflammatory cause, diagnostic vitrectomy may provide a definitive diagnosis, thereby allowing appropriate therapy to be instituted.5,8 In some cases of retinitis and choroiditis, a diagnosis can be made from vitreous sampling alone, particularly if marked vitritis is present. The vitrectomy also removes or debulks infectious material within the eye, reduces the intraocular load of inflammatory cells and associated biologically active material and debris, effectively removes material (e.g. retained lens material and blood) that may be part of the pathogenesis of the ocular disorder, allows clinicopathologic correlation in the active stages of disease for some conditions, and helps to define the pathophysiologic basis of many disorders.5 Cytopathologic diagnosis has proved valuable in reported series or case reports of patients with: endogenous and exogenous bacterial and fungal infections (Fig. 124.6); nematode endophthalmitis; inflammatory pseudotumor of the iris and ciliary body; pars planitis; phacoanaphylaxis; intraocular lymphoma; leukemia; metastatic melanoma; metastatic carcinoma; medulloepithelioma of the ciliary body; secondary glaucoma (including blood-induced, phacolytic, and melanomalytic glaucoma); epithelial ingrowth; proliferative vitreoretinopathy, and miscellaneous conditions with vitreous opacities (including chronic inflammation in patients with suspected lymphoma, acute retinal necrosis, birdshot chorioretinopathy, toxoplasmosis, Whipple’s disease, amyloidosis, and asteroid hyalosis).4,5,16,17 Vitrectomy may also help to clear vitreous opacities, remove scaffolding on which postoperative cicatricial proliferation can grow, relieve traction, and allow for improved intraocular penetration of antibiotics.5,15 Table 124.2 lists a sample of vitreous findings in major disease categories.
Surgical technique
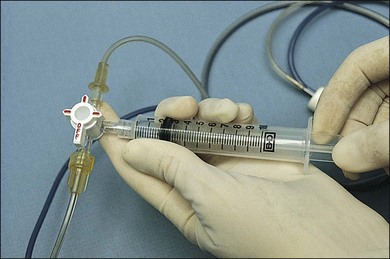
To obtain an undiluted specimen, a variety of vitrectomy techniques (with either 20-, 23-, 25-, or 27G instrumentation) may be used. If a large sample (>0.5 mL) of vitreous is needed, a standard three-port vitrectomy system should be utilized. A 10 mL Luer–Lok syringe is spliced via a three-way stopcock into the aspiration line (Fig. 124.7). Manual aspiration and specimen collection are carried out for retrieval of an undiluted specimen from the most intensely involved area (usually posteriorly, inferiorly, or over the area of retinitis or chorioretinitis).
Alternatively, a vitreous “trap” method may also be performed using two 18G needles, a male adaptor, and a sterile red-topped test tube.18 In the vitreous trap method, the male adaptor is attached to one of the needles, and the needles are attached to the ends of the aspiration tubing. The two needles are then inserted into the test tube. The diagnostic vitrectomy is performed and the pure vitreous sample is directed into the test tube.
After a sufficient amount of sample is obtained, the infusion is turned on and either fluid or air is infused before large choroidals form and the eye begins to collapse.14 The vitrector is withdrawn from the eye, and the vitreous specimen is aspirated into the syringe. Vitrectomy can then be completed. At completion of the surgery, the cassette with the dilute residual vitreous is also sent to the pathology laboratory, where the specimen is concentrated, using a micropore filter, or cytospin smears are made.4,19
If a small sample (≤0.5 mL) of vitreous is needed, a 27G vitrectomy with a single incision may be utilized.20,21 In this technique, the 27G vitrector is inserted perpendicular to the pars plana. Using a cut rate of 800 cuts/minute and an aspiration rate of 600 mmHg, a small amount of undiluted vitreous may be safely removed from the eye without the need of an additional infusion line or endoillumination port. The 27G scleral wound is also self-sealing and does not require sutures.
Histologic technique and preparations
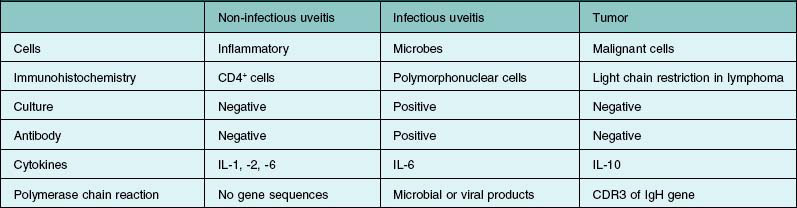
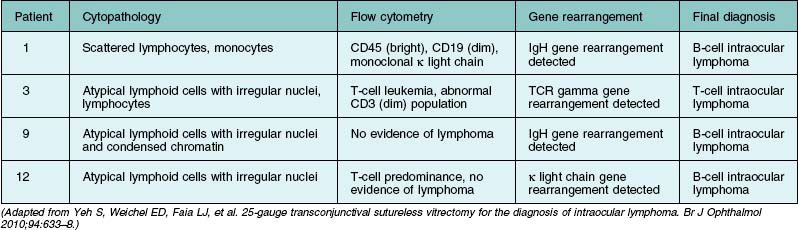
If primary intraocular lymphoma is suspected, obtaining a sufficient vitreous sample for cytologic testing is essential. In addition, consultation with an experienced cytopathologist is recommended because correct identification of lymphoma cells in the biopsy sample is essential for the diagnosis. In addition to characterizing cells from the vitreous biopsy, testing for immunohistochemical markers for leukocytes (e.g., CD45), B cells (e.g., CD 20), and T cells (e.g., CD45RO) can also aid in the diagnosis.14,22,23 Other diagnostic modalities that can be useful include flow cytometry, PCR to detect V–J immunoglobulin gene rearrangements, and measurements of IL-6 and IL-10 in aqueous or vitreous samples.22–24 Table 124.3 summarizes the key laboratory features of several patients who underwent 25G diagnostic vitrectomy for suspected intraocular lymphoma. Additional information on the diagnosis and management of primary intraocular lymphoma is available in Chapter 155, Leukemias and lymphomas.
Results
In cases of infectious endophthalmitis, vitreous samples can often reveal the presence of microbes. Unfortunately, these may not be found in all infectious cases, principally because of suboptimal sampling, inadequate culturing, or pretreatment with antimicrobials that render microbes nonculturable on routine media.10 In the Endophthalmitis Vitrectomy Study, confirmed microbiologic growth was demonstrated in intraocular specimens from 291 of 420 patients (69.3%).25 More recently, PCR testing has been applied to identify bacterial causes of endophthalmitis. Very little tissue is needed for PCR. Usually 50–100 µL samples are sufficient, but even a sample as small as 1 µL can be processed for PCR testing.26 Melo and co-authors evaluated real-time PCR to diagnose bacterial endophthalmitis after cataract surgery and demonstrated that PCR assays were positive in 91% of patients with a clinical diagnosis of endophthalmitis using either aqueous and/or vitreous samples.27 Lohmann and colleagues28 used PCR to analyze 25 patients with late-onset endophthalmitis, and they showed that bacterial DNA (belonging to P. acnes, Staphylococcus epidermis, or Actinomyces israelii) was obtained by PCR from 92% of vitreous samples. By comparison, bacterial culture was positive in only 24% of these cases. PCR can also be helpful for suspected fungal endophthalmitis. Salman and colleagues29 demonstrated that PCR was highly sensitive and specific in the diagnosis of post-traumatic fungal endophthalmitis. In their study of 26 endophthalmitis cases, PCR correctly identified Aspergillus organisms, while culture on blood agar was negative in all cases.
PCR is also a useful tool in the diagnosis of posterior segment infectious uveitis. Harper and colleagues conducted a retrospective review of 133 patients who underwent PCR testing of aqueous or vitreous for possible infectious chorioretinitis.30 PCR tests for herpes virus, varicella zoster virus, and CMV were performed in the majority of cases. The predictive value of positive and negative tests was 98.7% and 67.9% in this population. In addition, an alteration in treatment based on the PCR result led to a resolution in 25 of 26 patients after treatment was changed, suggesting that PCR is a useful test to aid in the diagnosis and management of challenging cases of infectious uveitis.
Several studies have shown that PCR has sensitivities exceeding 90% for varicella-zoster virus (VZV), herpes simplex virus (HSV), and cytomegalovirus (CMV), with specificities in excess of 95% for these organisms.26 Sensitivity approaches 95% in detecting untreated CMV retinitis, dropping to 48% in treated eyes.31–33 Moreover, false-positive rates are low.34 In addition, PCR can aid in detecting antiviral-resistant strains of CMV, which have mutations in the UL97 polymerase gene.26 Information on possible antiviral resistance is essential in the management of viral retinitis and may influence the choice of agent for a particular patient. PCR inhibitor has been detected in normal aqueous and vitreous fluids, but it can be removed by diluting the specimen, by chloroform extraction, or by thermostable DNA polymerases.35
Palexas and colleagues36 reported on 405 consecutive vitreous biopsy cases studied at the Wilmer Eye Pathology laboratory. Vitrectomy had been performed in five categories of eye disease: post-traumatic infections (8.4%); postoperative endophthalmitis (38.5%); endogenous endophthalmitis (6.2%); idiopathic inflammation (25.4%); intraocular neoplasm (14.3%); and miscellaneous (7.2%). Diagnostic vitrectomy was performed in 215 cases (53%) for suspected endophthalmitis. Overall, 60 of these (28.8%) had microbial organisms present, and 36 (16.7%) were culture-positive. In the postoperative endophthalmitis group, microorganisms were observed microscopically in 31 (20%) of 156 eyes, 74% of these Gram-positive. Bacteria were found in six (18%) of 34 post-traumatic cases of endophthalmitis and in 23 (92%) of 25 endogenous cases. Patients with endogenous infections had the most fungal infections. Neoplasms were diagnosed in 58 (14%) of the 405 vitrectomy specimens, the most common being ocular lymphoma in 42 (72%) (Fig. 124.8). However, only 42 (48.3%) of 87 patients clinically suspected of having ocular lymphoma had a vitreous biopsy that was positive for the disease. Other malignancies diagnosed cytopathologically included metastatic squamous cell carcinoma and acute lymphoblastic leukemia.
Atypical toxoplasmic chorioretinitis that mimics viral retinitis can also be diagnosed by vitreous biopsy.37 In a series of 22 patients with widespread chorioretinitis, 16 individuals were diagnosed with toxoplasmosis using PCR, culture, antibody detection, histopathologic examination, or a combination of the aforementioned techniques. Bottos and colleagues have also demonstrated the utility of PCR in diagnosing an atypical strain of Toxoplasma gondii as the causative agent in an unusual bilateral retinochoroiditis.38 The correct diagnosis is important in challenging cases since treatment with proper medicines may help avoid vision loss.
Case 1
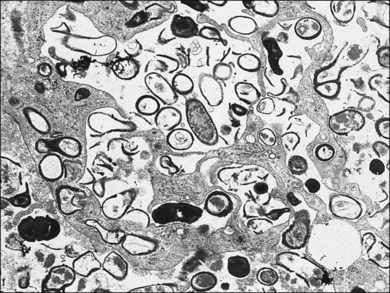
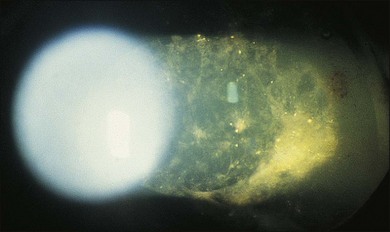
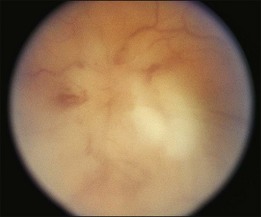
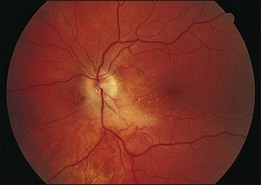
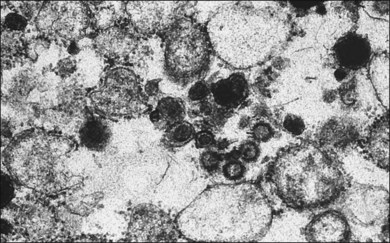
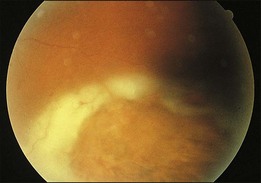
A 29-year-old white woman was referred for evaluation of blurred vision OD. Her acute myelocytic lymphoma had been treated with an allogeneic bone marrow transplant 2 months earlier. She had recently been diagnosed with CMV colitis, but ganciclovir had been discontinued because of thrombocytopenia. Visual acuity was 20/320 OD. A serous retinal detachment of the macula was present with white flocculent material beneath it (Fig. 124.9). A chest X-ray examination at this time revealed a right lower lobe infiltrate interpreted as pulmonary aspergillosis. She was treated with systemic antibiotics, including amphotericin B and flucytosine, but 3 days later the ocular lesion was substantially larger (Fig. 124.10). A diagnostic vitrectomy was performed, and 5 µg of intravitreal amphotericin B was given at the end of surgery. The vitreous specimen revealed branching septate hyphae and vitreous culture grew Aspergillus flavus.
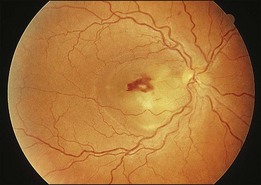
Fig. 124.9 Retinitis that developed from Aspergillus infection after bone marrow transplantation.
(Reproduced with permission from Coskuncan NM, Jabs DA, Dunn JP, et al. The eye in bone marrow transplantation. VI. Retinal complications. Arch Ophthalmol 1994;112:372–9.
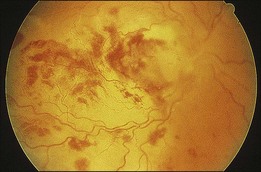
Fig. 124.10 Same eye as in Figure 124.9, shown 3 days later, despite intravenous amphotericin B and systemic flucytosine, with progressive fulminant infection. Histopathologic study of the vitreous biopsy disclosed branching, septate hyphae, and culture positivity for Aspergillus flavus.
(Reproduced with permission from Coskuncan NM, Jabs DA, Dunn JP, et al. The eye in bone marrow transplantation. VI. Retinal complications. Arch Ophthalmol 1994;112:372–9.
Case 2
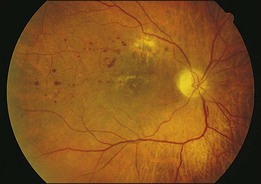
A 36-year-old white man was seen with a 2-week history of blurred vision in the left eye. Past history was positive for systemic lymphoma stage IIIA, which was treated with total nodal irradiation and chemotherapy. A relapse was successfully treated with chemotherapy, resulting in remission for 3 years, until a bone marrow biopsy showed Burkitt’s lymphoma. He was treated with chemotherapy, including seven intrathecal methotrexate injections for central nervous system relapse, and was referred for bone marrow transplantation. He had undergone bone marrow harvesting and had started cytoreductive therapy before transplantation. Vision was counting fingers. Fundus examination disclosed a retinitis involving the macula and optic nerve head with white vitreous opacities (Fig. 124.11). Diagnostic vitrectomy revealed atypical lymphocytes characteristic of lymphoma. The patient was treated with ocular irradiation, and his vision improved to 20/30 one month later (Fig. 124.12).
Transvitreal retinal biopsy
Vitreous biopsy is limited by its dependency on the spillover of cells from the diseased tissue in question into the vitreous cavity to allow a diagnosis. Some ocular pathologic processes are primarily confined to the retina, choroid, or both, and only histologic examination of these areas can yield a diagnosis. The transvitreal approach to retinal biopsy is of particular value in lesions located posterior to the equator, although more peripheral lesions are also accessible, particularly in pseudophakic and aphakic eyes. This approach also allows concomitant vitreous sampling.39–43
Surgical technique
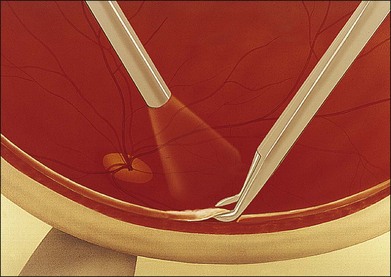
The specimen should include the advancing edge of the retinitis because this is where actively replicating, viable organisms are most likely to be found. Central areas of the lesion may contain only necrotic tissue. Cautery at the area of the biopsy site is occasionally needed if large vessels are present. The tissue is excised with scissors, leaving a small area of anchoring attachment (Fig. 124.13). The biopsied retina is grasped securely with forceps so that as little as possible of the specimen is crushed and is removed from the eye (Fig. 124.14). Laser is not necessary at the biopsy edges involved by inflammation, but it is placed at the edges of normal retina. An air–fluid exchange is performed, and occasionally a long-acting gas is injected.
Results
Rutzen et al.44 reported a series of 24 transvitreal retinal biopsies, 19 from eyes with clinical signs suggestive of viral retinitis. The clinical diagnosis was confirmed by electron microscopy, immunohistochemical staining, in situ DNA hybridization, and/or PCR in ten of the 19 eyes (53%). Virus was identified in seven of ten cases of suspected CMV retinitis, in one of seven cases of acute retinal necrosis, and in two of two cases of progressive outer retinal necrosis. The remaining five biopsies disclosed Candida organisms in one specimen, subretinal fibrosis in one, and chronic inflammation in three.
Johnston and colleagues45 performed a retrospective review of retinal and choroidal biopsies undertaken in cases of unclear uveitis of suspected infectious or malignant origin. In this series, 13 patients underwent either a retinal or choroidal biopsy. The pathologic diagnosis differed from the initial clinical diagnosis in five (38%) cases, and helped to direct treatment in seven (54%) cases. Retinal biopsies were invaluable in making the proper diagnosis in these cases.
Case 3
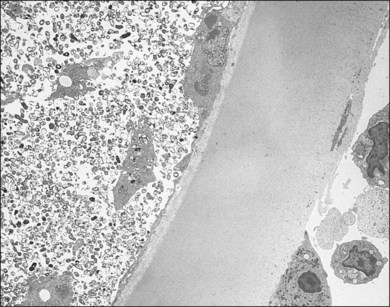
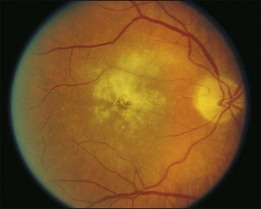
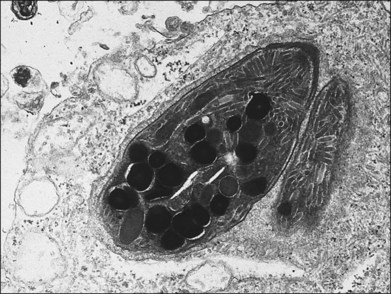
A 65-year-old white man had metamorphopsia and a gray scotoma. His history was positive for non-Hodgkin lymphoma and two courses of chemotherapy, followed by autologous bone marrow transplantation 7 months previously. Vision was 20/400, and white macular retinitis was seen with minimal vitreous inflammation (Fig. 124.15). A retinal biopsy disclosed intracytoplasmic nucleocapsids measuring 120–140 nm and several intracytoplasmic spherical election dense bodies measuring 120–350 nm, consistent with CMV retinitis (Fig. 124.16). The PCR analysis of the vitreous was also positive for CMV. The infection responded well to ganciclovir (Fig. 124.17).
Case 4
A 34-year-old white man developed blurred vision and floaters in his left eye. He had a history of bone marrow transplantation, with graft-versus-host disease requiring persistent immunosuppression. At first the white peripheral retinitis was thought to represent CMV infection, and he was unsuccessfully treated with ganciclovir (Fig. 124.18). Because of the atypical clinical picture (including no old toxoplasmic scars on earlier ophthalmologic evaluation), a retinal biopsy was performed, disclosing Toxoplasma organisms (Fig. 124.19).
Transvitreal and transscleral choroidal biopsy
In certain ocular disease processes, the pathologic evidence is primarily located at the level of the choroid. In such cases, the cells recovered by vitrectomy may be nonspecifically inflammatory in nature and not representative of the actual disorder.46,47 Therefore the biopsy of the choroid (and retina) may be informative and useful.8,39,48 Choroidal and chorioretinal biopsies have been performed to investigate nonspecific uveitis, acute retinal necrosis, tuberculous choroiditis, and retinal pigment epitheliopathies and to identify malignant incursion into the choroid.8,39
Transscleral chorioretinal biopsy was pioneered by Foulds, Peyman, and others who developed procedures that allow choroidal tissue sampling but minimize associated complications, particularly retinal detachment.49–55 Cole and colleagues have reported on the success of transvitreal chorioretinal biopsy using 20G instrumentation and vertical-cutting scissors to remove a large specimen (at least 2 × 2 mm) from the biopsy site.56,57 Among the nine eyes which underwent biopsy, a positive histologic diagnosis was made in five cases (55.6%).
Surgical technique
Transvitreal biopsy
A standard three-port pars plana vitrectomy is performed and endolaser is applied around the margins of the intended biopsy site, which should measure at least 2 × 2 mm.56,57 Vertical cutting intraocular scissors are used to isolate the retinal and choroidal biopsy specimen by cutting within the margins of the laser burns. The blade of the scissor should penetrate the choroid until clear white sclera is visible. In order to prevent intraocular hemorrhage, the infusion bottle should be raised to elevate the intraocular pressure. After obtaining the specimen, the sclerostomy site should be enlarged to allow for removal of the specimen from the eye. The specimen should be immediately sent for processing and analysis. After fluid/gas exchange, 20% SF6 gas can be injected into the eye to tamponade the retina.
Recently, a surgical technique using a newly developed instrument, the Essen biopsy forceps, was reported to be effective in the diagnosis of choroidal tumors in 20 patients.58 After performing a standard 23G vitrectomy, Akgul and colleagues used a modified version of a CE-certified intraocular forceps, which was re-designed to capture and hold a sufficient tissue sample, to grasp a biopsy of the tumor. Laser is applied prophylactically to the retinotomy site but no fluid–gas exchange was performed.
Transscleral biopsy
The conjunctiva is incised, and the rectus muscles in the involved quadrant are isolated with silk sutures.59 In biopsies of the choroid and retinal pigment epithelium, the retina tends to bulge into the biopsy site with a risk of retinal tear or incarceration. This risk is reduced by performing a pars plana vitrectomy first.55 If visualization is adequate, a heavy barrier of photocoagulation, cryotherapy, or diathermy is applied some days preoperatively around the planned biopsy area. Otherwise, endolaser therapy or cryotherapy is applied at the time of vitrectomy. The biopsy site is marked on the sclera, and a 6 × 6 mm scleral flap, nearly full-thickness and hinged (usually posteriorly), is dissected beginning about 5–6 mm posterior to the limbus, depending on the lesion site. The flap is retracted, exposing a near-bare choroid with a few remaining thin fibers of overlying sclera. Diathermy or cautery is done along the outer margin of the inner choroidal bed. A sharp blade is used to make an incision, or two parallel incisions, through the choroid (and retina, if retina is to be removed). One blade of a 0.12 forceps is placed through the incision, and the biopsy specimen is grasped at one edge. The block excision is then completed with Vannas scissors (Fig. 124.20). During removal of the specimen, particular attention is directed at grasping the tissue only once and ensuring that the entire specimen is delivered in one piece. If the retina is not being removed, it is carefully separated from the choroid and left intact. The biopsy specimen is then placed in fixative or handled as planned with the pathologist. Any prolapsed vitreous is then removed from the wound, and the scleral flap is sutured with interrupted 9–0 nylon or 7–0 Vicryl. Fluid–gas exchange is performed.
Another technique for obtaining a specimen of sclera, choroid, retinal pigment epithelium, and retina from the eye is to use a corneal trephine and to reconstitute the eye wall with a full-thickness donor scleral graft. In this situation, hemostasis may be improved by systemic hypotensive anesthesia.49,50,60
Cyanoacrylate tissue glue can be used to stabilize the choroidal specimen before its removal from the eye.49,50 A drop of cyanoacrylate glue is applied to the exposed choroid, forming a dense plaque that can be grasped or glued to an arrowhead sponge.
Histologic technique and preparations
Handling of the choroidal or chorioretinal biopsy specimen depends greatly on the amount of tissue harvested. Consultation with the pathologist is important to prioritize the tests that can be performed. Ideally, the tissue can be divided into three parts in a sterile manner under a dissecting microscope for microbiology, tissue culture, routine light histologic and immunopathologic studies, and electron microscopy.1
Results
Martin et al.59 reported their results with chorioretinal biopsy performed on seven eyes with progressive chorioretinal lesions of unknown cause. Chorioretinal biopsy provided useful information for determining the diagnosis (multifocal choroiditis with subretinal fibrosis, sarcoidosis, and viral retinitis) and led to change of therapy in five patients. Final visual acuity was unchanged or improved in five eyes.
Foulds and colleagues8,39,49,50 reported on 34 transscleral biopsies of the choroid and retina for the diagnosis of choroidal melanoma, acute retinal necrosis, chronic uveitis, and progressive retinal pigment epitheliopathy. An adverse event occurred in only one case: a retinal break developed, with associated vitreous hemorrhage and resultant proliferative vitreoretinopathy. Initial vitrectomy might have prevented this complication.8,39,49,50
Case 5
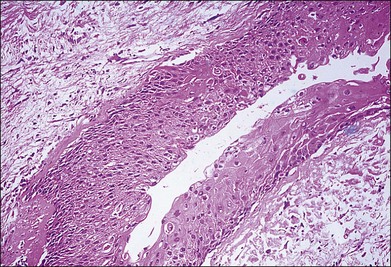
A 58-year-old man with a history of prostate cancer, thought to be in remission, had an amelanotic intraocular mass OS at presentation. Echographic study showed a plaque on the surface of the mass similar to a previously reported benign fibrous tumor. Needle aspiration biopsy was nondiagnostic. Because of tumor enlargement, choroidal biopsy was performed and disclosed features diagnostic of metastatic carcinoma of the prostate (Fig. 124.21).
Fine-needle biopsy
Fine-needle aspiration biopsy techniques have been used extensively in the diagnostic evaluation of many human tumors, including tumors of the orbit and eye.61 Drawbacks to the technique include the possibility of dissemination of malignant cells, intraocular complications, and misdiagnosis caused by sampling errors and lack of familiarity with fine-needle aspiration biopsy technique and methods of cytopathologic diagnosis of aspiration biopsy specimens. Although there is a theoretical risk of tumor dissemination along the needle track, no such occurrence has been documented with a needle of 25G or finer; nor has vitreous hemorrhage or retinal detachment been a problem.62,63
Surgical technique
The technique involves 22G–30G disposable needles for intraocular aspiration.64,65 The length of the needle selected depends on the intraocular location of the tumor and the planned biopsy route. The biopsy needle is connected to a 10 mL plastic disposable aspirating syringe via a standard plastic tubing. The connector tubing is used so that there will be no induced movement in the needle tip during the biopsy as the assistant exerts suction in the line for aspiration (Fig. 124.22).
Results
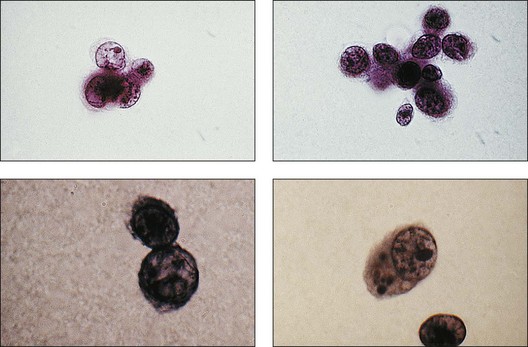
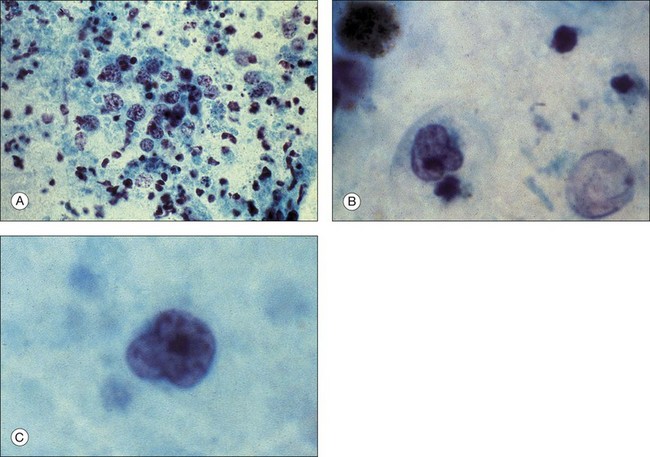
Transscleral fine-needle aspiration biopsy can be feasible in diagnosing choroidal melanoma. In addition, aspiration biopsy may assist in assessing cytogenetics of these tumors. In a prospective, interventional case series, 30G fine-needle aspiration biopsy diagnosed macular choroidal melanoma in 17 of 24 (71%) eyes.61 Adverse events such as submacular hemorrhage (nine eyes) and vitreous hemorrhage (five eyes) resolved spontaneously within 1 month of the biopsy. In addition, four eyes had retinal perforation but this did not require treatment and did not result in retinal detachment. Examples of cellular specimens obtained by needle aspiration biopsy are seen in Figure 124.23.
Complications of intraocular biopsy
The risks of intraocular biopsy are those of vitreoretinal surgery in general. These include increased intraocular pressure, cataract progression, peripheral retinal tears, retinal detachment, choroidal hemorrhage, vitreous hemorrhage, endophthalmitis, exacerbation of the underlying inflammatory disease, and proliferative vitreoretinopathy.61 The most hazardous of the biopsy procedures is the transscleral choroidal or retinochoroidal resection.
1 Nussenblatt RB, Davis JL, Palestine AG. Chorioretinal biopsy for diagnostic purposes in cases of intraocular inflammatory disease. Dev Ophthalmol. 1992;23:133–138.
2 Henderly DE, Genstler AJ, Smith RE, et al. Changing patterns of uveitis. Am J Ophthalmol. 1987;103:131–136.
3 Park SS, D’Amico DJ, Foster CS. The role of invasive diagnostic testing in inflammatory eye diseases. Int Ophthalmol Clin. 1994;34:229–238.
4 Green WR. Diagnostic cytopathology of ocular fluid specimens. Ophthalmology. 1984;91:726–749.
5 Michels RG, Green WR, Engel HM, et al. Diagnostic vitrectomy. In: Jakobiec FA, Sigelman J. Techniques in ocular surgery. Philadelphia: WB Saunders, 1984.
6 Vertes D, Snyers B, De Potter P. Cytomegalovirus retinitis after low-dose intravitreous triamcinolone acetonide in an immunocompetent patient: a warning for the widespread use of intravitreous corticosteroids. Int Ophthalmol. 2010(5):595–597.
7 Parolini B, Prigione G, Romanelli F, et al. Postoperative complications and intraocular pressure in 943 consecutive cases of 23-gauge transconjunctival pars plana vitrectomy with 1-year follow-up. Retina. 2010:107–111.
8 Foulds WS. The uses and limitations of intraocular biopsy. Eye. 1992;6:11–27.
9 Stoflet ES, Koeberl DD, Sarkar G, et al. Genomic amplification with transcript sequencing. Science. 1988;239:491–494.
10 Hykin PG, Tobal K, McIntyre G, et al. The diagnosis of delayed post-operative endophthalmitis by polymerase chain reaction of bacterial DNA in vitreous samples. J Med Microbiol. 1994;40:408–415.
11 Katai N, Nuriowa S, Fujimori K, et al. Diagnosis of intraocular lymphoma by polymerase chain reaction. Graefes Arch Clin Exp Ophthalmol. 1997;235:431–436.
12 Lohmann CP, Linde HJ, Reischl U. Rapid diagnosis of infectious endophthalmitis using polymerase chain reaction (PCR) as supplement to conventional microbiological diagnostic methods. Klin Monatsbl Augenheilkd. 1997;211:22–27.
13 Norose K, Tokushima T, Yano A. Quantitative polymerase chain reaction in diagnosing ocular toxoplasmosis. Am J Ophthalmol. 1996;121:441–442.
14 Yeh S, Weichel ED, Faia LJ, et al. 25-gauge transconjunctival sutureless vitrectomy for the diagnosis of intraocular lymphoma. Br J Ophthalmol. 2010;94:633–638.
15 Engel HM, Green WR, Michels RG, et al. Diagnostic vitrectomy. Retina. 1981;1:121–149.
16 Michels RG, Knox DL. Reticulum cell sarcoma: diagnosis by pars plana vitrectomy. Arch Ophthalmol. 1975;93:1331–1335.
17 Piro P, Pappas HR, Erozan YE, et al. Diagnostic vitrectomy in metastatic breast carcinoma in the vitreous. Retina. 1982;2:182–188.
18 Malinowski SM. The vitreous trap: a simple, surgeon-controlled technique for obtaining undiluted vitreous and subretinal specimens during pars plana vitrectomy. Retina. 2010;30:828–829.
19 Engel H, de la Cruz Z, Jimenez-Abalahin LD, et al. Cytopreparatory techniques for eye fluid specimens obtained by vitrectomy. Acta Cytol. 1982;26:551–560.
20 Oshima Y, Wakabayashi T, Ohguro N, et al. A 27-gauge sharp-tip short-shaft pneumatic vitreous cutter for transconjunctival sutureless vitreous biopsy. Retina. 2011;31:419–421.
21 Oshima Y, Wakabayashi T, Sato T, et al. A 27-gauge instrument system for transconjunctival sutureless microincision vitrectomy surgery. Ophthalmology. 2010;117:93–102.
22 Rajagopal R, Harbour JW. Diagnostic testing and treatment choices in primary vitreoretinal lymphoma. Retina. 2011;31:435–440.
23 Intzedy L, Teoh Sc, Hoga A, et al. Cytopathological analysis of vitreous in intraocular lymphoma. Eye. 2008;22:289–293.
24 Grimm SA, McCannel CA, Omuro AM, et al. Primary CNV lymphoma with intraocular involvement: International PCNSL Collaborative Group Report. Neurology. 2008;71:1355–1360.
25 Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study. Arch Ophthalmol. 1995;113:1479–1496.
26 Van Gelder RN. Polymerase chain reaction diagnostics for posterior segment disease. Retina. 2003;23:445–452.
27 Melo GB, Bispo PJ, Campos Pignatari AC, et al. Real-time polymerase chain reaction test to discriminate between contamination and intraocular infection after cataract surgery. J Cataract Refract Surg. 2011;37:1244–1250.
28 Lohmann CP, Linde HJ, Reischl U. Improved detection of microorganisms by polymerase chain reaction in delayed endophthalmitis after cataract surgery. Ophthalmology. 2000;107:1047–1051.
29 Salman AG, Mansour DE, Radwan AA, et al. Polymerase chain reaction in pediatric post-traumatic fungal endophthalmitis among Egyptian children. Ocul Immunol Inflamm. 2010;18:127–132.
30 Harper TW, Miller D, Schiffman JC, et al. Polymerase chain reaction analysis of aqueous and vitreous specimens in the diagnosis of posterior segment infectious uveitis. Am J Ophthalmol. 2009;147:140–147.
31 Garweg J, Fenner T, Bohnke M, et al. An improved technique for the diagnosis of viral retinitis from samples of aqueous humor and vitreous. Graefes Arch Clin Exp Ophthalmol. 1993;231:508–513.
32 Mitchell SM, Fox JD. Aqueous and vitreous humor samples for the diagnosis of cytomegalovirus retinitis. Am J Ophthalmol. 1995;120:252–253.
33 Mitchell SM, Fox JD, Tedder RX, et al. Vitreous fluid sampling and viral genome detection for the diagnosis of viral retinitis in patients with AIDS. J Med Virol. 1994;43:336–340.
34 McCann JD, Margolis TP, Wong MG, et al. A sensitive and specific polymerase chain reaction-based assay for the diagnosis of cytomegalovirus retinitis. Am J Ophthalmol. 1995;120:219–226.
35 Wiedbrauk DL, Werner JC, Drevon AM. Inhibition of PCR by aqueous and vitreous fluids. J Clin Microbiol. 1995;33:2643–2646.
36 Palexas GN, Green WR, Goldberg MF, et al. Diagnostic pars plana vitrectomy: report of a 21-year retrospective study. Trans Am Ophthalmol Soc. 1995;93:623–684.
37 Moshfeghi DM, Dodds EM, Couto CA, et al. Diagnostic approaches to severe, atypical toxoplasmosis mimicking acute retinal necrosis. Ophthalmology. 2004;111:716–725.
38 Bottos J, Miller RH, Belfort RN, et al. Bilateral retinochoroiditis caused by an atypical strain of Toxoplasma gondii. UNIFESP Toxoplasmosis group, Belfort R Jr, Grigg ME. Br J Ophthalmol. 2009;93:1546–1550.
39 Foulds WS, Lee WR, Roxburg STD, et al. Can chorioretinal biopsy be justified? Trans. Ophthalmol Soc UK. 1985;104:866–868.
40 Freeman WR, Stern WH, Gross JG, et al. Pathologic observations made by retinal biopsy. Retina. 1990;10:195–204.
41 Freeman WR, Thomas AEL, Rao NA. Demonstration of herpes group virus in acute retinal necrosis syndrome. Am J Ophthalmol. 1986;102:701–709.
42 Freeman WR, Wiley CA, Cross JG, et al. Endoretinal biopsy in immunosuppressed and healthy patients with retinitis: indications, utility, and techniques. Ophthalmology. 1989;96:1559–1565.
43 Warren K, Goldstein E, Hung VS, et al. Use of retinal biopsy to diagnose Bartonella (formerly Rochalimaea) henselae retinitis in an HIV-infected patient. Arch Ophthalmol. 1998;116:937–940.
44 Rutzen AR, Ortega-Larrocea G, Dugel PU, et al. Clinicopathologic study of retinal and choroidal biopsies in intraocular inflammation. Am J Ophthalmol. 1995;119:597–611.
45 Johnston RL, Tufail A, Lightman S, et al. Retinal and choroidal biopsies are helpful in unclear uveitis or suspected infectious or malignant origin. Ophthalmology. 2004;111:522–528.
46 Lopez JS, Chan CC, Burnier M. Immunohistochemistry findings in primary intraocular lymphoma. (Letter). Am J Ophthalmol. 1991;112:472–474.
47 Ridley ME, McDonald HR, Sternberg P, Jr. Retinal manifestations of ocular lymphoma (reticulum cell sarcoma). Ophthalmology. 1992;99:1153–1161.
48 Barondes MJ, Sponsel WE, Stevens TS, et al. Tuberculous choroiditis diagnosed by chorio-retinal endobiopsy. Am J Ophthalmol. 1991;112:460–461.
49 Foulds WS. The local excision of choroidal melanomata. Trans Ophthalmol Soc UK. 1973;93:343–346.
50 Foulds WS, Damato BE, Burton RL. Local resection of choroidal melanoma. In: Bornfeld N, Gragoudas ES, Hopping W. Tumors of the eye. Amsterdam: Kugler, 1991.
51 Peyman GA. Internal retinal biopsy: surgical technique and results. Int Ophthalmol. 1990;14:101–104.
52 Peyman GA, Fishman GA, Sanders DR, et al. Biopsy of human chorioretinal tissues. Invest Ophthalmol Vis Sci. 1975;14:707–710.
53 Peyman GA, Juarez CP, Diamond JG, et al. Ten years’ experience with eye wall resection for uveal malignant melanomas. Ophthalmology. 1984;91:1720–1725.
54 Peyman GA, Maisel SH, Batko KA, et al. An experimental approach to the tissue diagnosis and study of choroidal and retinal lesions. Invest Ophthalmol Vis Sci. 1975;14:484–487.
55 Peyman GA, Raichand M. Full thickness eye wall resection of choroidal neoplasms. Ophthalmology. 1978;6:1024–1036.
56 Cole CJ, Kwan AS, Laidlaw DAH, et al. A new technique of combined retinal and choroidal biopsy. Br J Ophthalmol. 2008;92:1357–1360.
57 Westerfeld C, Mukai S. Retinal and Choroidal Biopsy. Int Ophthalmol Clin. 2009;49:145–154.
58 Akgul H, Otterbach F, Bornfeld N, et al. Intraocular biopsy using special forceps: a new instrument and refined surgical technique. Br J Ophthalmol. 2011;95:79–82.
59 Martin DF, Chi-Chao C, de Smet MD, et al. The role of chorioretinal biopsy in the management of posterior uveitis. Ophthalmology. 1993;100:705–714.
60 Constable IG, Chester GH, Horne R, et al. Human chorioretinal biopsy under controlled systemic hypotensive anaesthesia. Br J Ophthalmol. 1980;64:659–664.
61 Young TA, Burgess BL, Rao NP, et al. Transscleral fine-needle aspiration biopsy of macular choroidal melanoma. Am J Ophthalmol. 2008;145:297–302.
62 Henkes HE, Manschott WA. The danger of diagnostic biopsy in eyes suspected of an intraocular tumour. Ophthalmologica. 1963;145:467–469.
63 Reese AB. Tumors of the eye, 3rd ed. Hagerstown: Harper & Row; 1976.
64 Augsburger JJ, Shields JA. Fine-needle aspiration biopsy of solid intraocular tumors: indications, instrumentation and techniques. Ophthalmic Surg. 1984;15:34–40.
65 Czerniak B, Woyke S, Domagala W. Fine needle aspiration cytology of intraocular malignant melanoma. Acta Cytol. 1983;27:157–165.

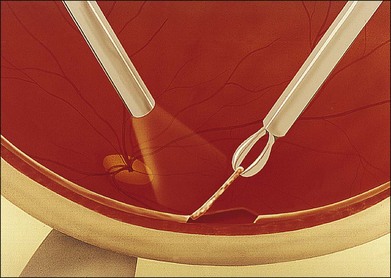
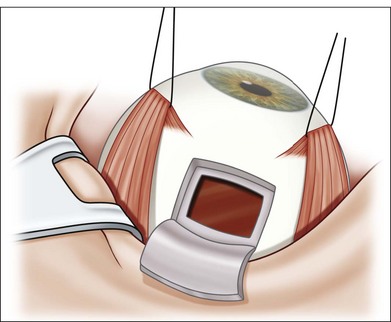
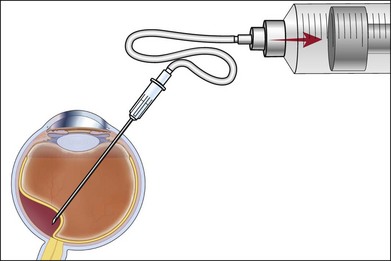
 -inch needle is inserted through the pars plana in this eye and into the tumor, which is located posteriorly. Once the needle is within the lesion to be biopsied, an assistant exerts forceful suction on a 10 mL syringe connected to the needle via tubing.
-inch needle is inserted through the pars plana in this eye and into the tumor, which is located posteriorly. Once the needle is within the lesion to be biopsied, an assistant exerts forceful suction on a 10 mL syringe connected to the needle via tubing.