Viruses in Human Disease
1. List the common human respiratory viruses and modes of transmission.
2. Differentiate between viral antigenic shift and antigenic drift. Explain how each occurs, its effect on the production of vaccine, and why is it an important consideration in the study of the influenza virus.
3. Define the term “pandemic” and identify historical pandemics within the past century, including the latest influenza pandemic.
4. List the serotypes of rhinovirus and explain how testing for rhinovirus is accomplished and how it differs from testing for the other respiratory viruses.
5. List some of the most common human arboviruses.
6. Define arbovirus and describe the mode of transmission.
7. List the viruses responsible for viral encephalitis.
8. Name the most common sexually transmitted viral diseases.
9. Define tissue tropism associated with human papillomavirus (HPV) and explain the relationship between HPV and cervical cancer.
10. Define skin exanthema and identify the most common types affecting children.
11. Compare human gastrointestinal viruses, stating the types that affect adults more frequently and those that affect children.
12. Define hanta pulmonary syndrome; identify the disease-causing virus and the mode of transmission.
13. Name the family of viruses responsible for the skin eruptions orf and molluscum contagiosum.
14. List the family of viruses responsible for outbreaks of severe disease among military recruits and describe the recommended preventive measures.
15. Define the viral proteins hemagglutinin and neuraminidase; explain how these proteins function to ensure the transmissibility and reproducibility of the influenza virus.
16. Correlate the agents of specific infections shown in the following box with diseases and pathologic manifestations, including routes of transmission and appropriate diagnostic tests.
Viruses in Human Disease
Viruses of medical importance to humans comprise seven families of deoxyribonucleic acid (DNA) viruses and fourteen families of ribonucleic acid (RNA) viruses. This chapter examines the specific families of viruses, including the diseases and the symptoms associated with the viral infection. Tables 66-1 and 66-2 present a quick reference to the viral families and syndromes caused by these viruses. Table 66-1 divides the virus families according to the makeup of the viral genome, either RNA or DNA. Table 66-2 lists some of the common human viral infections.
TABLE 66-1
DNA and RNA Viruses That Cause Serious Disease in Humans
| Family | Viral Members |
| DNA Viruses | |
| Adenoviridae | Human adenoviruses |
| Hepadnaviridae | Hepatitis B virus |
| Herpesviridae | HSV types I and II, VZV, CMV, EBV, human herpes viruses 6, 7, and 8 |
| Papillomaviridae | Human papilloma viruses |
| Parvoviridae | Parvovirus B-19 |
| Polyomaviridae | BK and JC polyomaviruses |
| Poxviridae | Variola, vaccinia, orf, molluscum contagiosum, monkeypox viruses |
| RNA Viruses | |
| Arenaviridae | Lymphocytic choriomeningitis virus, Lassa fever virus |
| Astroviridae | Gastroenteritis-causing astroviruses |
| Bunyaviridae | Arboviruses, including California encephalitis and Lacrosse viruses; nonarboviruses, including sin nombre and related hantaviruses |
| Caliciviridae | Noroviruses and hepatitis E virus |
| Coronaviridae | Coronaviruses, including SARS coronavirus |
| Filoviridae | Ebola and Marburg hemorrhagic fever viruses |
| Flaviviridae | Arboviruses, including yellow fever, dengue, West Nile, Japanese encephalitis, and St. Louis encephalitis viruses; nonarboviruses, including hepatitis C virus |
| Orthomyxoviridae | Influenza A, B, and C viruses |
| Paramyxoviridae | Parainfluenza viruses, mumps virus, measles virus, RSV, metapneumovirus, Nipah virus |
| Picornaviridae | Polio viruses, coxsackie A viruses, coxsackie B viruses, echoviruses, enteroviruses 68-71, enterovirus 72 (hepatitis A virus), rhinoviruses |
| Reoviridae | Rotavirus spp., Colorado tick fever virus |
| Retroviridae | HIV types 1 and 2, HTLV types 1 and 2 |
| Rhabdoviridae | Rabies virus |
| Togaviridae | Eastern, Western, and Venezuela equine encephalitis viruses, rubella virus |
CMV, Cytomegalovirus; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; HTLV, human T-lymphotropic viruses; RSV, respiratory syncytial virus; SARS, severe acute respiratory syndrome; VZV, varicella-zoster virus.
TABLE 66-2
Viral Syndromes and Common Viral Pathogens
| Viral Syndrome | Viral Pathogens |
| Infants and Children | |
| Upper respiratory tract infection | Rhinovirus, coronavirus, parainfluenza, adenovirus, RSV, influenza |
| Pharyngitis | Adenovirus, coxsackie A, HSV, EBV, rhinovirus, parainfluenza, influenza |
| Croup | Parainfluenza, RSV, metapneumovirus |
| Bronchitis | Parainfluenza, RSV, metapneumovirus |
| Bronchiolitis | RSV, parainfluenza, metapneumovirus |
| Pneumonia | RSV, adenovirus, influenza, parainfluenza |
| Gastroenteritis | Rotavirus, adenovirus 40-41, calicivirus, astrovirus |
| Congenital and neonatal disease | HSV-2, echovirus, and other enteroviruses, CMV, parvovirus B-19, VZV, HIV, hepatitis viruses |
| Adults | |
| Upper respiratory tract infection | Rhinovirus, coronavirus, adenovirus, influenza, parainfluenza, EBV |
| Pneumonia | Influenza, adenovirus, sin nombre virus (hantavirus), SARS coronavirus |
| Pleurodynia | Coxsackie B |
| Gastroenteritis | Noroviruses |
| All Patients | |
| Parotitis | Mumps, parainfluenza |
| Myocarditis/pericarditis | Coxsackie B and echoviruses |
| Keratitis/conjunctivitis | HSV, VZV, adenovirus, enterovirus 70 |
| Pleurodynia | Coxsackie B |
| Herpangina | Coxsackie A |
| Febrile illness with rash | Echoviruses and coxsackie viruses |
| Infectious mononucleosis | EBV, CMV |
| Meningitis | Echoviruses and coxsackie viruses; mumps, lymphocytic choriomeningitis viruses; HSV-2 |
| Encephalitis | HSV-1, togaviruses, bunyaviruses, flaviviruses, rabies virus, enteroviruses, measles virus, HIV, JC virus |
| Hepatitis | Hepatitis A, B, C, D (delta agent), E, and non-A, B, C, D, E viruses |
| Hemorrhagic cystitis | Adenovirus, BK virus |
| Cutaneous infection with or without rash | HSV types 1 and 2; VZV; enteroviruses; measles, rubella viruses; parvovirus B-19; human herpes virus 6 and 7; HPV; poxviruses, including smallpox, monkeypox, molluscum contagiosum, and orf |
| Hemorrhagic fever | Ebola, Marburg, Lassa, yellow fever, dengue, and other viruses |
| Generalized, no specific target organ | HIV-1, HIV-2, HTLV-1 |
CMV, Cytomegalovirus; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; HPV, human papillomavirus; HSV, herpes simplex virus; HTLV, human T-lymphotropic viruses; RSV, respiratory syncytial virus; SARS, severe acute respiratory syndrome; VZV, varicella-zoster virus.
Adenoviruses
Adenoviruses (Table 66-3) are medium-sized (70 to 90 nm), icosahedral, nonenveloped, double-stranded, linear DNA viruses. This virus was first isolated from cultures of human adenoids and tonsils in the early 1950s, hence the name adenovirus. The adenoviruses belong to the family Adenoviridae and are widely distributed in nature. However, only members of the genus Mastadenovirus cause human infection. Currently, 52 serotypes of human adenoviruses have been described. Most human disease is associated with one third of the viral types. These types are then divided into seven species, A through G, with species B subdivided into two subspecies; virus serotypes are then numbered within the species classification. The viruses can cause a broad range of disease in humans. Respiratory and gastrointestinal diseases are the most common clinical manifestation associated with adenovirus infection.
TABLE 66-3
| Family | Adenoviridae |
| Common name | Adenovirus |
| Virus | Adenovirus |
| Characteristics | Double-stranded DNA genome; icosahedral capsid, no envelope; approximately 50 human serotypes |
| Transmission | Respiratory, fecal-oral, and direct contact (eye) |
| Site of latency | Replication in oropharynx |
| Disease | Pharyngitis, pharyngoconjunctival fever, keratoconjunctivitis, pneumonia, hemorrhagic cystitis, disseminated disease, and gastroenteritis in children |
| Diagnosis | Cell culture (HEp-2 and other continuous human epithelial lines), enzyme immunoassay (EIA) for gastroenteritis serotypes 40-41 |
| Treatment | Supportive |
| Prevention | Vaccine (adenovirus serotypes 4 and 7) for military recruits |
Arenaviruses
Arenaviruses, of the family Arenaviridae, include 29 spherical, enveloped RNA viruses that have T-shaped glycoprotein spikes 7 to 10 nm long surrounding the surface membrane of the virion (Table 66-4). The viruses can readily infect a variety of mammalian species, especially rodents and bats, often resulting in a deleterious effect on the reservoir rodent host. Human transmission usually occurs through inhalation of aerosols of infected rodent excrement (urine, saliva, feces, nasal secretions) or by direct contact with infected rodents. Disease in humans clinically displays a broad range of symptoms, from asymptomatic (no symptoms) to fever, prostration, headache and vomiting, to the more severe cases of meningitis and hemorrhagic fever.
TABLE 66-4
| Family | Arenaviridae |
| Common name | Arenavirus |
| Virus | Lymphocytic choriomeningitis (LCM) and Lassa fever (Lassa, Nigeria) viruses |
| Characteristics | Enveloped, irregular-shaped capsid containing a two-segmented (each segment is circular), single-stranded RNA genome |
| Transmission | From rodent to human through contamination of human environment with rodent urine; virus enters through skin abrasions or inhalation |
| Disease | LCM causes asymptomatic to influenza-like to aseptic meningitis–type disease; Lassa fever virus causes influenza-like disease to severe hemorrhagic fever |
| Diagnosis | Serology, polymerase chain reaction |
| Treatment | Supportive for LCM; ribavirin and immune plasma for Lassa fever |
| Prevention | Avoid contact with virus, institute rodent control; isolation and barrier nursing prevent nosocomial spread |
Bunyaviruses
Bunyaviruses, first detected in Bunyamwera, Uganda, belong to the family Bunyaviridae (Table 66-5). The virus is an RNA virus consisting of three, single-stranded RNA segments enclosed in a helical nucleocapsid that is surrounded by a lipid envelope. A unique feature of this family of viruses is their tripartite genome. The genomic structure provides a mechanism for genetic reassortment in nature, much like the orthomyxovirus family of viruses. Bunyaviruses comprise a large, diverse group of viruses (approximately 300 total members with 12 human pathogens), most of which are transmitted by mosquitoes (arboviruses).
TABLE 66-5
| Family | Bunyaviridae |
| Common name | Bunyavirus |
| Virus | Arboviruses,* including the California encephalitis group containing Lacrosse virus, and non–arthropod-borne viruses, including hantaviruses (containing sin nombre virus) |
| Characteristics | Segmented, single-stranded, RNA genome; spherical or pleomorphic capsid with envelope |
| Transmission | Mosquito, tick, and sandfly vectors, except for hantaviruses, which are zoonoses transmitted by contact with rodent host and/or their excretions |
| Disease | Encephalitis for arboviruses; pneumonia or hemorrhagic fever for hantaviruses |
| Diagnosis | Serology and antibody detection in cerebrospinal fluid, reverse transcriptase polymerase chain reaction (RT-PCR) for hantaviruses (serology [IgM and IgG]) also available for hantavirus (sin nombre virus) |
| Treatment | Supportive |
| Prevention | Avoid contact with arthropod vector. Vector control programs; hantaviruses, avoid rodent urine and feces |
Caliciviruses
Caliciviruses are small (30 to 38 nm), rounded, nonenveloped, single-stranded, positive RNA viruses that cause acute gastroenteritis in humans. Caliciviruses (Table 66-6) have been previously recognized as major animal pathogens and have a broad host range and disease manifestation. The virus causes respiratory disease in cats, a vesicular disease in swine, and a hemorrhagic disease in rabbits. Not until the 1990s did the taxonomic status of noroviruses (formerly known as Norwalk-like viruses, named after Norwalk, Ohio) and hepatitis E virus result in classification in the family Caliciviridae. Hepatitis E virus has since been removed from the calicivirus family and included in a new family, the Hepeviridae. (Hepatitis E virus is discussed later in this chapter.)
TABLE 66-6
| Family | Caliciviridae |
| Common name | Calicivirus |
| Virus | Noroviruses |
| Characteristics | Nonenveloped, icosahedral capsid surrounding single-stranded RNA genome |
| Transmission | Fecal-oral |
| Disease | Nausea, vomiting, and diarrhea |
| Diagnosis | EM, RT-PCR, EIA for noroviruses |
| Treatment | Supportive |
| Prevention | Avoid contact with virus |
EIA, Enzyme immunoassay; EM, electron microscopy; RT-PCR, reverse transcriptase polymerase chain reaction.
Coronaviruses
The family Coronaviridae includes the genera Torovirus and Coronavirus (CoV) and contains many species of both human and animal origin (Table 66-7). Once considered a harmless virus capable of causing the human “cold,” the CoVs cause a wide variety of disease in animals and birds. Interest in this virus and its relationship with animals and humans was renewed after the global outbreak of the novel coronavirus severe acute respiratory syndrome (SARS) in 2002 that resulted in severe respiratory distress in the human population. (The SARS outbreak is discussed in detail later in this chapter.) Coronaviruses are pleomorphic, roughly spherical, medium-sized, enveloped RNA viruses. The prefix corona- results from the viral structure and the crownlike surface projections on the external surface of the virus that can be seen with electron microscopy. Human respiratory coronaviruses cause colds and occasionally pneumonia in adults. Together the rhinoviruses and coronaviruses cause more than 55% of the “common colds” in the human populations. Viral transmission is person to person via contaminated respiratory secretions or aerosols. The virus is present in the highest concentration in the nasal passages, where it infects the nasal epithelial cells. Coronaviruses are thought to cause diarrhea in infants based on the presence (as seen with electron microscopy) of coronavirus-like particles in the stool of symptomatic patients. Although antigen detection is available, the technique lacks sensitivity compared with nucleic acid–based testing. No practical diagnostic methods other than electron microscopy and RT-PCR are available. Many CoVs do not grow in routine cell culture. Modified cell cultures have been useful when confirmatory testing with antigen- or nucleic acid–based methods are used.
TABLE 66-7
| Family | Coronaviridae |
| Common name | Coronaviruses |
| Virus | Coronavirus |
| Characteristics | Single-stranded, RNA genome; helical capsid with envelope |
| Transmission | Unknown, probably direct contact or aerosol |
| Disease | Common cold; possibly gastroenteritis, especially in children; SARS |
| Diagnosis | EM, RT-PCR |
| Treatment | Supportive |
| Prevention | Avoid contact with virus |
EM, Electron microscopy; RT-PCR, reverse transcriptase polymerase chain reaction; SARS, severe acute respiratory syndrome.
Filoviruses
The Filoviridae family of viruses (Table 66-8) is considered the most pathogenic of the hemorrhagic fever viruses. The term filo means threadlike, referring to the virus’s long, filamentous structural morphology seen with electron microscopy. The viruses are pleomorphic, enveloped, nonsegmented, single-stranded, negative sense RNA viruses. The filamentous morphology appears in many forms or configurations under the electron microscope, such as the number “6,” “U,” or circular. Marburg hemorrhagic fever virus displays the characteristic “shepherd’s hook” morphology. The term “viral hemorrhagic fever” is used to describe a severe multisystem syndrome in which multiple organ systems are affected throughout the body. The patient’s vascular system becomes damaged, and the body’s ability to regulate itself is impaired. Infection with the Marburg or Ebola virus, endemic in Africa, results in severe hemorrhages, vomiting, abdominal pain, myalgia, pharyngitis, conjunctivitis, and proteinuria. Human case fatality rates for Ebola virus infection exceed 80%; the toll for Marburg virus infection is somewhat lower, with a case fatality rate of 23% to 25%. These diseases have no cure or established drug treatment.
TABLE 66-8
| Family | Filoviridae |
| Common name | Filovirus |
| Virus | Ebola (or Ebola-Reston) and Marburg viruses |
| Characteristics | Enveloped, long, filamentous and irregular capsid forms with single-stranded RNA |
| Transmission | Transmissible to humans from monkeys and, presumably, other wild animals; human-to-human transmission via body fluids and respiratory droplets |
| Disease | Severe hemorrhage and liver necrosis; mortality as high as 90% |
| Diagnosis | Electron microscopy, cell culture in monkey kidney cells; Biosafety Level 4 required |
| Treatment | Supportive |
| Prevention | Avoid contact with virus; export prohibitions on wild monkeys |
Flaviviruses
The flaviviruses (family Flaviviridae; Table 66-9) include viruses that cause arbovirus diseases, such as yellow fever, dengue, West Nile viral encephalitis, and Japanese and St. Louis encephalitis. Hepatitis C virus (HCV) is a flavivirus but not an arbovirus. Flaviviruses are small, single-stranded, positive sense RNA, enveloped, icosahedral viruses. The name is derived from the Latin word flavus, which means yellow. The first disease identified in this group was yellow fever, which causes yellow jaundice in humans. Diseases in this viral group are transmitted to humans through the bite of an infected arthropod, usually the mosquito.
TABLE 66-9
| Family | Flaviviridae |
| Common name | Flavivirus |
| Characteristics | Single-stranded RNA genome surrounded by spherical and icosahedral capsid with envelope |
| Virus | Arboviruses,* including yellow fever, dengue, West Nile, Japanese encephalitis, and St. Louis encephalitis viruses |
| Transmission | Arthropod vector, usually mosquito |
| Disease | St. Louis and West Nile encephalitis, dengue and yellow fever |
| Diagnosis | Serology and antibody detection in cerebrospinal fluid; reverse transcriptase polymerase chain reaction (RT-PCR) for dengue and yellow fever |
| Treatment | Supportive |
| Prevention | Avoid contact with vector; vector control programs |
| Virus | Hepatitis C virus |
| Transmission | Parenteral or sexual |
| Disease | Acute and chronic hepatitis; strong correlation between chronic HCV infection and hepatocellular carcinoma |
| Diagnosis | Serology, RT-PCR and viral genotyping |
| Treatment | Supportive, interferon |
| Prevention | Avoid contact with virus; blood supply screened for antibody to hepatitis C virus |
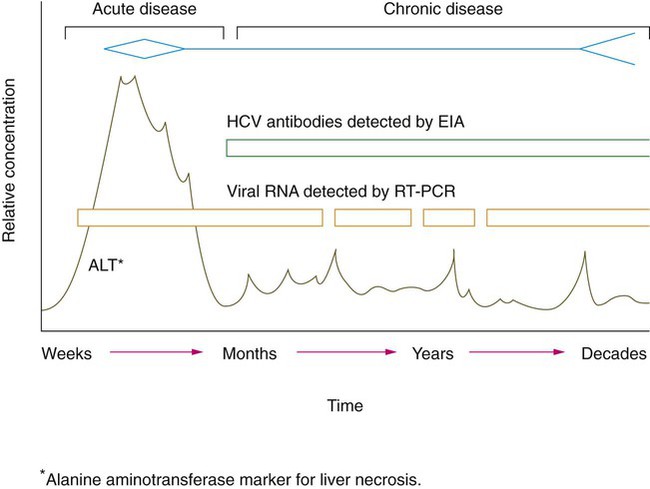
The hepatitis C virus causes hepatitis. Worldwide, an estimated 170 million people are HCV carriers, and about 4 million of those live in the United States. Acute infection with HCV progresses to a chronic infection in 50% to 90% of infected individuals (Figure 66-1). The acute infection with HCV often goes undiagnosed, because it is often asymptomatic. When clinical illness is present, it is generally mild. Chronic infection with HCV is an important cause of liver disease and is associated with the development of end-stage liver disease and hepatocellular carcinoma. The virus is transmitted predominantly by exposure to infected blood, such as during intravenous drug use and administration of contaminated blood products. The screening of blood products for HCV has eliminated the risk of transmission through contaminated blood products. Less efficient modes of transmission include sexual contact with infected partners, acupuncture, tattooing, and sharing of razors.
Hepevirus
Hepatitis E virus (HEV) is the type species of the new genus Hepevirus, in the family Hepeviridae (Table 66-10). Previously classified in the family of caliciviruses, HEV is a small, nonenveloped virus with a single-stranded RNA genome. The only other member of this virus family is an avian HEV known to cause enlarged liver and spleen disease in chickens. Several genetic and antigenic variants or strains of HEV exist and are referred to as genotypes. The different viral strains are common to different geographic locations. Genotype 3 is the strain found in the United States. HEV has also been isolated from swine worldwide and from wild deer in Japan. This indicates that the potential for transmission from animal to humans, resulting in a zoonotic human infection.
TABLE 66-10
| Family | Hepeviridae |
| Common name | Hepatitis E |
| Virus | Hepevirus |
| Characteristics | Nonenveloped, icosahedral capsid surrounding single-stranded RNA genome |
| Transmission | Fecal-oral |
| Disease | Hepatitis similar to that caused by hepatitis A virus except for extraordinarily high case fatality rate (10% to 20%) among pregnant women |
| Diagnosis | Serology |
| Treatment | Supportive |
| Prevention | Avoid contact with virus |
Hepadnaviruses
Hepatitis B virus (HBV) (Table 66-11) is the prototype virus of the Hepadnaviridae family (hepa- from hepatitis and dna from the genome type). The virus has long been recognized as a significant cause of liver damage associated with morbidity and mortality. Other mammalian and avian hepadnaviruses are known to exist. Hepadnavirus is a pleomorphic, enveloped virus containing circular, partially double-stranded DNA that replicates through an RNA intermediate. Replication occurs by means of reverse transcription and then DNA replication.
TABLE 66-11
| Family | Hepadnaviridae |
| Common name | Hepadnavirus |
| Virus | Hepatitis B virus (HBV) |
| Characteristics | Partly double-stranded DNA genome; icosahedral capsid with envelope; virion (also called Dane particle); surface antigen originally termed “Australia antigen” |
| Transmission | Humans are reservoir and vector; spread by direct contact, including exchange of body secretions, recipient of contaminated blood products, percutaneous injection of virus, and perinatal exposure |
| Site of latency | Liver |
| Disease | Acute infection with resolution (90%); fulminant hepatitis, most co-infected with delta virus (1%); chronic hepatitis, persistence of hepatitis B surface antigen (HBsAg) (9%) followed by resolution (disappearance of HBsAg), asymptomatic carrier state, chronic persistent (systemic disease without progressive liver disease), or chronic active disease (progressive liver damage) |
| Diagnosis | Serology, viral antigen detection, and polymerase chain reaction (PCR) |
| Oncogenic | Liver cancer |
| Treatment | Antivirals and liver transplant for fulminant disease |
| Prevention | HBV vaccine; hepatitis B immune globulin |
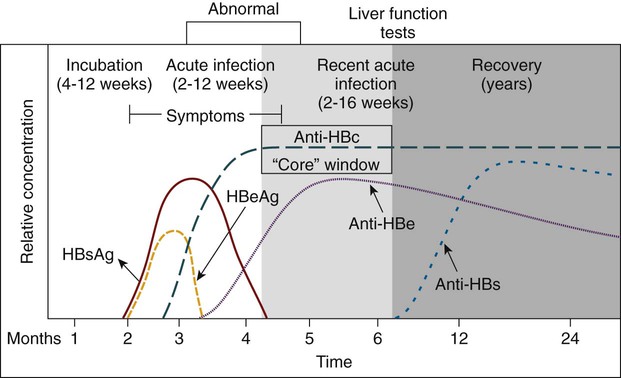
The incubation period for an acute HBV infection usually is 1 to 3 months but may be considerably longer. The initial symptoms of acute infection often are nonspecific, much like mild, flulike symptoms (Figure 66-2). Many cases are asymptomatic, especially in children. The infection presents as an acute or chronic hepatitis with a pathologic effect on the liver, resulting in self-limited or fatal outcomes. Fatal disease is most likely to occur in people co-infected with the hepatitis D virus (delta agent), a deficient RNA virus capable of replication in cells infected with HBV. Chronic HBV infection remains a significant worldwide cause of liver cirrhosis and hepatocellular carcinoma despite the availability of an effective vaccine.
Herpes Viruses
Eight human herpes group viruses have been described (Table 66-12). Herpes viruses are widely disseminated among animal species. However, the zoonotic forms of herpes do not infect humans, except for herpes B virus from nonhuman primates (not counted among the eight human herpes viruses). Herpes B virus causes a severe, usually fatal encephalitis in humans. Human herpes viruses include HSV types 1 and 2 (HSV-1 and HSV-2), varicella-zoster virus (VZV), Epstein-Barr virus (EBV), and cytomegalovirus (CMV). More recently detected herpes viruses include human herpes virus (HHV) types 6 (HHV-6), 7 (HHV-7), and 8 (HHV-8). HHV-6 and HHV-7 are lymphotropic viruses acquired early in life. HHV-8, Kaposi’s sarcoma–associated herpes virus (KSHV), causes a tumor of the connective tissue. HHV-6 and HHV-7 are associated with the childhood disease roseola (exanthem subitum). The disease is characterized by a short period of fever and a skin rash.
TABLE 66-12
| Family | Herpesviridae |
| Common name | Herpesvirus |
| Characteristics | Double-stranded DNA genome; icosahedral capsid with envelope; at least eight human herpes viruses known: HSV-1, HSV-2, VZV, Epstein-Barr virus (EBV), CMV, HHV-6, HHV-7, and HHV-8 |
| Virus | Herpes simplex virus types I and II (HSV-1 and HSV-2) |
| Transmission | Direct contact with infected secretions |
| Site of latency | Sensory nerve ganglia |
| Disease | Predominant virus in parentheses. Gingivostomatitis (HSV-1), pharyngitis (HSV-1), herpes labialis (HSV-1), genital infection (HSV-2), conjunctivitis (HSV-1), keratitis (HSV-1), herpetic whitlow (HSV-1 and HSV-2), encephalitis (HSV-1 in adults), disseminated disease (HSV-1 or HSV-2 in neonates) |
| Detection | Cell culture (HDF, others), EIA, FA stain, IH stain, PCR |
| Treatment | Acyclovir, valacyclovir, famciclovir |
| Prevention | Avoid contact |
| Virus | Varicella-zoster virus (VZV) |
| Transmission | Close personal contact, especially respiratory |
| Site of latency | Dorsal root ganglia |
| Disease | Chicken pox (varicella), shingles (zoster) |
| Detection | FA stain, cell culture (HDF), shell vial culture, PCR |
| Treatment | Acyclovir and famciclovir |
| Prevention | Vaccine |
| Virus | Epstein-Barr virus (EBV) |
| Transmission | Close contact with infected saliva |
| Site of latency | B lymphocytes |
| Disease | Infectious mononucleosis, progressive lymphoreticular disease, oral hairy leukoplakia in patients with HIV |
| Detection | Serology, PCR |
| Oncogenic | Burkitt’s lymphoma, nasopharyngeal carcinoma |
| Treatment | Supportive |
| Prevention | Avoid contact |
| Virus | Cytomegalovirus (CMV) |
| Transmission | Close contact with infected secretions, blood transfusions (WBCs), organ transplants, transplacental |
| Site of latency | WBCs, endothelial cells, cells in a variety of organs |
| Disease | Asymptomatic infection, congenital disease of newborn, symptomatic disease of immunocompromised host, heterophile-negative infectious mononucleosis |
| Diagnosis | Cell culture (HDF), shell vial culture, CMV antigenemia, FA stain, PCR |
| Treatment | Supportive; decrease immune suppression; ganciclovir and foscarnet |
| Prevention | Use CMV antibody-negative blood and tissue for transfusion and transplantation, respectively |
| Virus | Human herpesviruses 6 and 7 (HHV-6 and HHV-7) |
| Transmission | Most likely close contact via respiratory route; almost all children infected by age 2 to 3 years |
| Site of latency | T lymphocytes (CD4 cells) |
| Disease | Roseola (exanthem subitum), fever, malaise, rash, leukopenia, and interstitial pneumonitis in organ transplant recipients |
| Detection | Detection of virus in peripheral blood specimens by PCR, cell culture using lymphocyte lines |
| Treatment | Susceptible to ganciclovir and foscarnet |
| Prevention | None practical |
| Virus | Human herpesvirus 8 (HHV-8) |
| Transmission | Not known; much less widely disseminated than other herpes viruses |
| Site of latency | Viral genome found in Kaposi’s tumor cells, endothelial cells, and tumor-infiltrating leukocytes |
| Disease | Kaposi’s sarcoma |
| Detection | PCR or in situ by hybridization |
| Treatment | None known |
| Prevention | Avoid contact with virus |
EIA, Enzyme immunoassay; FA, fluorescent antibody; HDF, human diploid fibroblast; HIV, human immunodeficiency virus; IH, iron hematoxylin; PCR, polymerase chain reaction; WBCs, white blood cells.
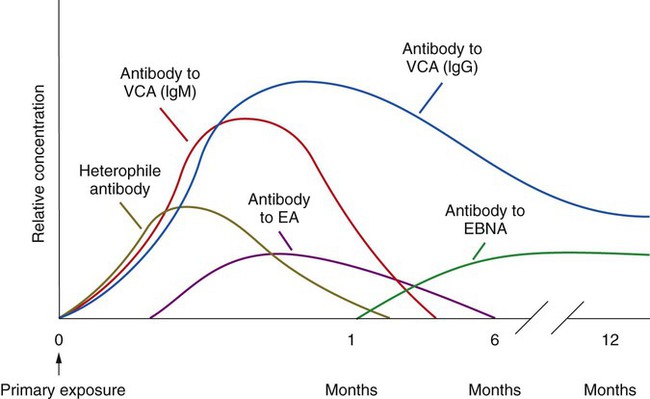
Epstein-Barr virus was discovered four decades ago during a search for the cause of Burkitt’s lymphoma, a disease that predominately affects children in Africa. EBV is responsible for the disease infectious mononucleosis (IM). The virus, which is transmitted in the saliva of infected patients, typically affects adolescents and young adults. The major symptoms include fever, sore throat, headache, malaise, and fatigue. Lymphadenopathy (swollen lymph nodes) and splenomegaly also may result during the disease. Mononucleosis typically is diagnosed through serologic methods that detect antibodies to EBV (Figure 66-3). Nonspecific heterophile antibodies (also referred to as Paul-Bunnell antibodies) appear early on during the disease, making the diagnosis difficult. Antibody production to the virus typically follows the classic immune response, resulting in specific IgM production followed by IgG production. Antibody to the viral capsid (IgM) appears within 4 weeks after infection. This is followed by IgG and IgA antibody to the early antigen (EA), indicating acute or recent infection. Both the EA-IgG and IgA may be undetectable after approximately 6 months. Some Anti-EA IgG antibodies may persist in the patient’s serum for life. These persistent antibodies typically are elevated in patients with Burkitt’s lymphoma. The final diagnostic serologic marker is the antibody to the nuclear antigen (EBNA) that appears within 1 month of infection and peaks approximately 6 to 12 months after infection. In addition to Burkitt’s lymphoma, other cancers have been associated with EBV infection (e.g., nasopharyngeal carcinoma), and the virus is recognized as an important agent in the development of lymphoma or other lymphoproliferative disorders in transplant recipients.
Molecular assays have become instrumental in the diagnosis of herpes viruses. Box 66-1 provides an outline for the basic procedure for EBV PCR amplification.
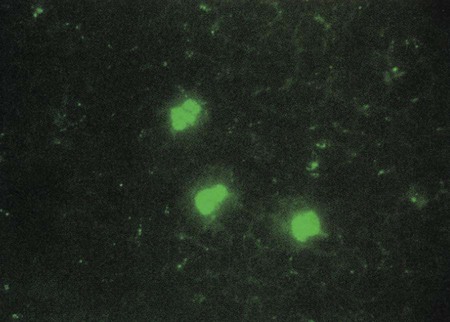
CMV infection is a common cause of congenital birth defects. The virus is included in the TORCH panel for disease screening in infants. (TORCH is an acronym for toxoplasma, rubella, CMV and HSV-1). Besides being the cause of congenital infection in infants, CMV has been found to cause an infectious mononucleosis–like illness in immunocompromised patients. The disease may be extremely serious in immunosuppressed organ transplant recipients. CMV may be identified using viral cell culture, serologic tests for IgM and IgG antibodies, direct antigen detection, and nucleic acid testing. Although the virus grows in cell culture using human fibroblasts, it is a slow-growing virus that requires 1 to 2 weeks of incubation before CPE is evident, and in some cases CPE may not be visible for a month. Centrifugation-amplified shell vials, a modification of conventional cell culture, can provide a diagnosis within 24 to 48 hours. CMV antigenemia (see Chapter 65 and Procedure 65-2) is routinely used to monitor therapy for CMV infection. A positive CMV antigenemia result is shown in Figure 66-4. Molecular qualitative and quantitative PCR amplification using analyte-specific reagents is available in some clinical laboratories. In addition, a fully automated quantitative CMV assay is available (COBAS AmpliPrep/COBAS TaqMan CMV Test, Roche Molecular Systems, Inc; Pleasanton, CA). Research studies indicate that nucleic acid-based methods are more sensitive for the detection of CMV in symptomatic and asymptomatic patients.
Orthomyxoviruses
The influenza virus is a member of the family Orthomyxoviridae (Table 66-13). The members of this family are pleomorphic, spherical, enveloped, single-stranded, segmented, negative sense RNA viruses. Of all the respiratory viruses known to infect humans, influenza is the cause of the greatest number of serious acute illnesses; more than 200,000 hospitalizations and more than 30,000 deaths occur in the United States every year. Although three types of influenza viruses are known to infect humans (A, B, and C), type C usually causes subclinical infections and is not known to pose a threat to human health. The three genera Influenza virus A, Influenza virus B, and Influenza virus C can be distinguished based on the antigenic differences in the matrix protein (M) and the nucleoprotein (NP). Influenza virus A is further subdivided based on the major surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA). Influenza A naturally infects many bird species, swine, seals, felines, and horses. Influenza B and C are only known to infect humans.
TABLE 66-13
| Family | Orthomyxoviridae |
| Common name | Orthomyxovirus |
| Characteristics | Segmented (eight separate molecules), single-stranded, RNA genome; helical capsid with envelope; three major antigenic types, influenza A, B, and C; types A and B cause nearly all human disease |
| Virus | Influenza A |
| Transmission | Contact with respiratory secretions |
| Disease | Influenza (fever, malaise, headache, myalgia, cough); primary influenza pneumonia; in children, bronchiolitis, croup, otitis media |
| Detection | Cell culture (PMK), EIA, FA stain, RT-PCR |
| Epidemiology | Viral subtypes based on hemagglutinin and neuraminidase glycoproteins abbreviated “H” and “N,” respectively (e.g., H1N1 or H3N2); infects humans and other animals; antigenic drift, resulting in minor antigenic change, causes local outbreaks of influenza every 1-3 years; antigenic shift, resulting in major antigenic change, causes periodic worldwide outbreaks |
| Treatment | Supportive; antivirals amantadine and rimantadine (influenza A only), and zanamivir and oseltamivir influenza A and B |
| Prevention | Influenza vaccine or antiviral prophylaxis |
| Virus | Influenza B |
| Transmission | Contact with respiratory secretions |
| Disease | Similar to “mild” influenza |
| Detection | Cell culture (PMK), EIA, FA stain, RT-PCR |
| Epidemiology | Antigenic drift only, resulting in local outbreaks every 1-3 years |
| Treatment | Supportive; antivirals zanamivir and oseltamivir |
| Prevention | Influenza vaccine or antiviral prophylaxis |
| Virus | Influenza C |
| Transmission | Contact with respiratory secretions |
| Disease | Mild form of influenza causing URTIs |
| Detection | Testing not routinely requested, so virus is infrequently detected; only valid test is NAAT |
| Epidemiology | Most cases occur in children; occurs sporadically or as localized outbreaks |
| Treatment | Supportive |
| Prevention | Avoid contact with virus |
EIA, Enzyme immunoassay; FA, fluorescent antibody; NAAT, nucleic acid amplified testing; RT-PCR, reverse transcriptase polymerase chain reaction; PMK, primary monkey kidney; URTIs, upper respiratory tract infections.
In addition to immunization, antiviral treatment of influenza has proven to be effective in limiting the duration and severity of the disease. Treatment options are discussed in Chapter 67.
Papillomaviruses
Papilloma viruses are small, nonenveloped, circular, double-stranded DNA viruses. These viruses are abundant in nature and cause infections in humans, dogs, cattle, monkeys, and many other species. The Papillomaviridae family (Table 66-14) includes the human papillomaviruses (HPVs). HPVs cause human warts. They exhibit a tissue tropism for either cutaneous or mucosal tissue. The viruses have not been cultivated in cell culture, which prevents the production of type-specific antigens and corresponding typing antisera. HPVs are divided into more than 200 genotypes based on the viral DNA sequences; approximately 80 of those have been well characterized. Much attention has been focused on the more than 30 sexually transmitted genotypes and their role in the pathogenesis of cancer. The various HPV genotypes have differing cellular tropisms, resulting in defined variation in the clinical presentation of the warts. For example, HPV-1 is associated with plantar warts; HPV-2 and HPV-4 are associated with common warts of the hands; and HPV-6, HPV-11, and others are associated with genital warts. Fifteen to 20 types of HPV cause virtually all cases of cervical cancer, with types 16 and 18 causing more than 60% of cases. Type 16 is also responsible for a subset of cancers of the oropharynx and penile cancer in men (Table 66-14).
TABLE 66-14
| Family | Papovaviridae |
| Common name | Papillomavirus |
| Characteristics | Double-stranded DNA genome; icosahedral capsid, no envelope; includes papilloma viruses |
| Virus | Human papilloma virus (HPV) |
| Characteristics | Contains more than 200 DNA types |
| Transmission | Direct contact, sexual contact for genital warts |
| Site of latency | Epithelial tissue |
| Disease | Skin and genital warts, benign head and neck tumors, anogenital warts |
| Diagnosis | Cytology, DNA probes |
| Oncogenic | Cervical and penile cancer (especially HPV types 16 and 18) |
| Treatment | Spontaneous disappearance the rule; surgical or chemical removal may be necessary |
| Prevention | Avoid contact with infected tissue, vaccination |
Paramyxoviruses
The Paramyxoviridae family (Table 66-15) includes many pathogenic viruses. Many of these viruses are identified more often in young children, including measles, mumps, parainfluenza viruses, and respiratory syncytial virus (RSV). Recently, human metapneumovirus and Nipah virus (Nipah is the area in Malaysia where the virus first was isolated) have been recognized as disease-causing paramyxoviruses. Paramyxoviruses do not have a segmented genome, as do the orthomyxoviruses, and therefore do not undergo antigenic shift. Paramyxoviruses are spherical, enveloped RNA viruses, and all members of this group can cause respiratory disease.
TABLE 66-15
| Family | Paramyxoviridae |
| Common name | Paramyxoviruses |
| Characteristics | Single-stranded, RNA genome; helical capsid with envelope; no segmented genome (e.g., orthomyxoviruses) |
| Virus | Measles virus |
| Transmission | Contact with respiratory secretions; extremely contagious |
| Disease | Measles, atypical measles (occurs in those with waning “vaccine” immunity), and subacute sclerosing panencephalitis |
| Detection | Cell culture (PMK) and serology |
| Treatment | Supportive; immunocompromised patients can be treated with immune serum globulin |
| Prevention | Measles vaccine |
| Virus | Mumps virus |
| Transmission | Person-to-person contact, presumably respiratory droplets |
| Disease | Mumps |
| Detection | Cell culture (PMK) and serology |
| Treatment | Supportive |
| Prevention | Mumps vaccine |
| Virus | Parainfluenza virus |
| Transmission | Contact with respiratory secretions |
| Disease | Adults: Upper respiratory disease, rarely pneumonia Children: Respiratory including croup, bronchiolitis, and pneumonia |
| Detection | Cell culture (PMK), shell vial culture, and FA stain |
| Epidemiology | Four serotypes, disease occurs year-round |
| Treatment | Supportive |
| Prevention | Avoid contact with virus |
| Virus | Respiratory syncytial virus (RSV) |
| Transmission | Person-to-person by hand and respiratory contact |
| Disease | Primarily in infants and children. Infants: Bronchiolitis, pneumonia, and croup Children: Upper respiratory disease |
| Detection | Cell culture (HEp-2), EIA, and FA stain |
| Epidemiology | Disease occurs annually late fall through early spring; nosocomial transmission can occur readily |
| Treatment | Supportive; treat severe disease in compromised infants with ribavirin |
| Prevention | Avoid contact with virus; immune globulin for infants with underlying lung disease; prevent nosocomial transmission with isolation and cohorting |
| Virus | Metapneumovirus |
| Transmission | Person to person |
| Disease | Primarily in infants and children; bronchiolitis and pneumonia |
| Detection | RT-PCR |
| Epidemiology | Winter epidemics, severity varies from year to year |
| Treatment | Supportive |
| Prevention | Avoid contact with virus |
EIA, Enzyme immunoassay; FA, fluorescent antibody; HEp-2, human epidermoid carcinoma; PMK, primary monkey kidney; RT-PCR, reverse transcriptase polymerase chain reaction.
Parvoviruses
Parvoviruses (the Latin term parvus means small) have a wide distribution among warm-blooded animals (Table 66-16). Parvovirus B-19 is the single human pathogen among the Parvoviridae. The virus is a nonenveloped, icosahedral, single-stranded DNA virus that may appear spherical on electron microscopy. Because its replication in human cells is largely restricted to erythroid progenitor cells, adult bone marrow and fetal liver cells (the site of erythropoiesis during fetal development) are the major sites of viral replication. Important diseases associated with parvovirus B-19 infection are fifth disease (the fifth of the childhood exanthems), aplastic crisis in patients with underlying hemoglobinopathies, and fetal infection (hydrops fetalis) resulting from transplacental inoculation. Parvovirus causes a biphasic illness in humans. The first phase, marked fever, malaise, myalgia, and chills, corresponds to peak levels of virus and destruction of erythroblasts. This phase, when mild, may be overlooked or considered a nonspecific viral disease. The second phase involves rash and arthralgia, which occur after the virus has disappeared but at a time when parvovirus-specific antibody can be detected. This is consistent with the appearance of the rash caused by immune complex deposition in the capillaries of the skin. IgM antibodies appear within 7 days after infection, followed by IgG at approximately 14 days. Laboratory diagnosis is accomplished using parvovirus-specific IgM or virus-specific IgG antibody testing with paired acute and convalescent sera or by detection of viral DNA using PCR. Parvovirus cannot be cultivated in the typical cells available in clinical virology laboratories.
TABLE 66-16
| Family | Parvoviridae |
| Common name | Parvovirus |
| Virus | Parvovirus B-19 |
| Characteristics | Single-stranded DNA virus; icosahedral capsid, no envelope; parvovirus B-19 is the only known human parvovirus |
| Transmission | Close contact, probably respiratory |
| Disease | Erythema infectiosum (fifth disease), aplastic crises in patients with chronic hemolytic anemias, and fetal infection and stillbirth |
| Detection | Serology, polymerase chain reaction (PCR), histology |
| Treatment | Supportive |
| Prevention | Avoid contact |
Picornaviruses
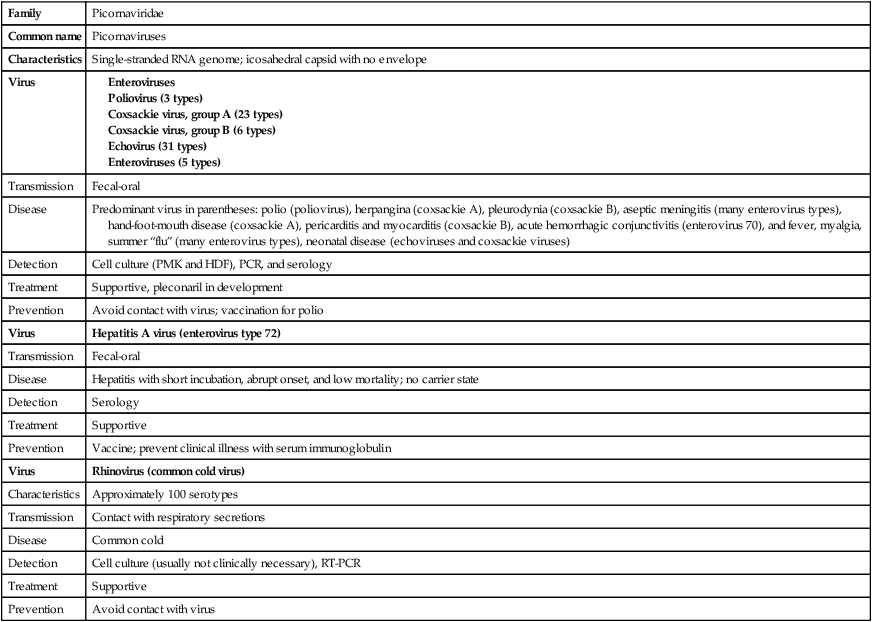
Picornaviruses (Table 66-17) are small (from the Italian word piccolo, meaning small), nonenveloped, single-stranded RNA viruses. They are among the simplest of the RNA viruses, with a highly structured capsid that has limited surface elaboration. This family of viruses includes the enteroviruses, rhinoviruses, and HAV. Enterovirus infections are among the most common human viral infections (Table 66-18), and although these infections often are mild, the viruses also can cause serious disease. Enteroviruses are responsible for a variety of diseases and conditions, including aseptic meningitis, paralytic poliomyelitis, and encephalitis, in addition to respiratory illness, myocarditis, and pericarditis. Enteroviruses are the most common cause of aseptic meningitis, an inflammation of the brain parenchyma, and have been isolated from more than 40% of patients with this disease.
TABLE 66-17
| Family | Picornaviridae |
| Common name | Picornaviruses |
| Characteristics | Single-stranded RNA genome; icosahedral capsid with no envelope |
| Virus |
EIA, Enzyme immunoassay; FA, fluorescent antibody; HDF, human diploid fibroblasts; HEp-2, human epidermoid carcinoma; PCR, polymerase chain reaction; PMK, primary monkey kidney; RT-PCR, reverse transcriptase polymerase chain reaction.
TABLE 66-18
| Central nervous system | Aseptic meningitis, encephalitis, flaccid paralysis |
| Respiratory | Mild upper respiratory tract (URT) illness (common cold), lymphonodular pharyngitis, bronchiolitis, bronchitis, pneumonia |
| Exanthems | Hand-foot-mouth disease, herpangina |
| Cardiac | Myocarditis, pericarditis |
| Other | Pleurodynia, acute hyperemia conjunctivitis (AHC), neonatal disseminated disease, chronic infection of agammaglobulinemic patients |
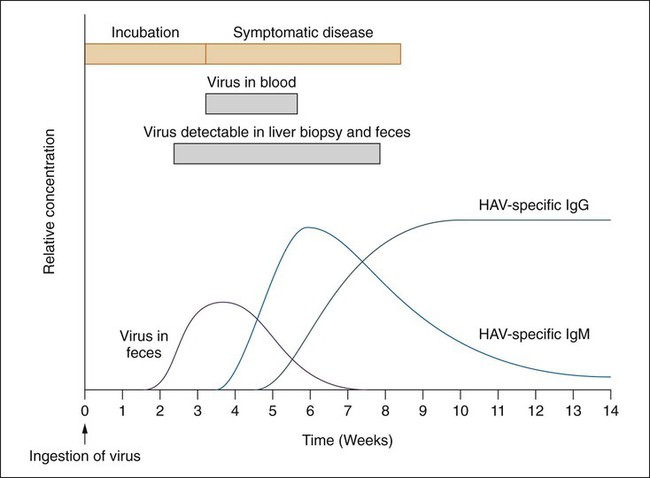
Hepatitis A virus, another member of the picornaviruses, causes an infectious nonchronic hepatitis. HAV is usually transmitted through contaminated food or water or household contact with an infected person. Other transmission routes include sharing of contaminated needles (illicit drug use), travel to endemic countries, and homosexual male intercourse. The virus is significantly different from the other picornavirus based on the liver tissue tropism, high thermal stability, and viral assembly. This is the only hepatitis group of viruses capable of growth in cell culture. However, currently diagnosis is still completed using a serologic assay to identify the IgM antibody (Figure 66-5). A vaccine against this virus for adults and for children older than 2 years of age became available during the 1990s.
Polyomaviruses
The polyomaviruses (Table 66-19) are small, nonenveloped, circular, double-stranded DNA viruses that have been isolated from many species, including humans. The first human viruses included the JC and BK viruses, named with the initials of the patients from whom the viruses were first isolated. Infection with these viruses usually occurs during childhood and has little clinical significance. These viral infections include latent states in the kidney and B lymphocytes and can result in symptomatic reactivation during periods of immune suppression.
TABLE 66-19
| Family | Polyomaviridae |
| Common name | Polyomavirus |
| Virus | Polyomavirus (BK virus [BKV] and JC virus [JCV] infect humans) |
| Characteristics | Double-stranded DNA genome; icosahedral capsid, no envelope; includes BK and JC polyomaviruses |
| Transmission | Probably direct contact with infected respiratory secretions; both viruses are ubiquitous in humans |
| Site of latency | Kidney |
| Disease | Mild or asymptomatic primary infection, virus remains dormant in kidneys; reactivation in immunocompromised patients causes hemorrhagic cystitis (BKV) or progressive multifocal leukoencephalopathy (JCV) |
| Detection | JCV by polymerase chain reaction (PCR) (cerebrospinal fluid) or electron microscopy (EM) (brain tissue); BKV by PCR or cytology (urine) |
| Treatment | Supportive; decrease immune suppression |
| Prevention | Avoid contact with virus; prevention of acquisition of virus unlikely |
Poxviruses
The poxviruses (Table 66-20) are the largest and most complex of all viruses. The virions consist of a double-stranded DNA genome. The virions appear as oval or brick-shaped structures 200 to 400 nm in length. Because of their large size, poxvirus virions may be visualized through a light microscope.
TABLE 66-20
| Family | Poxviridae |
| Common name | Poxvirus |
| Virus | Smallpox, molluscum contagiosum, orf, and monkeypox viruses |
| Characteristics | Largest and most complex of all viruses; brick-shaped virion with nonconforming symmetry, referred to as complex; double-stranded DNA genome |
| Transmission | Respiratory droplets (smallpox); direct contact (molluscum contagiosum, orf, monkeypox) |
| Disease | All are diseases of the skin; smallpox is a generalized infection with pustular rash (10% to 25% fatal); molluscum contagiosum manifests as benign nodules; orf manifests as localized papules/vesicles; monkeypox manifests as a generalized infection that includes the skin |
| Detection | Electron microscopy (EM) of material from a skin lesion; polymerase chain reaction (PCR) |
| Epidemiology | Smallpox was eradicated in 1977; smallpox and molluscum contagiosum are limited to humans; orf and monkeypox are zoonoses |
| Treatment | Supportive |
| Prevention | Vaccine for smallpox; avoid contact for all viruses |
Reoviruses
The reoviruses (Table 66-21) were first isolated from respiratory and enteric specimens and therefore are referred to as respiratory-enteric-orphan viruses (reoviruses). The term “orphan” originally was included in the description of the virus as a result of the absence of an associated disease when the viruses were first described. Reoviruses infect most mammalian species and are readily detected in water contaminated with animal feces. Common human pathogens of this family include the rotavirus and the agent of Colorado tick fever. Rotaviruses are nonenveloped, double-stranded RNA viruses composed of three concentric protein shells, the outer shell, the inner shell, and the core. Based on the proteins present in these shells, rotavirus is further classified into seven distinct groups, A through G; groups A, B and C cause human disease. Rotavirus is now recognized as the major causative agent of infantile severe gastroenteritis throughout the world. Worldwide, rotavirus is responsible for more than 111 million cases per year, resulting in more than 2 million hospitalizations and 352,000 to 592,000 deaths. Gastroenteritis caused by rotavirus can occur in children of all ages but is most common in infants from 6 months to 3 years old. The disease is characterized by sudden onset of vomiting, followed by explosive, watery diarrhea and moderate to high fever, often accompanied by dehydration. The severity of the disease often is worse for children in developing countries because of malnutrition and limited or delayed health care. Rotaviruses are transmitted by the fecal-oral route, although airborne transmission has been suspected as the cause of nosocomial infections and outbreaks in nursing homes, hospitals, and day care centers. Rotavirus occurs more frequently in the winter months in temperate climates.
TABLE 66-21
| Family | Reoviridae |
| Common name | Reovirus |
| Virus | Rotavirus |
| Characteristics | Segmented, double-stranded, RNA genome; icosahedral capsid with no envelope |
| Transmission | Fecal-oral; survives well on inanimate objects |
| Disease | Gastroenteritis in infants and children 6 months to 2 years |
| Detection | Enzyme immunoassay (EIA), latex agglutination (LA) |
| Epidemiology | Winter-spring seasonality in temperate climates; nosocomial transmission can occur easily |
| Treatment | Supportive, especially fluid replacement |
| Prevention | Avoid contact with virus; vaccination |
Retroviruses
The retrovirus family Retroviridae (Table 66-22) constitutes a large group of viruses that primarily infect vertebrates. They are enveloped RNA viruses, and each virion contains two identical copies of single-stranded RNA. The viral nucleic acid strands are surrounded by the structural proteins that form the nucleocapsid and the matrix shell. On the outer surface of the nucleocapsid and matrix protein is the lipid envelope derived from the host cell membrane. Proteins that mediate adsorption and penetration into the host cell membrane are inserted into the viral envelop. Retroviruses are unique, because they have the enzyme reverse transcriptase. Reverse transcriptase allows the viral RNA genome to be replicated into DNA and then RNA rather than directly into RNA.
TABLE 66-22
| Family | Retroviridae |
| Common name | Retroviruses |
| Characteristics | Single-stranded, RNA genome; icosahedral capsid with envelope; reverse transcriptase converts genomic RNA into DNA |
| Virus | Human immunodeficiency virus types 1 and 2 (HIV-1, HIV-2) |
| Transmission | Sexual contact, blood and blood product exposure, and perinatal exposure |
| Site of latency | CD4 T lymphocytes |
| Disease | Most disease in humans caused by HIV-1; infected cells include CD4+ (helper) T lymphocytes, monocytes, and some cells of the central nervous system; asymptomatic infection, acute flulike disease, acquired immunodeficiency syndrome (AIDS)–related complex, and AIDS-associated infections and malignancies |
| Detection | Serology, antigen detection, reverse transcriptase polymerase chain reaction (RT-PCR) |
| Epidemiology | Those at risk of infection are homosexual or bisexual males, intravenous drug abusers, sexual contacts of individuals infected with HIV, and infants of infected mothers |
| Treatment | Many, including nucleoside reverse transcriptase inhibitors, nonnucleoside reverse transcriptase inhibitors, protease inhibitors, and inhibitors of viral entry into host cells; treat infections resulting from immunosuppression |
| Prevention | Avoid contact with infected blood/blood products and secretions; blood for transfusion is screened for antibody to HIV-1 and -2 |
| Virus | Human T-lymphotropic viruses types 1 and 2 (HTLV-1, HTLV-2) |
| Transmission | Known means of transmission are similar to those for HIV |
| Disease | T-cell leukemia and lymphoma, and tropical spastic paraparesis for HTLV-1; no known disease associations for HTLV-2 |
| Detection | Serology |
| Epidemiology | HTLV-1 is present in 0.025% of volunteer blood donors in the United States. Blood is screened for antibody to HTLV-1 and HTLV-2; rates of HTLV-1 infection in areas of Japan and the Caribbean are considerably higher than those in the United States. |
| Oncogenic | T-cell lymphoma (HTLV-1) |
| Treatment | Supportive |
| Prevention | Avoid contact with virus |
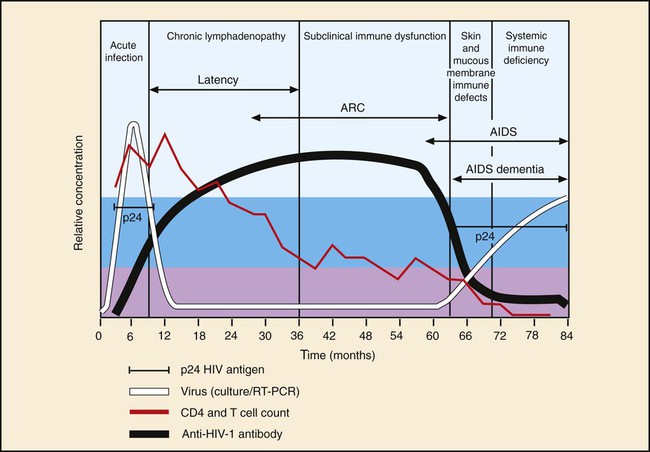
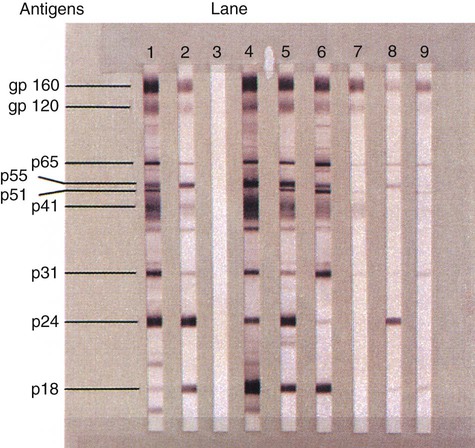
Amino acid sequencing of the reverse transcriptase protein divides the retrovirus family into groups. The human immunodeficiency viruses types 1 and 2 (HIV-1 and HIV-2) are members of this family as are the human T cell lymphoma viruses types 1 and 2 (HTLV-1 and HTLV-2). HIV-1 (Figure 66-6) is the more aggressive virus and is responsible for the acquired immunodeficiency syndrome (AIDS) pandemic. The virus was first isolated in 1983, and a year later was proven to be associated with early and late stages of AIDS. HIV-2 was discovered in 1986 and is less pathogenic. AIDS is the end stage of a process in which the immune system and its ability to control infections and malignant proliferation is destroyed. The virus has an affinity for the CD4+ surface marker of T lymphocytes. As the number of CD4+ T lymphocytes decreases, the risk and severity of opportunistic infections increases. Some of the most common opportunistic infections associated with HIV infection include disseminated coccidioidomycosis, cryptococcosis, cryptosporidiosis, histoplasmosis, recurrent pneumonia, and pneumocystis pneumonia. Detection of the HIV antibody is the mainstay of clinical diagnosis. Repeatedly reactive antibody tests done with EIA should be confirmed using Western blot testing (Figure 66-7). Clinical management of infected individuals involves the use of highly active antiretroviral therapy (HAART) and depends on the measurement of CD4+ lymphocytes and the viral load. Molecular methods often are used to quantify the viral load. Diagnosis of HIV infection in babies born to HIV-positive mothers is problematic because of maternal IgG in the baby’s blood; therefore, PCR for identification of viral DNA or RNA is recommended. Genome sequencing is used to establish susceptibility to antiviral agents.
Rhabdoviruses
Rhabdoviruses (Table 66-23) infect plants, arthropods, fish, and mammals. The virion consists of single-stranded RNA with a helical nucleocapsid surrounded by a lipid bilayer envelope. Spikelike projections approximately 10 nm long extend from the surface of the lipid bilayer. Electron microscopy has shown that the virion has a bullet-shaped or conical appearance. The rabies virus is a neurotropic virus that infects all mammals; with very few exceptions, infection terminates in the death of the infected mammal. The rabies virus is transmitted through the saliva of infected animals, usually by a bite. After inoculation, the virus may invade the peripheral nerves or nerve endings directly. Following infection of the nerve cells, the viral genome progresses centripetally transneuronally, through retrograde axoplasmal flow to the central nervous system. In the CNS it proceeds from first-order neurons to second-order neurons. the neurons are the site of viral replication, mainly in the brain and spinal cord; from there the virus spreads to peripheral nerves and to some nonnervous tissue, including the salivary glands. After a variable incubation period, human disease usually begins with generalized symptoms of malaise, fever, fatigue, anorexia, and headache. Frequently (and characteristically for this disease), symptoms include pain and sometimes “tingling” at the site of exposure, which can be the first “rabies-specific” symptom. After this prodromal phase, behavioral changes may start to manifest, followed by rapidly progressing neurologic symptoms that lead to coma and death.
TABLE 66-23
| Family | Rhabdoviridae |
| Common name | Rhabdovirus |
| Virus | Rabies virus |
| Characteristics | Single-stranded, RNA genome; helical capsid with envelope, bullet-shaped |
| Transmission | Bite of rabid animal most common; 20% of human rabies cases have no known exposure to rabid animal |
| Disease | Rabies |
| Detection | Fluorescent antibody (FA) staining, polymerase chain reaction (PCR) |
| Treatment | Supportive |
| Prevention | Avoid contact with rabid animals; vaccinate domestic animals; postexposure prophylaxis with hyperimmune antirabies globulin and immunization with rabies vaccine |
Only six cases of survival of a rabies infection have been documented worldwide. These cases include patients who survived without any complications; other patients have experienced significant neurologic impairment. In 2004, Randy Willoughby developed a treatment protocol for rabies referred to as “The Milwaukee Protocol.” This protocol requires that the patient remain in a prolonged state of generalized anesthesia, anti-viral drugs, and supportive, life-sustaining care until the individual’s natural active immunity is capable of clearing and/or fighting the infection. Updated protocol and statistics related to patient treatment and survival are maintained by the Medical College of Wisconsin and can be accessed at mcw.edu/Pediatrics/Infectious Diseases/PatientCare/Rabies.htm
Togaviruses
The Togaviridae family (Table 66-24) includes rubella virus and the alpha viruses, a large group of mosquito-borne arboviruses. Rubella is found only in the human population and is transmitted through direct contact with nasopharyngeal secretions or by congenital transmission. Rubella, sometimes called the “German measles,” is usually a benign disease characterized by fever and rash. Before the trivalent vaccine, MMR (measles, mumps, and rubella), was developed, rubella was an epidemic disease. A risk associated with this disease is exposure and infection of pregnant women. The virus can infect the developing fetus, causing multiple congenital anomalies. Intrauterine infection during the first trimester may result in low birth weight, mental retardation, deafness, congenital heart disease, and neurologic defects. Infection that occurs later in pregnancy may result in splenomegaly or osteomyelitis, among other birth deficiencies. Fetal infection can be prevented through vaccination of all women before pregnancy.
TABLE 66-24
| Family | Togaviridae |
| Common name | Togaviruses |
| Characteristics | Single-stranded RNA genome and icosahedral capsid with envelope; family contains arboviruses and non–arthropod-borne rubella virus |
| Virus | Rubella virus |
| Transmission | Respiratory, transplacental |
| Disease | Rubella (mild exanthematous disease), congenital rubella |
| Detection | Serology |
| Treatment | Supportive |
| Prevention | Rubella vaccine |
| Virus | Arboviruses referred to as alphaviruses* |
| Transmission | Arthropod vector, usually mosquito |
| Disease | Eastern, Western, and Venezuelan equine encephalitis |
| Detection | Serology and antibody detection in cerebrospinal fluid (CSF) |
| Treatment | Supportive |
| Prevention | Avoid contact with vector; vector control programs |
Interpretation of Laboratory Test Results
Detection of Epstein-Barr Virus
Disease caused by EBV is established by detecting antibody to multiple antigens (see Figure 66-3). Detection of antibody to viral capsid antigen, early antigen, and Epstein-Barr nuclear antigen is interpreted as shown in Table 66-25.
TABLE 66-25
Interpretation of Serology Results for Epstein-Barr Virus Infection
| Clinical Situation | Heterophile Antibody | IgG-VCA | IgM-VCA | EA | EBNA |
| No past infection | Usually negative | – | – | – | – |
| Acute infection | Usually positive | + | + | + | – |
| Convalescence phase | +/– | + | + or – | + or – | + |
| Past infection | Usually negative | + | – | – or W+ | + |
| Chronic or reactivation | Not useful | + | – | + | + |
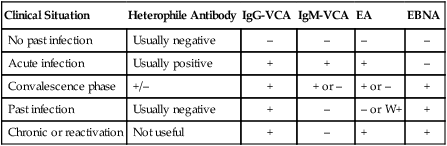
EA, Early antigen; EBNA, Epstein-Barr nuclear antigen; VCA, viral capsid antigen; W+, weakly positive.
Detection of Hepatitis Viruses
Disease caused by HAV is detected using serology tests specific for viral-induced IgM and IgG (see Figure 66-5; Table 66-26). In addition to clinical findings consistent with disease, the presence of HAV-specific IgM is diagnostic of current, active disease. HBV requires detection of antigen and antibody to multiple antigens to classify the disease type. HDV co-infection with HBV relies on detection of anti-HDV antibodies. Diagnostic tests for HCV include RT-PCR and antibody detection. PCR is used to detect early acute hepatitis C disease, because antibody detection tests may be negative. ELISA testing for antibody is used to detect chronic hepatitis C disease (see Figure 66-1). HCV RNA levels in serum, detected by a number of highly sensitive molecular biopsy methods, are used to differentiate patients who are likely to respond to therapy from those with lower response rates. All patients with sustained response to therapy became negative for HCV RNA within 6 months. HCV genotyping is used to identify genotypes more or less likely to respond to therapy. For example, patients with HCV genotype 1 have significantly lower response rates to therapy. Antibody tests are used to detect patients infected with hepatitis E; however, disease is rare in the United States.
TABLE 66-26
Serologic Profiles After Typical Hepatitis B Virus (HBV) Infection
| HBsAg | Anti-HBsAg | HBeAg | Anti-HBeAg | Anti-HBcAg | Most Likely Interpretation |
| – | – | – | – | – | No (or very early) exposure to HBV |
| + | – | +/– | – | – | Early acute hepatitis B (HB) |
| + | – | + | – | + | Acute or chronic HB |
| + | – | – | + | + | Chronic HBV carrier state |
| – | – | – | + | + | Early recovery phase from acute HB |
| – | + | – | + | + | Recovery from HB with immunity |
| – | + | – | – | – | Distant HBV infection or HB vaccine |
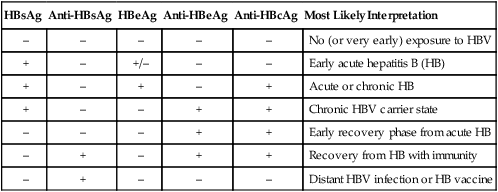
Detection of Human Immunodeficiency Virus
The HIV-1 Western blot test provides a method of antibody-specific identification of several HIV antigens (see Figure 66-7). The presence of antibody to HIV p24 and to either gp41 or gp160 is sufficient to confirm HIV-1 infection. HIV-1 p24 antigen testing is used to detect acutely infected patients before the appearance of antibody. PCR testing for HIV is useful for newborns, whose maternal HIV antibody may confound interpretation of serology tests, and for all patients because detectable antibody may not be produced for months after primary infection. The quantitative plasma RNA test (viral load) is used to measure the amount of HIV in the blood. As many as 10 billion new HIV virions may be produced daily in the blood of untreated patients. Viral load testing has become an essential parameter in guiding decisions to begin or change antiviral therapy. Plasma HIV RNA can be quantified with various assays approved by the U.S. Food and Drug Administration, including the Roche Monitor RT-PCR (Roche Molecular Diagnostics), Bayer Versant HIV-1 (bDNA) Assay (Bayer Diagnostics), and NucliSense EasyQ HIV-1 (NASBA) Assay (bioMérieux). Viral load testing is performed at the time of diagnosis of HIV infection and periodically thereafter. Successful antiretroviral therapy should reduce plasma RNA to undetectable levels (less than 50 copies/mL).
1. Which family of viruses produces one of the most lethal hemorrhagic fevers?
2. A pulmonary syndrome, referred to as hanta pulmonary syndrome, that often results in death is carried in the excreta of:
3. The type of influenza A virus most often associated with greatest human morbidity and mortality is:
4. The three respiratory agents most often responsible for causing croup in pediatric patients are:
a. Parainfluenza, RSV, and rhinovirus
b. Influenza A, RSV, and parainfluenza 3
5. The filovirus that has a characteristic “shepherd’s hook” morphology when viewed by electron microscopy is:
6. Which of the following factors does not contribute to the rapid spread and transmission of noroviruses?
a. Transmission by the fecal-oral route
b. Nucleic acid segmented genome
7. Congenital infections passed from mother to infant often are a cause of severe disease. Which viral agents are responsible for causing congenital infections? (Select all that apply.)
8. Rotaviruses are most readily detected using which type of laboratory testing?
a. Cell culture with IFA confirmation
b. Cell culture using hemadsorption
_____ A virus has a metabolism.
_____ Exposure to hepatitis A results in a life-long illness.
_____ Dengue virus is the most prevalent arbovirus in the world.
_____ Measles infection is often accompanied by a maculopapular rash and swollen parotid glands.
_____ HIV-2 is the more aggressive of the HIVs and is responsible for AIDS.
_____ Adenovirus can be readily grown in cell culture and produces a CPE of round, refractile grapelike cell clusters.
_____ Norovirus is a significant cause of gastrointestinal infection in infants.
_____ Another name for the virus causing hanta pulmonary syndrome is sin nombre virus.
_____ HCV chronic infection often results in end-stage liver disease or hepatocellular carcinoma.
_____ Mosquitoes are the natural reservoir for West Nile virus.
_____ Enteroviruses are the most frequent cause of aseptic meningitis.
_____ Rabies is a neurotrophic virus and affects all mammals.
a. Number of cases of disease ending in death
b. Infective disease passed from animals to humans
c. Outbreak of disease in animal population
d. A carrier that transmits an infective agent from one host to another
e. A fine mist containing minute particles
f. Viral infection in the blood
g. A person or animal that harbors an infectious agent without showing signs of disease
h. Disease that is widespread in a given population
i. A skin infection transmitted to humans by handling sheep
j. An infection acquired at a hospital or health care facility
k. Any person, animal, arthropod, plant, soil, or substance in which an infectious agent lives and multiplies
m. One of the childhood exanthems
n. An organism in which a parasite lives and is nourished
o. The occurrence of cases of an illness in a community or region that exceeds the number of cases expected
p. Causative agent of infection mononucleosis and Burkitt’s lymphoma

(1) Explain the transmissibility of a vector-borne virus. List some of the viruses transmitted through vectors and the disease associated with the virus.
(2) Human papillomavirus causes genital warts; what is a unique feature of the virus and what strains of this virus are frequently responsible for causing cancers?
(3) Explain the difference between antigenic shift and antigenic drift and describe how they affect yearly production of the influenza vaccine.
(4) Explain viral latency; also, list some of the viruses capable of it and the diseases associated with viral reactivation.
(5) Explain how an influenza pandemic can occur. What viral property makes genetic reassortment possible?
(6) What unusual clinical characteristic associated with SARS coronavirus infection distinguishes this virus from other respiratory viruses?
(7) How did the coronavirus acquire its name?
(8) Which historic individual studied “yellow fever” in the tropics? What was the significance of this study?
(9) List the six childhood rashes or exanthemas and the etiologic agents responsible.
(10) Two proteins on the surface of the influenza virus give this virus its unique properties; what are these proteins and what is their function?
(11) What are Koplik’s spots and for what viral disease are they diagnostic?