Obligate Intracellular and Nonculturable Bacterial Agents
1. Define the following: bubo, proctitis, bartholinitis, salpingitis, elementary body, reticulate body, Whipple’s disease, morulae, and Donovan body.
2. Describe the general characteristics for the organisms included in this chapter including gram stain characteristics, cultivation methods (media and growth conditions), transmission and clinical significance.
3. Explain the mechanism and location for the replication of Chlamydia spp.
4. Compare the clinical manifestations and diagnosis of trachoma and other oculogenital infections associated with Chlamydia spp.
5. List the appropriate specimens used for the isolation of the organisms included in this chapter.
6. Describe the correct collection method for a specimen to be submitted for C. trachomatis screening from the female genital tract.
7. Explain the three stages associated with lymphogranuloma venereum, and compare the disease with other genital infections.
8. Describe the laboratory methods used for the diagnosis of Chlamydia infections, including sensitivity, limitations and appropriate use for culture, cytology, antigen (DFA), and nucleic acid testing (NAAT).
9. Compare hybridization and amplification nucleic acid–based testing for chlamydia.
10. Describe the triad of symptoms associated with Rickettsia spp.
11. Compare human monocytic ehrlichiosis (HME) and granulocytic anaplasmosis (HGA).
12. Distinguish and describe the three groups of Rickettsia based on mode of transmission, clinical manifestations, and intracellular growth characteristics.
13. Describe the Weil-Felix reaction, including chemical principle and limitations.
14. Describe the clinical significance for Coxiella burnetii phase I and phase II forms, including laboratory diagnosis.
15. Explain the limitations of the laboratory tests used to diagnose disease caused by the obligate intracellular and nonculturable bacteria.
16. Correlate signs, symptoms, and laboratory data for the identification of the organisms included in this chapter.
The organisms addressed in this chapter are obligate intracellular bacteria or are considered either extremely difficult to culture or unable to be cultured. Organisms of the genera Chlamydia, Rickettsia, Orientia, Anaplasma, and Ehrlichia are prokaryotes that differ from most other bacteria with respect to their very small size and obligate intracellular parasitism. Three other organisms, Coxiella, Calymmatobacterium granulomatis, and Tropheryma whipplei, are discussed in this chapter because they are also difficult to cultivate or are noncultivable.
Chlamydia
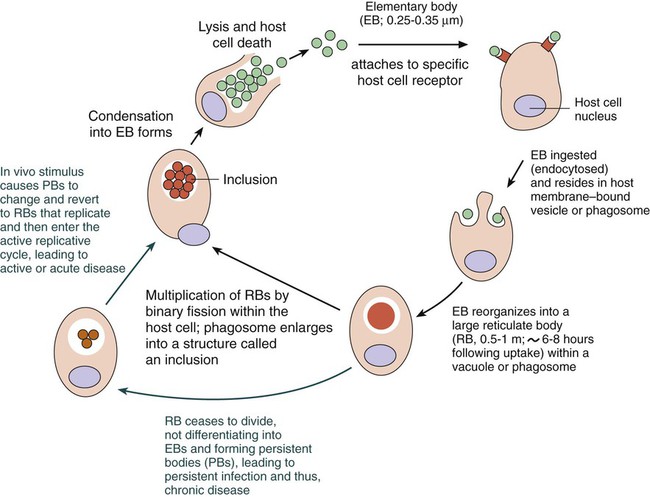
Chlamydiae have a unique developmental life cycle reminiscent of parasites, with an intracellular, replicative form, the reticulate body (RB), and an extracellular, metabolically inert, infective form, the elementary body (EB). The EB cannot survive outside of a host cell for an extended period. Following infection of a host cell, the EB differentiates into a RB. The RB divides by binary fission within vacuoles. As the numbers of RB increase, the vacuole expands forming an intracytoplasmic inclusion. The RB then revert to EB, and 48 to 72 hours postinfection, the EB are released from the host cell (Figure 44-1). In addition to the replicative cycle associated with acute chlamydial infections, there is evidence that Chlamydia can persist in an aberrant form in vitro depending on the amount of interferon-gamma (IFN-γ) and tryptophan in the host cell as well as the function of the tryptophan synthase encoded by the organism. Removal of the IFN-γ or increase in tryptophan will result in the differentiation of the chlamydiae into an active EB infection. The therapeutic implications of this persistence in vivo has not yet been completely defined; however, evidence suggests that the activity of the tryptophan synthase gene in C. trachomatis differs between isolates recovered from the eye versus the genital tract.
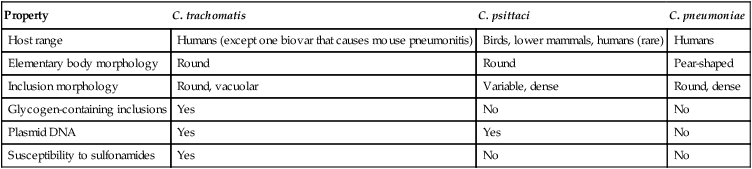
C. trachomatis, C. pneumoniae, and C. psittaci are important causes of human infection; C. psittaci and C. pecorum are common pathogens among animals. The three species that infect humans differ with respect to their antigens, host cell preference, antibiotic susceptibility, EB morphology, and inclusion morphology (Table 44-1).
TABLE 44-1
Differential Characteristics among Chlamydiae That Cause Human Disease
| Property | C. trachomatis | C. psittaci | C. pneumoniae |
| Host range | Humans (except one biovar that causes mouse pneumonitis) | Birds, lower mammals, humans (rare) | Humans |
| Elementary body morphology | Round | Round | Pear-shaped |
| Inclusion morphology | Round, vacuolar | Variable, dense | Round, dense |
| Glycogen-containing inclusions | Yes | No | No |
| Plasmid DNA | Yes | Yes | No |
| Susceptibility to sulfonamides | Yes | No | No |
Chlamydia Trachomatis
General Characteristics
C. trachomatis infects humans almost exclusively and is responsible for various clinical syndromes. Based on MOMP antigenic differences, C. trachomatis is divided into 18 different serovars that are associated with different primary clinical syndromes (Table 44-2).
TABLE 44-2
Primary Syndromes Caused by C. trachomatis
| Serovars | Clinical Syndrome | Route(s) of Transmission |
| A, B, Ba, C | Endemic trachoma (multiple or persistent infections that ultimately lead to blindness) | Hand to eye from fomites, flies |
| L1, L2, L2a, L3 | Lymphogranuloma venereum | Sexual |
| D-K | Urethritis, cervicitis, pelvic inflammatory disease, epididymitis, infant pneumonia, and conjunctivitis (does not lead to blindness) | Sexual, hand to eye by autoinoculation of genital secretions; eye to eye by infected secretions; neonatal |
Epidemiology and Pathogenesis
C. trachomatis causes significant infection and disease worldwide. In the United States, C. trachomatis is the most common sexually transmitted bacterial pathogen and a major cause of pelvic inflammatory disease (PID), ectopic pregnancy, and infertility (see Chapter 74 for more information on PID). An estimated 3 million cases of C. trachomatis infection occur annually in the United States. In 2010, more than 1.3 million cases of C. trachomatis infection were reported to the Centers for Disease Control and Prevention (CDC), corresponding to a rate of infection of 426 per 100,000 population and a 5.1% increase over the cases reported in 2009. In fact, of all the organisms causing sexually transmitted disease reported to the CDC, only C. trachomatis cases have increased every year. Genital tract infections caused by C. trachomatis were identified most frequently in women between the ages of 15 and 24 years. It is important to note, however, that data reported to the CDC, especially with regards to Chlamydia, come as a result of screening programs that primarily target women between the ages of 15 and 24 years.
C. trachomatis infections are primarily transmitted from human to human by direct contact with infected secretions. Some infections, such as neonatal pneumonia or inclusion conjunctivitis, are transmitted from mother to infant during birth. The various routes of transmission for C. trachomatis infection are summarized in Table 44-2.
Laboratory Diagnosis
Cultivation.
Although its specificity approaches 100%, the sensitivity of culture has been estimated at between 70% and 90% in experienced laboratories. Limitations of Chlamydia culture contributing to the lack of sensitivity include prerequisites to maintain viability of patient specimens by either rapid or frozen transport and to ensure the quality of the specimen submitted for testing (i.e., endocervical specimens devoid of mucus and containing endocervical epithelial or metaplastic cells or urethral epithelial cells). In addition, successful culture requires a sensitive cell culture system and a minimum of at least 2 days turnaround time between specimen receipt and the availability of results. Despite these limitations, culture is still recommended as the test of choice in some situations (Table 44-3). As of this writing, only chlamydia cultures should be used in situations with legal implications (e.g., sexual abuse) when the possibility of a false-positive test is unacceptable. Local and state requirements may vary.
TABLE 44-3
Use of Different Laboratory Tests to Diagnose C. trachomatis Infections
| Patient Population | Specimen Type | Acceptable Diagnostic Test |
| Prepubertal girls | Vaginal | Culture (if culture is unavailable, certain specialists accept NAAT) |
| Neonates and infants | Nasopharyngeal | Culture, DFA |
| Rectal | Culture | |
| Conjunctiva | Culture, DFA, EIA, NAAT | |
| Women | Cervical | NAAT*, culture, DFA, EIA, NAH, NAAT |
| Vaginal | NAAT* | |
| Urethral | NAAT, culture, DFA, EIA, NAH | |
| Urine | NAAT* | |
| Children, women and men | Rectal | Culture, DFA, NAAT* |
| Men | Urethral | NAAT* (DFA, EIA, NAH recommended when NAAT is unavailable) |
| Urine† | NAAT* |
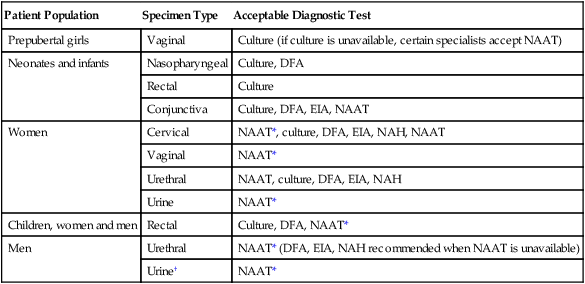
DFA, direct fluorescent antibody staining; EIA, enzyme immunoassay; NAH, nucleic acid hybridization; NAAT, nucleic acid amplification test.
*Must be confirmed in a population with a low prevalence (<5%) of C. trachomatis infection.
†EIA can be used on urine from symptomatic men but not on urine from older men. Also, a positive result must be confirmed in a population with a low prevalence of C. trachomatis infection.
Modified from Centers for Disease Control and Prevention: Recommendations for the prevention and management of Chlamydia trachomatis infections, MMWR 42(RR-12):1-39, 1993; Centers for Disease Control and Prevention: Screening tests to detect Chlamydia trachomatis and Neisseria gonorrhoeae infections, MMWR 51(RR-15):1-27, 2002; Centers for Disease Control and Prevention: Sexually transmitted diseases treatment guidelines, 2010, MMWR 59(RR-12):1, 2010.
Direct Detection Methods
Antigen Detection and Nucleic Acid Hybridization.
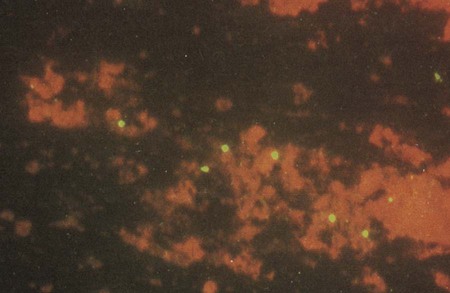
Direct fluorescent antibody (DFA) staining methods use fluorescein-isothiocyanate conjugated monoclonal antibodies to either MOMP or LPS of C. trachomatis to detect elementary bodies in smears of clinical material (Figure 44-2). The sensitivity and specificity of DFA are similar to those of culture. Chlamydial antigen can also be detected by enzyme immunoassays (EIA). Numerous U.S. Food and Drug Administration (FDA)-approved kits are commercially available. These assays use polyclonal or monoclonal antibodies that detect chlamydial LPS. These tests are not species-specific for C. trachomatis and may cross-react with LPS of other bacterial species present in the vagina or urinary tract and thereby produce a false-positive result.
• Performing a second nonculture test that identifies a C. trachomatis antigen or nucleic acid sequence that is different from that used in the screening test
• Using a blocking antibody or competitive probe that verifies a positive test result by preventing attachment of a labeled antibody or probe used in the standard assay
Nucleic Acid Amplification Tests.
FDA-approved nucleic acid amplification tests (NAATs) for the laboratory diagnosis of C. trachomatis infection use three different formats: polymerase chain reaction (PCR), strand displacement amplification (SDA), and transcription-mediated amplification (TMA). The first two assay formats amplify DNA sequences present in the cryptic plasmid that is present in 7 to 10 copies in the chlamydial EB, whereas the last format amplifies 23S ribosomal RNA sequences. Studies clearly indicate that NAATs are more sensitive than culture and other non-nucleic acid amplification assays. Because of the increased sensitivity of detection, first-voided urine specimens from symptomatic and asymptomatic men and women are acceptable specimens to detect C. trachomatis, thereby affording a noninvasive means of chlamydia testing. NAATs are the preferred methodology for detecting C. trachomatis in most clinical situations because of increased sensitivity, ease of specimen collection, and the availability of automated high volume methods. Table 44-3 summarizes the possible uses of the different methodologies available for the detection of C. trachomatis; however, NAATs are used almost exclusively for the laboratory detection of C. trachomatis.
Chlamydia Pneumoniae
General Characteristics
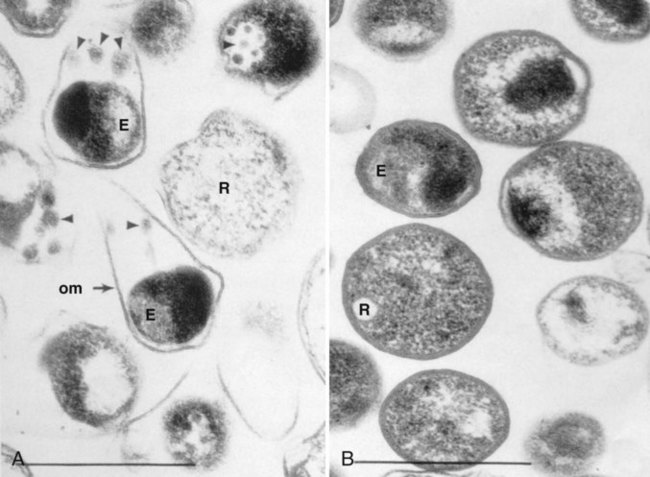
C. pneumoniae is considered more homogeneous than either C. trachomatis or C. psittaci, because all isolates tested are immunologically similar because of the homogeneity of the MOMP. One significant difference between C. pneumoniae and the other chlamydiae is the pear-shaped appearance of its EB (Figure 44-3).
Rickettsia, Orientia, Anaplasma, and Ehrlichia
Coxiella and Bartonella, two other genera of intracellular bacteria causing human disease, were at one time included in the Rickettsiaceae family. However, based on phylogenetic differences, these two genera were removed from the Rickettsiaceae family and separated into two families, Coxiellaceae and Bartonellaceae. Bartonella spp. can be cultured on standard bacteriologic media; therefore, this group of organisms is addressed in Chapter 33. Because Coxiella burnetii can survive extracellularly, unlike the rickettsiae, yet requires cultivation in cell culture similar to the rickettsiae, this organism is discussed separately in this chapter.
Epidemiology and Pathogenesis
This group of organisms infects wild animals, with humans acting as accidental hosts in most cases. Most of these organisms are passed between animals by an insect vector. Similarly, humans become infected following the bite of an infected arthropod vector or by inhalation of infectious aerosols. Characteristics, including the respective arthropod vector of the prominent species of Rickettsia, Orientia, Anaplasma, and Ehrlichia, are summarized in Table 44-4.
TABLE 44-4
Characteristics of Prominent Rickettsia* Orientia, Anaplasma, and Ehrlichia spp.
| Agent | Disease | Vector | Distribution | Diagnostic Tests |
| Spotted Fever Group | ||||
| R. conorii | Mediterranean and Israeli spotted fevers; Indian tick typhus; Kenya tick typhus | Ticks | Southern Europe, Middle East, Africa | Serology, immunohistology, PCR with sequencing |
| R. rickettsii | Rocky Mountain spotted fever | Ticks (Dermacentor spp.) | North and South America; particularly in southeastern states and Oklahoma in the United States | Serology, immunohistology, PCR with sequencing |
| Typhus Group | ||||
| R. prowazekii | Epidemic typhus | Lice | Worldwide | Serology, PCR with sequencing |
| Brill-Zinsser disease | None; recrudescent disease | Worldwide | Serology, PCR with sequencing | |
| R. typhi | Murine typhus | Fleas | Worldwide | Serology, PCR with sequencing |
| Scrub Typhus Group | ||||
| O. tsutsugamushi | Scrub typhus | Chiggers | South and Southeast Asia, South Pacific, | Serology, PCR with sequencing |
| Ehrlichia/Anaplasma/Neorickettsia | ||||
| Ehrlichia chaffeensis | Human monocytic ehrlichiosis | Ticks (Amblyomma americanum—Lone Star Tick) | Southeast, South Central, and mid-Atlantic United States | Serology, PCR, immunohistology, immunocytology |
| E. ewingii | Ticks (Amblyomma americanum—Lone Star Tick) | United States (overlapping with E. chaffeensis) | PCR with species-specific primers or DNA sequencing of amplicons | |
| Anaplasma | Human granulocytic anaplasmosis | Ticks (Ixodes spp.) | United States, Europe | Serology, PCR, immunohistology, phagocytophilum peripheral blood smear, immunocytology |
| Neorickettsia sennetsu | Sennetsu fever | Ticks | Southeast Asia (primarily Japan) | Serology |
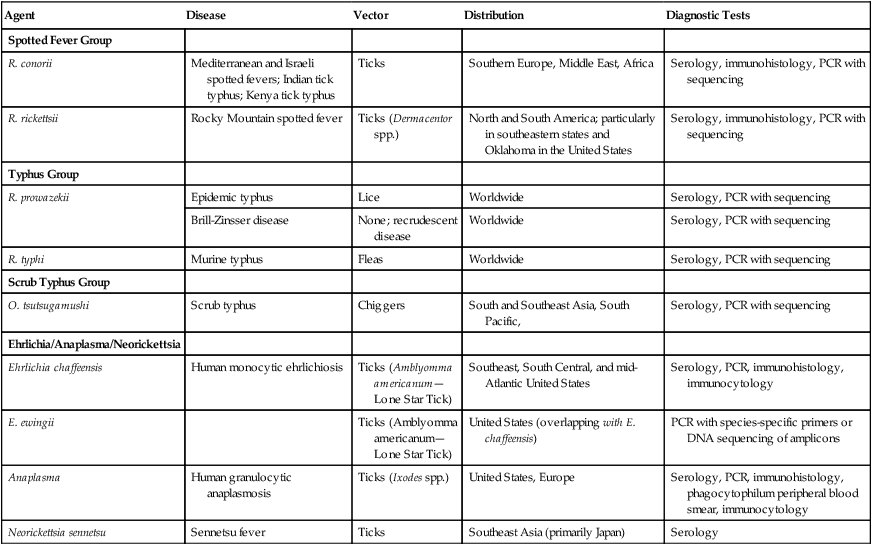
*Other Rickettsia species recognized as emerging human pathogens include R. africae, R. sibirica, R. japonica, R. honei, R. australis, R. slovaca, R. aeschimannii, R. helvetica, R. heilongjiangensis, and R. parkeri; all belong to the spotted fever group.
Spectrum of Disease
Species in the genus Rickettsia are divided into three groups: the spotted fever group, the typhus group, and the scrub typhus group (O. tsutsugamushi), based on the arthropod mode of transmission, clinical manifestations, rate of intracellular growth, rate of intracellular burden, and extent of intracellular growth (see Table 44-4). Rickettsias are suspected when the triad of fever, headache, and rash is the primary clinical manifestation in patients with an exposure to insect vectors. Infections caused by these organisms may be severe and are sometimes fatal.
Laboratory Diagnosis
Direct Detection Methods
Immunohistology and conventional and real-time PCR have been used to diagnose rickettsial and ehrlichial infections. Biopsy of skin tissue from the rash caused by the spotted fever group rickettsiae is the preferred specimen. Organisms are identified using polyclonal antibodies and are detected with the use of fluorescein-labeled antibodies or enzyme-labeled indirect procedures. The sensitivity of these techniques is about 70% and depends on correct tissue sampling, examination of multiple tissue levels, and biopsy before or during the first 24 hours of therapy (see Table 44-4).
Serodiagnosis
Although it is not fast, the diagnosis of rickettsial disease and ehrlichiosis is primarily accomplished serologically. Serologic assays for the diagnosis of rickettsial infections include the indirect immunofluorescence assay (IFA), enzyme immunoassay (EIA), Proteus vulgaris OX-19 and OX-2 and Proteus mirabilis OX-K strain agglutination (the Weil-Felix reaction), line blot, and Western immunoblotting. The Weil-Felix reaction (see Procedure 44-1 on the Evolve site), the fortuitous agglutination of certain strains of P. vulgaris by serum from patients with rickettsial disease, may still be performed in developing countries, but because false-positive and false-negative tests are a continuing problem, these tests have been replaced by more accurate serologic methods such as IFA.
Coxiella
Spectrum of Disease
After the incubation period, initial clinical manifestations of C. burnetii infections are systemic and nonspecific: headache, fever, chills, and myalgias. In contrast to rickettsial infections, a rash does not develop. Both acute and chronic forms of the disease are recognized. Possible clinical manifestations are listed in Box 44-1.
1. Which organism causes Rocky Mountain spotted fever?
2. Which serovar of Chlamydia trachomatis causes lymphogranuloma venereum?
3. Which of the following organisms is acquired via exposure to infected birds?
4. A 24-year-old man presented to his physician in India because of a painless ulcer on his penis. Upon examination, the ulcer was erythematous, large and “beefy.” When the lesion was sampled with a swab, it readily started bleeding. In addition, inguinal lymph nodes were swollen. Wright-Giemsa stain of material from the lesion revealed the presence of blue rods within the mononuclear cells that were stained more prominently on the ends than in the middle. What organism is most likely causing this lesion?
5. A physician calls the laboratory and asks the medical laboratory scientist which test to order to rule out Chlamydia pneumoniae as a cause of pneumonia in a 17-year-old patient. What does the medical laboratory scientist tell the physician?
a. sputum culture for routine bacterial pathogens
b. Wright-Giemsa staining of bronchoalveolar lavage fluid
c. real-time PCR on a nasopharyngeal aspirate
d. serum tested for agglutination with Proteus OX-K, OX-19, and OX-2
6. Transmission of Orientia tsutsugamushi is associated with what vector?
7. Which triad of symptoms is associated with rickettsial infections?
a. urethral discharge, fever, and dysuria
b. coughing, production of sputum, and chest pain
d. genital lesion, swollen inguinal lymph nodes, and headache
_____ The phase II form (small-cell variant) of C. burnetii is like a spore and is not infectious.
_____ A woman presents to her nurse practitioner complaining of a vaginal discharge. The nurse practitioner suspects Chlamydia trachomatis and collects a specimen in the following manner: A large swab was used to remove all secretions from the cervix and then placed into the swab sheath and sent to the laboratory. This specimen was collected appropriately for C. trachomatis.

2. Match the correct specimen type to collect for the diagnosis of each disease (more than one may apply).

3. Match the disease with its causative agent (more than one may apply).