Chapter 160 Video-Assisted Thoracoscopic Discectomy
Indications and Techniques
History
Thoracic Disc Disease
The surgical management of disorders of the thoracic spine began in 1814 when H. J. Cline attempted to treat a fracture of the thoracic spine by laminectomy.1 In 1911, Middleton and Teacher undertook the first surgical procedure for a thoracic disc herniation, which was later described by Benjamin.2 In this case, the patient was paraplegic and subsequently died.
Thoracic disc herniation is a relatively infrequent clinical diagnosis, accounting for 0.25% to 0.75% of all disc herniations and approximately 4% of surgical cases.3,4 The symptoms of thoracic disc herniation are variable and often nonspecific, and many patients experience a protracted clinical course with a delay in diagnosis. Radiologic studies focused on computed tomography myelography and magnetic resonance imaging have demonstrated an 11% to 14.5% incidence of thoracic disc herniations.5,6 Woods and colleagues7 found disc herniations in 37% of the 60 asymptomatic patients evaluated by magnetic resonance imaging.
There has been a significant evolution in the surgical management of thoracic disc herniation. Early surgical therapy consisted of laminectomy, which often resulted in paraplegia and carried a combined operative mortality as high as 10%.8 In 1969, Perot and Munro reviewed 91 laminectomies for thoracic disc herniations and found no neurologic improvement in 40 of the patients and progressive paraplegia in 16 of these patients.9 Others have also reported similarly poor outcomes with laminectomies for thoracic disc herniation.10–12 Variations on the surgical technique were employed with laminectomies, including the use of decompression alone, decompression and transdural removal of disc material, and decompression with transdural rhizotomy and sectioning of the dentate ligaments, but all these successive approaches had similarly poor outcomes. The only exception to this pattern of poor surgical outcomes was the series reported by Horwitz and colleagues13 in 1955 in which five consecutive cases of thoracic disc herniation treated with laminectomy resulted in a good outcome. In 1998, Fessler and Sturgell14 reviewed and reported on 60 years of the literature in which they compared the mortality and morbidity rates with the various surgical approaches to the thoracic spine. They concluded that laminectomy does not provide adequate access to safely treat thoracic disc herniations.
In response to the uniformly poor outcomes after midline dorsal approaches to thoracic disc disease, surgical alternatives were developed using the anterior or extended posterolateral approaches with the costotransversectomy, transpedicular, and the lateral extracavitary approach.9,15–17 Maiman and colleagues18 in their report on the lateral extracavitary approach for thoracic disc herniation reviewed 23 cases. None of the patients in the review experienced any new deficits postoperatively. This surgical technique, however, required significantly more soft-tissue dissection and manipulation, with the paraspinous muscles being mobilized medially, resulting in devascularization and denervation. This was found to contribute to poor wound healing and increase in perioperative kyphosis. In addition, the lateral parascapular extrapleural approach, as developed by Fessler and colleagues, provided exposure to the upper thoracic spine, which was comparable with the lateral extracavitary approach. However, it presents the risk of significant shoulder girdle dysfunction due to lateral scapular mobilization.19 These approaches, despite their complexity, yielded significantly improved surgical and neurologic outcomes when compared with laminectomy for thoracic disc herniations.
The transpleural approach to the thoracic spine dates to 1958 when Craffoord and colleagues20 reported the use of this technique for herniated thoracic discs. In 1969, Perot and Munro9 reported the use of this approach in two patients, and in the same year Ranasohoff and colleagues15 reported the results of a similar approach for three patients. Since that time, the benefits of the anterior transthoracic approach have been supported by other published series.21–23
In 1988, Bohlmann and Zdeblick21 recommended the anterior transthoracic approach over the costotransversectomy to treat herniated thoracic discs. This anterior transthoracic approach required a thoracotomy with rib resection and often resulted in significant perioperative morbidity including pulmonary dysfunction, intercostal neuralgia, and shoulder girdle dysfunction.24–26
Spinal Endoscopy
The beginnings of endoscopic surgery date to 1807 when Philip Bozzini developed an endoscopic device that he called the Lichtleiter.27 This, like other earlier endoscopic devices, was primarily used to explore body orifices using reflected light. Later, optical lenses were incorporated, and in 1910, Jacobeus28,29 reported the first use of an endoscope to explore the thoracic cavity. He used a cystoscope to examine the pleural cavity in the treatment of tuberculosis and eventually expanded its use to the diagnosis of malignant and benign pulmonary diseases.30,31 Since that time, thoracoscopes have been adapted to treat a variety of pulmonary disorders including penetrating chest injuries.32–34
Spinal endoscopy began in 1931 when Burman35 published a report on a technique he called myeloscopy. He described the use of an arthroscope to examine cadaveric spines and explore the lumbar thecal sac. In 1938, Pool36,37 expanded on Burman’s work and used a hot lamp system with improved visualization of the thecal space to examine more than 400 patients between 1938 and 1942. Despite the initial success of Barman and Pool, spinal endoscopy did not immediately gain widespread acceptance. Optical resolution and the light intensity were poor, and the instruments were far too large to easily explore and work in the confines of this small surgical space. Advances in fiber optics and the development of modern video technology have led to resurgence in interest in endoscopic approaches to the spine. Small cold light sources and video display monitors have replaced the older hot reflected light and lens tube systems.
In 1983, Hausmann and Forst38 used a nucleoscope to inspect the disc space for loose fragments after an open discectomy, and in 1992, Schreiber and Leu39 successfully performed a percutaneous discoscopy. The procedure was rapidly applied to surgery for thoracic disc herniations.40,41
The anterior approaches provide an unsurpassed exposure of the ventral aspect of the spinal column. It not only provides a large working area in which the adjacent anatomic structures became clearly identified but also provides the optimal angle for removal of intervertebral discs and allows easy inspection of the spinal cord. If necessary, repair of the dura in cases of intradural disc herniations can be performed via this approach. The anterior approach has become the preferred approach for most thoracic spinal pathology other than far lateral lesions.2,9,15
The risk associated with the anterior transpleural approach is that of injury to the adjacent vascular and visceral structures. There is additional associated morbidity with prolonged pulmonary dysfunction, incisional pain, and pain associated with thoracostomy tube drainage that contributes to the potential adverse consequences. Comparative studies have shown a lower rate of pulmonary morbidity with thoracoscopic procedures when compared with open thoracotomy. Thoracoscopy minimizes the incidence of intercostal neuralgia and avoids shoulder girdle dysfunction. In addition, there are reduced blood loss and a proven reduction in hospital length of stay.42–44
The dramatically improved optics and lighting of rigid glass endoscopes as developed by physicist Harold Hopkins in 1970 nurtured the rapid growth of endoscopic surgical techniques.45 Landreneau and colleagues44 reported 106 such cases in 1993 in which they compared video-assisted thoracoscopic surgery (VATS) with thoracotomy. The patients who underwent VATS had less pain, improved pulmonary function, and superior shoulder girdle function when compared with thoracotomy patients. That year, Mack and colleagues published a report demonstrating the potential of VATS to provide reliable access to the ventral surface of the thoracic spine.46 In 1995, Caputy and colleagues47 demonstrated the successful use of VATS in performing thoracic discectomy on both cadaveric and porcine models. In that study, the clinical use of thoracoscopic dissection was also reported.
Although the benefit of VATS is usually compared only with the alternative thoracotomy, data also suggest that it is a less morbid procedure than a costotransversectomy. Rosenthal and Dickman48 reported a series of 55 patients who underwent thoracoscopic discectomy and compared the rate of complications of the thoracoscopic procedures with both the patients undergoing open thoracotomy and the patients undergoing costotransversectomy for thoracic disc herniations. There were no instances of postoperative neurologic deterioration in either the thoracoscopic or thoracotomy group, but of those patients undergoing costotransversectomies, 7% experienced new neurologic deficits after surgery. Intercostal neuralgia, both temporary and permanent, has been a significant problem associated with thoracotomy. The use of VATS has significantly reduced the incidence of this painful disorder. In that series, there was a 16% rate of intercostal neuralgia in the VATS group compared with 50% in patients who had a thoracotomy. In all patients in the thoracoscopic group with intercostal neuralgia, the condition was temporary and resolved completely within 1 to 2 weeks. In patients undergoing costotransversectomy, there was a 20% rate of intercostal neuralgia.48
Surgical Anatomy
Thoracic Cavity Anatomy
A thorough knowledge of the anatomy of the thoracic cavity is critical for a successful procedure as well as for avoiding complications. The muscles of the chest wall, primarily the serratus anterior, pectoralis major, and latissimus dorsi, form important landmarks for thoracoscopic port placement. The serratus anterior forms the medial wall of the axilla. The pectoralis major demarcates the anterior axillary line and serves as the anterior border for trocar insertion, whereas the latissimus dorsi denotes the posterior axillary line and the posterior border for trocar placement. Attention should also be paid to the mammary gland overlying the anterior and lateral thoracic wall. Its origin just anterior to the midaxillary line, from the second to the sixth rib, is at risk during trocar introduction.49
Inside the thoracic cavity, transparent parietal pleura covers the anterior, posterior, and superior aspects of the chest cavity. It reflects over the great vessels, trachea, esophagus, and spinal column and is easily separated from these structures. Commonly, the parietal pleura is studded with anthracotic pigment, indicating exposure to smoke or other inhaled pollution over the patient’s lifetime. Chronic inflammation of the pleura can render it opaque and prevent visualization of the underlying structures.
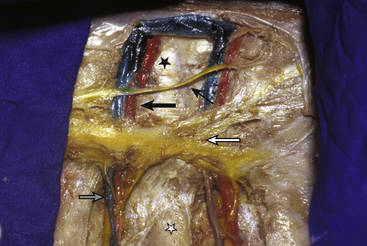
The complex vascular anatomy of the paravertebral area as demarcated from the intrathoracic perspective requires a detailed understanding before embarking on thoracoscopic procedures. The posterior intercostal arteries of the first two vertebral segments arise from the superior intercostal artery branch of the costocervical trunk of the subclavian artery. The lower posterior intercostal arteries arise segmentally directly from the aorta. The segmental branches on the right are longer and traverse a greater distance than segmental branches on the left. These arteries leave the aorta and travel on the side of the vertebral body between the intravertebral discs. These arteries are crossed, immediately anterior to the rib head articulation, by the sympathetic chain. The arteries then course superiorly under the tip of the transverse process, merging with the vein and nerve in the costal groove. At this point, the artery gives off a branch that continues in a posterior course over the transverse process to supply the muscles of the back. Before passing over the transverse process, however, it sends a spinal branch through the intravertebral foramen, which supplies the spinal cord (Fig. 160-1).
The primary blood supply to the lower thoracic spinal cord is via the great radicular anastomotic artery of Adamkiewicz. This vessel most often enters from the left side between T8 and L3. Disruption of this radicular artery can lead to spinal cord infarction and paraplegia. Spinal angiography should be considered for locating this vessel before exposure of the lower thoracic spine.50 However, angiography is generally unnecessary when a thoracoscopic technique is used.
The sympathetic chain and ganglia lie in the retropleural space over the rib heads in the upper chest cavity, cross the segmental vessels, and move medially to lie over the vertebral bodies in the caudal portion. The sympathetic chain is made up of ganglia linked by interganglionic cords. This chain is located anterior to the rib head of the thoracic vertebrae and crosses the segmental vessels. The medial branches of the upper five ganglia supply the thoracic aorta via the thoracic aortic plexus. The medial branches of the lower ganglia coalesce to form the splanchnic nerves. The anterior rami of the thoracic nerves form an intercostal nerve. Each nerve is connected to the ganglion of the adjacent sympathetic trunk by a gray and a white communicating ramus. They pass forward in the intercostal space below the intercostal vessels. The sympathetic preganglionic nerve fibers are conveyed through white rami to the sympathetic trunk. They in turn synapse with the cells of the sympathetic ganglia. These ganglion cells of the sympathetic chain send out postganglionic fibers through the gray rami, which return to join the spinal nerves.
The vagus nerves and their recurrent branches also lie within the thoracic cavity. The left vagus nerve runs between the left common carotid artery and subclavian artery, then passes between the left pulmonary artery and the aortic arch. It continues in close proximity to the esophagus, where it forms the anterior vagal trunk. The left recurrent laryngeal nerve arises below the aortic arch and ascends into the neck in the tracheoesophageal groove. The right vagus nerve runs anterior to the right subclavian artery and deep to the brachiocephalic vein. It then gives off its recurrent branch and continues along the trachea and ends as the posterior vagal trunk along the esophagus.51
Preoperative Evaluation
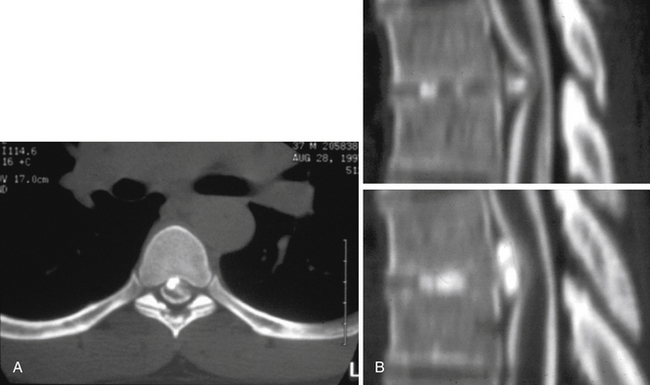
The preoperative evaluation involves an anatomic and a functional evaluation of the patient’s pathology. An anatomic evaluation is used to define the structural spinal pathology and to correlate the physical findings with that pathology. An initial radiographic evaluation is done using magnetic resonance imaging, which delineates the degree of compromise of the spinal cord and exiting nerve roots. Bright signal on T2-weighted sequences, indicating myelomalacia, may be appreciated within the spinal cord. Sagittal views permit the vertebrae to be counted, localizing the pathologic level. The axial images aid in planning which side is optimal in approaching the pathology and can provide information on the adjacent vascular structures. Myelography with postmyelogram computed tomography scanning has been invaluable in defining the bony anatomy of the thoracic spine and in localizing the pathologic changes within the context of the broader anatomy of the thoracic cavity. It allows a determination as to whether the disc pathology is soft or calcified, and it can provide a preoperative indication of the involvement of the dura with an adherent calcified disc (Fig. 160-2).
In at-risk patients, further screening may be obtained by the use of pulmonary function tests. A forced vital capacity that is 50% of predicted and a maximal ventilatory ventilation of 50% of predicted may also be associated with postoperative pulmonary complications.
Anesthesia
Patients undergoing a thoracoscopic procedure require single-lung ventilation to facilitate the surgical exposure. Single-lung ventilation is routinely achieved by the use of either a double-lumen endotracheal tube or the bronchial blocker technique. The preferred method of single-lung ventilation is with the use of a double-lumen endotracheal tube; however, this endotracheal tube may be too large for patients weighing less than 50 kg, and in that instance, bronchial blockers are used. Double-lumen endotracheal tubes are formed by two catheters attached to each other side by side (Fig. 160-3). Each catheter independently ventilates one of the lungs. Bronchial blockers are catheters that are placed within the lumen of a single endotracheal tube. The blocker is placed fiberoptically to block one of the main stem bronchi, and the endotracheal tube is positioned in the trachea and ventilates the nonblocked lung. This blocking technique has many disadvantages including the tendency of the balloon blocker to back out of the bronchus and partially obstruct the trachea. Bronchoscopy should be used to confirm proper positioning of the endotracheal tube and reconfirm once positioning is complete. Invasive blood pressure monitoring and somatosensory evoked potentials are other techniques employed in this procedure.
Surgical Technique
Endoscopic Instruments
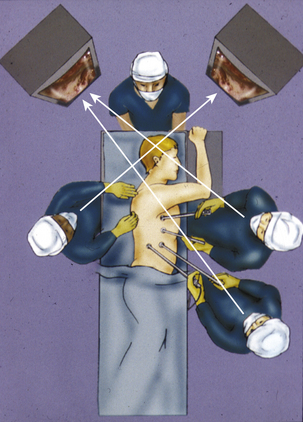
Three or four ports are needed to provide a surgical working area for a VATS discectomy. These ports are arranged in a reverse L pattern (Fig. 160-4). The first port is placed in the anterior axillary line at the sixth or seventh intercostal space to avoid injury to the diaphragm. After the initial lung inspection, two or three more ports are inserted under direct inspection. These ports are centered on the area of interest. Two additional ports are placed, one rostral and the other caudal to this initial port. A fourth port may be placed at the caudal corner of the reverse L pattern if further lung or diaphragm retraction is required in the case of lower thoracic lesions. One of the ports is used for the endoscope. It is usually the more posterior port, along the posterior axillary line. The remaining ports along the anterior axillary line serve as the working channels. Proper placement of the ports is critical to ensure that the surgical instruments inserted through any given port are not inhibiting the use of a second instrument inserted through a second port. Ports that are placed too close together can cause fencing when the instruments crowd the operative area and make contact with the shaft of the other instruments, thereby inhibiting the free movement of the primary surgical instrument.
The imaging equipment consists of the endoscope, light source, camera, video monitors, and video recorders. The endoscopes may be either rigid or flexible, but the most commonly used scope is a rigid rod-lens system in which the image is transmitted through a series of quartz rods to a camera mounted at the end. The field of view through the lens can vary from 0 to 70 degrees, but the 30-degree lens is preferred because it affords the greatest visualization of the operative field. In addition, better angles of inspection of the surgical area and the thoracic cavity are afforded by a 30-degree scope.
Instruments that are used by the thoracic surgeons for VATS procedures have become the mainstay for VATS discectomy. These instruments are longer and have been adapted with pistol grips. They include scissors and dissectors with articulating heads that allow the tip to be repositioned for greater visualization during the surgical dissection (Fig. 160-5). Other instruments have been developed to aid in the specific needs unique to the thoracic cavity, such as fan retractors and suction irrigation devices.
The existing instruments common to spinal surgery have been modified by lengthening and by providing a greater variety of tip angles. These include a variety of periosteal elevators, curets, rongeurs, and dissectors. Modified long drill bits with coarse diamond bits are available (Midas Rex, Fort Worth, TX) (Fig. 160-6).
Description of Procedure
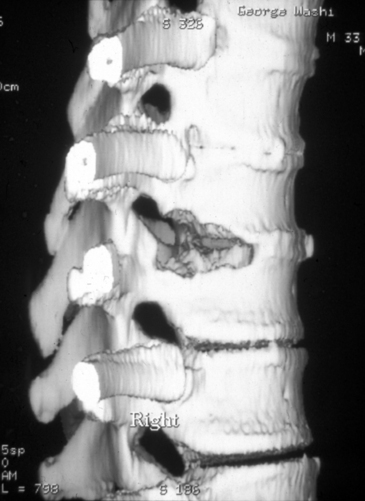
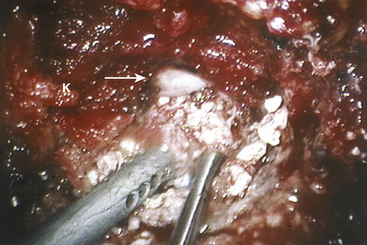
Once the rib head has been removed and the lateral disc space is clearly identified, the spinal canal must be defined. To accomplish this, the pedicle immediately posterior to the costovertebral articulation is exposed. The upper one third of the pedicle is typically removed using the diamond bur and a Kerrison rongeur. An extended rostral removal of the pedicle may be necessary if the disc herniation is large or calcified. Removing this portion of the pedicle exposes the anterior portion of the vertebral canal and the posterior longitudinal ligament. The normal dura and the epidural space are identified. Epidural venous bleeding may be encountered, and it is generally controlled with the use of the bipolar cautery or Avitene (CR Bard, Murray Hill, NJ). The decompression of the spinal canal and the removal of the disc material may now take place under direct visualization. A portion of the vertebral body on either side of the disc is removed to create a working channel (Fig. 160-7). This is accomplished using a shielded diamond bur to fashion a pyramid-shaped space. The disc material is pulled away from the thecal sac into this cavity (Fig. 160-8).
The amount of bone removed to create this space is determined by the size of the disc herniation and the amount of associated canal compromise. The working channel must be large enough to afford visualization of the normal dura rostral and caudal to the disc herniation. For large calcified discs or when an intradural fragment is identified, an extended vertebral body resection may be necessary. After the spinal canal has been exposed through this working channel, the disc material may be pulled away from the spinal cord into this created working space. If the disc material is soft, then it may be removed with a pituitary forceps or a curet. In the case of a calcified disc, however, care must be taken to sequentially dissect it away from the dura. The decompressed spinal canal is inspected and palpated to the level of the contralateral pedicle. If an intradural fragment is encountered, then it should be carefully dissected from the adjacent pia-arachnoid. A dural repair is accomplished with a tissue patch and fibrin glue. A lumbar drain is placed postoperatively.
Outcomes and Complications
Outcome results for patients undergoing thoracoscopic discectomy have been widely available since the late 1990s. In 1999, Rosenthal and Dickman48 reported on their experience with 55 patients undergoing thoracoscopic discectomy and compared their patients with a smaller subset undergoing thoracotomy. They reported a 1-hour reduction in operative time and a 50% reduction in blood loss, narcotics use, and hospital length of stay. They also noted a 16% incidence of intercostal neuralgia in the thoracoscopy group as compared with the thoracotomy group, who experienced a 50% incidence. Regarding neurologic outcome, of the 36 patients with preoperative myelopathy, 27 had neurologic improvement, and all patients’ neurologic examinations stabilized. Nineteen patients were treated for radiculopathy, and all 19 experienced improvement.48
In 2000, Johnson and colleagues52 reported their prospective series of 36 patients undergoing thoracoscopic discectomy compared with 8 patients undergoing thoracotomy. Their results matched those reported earlier, demonstrating a statistically significant reduction in hospital length of stay and narcotic use in the thoracoscopy group. They also reported a 30% complication rate in the thoracoscopy group versus a greater than 100% (i.e., more than one complication per patient) complication rate in the thoracotomy group. However, they were not able to show a statistically significant difference in ultimate neurologic outcome between the two groups.
Anand and Regan53 have evaluated the long-term outcome after thoracoscopic discectomy for thoracic disc disease. They examined 100 consecutive patients who underwent thoracoscopic discectomy with an average of 4 years of follow-up. Five percent of patients required a reoperation during the follow-up interval, including one nonunion. Seventy percent of those treated had achieved a 20% improvement on their Oswestry score (their definition of a clinical success) at their last visit, and 84% of the patients were satisfied with their results.
More recently, Oskouian and Johnson54 reported their experience with 46 consecutive patients undergoing thoracoscopic discectomy. They reported a 75% improvement in postoperative Ostwestry scores for radiculopathy, with 40% of patients with myelopathy achieving a grade improvement in Frankel scores.
In 1995, McAffe and colleagues55 addressed the issue of complications related to the thoracoscopic approach. They reported on 78 patients undergoing thoracoscopic discectomy. They found an 8% incidence of intercostal neuralgia and a 6% incidence of postoperative atelectasis; both figures are significantly less than those reported for thoracotomy. Only one case required conversion to thoracotomy for significant pleural adhesions. Injury to the thoracic duct and development of chylothorax is another potential risk of the procedure.54,56
Anand N., Regan J.J. Video assisted thoracoscopic surgery for thoracic disk disease: classification and outcome study of 100 consecutive cases with a 2-year minimum follow-up period. Spine. 2002;27:871-879.
Awwad E.E., Martin D.S., Smith K.R., et al. Asymptomatic versus symptomatic herniated thoracic discs: their frequency and characteristics as detected by computed tomography after myelography. Neurosurgery. 1991;28:180-186.
Benson M.D., Byrnes D.P. The clinical syndromes and surgical treatment of thoracic intervertebral disc prolapse. J Bone Joint Surg Am. 1988;70:1038-1047.
Bohlmann H.H. Zdeblick: Anterior excision of herniated thoracic discs. J Bone Joint Surg Am. 1988;70:1038-1047.
Caputy A., Starr J., Riedel C. Video-assisted endoscopic spinal surgery: thorascopic diskectomy. Acta Neurochir (Wien). 1995;134:196-199.
Fessler R.G., Sturgill M. Review: complications of surgery for thoracic disc disease. Surg Neurol. 1998;49:609-618.
Horowitz M.B., Moosey J.J., Julian T., et al. Thoracic diskectomy using video-assisted thorascopy. Spine. 1994;9:1082-1086.
Landreneau R.J., Hazelrigg S.R., Mack M.J., et al. Post operative pain related morbidity: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 1993;56:1285-1289.
Maiman D.J., Larson S.J., Luck E., Elghatit A. Lateral extracavitary approach to the spine for thoracic disk herniation: report of 23 cases. Neurosurgery. 1984;41:178-182.
Patterson R.H., Arbit E. A surgical approach through the pedicle to protruded thoracic disks. J Neurosurg. 1978;48:768-772.
Rosenthal D., Dickman C.A. Thorascopic microsurgical excision of herniated thoracic discs. Neurosurg Focus. 1999;6:4.
1. Hayward G. An account of a case of fracture and dislocation of the spine. J Med Sci. 1815;4:1-3.
2. Benjamin V. Diagnosis and management of thoracic disc disease. Clin Neurosurg. 1983;38:577-605.
3. Arce C.A., Dohrmann G.J. Herniated thoracic disks. Neurol Clin. 1985;3:383-392.
4. Ridenore T.R., Haddad P.W., Hichson P.W., et al. Herniated thoracic disks: treatment and outcome. J Spinal Disord. 1993;3:218-224.
5. Awwad E.E., Martin D.S., Smith K.R., et al. Asymptomatic versus symptomatic herniated thoracic discs: their frequency and characteristics as detected by computed tomography after myelography. Neurosurgery. 1991;28:180-186.
6. Williams M.P., Cherryman G.R., Husband J.E. Significance of thoracic disc herniation demonstrated by MR imaging. J Comput Assist Tomogr. 1989;13:211-214.
7. Woods K.B., Schellhas K.P., Garvey T.A., et al. Thoracic discography in healthy individuals: a controlled prospective study of magnetic resonance imaging and discography in asymptomatic and symptomatic individuals. Spine. 1999;24:1548-1555.
8. Horwitz N.H., Rizzoli N.V. Postoperative Complications of Extracranial Neurological Surgery. Baltimore: Williams & Wilkins; 1987.
9. Perot P.L., Munro D.D. Transthoracic removal of midline thoracic protrusions causing spinal cord compression. J Neurosurg. 1969;31:452-458.
10. Benson M.D., Byrnes D.P. The clinical syndromes and surgical treatment of thoracic intervertebral disc prolapse. J Bone Joint Surg Am. 1988;70:1038-1047.
11. Logue V. Thoracic intervertebral disc prolapse with spinal cord compression. J Neurol Neurosurg Psychiatry. 1952;15:221-247.
12. Mixter W.J., Barr J.S. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med. 1934;221:210-215.
13. Horwitz N.H., Whitcomb B.B., Reilly F.G. Ruptured thoracic discs. Yale J Biol Med. 1955;28:322-330.
14. Fessler R.G., Sturgill M. Review: complications of surgery for thoracic disc disease. Surg Neurol. 1998;49:609-618.
15. Ranasohoff J., Spencer F., Siew F., Gade L.Jr. Transthoracic removal of thoracic disks: report of three cases. J Neurosurg. 1969;31:459-461.
16. Huhme A. The surgical approach to intervertebral disk protrusions. J Neurol Neurosurg Psychiatry. 1960;23:133-137.
17. Patterson R.H., Arbit E. A surgical approach through the pedicle to protruded thoracic disks. J Neurosurg. 1978;48:768-772.
18. Maiman D.J., Larson S.J., Luck E., Elghatit A. Lateral extracavitary approach to the spine for thoracic disk herniation: report of 23 cases. Neurosurgery. 1984;41:178-182.
19. Fessler R.G., Dietze D.D.Jr., MacMillan M. Lateral parascapular extrapleural approach to the upper thoracic spine. J Neurosurg. 1991;75:349-355.
20. Craffoord C., Hiertonn T., Lindblom K., Olsson S.E. Spinal cord compression caused by a protruded thoracic disc: report of a case treated with antero-lateral fenestration of the disc. Acta Orthop Scand. 1958;28:103-107.
21. Bohlmann H.H., Zdeblick T.A. Anterior excision of herniated thoracic discs. J Bone Joint Surg Am. 1988;70:1038-1047.
22. Otani K., Yoshida M., Fujii E., et al. Thoracic disc herniation: surgical treatment in 23 patients. Spine. 1998;13:1262-1267.
23. Otani K., Nakai S., Fujimura Y., et al. Surgical treatment of thoracic disc herniation using the anterior approach. J Bone Joint Surg Br. 1982;64:340-343.
24. Faciszewski T., Winter R.B., Lonstein J.E., et al. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults: a review of 1223 procedures. Spine. 1995;20:1592-1599.
25. Naunheim K.S., Barnett M.G., Crandall D.G., et al. Anterior exposure of the thoracic spine. Ann Thorac Surg. 1994;57:1436-1439.
26. Sundaresan N., Shah J., Foley K.M., et al. An anterior surgical approach to the upper thoracic vertebrae. J Neurosurg. 1984;61:686-690.
27. Bozzini P.H. Lichtleiter, eine Erfindung zur Anschauung innerer Teile und Krankheiten. J Prak Heilk. 1806;24:107.
28. Nitze M. Beobachtungs and Untersuchungsmethode für Harnrohre Harnblase und Rectum. Wien Med Wochenschr. 1879;24:649-652.
29. Jacobeus H.C. Über die Möglichkeit die Zystoskopie bei Untersuchung seröser Höhlungen anzuwenden. Munch Med Wochenschr. 1910;57:2090-2092.
30. Jacobeus H.C. The cauterization of adhesions in pneumothorax treatment of tuberculosis. Surg Gynecol Obstet. 1921;32:493-500.
31. Jacobeus H.C. The practical importance of thorascopy in surgery of the chest. Surg Gynecol Obstet. 1922;34:289-296.
32. Branco J.M.C. Thorascopy as a method of exploration in penetrating injuries of the chest. Dis Chest. 1946;12:330.
33. Kux M. Thoracic endoscopic sympathectomy in palmar and axillary hyperhydrosis. Arch Surg. 1978;113:264-266.
34. Hatch H.B., DeCamp P.T. Diagnostic thorascopy. Surg Clin North Am. 1966;46:1405-1410.
35. Burman M.S. Myeloscopy or the direct visualization of the spinal canal and its contents. J Bone Joint Surg. 1931;13:695-696.
36. Pool J.L. Direct visualization of the dorsal nerve roots of the cauda equine by means of the myeloscope. Arch Neurol Psychol. 1938;39:1308-1312.
37. Pool J.L. Myeloscopy, intrathecal endoscopy. Surgery. 1942;11:169-182.
38. Hausmann B., Forst R. Nucleoscope instrumentarium for endoscopy of the intervertebral disc space. Arch Orthop Trauma Surg. 1983;102:37-59.
39. Schreiber A., Leu H.J. Percutaneous nucleotomy: technique with discoscopy. Orthopedics. 1991;14:439-444.
40. Horowitz M.B., Moosey J.J., Julian T., et al. Thoracic diskectomy using video-assisted thorascopy. Spine. 1994;9:1082-1086.
41. Rosenthal D., Rosenthal S., Somone A. Removal of a protruded thoracic disc using microsurgical endoscopy: a new technique. Spine. 1994;19:1087-1091.
42. Ferson P.F., Landreneau R.J., Dowling R.D., et al. Comparison of open versus thorascopic lung biopsy for diffuse infiltrating pulmonary disease. J Thoracic Cardiovasc Surg. 1993;106:194-199.
43. Kaiser L.R. Video assisted thoracic surgery current state of the art. Ann Surg. 1994;220:720-734.
44. Landreneau R.J., Hazelrigg S.R., Mack M.J., et al. Post operative pain related morbidity: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 1993;56:1285-1289.
45. Gow J.G. Harold Hopkins and optical systems for urology—an appreciation. Urology. 1998;52:152-157.
46. Mack M.J., Regan J.J., Bobechko W.P., Acuff T.E. Application of thoracoscopy for diseases of the spine. Ann Thorac Surg. 1993;56:736-738.
47. Caputy A., Starr J., Riedel C. Video-assisted endoscopic spinal surgery: thorascopic diskectomy. Acta Neurochir (Wien). 1995;134:196-199.
48. Rosenthal D., Dickman C.A. Thorascopic microsurgical excision of herniated thoracic discs. Neurosurg Focus. 1999;6:4.
49. Liljenqvist U. Anatomic principles of thoracoscopic spine surgery. In: Mayer H.M., editor. Minimally Invasive Spine Surgery. New York: Springer, 2000.
50. Benaventre O.R., Barnett H.M. Spinal cord infarction. In: Carter L.P., Spetzler R.F., Hamilton M.G. Neurovascular Surgery. New York: McGraw-Hill, 1995.
51. Jasuja M.L. Intrathoracic anatomy: an endoscopic perspective. In: Dieter R.A., editor. Thoracoscopy for Surgeons: Diagnostic and Therapeutic. New York: Igaku-Shoin, 1995.
52. Johnson J.P., Filler A.G., McBride D.Q. Endoscopic thoracic diskectomy. Neurosurg Focus. 9, 2000.
53. Anand N., Regan J.J. Video assisted thoracoscopic surgery for thoracic disk disease: classification and outcome study of 100 consecutive cases with a 2-year minimum follow-up period. Spine. 2002;27:871-879.
54. Oskouian R.J., Johnson J.P. Endoscopic thoracic microdisectomy. J Neurosurg Spine. 2005;3:459-464.
55. McAffe P.C., Regan J.R., Zdeblick T., et al. The incidence of complications in endoscopic anterior thoracolumbar spinal reconstructive surgery: a prospective multicenter study comprising the first 100 consecutive cases. Spine. 1995;20:1624-1632.
56. Amini A., Apfelbaum R.I., Schmidt M.H. Chylorrhea: a rare complication of thoraciscopic discectomy of the thoraclumbar junction. Case report. J Neurosurg Spine. 2007;6:563-566.