Chapter 137 Surgical Management of Chronic Subdural Hematoma in Adults
Epidemiology
Chronic subdural hematoma (CSDH) is one of the commonest neurosurgical disorders and is especially prevalent among the elderly. Its incidence is about five per 100,000 per year in the general population but is higher for those aged 70 years and older (58 per 100,000 per year).1 Because the proportion of people aged 65 years and older is expected to double worldwide between 2000 and 2030, a substantial rise in incidence is expected.1 CSDH has a strong male preponderance, with a male-to-female ratio approximately 3:1.2 Age distributions vary among published series depending on the populations from which they were derived. Similarly to most published series,3–5 our published cohort of 215 patients had a mean age of 76.8 years, ranging from 36 to 95 years.2
Pathogenesis
CSDH arises in the dural border cell (DBC) layer, a loose cellular layer devoid of intercellular collagen and tight junctions, located between two firm meninges: the dura mater wired with abundant intercellular collagen on one side and the arachnoid matter with cells anchored to a basal membrane and clamped together with tight junctions on the other (Fig. 137-1).6 Traversing veins are being increasingly stretched by the shrinking brain until only a minor additional force is sufficient to cause rupture through stretching or shearing.6 The extravasated blood dissects the DBC layer, creating a subdural cavity. Similarly, a traumatic tear of the arachnoid mater can cause a hygroma, which can later transform into a CSDH.4,7,8 Whatever the initial mechanism, a failure of complex reparatory processes to heal the injured tissues results in CSDH. In contrast to young and healthy persons in whom healing is often successfully completed, CSDHs are more likely to arise in elderly patients with atrophic brain, impaired coagulation, repeated falls, and conditions resulting in intracranial hypotension, such as ventriculoperitoneal shunt. However, rarer causes of a CSDH such as vascular malformations, arachnoid cysts, and neoplasms have to be borne in mind.9–11 CSDH is characterized by the formation of neomembranes (the inner, visceral, membrane is relatively less vascular and usually thinner than the outer, parietal, membrane) with growth of leaky neocapillaries and enzymatic hyperfibrinolysis.12
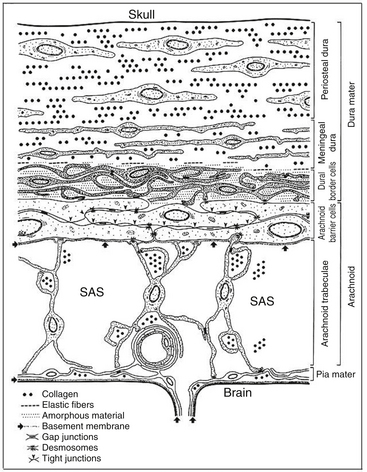
Figure 137-1 Schematic representation of the ultrastructure of the meninges (from Haines et al6). The dura mater is composed of fibroblasts and a large amount of collagen. The arachnoid barrier cells are supported by a basement membrane and bound together by numerous tight junctions. The dural border cells layer is formed by flattened fibroblasts, with no tight junctions and no intercellular collagen. It is therefore a relatively loose layer positioned between firm dura mater and arachnoid. The subdural space is a potential space that can form within the dural border cell layer. SAS, subarachnoid space.
Clinical Features
Patients present with a variety of complaints, usually following a subacute or chronic course. In our published cohort the most common symptoms in order of decreasing frequency were gait disturbance and falls, mental deterioration, hemiparesis, headache, speech impairment, and drowsiness or coma.2 A history of head injury was established in 119 of 196 cases (61%). On admission, 86 (42%) patients had a Glasgow Coma Score (GCS) of 15, 102 (50%) had scores of 9 to 14, and 14 (7%) had scores less than 8. Limb weakness was recorded in 111 of 184 (63%), and dysphasia was found in 62 of 191 (33%).
Radiologic Evaluation
Most CSDHs can be diagnosed with a computed tomography (CT) or magnetic resonance imaging (MRI) scan. CT is currently the investigation of choice in CSDH. The density of a subdural hematoma depends on the interval(s) between the bleeding episode(s) and the time of examination.13 A hyperdense, crescent-shaped collection lying between the brain and the inner skull table represents an acute subdural hematoma (60 to 80 Hounsfield units). A subacute subdural hematoma can be nearly isodense with the adjacent cortex (30 Hounsfield units). This phase occurs 1 to 3 weeks after the acute event.14 Isodense hematomas may be difficult to differentiate from normal brain tissue, and MRI may be helpful in establishing the diagnosis.14 Certain features on CT such as medial displacement of the gray–white matter interface and a failure of cortical sulci to reach the inner skull table can point toward the presence of an isodense subdural hematoma.14 CSDHs more than 3 weeks old are encapsulated, crescent-shaped, hypodense collections (0 to 25 Hounsfield units). As the capsule of a CSDH undergoes neoangiogenesis, it enhances with contrast administration. A mixed-density pattern or a fluid level indicate rebleeding into a preexisting CSDH or hygroma.13
Nomura and colleagues15 divided subdural hematomas into five types based on CT appearances: hyperdense, isodense, hypodense, mixed-density, and layering types. The authors demonstrated that the layering type is characterized by a high hyperfibrinolytic activity. The mixed-density type has a lower hyperfibrinolytic activity than the layering type, and the hypodense hematoma has the lowest fibrinolytic activity. Nakaguchi and colleagues16 grouped subdural hematomas into four types: homogeneous-density (hyperdense, hypodense, isodense), laminar (known as mixed-density), separated (known as layering) including gradation subtype, and trabecular (Fig. 137-2). They showed that the separated type of CSDH had a higher recurrence rate (36%) compared to the laminar (19%) and homogeneous (15%) types. Stanisic and colleagues17 confirmed the high recurrence rate of the separated (29%) and laminar (27%) types.
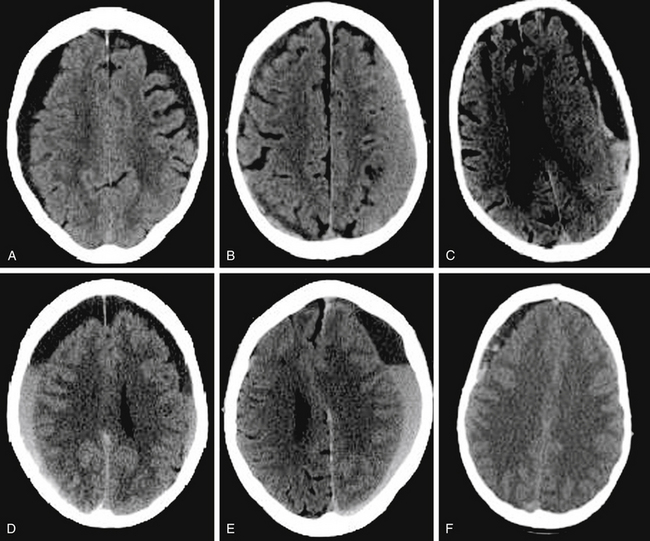
Figure 137-2 Computed tomography scans demonstrating classification of chronic subdural hematomas according to their internal architecture (as proposed by Nakaguchi et al16): A, Homogeneous hypodense. B, Homogeneous isodense. C, Laminar. D, Gradation. E. Separated. F, Trabecular.
MRI of CSDH can better define the multicompartmental nature of certain CSDHs.18 The MRI signal intensity changes for a subdural hematoma follow the pattern of changes of an intracranial hematoma.13 Tsutsumi and colleagues19 attempted to classify CSDHs into five groups according to the intensity of their appearance on T1-weighted MRI: high, mixed high/isointense, isointense, mixed isointense/low, and low. The authors could not demonstrate a correlation between CT classifications and T1-weighted MRI appearances. However, they showed that the recurrence rate of CSDHs that exhibited homogeneous high intensity on T1-weighted images was significantly lower (3.4%) than that in the non–high-intensity groups (11.6%). Tanikawa and colleagues20 found that subdural hematomas divided into multiple layers by an intrahematomal membrane (as demonstrated on T2-weighted MRI) are more effectively treated with craniotomy than burr-hole craniostomy. However, the need for division of membranous septae remains controversial (see the section on craniotomy), and the role of imaging in selecting a surgical technique has not been sufficiently studied.
Treatment
Conservative management is usually reserved for moribund patients with significant comorbidities or asymptomatic patients with small collections.21 Most surgeons would agree that surgical evacuation of a CSDH is indicated for symptomatic patients or collections exerting significant mass effect. Preoperatively, iatrogenic anticoagulation and coagulopathies need to be reversed. With respect to the use of prophylactic antiepileptic medications, there is still not a sufficient evidence base to support their routine use. The many surgical techniques used in the treatment of CSDH can be broadly classified into the following three categories: twist-drill craniostomy (diameter less than 5 mm), burr-hole craniostomy (5 to 30 mm), and craniotomy (more than 30 mm).22 Burr-hole craniostomy is the most popular surgical technique worldwide.22
Burr-Hole Craniostomy
Burr-hole craniostomy is a widely practiced technique with an overall morbidity and recurrence rates of 0% to 9% and 5% to 30%, respectively.1,22 Both the systematic review by Weigel and colleagues22 and a decision analysis model based on the data reported in the literature by Lega and colleagues23 have identified burr-hole craniostomy as the most efficient choice to treat a primary and uncomplicated CSDH because it balances a low recurrence rate against a better morbidity and mortality profile compared to craniotomy and twist-drill craniostomy.
Technique
Two 14-mm burr holes approximately 7 cm apart are drilled over the maximum width of the hematoma (usually one frontal and one parietal, with the exact location determined from the CT scan). The dura mater is opened with a cruciate incision and coagulated with bipolar diathermy. The subdural collection is washed out with warmed Ringer’s solution using a 50-ml syringe until the subdural fluid runs clear. A soft catheter may be used to irrigate distant areas of the hematoma cavity, although we do not consider it always necessary. The subdural membrane loculations accessible via the burr holes are disrupted. A soft silicon drain with three smooth side holes and a blunt tip (external diameter 4.7 mm and length 90 cm) is inserted into the subdural space through the burr hole overlying the large part of the subdural cavity, and the drain is tunneled for a minimum of 5 cm away from the scalp incision.
First, the parietal burr hole is closed in layers to achieve watertight closure. The burr hole may be plugged with a thick wafer of an absorbable sponge (e.g., gelatin sponge) to aid hemostasis and improve seal. The head is manipulated so that the frontal burr hole is at the highest point, the subdural cavity is filled with Ringer’s solution, and the wound is closed, trying to minimize air trapping. The drain is connected to a soft collection bag (subdural-external, no-suction, closed drainage system) that is kept in a dependent position for 48 hours. The drain is then carefully removed and a single stitch is applied at the drain insertion site.2
Discussion
Since the 1990s, evidence has been emerging that the use of drains with burr-hole craniostomy is associated with lower recurrence rates.3,24–27 In the review by Weigel and colleagues, the use of drains was endorsed with type B recommendation.22 The results of a Monte Carlo simulation in the paper by Lega and colleagues suggests a trend toward better outcomes with insertion of an indwelling drain.23
In 2009, we published the results of a randomized, controlled trial of the use of drains versus no drains after burr-hole craniostomy.2 Hematoma recurred in 10 of 108 (9.3%) patients with a drain and in 26 of 107 (24%) without one (p = 0.003). At 6 months, mortality was 9 of 105 (8.6%) and 19 of 105 (18.1%), respectively (p = 0.042). Medical and surgical complications did not differ between the two arms. In accordance with the aforementioned studies, the results of this trial provide strong evidence for the use of drains with burr-hole craniostomy.
Several groups have attempted to address the role of irrigation in the treatment of CSDH and have demonstrated a trend toward reduced recurrence with the use of intraoperative irrigation.28–31 Only Kuroki and colleagues32 found more than six times higher recurrence (p = 0.49) in cases with irrigation (5/45, 11.1%) than without irrigation (1/55, 1.8%). Conversely, Aoki33 in a study of twist-drill craniostomies found a lower recurrence rate in the cases where intraoperative irrigation was used (1/15 vs 7/24, p = 0.096). None of the studies cited here were sufficiently powered to demonstrate significant differences.
Most surgeons use two burr holes, mainly because this allows better wash out of the subdural cavity. Taussky and colleagues34 observed a higher recurrence rate if one rather than two burr holes was used. By contrast, in the series by Han and colleagues35 the recurrence rate was 1/51 (2%) with one burr hole and 9/129 (7%) with two (p = 0.186). Both were retrospective studies, and a number of factors, including the decision making as to when one or two burr holes should be drilled, may have been responsible for obtaining such disparate results. If drains are used it seems more advantageous to insert them via a frontal burr hole.22
Twist-Drill Craniostomy
Since 1977, when Tabaddor and Shulman36 presented their results with bedside twist-drill craniostomy, 16 more publications have reported on the efficacy and safety of twist-drill craniostomy or modifications of it for treating CSDH.37 In those studies, the percentage of patients requiring a subsequent procedure to treat recurrent CSDH ranged from 1.5% to 63.6%. In total, 84 of 556 patients (15.1%) required a subsequent procedure.37 The systematic review by Weigel and colleagues22 demonstrated that twist-drill craniostomy and burr-hole craniostomy have a comparable morbidity and mortality profile, but twist-drill craniostomy is less effective because it has a higher recurrence rate. However, an advantage of twist-drill craniostomy is the possibility of performing it at the bedside.
Technique
The technique described here is based on the original reports by Tabaddor and Shulman36 and by Camel and Grubb,38 although many modifications exist.39–44 The site for the burr hole is selected so that the tip of the catheter can be placed in the most rostral part of the subdural collection. After infiltrating the scalp with a local anesthetic, a 1-cm incision is made. A burr hole is drilled at an angle approximately 45 degrees to the skull surface. The dura is perforated with the drill or a spinal needle. A standard ventricular catheter is passed into the most rostral part of the subdural cavity and tunneled at least 5 cm. It is then connected to a reservoir placed approximately 40 cm under the head level. The drain is kept for 2 to 7 days, depending on the amount of drainage. In 2003, Asfora and Schwebach published a modified TDC method (the subdural evacuating port system), which, in comparison with existing TDC methods, offers the advantage of a closed-system drainage without the need to insert a subdural catheter blindly, thereby avoiding the risks of brain laceration.39
Craniotomy
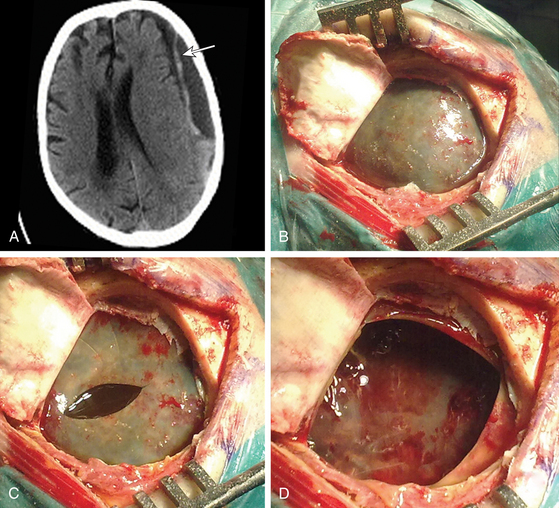
Until Markwalder’s review paper in 1981, craniotomy and evacuation of the hematoma together with removal of the membranes was widely practiced, often as the primary treatment.45 However, that paper led to a wider appreciation that burr-hole craniostomy is sufficient for most patients, and this became the procedure of choice in many centers. Weigel and colleagues demonstrated that craniotomy and burr-hole craniostomy have a comparable recurrence rate, but craniotomy carries higher morbidity.22 The Monte Carlo simulation used by Lega and colleagues revealed that burr-hole craniostomy results in significantly better outcomes than twist-drill craniostomy or craniotomy. They performed one-way sensitivity analyses, which indicated that the variables with the greatest influence on outcomes are the probability of death, the probability of recurrence, and the utility of major complications.23 Hamilton and colleagues retrospectively reviewed 92 consecutive cases of CSDH, of which 49 underwent craniotomy and 43 underwent burr-hole craniostomy, in an attempt to reevaluate the safety and efficacy of craniotomy.46 They found no significant difference in the incidence of postoperative complications, hematoma recurrence, or operative mortality between these two groups. Indeed, some surgeons use mini-craniotomy as the method of choice for evacuating CSDH, believing that these are associated with lower recurrence rate. However, craniotomy and burr-hole craniostomy have not been compared in an appropriately powered clinical trial. Hence, most surgeons would agree with Markwalder’s conclusion that craniotomy is indicated for multiple recurrences, for solid hematoma, or if disruption of hematoma membranes is desired.45 Although we tend to perform mini-craniotomy for a laminar type of CSDH to open the inner sagittal septum of the parietal membrane (Fig. 137-3), the benefit of membranectomy and disruption of membranes remains uncertain.4,47–50
Outcome
Tabaddor and Shulman in their study of twist-drill craniostomy with closed-system drainage demonstrated that the subdural pressure dropped to zero after the initial 20% of the subdural collection was removed. This initial 20% was usually enough to lead to marked clinical improvement.36 Markwalder and colleagues in their study of burr-hole craniostomy with closed-system drainage demonstrated that 78% of the patients had a persistent subdural collection on CT, on the 10th day after surgery, that did not interfere with recovery. Forty days following surgery, 85% of the patients had complete resolution of the subdural collection on CT. Their recommendation was therefore not to treat persistent fluid collections with additional procedures unless the patient fails to improve or, indeed, deteriorates.51 In accordance with this reasoning, most surgeons in the United Kingdom and Ireland do not routinely obtain postoperative imaging in asymptomatic patients.21
However, recurrence of CSDH is not a trivial problem, especially because patients suffering from CSDH are usually elderly. Recurrence is usually defined by a clinical deterioration or failure to improve after a primary procedure, along with consistent radiologic findings.22 Recurrence rates range from 5% to 30%.1,22 Twist-drill craniostomy has a higher recurrence rate compared to burr-hole craniostomy and craniotomy,22 and the use of closed-system drainage after burr-hole craniostomy results in a markedly reduced recurrence rate compared to burr-hole craniostomy alone.2 A variety of factors such as age, sex, treatment with anticoagulant or antiplatelet agents, brain reexpansion, pneumocephalus, states of intracranial hypotension, and preoperative CT and MRI findings have been put forward as predisposing to the development of a recurrence. A review of 500 consecutive patients treated with burr-hole craniostomy and closed-system drainage found that the brain reexpansion rate (as measured by CT) in those with recurrence was significantly lower than the reexpansion rate in those without recurrence. Factors resulting in reduced brain reexpansion after surgery were age older than 70 years, preexisting cerebral infarction, anticoagulant therapy, and presence of air in the subdural space.52 It is possible that postoperative bed rest after CSDH evacuation facilitates brain expansion. However, currently available evidence is conflicting.53,54 This is reflected in the current practice in Canada, Ireland and the United Kingdom, where just over half (55%) of surgeons prescribe postoperative bed rest.21,55
Morbidity is related to general (e.g., pneumonia, venous thromboembolism) and central nervous system complications. Weigel and colleagues,22 who defined morbidity as any complication happening during or after surgery other than recurrence, found that craniotomy had a higher morbidity rate (12.3%) than twist-drill craniostomy (3%) or burr-hole craniostomy (3.8%). Central nervous system complications can take the form of seizures, acute subdural and intracerebral hemorrhage, tension pneumocephalus, subdural empyema, and wound infection.23 Ogasawara and colleagues observed transient hyperemia in the cortex beneath the CSDH immediately after surgical evacuation. This was observed in elderly patients and may be related to the complications of acute intracranial hemorrhage and seizures.56 Weigel and colleagues, who defined mortality as any death reported between surgery and discharge from hospital, showed that the mortality rates for burr-hole craniostomy (2.7%), twist-drill craniostomy (2.9%), and craniotomy (4.6%) do not differ significantly.22
Restarting anticoagulation after CSDH evacuation is a complex issue. Not surprisingly, the published data are insufficient to provide specific guidance. Essentially it is a matter of balancing the estimated risk of adverse effects resulting from recurrence on the one hand and complication of thromboembolic events on the other. Because of this, each case must be treated with consideration of all pertinent individual patient factors. Factors such as the indication for anticoagulation, age, estimated degree of brain atrophy and postoperative brain expansion, and the likelihood of repeated head trauma need to be taken into account.57 Routine postoperative thromboprophylaxis, such as low-molecular weight heparin, can be commenced the day after surgery. Compression stockings should be prescribed upon admission to the hospital, unless they are contraindicated.
Baglin T.P., Keeling D.M., Watson H.G. Guidelines on oral anticoagulation (warfarin): third edition—2005 update. Br J Haematol. 2006;132:277-285.
Haines D.E., Harkey H.L., al-Mefty O. The “subdural” space: a new look at an outdated concept. Neurosurgery. 1993;32:111-120.
Hamilton M.G., Frizzell J.B., Tranmer B.I. Chronic subdural hematoma: the role for craniotomy reevaluated. Neurosurgery. 1993;33:67-72.
Horn E.M., Feiz-Erfan I., Bristol R.E., et al. Bedside twist drill craniostomy for chronic subdural hematoma: a comparative study. Surg Neurol. 2006;65:150-153.
Lee J.Y., Ebel H., Ernestus R.I., et al. Various surgical treatments of chronic subdural hematoma and outcome in 172 patients: is membranectomy necessary? Surg Neurol. 2004;61:523-527.
Lee K.S. Natural history of chronic subdural haematoma. Brain Inj. 2004;18:351-358.
Lega B.C., Danish S.F., Malhotra N.R., et al. Choosing the best operation for chronic subdural hematoma: a decision analysis. J Neurosurg, 2010;3(113):615-621
Lind C.R., Lind C.J., Mee E.W. Reduction in the number of repeated operations for the treatment of subacute and chronic subdural hematomas by placement of subdural drains. J Neurosurg. 2003;99:44-46.
Markwalder T.M., Steinsiepe K.F., Rohner M., et al. The course of chronic subdural hematomas after burrr-hole craniostomy and closed-system drainage. J Neurosurg. 1981;55:390-396.
Markwalder T.M. Chronic subdural hematomas: a review. J Neurosurg. 1981;54:637-645.
Mori K., Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo). 2001;41:371-381.
Nakaguchi H., Tanishima T., Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg. 2001;95:256-262.
Nomura S., Kashiwagi S., Fujisawa H., et al. Characterization of local hyperfibrinolysis in chronic subdural hematomas by SDS-PAGE and immunoblot. J Neurosurg. 1994;81:910-913.
Parizel P.M., Makkat S., Van M.E., et al. Intracranial hemorrhage: principles of CT and MRI interpretation. Eur Radiol. 2001;11:1770-1783.
Rocchi G., Caroli E., Salvati M., et al. Membranectomy in organized chronic subdural hematomas: indications and technical notes. Surg Neurol. 2007;67:374-380.
Rughani A.I., Lin C., Dumont T.M., et al. A case-comparison study of the subdural evacuating port system in treating chronic subdural hematomas. J Neurosurg. 2010;113:609-614.
Santarius T., Hutchinson P.J. Chronic subdural haematoma: time to rationalize treatment? Br J Neurosurg. 2004;18:328-332.
Santarius T., Kirkpatrick P.J., Ganesan D., et al. Use of drains versus no drains after burrr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet. 2009;374:1067-1073.
Santarius T., Kirkpatrick P.J., Kolias A.G., Hutchinson P.J. Working towards rational and evidence-based treatment of chronic subdural haematoma. Clin Neurosurg. 2010;57:112-122.
Santarius T., Lawton R., Kirkpatrick P.J., et al. The management of primary chronic subdural haematoma: a questionnaire survey of practice in the United Kingdom and the Republic of Ireland. Br J Neurosurg. 2008;22:529-534.
Sucu H.K., Gokmen M., Ergin A., et al. Is there a way to avoid surgical complications of twist drill craniostomy for evacuation of a chronic subdural hematoma? Acta Neurochir (Wien). 2007;149:597-599.
Suzuki K., Sugita K., Akai T., et al. Treatment of chronic subdural hematoma by closed-system drainage without irrigation. Surg Neurol. 1998;50:231-234.
Tabaddor K., Shulman K. Definitive treatment of chronic subdural hematoma by twist-drill craniostomy and closed-system drainage. J Neurosurg. 1977;46:220-226.
Tanikawa M., Mase M., Yamada K., et al. Surgical treatment of chronic subdural hematoma based on intrahematomal membrane structure on MRI. Acta Neurochir (Wien). 2001;143:613-618.
Weigel R., Schmiedek P., Krauss J.K. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry. 2003;74:937-943.
1. Santarius T., Hutchinson P.J. Chronic subdural haematoma: time to rationalize treatment? Br J Neurosurg. 2004;18:328-332.
2. Santarius T., Kirkpatrick P.J., Ganesan D., et al. Use of drains versus no drains after burrr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet. 2009;374:1067-1073.
3. Lind C.R., Lind C.J., Mee E.W. Reduction in the number of repeated operations for the treatment of subacute and chronic subdural hematomas by placement of subdural drains. J Neurosurg. 2003;99:44-46.
4. Mondorf Y., Abu-Owaimer M., Gaab M.R., et al. Chronic subdural hematoma—craniotomy versus burrr hole trepanation. Br J Neurosurg. 2009;23:612-616.
5. Zumofen D., Regli L., Levivier M., et al. Chronic subdural hematomas treated by burrr hole trepanation and a subperiostal drainage system. Neurosurgery. 2009;64:1116-1121.
6. Haines D.E., Harkey H.L., al-Mefty O. The “subdural” space: a new look at an outdated concept. Neurosurgery. 1993;32:111-120.
7. Lee K.S. Natural history of chronic subdural haematoma. Brain Inj. 2004;18:351-358.
8. Park C.K., Choi K.H., Kim M.C., et al. Spontaneous evolution of posttraumatic subdural hygroma into chronic subdural haematoma. Acta Neurochir (Wien). 1994;127:41-47.
9. Maiuri F., Iaconetta G., Sardo L., et al. Dural arteriovenous malformation associated with recurrent subdural haematoma and intracranial hypertension. Br J Neurosurg. 2001;15:273-276.
10. Di R.F., Mannino S., Puca A., et al. Intracranial meningiomas associated with non-traumatic chronic subdural hematoma. Acta Neurochir (Wien). 2006;148:1097-1102.
11. Domenicucci M., Russo N., Giugni E., et al. Relationship between supratentorial arachnoid cyst and chronic subdural hematoma: neuroradiological evidence and surgical treatment. J Neurosurg. 2009;110:1250-1255.
12. Santarius T., Kirkpatrick P.J., Kolias A.G., Hutchinson P.J. Working towards rational and evidence-based treatment of chronic subdural haematoma. Clin Neurosurg. 2010;57:112-122.
13. Parizel P.M., Makkat S., Van M.E., et al. Intracranial hemorrhage: principles of CT and MRI interpretation. Eur Radiol. 2001;11:1770-1783.
14. Besenski N. Traumatic injuries: imaging of head injuries. Eur Radiol. 2002;12:1237-1252.
15. Nomura S., Kashiwagi S., Fujisawa H., et al. Characterization of local hyperfibrinolysis in chronic subdural hematomas by SDS-PAGE and immunoblot. J Neurosurg. 1994;81:910-913.
16. Nakaguchi H., Tanishima T., Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg. 2001;95:256-262.
17. Stanisic M., Lund-Johansen M., Mahesparan R. Treatment of chronic subdural hematoma by burrr-hole craniostomy in adults: influence of some factors on postoperative recurrence. Acta Neurochir (Wien). 2005;147:1249-1256.
18. Parizel P.M., Van Goethem J.W., Ozsarlak O., et al. New developments in the neuroradiological diagnosis of craniocerebral trauma. Eur Radiol. 2005;15:569-581.
19. Tsutsumi K., Maeda K., Iijima A., et al. The relationship of preoperative magnetic resonance imaging findings and closed system drainage in the recurrence of chronic subdural hematoma. J Neurosurg. 1997;87:870-875.
20. Tanikawa M., Mase M., Yamada K., et al. Surgical treatment of chronic subdural hematoma based on intrahematomal membrane structure on MRI. Acta Neurochir (Wien). 2001;143:613-618.
21. Santarius T., Lawton R., Kirkpatrick P.J., et al. The management of primary chronic subdural haematoma: a questionnaire survey of practice in the United Kingdom and the Republic of Ireland. Br J Neurosurg. 2008;22:529-534.
22. Weigel R., Schmiedek P., Krauss J.K. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry. 2003;74:937-943.
23. Lega B.C., Danish S.F., Malhotra N.R., et al. Choosing the best operation for chronic subdural hematoma: a decision analysis. J Neurosurg. 2010;113(3):615-621.
24. Wakai S., Hashimoto K., Watanabe N., et al. Efficacy of closed-system drainage in treating chronic subdural hematoma: a prospective comparative study. Neurosurgery. 1990;26:771-773.
25. Okada Y., Akai T., Okamoto K., et al. A comparative study of the treatment of chronic subdural hematoma—burrr hole drainage versus burrr hole irrigation. Surg Neurol. 2002;57:405-409.
26. Ramachandran R., Hegde T. Chronic subdural hematomas—causes of morbidity and mortality. Surg Neurol. 2007;67:367-372.
27. Asano Y., Hasuo M., Takahashi I., et al. Recurrent cases of chronic subdural hematoma—its clinical review and serial CT findings. No To Shinkei. 1992;44:827-831.
28. Suzuki K., Sugita K., Akai T., et al. Treatment of chronic subdural hematoma by closed-system drainage without irrigation. Surg Neurol. 1998;50:231-234.
29. Matsumoto K., Akagi K., Abekura M., et al. Recurrence factors for chronic subdural hematomas after burrr-hole craniostomy and closed system drainage. Neurol Res. 1999;21:277-280.
30. Gurelik M., Aslan A., Gurelik B., et al. A safe and effective method for treatment of chronic subdural haematoma. Can J Neurol Sci. 2007;34:84-87.
31. Zakaraia A.M., Adnan J.S., Haspani M.S., et al. Outcome of 2 different types of operative techniques practiced for chronic subdural hematoma in Malaysia: an analysis. Surg Neurol. 2008;69:608-615.
32. Kuroki T., Katsume M., Harada N., et al. Strict closed-system drainage for treating chronic subdural haematoma. Acta Neurochir (Wien). 2001;143:1041-1044.
33. Aoki N. Subdural tapping and irrigation for the treatment of chronic subdural hematoma in adults. Neurosurgery. 1984;14:545-548.
34. Taussky P., Fandino J., Landolt H. Number of burrr holes as independent predictor of postoperative recurrence in chronic subdural haematoma. Br J Neurosurg. 2008;22:279-282.
35. Han H.J., Park C.W., Kim E.Y., et al. One vs. two burrr hole craniostomy in surgical treatment of chronic subdural hematoma. J Korean Neurosurg Soc. 2009;46:87-92.
36. Tabaddor K., Shulman K. Definitive treatment of chronic subdural hematoma by twist-drill craniostomy and closed-system drainage. J Neurosurg. 1977;46:220-226.
37. Rughani A.I., Lin C., Dumont T.M., et al. A case-comparison study of the subdural evacuating port system in treating chronic subdural hematomas. J Neurosurg. 2010;113(3):609-614.
38. Camel M., Grubb R.L.Jr. Treatment of chronic subdural hematoma by twist-drill craniotomy with continuous catheter drainage. J Neurosurg. 1986;65:183-187.
39. Asfora W.T., Schwebach L. A modified technique to treat chronic and subacute subdural hematoma: technical note. Surg Neurol. 2003;59:329-332.
40. Carlton C.K., Saunders R.L. Twist drill craniostomy and closed system drainage of chronic and subacute subdural hematomas. Neurosurgery. 1983;13:153-159.
41. Horn E.M., Feiz-Erfan I., Bristol R.E., et al. Bedside twist drill craniostomy for chronic subdural hematoma: a comparative study. Surg Neurol. 2006;65:150-153.
42. Miele V.J., Sadrolhefazi A., Bailes J.E. Influence of head position on the effectiveness of twist drill craniostomy for chronic subdural hematoma. Surg Neurol. 2005;63:420-423.
43. Ramnarayan R., Arulmurugan B., Wilson P.M., et al. Twist drill craniostomy with closed drainage for chronic subdural haematoma in the elderly: an effective method. Clin Neurol Neurosurg. 2008;110:774-778.
44. Sucu H.K., Gokmen M., Ergin A., et al. Is there a way to avoid surgical complications of twist drill craniostomy for evacuation of a chronic subdural hematoma? Acta Neurochir (Wien). 2007;149:597-599.
45. Markwalder T.M. Chronic subdural hematomas: a review. J Neurosurg. 1981;54:637-645.
46. Hamilton M.G., Frizzell J.B., Tranmer B.I. Chronic subdural hematoma: the role for craniotomy reevaluated. Neurosurgery. 1993;33:67-72.
47. Svien H.J., Gelety J.E. On the surgical management of encapsulated subdural hematoma. A comparison of the results of membranectomy and simple evacuation. J Neurosurg. 1964;21:172-177.
48. Rocchi G., Caroli E., Salvati M., et al. Membranectomy in organized chronic subdural hematomas: indications and technical notes. Surg Neurol. 2007;67:374-380.
49. Lee J.Y., Ebel H., Ernestus R.I., et al. Various surgical treatments of chronic subdural hematoma and outcome in 172 patients: is membranectomy necessary? Surg Neurol. 2004;61:523-527.
50. Tyson G., Strachan W.E., Newman P., et al. The role of craniectomy in the treatment of chronic subdural hematomas. J Neurosurg. 1980;52:776-781.
51. Markwalder T.M., Steinsiepe K.F., Rohner M., et al. The course of chronic subdural hematomas after burrr-hole craniostomy and closed-system drainage. J Neurosurg. 1981;55:390-396.
52. Mori K., Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo). 2001;41:371-381.
53. Nakajima H., Yasui T., Nishikawa M., et al. The role of postoperative patient posture in the recurrence of chronic subdural hematoma: a prospective randomized trial. Surg Neurol. 2002;58:385-387.
54. Abouzari M., Rashidi A., Rezaii J., et al. The role of postoperative patient posture in the recurrence of traumatic chronic subdural hematoma after burrr-hole surgery. Neurosurgery. 2007;61:794-797.
55. Cenic A., Bhandari M., Reddy K. Management of chronic subdural hematoma: a national survey and literature review. Can J Neurol Sci. 2005;32:501-506.
56. Ogasawara K., Koshu K., Yoshimoto T., et al. Transient hyperemia immediately after rapid decompression of chronic subdural hematoma. Neurosurgery. 1999;45:484-488.
57. Baglin T.P., Keeling D.M., Watson H.G. Guidelines on oral anticoagulation (warfarin): third edition—2005 update. Br J Haematol. 2006;132:277-285.