Chapter 136 Management of Skull Base Trauma
Skull base trauma encompasses injury to the neurovascular and bony elements of the orbital plate of the frontal bone, the cribriform plate of the ethmoid bone, the sphenoid bone, the squamous and petrous temporal bone, and the suboccipital bone. Biomechanical studies have shown that the skull base is surprisingly elastic. Strong ligaments within the skull base and tough connective tissue in the suture lines are resilient and often may not be disrupted by the forceful impact. However, fractures within the bony elements of the skull base occur in approximately 25% of all blunt head injuries.1 A basilar skull fracture does not necessarily imply that the site of impact was to the skull base. Severe forces anywhere to the cranium can radiate down and disrupt the bony integrity of the base of skull.2 Most commonly, skull base fractures result from direct trauma either to the frontal and supraorbital regions or to the occiput. Cranial nerve and major arterial and venous injuries are more common in traumatic brain injury (TBI) accompanied by a skull base fracture.3
General Management
A dedicated, multidisciplinary craniofacial–trauma team is best equipped to manage patients with skull base trauma. Collaborative input with expertise from neurosurgeons; maxillofacial, plastics, and trauma surgeons; and intensivists is required. A multidisciplinary subspecialty team has been shown to reduce costs of care for trauma patients with complex skull base injury through several mechanisms that include a decreased number of consultations, decreased number of transfers to other hospitals, reduced time to operative treatment, and reduced overall length of hospital stay.4 A universal classification system for skull base fractures is needed to facilitate communication between subspecialty groups and centers, to provide a framework for protocols that guide medical and surgical management, and to enable a systematic approach to prognostication for patients with these injuries.
Graft Repair Options
A variety of graft options are available for surgical repair of skull base defects. Autologous grafts are preferred and include vascularized pericranium and rotational flaps of temporalis muscle and fascia. Nonvascularized, free grafts of pericranium, temporalis, rectus femoris, and abdominal muscle or fascia lata can also be used. Vascularized pedicle grafts resist infection and incorporate more quickly into the recipient site than free flaps, and they give a watertight seal that is durable for years.5,6 In the setting of trauma, defects in the pericranium are common and repair techniques have been described to salvage these grafts.7 The dura of the anterior falx can also be used to close dural defects.8 The accessibility of vascularized, septal mucosal flaps is a valuable adjunct to skull base repairs during endoscopic approaches.9 Dead space invites infection, and the presence of viable tissue in the vicinity chosen for graft placement is important to facilitate graft survival. Fat may be used to fill large defects and is preferred over nonvascularized muscle, which can fibrose and atrophy. While fat is preferred over muscle for its longer durability, free fat grafts rely on their ability to parasitize blood supply from adjacent soft tissue, and they may resorb when juxtaposed to devitalized bone and dura.10,11
Large bone defects from comminuted fractures in the skull base are best repaired using the patient’s own bone. Large fracture fragments may be debrided, soaked in antibiotic solution, and reapproximated with a titanium craniofacial plating system. Reconstruction by plating of multiple small fragments and of the thin bones of the nose and orbit is not recommended, because it carries significant risk of bony resorption of the repair. Fracture defects in the orbital roof should always be reconstructed, either with titanium mesh or with a synthetic prosthesis, to prevent pulsatile exophthalmos. Split calvarium is preferred for autologous repair when large defects cannot be primarily reconstructed with the fracture fragments. Bone can also be harvested from iliac crest and rib. Calvaria bone has a low resorption rate (17%-19%), whereas the rate of resorption for iliac crest and rib may approach 60% to 80%.12–15 Use of oblique angles in the bone cuts can reduce the number of screws and plates required for reapproximation of the bone flap. Septal cartilage can also be used for reconstruction of the ethmoid and sphenoid skull base. Inorganic implants, including both titanium mesh and porous, polyethylene implants, yield excellent cosmetic results for reconstruction of soft tissue and bony defects with a low risk of complications.16 However, methyl methacrylate and other cranioplasty cements should not be used to repair fracture defects, because they do not give a watertight seal and are predisposed to entrapping microbes leading to delayed infectious complications, especially when placed in the vicinity of the paranasal sinuses. Use of monocryl suture, an absorbable copolymer of glycolide and caprolactone, minimizes tissue reaction during healing and slowly hydrolyses over a period of 3 to 4 months.
Skull Base Fractures
Clinical signs of skull base fracture include anosmia, bilateral periorbital ecchymosis, hemotympanum and/or perforation of the tympanic membrane, facial palsy, hearing loss, nystagmus, vertigo, cerebrospinal fluid (CSF) rhinorrhea or otorrhea, and ecchymosis over the mastoid prominence, also known as a Battle sign.17,18 Techniques of surgical repair of associated facial, mandibular, and orbital fractures that frequently accompany skull base fractures are beyond the scope of this review. CSF leaks occur in 10% to 20% of skull base fractures.8,19–21 The proximity of basilar skull fractures to the paranasal sinuses raises concern for meningitis if the dura is torn in the vicinity of the fracture. Prophylactic antibiotics do not decrease the incidence of meningitis in basilar skull fractures22–25; rather, they may predispose the patient to a higher risk of superinfection with resistant organisms.26,27 The risk of meningitis persists even years after the trauma, independent of whether or not the patient developed a CSF fistula with the injury.28,29
Anterior Skull Base Fractures
Indications
Anterior skull base fractures of the orbital and cribriform plates frequently extend to involve the frontal sinus. Frontal sinus fractures may be open or closed and displaced or nondisplaced. In 40% to 60% of cases, both walls of the sinus are fractured, although isolated fractures of either the anterior or, less commonly, the posterior wall may occur.30–32 There is a 15% to 30% incidence of CSF leak associated with frontal sinus fractures.33 In particular, anterior wall fractures that extend into the base of the anterior fossa or those involving the posterior sinus wall should be observed closely for CSF leak. Fractures of the anterior skull base are an absolute contraindication to passage of a nasogastric feeding tube or nasopharyngeal airway.34
Obstruction of the nasofrontal outflow tracts is common, occurring in approximately 70% of frontal sinus fractures.32 These tracts connect the frontal and ethmoid sinuses, and the status of their patency is a key criterion for surgical intervention.32,35–38 Indirect signs of nasofrontal outflow obstruction include computed tomography (CT) evidence of fluid in the frontal sinus and fractures of the medial frontal sinus floor.39–41 Nasoethmoidal or supraorbital fractures, especially those medial to the supraorbital notch, raise suspicion for nasofrontal outflow obstruction.42,43 Facial fractures, most commonly orbital floor, naso-orbitoethmoidal complex, zygomatic, and Le Fort fractures are three times more likely in patients with nasofrontal outflow tract involvement.32 Complications of missed outflow obstruction include chronic sinusitis and mucocele formation.44–47 Mucoceles have a high likelihood of becoming infected, thereby giving rise to frontal osteomyelitis or Pott’s puffy tumor, in addition to epidural and subdural empyemas.
Operative indications for frontal sinus fractures include (1) anterior table displacement with cosmetic deformity; (2) fractures with evidence of nasofrontal outflow obstruction; (3) displacement of the posterior table greater than the thickness of the skull, because this predicts likely dural laceration; and (4) presence of refractory CSF leak.32,36,42 Closed, depressed anterior wall fractures frequently cause cosmetic deformity and may require surgical repair for cosmesis. Some have argued that fractures that do not involve the nasofrontal outflow tract are rarely displaced enough to require cosmetic realignment.32 The management of posterior wall frontal sinus fractures is complex and varied.48–51 Extensive comminution of the posterior sinus wall,52 fracture dislocation greater than the width of the posterior table,36,44 or accompanying CSF leak48,53 is an indication for surgical repair. Rodriguez et al.32 suggest that, as for anterior frontal sinus fractures, posterior frontal wall fractures with nasofrontal outflow obstruction should undergo surgical repair with either obliteration or cranialization of the frontal sinus. In their series of 31 nondisplaced posterior wall fractures, they had 3 complications following conservative management, and all 3 occurred in patients with nasofrontal outflow obstruction.32 In patients managed conservatively, a follow-up CT should be considered to check that there is no residual fluid level and that the frontal sinus is draining normally.
Methods
Repair options for frontal sinus fractures include reconstruction, obliteration, and cranialization.32,51,54 Simple reconstruction involves in situ elevation and plating of the sinus wall fracture with preservation of the nasofrontal ducts and sinus mucosa. Fractures that impair the nasofrontal duct should be treated by obliteration or cranialization procedures in which the frontal sinus mucosa is removed completely and the nasofrontal ducts are blocked. The frontal sinus is filled and closed off in an obliteration procedure. In a cranialization procedure, the posterior wall of the frontal sinus is removed and the sinus cavity is excluded from the cranial compartment by a pericranial graft.
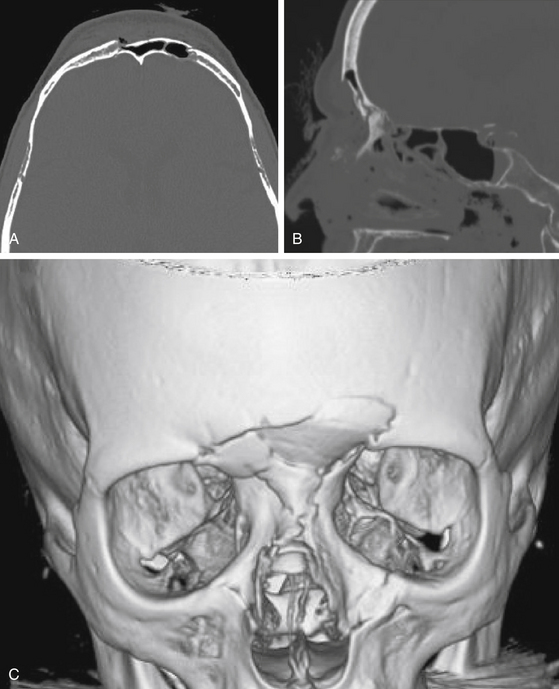
Reconstruction should be reserved for fractures of the anterior frontal sinus with cosmetic deformity and without signs of nasofrontal duct obstruction (Fig. 136-1). Both endoscopic and open techniques may be employed to reconstruct simple anterior frontal sinus fractures.55,56 Intraoperatively, the patency of the nasofrontal outflow tract can be verified by instilling fluorescein or methylene blue medially at the posterior aspect of the sinus floor and checking for staining of Cottonoids inserted at the level of the middle meatus. Attempts to reconstruct the nasofrontal outflow tract with stents57–59 is associated with a high rate of failure due to restenosis and other complications.36,54
Obliteration procedures are employed for complex anterior displaced frontal sinus fractures with obstruction of the nasofrontal outflow tracts. These complex fractures are best approached through a bicoronal scalp incision and bifrontal craniotomy with removal of the sinus mucosa, blockage of the nasofrontal ducts, reduction or fixation of the fracture, and obliteration of the sinus cavity.60,61 The fracture fragments are elevated and stabilized with low-profile craniofacial miniplates. During frontal sinus repair, complete removal of the frontal sinus mucosa is difficult due to the numerous small excrescences of mucosa that line the small cavities and divets within the sinus and follow the paths of the exiting veins of Breschet.62 Drilling 1 to 2 mm of bone from the sinus wall facilitates removal of these small excrescences of mucosa.62 The nasofrontal ducts are blocked with a combination of small pieces of temporalis muscle and small bone chips to prevent ascending infection or mucocele “in growth” from the nasal sinuses. In an obliteration procedure, the sinus cavity is filled with muscle, bone, or alloplasts such as Gelfoam (Pfizer, New York, NY). Bone dust from the cancellous bone of the calvarium resists resorption and facilitates ossification of the sinus.56,63,64 Fat should be avoided if possible. Survival of fat grafts depends on a vascular bed. Especially in the setting of comminuted fractures, there is a high rate of complication (22%) with resorption and fibrous replacement of the fat graft.32 Alloplastic biomaterials such as hydroxyapatite, bioactive glass, methyl methacrylate, calcium phosphate bone cement, and oxidized regenerated cellulose have also been used to obliterate the sinus, but most surgeons advocate autogenous materials.32 If the sinus cavity is not filled, spontaneous obliteration occurs by a process of osteoneogenesis in which scar tissue and bone slowly form and thereby obliterate the sinus cavity. Obliteration of the frontal sinus cavity by the process of osteoneogenesis is associated with a high risk of complications (49%).32
Open depressed fractures of the anterior sinus wall are also often best repaired through a bicoronal scalp incision, because the exposure offered through the traumatic laceration is seldom large enough to enable elevation of the fractured bone fragments, debridement of sinus mucosa, and blockage of the nasofrontal ducts. Fracture fragments from open depressed injuries should be debrided, but they may be incorporated into the reconstruction without increased risk of infection.65,66 Large defects can be repaired using split calvarium. Ideally, open depressed fractures should be repaired within hours of the injury.
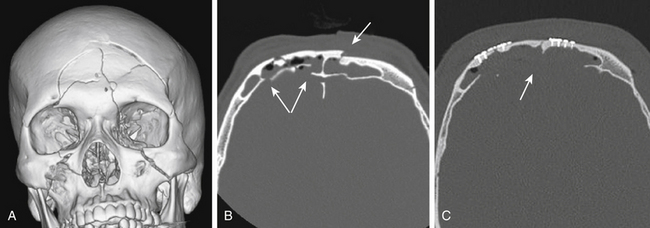
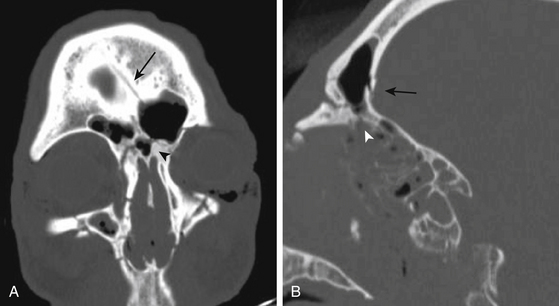
Fractures of the posterior sinus wall are generally best repaired by a cranialization procedure through an open craniotomy following the principles for anterior sinus wall fractures. In a cranialization procedure, the posterior sinus wall is removed so that the frontal sinus becomes part of the intracranial cavity (Fig. 136-2). With cranialization, the sinus does not need to be obliterated, a process reliant on the variable survival of fat, muscle, or bone used to obliterate the sinus. The frontal sinus should be cranialized, with removal of the posterior wall of the sinus, if more than 25% of the posterior sinus wall is fractured. However, some reserve cranialization procedures for only the most complex frontal sinus fractures with open depressed injuries and comminuted depressed posterior wall fragments. Cranialization entails expansion of the cranial compartment into the sinus with complete removal of the frontal sinus mucosa and obliteration of the communication to the nasal sinuses. The dura is carefully inspected for lacerations, which are repaired. As for obliteration procedures, the sinus mucosa is completely removed and the nasofrontal ducts are blocked to exclude the intracranial cavity from the nasal sinuses.52,67 A vascularized pericranial graft is harvested, reflected over the preexisting frontal sinus, and tacked to the dura (Fig. 136-3).
Posterior frontal sinus wall fractures that are non-displaced wall fractures can be treated conservatively.36,48 For small posterior sinus wall defects, the frontal sinus can be obliterated with exenteration of the mucosa, blockage of the nasofrontal ducts, and packing of the sinus.20 Removal of a small amount of the posterior wall in the area of the fracture allows exploration and repair of underlying dural lacerations. Reconstruction, rather than cranialization, of the fractured posterior sinus wall has been advocated by some surgeons, but this has not been widely adopted.68,69 Others have suggested that removal of the mucosa is the most important and the nasofrontal ducts do not need to be blocked.70 Leaving the nasofrontal ducts open can only be considered when the surgeon is certain that there is no risk of CSF leak from the sinus fracture. If the nasofrontal ducts are not blocked, there is a risk of delayed mucocele formation, as the mucosal cells of the nasal sinus can migrate up through the duct and repopulate the frontal sinus.32 High rates of complication accompany posterior sinus wall fractures with nasofrontal outflow obstruction that are treated either conservatively or by reconstruction alone, without obliteration of the sinus mucosa and blockage of the nasofrontal ducts; complication rates were 73% for reconstruction and 63% following conservative observation.32 By comparison, overall complications rates for repair of frontal sinus fractures with nasofrontal outflow obstruction were 9% by obliteration and 9% by cranialization.32
Temporal Bone Skull Base Fractures
Indications
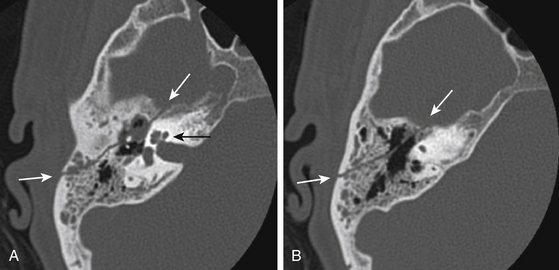
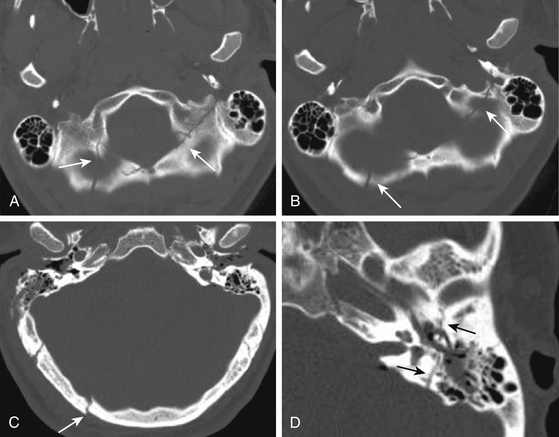
Clinical signs of trauma to the middle cranial skull base include hemotympanum, otorrhea, postauricular hemorrhage (Battle sign), facial palsy, vertigo, and tinnitus. Temporal bone fractures are classified relative to the long axis of the petrous pyramid. Longitudinal temporal bone fractures, comprising 70% to 90% of temporal bone fractures, result from direct trauma to the lateral temporoparietal skull and run lengthwise through the petrous bone (Fig. 136-4).71–73 Conductive hearing loss due to disruption of the ossicles is more common with longitudinal as compared to transverse temporal bone fractures. Facial nerve injury occurs in approximately 10% to 25% of longitudinal fractures. The site of facial nerve injury in longitudinal fractures most often localizes to the region of the geniculate ganglion.74
By contrast, transverse temporal bone fractures comprise 10% to 30% of temporal bone fractures and occur perpendicular to the petrous axis (Fig. 136-5). They arise as a result of significant trauma to the occipital skull. A typical fracture begins in the suboccipital bone at the site of impact and extends toward the foramen magnum coursing forward at right angles through the petrous temporal bone, often to the foramen lacerum. Damage to the labyrinth, cochlea, or cranial nerve VIII commonly leads to sensorineural hearing loss. Immediate facial nerve paralysis occurs in 30% to 50% of transverse temporal fractures. The facial nerve is typically injured in the proximal region of the labyrinthine segment or internal meatus.74 Extension of a transverse fracture to the jugular foramen may also cause lower cranial nerve dysfunction.
Methods
Fractures of the temporal bone may be exposed by two main approaches: an extradural, subtemporal exposure and a transmastoid approach. The specifics of the fracture, site of facial nerve injury, or both determine the approach. For longitudinal fractures, a middle fossa subtemporal approach is generally used. The patient’s head is positioned parallel to the floor, and a temporal craniotomy is performed through an incision anterior to the ear, above the zygoma. Dura is elevated from the floor of the middle cranial fossa, and the greater petrosal nerve and arcuate eminence are identified. The greater petrosal nerve is followed posteriorly to the geniculate ganglion and the site of facial nerve injury. If necessary, the tegmen tympani and internal auditory canal are opened, enabling exposure and decompression of the meatal, labyrinthine, and proximal tympanic segments of cranial nerve VII.75
For transverse temporal bone fractures, a transmastoid approach is generally indicated if hearing is preserved. A transmastoid approach through a retroauricular incision enables decompression of the facial nerve along its distal tympanic and mastoid segments.75,76 If serviceable hearing is absent, the labyrinthine and geniculate courses of cranial nerve VII can be decompressed through a translabyrinthine approach. Some believe, however, that even trace cochlear function justifies a labyrinthine-sparing approach.75 The nerve is decompressed or repaired by a primary or interposition anastomosis. Fat and fibrin glue are used to fill the mastoidectomy cavity. If a proximal segment of normal facial nerve is not identified, the mastoid exposure can be extended to open the posterior fossa and a proximal stump of the facial nerve can be identified at the brain stem and sutured to an interposition sural nerve graft.77
Suboccipital Skull Base Fractures
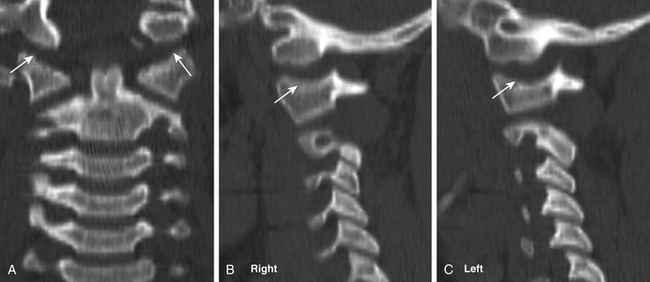
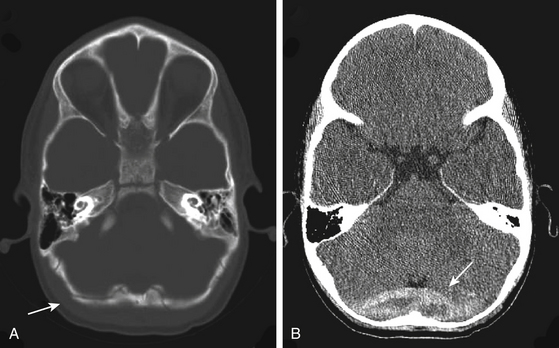
In contrast to anterior fossa floor and petrous temporal skull base fractures, wherein the site of impact may be remote from the fracture site, fractures of the suboccipital bone generally arise at the site of direct impact. Falls backward from standing and assaults are the most common mechanisms of injury. Displaced and nondisplaced fractures of the suboccipital bone may be associated with injury to the underlying major venous sinuses. Trauma of force sufficient to fracture the suboccipital bone and skull base should flag a careful assessment of cranial–cervical stability.76,78 Occipital–cervical dislocation injuries are usually fatal (Fig. 136-6).78–80 Suboccipital fractures should also heighten awareness for possible vertebral artery injury.
Fractures of the suboccipital bone may extend to involve the occipital condyles. Occipital condyle fractures are classified into three types.81 They are associated with cranial nerve deficits in upward of one third of cases.82 Dynamic flexion–extension views and magnetic resonance imaging are useful adjuncts to evaluating the stability of occipital–condyle fractures.83 Most are stable and can be managed in a cervical orthosis without need for operative intervention.84,85
Decompressions for Skull Base Trauma
Decompressive Hemicraniectomy for Supratentorial TBI with Associated Skull Base Injury
Indications
Skull base trauma entails transmission of large forces to the cranium, and not surprisingly these patients frequently require surgery for supratentorial injuries. Decompressive hemicraniectomy is a surgical procedure in which a large area (about 12 × 15 cm) of skull bone is removed to treat brain swelling and raised ICP.86–89 An emergent or primary decompressive craniectomy is performed when the initial CT scan in the emergency department shows a mass lesion with indications for operative intervention. At the time of surgical evacuation, the bone is not replaced to accommodate anticipated postoperative brain swelling. By comparison, a delayed decompressive craniectomy is performed to relieve raised ICP that has become refractory to medical management in the ICU.
Methods
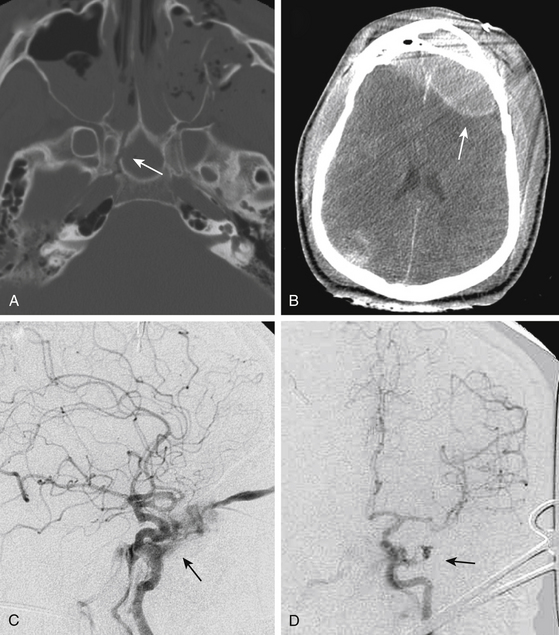
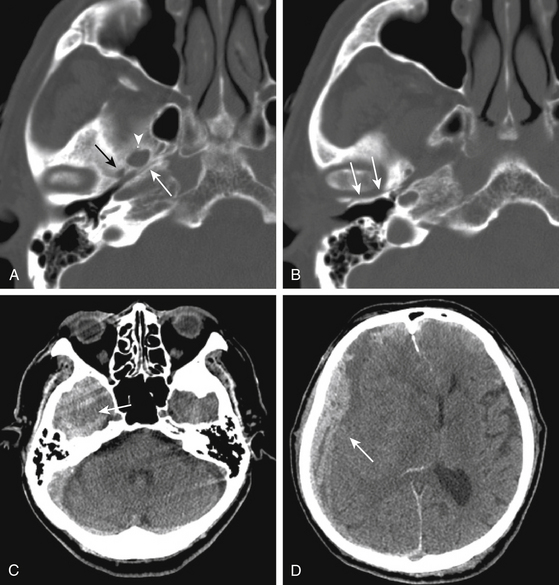
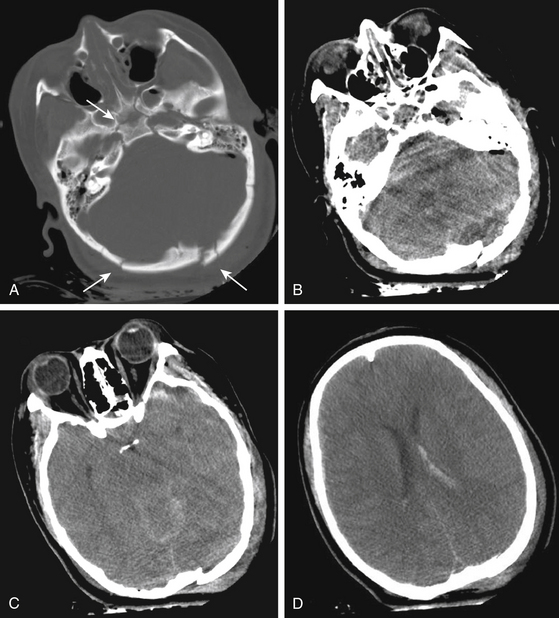
The techniques of a decompressive craniectomy have been reviewed elsewhere.90 Additional considerations and risks accompany a decompressive craniectomy performed in the setting of associated skull base trauma. Upon elevation of the craniectomy bone flap, inaccessible and uncontrolled bleeding may be evident from the depths of a skull base fracture. This is especially common in the area of the sphenoid wing and the middle cranial fossa floor. The source of the bleeding may be the fracture, or it may signify the presence of a vascular injury within the skull base, such as an injured carotid artery (Fig. 136-7) or middle meningeal artery (Fig. 136-8). The presence of a hematoma along the floor of the middle cranial fossa on CT scan should alert the surgeon to the possibility of active bleeding from the skull base (Fig. 136-8). In addition, an important step in a decompressive craniectomy is to decompress the midbrain through removal of bone from the sphenoid wing, as well as the anterior and inferior aspects of the middle cranial fossa above the zygoma. In the presence of a temporal bone fracture that extends to the skull base, torque or twist maneuvers with the bone rongeurs may provoke uncontrolled bleeding from further disruption of an injured carotid artery within a fractured petrous bone or a middle meningeal artery lacerated from a fracture involving the foramen spinosum.
Arterial bleeding without an obvious source may continue to well up from the skull base, persistent and brisk enough to prohibit closure of the wound. When routine methods to achieve hemostasis fail, the dura should be elevated and the middle fossa floor explored for the possibility of middle meningeal artery injury at the foramen spinosum (Fig. 136-8). Bleeding from an injured carotid artery in the petrous bone is substantially more problematic to control. In the setting of an injured and swollen brain, attempts to expose the carotid canal to achieve proximal control and repair the injured vessel are heroic measures, especially in the setting of brisk, arterial bleeding. Furthermore, without a prior CT angiogram or intra-arterial cerebral angiogram to evaluate the patency of collateral filling through the Circle of Willis, proximal occlusion of the carotid artery risks massive cerebral infarction in return for control of the bleeding. In general, the best approach may be to pack the area as firmly as possible and to expedite a cerebral angiogram, wherein a carotid stent or proximal balloon occlusion of the artery may be more safely achieved.
Suboccipital Decompression for Posterior Fossa Trauma
Most posterior fossa trauma arises from direct trauma to the suboccipital region. Suboccipital fractures may be associated with venous epidural hematomas arising from injury to the transverse, sigmoid, and occasionally occipital draining sinuses. Posterior fossa venous epidurals may be occult on an initial CT scan and may only be detected following enlargement several hours after injury. In the posterior fossa, epidural hematomas are more common than either subdural hematomas or contusions. Subdural hematomas in the posterior fossa are more likely related to injury of the cerebellar folia than with tearing of a subdural vein.91 Intraparenchymal hematomas from posterior fossa trauma are rare.91
Indications
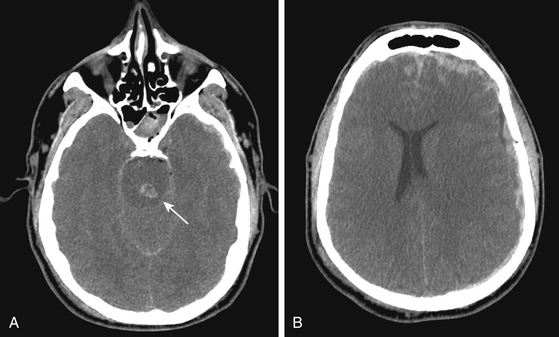
Conservative management of posterior fossa trauma is favored for patients who are neurologically stable with minimal deficit. The primary indication for suboccipital decompression following TBI is for a hematoma with mass effect in the posterior fossa. Clinical signs of depressed consciousness from mass effect in the posterior fossa may be difficult to differentiate from altered consciousness that accompanies concomitant supratentorial trauma. Decompression of traumatic posterior fossa injury with mass effect should be considered for patients with impaired consciousness in the absence of elevated ICP and without evidence of primary brain stem injury (Fig. 136-9). Effacement, shift, or both of the fourth ventricle; interval scans with enlarging hematoma or other mass lesion; tonsillar herniation; and compressed basal cisterns associated with upward herniation of the cerebellar vermis through the tentorial incisura are signs of posterior fossa traumatic mass effect that require surgical evaluation. Patient selection to distinguish surgical candidates with reversible mass effect from those with devastating primary brain stem injury is difficult in the acute trauma setting (Fig. 136-10). The presence of a depressed skull fracture overlying a major venous sinus significantly increases the operative risks and raises the threshold for surgical intervention.
Methods
Patients are positioned prone with a central venous line and precordial Doppler monitoring. A midline incision extends from above the external occipital protuberance to the level of C2. The occiput, foramen magnum, and arch of C1 are exposed. Muscular attachments to C2 are preserved. A craniotomy is performed based on bur holes made safely below the transverse sinus. The craniotomy may be taken directly down to the foramen magnum or alternatively brought low across the occipital bone above the foramen magnum, with subsequent piecemeal removal of bone from the foramen magnum with bone and Kerrison rongeurs. Elevation of depressed fragments overlying the torcula and the transverse and sigmoid sinuses should be avoided, if possible. Weck clips facilitate control of bleeding from an occipital sinus. The epidural hematoma is evacuated. If subdural or intraparenchymal blood needs to be evacuated, the dura should be opened widely in a Y fashion, with the base along the transverse sinus. A generous duroplasty should be performed to accommodate postoperative swelling in the posterior fossa. In the setting of trauma, it is often prudent to not replace the bone.
Major outflow obstruction of a critical draining sinus with neurologic deficit directly attributable to the venous obstruction may necessitate elevation of a compressive fracture fragment. Preparations should include measures to control the possibility of major blood loss or air embolus. An area of normal bone should be removed, allowing the dura to be exposed on either side of the sinus, proximal and distal to the fracture. This provides exposure for temporary occlusion of the sinus. Control of blood loss, with a temporary aneurysm clip or encircling silk suture, enables primary suture or patch graft repair of the laceration in the sinus wall. Occasionally, an epidural hematoma may extend from a skull base fracture, stripping dura from bone and leading to compression of a major draining sinus. In these cases, there is no injury to the sinus itself, and the epidural hematoma can be evacuated safely without major risk (Fig. 136-11).
Repair of Traumatic Skull Base CSF Fistula
In the setting of an associated dural tear, fractures of the skull base traversing the paranasal sinuses or middle ear may be accompanied by a CSF fistula. In general, approximately 10% to 20% of patients with skull base fractures develop a CSF fistula.1,92,93 In a series of 625 patients with severe skull base trauma, the incidence of CSF leak was 12% (75 patients).76 In young children, a CSF fistula is unusual due to the late development and maturation of the paranasal sinuses. CSF leaks are more common in anterior as compared to middle fossa and posterior skull base fractures.94–96 The site of the CSF fistula most commonly localizes to a fracture through the ethmoid–cribriform plate and manifests as rhinorrhea.94 Less frequently, CSF may arise from fracture of the frontal sinus, ethmoidal cells, or sphenoid sinus. CSF may also leak through the foramina in the cribriform plate following olfactory nerve avulsion.94 Rhinorrhea can also occur from a temporal bone fracture with an intact tympanic membrane. In this setting, CSF escapes through the fracture and along the eustachian tube into the posterior nasopharynx.97 Rhinorrhea may present in a delayed fashion, possibly due to unmasking of fistula sites as brain swelling from the injury resolves. The risk of meningitis from untreated CSF leakage is as high as 85% in long-term follow-up.92,98 Dural tears, if masked or occult, may not be diagnosed until they present with meningitis, CSF rhinorrhea, or a mucocele, sometimes years following the injury.
Otorrhea, drainage of CSF from the ear, requires both a dural laceration with an underlying fracture in the petrous bone and a perforation of the tympanic membrane. Otorrhea occurs in approximately 15% to 25% of all temporal bone fractures.25,99 In longitudinal fractures, CSF may leak through a bony fracture that directly communicates with the external auditory meatus, or it may egress through a disrupted tympanic membrane. By comparison, in transverse temporal bone fractures, CSF passes down the eustachian tube, leading to rhinorrhea, because the tympanic membrane is often intact. Otorrhea usually resolves spontaneously and rarely persists beyond 7 days from time of injury. Delayed otorrhea is uncommon.
Most CSF leaks resolve spontaneously. Approximately 85% of patients with rhinorrhea and more than 90% with CSF otorrhea resolve spontaneously within 1 week after injury.25,100 In a series of 54 patients with anterior fossa CSF rhinorrhea, Mincy found that 35% resolved spontaneously within 24 hours, 68% within 48 hours, and 85% within 7 days.100 In the first week of CSF leakage, the risk of meningitis is low.100 The risk of meningitis increases 8- to 10-fold,101 with an incidence of more than 20%, if the CSF leakage persists beyond 7 to 10 days.25,100,102 To facilitate spontaneous closure, the patient should be maintained with elevation of the head of the bed, stool softeners, antiemetics, and avoidance of valsalva maneuvers such as nose blowing or drinking through a straw. If the CSF leak does not resolve spontaneously within approximately 72 hours with these conservative measures, and if there are no risks of cerebral herniation, then a lumbar drain should be inserted with removal of CSF (10-15 ml/hr). Reduction and repair of concomitant nasoethmoid, supraorbital, and glabellar fractures can facilitate spontaneous resolution of CSF leaks from the anterior frontal floor and cribriform region.
Prophylactic antibiotics for CSF fistula are controversial and generally are not advised.23,101,103 A recent Cochrane review of antibiotic prophylaxis for patients with basilar skull fractures evaluated 208 patients in four randomized, controlled trials by meta-analysis and a further 2168 patients in nonrandomized, controlled studies.24 This review concluded that prophylactic antibiotics were not supported, whether or not CSF leakage was present.24 In this analysis, early surgical repair was found to reduce the risk of meningitis.
Indications
Indications for operative repair of CSF fistula include (1) failure of conservative therapy to resolve the CSF leak; (2) penetrating injury with CSF leak; (3) pneumocephalus with persistent CSF leak; (4) development of meningitis during conservative treatment of a CSF leak; (5) a CSF leak that presents in a delayed fashion; (6) a recurrent, intermittent CSF leak, especially one timed to clamping of a lumbar drain; and (7) a severe anterior skull base defect with evidence on CT scan or endoscopy that the brain is externally herniating through the skull defect.20,104 Persistent or recurrent CSF leak despite lumbar drainage beyond 7 to 10 days from the time of injury is deemed long enough to establish that conservative therapy has failed. Early surgery should be considered for patients with high-risk features for development of meningitis during a trial of conservative therapy. CSF leakage that presents in a delayed manner; pneumocephalus that persists on serial CT scan, indicating a large dural tear; and patients with external brain herniation through the skull defect are less likely to resolve their CSF fistulae spontaneously and should be considered for early surgical repair. Penetrating injuries with CSF leak carry a very high risk of developing gram-negative meningitis and should not be managed conservatively.105,106
Methods
There are two surgical approaches to the operative repair of traumatic skull base CSF fistulae: (1) craniotomy with intradural and extradural exposure and (2) extracranial paranasal sinus exposure. In addition, consideration should be given to the possible need for ancillary CSF diversion procedures. Localization of the site of the dural fistula helps guide the surgical approach.107 Metrizamide CT cisternography and radioactivity studies can be used to localize the fistula site. Metrizamide CT requires an actively leaking fistula. Radioactive studies are more labor intensive and require placement and radioactive counting of pledgets in different sites within the paranasal sinuses following lumbar puncture and CSF circulation of a radiolabeled substance. For complex fractures of the anterior skull base involving the cribriform plate and ethmoid sinus, a bifrontal craniotomy is the most effective approach (Fig. 136-12).108 Extradural dissection is associated with less brain retraction and is employed for simple anterior skull base, as well as for middle cranial fossa dural repairs. Direct paranasal sinus approaches are best suited for localized fractures of the frontal, ethmoid, and sphenoid sinuses.107
Perioperative Considerations
Perioperative antibiotics are generally administered and continued for 5 to 7 days postoperatively. If not already present, a lumbar drain with CSF diversion for a period of approximately 1 week postoperatively facilitates healing of the repair site.109 With an open CSF fistula, intracranial air can exchange freely and does not build up pressure. As the fistula site closes down, tension pneumocephalus can develop and be exacerbated by negative pressures induced by CSF drainage through a lumbar drain. During closure of the fistula and weaning of the lumbar drain, patients should be observed for signs of hydrocephalus that may have been masked earlier by the ongoing leakage of CSF, by lumbar CSF drainage, or both.
Craniotomy for Open Repair of Traumatic Anterior Cranial Skull Base CSF Fistula
Open craniotomy allows both intradural and extradural exploration of the fistula site, treatment of associated intracranial mass lesions, and repair of cranial fractures in a single procedure. A dural tear at the fistula site can be repaired by direct suture more easily through an intradural exposure. The extradural approach offers the advantages of less brain retraction. For complex skull base fistula, some surgeons recommend first exploring and repairing the fistula site intradurally. If both olfactory nerves have been disrupted by the trauma, the intradural procedure can then be augmented with extradural repair over the cribriform and olfactory fossa.8,94
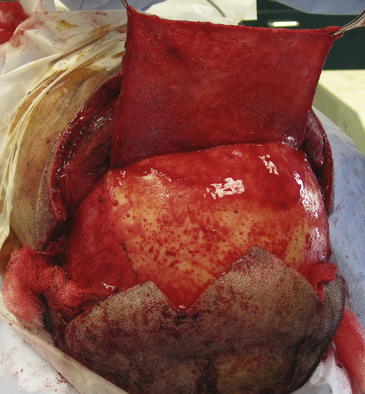
If the cervical spine has been cleared of injury, the patient’s head is extended 15 to 20 degrees to enable optimal visualization along the anterior frontal floor. A Souttar bifrontal incision is made from zygoma to zygoma, with preservation of the superficial temporal arteries. The scalp is reflected forward to the supraorbital rim, taking care to protect the supraorbital nerves. A large pericranial graft is delineated on three sides, preserving the vascularized base along the anterior frontal limb (see Fig. 136-3). The pericranial graft is reflected forward and protected between moist Telfa. A bifrontal craniotomy is fashioned to the margins of the temporalis muscle and as far forward as possible to minimize the need for frontal lobe retraction. Air embolus precautions are employed during passage over the sagittal sinus and upon elevation of the bone. The edges of the bone flap are inspected, and any sinus mucosa is debrided. As described for frontal sinus fracture repairs, the mucosa of the frontal sinus is completely debrided, the nasofrontal ducts are blocked, and the posterior wall of the sinus is craniectomized.
For simple dural defects, extradural exposure and primary repair of the dura with or without a pericranial graft may be adequate to treat the CSF fistula. Simple lacerations are repaired primarily. Free pericranial grafts should extend 2 cm beyond the dural defect. Tacked to the dura and reinforced with fibrin glue, they incorporate well into the repair.8 Elevation of the dura for repair of the anterior fossa floor risks disruption of the olfactory hair cells at the level of the cribriform plate.76 A subfrontal extradural approach should be undertaken only if the patient is known to be anosmic from traumatic disruption of the olfactory bulbs. Traumatic avulsion of the olfactory nerves from the cribriform plate may be the source of the CSF leak. Dura is elevated from both sides of the cristae galli and elevated from the cribriform plate. A split thickness bone graft may be used to repair open bony defects. If the olfactory nerves are found to be completely avulsed, the olfactory fossa area is plugged with bone chips, bone dust, and fibrin glue.94 A generous vascularized pericranial graft should be laid over the anterior skull base to both cover the cribriform plate and seal off the frontal sinus.8,76
For more complex lacerations or if the site of the CSF leak cannot be localized preoperatively, an intradural approach enables better visualization and repair of the dural defect. For intradural exposure, the dura is opened parallel to the orbital rim with sacrifice of the anterior superior sagittal sinus as far anterior as possible. The falx is cut. Careful frontal lobe retraction reduces risk of iatrogenic olfactory nerve injury. If the olfactory nerves are intact, they can be carefully dissected from the brain surface during elevation of the frontal lobes. Intradural exposure of the skull base dura entails moderate brain retraction that can be assisted by a ventriculostomy, opening of the lamina terminalis, or a lumbar drain. Debridement and repair of the fracture and CSF fistula site are followed by a multilayered closure. Simple dural lacerations may be repaired primarily and reinforced with a free pericranial graft tacked to the site, overlaid with Tisseel fibrin glue (Baxter, Deerfield, IL). For more complex dural injuries, a vascularized pericranial graft is mobilized to generously cover the fracture and dural defect. Slits are made in the pericranial graft around the olfactory nerves. The flap is tacked to the native dura, and the repair is reinforced with fibrin glue. The durotomy is closed, and the vascularized base of the pericranial graft is tacked to the dura, closing over the sinus completely. The repair over the frontal sinus is reinforced with Tisseel fibrin sealant. The bone flap is replaced without compression across its anterior aspect to allow continued perfusion of the intervening pericranial graft. The scalp is closed with meticulous approximation of layers. Complications include anosmia, intracranial hemorrhage, and cerebral edema. There is an approximately 2% to 4% chance of failure with recurrence of CSF leak following an extensive intradural repair.8,94
Craniotomy for Open Repair of Traumatic Middle Cranial Skull Base CSF Fistula
Operative repair of middle fossa fractures for otorrhea commonly employ a subtemporal craniotomy and extradural exposure. In cases of refractory otorrhea in which hearing is preserved, combined mastoid and middle cranial fossa exploration is generally required. A horseshoe craniotomy flap is positioned over the middle temporal fossa, two thirds anterior and one third posterior to the external auditory meatus. An extradural approach minimizes risk to the Labbé vein. However, during extradural exposure along the anterior surface of the petrous pyramid, the geniculate ganglion and greater petrosal nerve lie under the dura and are not protected by bone. An anterior to posterior dissection minimizes traction injury to the greater superficial petrosal nerve. A subtotal petrosectomy with exenteration of the temporal bone air cells may be filled with a fat graft.110,111 A split thickness bone graft from the free craniotomy bone flap may be used to repair a fracture defect. A vascularized pedicle of temporalis muscle and fascia can be laid down between the dura and the fracture. Alternatively, free fascia grafts are easier to work with than vascularized pedicle grafts in the middle fossa. In the setting of a ruptured tympanic membrane, a tympanoplasty or obliteration of the eustachian tube may also be considered as adjunctive measures to control the CSF leak.112 A mastoidectomy, through a posterior auricular incision, should be performed by an otologic surgeon. The mastoid is drilled, exposing the fracture site, and the fracture defect is covered with a fascia graft held in place with fat and Tisseel fibrin glue. If hearing loss is not salvageable, then closure of the CSF defect can be done through a mastoidectomy and a translabyrinthine approach with obliteration of the eustachian tube and closure of the external ear canal.
Extracranial Paranasal Repair of Traumatic Skull Base CSF Fistula
Direct extracranial approaches to repair CSF fistula through the paranasal sinuses are most favorable for simple CSF leaks without major defects in the skull base and in which the site of the CSF leak has been definitively identified preoperatively.113–116 They are better suited for localized fractures. As compared to a craniotomy, extracranial approaches through the sinuses do not provide as wide an exposure of the anterior skull base, although extended subcranial exposures may provide wider visualization.117–119 The major advantage of the extracranial paranasal approach is that it avoids brain retraction and injury to the olfactory nerves.120 In addition, a direct approach through the sinuses enables more extensive debridement of the sinus mucosa. These approaches are appropriate for leaks due to nondisplaced defects in the posterior wall of the frontal sinus that do not require cranialization, those of the posterior two thirds of the roof of the ethmoid sinus, and defects in the sphenoid sinus. The medial wall of the optic canal is bounded by the sphenoid sinus and occasionally by posterior ethmoidal air cells.121 During extracranial repair of cribriform and posterior ethmoidal CSF leaks, the posterior ethmoidal artery is a useful landmark. The ethmoidal foramen lies 5 mm (range 1-11 mm) rostral to the orbital opening of the optic canal.121
Direct repair of CSF fistula via the paranasal sinuses can be done by open microsurgery or endoscopically.114,122–124 These procedures are typically performed through the nose, obviating an incision on the face. For CSF leaks associated with fractures of the cribriform plate and ethmoid sinus, a transethmoidectomy is performed. The sinus mucosa is exenterated, and the ethmoid air cells are drilled with a diamond bur to expose the site of the fracture and CSF leak.125,126 Dura is reflected from the adjacent bone to expose normal dura circumferentially around the leak site. A multilayer repair is performed in the extradural space. A rotated flap based on a vascularized pedicle from the middle turbinate or septum or a free fascia graft can be used to repair the fistula site.127 Tisseel fibrin glue and a piece of cartilage or a titanium plate may further reinforce the repair. The sinus is packed for 5 to 7 days to prevent the graft from moving, and antibiotics and lumbar drainage are used to augment healing.
Steps in a sphenoid CSF repair are similar to those in an ethmoidal repair, except that the multilayer repair is performed on the sinus aspect, rather than the extradural (sellar) aspect of the fracture. Insertion of a graft on the sellar aspect, between the bone and the dura, carries risk of injury to the carotid arteries. Fat, harvested from the abdomen, is placed in the sinus, and the packing is reinforced with Tisseel fibrin glue. Free septal cartilaginous grafts can be used to repair a large defect in the floor. A small amount of bone left on the inner margins of the sinus provides a buttress for placement of a cartilaginous graft or titanium plate to support packing placed within the sinus.
Ancillary CSF Diversion Procedures
Traumatic CSF leaks do not resolve spontaneously, and repair of the dural and fracture defects fails in the setting of concomitant, unrecognized hydrocephalus.128 Hydrocephalus may be clinically occult, masked by the ongoing leakage of CSF, and may not become evident until the CSF fistula reaches the final stages of closure. CSF leaks that recur timed to clamping of a lumbar drain should raise suspicion for underlying hydrocephalus. These are indications that insertion of a ventriculoperitoneal or lumboperitoneal shunt may be necessary. Shunts should not be used as stand-alone procedures for control of CSF fistula. Without concomitant fistula repair, the negative cranial–spinal pressure gradient that accompanies shunting decreases spontaneous closure of the fistula and predisposes to the development of pneumocephalus.129,130 CSF should be sampled prior to shunting in patients with a history of a CSF fistula to rule out the possibility of infection.
Aarabi B., Hesdorffer D.C., Ahn E.S., et al. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006;104:469-479.
Asano T., Ohno K., Takada Y., et al. Fractures of the floor of the anterior cranial fossa. J Trauma. 1995;39:702-706.
Brodie H.A. Prophylactic antibiotics for posttraumatic cerebrospinal fluid fistulae. A meta-analysis. Arch Otolaryngol Head Neck Surg. 1997;123:749-752.
Brodie H.A., Thompson T.C. Management of complications from 820 temporal bone fractures. Am J Otol. 1997;18:188-197.
Cooper D.J., Rosenfeld J.V., Murray L., et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364:1493-1502.
Donald P.J. The tenacity of the frontal sinus mucosa. Otolaryngol Head Neck Surg. 1979;87:557-566.
Donath A., Sindwani R. Frontal sinus cranialization using the pericranial flap: an added layer of protection. Laryngoscope. 2006;116:1585-1588.
Ernst A., Herzog M., Seidl R. Head and Neck Trauma. New York: Thieme Verlag; 2006.
Fisch U. Facial paralysis in fractures of the petrous bone. Laryngoscope. 1974;84:2141-2154.
Hegazy H.M., Carrau R.L., Snyderman C.H., et al. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhea: a meta-analysis. Laryngoscope. 2000;110:1166-1172.
Hutchinson P.J., Corteen E., Czosnyka M., et al. Decompressive craniectomy in traumatic brain injury: the randomized multicenter RESCUEicp study, www.RESCUEicp.com Acta Neurochir Suppl, 2006;96:17-20
Kassam A.B., Thomas A., Carrau R.L., et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery. 2008;63:ONS44-ONS52.
Manolidis S., Hollier L.H.Jr. Management of frontal sinus fractures. Plast Reconstr Surg. 2007;120:32S-48S.
Piek J. Surgical treatment of complex traumatic frontobasal lesions: personal experience in 74 patients. Neurosurg Focus. 2000;9:e2.
Probst C. Neurosurgical treatment of traumatic frontobasal CSF fistulae in 300 patients (1967-1989). Acta Neurochir (Wien). 1990;106:37-47.
Ratilal B., Costa J., Sampaio C. Antibiotic prophylaxis for preventing meningitis in patients with basilar skull fractures. Cochrane Database Syst Rev. 2006:CD004884.
Rodriguez E.D., Stanwix M.G., Nam A.J., et al. Twenty-six-year experience treating frontal sinus fractures: a novel algorithm based on anatomical fracture pattern and failure of conventional techniques. Plast Reconstr Surg. 2008;122:1850-1866.
Samii M., Tatagiba M. Skull base trauma: diagnosis and management. Neurol Res. 2002;24:147-156.
Scholsem M., Scholtes F., Collignon F., et al. Surgical management of anterior cranial base fractures with cerebrospinal fluid fistulae: a single-institution experience. Neurosurgery. 2008;62:463-469.
Tiwari P., Higuera S., Thornton J., et al. The management of frontal sinus fractures. J Oral Maxillofac Surg. 2005;63:1354-1360.
Ulug T., Arif Ulubil S. Management of facial paralysis in temporal bone fractures: a prospective study analyzing 11 operated fractures. Am J Otolaryngol. 2005;26:230-238.
1. Brawley B.W., Kelly W.A. Treatment of basal skull fractures with and without cerebrospinal fluid fistulae. J Neurosurg. 1967;26:57-61.
2. Yoganandan N., Pintar F.A., Sances A.Jr., et al. Biomechanics of skull fracture. J Neurotrauma. 1995;12:659-668.
3. Resnick D.K., Subach B.R., Marion D.W. The significance of carotid canal involvement in basilar cranial fracture. Neurosurgery. 1997;40:1177-1181.
4. Mathiasen R.A., Eby J.B., Jarrahy R., et al. A dedicated craniofacial trauma team improves efficiency and reduces cost. J Surg Res. 2001;97:138-143.
5. Costa H., Cerejo A., Baptista A., et al. The galea frontalis myofascial flap in anterior fossa CSF leaks. Br J Plast Surg. 1993;46:503-507.
6. Rocchi G., Caroli E., Belli E., et al. Severe craniofacial fractures with frontobasal involvement and cerebrospinal fluid fistula: indications for surgical repair. Surg Neurol. 2005;63:559-563.
7. Ross D.A. Use of modified pericranial flaps in anterior skull base reconstruction: technical note. Skull Base Surg. 1992;2:83-86.
8. Piek J. Surgical treatment of complex traumatic frontobasal lesions: personal experience in 74 patients. Neurosurg Focus. 2000;9:e2.
9. Kassam A.B., Thomas A., Carrau R.L., et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery. 2008;63:ONS44-ONS52.
10. Karacaoglu E., Kizilkaya E., Cermik H., Zienowicz R. The role of recipient sites in fat-graft survival: experimental study. Ann Plast Surg. 2005;55:63-68. discussion 68
11. Hirase Y., Valauri F.A., Buncke H.J. Neovascularized free fat flaps: an experimental model. J Reconstr Microsurg. 1988;4:197-201.
12. Zins J.E., Whitaker L.A. Membranous versus endochondral bone: implications for craniofacial reconstruction. Plast Reconstr Surg. 1983;72:778-785.
13. Ernst A., Herzog M., Seidl R. Head and Neck Trauma. New York: Thieme Verlag; 2006.
14. Arden R.L., Burgio D.L. Bone autografting of the craniofacial skeleton: clinical and biological considerations. Am J Otolaryngol. 1992;13:328-341.
15. Alonso N., Machado de Almeida O., Jorgetti V., et al. Cranial versus iliac onlay bone grafts in the facial skeleton: a macroscopic and histomorphometric study. J Craniofac Surg. 1995;6:113-118.
16. Janecka I.P. New reconstructive technologies in skull base surgery: role of titanium mesh and porous polyethylene. Arch Otolaryngol Head Neck Surg. 2000;126:396-401.
17. Alvi A. Battle’s sign in temporal bone trauma. Otolaryngol Head Neck Surg. 1998;118:908.
18. Pretto Flores L., De Almeida C.S., Casulari L.A. Positive predictive values of selected clinical signs associated with skull base fractures. J Neurosurg Sci. 2000;44:77-82.
19. Lewin W. Cerebrospinal fluid rhinorrhea in nonmissile head injuries. Clin Neurosurg. 1964;12:237-252.
20. Marentette L.J., Valentino J. Traumatic anterior fossa cerebrospinal fluid fistulae and craniofacial considerations. Otolaryngol Clin North Am. 1991;24:151-163.
21. Yilmazlar S., Arslan E., Kocaeli H., et al. Cerebrospinal fluid leakage complicating skull base fractures: analysis of 81 cases. Neurosurg Rev. 2006;29:64-71.
22. Ignelzi R.J., VanderArk G.D. Analysis of the treatment of basilar skull fractures with and without antibiotics. J Neurosurg. 1975;43:721-726.
23. Choi D., Spann R. Traumatic cerebrospinal fluid leakage: risk factors and the use of prophylactic antibiotics. Br J Neurosurg. 1996;10:571-575.
24. Ratilal B., Costa J., Sampaio C. Antibiotic prophylaxis for preventing meningitis in patients with basilar skull fractures. Cochrane Database Syst Rev. 2006:CD004884.
25. Brodie H.A., Thompson T.C. Management of complications from 820 temporal bone fractures. Am J Otol. 1997;18:188-197.
26. Stoikes N.F., Magnotti L.J., Hodges T.M., et al. Impact of intracranial pressure monitor prophylaxis on central nervous system infections and bacterial multi-drug resistance. Surg Infect (Larchmt). 2008;9:503-508.
27. May A.K., Fleming S.B., Carpenter R.O., et al. Influence of broad-spectrum antibiotic prophylaxis on intracranial pressure monitor infections and subsequent infectious complications in head-injured patients. Surg Infect (Larchmt). 2006;7:409-417.
28. Schick B., Weber R., Kahle G., et al. Late manifestations of traumatic lesions of the anterior skull base. Skull Base Surg. 1997;7:77-83.
29. Okada J., Tsuda T., Takasugi S., et al. Unusually late onset of cerebrospinal fluid rhinorrhea after head trauma. Surg Neurol. 1991;35:213-217.
30. Olson E.M., Wright D.L., Hoffman H.T., et al. Frontal sinus fractures: evaluation of CT scans in 132 patients. AJNR Am J Neuroradiol. 1992;13:897-902.
31. Bell R.B., Dierks E.J., Brar P., et al. A protocol for the management of frontal sinus fractures emphasizing sinus preservation. J Oral Maxillofac Surg. 2007;65:825-839.
32. Rodriguez E.D., Stanwix M.G., Nam A.J., et al. Twenty-six-year experience treating frontal sinus fractures: a novel algorithm based on anatomical fracture pattern and failure of conventional techniques. Plast Reconstr Surg. 2008;122:1850-1866.
33. Wallis A., Donald P.J. Frontal sinus fractures: a review of 72 cases. Laryngoscope. 1988;98:593-598.
34. Fremstad J.D., Martin S.H. Lethal complication from insertion of nasogastric tube after severe basilar skull fracture. J Trauma. 1978;18:820-822.
35. Rohrich R.J., Hollier L. The role of the nasofrontal duct in frontal sinus fracture management. J Craniomaxillofac Trauma. 1996;2:31-40.
36. Rohrich R.J., Hollier L.H. Management of frontal sinus fractures. Changing concepts. Clin Plast Surg. 1992;19:219-232.
37. McLaughlin R.B.Jr., Rehl R.M., Lanza D.C. Clinically relevant frontal sinus anatomy and physiology. Otolaryngol Clin North Am. 2001;34:1-22.
38. Lee D., Brody R., Har-El G. Frontal sinus outflow anatomy. Am J Rhinol. 1997;11:283-285.
39. Harris L., Marano G.D., McCorkle D. Nasofrontal duct: CT in frontal sinus trauma. Radiology. 1987;165:195-198.
40. Heller E.M., Jacobs J.B., Holliday R.A. Evaluation of the frontonasal duct in frontal sinus fractures. Head Neck. 1989;11:46-50.
41. Landsberg R., Friedman M. A computer-assisted anatomical study of the nasofrontal region. Laryngoscope. 2001;111:2125-2130.
42. Tiwari P., Higuera S., Thornton J., Hollier L.H. The management of frontal sinus fractures. J Oral Maxillofac Surg. 2005;63:1354-1360.
43. Stanley R.B.Jr., Becker T.S. Injuries of the nasofrontal orifices in frontal sinus fractures. Laryngoscope. 1987;97:728-731.
44. Donald P.J., Montgomery W.W., Calcaterra T. Frontal bone defect with frontal sinus mucopyocele. Head Neck Surg. 1987;10:59-62.
45. Weitzel E.K., Hollier L.H., Calzada G., Manolidis S. Single stage management of complex fronto-orbital mucoceles. J Craniofac Surg. 2002;13:739-745.
46. Abrahamson I.A.Jr., Baluyot S.T., Tew J.M.Jr., Scioville G. Frontal sinus mucocele. Ann Ophthalmol. 1979;11:173-178.
47. LaRossa D.D., Noone R.B., Jackson P. Facial deformity from frontal sinus mucocele: single stage surgical correction. Case report. Plast Reconstr Surg. 1977;60:917-919.
48. Yavuzer R., Sari A., Kelly C.P., et al. Management of frontal sinus fractures. Plast Reconstr Surg. 2005;115:79e-93e.
49. Donald P.J. Frontal sinus ablation by cranialization. Report of 21 cases. Arch Otolaryngol. 1982;108:142-146.
50. Donald P.J. Obliteration of compressed frontal sinus. Plast Reconstr Surg. 1986;78:832-833.
51. Manolidis S., Hollier L.H.Jr. Management of frontal sinus fractures. Plast Reconstr Surg. 2007;120:32S-48S.
52. Donath A., Sindwani R. Frontal sinus cranialization using the pericranial flap: an added layer of protection. Laryngoscope. 2006;116:1585-1588.
53. Chen K.T., Chen C.T., Mardini S., et al. Frontal sinus fractures: a treatment algorithm and assessment of outcomes based on 78 clinical cases. Plast Reconstr Surg. 2006;118:457-468.
54. Luce E.A. Frontal sinus fractures: guidelines to management. Plast Reconstr Surg. 1987;80:500-510.
55. Rontal M.L. State of the art in craniomaxillofacial trauma: frontal sinus. Curr Opin Otolaryngol Head Neck Surg. 2008;16:381-386.
56. Shumrick K.A. Endoscopic management of frontal sinus fractures. Facial Plast Surg Clin North Am. 2006;14:31-35.
57. Dedo H.H., Broberg T.G., Murr A.H. Frontoethmoidectomy with Sewall-Boyden reconstruction: alive and well, a 25-year experience. Am J Rhinol. 1998;12:191-198.
58. Rains B.M.3rd. Frontal sinus stenting. Otolaryngol Clin North Am. 2001;34:101-110.
59. Murr A.H., Dedo H.H. Frontoethmoidectomy with Sewall-Boyden reconstruction: indications, technique, and philosophy. Otolaryngol Clin North Am. 2001;34:153-165.
60. Tedaldi M., Ramieri V., Foresta E., et al. Experience in the management of frontal sinus fractures. J Craniofac Surg. 2010;21:208-210.
61. Kienstra M.A., Van Loveren H. Anterior skull base fractures. Facial Plast Surg. 2005;21:180-186.
62. Donald P.J. The tenacity of the frontal sinus mucosa. Otolaryngol Head Neck Surg. 1979;87:557-566.
63. Shumrick K.A., Smith C.P. The use of cancellous bone for frontal sinus obliteration and reconstruction of frontal bony defects. Arch Otolaryngol Head Neck Surg. 1994;120:1003-1009.
64. Grahne B. Chronic frontal sinusitis treated by autogenous osteoplasty. Acta Otolaryngol. 1971;72:215-219.
65. Nadell J., Kline D.G. Primary reconstruction of depressed frontal skull fractures including those involving the sinus, orbit, and cribriform plate. J Neurosurg. 1974;41:200-207.
66. Donald P.J., Bernstein L. Compound frontal sinus injuries with intracranial penetration. Laryngoscope. 1978;88:225-232.
67. Spinelli H.M., Irizarry D., McCarthy J.G., et al. An analysis of extradural dead space after fronto-orbital surgery. Plast Reconstr Surg. 1994;93:1372-1377.
68. Stanley R.B.Jr. Management of frontal sinus fractures. Facial Plast Surg. 1988;5:231-235.
69. Stanley R.B.Jr. Fractures of the frontal sinus. Clin Plast Surg. 1989;16:115-123.
70. Onishi K., Nakajima T., Yoshimura Y. Treatment and therapeutic devices in the management of frontal sinus fractures. Our experience with 42 cases. J Craniomaxillofac Surg. 1989;17:58-63.
71. Cannon C.R., Jahrsdoerfer R.A. Temporal bone fractures. Review of 90 cases. Arch Otolaryngol. 1983;109:285-288.
72. Aguilar E.A.3rd, Yeakley J.W., Ghorayeb B.Y., et al. High resolution CT scan of temporal bone fractures: association of facial nerve paralysis with temporal bone fractures. Head Neck Surg. 1987;9:162-166.
73. Nosan D.K., Benecke J.E.Jr., Murr A.H. Current perspective on temporal bone trauma. Otolaryngol Head Neck Surg. 1997;117:67-71.
74. Fisch U. Facial paralysis in fractures of the petrous bone. Laryngoscope. 1974;84:2141-2154.
75. Ulug T., Arif Ulubil S. Management of facial paralysis in temporal bone fractures: a prospective study analyzing 11 operated fractures. Am J Otolaryngol. 2005;26:230-238.
76. Samii M., Tatagiba M. Skull base trauma: diagnosis and management. Neurol Res. 2002;24:147-156.
77. Samii M. Intra-cranial reconstruction of facial nerve after lateral basal fracture. In: Samii M., Brihaye J. Traumatology of the skull base. Berlin Heidelberg: Springer Verlag; 1983:164-170.
78. Harris J.H.Jr., Carson G.C., Wagner L.K., Kerr N. Radiologic diagnosis of traumatic occipitovertebral dissociation: 2. Comparison of three methods of detecting occipitovertebral relationships on lateral radiographs of supine subjects. AJR Am J Roentgenol. 1994;162:887-892.
79. Levine A.M., Edwards C.C. Traumatic lesions of the occipitoatlantoaxial complex. Clin Orthop Relat Res. 1989:53-68.
80. Kenter K., Worley G., Griffin T., Fitch R.D. Pediatric traumatic atlanto-occipital dislocation: five cases and a review. J Pediatr Orthop. 2001;21:585-589.
81. Anderson P.A., Montesano P.X. Morphology and treatment of occipital condyle fractures. Spine (Phila Pa 1976). 1988;13:731-736.
82. Tuli S., Tator C.H., Fehlings M.G., Mackay M. Occipital condyle fractures. Neurosurgery. 1997;41:368-376.
83. Mann F.A., Cohen W. Occipital condyle fracture: significance in the assessment of occipitoatlantal stability. AJR Am J Roentgenol. 1994;163:193-194.
84. Noble E.R., Smoker W.R. The forgotten condyle: the appearance, morphology, and classification of occipital condyle fractures. AJNR Am J Neuroradiol. 1996;17:507-513.
85. Maserati M.B., Stephens B., Zohny Z., et al. Occipital condyle fractures: clinical decision rule and surgical management. J Neurosurg Spine. 2009;11:388-395.
86. Aarabi B., Hesdorffer D.C., Ahn E.S., et al. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006;104:469-479.
87. Cooper D.J., Rosenfeld J.V., Murray L., et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364:1493-1502.
88. Timofeev I., Kirkpatrick P.J., Corteen E., et al. Decompressive craniectomy in traumatic brain injury: outcome following protocol-driven therapy. Acta Neurochir Suppl. 2006;96:11-16.
89. Hutchinson P.J., Corteen E., Czosnyka M., et al. Decompressive craniectomy in traumatic brain injury: the randomized multicenter RESCUEicp study, www.RESCUEicp.com Acta Neurochir Suppl, 2006;96:17-20
90. Munch E., Horn P., Schurer L., et al. Management of severe traumatic brain injury by decompressive craniectomy. Neurosurgery. 2000;47:315-322.
91. Overgaard J. Traumatic lesions of the planum occipitale and posterior fossa brain parenchyma. In: Samii M., Brihaye J. Traumatology of the skull base. Berlin Heidelberg: Springer-Verlag; 1983:88-93.
92. Dagi T.F., Meyer F.B., Poletti C.A. The incidence and prevention of meningitis after basilar skull fracture. Am J Emerg Med. 1983;1:295-298.
93. Park J.I., Strelzow V.V., Friedman W.H. Current management of cerebrospinal fluid rhinorrhea. Laryngoscope. 1983;93:1294-1300.
94. Scholsem M., Scholtes F., Collignon F., et al. Surgical management of anterior cranial base fractures with cerebrospinal fluid fistulae: a single-institution experience. Neurosurgery. 2008;62:463-469.
95. Asano T., Ohno K., Takada Y., et al. Fractures of the floor of the anterior cranial fossa. J Trauma. 1995;39:702-706.
96. Friedman J.A., Ebersold M.J., Quast L.M. Post-traumatic cerebrospinal fluid leakage. World J Surg. 2001;25:1062-1066.
97. Henry R.C., Taylor P.H. Cerebrospinal fluid otorrhoea and otorhinorrhoea following closed head injury. J Laryngol Otol. 1978;92:743-756.
98. Eljamel M.S., Foy P.M. Acute traumatic CSF fistulae: the risk of intracranial infection. Br J Neurosurg. 1990;4:381-385.
99. Nageris B., Hansen M.C., Lavelle W.G., Van Pelt F.A. Temporal bone fractures. Am J Emerg Med. 1995;13:211-214.
100. Mincy J.E. Posttraumatic cerebrospinal fluid fistula of the frontal fossa. J Trauma. 1966;6:618-622.
101. Brodie H.A. Prophylactic antibiotics for posttraumatic cerebrospinal fluid fistulae. A meta-analysis. Arch Otolaryngol Head Neck Surg. 1997;123:749-752.
102. Leech P.J., Paterson A. Conservative and operative management for cerebrospinal-fluid leakage after closed head injury. Lancet. 1973;1:1013-1016.
103. Klastersky J., Sadeghi M., Brihaye J. Antimicrobial prophylaxis in patients with rhinorrhea or otorrhea: a double-blind study. Surg Neurol. 1976;6:111-114.
104. Eftekhar B., Ghodsi M., Nejat F., et al. Prophylactic administration of ceftriaxone for the prevention of meningitis after traumatic pneumocephalus: results of a clinical trial. J Neurosurg. 2004;101:757-761.
105. Arendall R.E., Meirowsky A.M. Air sinus wounds: an analysis of 163 consecutive cases incurred in the Korean War, 1950-1952. Neurosurgery. 1983;13:377-380.
106. Aarabi B., Taghipour M., Alibaii E., Kamgarpour A. Central nervous system infections after military missile head wounds. Neurosurgery. 1998;42:500-507.
107. Locatelli D., Rampa F., Acchiardi I., et al. Endoscopic endonasal approaches for repair of cerebrospinal fluid leaks: nine-year experience. Neurosurgery. 2006;58:ONS-246-ONS-256.
108. Probst C. Neurosurgical treatment of traumatic frontobasal CSF fistulae in 300 patients (1967-1989). Acta Neurochir (Wien). 1990;106:37-47.
109. Hegazy H.M., Carrau R.L., Snyderman C.H., et al. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhea: a meta-analysis. Laryngoscope. 2000;110:1166-1172.
110. Fisch U., Mattox D. Microsurgery of the Skull Base. New York: Thieme; 1988.
111. Magliulo G., Celebrini A., Cuiuli G., Parlotto D. Cranial base fracture and rhino cerebrospinal fluid leakage. A case report. An Otorrinolaringol Ibero Am. 2007;34:35-44.
112. Orlandi R.R., Shelton C. Endoscopic closure of the eustachian tube. Am J Rhinol. 2004;18:363-365.
113. Calcaterra T.C. Extracranial surgical repair of cerebrospinal rhinorrhea. Ann Otol Rhinol Laryngol. 1980;89:108-116.
114. McCormack B., Cooper P.R., Persky M., Rothstein S. Extracranial repair of cerebrospinal fluid fistulas: technique and results in 37 patients. Neurosurgery. 1990;27:412-417.
115. Martin T.J., Loehrl T.A. Endoscopic CSF leak repair. Curr Opin Otolaryngol Head Neck Surg. 2007;15:35-39.
116. Zimmer L.A., Theodosopoulos P.V. Anterior skull base surgery: open versus endoscopic. Curr Opin Otolaryngol Head Neck Surg. 2009;17:75-78.
117. Schaller B. Subcranial approach in the surgical treatment of anterior skull base trauma. Acta Neurochir (Wien). 2005;147:355-366. discussion 366
118. Gliklich R.E., Lazor J.B. The subcranial approach to trauma of the anterior cranial base: preliminary report. J Craniomaxillofac Trauma. 1995;1:56-62.
119. Fliss D.M., Zucker G., Cohen J.T., Gatot A. The subcranial approach for the treatment of cerebrospinal fluid rhinorrhea: a report of 10 cases. J Oral Maxillofac Surg. 2001;59:1171-1175.
120. Thumfart W. Olfaction after frontobasal trauma with and without surgery. In: Samii M., Brihaye J. Traumatology of the skull base. Berlin Heidelberg: Springer Verlag; 1983:101-107.
121. Lang J. Anatomy of the skull base related to trauma. In: Samii M., Brihaye J. Traumatology of the Skull Base. New York: Springer-Verlag; 1983:3-34.
122. Lee T.J., Huang C.C., Chuang C.C., Huang S.F. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhea and skull base defect: ten-year experience. Laryngoscope. 2004;114:1475-1481.
123. Papay F.A., Benninger M.S., Levine H.L., Lavertu P. Transnasal transseptal endoscopic repair of sphenoidal cerebral spinal fluid fistula. Otolaryngol Head Neck Surg. 1989;101:595-597.
124. Mattox D.E., Kennedy D.W. Endoscopic management of cerebrospinal fluid leaks and cephaloceles. Laryngoscope. 1990;100:857-862.
125. Weisman R.A. Septal chondromucosal flap with preservation of septal integrity. Laryngoscope. 1989;99:267-271.
126. Yessenow R.S., McCabe B.F. The osteo-mucoperiosteal flap in repair of cerebrospinal fluid rhinorrhea: a 20-year experience. Otolaryngol Head Neck Surg. 1989;101:555-558.
127. McCabe B.F. The osteo-mucoperiosteal flap in repair of cerebrospinal fluid rhinorrhea. Laryngoscope. 1976;86:537-539.
128. Komisar A., Weitz S., Ruben R.J. Cerebrospinal fluid dynamics and rhinorrhea: the role of shunting in repair. Otolaryngol Head Neck Surg. 1983;91:399-403.
129. Laun A. Traumatic cerebrospinal fluid fistulas in the anterior and middle cranial fossae. Acta Neurochir (Wien). 1982;60:215-222.
130. Komisar A., Weitz S., Ruben R.J. Rhinorrhea and pneumocephalus after cerebrospinal fluid shunting. The role of lateral extensions of the sphenoid sinus. Otolaryngol Head Neck Surg. 1986;94:194-197.