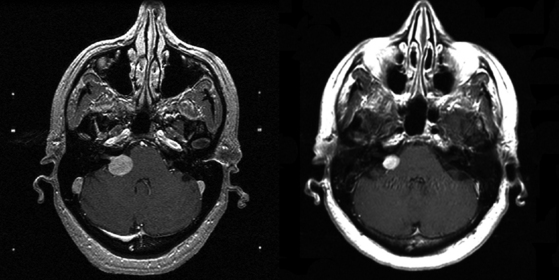
Chapter 103 Vestibular Schwannomas
The Role of Stereotactic Radiosurgery
Acoustic neuromas (vestibular schwannomas) are generally slow-growing, intracranial extra-axial benign tumors that usually develop from the vestibular portion of the eighth nerve.1 Bilateral vestibular schwannomas are usually associated with neurofibromatosis 2 (NF2). Both unilateral and bilateral vestibular schwannomas may form due to malfunction of a gene on chromosome 22, which produces a protein (schwannomine/merlin) that controls the growth of Schwann cells. In NF2 patients, the faulty gene on chromosome 22 is inherited and is present in all or most somatic cells. However, in individuals with unilateral vestibular schwannoma, for unknown reasons this gene loses its ability to function properly and is present only in the schwannoma cells.2
A progressive unilateral hearing decline is the most common symptom that leads to the diagnosis of a vestibular schwannoma.3 In years past it was rare for patients to present with intact hearing, but this is becoming more common due to earlier diagnosis and higher-quality magnetic resonance imaging (MRI). Overall, three separate growth patterns can be distinguished1: no or very slow growth,2 slow growth (i.e., 2 mm/year linear growth on imaging studies),3 and fast growth (i.e., >8 mm/year). Although most tumors grow slowly, some grow quickly and can double in volume within 6 months to a year.4 Cystic vestibular schwannomas are sometimes capable of relatively rapid enlargement of their cystic component. Although rare, other tumors may hemorrhage spontaneously.5 Stereotactic radiosurgery has greatly expanded the management options since patients no longer have to choose simply between resection and observation.
Observation with Serial Imaging
In some cases, usually elderly or medically infirm patients or individuals with very small tumors, it may be reasonable to “watch” the tumor for potential growth.6 Repeat MRI scans over time are used to carefully monitor the tumor for any growth.7 The object of serial observation is to obviate treatment unless signs of growth are confirmed.6,8 In our 20-year experience 70% of tumors under observation have measurable growth in 5 years and almost all by 10 years. Recent published series continue to note annual tumor growth rates in the 1 to 3 mm/year range, with some evidence that extracanalicular tumors grow at a faster rate. This could be due to an easier appreciation of volumetric growth with a larger lesion.
Considering Radiosurgery Versus Surgical Resection
Resection is indicated for larger tumors with disabling brainstem compression, hydrocephalus, intractable headache, or trigeminal neuralgia. The majority of patients have smaller tumors and do not have these problems. The three main surgical avenues include the retrosigmoid, translabyrinthine, and middle fossa approaches.9,10 As discussed elsewhere in this text, several factors help in the decision on which approach is optimal. Although the outcomes of surgical removal at centers of excellence have improved markedly over the last two decades, patients increasingly seek lesser invasive options. It is important to understand the differences between approaches.
First, preservation of facial function varies according to tumor size and the surgeon’s experience.11 When tumors are smaller than 1.5 cm, good facial nerve function can be expected (House-Brackmann grades I–II) in more than 90% of patients who have surgery at centers of excellence. Only 3.2% to 6.7% of patients with smaller tumors have poor facial nerve outcomes (House-Brackmann grades III–V). In addition to tumor size, intraoperative electrophysiologic facial nerve monitoring assists the surgeon to save the nerve.12 The overall facial nerve anatomic preservation rate is 80%.13 However, facial nerve function (grades I and II) can be preserved in only 40% to 50% of patients with large (>4-cm diameter) tumors.14 Injuries of the nervus intermedius are underestimated because this nerve is rarely assessed preoperatively.15
Second, hearing preservation rates have also improved. Depending on the criteria used for reporting successful hearing conservation, preservation has been reported in 30% to 80% of patients considered eligible for hearing preservation surgery.16 A meta-analysis performed by Gardner and Robertson in 1988 revealed an overall average success rate of about 33%.17 Delayed hearing deterioration may occur days to years after surgery in 30% to 50% of patients who originally had successful hearing preservation.18–21 In various studies, serviceable hearing preservation rates vary from 8% to 57%18,22,23 using the retrosigmoid approach and from 32% to 68%23 using the middle fossa approach.
Cerebrospinal fluid leakage through either the surgical incision or the Eustachian tube and middle ear occurs in 2% to 20% of patients.20,21,24–26 Although in individual published reports the cerebrospinal fluid leak rate appears higher with the retrosigmoid approach (2.9%–18%),23 a recent meta analysis suggests similar rates of cerebrospinal fluid leak for all surgical approaches (10.6% of 2273 retrosigmoid surgeries; 9.5% of 3118 translabyrinthine surgeries; and 10.6% of 573 middle fossa surgeries). The adjunctive use of endoscopy may assist the surgeon to avoid or to detect a CSF leakage.27 Other rarer perioperative complications include death (0%–3%),28,29 intracranial hematomas (1%–2%), wound hematoma (3%), cerebellar and brainstem edema, hemiparesis, meningitis (1.2%), wound infections (1.2%), abducens nerve paresis (1%–2%), and other lower cranial nerve injuries.25,29
Overall, tumor recurrence rates of 5% to 10% are found in the published literature, although a few studies report no long term recurrence after translabyrinthine approach.30 However, incomplete resection of vestibular schwannomas is associated with a significant risk of tumor progression requiring subsequent intervention.31
The Patients’ Perspective on Resection
A variety of complications have been reported after vestibular schwannoma surgery.32–38 Studies from the Acoustic Neuroma Association are a good resource.39 Bateman et al., described patients’ subjective condition after vestibular schwannoma surgery as impairment (141, 51%), disability (95, 34%) or handicap (43, 15%).40 Most of the impairments were related to problems with facial nerve function. The other most common issues were “balance problems” (19/141, 13%) followed by “hearing loss” (17/141, 12%) and “difficulty with background noise” (14/141, 10%). Tinnitus accounted for 5 of 141 responses (4%). Disabilities resulting from facial nerve dysfunction accounted for most of the disabilities reported by patients. A significant number of disabilities were associated with balance problems (e.g., “unable to drive,” “problems changing direction,” “unable to swim, cycle, run, climb steps, do aerobics,” and “problems bending down”) and with hearing loss (e.g., “difficulty locating the source of sounds,” “difficulty following conversations in a crowd,” “unable to hear people to one side” and “unable to hear doorbell/telephone”). Some patients reported symptoms of social isolation after the surgery. Fifteen out of forty-three responses (35%) were “reluctance to attend large social gatherings.” Employment-related problems were also important with 7 of 43 responses (16%).
Stereotactic Radiosurgery
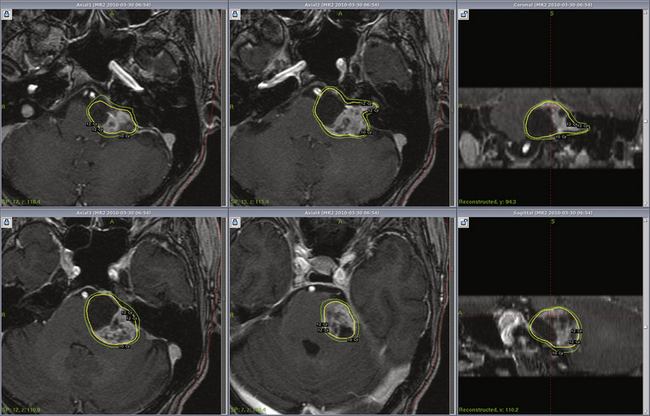
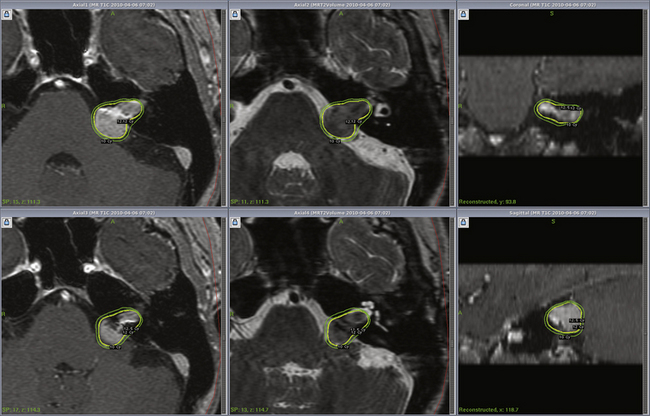
Vestibular schwannoma stereotactic radiosurgery using the gamma knife was first performed by Leksell in 1969.41 During the past two decades radiosurgery has emerged as an effective alternative to surgical removal of small- to moderate-sized vestibular schwannomas (Figs. 103-1 and 103-2). Long-term results have established radiosurgery as an important minimally invasive alternative to resection. Advanced multi-isocenter dose-planning software, high-resolution MRI for targeting, dose optimization, and robotic delivery reflect the evolution of this technology. Other image-guided linear accelerator (LINAC) devices (Trilogy, Synergy S, Novalis and CyberKnife) generally can be used to fractionate radiation delivery in 5 to 30 sessions. Proton beam technology is also used to deliver fractionated radiation therapy. The goals of vestibular schwannoma radiosurgery are to prevent further tumor growth, preserve neurologic function where possible, avoid the risks associated with open resection, and in selected patients to improve pre-existing symptoms.
Radiosurgery Technique for Vestibular Schwannomas
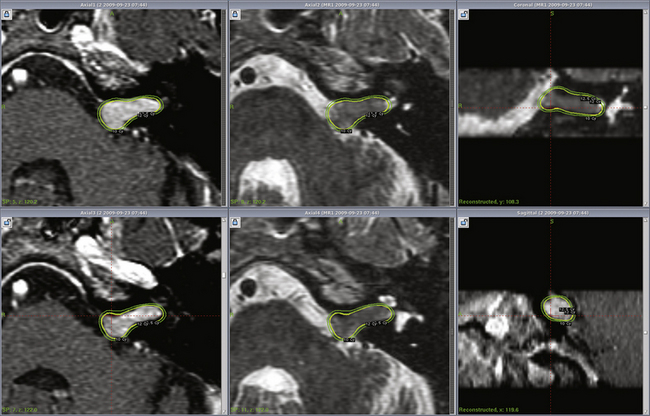
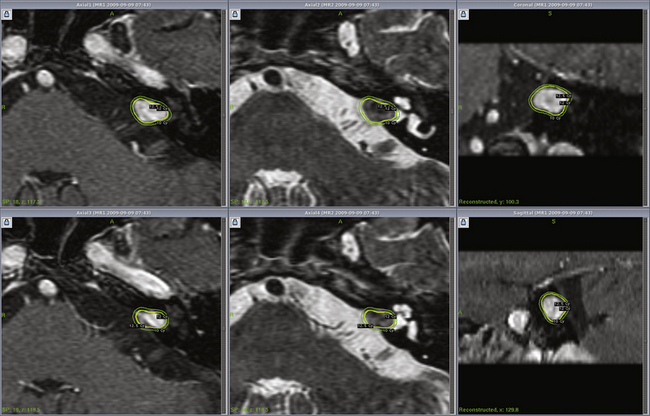
Radiosurgery can be performed using the gamma knife, modified LINACs, or the proton beam. Techniques of head frame fixation, stereotactic imaging, dose planning, and dose delivery are different for these three modalities. In gamma knife radiosurgery the procedure begins with rigid fixation of an MRI-compatible Leksell stereotactic frame (Model G, Elekta Instruments, Atlanta, GA) to the patient’s head. Local anesthetic scalp infiltration (5% Marcaine and 1% Xylocaine) is used, supplemented by mild intravenous sedation as needed. High-resolution images are acquired with a fiducial system attached to the stereotactic frame. For vestibular schwannoma radiosurgery, a three-dimensional (3-D) volume acquisition MRI using a gradient pulse sequence (divided into 1- or 1.5-mm thick, 28–36 axial slices) is performed in order to cover the entire lesion and surrounding critical structures. A T2-weighted, 3-D volume sequence is performed to visualize cranial nerves and delineate inner ear structures (the cochlea and semicircular canals) (Figs. 103-3 and 103-4). Planning is performed on narrow-slice thickness, axial MR images with coronal and sagittal reconstructions. Centers using LINAC or proton-beam systems may use mask immobilization of the patient’s head along with image guidance and typically deliver the radiation dose in five or more fractions over many days. CT is used for planning at most LINAC sites but may be fused to MRI scans.
Radiosurgical Dose Planning
Dose planning is a critical aspect of radiosurgery. Complete coverage of the tumor and preservation of facial, cochlear, and trigeminal nerve function is given priority during dose planning. For large tumors, preservation of brain-stem function is also a consideration. Conformality and selectivity is necessary for hearing and facial nerve preservation.42 Specific gamma knife radiosurgery techniques include accurate definition of the tumor volume, use of multiple isocenters, beam weighting, and selective use of plug patterns to reduce dose to critical structures. This degree of conformality can be achieved through complex multi-isocenter planning (see all figures). Vestibular schwannoma planning is usually performed using a combination of small-beam-diameter (4- and 8-mm) collimators. For large tumors, 14-, 16-, or 18-mm collimators are used. A series of 4-mm isocenters are used to create a tapered isodose plan to conform to the intracanalicular portion of the tumor.
Dose Selection
After optimizing the plan, a maximum dose inside target is determined as well as the dose to the tumor edge. The treatment isodose, maximum dose, and dose to the margin (edge) are jointly decided by a neurosurgeon, radiation oncologist, and medical physicist and, in some centers, a neurotologist. In gamma knife radiosurgery, a dose of 12 to 13 Gy is typically prescribed to the 50% (or other) isodose line that conforms to the tumor margin. The commonest dose is 12.5 Gy, used for hearing preservation in smaller tumors. Larger tumors may receive 12 Gy, and in those with hearing loss or prior resection, 13 Gy. Dose prescription for vestibular schwannomas changed significantly during the first 10 years experience at our center. This margin dose range is associated with a low complication rate and yet maintains a high rate of tumor control as we have found in our most recent 10-years-plus experience using these doses. We suspect that further dose reduction is unwarranted. Most centers are reluctant to prescribe lower margin doses (such as 12 Gy) for vestibular schwannomas. Similar doses are also used for patients with bilateral (NF2 related) vestibular schwannomas and for patients with contralateral deafness from other causes, for whom hearing preservation is highly desirable. After prescribing the margin dose, the mean dose to the cochlea, semicircular canals, and brain stem are assessed. A mean cochlear dose less than 4.2 Gy may be important for hearing preservation (Fig. 103-5). The majority of the tumor volume is receiving a radiobiologic dose in excess of a biologically equivalent dose delivered by fractionated image-guided radiation therapy. The maximum radiosurgical dose of 25 Gy may be radiobiologically equivalent to 100 Gy of fractionated radiation. While many radiosurgical centers have evolved toward similar dose selection parameters, the doses and regimens chosen for fractionated radiotherapy continue to vary widely.
Gamma Knife Radiosurgery: Clinical Results
Long-term results of gamma knife radiosurgery for vestibular schwannomas have been documented.43–48 Recent reports suggest a tumor control rate of 93% to 100% after radiosurgery.42–64 Kondziolka et al. studied 5- to 10-year outcomes in 162 vestibular schwannoma patients who had radiosurgery at the University of Pittsburgh.58 In this study a long-term 98% tumor control rate was reported. Sixty-two percent of tumors became smaller, 33% remained unchanged, and 6% became slightly larger. Some tumors initially enlarged 1 to 2 mm during the first 6 to 12 months after radiosurgery as they lost their central contrast enhancement. Such tumors generally regressed in volume compared to their preradiosurgery size. Only 2% of patients required tumor resection after radiosurgery. Norén, in his 28-year experience with vestibular schwannoma radiosurgery, reported a 95% long-term tumor control rate. Litvack et al. reported a 98% tumor control rate at a mean follow-up of 31 months after radiosurgery using a 12-Gy margin dose.65 Niranjan et al. analyzed the outcome of intracanalicular tumor radiosurgery performed at the University of Pittsburgh.66 All patients (100%) had imaging-documented tumor growth control.
Flickinger et al. performed an outcome analysis of acoustic neuroma patients treated between August 1992 and August 1997 at the University of Pittsburgh. The actuarial 5-year clinical tumor control rate (no requirement for surgical intervention) was 99.4 ± 0.6%.44,50 The long-term (10–15 years) outcome of benign tumor radiosurgery also has been evaluated. In a study which included 157 patients with vestibular schwannomas, the median follow-up for the patients still living at the time of the study (n = 136) was 10.2 years. Serial imaging studies after radiosurgery (n = 157) showed a decrease in tumor size in 114 patients (73%), no change in 40 patients (25.5%), and an increase in three patients who later had resection (1.9%).47 No patient developed a radiation-associated malignant or benign tumor (defined as a histologically confirmed and distinct neoplasm arising in the initial radiation field after at least 2 years have passed). In patients under 40 years of age, with minimum 4-year follow-up, all remained employed and active.65
Hearing Preservation
Preradiosurgery hearing can now be preserved in 60% to 90% of patients, with higher preservation rates found for smaller tumors. In a long-term (5–10-year follow-up) study conducted at the University of Pittsburgh, 51% of patients had no change in hearing ability.50,58 All patients (100%) who were treated with a margin dose of 14 Gy or less maintained a serviceable level of hearing after intracanalicular tumor radiosurgery.66 Among patients treated after 1992, the 5-year actuarial rates of hearing level preservation and speech preservation were 75.2% and 89.2%, respectively, for patients (n = 89) treated with a 13-Gy tumor margin dose. However, in a longer-term assessment at a median of 6 years, the same Gardner/Robertson level was preserved in 71%, serviceable hearing in 74%, and any testable hearing in 95%. For intracanalicular tumors, these rates were 84%, 92%, and 100%.
Our recent research has shown that the mean cochlear dose is important for hearing preservation. A dose of less than 4.2 Gy was associated with better hearing,67 a finding similar to the dose of 4 Gy noted from Marseille. Age is also important, as those under 60 years old fare better.67 Long-relaxation-time (T2) volumetric images are important to identify the cochlea for dose planning.
Facial Nerve and Trigeminal Nerve Preservation
Facial and trigeminal nerve function can now be preserved in the majority of patients (>95%). In a study using MR-based dose planning, a 13-Gy tumor margin dose was associated with 0% risk of new facial weakness and 3.1% risk of facial numbness (5-year actuarial rates). A margin dose of greater than 14 Gy was associated with a 2.5% risk of new onset facial weakness and a 3.9% risk of facial numbness (5-year actuarial rates).44 None of the patients who had radiosurgery for intracanalicular tumors developed new facial or trigeminal neuropathies. At the current 12- to 13-Gy dose range, any degree of facial weakness is exceedingly rare.
Neurofibromatosis 2
Patients with vestibular schwannomas associated with neurofibromatosis 2 represent a special challenge because of the risk of complete deafness. Unlike the solitary sporadic tumors that tend to displace the cochlear nerve, tumors associated with NF2 tend to form nodular clusters that engulf or even infiltrate the cochlear nerve. Complete resection may not always be possible. Radiosurgery has been performed for patients with NF2. Subach et al. studied our first 40 patients (with 45 tumors) who were treated with radiosurgery for NF2. Serviceable hearing was preserved in 6 of 14 patients (43%), and this rate improved to 67% after modifications made to the technique in 1992. The tumor control rate was 98%.68 Only one patient showed imaging-documented growth. Normal facial nerve function and trigeminal nerve function were preserved in 81% and 94% of patients, respectively. In two recent series,69,70 serviceable hearing was preserved in only 30%69 and 40%70 of cases, respectively. The tumor control rate was respectively 71%69 and 79%.70 Mathieu et al. updated outcomes of our NF2 series over the year 2007.71 The tumor control rate was 87.5%. The rate of serviceable hearing preservation using current technique was 52.6%. It now appears that preservation of serviceable hearing in patients with NF2 is an attainable goal using gamma-knife radiosurgery. However, longer-term results from Sheffield showed a drop off in hearing over many years. We believe that early radiosurgery when the hearing level is still excellent may become an appropriate strategy in the future. At present we generally delay radiosurgery in NF2 patients until we see hearing deterioration or tumor growth.
Proton Beam Radiosurgery: Clinical Results
Weber et al. evaluated 88 patients with vestibular schwannomas treated with proton beam stereotactic radiosurgery, in which two to four convergent fixed beams of 160-MeV protons were applied.72 A median dose of 12 cobalt Gy equivalents was prescribed to the 70% to 108% isodose lines (median, 70%). The median follow-up period was 38.7 months. The actuarial 2- and 5-year tumor control rates were 95.3% and 93.6%, respectively. Serviceable hearing was preserved in 33.3% of patients. Actuarial 5-year normal facial and trigeminal nerve function preservation rates were 91.1% and 89.4%, respectively. Harsh et al. evaluated 68 patients with vestibular schwannomas who were treated with proton beam using a marginal dose of only 12 Gy.53 After a mean clinical follow-up of 44 months and imaging follow-up of 34 months, actuarial control rates of 94% at 2 years and 84% at 5 years were reported. Cranial neuropathies included persistent facial hypoesthesia (4.7%), intermittent facial paresthesias (9.4%), persistent facial weakness (4.7%) requiring oculoplasty, transient partial facial weakness (9.4%), and synkinesis (9.4%).
Linac Radiosurgery: Clinical Results
Suh et al. evaluated 29 patients treated with a modified linear accelerator stereotactic radiosurgery system.73 The median margin dose was 1600 cGy. The 5-year local disease control rate was 94%. Long-term complications included new or progressive trigeminal and facial nerve deficits with estimated 5-year incidences of 15% and 32%, respectively. Subjective hearing reduction or loss occurred in 14 of the 19 patients (74%) who had useful hearing prior to treatment. Since there was a high risk of cranial nerve neuropathy, these authors did not recommend using only computed tomography-based planning and high prescription doses. Spiegelmann et al. reported their results of LINAC radiosurgery for 44 patients with vestibular schwannomas.74 After a mean follow-up period of 32 months (range, 12–60 months), 98% of the tumors were controlled. The actuarial hearing preservation rate was 71%. New transient facial neuropathy developed in 24% of the patients and persisted to a mild degree in 8%. The University of Florida group published clinical outcomes in a series of 390 patients, with a high control rate and a facial neuropathy rate of 0.7% using current techniques and dose.52
Stereotactic Radiation Therapy: Clinical Results
Stereotactic radiation therapy (SRT) or fractionated stereotactic radiation therapy (FSRT) refers to the delivery of a standard fractionation scheme of radiation, used with rigidly applied or relocatable stereotactic-guiding devices. Some LINAC based radiosurgery centers (driven by the desire to reduce complication rates) have shifted to fractionated stereotactic radiotherapy for vestibular schwannomas.56,73,75–80 Ishihara et al. reported 94% tumor control rate at a median follow-up of 31.9 months in a series of 38 patients who had CyberKnife SRT for vestibular schwannoma. One patient developed transient facial paresis (2.6%) and one developed trigeminal nerve neuropathy (2.6%).56 Fuss et al. described 51 patients with vestibular schwannomas who were treated with SRT.81 The mean follow-up was 42 months and the actuarial 5-year tumor control rate was 95%. One patient developed a transient facial nerve paresis and two noted new trigeminal dysesthesias. Chung et al., using SRT for 25 patients with useful hearing, reported 57% hearing preservation at 2 years.82 The mean pre- and post-SRT speech recognition threshold was 20 and 38 dB, respectively. The mean proportion of pre- and post-SRT speech discrimination was 91% and 59%, respectively.
Sawamura et al. treated 101 patients with vestibular schwannoma using fractionated SRT to a total dose of 40 to 50 Gy, administered in 20 to 25 fractions over a 5- to 6-week period.78 The median follow-up period was 45 months, and the actuarial 5-year rate of tumor control was 91.4%. The actuarial 5-year rate of useful hearing preservation (Gardner-Robertson class I or II) was 71%. The complications of fractionated SRT included transient facial nerve palsy (4%), trigeminal neuropathy (14%), and balance disturbance (17%). Eleven patients (11%) who had progressive communicating hydrocephalus after FSRT required a shunt.
Meijer et al. performed a single-institution trial to study whether fractionated stereotactic radiation therapy is superior to single-session LINAC-based radiosurgery with respect to treatment-related toxicity and local control in patients with vestibular schwannomas.83 These authors analyzed 129 vestibular schwannoma patients who were treated at a LINAC-based radiosurgery facility. Stereotactic radiation therapy was performed on 80 patients with a relocatable guidance device using 5 × 4 Gy and later 5 × 5 Gy at the 80% isodose. Forty-nine patients had stereotactic radiosurgery of 1 × 10 Gy and later 1 × 12.5 Gy at the 80% isodose using a stereotactic frame. There was no statistically significant difference between the single-fraction group and the fractionated group with respect to mean tumor diameter (2.6 vs. 2.5 cm) or mean follow-up time (both 33 months). Outcome differences between the single-session group and the fractionated treatment group with respect to 5-year local control probability (100% vs. 94%), 5-year facial nerve preservation probability (93% vs. 97%), and 5-year hearing preservation probability (75% vs. 61%) were not statistically significant. The difference in 5-year trigeminal nerve preservation (92% vs. 98%) reached statistical significance (p = 0.048). These authors concluded that LINAC-based radiosurgery was as good as LINAC-based fractionated stereotactic radiation therapy in vestibular schwannoma patients, except for a small difference in trigeminal nerve preservation rate in favor of a fractionated schedule.
Andrews et al. published their outcomes using stereotactic radiotherapy at total dose of 50.4 or 46.8 Gy. In patients with grade 1 or 2 hearing, the median follow-up was 65 weeks. Although no patient had later tumor growth, the hearing preservation rates were better at the lower dose. At 3 years, the hearing preservation rate was 55% to 60%, and no patient with grade 2 hearing preserved it at the 50-Gy dose.84
At the present time there are limited data on SRT for vestibular schwannomas.85 There are no compelling radiobiological principles supporting the use of SRT over radiosurgery for achieving an optimal therapeutic response for the slowly proliferating, late-responding tissue of a schwannoma. The long-term results (5–10 years) of SRT are not yet available. For those centers who cannot achieve the necessary conformal plan to permit radiosurgery, SRT may be an option for vestibular schwannomas if they have a higher complication rate using LINAC radiosurgery.
Comparison of Radiosurgery and Microsurgery Options
It is likely that a randomized clinical trial will probably never be completed to compare surgical resection with radiosurgery for vestibular schwannoma.86 However, there are several well-matched cohort studies that compare outcomes for patients with tumors less than 2.5 cm in extracanalicular diameter. Karpinos et al. analyzed 96 patients with unilateral acoustic neuromas treated with the Leksell gamma knife or microsurgery and concluded that radiosurgery was associated with a lower rate of immediate and long-term development of facial and trigeminal neuropathy, postoperative complications, and hospital stay. Radiosurgery yielded better measurable hearing preservation than microsurgery and equivalent serviceable hearing preservation rate and tumor growth control.87
Pollock et al. studied 87 patients with unilateral, previously unoperated vestibular schwannomas with an average diameter of less than 3 cm treated at the University of Pittsburgh between 1990 and 1991.88 In this matched cohort trial preoperative patient characteristics and average tumor size were similar between the treatment groups. Microsurgical or radiosurgical techniques were used by experienced surgeons in both treatment groups. The treatment groups were compared based on cranial nerve preservation, tumor control, postoperative complications, patient symptomatology, length of hospital stay, total management charges, effect on employment status and overall patient satisfaction. Stereotactic radiosurgery was more effective in preserving normal postoperative facial function and hearing preservation with less treatment associated morbidity. Effects on preoperative symptoms were similar between the treatment groups. Postoperative functional outcomes and patients’ satisfaction were greater after radiosurgery when compared to microsurgery. Patients returned to independent functioning sooner after radiosurgery. Hospital length of stay and total management charges were less in the radiosurgical group.
In a similar study of vestibular schwannoma patients, Régis et al. used objective results and questionnaire answers to compare the results of radiosurgery (97 consecutive patients) with a microsurgery group (110 patients who fulfilled the inclusion criteria).89 Questionnaire answers indicated that 100% of patients who underwent gamma knife® radiosurgery compared with 63% of patients who underwent microsurgery had no new facial motor disturbance. Ninety-one percent of patients treated with gamma knife® radiosurgery, and 61% in the microsurgery study, had no functional deterioration after treatment. The mean hospital stay was 3 days after gamma knife® radiosurgery and 23 days after microsurgery. All working patients who underwent gamma knife® radiosurgery kept the same professional activity, compared to 56% in the microsurgery arm. The mean time away from work was 7 days for gamma knife® radiosurgery compared to 130 days for the microsurgery group. Among patients whose preoperative hearing level was Class 1 according to the Gardner and Robertson scale, 70% had preserved functional hearing after gamma knife radiosurgery (class 1 or 2), compared with only 37.5% in the microsurgery group. At 4 years of follow-up, gamma knife radiosurgery provided better functional outcomes than microsurgery. It was concluded that stereotactic radiosurgery was an effective and less costly management strategy for unilateral vestibular schwannomas less than 3 cm in diameter, and should be considered a primary management option.
In a recently published study, Myrseth et al. compared the quality of life outcomes for 189 acoustic neuroma patients with tumors less than 30 mm in diameter who were treated with either microsurgery or radiosurgery.13 The outcome analysis included assessments of tumor control, cranial nerve preservation rates, and complications. The results showed that cranial nerve function and overall patient outcomes were better in the radiosurgery group. The results reveal that from the patients’ perspective, radiosurgery provides a more desirable outcome than microsurgery. Pollock et al. prospectively collected data on patients undergoing either resection or gamma knife radiosurgery and found similar or better outcomes after radiosurgery, including quality of life measures.90
Summary
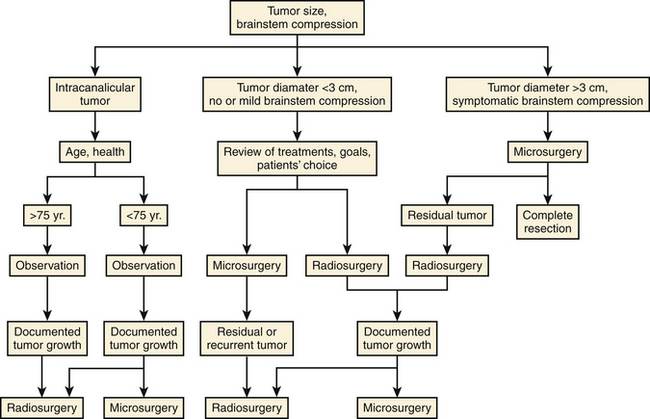
Radiosurgery has become a well documented management option for patients with vestibular schwannomas. As a minimally invasive strategy, we now know the expected success rate and risks. Long-term data are now published, and for the first time, systematic, serially collected outcomes data are available on patients with this tumor. Like microsurgical resection, not all radiosurgery technologies are the same. The evolution of radiosurgery has led to enhanced outcomes for patients diagnosed with such tumors. It may currently be the most common treatment choice for patients. An algorithm for management is shown (Fig. 103-6).
Andrews D., Werner-Wasik M., Den R., et al. Toward dose optimization for fractionated stereotactic radiotherapy for acoustic neuromas: comparison of two dose cohorts. Int J Radiat Oncol Biol Phys. 2009;74:419-426.
Chung W.Y., Liu K.D., Shiau C.Y., et al. Gamma knife surgery for vestibular schwannoma: 10-year experience of 195 cases. J Neurosurg. 2005;102(Suppl):87-96.
Delbrouck C., Hassid S., Massager N., et al. Preservation of hearing in vestibular schwannomas treated by radiosurgery using Leksell gamma knife: preliminary report of a prospective Belgian clinical study. Acta Otorhinolaryngol Belg. 2003;57:197-204.
Flickinger J.C., Kondziolka D., Niranjan A., Lunsford L.D. Results of acoustic neuroma radiosurgery: an analysis of 5 years’ experience using current methods [see comment]. J Neurosurg. 2001;94:1-6.
Flickinger J.C., Kondziolka D., Niranjan A., et al. Acoustic neuroma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2004;60:225-230.
Friedman W.A., Bradshaw P., Myers A., Bova F.J. Linear accelerator radiosurgery for vestibular schwannomas. J Neurosurg. 2006;105:657-661.
Hasegawa T., Kida Y., Kobayashi T., Yoshimoto M., et al. Long-term outcomes in patients with vestibular schwannomas treated using gamma knife surgery: 10-year follow up. J Neurosurg. 2005;102:10-16.
Kano H., Kondziolka D., Flickinger J.C., Lunsford L.D. Predictors of hearing preservation after stereotactic radiosurgery for acoustic neuromas. J Neurosurg. 2009;111:863-873.
Kondziolka D., Lunsford L.D., McLaughlin M.R., Flickinger J.C. Long-term outcomes after radiosurgery for acoustic neuromas [see comment]. N Engl J Med. 1998;339:1426-1433.
Kondziolka D., Nathoo N., Flickinger J.C., et al. Long-term results after radiosurgery for benign intracranial tumors [see comment]. Neurosurgery. 2003;53:815-821. discussion 821-822
Lee D.J., Westra W.H., Staecker H., et al. Clinical and histopathologic features of recurrent vestibular schwannoma (acoustic neuroma) after stereotactic radiosurgery. Otol Neurotol. 2003;24:650-660. discussion 660
Linskey M.E., Johnstone P.A. Radiation tolerance of normal temporal bone structures: implications for gamma knife stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;57:196-200.
Lobato-Polo J., Kondziolka D., Zorro O., et al. Gamma knife radiosurgery in younger patients with vestibular schwannomas. Neurosurgery. 2009;65:294-301.
Lunsford L.D., Niranjan A., Flickinger J.C., et al. Radiosurgery of vestibular schwannomas: summary of experience in 829 cases. J Neurosurg. 2005;102(Suppl):195-199.
Mathieu D., Kondziolka D., Flickinger J.C., et al. Stereotactic radiosurgery for vestibular schwannomas in patients with neurofibromatosis type 2: an analysis of tumor control, complications, and hearing preservation rates. Neurosurgery. 2007;60:460-468. discussion 468-470
Matthies C., Samii M. Management of vestibular schwannomas (acoustic neuromas): the value of neurophysiology for intraoperative monitoring of auditory function in 200 cases. Neurosurgery. 1997;40:459-466. discussion 466-468
McIver J.I., Pollock B.E. Radiation-induced tumor after stereotactic radiosurgery and whole brain radiotherapy: case report and literature review. J Neurooncol. 2004;66:301-305.
Myrseth E., Moller P., Pedersen P.H., et al. Vestibular schwannomas: clinical results and quality of life after microsurgery or gamma knife radiosurgery. Neurosurgery. 2005;56:927-935. discussion 927-935
Niranjan A., Lunsford L.D., Flickinger J.C., et al. Dose reduction improves hearing preservation rates after intracanalicular acoustic tumor radiosurgery. Neurosurgery. 1999;45:753-762. discussion 762-765
Noren G. Long-term complications following gamma knife radiosurgery of vestibular schwannomas. Stereotact Funct Neurosurg. 1998;70(Suppl 1):65-73.
Petit J.H., Hudes R.S., Chen T.T., et al. Reduced-dose radiosurgery for vestibular schwannomas. Neurosurgery. 2001;49:1299-1306. discussion 1306-1307
Pollock B.E., Driscoll C.L., Foote R.L., et al. Patient outcomes after vestibular schwannoma management: a prospective comparison of microsurgical resection and stereotactic radiosurgery. Neurosurgery. 2006;59:77-85.
Pollock B.E., Lunsford L.D., Flickinger J.C., et al. Vestibular schwannoma management. Part I. Failed microsurgery and the role of delayed stereotactic radiosurgery. J Neurosurg. 1998;89:944-948.
Pollock B.E., Lunsford L.D., Kondziolka D., et al. Outcome analysis of acoustic neuroma management: a comparison of microsurgery and stereotactic radiosurgery. Neurosurgery. 1995;36:215-224. discussion 224-229 [erratum Neurosurgery. 1995;36:427]
Pollock B.E., Lunsford L.D., Kondziolka D., et al. Vestibular schwannoma management. Part II. Failed radiosurgery and the role of delayed microsurgery. J Neurosurg. 1998;89:949-955.
Regis J., Pellet W., Delsanti C., et al. Functional outcome after gamma knife surgery or microsurgery for vestibular schwannomas. J Neurosurg. 2002;97:1091-1100.
Rowe J.G., Radatz M.W., Walton L., et al. Clinical experience with gamma knife stereotactic radiosurgery in the management of vestibular schwannomas secondary to type 2 neurofibromatosis. J Neurol Neurosurg Psychiatry. 2003;74:1288-1293.
1. Tos M., Stangerup S.E., Caye-Thomasen P., et al. What is the real incidence of vestibular schwannoma? Arch Otolaryngol Head Neck Surg. 2004;130:216-220.
2. Lanser M.J., Sussman S.A., Frazer K. Epidemiology, pathogenesis, and genetics of acoustic tumors. Otolaryngol Clin North Am. 1992;25:499-520.
3. Kentala E., Pyykko I. Clinical picture of vestibular schwannoma. Auris Nasus Larynx. 2001;28:15-22.
4. Charabi S., Tos M., Thomsen J., et al. Vestibular schwannoma growth—long-term results. Acta Otolaryngol Suppl. 2000;543:7-10.
5. Sugihara S., Kinoshita T., Matsusue E., et al. Multicystic acoustic schwannoma with intratumoral hemorrhage: a report of two cases. Magn Reson Med Sci. 2004;3:101-104.
6. Hoistad D.L., Melnik G., Mamikoglu B., et al. Update on conservative management of acoustic neuroma. Otol Neurotol. 2001;22:682-685.
7. Walsh R.M., Bath A.P., Bance M.L., et al. The role of conservative management of vestibular schwannomas. Clin Otolaryngol Allied Sci. 2000;25:28-39.
8. Raut V.V., Walsh R.M., Bath A.P., et al. Conservative management of vestibular schwannomas—second review of a prospective longitudinal study. Clin Otolaryngol Allied Sci. 2004;29:505-514.
9. Glasscock M.E.3rd, Kveton J.F., Jackson C.G., et al. A systematic approach to the surgical management of acoustic neuroma. Laryngoscope. 1986;96:1088-1094.
10. Gormley W.B., Sekhar L.N., Wright D.C., et al. Acoustic neuromas: results of current surgical management. Neurosurgery. 1997;41:50-58. discussion 58-60
11. Samii M., Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): the facial nerve—preservation and restitution of function. Neurosurgery. 1997;40:684-694. discussion 694-695
12. Matthies C., Samii M. Management of vestibular schwannomas (acoustic neuromas): the value of neurophysiology for intraoperative monitoring of auditory function in 200 cases. Neurosurgery. 1997;40:459-466. discussion 466-468
13. Myrseth E., Moller P., Pedersen P.H., et al. Vestibular schwannomas: clinical results and quality of life after microsurgery or gamma knife radiosurgery. Neurosurgery. 2005;56:927-935. discussion 927-935
14. Zhang X., Fei Z., Chen Y.J., et al. Facial nerve function after excision of large acoustic neuromas via the suboccipital retrosigmoid approach. J Clin Neurosci. 2005;12:405-408.
15. Kanzaki J., Tos M., Sanna M., et al. New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol Neurotol. 2003;24:642-648. discussion 648-649
16. Samii M., Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): hearing function in 1000 tumor resections. Neurosurgery. 1997;40:248-260. discussion 260-262
17. Gardner G., Robertson J.H. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988;97:55-66.
18. Chee G.H., Nedzelski J.M., Rowed D. Acoustic neuroma surgery: the results of long-term hearing preservation. Otol Neurotol. 2003;24:672-676.
19. Shelton C., Hitselberger W.E., House W.F., Brackmann D.E. Hearing preservation after acoustic tumor removal: long-term results. Laryngoscope. 1990;100:115-119.
20. Brennan J.W., Rowed D.W., Nedzelski J.M., Chen J.M. Cerebrospinal fluid leak after acoustic neuroma surgery: influence of tumor size and surgical approach on incidence and response to treatment. J Neurosurg. 2001;94:217-223.
21. Slattery W.H.3rd, Francis S., House K.C. Perioperative morbidity of acoustic neuroma surgery. Otol Neurotol. 2001;22:895-902.
22. Maw A.R., Coakham H.B., Ayoub O., Butler S.R. Hearing preservation and facial nerve function in vestibular schwannoma surgery. Clin Otolaryngol Allied Sci. 2003;28:252-256.
23. Sanna M., Khrais T., Russo A., et al. Hearing preservation surgery in vestibular schwannoma: the hidden truth. Ann Otol Rhinol Laryngol. 2004;113:156-163.
24. Bani A., Gilsbach J.M. Incidence of cerebrospinal fluid leak after microsurgical removal of vestibular schwannomas. Acta Neurochir (Wien). 2002;144:979-982. discussion 982
25. Sanna M., Taibah A., Russo A., et al. Perioperative complications in acoustic neuroma (vestibular schwannoma) surgery. Otol Neurotol. 2004;25:379-386.
26. Selesnick S.H., Liu J.C., Jen A., Newman J. The incidence of cerebrospinal fluid leak after vestibular schwannoma surgery. Otol Neurotol. 2004;25:387-393.
27. Wackym P.A., King W.A., Poe D.S., et al. Adjunctive use of endoscopy during acoustic neuroma surgery. Laryngoscope. 1999;109:1193-1201.
28. Elsmore A.J., Mendoza N.D. The operative learning curve for vestibular schwannoma excision via the retrosigmoid approach. Br J Neurosurg. 2002;16:448-455.
29. Samii M., Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery. 1997;40:11-21. discussion 21-23
30. Schmerber S., Palombi O., Boubagra K., et al. Long-term control of vestibular schwannoma after a translabyrinthine complete removal. Neurosurgery. 2005;57:693-698. discussion 693-698
31. El-Kashlan H.K., Zeitoun H., Arts H.A., et al. Recurrence of acoustic neuroma after incomplete resection. Am J Otol. 2000;21:389-392.
32. Blomstedt G.C., Katila H., Henriksson M., et al. Depression after surgery for acoustic neuroma. J Neurol Neurosurg Psychiatry. 1996;61:403-406.
33. Cabral R., King T.T., Scott D.F. Incidence of postoperative epilepsy after a transtentorial approach to acoustic nerve tumours. J Neurol Neurosurg Psychiatry. 1976;39:663-665.
34. Driscoll C.L., Beatty C.W. Pain after acoustic neuroma surgery. Otolaryngol Clin North Am. 1997;30:893-903.
35. Duane D.T., Howard S.J., Kraayenbrink M. Incidence and predictors of bulbar palsy after surgery for acoustic neuroma. J Neurosurg Anesthesiol. 1997;9:263-268.
36. Glasscock M.E.3rd, Hays J.W., Murphy J.P. Complications in acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1975;84:530-540.
37. House W.F. Fatalities in acoustic tumor surgery. Arch Otolaryngol. 1968;88:687-699.
38. Sterkers J.M. [Life-threatening complications and severe neurologic sequelae in surgery of acoustic neurinoma]. Ann Otolaryngol Chir Cervicofac. 1989;106:245-250.
39. Wiegand D.A., Fickel V. Acoustic neuroma—the patient’s perspective: subjective assessment of symptoms, diagnosis, therapy, and outcome in 541 patients. Laryngoscope. 1989;99:179-187.
40. Bateman N., Nikolopoulos T.P., Robinson K., O’Donoghue G.M. Impairments, disabilities, and handicaps after acoustic neuroma surgery. Clin Otolaryngol Allied Sci. 2000;25:62-65.
41. Leksell L. A note on the treatment of acoustic tumours. Acta Chir Scand. 1971;137:763-765.
42. Linskey M.E. Stereotactic radiosurgery versus stereotactic radiotherapy for patients with vestibular schwannoma: a Leksell Gamma Knife Society 2000 debate. J Neurosurg. 2000;93(Suppl 3):90-95.
43. Chung W.Y., Liu K.D., Shiau C.Y., et al. Gamma knife surgery for vestibular schwannoma: 10-year experience of 195 cases. J Neurosurg. 2005;102(Suppl):87-96.
44. Flickinger J.C., Kondziolka D., Niranjan A., et al. Acoustic neuroma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2004;60:225-230.
45. Hasegawa T., Kida Y., Kobayashi T., et al. Long-term outcomes in patients with vestibular schwannomas treated using gamma knife surgery: 10-year follow-up. J Neurosurg. 2005;102:10-16.
46. Kondziolka D., Lunsford L.D., Flickinger J.C. Acoustic neuroma radiosurgery. Origins, contemporary use and future expectations. Neuro-Chirurgie. 2004;50:427-435.
47. Kondziolka D., Nathoo N., Flickinger J.C., et al. Long-term results after radiosurgery for benign intracranial tumors [see comment]. Neurosurgery. 2003;53:815-821. discussion 821-822
48. Lunsford L.D., Niranjan A., Flickinger J.C., et al. Radiosurgery of vestibular schwannomas: summary of experience in 829 cases. J Neurosurg. 2005;102(Suppl):195-199.
49. Delbrouck C., Hassid S., Massager N., et al. Preservation of hearing in vestibular schwannomas treated by radiosurgery using Leksell gamma knife: preliminary report of a prospective Belgian clinical study. Acta Otorhinolaryngol Belg. 2003;57:197-204.
50. Flickinger J.C., Kondziolka D., Niranjan A., Lunsford L.D. Results of acoustic neuroma radiosurgery: an analysis of 5 years’ experience using current methods [see comment]. J Neurosurg. 2001;94:1-6.
51. Flickinger J.C., Kondziolka D., Pollock B.E., Lunsford L.D. Evolution in technique for vestibular schwannoma radiosurgery and effect on outcome. Int J Radiat Oncol Biol Phys. 1996;36:275-280.
52. Friedman W.A., Bradshaw P., Myers A., Bova F.J. Linear accelerator radiosurgery for vestibular schwannomas. J Neurosurg. 2006;105:657-661.
53. Harsh G.R., Thornton A.F., Chapman P.H., et al. Proton beam stereotactic radiosurgery of vestibular schwannomas. Int J Radiat Oncol Biol Phys. 2002;54:35-44.
54. Horstmann G.A., Van Eck A.T. Gamma knife model C with the automatic positioning system and its impact on the treatment of vestibular schwannomas. J Neurosurg. 2002;97:450-455.
55. Inoue H.K. Low-dose radiosurgery for large vestibular schwannomas: long-term results of functional preservation. J Neurosurg. 2005;102(Suppl):111-113.
56. Ishihara H., Saito K., Nishizaki T., et al. CyberKnife radiosurgery for vestibular schwannoma. Minim Invasive Neurosurg. 2004;47:290-293.
57. Kondziolka D., Lunsford L.D., Flickinger J.C. Gamma knife radiosurgery for vestibular schwannomas. Neurosurg Clin North Am. 2000;11:651-658.
58. Kondziolka D., Lunsford L.D., McLaughlin M.R., Flickinger J.C. Long-term outcomes after radiosurgery for acoustic neuromas [see comment]. N Engl J Med. 1998;339:1426-1433.
59. Linskey M.E., Johnstone P.A. Radiation tolerance of normal temporal bone structures: implications for gamma knife stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;57:196-200.
60. Linskey M.E., Lunsford L.D., Flickinger J.C. Tumor control after stereotactic radiosurgery in neurofibromatosis patients with bilateral acoustic tumors. Neurosurgery. 1992;31:829-838. discussion 838-839
61. Lunsford L.D. Vestibular schwannomas. Neuro-Chirurgie. 2004;50:151-152.
62. Meijer O.W., Wolbers J.G., Vandertop W.P., Slotman B.J. Stereotactische bestraling van het vestibulair schwannoom (acusticusneurinoom). Ned Tijdschr Geneeskunde. 2000;144:2088-2093.
63. Noren G. Long-term complications following gamma knife radiosurgery of vestibular schwannomas. Stereotact Funct Neurosurg. 1998;70(Suppl 1):65-73.
64. Petit J.H., Hudes R.S., Chen T.T., et al. Reduced-dose radiosurgery for vestibular schwannomas. Neurosurgery. 2001;49:1299-1306. discussion 1306-1307
65. Lobato-Polo J., Kondziolka D., Zorro O., et al. Gamma knife radiosurgery in younger patients with vestibular schwannomas. Neurosurgery. 2009;65:294-301.
66. Niranjan A., Lunsford L.D., Flickinger J.C., et al. Dose reduction improves hearing preservation rates after intracanalicular acoustic tumor radiosurgery. Neurosurgery. 1999;45:753-762. discussion 762-765
67. Kano H., Kondziolka D., Flickinger J.C., Lunsford L.D. Predictors of hearing preservation after stereotactic radiosurgery for acoustic neuromas. J Neurosurg. 2009;111:863-873.
68. Subach B.R., Kondziolka D., Lunsford L.D., et al. Stereotactic radiosurgery in the management of acoustic neuromas associated with neurofibromatosis Type 2 [see comment]. J Neurosurg. 1999;90:815-822.
69. Roche P.H., Robitail S., Thomassin J.M., et al. Radiochirurgie gamma knife des schwannomes vestibulaires associes a une neurofibromatose de type 2. Neuro-Chirurgie. 2004;50:367-376.
70. Rowe J.G., Radatz M.W., Walton L., et al. Clinical experience with gamma knife stereotactic radiosurgery in the management of vestibular schwannomas secondary to type 2 neurofibromatosis. J Neurol Neurosurg Psychiatry. 2003;74:1288-1293.
71. Mathieu D., Kondziolka D., Flickinger J.C., et al. Stereotactic radiosurgery for vestibular schwannomas in patients with neurofibromatosis type 2: an analysis of tumor control, complications, and hearing preservation rates. Neurosurgery. 2007;60:460-468. discussion 468-470
72. Weber D.C., Chan A.W., Bussiere M.R., et al. Proton beam radiosurgery for vestibular schwannoma: tumor control and cranial nerve toxicity. Neurosurgery. 2003;53:577-586. discussion 586-588
73. Suh J.H., Barnett G.H., Sohn J.W., et al. Results of linear accelerator-based stereotactic radiosurgery for recurrent and newly diagnosed acoustic neuromas. Int J Cancer. 2000;90:145-151.
74. Spiegelmann R., Lidar Z., Gofman J., et al. Linear accelerator radiosurgery for vestibular schwannoma [see comment]. J Neurosurg. 2001;94:7-13.
75. Meijer O.W., Wolbers J.G., Baayen J.C., Slotman B.J. Fractionated stereotactic radiation therapy and single high-dose radiosurgery for acoustic neuroma: early results of a prospective clinical study. Int J Radiat Oncol Biol Phys. 2000;46:45-49.
76. Poen J.C., Golby A.J., Forster K.M., et al. Fractionated stereotactic radiosurgery and preservation of hearing in patients with vestibular schwannoma: a preliminary report. Neurosurgery. 1999;45:1299-1305. discussion 1305-1307
77. Sakamoto T., Shirato H., Takeichi N., et al. Annual rate of hearing loss falls after fractionated stereotactic irradiation for vestibular schwannoma. Radiother Oncol. 2001;60:45-48.
78. Sawamura Y., Shirato H., Sakamoto T., et al. Management of vestibular schwannoma by fractionated stereotactic radiotherapy and associated cerebrospinal fluid malabsorption. J Neurosurg. 2003;99:685-692.
79. Shirato H., Sakamoto T., Takeichi N., et al. Fractionated stereotactic radiotherapy for vestibular schwannoma (VS): comparison between cystic-type and solid-type VS. Int J Radiat Oncol Biol Phys.. 2000;48:1395-1401.
80. Szumacher E., Schwartz M.L., Tsao M., et al. Fractionated stereotactic radiotherapy for the treatment of vestibular schwannomas: combined experience of the Toronto-Sunnybrook Regional Cancer Centre and the Princess Margaret Hospital. Int J Radiat Oncol Biol Phys.. 2002;53:987-991.
81. Fuss M., Debus J., Lohr F., et al. Conventionally fractionated stereotactic radiotherapy (FSRT) for acoustic neuromas. Int J Radiat Oncol Biol Phys. 2000;48:1381-1387.
82. Chung H.T., Ma R., Toyota B., et al. Audiologic and treatment outcomes after linear accelerator-based stereotactic irradiation for acoustic neuroma. Int J Radiat Oncol Biol Phys. 2004;59:1116-1121.
83. Meijer O.W., Vandertop W.P., Baayen J.C., Slotman B.J. Single-fraction vs. fractionated linac-based stereotactic radiosurgery for vestibular schwannoma: a single-institution study [see comment]. Int J Radiat Oncol Biol Phys. 2003;56:1390-1396.
84. Andrews D., Werner-Wasik M., Den R., et al. Toward dose optimization for fractionated stereotactic radiotherapy for acoustic neuromas: comparison of two dose cohorts. Int J Radiat Oncol Biol Phys. 2009;74:419-426.
85. Couldwell W.T., Mohan A.L. Enlargement of a vestibular schwannoma after stereotactic radiotherapy. Acta Neurochir. 2002;144:1319-1322.
86. Nikolopoulos T.P., O’Donoghue G.M. Acoustic neuroma management: an evidence-based medicine approach. Otol Neurotol. 2002;23:534-541.
87. Karpinos M., Teh B.S., Zeck O., et al. Treatment of acoustic neuroma: stereotactic radiosurgery vs. microsurgery. Int J Radiat Oncol Biol Phys. 2002;54:1410-1421.
88. Pollock B.E., Lunsford L.D., Kondziolka D., et al. Outcome analysis of acoustic neuroma management: a comparison of microsurgery and stereotactic radiosurgery. Neurosurgery. 1995;36:215-224. discussion 224-229 [erratum in Neurosurgery. 1995;36:427]
89. Regis J., Pellet W., Delsanti C., et al. Functional outcome after gamma knife surgery or microsurgery for vestibular schwannomas. J Neurosurg. 2002;97:1091-1100.
90. Pollock B.E., Driscoll C.L., Foote R.L., et al. Patient outcomes after vestibular schwannoma management: a prospective comparison of microsurgical resection and stereotactic radiosurgery. Neurosurgery. 2006;59:77-85.